Abstract
When pain or disability occurs after rotator cuff surgery, post-operative imaging is frequently performed. Post-operative complications and expected post-operative imaging findings in the shoulder are presented, with a focus on MRI, MR arthrography (MRA) and CT arthrography. MR and CT techniques are available to reduce image degradation secondary to surgical distortions of native anatomy and implant-related artefacts and to define complications after rotator cuff surgery. A useful approach to image the shoulder after surgery is the standard radiography, followed by MRI/MRA for patients with low “metal presence” and CT for patients who have a higher metal presence. However, for the assessment of patients who have undergone surgery for rotator cuff injuries, imaging findings should always be correlated with the clinical presentation because post-operative imaging abnormalities do not necessarily correlate with symptoms.
The complexity of the anatomy and function of the rotator cuff makes the rotator cuff tendons vulnerable to considerable morbidity, often necessitating surgical intervention. Optimal management of rotator cuff abnormalities depends on a variety of factors, such as the presence and severity of an impingement, the degree of tendon damage and individual functional demands.1 The goals of rotator cuff surgery are to reduce pain, while simultaneously improving the function. The latter is accomplished by two main types of surgical procedures: (1) subacromial decompression surgery alone, typically with an acromioplasty and/or Mumford procedure (distal clavicular resection); and (2) repair of the rotator cuff tear (open or arthroscopic), which is almost always accompanied by a subacromial decompression.
Post-operative imaging is performed when pain or disability occurs after a surgical procedure. Often, however, post-operative imaging is degraded by surgical distortions of the native anatomy and metallic artefacts related to implants. Nevertheless, it is imperative that clinicians have an accurate anatomical delineation of the operative site. It is also important for the radiologist to accurately diagnose complications that might occur after rotator cuff surgery to guide optimal treatment. Mansat et al2 examined 40 articles reporting the results of open rotator cuff repairs and determined that the overall mean complication rate was 10.5%.
The article addresses complications that occur after rotator cuff surgery and expected post-operative imaging findings, with a focus on MRI, MR arthrography (MRA) and CT arthrography (CTA). Because not all post-operative imaging findings result in disability or pain for the patient, we also emphasize our approach and experience regarding how best to define imaging abnormalities after rotator cuff surgery.
IMAGING APPROACH
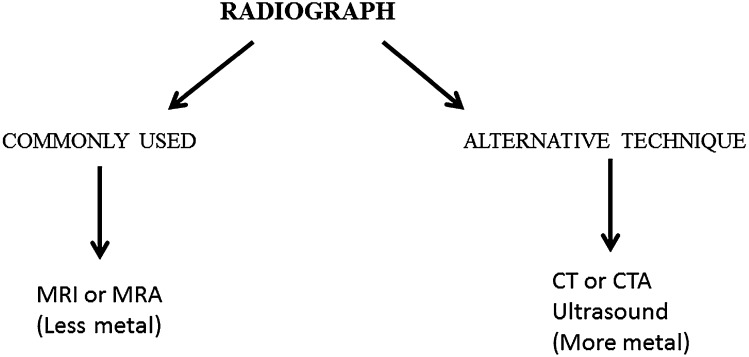
A protocol for the imaging of the post-operative shoulder always begins with radiography of the shoulder. The purpose of the radiograph is not only to detect obvious osseous complications, but also to identify the type of surgical procedure performed and to assess the amount of metallic implantation that may be present. The latter is important in guiding additional cross-sectional imaging toward MRI (for patients in whom little metallic hardware has been used) or toward CTA (for patients with more metallic hardware). Ultrasonography is an extremely valuable cross-sectional imaging alternative to the evaluation of the rotator cuff tendons3 and is discussed later; but, because ultrasonography does not provide a global view of the post-operative shoulder, it is performed less commonly than MRI or MRA for patients with shoulder issues at our institution (Johns Hopkins University School of Medicine, Baltimore, MD) (Figure 1).
Figure 1.
Approach to imaging after a rotator cuff surgery. CTA, CT angiography; MRA, MR arthrography.
MRI plays an active role in post-operative shoulder imaging because of its superior contrast resolution. As is done for the native shoulder, a surface coil should be used for maximum signal-to-noise ratio, and the imaging planes are similar to those of routine shoulder imaging, with axial, coronal oblique and sagittal oblique perspectives. For full-thickness (or complete) recurrent tears confirmed at surgery, Owen et al4 reported 86% sensitivity and 92% specificity for MRI. However, although the subacromial space and rotator cuff are exquisitely evaluated in the native shoulder, the presence of susceptibility artefact after subacromial decompression and surgical repair might sometimes limit the evaluation of the rotator cuff, especially when modifications to the MRI protocol to reduce artefacts related to metal are not made. The degree of artefact production in the presence of metal is related to the quantity of the metal, its geometry and MRI acquisition parameters. Artefacts are the greatest with the presence of ferromagnetic screws or staples (rather than titanium materials) and small metal shavings from the use of a burr during acromioplasty. Hence, alterations to the MRI protocol are needed to reduce artefacts in the post-operative shoulder (Table 1). For example, simple pulse sequence changes can be implemented with the substitution of gradient-echo sequences with fast spin-echo imaging. In addition, frequency-selective fat suppression might not produce uniform loss of fat signals in the setting of metal because of local changes in the magnetic field around the site of the metal; although frequency-selective fat suppression is still used in post-operative shoulders, a comparison with non-fat-suppressed techniques is optimal, and inversion-recovery sequences should be considered in the presence of extensive metal artefact. With regard to the magnetic field strength, clinical MRI at 3.0 T is finding increasing use, and it is feasible in the post-operative shoulder as long as the metal presence is not extensive. Along with the advantage of increased signal-to-noise ratio at 3.0 T, there are drawbacks for the post-operative shoulder because of increased susceptibility to artefacts.5 In particular, after extensive burr use for subacromial decompression, artefacts can substantially obstruct interpretation, and the scan may be better suited for 1.5 T. Otherwise, tendon sutures and anchors usually produce artefact that does not substantially interfere with image interpretation. A sample protocol would require approximately 30 min for completion (Table 2).
Table 1.
Potential modifications to CT and MRI technique in the presence of metal artefact after surgery for rotator cuff disease
| Technique | Modifications |
|---|---|
| CT | Acquisition factors to be considered:a decrease pitch; increase peak kilovoltage; increase milliamperage per second; use large focal spot |
| Reconstruction algorithms: use soft tissue filter; reconstruct thicker slices, iterative reconstruction | |
| Display: use wide display window; create multiplanar three-dimensional images to reduce the display of artefact | |
| MRI | 3 T is feasible, but with extensive metal artefact use 1.5 T |
| Avoid parallel imaging as there is a reduction in signal-to-noise ratio | |
| Pulse sequence choice: | |
| Fast spin echo favoured over gradient echo (although gradient echo is not as commonly used in shoulder protocols) | |
| Intermediate-weighted images are used for maximal signal-to-noise ratio, with echo times in the mid-30 s to mid-40 s | |
| Fat-suppression technique choice: | |
| Frequency selective fat suppression is feasible, but always compared with non-fat-suppressed images | |
| Inversion recovery favoured over frequency-selective fat suppression with the presence of heavy metal; however, in many cases, fat-suppressed imaging can still be used without sacrificing detail around the rotator cuff | |
| Pulse sequence parameter choices: | |
| Frequency and phase direction can be swapped | |
| Use longer echo train length | |
| Use shorter echo time | |
| Field of view, slice thickness, and matrix size altered to create small voxels (high spatial resolution) | |
| Increase bandwidth | |
| Increase signal averages | |
| Consider contrast (MR arthrography) | |
| Consider another modality: CT or ultrasound |
Other modifications to the reconstruction and display should be favoured over increasing the dose.
Modifications to acquisition parameters should be made sparingly, as these increase the radiation dose delivered to the patient.
Table 2.
Sample MRI protocol for assessment of the post-operative rotator cuffa
| Pulse sequence | Utilityb |
|---|---|
| Anatomic sequences (provide high SNR and spatial resolution) | |
| Axial intermediate weighted | Assess tendons (particularly subscapularis), acromioclavicular anatomy, heterotopic ossification |
| Coronal intermediate weighted (substitute T1 weighted imaging for MRA) | Assess tendons (particularly supraspinatus/infraspinatus), acromial arch anatomy, heterotopic ossification |
| Sagittal intermediate-weighted (substitute T1 weighted imaging for MRA) | Assess cuff tendons, acromial arch anatomy, adhesive capsulitis, heterotopic ossification, muscle bulk |
| Fluid-sensitive sequences (show fluid around abnormalities to best advantage) | |
| Coronal fat-suppressed intermediate-weighted images (or STIR if metal artefact is heavy) | Assess cuff tendons (supraspinatus, infraspinatus primarily), deltoid, joint fluid/synovitis, BME |
| Sagittal fat-suppressed intermediate-weighted images (or STIR if metal artefact is heavy) | Assess cuff tendons, deltoid tendon and muscle, BME |
| Optional: axial images in abduction–internal rotation | For assessment of the post-operative labrum |
| Optional: fat-suppressed T2 weighted images for added sensitivity to fluid signal, but results in decreased SNR | Assess cuff tendons and muscles, BME, joint fluid/synovitis |
BME, bone marrow oedema-like signal; MRA, MR arthrography; SNR, signal-to-noise ratio; STIR, short tau inversion recovery.
This table does not comprehensively describe the utility of these sequences for assessing all shoulder structures (such as the labrum and biceps tendon).
The utility column describes features of the post-operative rotator cuff, including the cuff tendons and acromial arch anatomy.
MRA may be advantageous in providing distension of the joint capsule, enabling further assessment of the articular surface of the rotator cuff and, on occasions, also the bursal surface, given that a contrast can enter the subacromial bursa because a repair of the rotator cuff tendon is not watertight, allowing intra-articular contrast to flow into the subacromial/subdeltoid space.1,6 With MRA, post-operative inflammation and scarring may be distinguished from recurrent partial articular-side tears,7 a distinction not easily made by non-contrast fluid-sensitive sequences. Hence, although MRA is not advocated at some centres, many authors suggest MRA as a useful tool to help image the post-operative patient successfully.8–10
CT plays an important role in identifying the position of the sutures and evaluating the osseous structures in the post-operative shoulder. CTA is an alternative to MRI and MRA. In particular, CTA may be performed when MRI or MRA is contraindicated or seriously restricted by metallic hardware (Figure 2). In the post-operative patient, CTA detects rotator cuff and capsulolabral complex injuries, with accuracies of 94% compared with arthroscopy, whereas the accuracies of MRI were approximately 25%, according to one study.11 CTA exploits the difference produced at the interface between intra-articular iodinated contrast injected into the joint and the adjacent lower density tendon. Like MRI and MRA, CTA provides a global assessment of the shoulder anatomy but has the important advantages of rapid scan times and submillimeter resolution.12 In patients with metal implants, several modifications can be made to CT protocols in terms of the acquisition and reconstruction options to reduce artefact associated with the presence of metal (Table 1).13,14 Indirect CTA or MRA can also be performed with intravenous (i.v.) contrast, but in our experience, the use of indirect CTA is limited to the detection of synovitis.8
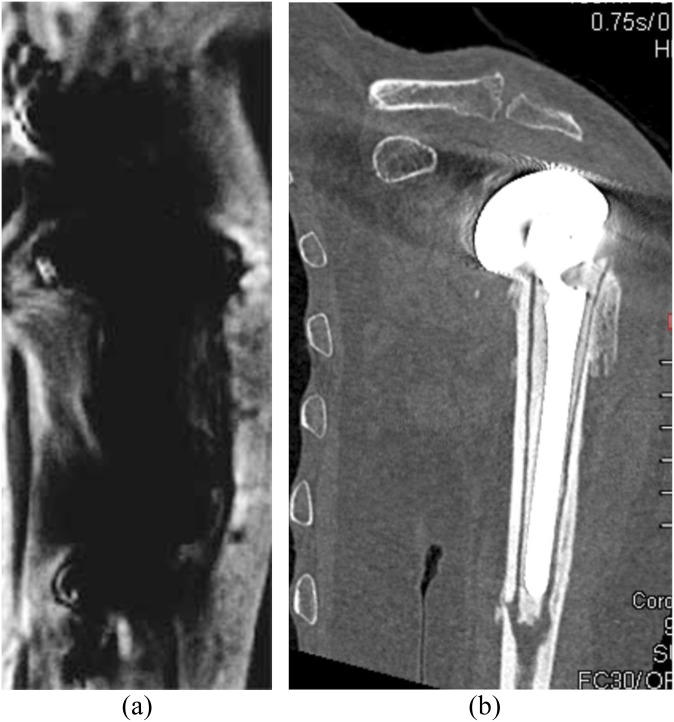
Figure 2.
A 95-year-old male with a shoulder prosthesis underwent MRI and CT for symptoms of pain after a non-syncopal fall. (a) The coronal MRI view shows extensive artefact, which rendered the study non-diagnostic. (b) The coronal CT was not affected with artefacts and shows the prosthesis and periprosthetic fractures.
Ultrasonography is an excellent alternative modality for evaluating the post-operative rotator cuff, because it is not subject to artefact from metallic implants.15 Prickett et al16 showed that ultrasonography has a sensitivity of 91%, a specificity of 89% and an accuracy of 89% for identifying rotator cuff integrity after surgery. The significance of an ultrasound abnormality may be correlated clinically by directly questioning the patient and observing tendon movement dynamically. However, it is an operator-dependent technique and is also limited because it does not provide a comprehensive assessment of other portions of the shoulder joint.17
EXPECTED POST-OPERATIVE FINDINGS
Osseous findings
By radiography, several osseous changes can be identified after surgery for the rotator cuff, including those related to subacromial decompression (such as distal clavicular resection or acromioplasty) and those related to rotator cuff repair (with the identification of the positions of metallic implants that may be present) (Figures 3–5).
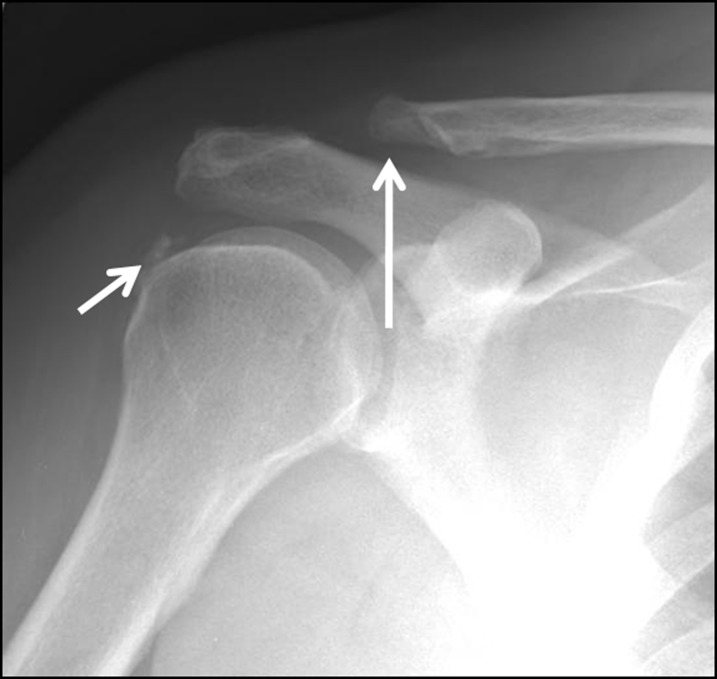
Figure 3.
The radiograph of a 59-year-old male after subacromial decompression surgery. There is lateral resection of the clavicle (Mumford procedure) (large arrow) accompanied by a partial acromioplasty. Note the calcific tendinitis of the infraspinatus tendon (small arrow).
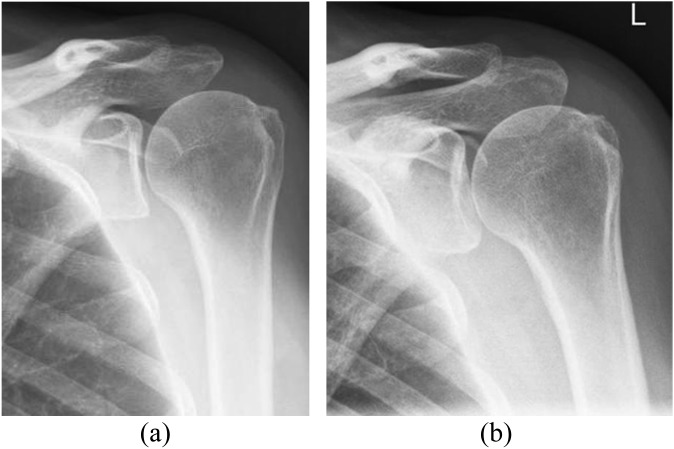
Figure 5.
Two frontal views obtained before (a) and after (b) subacromial decompression. Osseous changes are subtle, with subcortical lucency and slight irregularity at the anterolateral acromion. The distal clavicle has not been resected. L, left.
Figure 4.
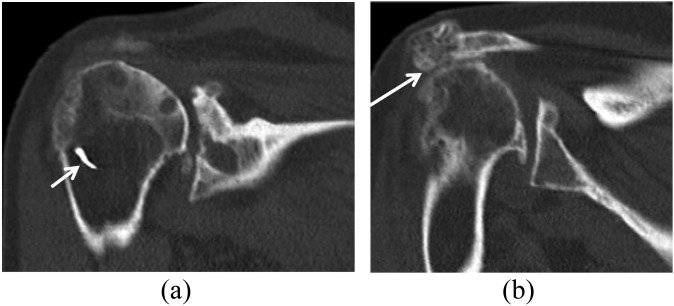
Radiographic findings of a 75-year-old female with a history of subacromial decompression and a failed rotator cuff repair. (a) Coronal CT image showing the position of the metallic suture anchor within the bone distal to the greater tuberosity (arrow) and superior migration of the humerus and glenohumeral joint osteoarthrosis, with extensive cyst formation along the articular surface of the acromion and humeral head. (b) Coronal CT image shows reformation of the lateral acromial osteophyte (arrow).
Figure 6.
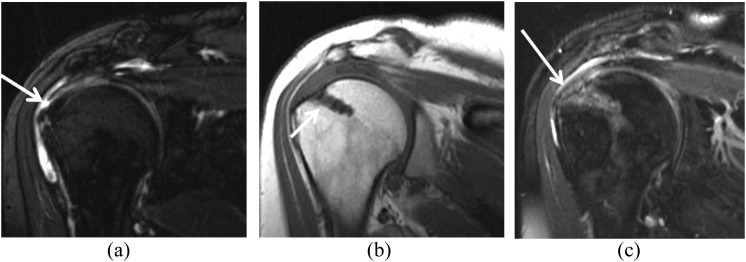
Common soft-tissue findings on MRI of a middle-aged male before and after rotator cuff repair and subacromial decompression. (a) Pre-operative fat-suppressed T2 weighted (repetition time/echo time, 3380/90 ms) coronal image showing a small full-thickness tear of the supraspinatus tendon (arrow). (b) After rotator cuff repair with subacromial decompression, T1 weighted (repetition time/echo time, 530/10 ms) coronal view shows a suture anchor (arrow) as a susceptibility artefact related to the presence of a bioabsorbable anchor. (c) Fat-suppressed T2 weighted (repetition time/echo time, 3380/90 ms) coronal image shows intermediate signal in the tendon, a common and expected finding after surgery (arrow).
Figure 7.
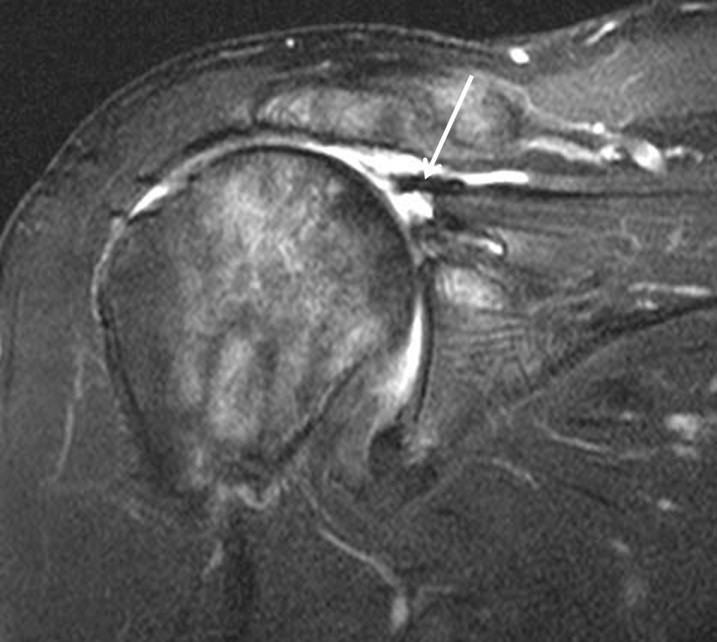
A clinically asymptomatic 54-year-old female after acromioplasty and rotator cuff repair. (a) T1 weighted (repetition time/echo time, 530/10 ms) sagittal image shows a diminutive undersurface of the acromion related to acromioplasty (arrow). An excessively thinned acromion is susceptible to fracture and can be further evaluated if necessary with CT. (b) Fat-suppressed intermediate-weighted (repetition time/echo time, 3200/45 ms) coronal image obtained after surgery shows fluid in the subacromial–subdeltoid bursa (arrow).
Several post-operative osseous changes are expected to be seen on cross-sectional imaging. First, mild superior subluxation of the humeral head can be the result of capsular tightening, scarring, rotator cuff atrophy or bursectomy. Second, along the humeral head in patients with tendon-to-bone repair, a surgically created trough with associated susceptibility artefacts may be observed in the insertion zone, proximal to the native insertion on the greater tuberosity.18 Bone marrow oedema-like signals might also be observed in the humeral head, sometimes from artefacts (secondary to a failure of fat suppression) or as a result of residual cystic changes in the greater tuberosity.19 Third, in the acromion, decreased signal intensity on both short echo-time (T1 or intermediate weighted) and long echo-time (T2 weighted) images represents fibrosis in the acromial marrow.1 An important cause of poor outcome after rotator cuff surgery is the failure to recognize pathology in other areas around the cuff, such as the acromioclavicular joint, which may be the aetiology of new post-operative symptoms. However, reactive bone marrow oedema in the distal clavicle or the acromion is a more reliable predictor of pathology in the acromioclavicular joint,20 marrow oedema around the acromioclavicular joint is not a predictor of rotator cuff pathology. Characteristic imaging findings after acromioplasty include a flattened acromial undersurface with loss of the anterior acromion. CTA will show the osseous changes of subacromial decompression with little artefact, but with MRI, low-signal artefacts from microscopic metallic fragments are common and may be exuberant, limiting interpretation of the rotator cuff.17 With a Mumford procedure, the amount of susceptibility artefact is often less.
Soft-tissue findings
On MRI, there are several findings that may be identified in the post-operative rotator cuff that are sequelae of a surgery and are not commonly associated with patient symptoms; in particular, the foci of increased signal intensity within the rotator cuff seen on short echo-time sequences are considered non-specific findings, which might represent frank tears or granulation tissues.19 For the distinction of the latter entities, techniques to separate T2 signal intensities might prove useful, but they have not been studied as yet in the post-operative shoulder. On fluid-sensitive sequences, frank fluid signal intensity within the post-operative rotator cuff tendons can be an asymptomatic finding:19 according to one study, the morphologic alterations of a post-operative rotator cuff might persist for several years after surgical repair, with 20%–50% of tendons having a visible tendon defect for years.21 Thus, the presence of a defect at the site of repair may not be clinically significant, and all post-operative tears found with MRI should be correlated with symptoms and an examination of the shoulder by a clinician.
The inspection of a shoulder for signs of muscle atrophy is an important part of the post-operative evaluation. First, the radiologist must be aware that the remaining rotator cuff muscle belly may be pulled laterally during reconstruction, resulting in the appearance of erroneous muscle enlargement on parasagittal images of the repaired rotator cuff compared with images of the rotator cuff muscles obtained before surgery.17 It should also be noted that muscle atrophy is typically not reversed with rotator cuff repair.22 However, the functional outcome after rotator cuff repair is determined by muscle atrophy and fatty infiltration of the muscle, which are in turn linked to the success of the repair procedure. A successful repair does not lead to improvement or reversal of muscle degeneration, and a failed repair might cause substantial progression of atrophy and fatty infiltration.23,24 As a result, observing the progression of atrophy after a rotator cuff repair is a helpful sign for the clinician who is trying to determine the success or failure of the repair. With MRI and CT, the presence of atrophy and fatty infiltration can be assessed reliably and quantitatively.25
Evaluation of the peritendinous region subacromial space after surgery often shows signal abnormalities that are related to the surgical procedure performed. The procedure is typically either a bursectomy alone or a bursectomy with partial acromioplasty. The indications for each are controversial, but acromioplasty has not been found to improve surgical results.26 With surgical disruption of the tissues around the rotator cuff, the absence of subacromial peribursal fat is a common but clinically irrelevant finding. Similarly, fluid in the subacromial bursa is a normal post-operative feature and cannot be used reliably as a secondary sign of a full-thickness tear or bursitis in the absence of bursectomy. Fluid in the subacromial bursal region might persist for many years. In one study of 14 asymptomatic patients with rotator cuff repair and a mean follow-up period of 40 months (range, 24–49 months), the prevalence of bursitis-like abnormalities was 100%.27 Similarly, a mild-to-moderate joint effusion is not uncommon in the post-operative shoulder, but large effusions might indicate other processes such as synovitis or infection.
COMPLICATIONS AFTER ROTATOR CUFF SURGERY
Complications after surgery are classified into general complications related to surgery and specific complications related to rotator cuff repair.28 Mansat et al2 identified the following risk factors for developing a complication: large (3–5 cm) rotator cuff tears; advanced age; pre-operative limitations in motion; weakness in abduction, internal rotation and flexion; and a diminished acromiohumeral interval. We address below the imaging features of complications specifically related to rotator cuff repair (Table 3).
Table 3.
Post-operative findings (expected) and complications after rotator cuff surgery
| Parameter | Imaging modality | Appearance on imaging |
|---|---|---|
| Expected post-operative finding | ||
| Presence of metal implants | Radiographs/CT | Identification of implants and sutures |
| Osseous changes of subacromial decompression | Radiographs/CT/MRI | Distal clavicle resection |
| Acromioplasty: flattened acromial undersurface with loss of the anterior acromion | ||
| Superior subluxation of humeral head | ||
| Bursectomy | CT/MRI | Absence of subacromial peribursal fat |
| Persistent fluid in subacromial bursal region | ||
| Expected post-operative rotator cuff tendon appearance | MRI | Foci of intermediate (less the fluid or intra-articular contrast) intensity in tissue secondary to granulation tissue |
| Tendon-to-bone repair | Radiographs/CT/MRI | Surgical trough in humeral head |
| Suture anchors at tendon–bone interface | ||
| Tendon-to-tendon repair | Radiographs/CT/MRI | Suture placement within tendons |
| Expected post-operative muscle changes | CT/MRI | Apparent muscle enlargement on parasagittal images as muscle pulled laterally during reconstruction |
| Muscle fatty infiltration/atrophy if present before surgery | ||
| Post-operative complication | ||
| Recurrent rotator cuff tear | MRI | Signal similar to fluid in tendon substance, usually at the tendon–bone interface or 1.5 cm medial to re-attachment site |
| MRA/CTA | Definite gap in tendon with signal similar to intra-articular contrast | |
| Suture displacement | Radiographs/CT/MRI | Suture anchor displacement into unexpected position |
| Subacromial spur reformation | Radiographs/CT/MRI | Subacromial spur evidence, best on sagittal and coronal views |
| Infection | MRI | Joint effusion, synovial thickening, adjacent soft-tissue oedema |
| Adhesive capsulitis | MRI/MRA | Loss of normal fat signal in subcoracoid triangle, thickening of inferior glenohumeral ligament |
| Deltoid detachment | MRI | Retraction of deltoid with fluid filling the defect, muscle atrophy with fatty infiltration |
| Heterotopic ossification | Radiographs/CT/MRI | Mature ossification in deltoid better assessed by CT |
| Acromial fracture | Radiographs/CT | Fracture line well shown on axillary view or axial CT |
| MRI | Increased fluid signal in acromion | |
CTA, CT angiography; MRA, MR arthrography.
Recurrent rotator cuff tear
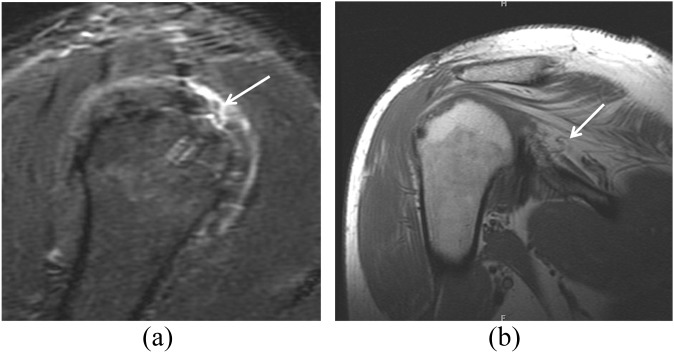
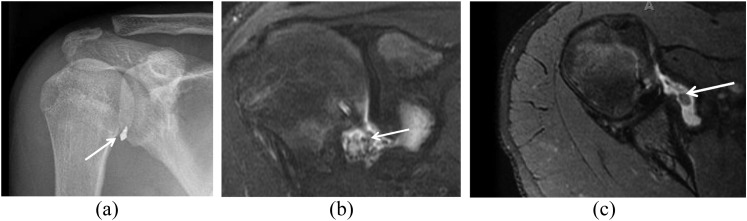
The development of a recurrent rotator cuff tear is often the most important concern in the patient who has post-operative pain or decreased function after a rotator cuff repair.29 As already described, the MRI appearance of the repaired rotator cuff tendons varies. Intrasubstance-increased T2 signal is seen in the vast majority of rotator cuff repairs and might be related to previously present tendinosis, post-operative granulation tissue and scarring or chemical shift artefact related to the presence of sutures.10 The repaired tendon might appear thickened or thinned depending on the stage of the disease process. Therefore, comparison with a pre-operative MRI scan may be useful for distinguishing residual and recurrent disease. The detection of a tear by MRI in the post-operative patient uses criteria similar to those used for identifying a native rotator cuff tendon tear, for example, the presence of fluid signal on a T2 weighted image extending into the tendon substance (Figures 8 and 9). In addition, location plays a role in the diagnostic process. Recurrent tears usually occur at the tendon–bone interface, but they can also occur approximately 1.5 cm medial to the reattachment site, possibly because of increased tension after reattachment in a tendon that contains intrinsic abnormal architecture.30 Gaenslen et al31 reported 84% sensitivity and 91% specificity for MRI in identifying full-thickness tears.
Figure 8.
A 56-year-old female with a history of subacromial decompression presented with a history of 1 week of left shoulder pain. This fat-suppressed intermediate-weighted (repetition time/echo time, 3245/48 ms) coronal image obtained 3 months later shows a partial-thickness articular-side tear of the supraspinatus (large arrow) and marked complex fluid within the subacromial/subdeltoid bursa (small arrow). Complex fluid may be the result of infection, inflammatory synovitis, joint bodies or blood products. In this case, extensive mixed fluid and blood products in the subacromial/subdeltoid bursa is an evolving feature after surgery, but this degree and complexity of fluid 3 months after surgery is usually symptomatic. Hence, in the differential diagnosis a post-operative infection must be also considered in the presence of persistent pain and loss of function.
Figure 9.
This middle-aged male had a history of rotator cuff repair. A fat-suppressed T2 weighted (repetition time/echo time, 3380/70 ms) coronal image shows a recurrent full-thickness tear of the rotator cuff with retracted sutures and tendon (arrow) to the level of the glenohumeral joint.
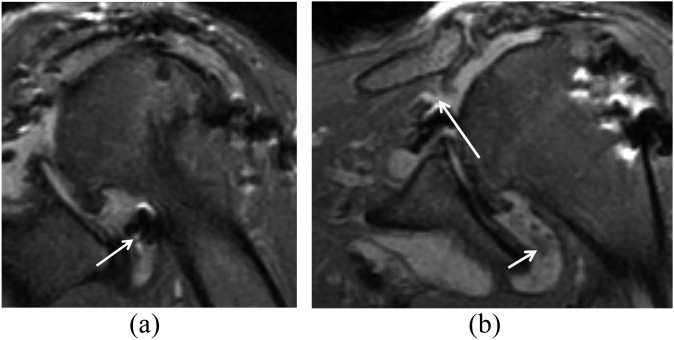
However, the presence of a recurrent tear might not generate symptoms, even when the tear is of full thickness in extent, although the size of the tear is probably related to the development of symptoms (Figure 10). Zanetti et al27 showed that full-thickness defects are substantially smaller in asymptomatic patients (<11 mm) than those in symptomatic patients. Similarly, one should be cautious about calling slight irregularities of the undersurface of the tendon partial undersurface tears, and they should be clinically well correlated.
Figure 10.
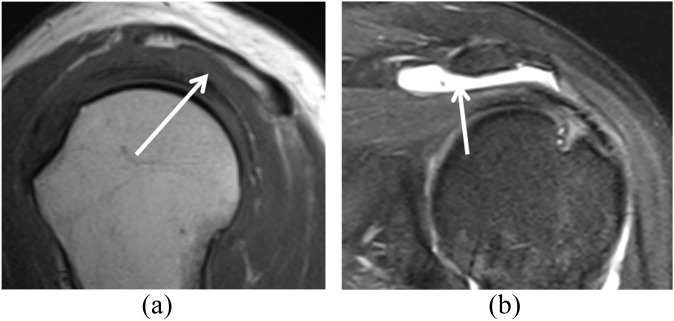
A 33-year-old male underwent rotator cuff repair surgery but had recurrent symptoms. (a) A sagittal short tau inversion–recovery (repetition time/echo time, 4450/42 ms) image shows a small full-thickness tear of the supraspinatus (arrow). (b) T1 weighted (repetition time/echo time, 733/9 ms) coronal view shows atrophy of the infraspinatus that was present before surgery (arrow). It is controversial whether rotator cuff repair can reverse fatty atrophy in the rotator cuff muscles.
MRA improves the identification of rotator cuff re-tears7 (Figure 11). However, on MRA, identifying contrast extending into the subacromial–subdeltoid space does not necessarily signify a recurrent full-thickness tear because repair of the rotator cuff tendon is not necessarily watertight, and a small amount of contrast leakage can be expected in the subacromial space.1,6 Conversely, the absence of contrast in the subacromial–subdeltoid space does not exclude a full-thickness tear because scar tissue might prevent contrast from extending through the entire defect.10,32 The presence of a definitive gap in the tendon makes the diagnosis most definitive.
Figure 11.
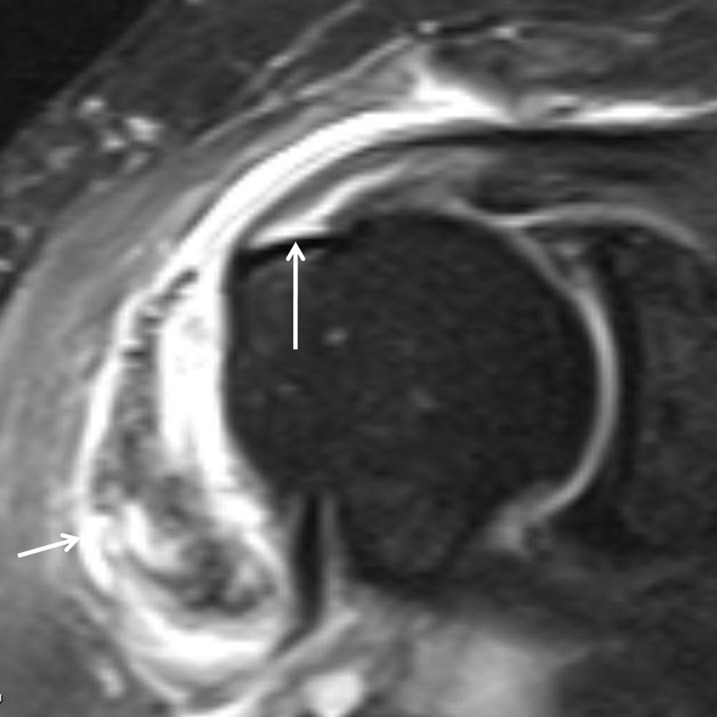
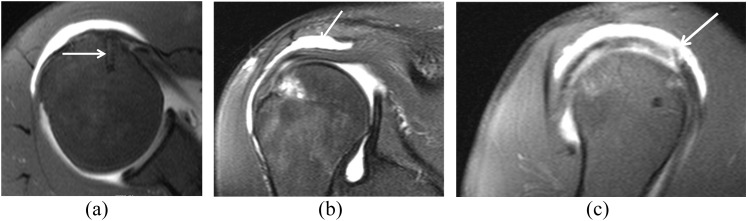
MR arthrography (MRA) views of a recurrent tear of rotator cuff in a 42-year-old male with a history of subacromial decompression and mini-open arthrotomy and rotator cuff repair, with recurrent complaints of pain in his shoulder. (a) Fat-suppressed T1 weighted (repetition time/echo time, 689/20 ms) axial MRA view shows the position of the suture anchor (arrow). (b) Fat-suppressed T1 weighted (repetition time/echo time, 610/20 ms) coronal view shows the fluid (arrow) in the bursa. (c) Fat-suppressed T1 weighted (repetition time/echo time, 610/20 ms) sagittal MRA view shows a small full-thickness tear (arrow). Subsequent arthroscopy confirmed a full-thickness tear of the supraspinatus.
On CTA, the detection of full-thickness recurrent tears, and of partial undersurface re-tears, can be made with filling of the tendon tear by an intra-articular contrast agent (Figure 12). CTA is useful for the evaluation of the post-operative rotator cuff, although evaluation can be performed mainly in regions where contrast extends; for example, if the rotator cuff repair is watertight, the bursal aspect will not be visualized, unlike with MRI or ultrasound.
Figure 12.
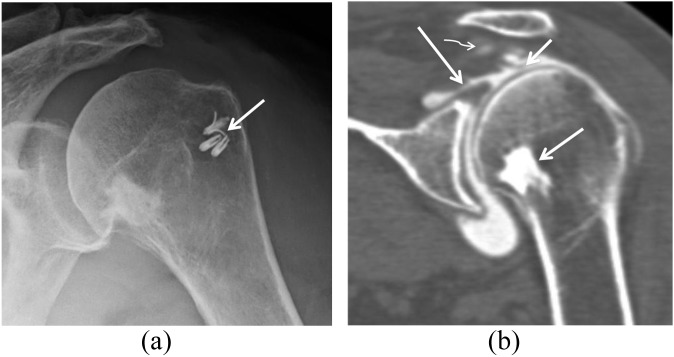
A 68-year-old male had a history of rotator cuff repair surgery 5 years previously; he presented with new symptoms 4 months after a new injury in a motor vehicle accident. (a) Radiograph shows the metallic suture anchor at the greater tuberosity (arrow). (b) Coronal CT arthrography shows a full-thickness tear of the supraspinatus tendon (large arrow) and retracted tendon fibres (small arrow). Incidentally noted is a bone island in the metaphysis of the humeral head (medium arrow). Curved arrow shows the contrast that has passed through the rotator cuff tear.
The factors that contribute to recurrent rotator cuff tearing can be divided into those extrinsic to the cuff (most notably, impingement) and those intrinsic to the cuff (age-related degeneration, hypovascularity and inflammation, among others).33 Some factors are identifiable by imaging, including suture failure, suture anchor displacement and re-formation of a subacromial spur, but the rotator cuff might also re-tear because of too aggressive or inappropriate physical therapy.2
Infection
As with any septic joint, MRI might depict a joint effusion, thickened synovium and surrounding soft-tissue enhancement with the administration of i.v. gadolinium. However, the diagnosis of infection is generally confirmed clinically through aspiration and culture of the joint fluid. Imaging can also serve as a guide to direct a needle to an optimal sight for aspiration. If confirmed, the patient is treated with surgical washout and possible hardware removal. If synovitis is identified by imaging, but the patient does not show clinical signs of infection, he or she may be treated non-operatively (Figures 13 and 14).
Figure 13.
A 16-year-old male complained of right shoulder pain after arthroscopic subscapularis repair. (a) Frontal radiograph shows a displaced metallic suture (arrow). (b) Fat-suppressed T2 weighted (repetition time/echo time, 3416/55 ms) coronal view shows numerous intra-articular bodies (arrow) in the axillary recess. (c) Fat-suppressed intermediate-weighted (repetition time/echo time, 2216/42 ms) axial view shows complex synovial thickening (arrow) and numerous intra-articular bodies, which may represent blood products. Laboratory findings indicated elevated C-reactive protein (60.4 mg l; normal, 0–11 mg l) and elevated white blood cell count (10.3; normal, 3.9–9.8 × 103 μl). The patient underwent arthroscopic debridement, removal of the suture anchor and synovectomy. Aseptic diffuse synovitis has been described after the use of absorbable suture anchors, but infection can also cause similar clinical and radiographic findings.
Figure 14.
An 80-year-old female underwent a subacromial decompression and repair of the rotator cuff several years previously and presented with pain of 3-week duration. (a) Fat-suppressed intermediate-weighted (repetition time/echo time, 1883/41 ms) coronal view shows suture anchors in the axillary pouch (arrow). (b) Fat-suppressed intermediate-weighted (repetition time/echo time, 1883/41 ms) coronal view more anteriorly shows a full-thickness, full-width tear of the supraspinatus with retraction (large arrow) and synovial thickening and with effusion in the glenohumeral joint (small arrow), possibly because of a foreign body reaction to the suture anchor. The patient was treated with steroids for her symptoms and reverse shoulder arthroplasty is planned.
Adhesive capsulitis and post-operative stiffness
This complication usually occurs in the short-term perioperative period. The incidence of adhesive capsulitis varies between 2.7% and 4.9%.34–37 With adhesive capsulitis, there is a general inflammatory response associated with a loss of motion and pain. According to Mansat et al,2 small cuff tears are more likely to develop stiffness after surgery, presumably because of a larger amount of tissue being available to participate in the inflammatory response. However, Brislin et al,38 found the tear size was not statistically significant with regard to the development of stiffness after surgery. MRI findings are usually non-specific, such as thickening of the inferior glenohumeral ligament. In the pre-operative shoulder, the diagnosis is suggested by thickening of the coracohumeral ligament (>4 mm), infiltration of the adjacent fat planes secondary to synovitis and capsular thickness of >7 mm.35,39 According to Mengiardi et al,35 thickening of the coracohumeral ligament and the joint capsule in the rotator cuff interval, and the complete obliteration of the fat triangle between the coracohumeral ligament and the coracoid process (subcoracoid triangle sign), are the characteristic MRI findings for adhesive capsulitis in the pre-operative shoulder. In the post-operative shoulder, features of adhesive capsulitis are probably similar, but to our knowledge, these have not been previously described. With arthrography, the patient is very uncomfortable and shows pain as the needle approaches the capsule. In addition, the volume of contrast that can be administered is reduced and, therefore, there is less joint distension. MRI will show the absence of contrast flowing into the subcoracoid portion of the subscapularis bursa. Clinically, such patients are often treated successfully with anti-inflammatory medications, corticosteroids and/or physical therapy. Many of these patients also improve with arthroscopic release.40
Displacement of the suture anchor
A common implant used to repair the rotator cuff is a suture anchor. Suture anchors come in a wide variety of shapes, sizes and materials. Anchors with threads are typically 1 cm long and 5–6 mm wide. Some anchors may be as small as 3 mm. Metallic anchors can be easily identified on a radiograph. Absorbable and polyether ether ketone anchors are not visualized radiographically, although they may be identified with MRI with little or no artefact.41 Malpositioning of a suture anchor can result in persistent pain after surgery, serious cartilage damage, decreased range of motion and failure of the reconstruction, mandating revision surgery.42 Bioabsorbable sutures and anchors might displace into the joint as they resorb, resulting in locking and catching. Resorption of the anchor is considered as a desirable course for bioabsorbable materials (unlike metallic sutures). However, exposure to the debris of absorbable anchors in the joint can cause synovitis and pain. MRI allows clear delineation of where the anchor is placed and whether it is dislodged, providing a pre-surgical map for the second-look arthroscopy to assess a re-tear of the tendon; MRI also aids in the retrieval of dislodged rotator cuff anchors. Displaced suture anchors can be a sign of cuff abnormality and should be reported in association with cuff defects (Figures 13 and 14).17
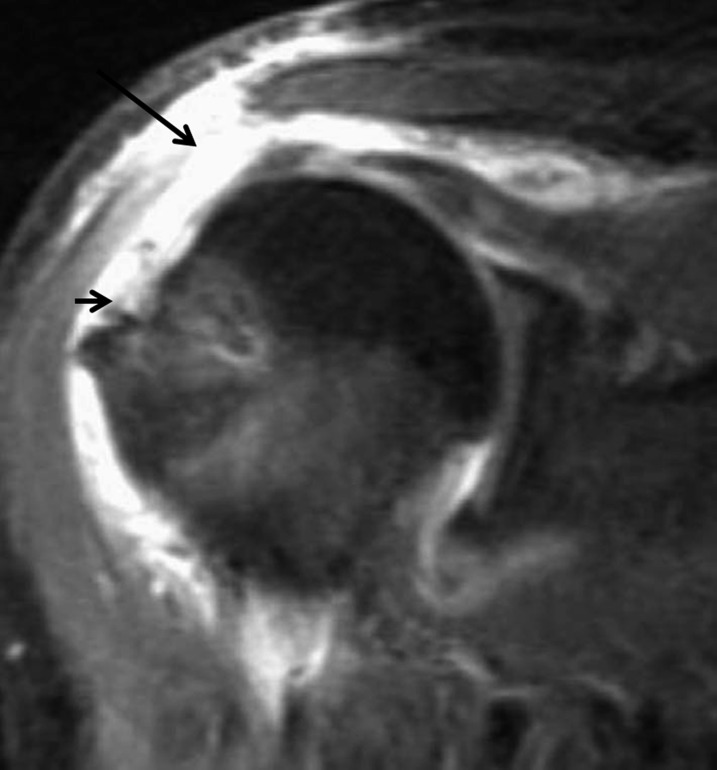
Deltoid dehiscence
Detachment of the deltoid from its attachment to the acromion is a rare, but devastating, complication of rotator cuff surgery. Injury to the deltoid tendon origin is poorly tolerated and can result in severe shoulder dysfunction and pain. Most cases of loss of deltoid function result from iatrogenic injury during shoulder operations, including open rotator cuff repair, acromioplasty, lateral acromionectomy, arthroscopic decompression and arthroscopically assisted mini-open procedures.43 On MRI, this dehiscence is identified by retraction of the deltoid, with fluid filling the defect (Figure 15). If the detachment is chronic, atrophy is manifested by a decrease in muscle bulk and fat replacement (T1 hyperintensity).
Figure 15.
Fat-suppressed intermediate-weighted (repetition time/echo time, 1883/41 ms) coronal view of an elderly female with continued weakness that shows complete detachment of the deltoid at the acromial attachment site with a fluid-filled defect (large arrow). There is also a recurrent full-thickness tear of the supraspinatus (small arrow).
Synovitis
Biodegradable implants lead to other problems, such as cyst formation, soft-tissue inflammation, and local osteolysis or foreign body granulomas.44,45 The causal factors associated with these phenomena appear to be related to the biochemical design and degradation time. On MRI, synovitis and related conditions appear as synovial thickening with enhancement and joint effusion. After i.v. contrast administration, there is an early extravasation of contrast into the joint (Figures 13 and 14).
Heterotopic ossification of the deltoid
Heterotopic ossification of the deltoid fascia is a complication after rotator cuff reconstruction surgery that is easily identified by CT.46 Erggelet et al47 reported an incidence of approximately 26.7% in 131 cases of open rotator cuff reconstruction and acromioplasty without major effects on functional outcome. The underlying pathomorphogenetic processes are not yet fully understood. It is important that this be distinguished from avulsion of the deltoid with small portions of the acromion (Figure 15). Although patients often become concerned about heterotopic ossification, it is rarely clinically significant enough to require treatment.
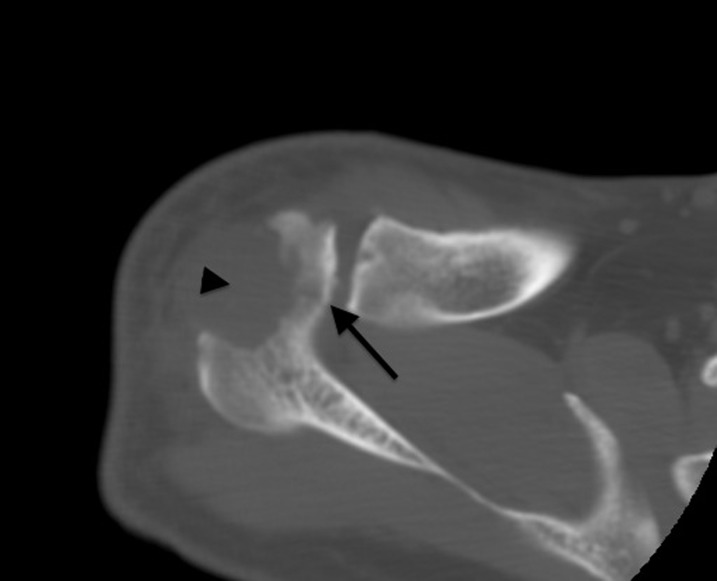
Iatrogenic acromial fracture
Iatrogenic acromial fracture after acromioplasty is an uncommon complication, but it can be devastating for the patient because of pain and shoulder dysfunction. These fractures occur when the bone removed for the acromioplasty thins out the acromion to the point that it fractures secondary to forces applied to it by the upper extremity. Iatrogenic acromial fracture is more common after arthroscopic surgery than after open acromioplasty.48 Although the best radiograph for detection is an axillary view, these fractures can be difficult to see with conventional radiography. These fractures can be detected with MRI, but they are best visualized with CT scanning (Figure 16).
Figure 16.
An axial CT scan obtained in an elderly patient who had undergone subacromial decompression with acromioplasty and was experiencing post-operative pain. A fracture line (arrow) is present on the medial side of the acromion as a result of a previous vigorous acromioplasty, with a large defect (arrowhead).
Other causes of pain in the rotator cuff after surgery
In patients with failed rotator cuff surgery, it is possible that other pathologies are generating pain and may be the primary source of symptoms, rather than the rotator cuff. Specifically, the biceps tendon has been recognized as a potential source of pain in patients with rotator cuff disease.49 Therefore, when using MRI in patients after rotator cuff repair, it remains important to inspect the images for biceps subluxation and biceps tendon tears, as well as for superior labrum anterior posterior (SLAP) lesions.50 Lesions of the labrum in this patient population are typically degenerative SLAP lesions, and lesions of other portions of the labrum are uncommon. Occasionally, when repairing a rotator cuff tear, a co-existing SLAP lesion may be encountered. In this setting, the rotator cuff tear is repaired, but often a concomitant biceps tenodesis or tenotomy will be performed. In the hands of most orthopaedic surgeons, a SLAP repair will not be performed in patients who have co-existing rotator cuff tears and SLAP lesions because symptomatic unrepaired SLAP lesions are rarely a cause of failed rotator cuff repair. Finally, it is very uncommon to have an anterior–inferior labrum tear and a rotator cuff tear at the same time without a history of shoulder instability. In elderly patients, dislocation of the shoulder may tear the rotator cuff, but in most cases, the rotator cuff tear is chronic and there is increased shoulder laxity, which pre-disposes the shoulder to instability. In the latter situation, repair of the anterior–inferior labrum is not typically necessary because recurrent instability in such patients is rare.
CONCLUSION
Knowledge of common surgical procedures, expected post-operative findings on various imaging techniques and potential complications are important for the imaging evaluation of the shoulder after rotator cuff surgery. Our approach to the assessment of patients who have had rotator cuff surgery includes a radiographic examination followed by MRI (and sometimes MRA) for patients with low “metal presence” and by CTA for patients who have a higher metal presence. Ultrasound is also a useful modality, when targeted to identifying rotator cuff recurrent tears; however, it is limited in providing a comprehensive view of the shoulder. On MRI, the detection of a tear in the post-operative patient uses criteria similar to those used for identifying a native rotator cuff tendon tear, that is, the presence of fluid signal on a fluid-sensitive image extending into the tendon substance. In addition, location plays a role in the diagnostic process. Recurrent tears usually occur at the tendon–bone interface, but they can also occur approximately 1.5 cm medial to the reattachment site, possibly because of increased tension after reattachment in a tendon that contains intrinsic abnormal architecture. On CTA, the detection of full-thickness recurrent tears, and of partial undersurface re-tears, can be made by filling the tendon tear with an intra-articular contrast agent.
CONFLICTS OF INTEREST
One author (LMF) has conflicts to declare: General Electric Radiology Research Fellowship 2008–2010 and Siemens Medical Systems grant 2011–2012 for the study of musculoskeletal tumours by proton MR spectroscopy.
REFERENCES
- 1.Mohana-Borges AVR, Chung CB, Resnick D. MR imaging and MR arthrography of the postoperative shoulder: spectrum of normal and abnormal findings. Radiographics 2004; 24: 69–85. doi: 10.1148/rg.241035081 [DOI] [PubMed] [Google Scholar]
- 2.Mansat P, Cofield RH, Kersten TE, Rowland CM. Complications of rotator cuff repair. Orthop Clin North Am 1997; 28: 205–13. [DOI] [PubMed] [Google Scholar]
- 3.Nazarian LN, Jacobson JA, Benson CB, Bancroft LW, Bedi A, McShane JM, et al. Imaging algorithms for evaluating suspected rotator cuff disease: Society of Radiologists in Ultrasound consensus conference statement. Radiology 2013; 267: 589–95. doi: 10.1148/radiol.13121947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owen RS, Iannotti JP, Kneeland JB, Dalinka MK, Deren JA, Oleaga L. Shoulder after surgery: MR imaging with surgical validation. Radiology 1993; 186: 443–7. doi: 10.1148/radiology.186.2.8421748 [DOI] [PubMed] [Google Scholar]
- 5.Bernstein MA, Huston J3rd, Ward HA. Imaging artifacts at 3.0T. J Magn Reson Imaging 2006; 24: 735–46. doi: 10.1002/jmri.20698 [DOI] [PubMed] [Google Scholar]
- 6.Rand T, Trattnig S, Breitenseher M, Wurnig C, Marschner B, Imhof H. The postoperative shoulder. Top Magn Reson Imaging 1999; 10: 203–13. [DOI] [PubMed] [Google Scholar]
- 7.Rand T, Freilinger W, Breitenseher M, Trattnig S, Garcia M, Landsiedl F, et al. Magnetic resonance arthrography (MRA) in the postoperative shoulder. Magn Reson Imaging 1999; 17: 843–50. [DOI] [PubMed] [Google Scholar]
- 8.Vahlensieck M, Peterfy CG, Wischer T, Sommer T, Lang P, Schlippert U, et al. Indirect MR arthrography: optimization and clinical applications. Radiology 1996; 200: 249–54. doi: 10.1148/radiology.200.1.8657921 [DOI] [PubMed] [Google Scholar]
- 9.Zanetti M, Hodler J. MR imaging of the shoulder after surgery. Magn Reson Imaging Clin N Am 2004; 12: 169–83. doi: 10.1016/j.mric.2004.01.003 [DOI] [PubMed] [Google Scholar]
- 10.Zlatkin MB. MRI of the postoperative shoulder. Skeletal Radiol 2002; 31: 63–80. doi: 10.1007/s00256-001-0460-1 [DOI] [PubMed] [Google Scholar]
- 11.De Filippo M, Bertellini A, Sverzellati N, Pogliacomi F, Costantino C, Vitale M, et al. Multidetector computed tomography arthrography of the shoulder: diagnostic accuracy and indications. Acta Radiol 2008; 49: 540–9. doi: 10.1080/02841850801935559 [DOI] [PubMed] [Google Scholar]
- 12.Lecouvet FE, Simoni P, Koutaïssoff S, Vande Berg BC, Malghem J, Dubuc JE. Multidetector spiral CT arthrography of the shoulder. Clinical applications and limits, with MR arthrography and arthroscopic correlations. Eur J Radiol 2008; 68: 120–36. doi: 10.1016/j.ejrad.2008.02.025 [DOI] [PubMed] [Google Scholar]
- 13.Calhoun PS, Kuszyk BS, Heath DG, Carley JC, Fishman EK. Three-dimensional volume rendering of spiral CT data: theory and method. Radiographics 1999; 19: 745–64. doi: 10.1148/radiographics.19.3.g99ma14745 [DOI] [PubMed] [Google Scholar]
- 14.Fritz J, Fishman EK, Small KM, Winalski CS, Horger MS, Corl F, et al. MDCT arthrography of the shoulder with datasets of isotropic resolution: indications, technique, and applications. AJR Am J Roentgenol 2012; 198: 635–46. doi: 10.2214/AJR.11.7078 [DOI] [PubMed] [Google Scholar]
- 15.Adler RS. Postoperative rotator cuff. Semin Musculoskelet Radiol 2013; 17: 12–19. doi: 10.1055/s-0033-1333909 [DOI] [PubMed] [Google Scholar]
- 16.Prickett WD, Teefey SA, Galatz LM, Calfee RP, Middleton WD, Yamaguchi K. Accuracy of ultrasound imaging of the rotator cuff in shoulders that are painful postoperatively. J Bone Joint Surg Am 2003; 85: 1084–9. [DOI] [PubMed] [Google Scholar]
- 17.McMenamin D, Koulouris G, Morrison WB. Imaging of the shoulder after surgery. Eur J Radiol 2008; 68: 106–19. doi: 10.1016/j.ejrad.2008.02.050 [DOI] [PubMed] [Google Scholar]
- 18.Gusmer PB, Potter HG, Donovan WD, O'Brien SJ. MR imaging of the shoulder after rotator cuff repair. AJR Am J Roentgenol 1997; 168: 559–63. doi: 10.2214/ajr.168.2.9016248 [DOI] [PubMed] [Google Scholar]
- 19.Spielmann AL, Forster BB, Kokan P, Hawkins RH, Janzen DL. Shoulder after rotator cuff repair: MR imaging findings in asymptomatic individuals–initial experience. Radiology 1999; 213: 705–8. doi: 10.1148/radiology.213.3.r99dc09705 [DOI] [PubMed] [Google Scholar]
- 20.Shubin Stein BE, Ahmad CS, Pfaff CH, Bigliani LU, Levine WN. A comparison of magnetic resonance imaging findings of the acromioclavicular joint in symptomatic versus asymptomatic patients. J Shoulder Elbow Surg 2006; 15: 56–9. doi: 10.1016/j.jse.2005.05.013 [DOI] [PubMed] [Google Scholar]
- 21.Gulotta LV, Nho SJ, Dodson CC, Adler RS, Altchek DW, MacGillivray JD, et al. Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I–functional outcomes and radiographic healing rates. J Shoulder Elbow Surg 2011; 20: 934–40. [DOI] [PubMed] [Google Scholar]
- 22.Itoi E, Minagawa H, Sato T, Sato K, Tabata S. Isokinetic strength after tears of the supraspinatus tendon. J Bone Joint Surg Br 1997; 79: 77–82. [DOI] [PubMed] [Google Scholar]
- 23.Gladstone JN, Bishop JY, Lo IKY, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 2007; 35: 719–28. doi: 10.1177/0363546506297539 [DOI] [PubMed] [Google Scholar]
- 24.Liem D, Lichtenberg S, Magosch P, Habermeyer P. Magnetic resonance imaging of arthroscopic supraspinatus tendon repair. J Bone Joint Surg Am 2007; 89: 1770–6. doi: 10.2106/JBJS.F.00749 [DOI] [PubMed] [Google Scholar]
- 25.Bancroft LW, Wasyliw C, Pettis C, Farley T. Postoperative shoulder magnetic resonance imaging. Magn Reson Imaging Clin N Am 2012; 20: 313–25. doi: 10.1016/j.mric.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 26.Henkus HE, de Witte PB, Nelissen RGHH, Brand R, van Arkel ERA. Bursectomy compared with acromioplasty in the management of subacromial impingement syndrome: a prospective randomised study. J Bone Joint Surg Br 2009; 91: 504–10. [DOI] [PubMed] [Google Scholar]
- 27.Zanetti M, Jost B, Hodler J, Gerber C. MR imaging after rotator cuff repair: full-thickness defects and bursitis-like subacromial abnormalities in asymptomatic subjects. Skeletal Radiol 2000; 29: 314–19. [DOI] [PubMed] [Google Scholar]
- 28.Randelli P, Spennacchio P, Ragone V, Arrigoni P, Casella A, Cabitza P. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg 2012; 96: 9–16. doi: 10.1007/s12306-011-0175-y [DOI] [PubMed] [Google Scholar]
- 29.Kyrölä K, Niemitukia L, Jaroma H, Väätäinen U. Long-term MRI findings in operated rotator cuff tear. Acta Radiol 2004; 45: 526–33. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Covey A, Katz LD. MRI of the postoperative shoulder. Clin Sports Med 2006; 25: 445–64. doi: 10.1016/j.csm.2006.03.003 [DOI] [PubMed] [Google Scholar]
- 31.Gaenslen ES, Satterlee CC, Hinson GW. Magnetic resonance imaging for evaluation of failed repairs of the rotator cuff. Relationship to operative findings. J Bone Joint Surg Am 1996; 78: 1391–6. [DOI] [PubMed] [Google Scholar]
- 32.Magee TH, Gaenslen ES, Seitz R, Hinson GA, Wetzel LH. MR imaging of the shoulder after surgery. AJR Am J Roentgenol 1997; 168: 925–8. doi: 10.2214/ajr.168.4.9124141 [DOI] [PubMed] [Google Scholar]
- 33.Yadav H, Nho S, Romeo A, MacGillivray JD. Rotator cuff tears: pathology and repair. Knee Surg Sports Traumatol Arthrosc 2009; 17: 409–21. doi: 10.1007/s00167-008-0686-8 [DOI] [PubMed] [Google Scholar]
- 34.Curtis AS, Snyder SJ, Del Pizzo W, Friedman MJ, Ferkel RD, Karzel RP. Complications of shoulder arthroscopy. Arthroscopy 1992; 8: 395. [Google Scholar]
- 35.Mengiardi B, Pfirrmann CWA, Gerber C, Hodler J, Zanetti M. Frozen shoulder: MR arthrographic findings. Radiology 2004; 233: 486–92. doi: 10.1148/radiol.2332031219 [DOI] [PubMed] [Google Scholar]
- 36.Muller D, Landsiedl F. Arthroscopy of the shoulder joint: a minimal invasive and harmless procedure? Arthroscopy 2000; 16: 425. [Google Scholar]
- 37.Vezeridis PS, Goel DP, Shah AA, Sung SY, Warner JP. Postarthroscopic arthrofibrosis of the shoulder. Sports Med Arthrosc 2010; 18: 198–206. doi: 10.1097/JSA.0b013e3181ec84a5 [DOI] [PubMed] [Google Scholar]
- 38.Brislin KJ, Field LD, Savoie FH3rd. Complications after arthroscopic rotator cuff repair. Arthroscopy 2007; 23: 124–8. doi: 10.1016/j.arthro.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 39.Emig EW, Schweitzer ME, Karasick D, Lubowitz J. Adhesive capsulitis of the shoulder: MR diagnosis. AJR Am J Roentgenol 1995; 164: 1457–9. doi: 10.2214/ajr.164.6.7754892 [DOI] [PubMed] [Google Scholar]
- 40.Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy 2009; 25: 880–90. doi: 10.1016/j.arthro.2009.01.018 [DOI] [PubMed] [Google Scholar]
- 41.Abrams JS. Management of the failed rotator cuff surgery: causation and management. Sports Med Arthrosc 2010; 18: 188–97. doi: 10.1097/JSA.0b013e3181eb6cc1 [DOI] [PubMed] [Google Scholar]
- 42.Lorbach O, Wilmes P, Brogard P, Seil R. Complications related to implants in arthroscopic shoulder surgery. [In German.] Orthopade 2008; 37: 1073–9. [DOI] [PubMed] [Google Scholar]
- 43.Chebli CM, Murthi AM. Deltoidplasty: outcomes using orthobiologic augmentation. J Shoulder Elbow Surg 2007; 16: 425–8. doi: 10.1016/j.jse.2006.09.017 [DOI] [PubMed] [Google Scholar]
- 44.Hotta T, Yamashita T. Osteolysis of the inferior surface of the acromion caused by knots of the suture thread after rotator cuff repair surgery: knot impingement after rotator cuff repair. J Shoulder Elbow Surg 2010; 19: e17–23. doi: 10.1016/j.jse.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 45.Nusselt T, Freche S, Klinger HM, Baums MH. Intraosseous foreign body granuloma in rotator cuff repair with bioabsorbable suture anchor. Arch Orthop Trauma Surg 2010; 130: 1037–40. doi: 10.1007/s00402-010-1125-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders BS, Wilcox RB3rd, Higgins LD. Heterotopic ossification of the deltoid muscle after arthroscopic rotator cuff repair. Am J Orthop (Belle Mead NJ) 2010; 39: E67–71. [PubMed] [Google Scholar]
- 47.Erggelet C, Eggensperger G, Steinwachs M, Lahm A, Reichelt A. Postoperative ossifications of the shoulder. Incidence and clinical impact. Arch Orthop Trauma Surg 1999; 119: 168–70. [DOI] [PubMed] [Google Scholar]
- 48.Matthews LS, Burkhead WZ, Gordon S, Racanelli J, Ruland L. Acromial fracture: a complication of arthroscopic subacromial decompression. J Shoulder Elbow Surg 1994; 3: 256–61. doi: 10.1016/S1058-2746(09)80044-X [DOI] [PubMed] [Google Scholar]
- 49.Tonino PM, Gerber C, Itoi E, Porcellini G, Sonnabend D, Walch G. Complex shoulder disorders: evaluation and treatment. J Am Acad Orthop Surg 2009; 17: 125–36. [DOI] [PubMed] [Google Scholar]
- 50.Forsythe B, Guss D, Anthony SG, Martin SD. Concomitant arthroscopic SLAP and rotator cuff repair. J Bone Joint Surg Am 2010; 92: 1362–9. doi: 10.2106/JBJS.H.01632 [DOI] [PubMed] [Google Scholar]