Abstract
Objective:
To retrospectively evaluate the association of MRI findings with local control of nasopharyngeal carcinoma (NPC) treated with radiation therapy and chemotherapy (chemoradiotherapy).
Methods:
Pre-treatment MRIs of 101 patients (78 males and 23 females, 23–79 years of age) who had NPC treated with chemoradiotherapy were retrospectively reviewed to evaluate tumour involvement of nasopharyngeal anatomic subsites, tumour volume and MRI appearance. Local control rates were evaluated with respect to these MRI findings.
Results:
Univariate analysis (using the Kaplan–Meier method) showed that invasion of the skull base as determined by MRI was a significant predictor of local control. In terms of clinical characteristics, T stage and pathological subtype were significant predictors of local control. Multivariate analysis (Cox regression model) of the radiologic findings and clinical characteristics revealed that invasion of the skull base (p = 0.003) and pathological subtype (p < 0.001) were independent prognostic factors for local control.
Conclusion:
Invasion of the skull base as determined by MRI predicts the likelihood of local failure and may be helpful in identifying a subset of patients with tumours at risk of local recurrence within 3 years after primary chemoradiotherapy.
Advances in knowledge:
It has now become common practice to use MRI for pre-treatment evaluation of patients with NPC. The potential role for MRI findings in predicting local control and prognosis in patients with NPC has implications for treatment planning.
The “local control” rate of nasopharyngeal carcinoma (NPC) has improved significantly in recent years owing to advances in imaging, improved radiotherapy techniques and the use of combined chemotherapy. Unfortunately, 15–20% of such patients still develop recurrence.1,2 Local recurrence represents a major cause of mortality and morbidity in advanced-stage disease, and the management of local failure remains a challenging issue in NPC.3 Therefore, early identification of patients who are at risk of such failures is an essential step towards improving existing treatments.
Several factors have been linked to an increased risk of local recurrence, including advanced tumour (T)-stage, nodal (N)-stage, histological classification, parapharyngeal tumour invasion, tumour volume and treatment choice.4–7
MRI provides excellent soft tissue contrast and has been shown to be effective for diagnosis, characterization, staging and response evaluation in patients with NPC. However, to our knowledge, the association of pre-treatment MRI findings of NPC with the risk of local recurrence has not been well studied.
Therefore, the purpose of our study is to retrospectively evaluate the prognostic significance of MRI findings, regarding the local control of NPC treated with chemoradiotherapy.
METHODS AND MATERIALS
Patient selection
Patients enrolled in this study had pathologically proven NPC treated with concurrent radiation therapy and chemotherapy between 2005 and 2010. All patients underwent pre-treatment nasopharyngoscopy and MR scanning of the head and neck area. The study was performed with the approval of our institutional review board and direct patient consent was waived.
Tumour stage was determined according to the 6th edition of the American Joint Commitee on Cancer (AJCC) staging system.8 In this study, all patients had an initial stage higher than IIA and were treated with concurrent chemoradiotherapy. The histological classification of NPC was determined according to the World Health Organization (WHO) protocol.
MRI technique
MRI of the nasopharyngeal region was performed with a 1.5-T unit (Magnetom SP 63; Siemens Medical Systems, Erlangen, Germany). In all patients, the following four sequences were performed: axial T1 weighted spin-echo (SE), coronal T1 weighted SE, axial T2 weighted fast SE (FSE) and axial T1 weighted contrast material-enhanced SE. Axial T1 weighted SE images were obtained with a repetition time/echo time of 600/15 ms, 23-mm field of view (FOV), 256 × 256 matrix, 5 mm-thick section. Coronal T1 weighted SE images were obtained with a repetition time/echo time of 608/14 ms, 23-mm FOV, 256 × 256 matrix, 4 mm-thick section. Axial T2 weighted FSE images were obtained with 6000/90 ms, 23 mm FOV, 256 × 256 matrix, 5 mm-thick section. The axial T1 weighted contrast-enhanced SE images were obtained with the same parameters as the pre-contrast T1 weighted SE images. Images in the sagittal plane were obtained if infiltration of the pharyngobasilar fascia or the clivus was suspected. An intravenous bolus injection (0.1 mmol kg−1 of Gadopentetate Dimeglumine/Magnevist®; Bayer Schering Pharma AG, Berlin, Germany) was administered for the contrast-enhanced series.
To evaluate cervical lymph node metastases, MR studies were also performed with the same MR scanner. Images were obtained with 5 mm-thick contiguous sections in two planes (axial and coronal).
Chemoradiotherapy and clinical follow-up
All radiation treatment was administered using a linear accelerator by two-dimensional techniques. Patients received 2.0 Gy per fraction five times a week for a total dose of 66–70 Gy to the primary tumour, 60–66 Gy to the neck region with neck nodal involvement and 50–60 Gy to the neck region without neck nodal involvement. The concurrent chemotherapy regimen consisted of 40 mg m−2 cisplatin given as a 2-h intravenous infusion every week for a maximum of seven cycles, beginning on the first day of radiotherapy.
After the completion of treatment, post-treatment baseline radiographic studies were performed 2–3 months after completion of chemoradiotherapy. After the baseline evaluation, further imaging follow-up was obtained every 3–6 months for the first 3 years after treatment. After the completion of chemoradiotherapy, clinical follow-up included flexible fibreoptic endoscopy every 1–2 months for the first year, every 3 months for the next year, then every 6 months thereafter, supplemented by biopsies as necessary. The follow-up period was defined as the total follow-up time, ending either at local recurrence or at the last patient contact without local recurrence, with a minimum of 3 years (mean follow-up time, 38 months; range, 10–53 months). Tumour recurrence was defined as a lesion detected after a documented tumour-free period.9
MRI analysis
MRI was interpreted by consensus agreement of two head and neck radiologists. The following MRI parameters were assessed: maximal diameter of the primary tumour (0–30 mm vs >30 mm); uniformity of the T2 signal intensity (homogeneous or heterogeneous); enhancement uniformity (homogeneous or heterogeneous); invasion of the oropharynx, the nasal fossa, the parapharyngeal space, the skull base, the sinus, the orbit, the cranium and the infratemporal fossa (present or absent); metastases to the lymph nodes in the cervical part and retropharyngeal spaces (present or absent).
The nasal fossa extension was defined as a tumour invading the posterior edge of nasal septum or a tumour crossing the line connecting bilateral pterygopalatine fossa.
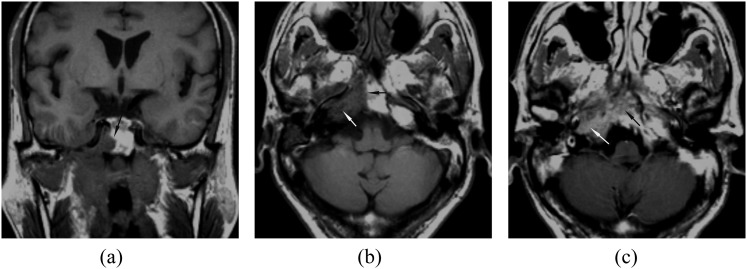
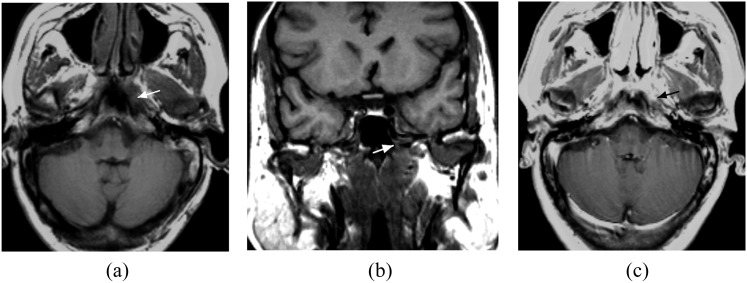
Skull-base bone involvement on MRI was evaluated at the sites of the petrous bone, the sphenoid bone and/or sphenoid sinus, ethmoid bone and/or ethmoid sinus, basilar part of occipital bone, the clivus; skull-base foramen involvement of the foramen ovale, the foramen lacerum and the jugular foramen was also recorded. MRI criteria for skull base bony abnormalities were the presence of erosions of the bone cortex, which could be seen as a defect in the low signal intensity cortex; low-intensity tissue in the high-signal bone marrow on T1 weighted images and gadopentetate dimeglumine (Gd-DTPA) enhancement of the abnormal tissue10 (Figure 1). Infiltration of the foramen was identifying tumour within the foramen, with or without traversing it (Figure 2). All the invasions of the skull base on MRI were determined by at least two different sequences or planes.
Figure 1.
(a–b) Axial and coronal Tl weighted MRI showing marrow infiltration in the clivus (black arrow) and the right petrous apex (white arrow). (c) Axial contrast-enhanced MRI showing tumour enhancement in the clivus (black arrow) and the right petrous apex (white arrow).
Figure 2.
(a–b) Axial and coronal Tl weighted MRI showing tumour invasion in the left foramen lacerum (white arrows) without traversing it and without infiltration of the adjacent clivus and petrous apex. (c) Axial contrast-enhanced MRI showing tumour enhancement in the left foramen lacerum (black arrow).
The intracranial extension included meningeal involvement, masses within the middle and/or posterior cranial fossa and perineural tumour spread.
Cervical lymph node metastases were defined as the following: (1) gadolinium enhancement of a cervical lymph node; (2) short axis of a lymph node ≥1 cm (unless in the retropharyngeal space or Level I, where size cut-offs were ≥0.5 cm and ≥1.5 cm, respectively); and (3) central necrosis.11
Statistical analysis
Local control was defined as the absence of primary tumour recurrence. Tumour recurrence was confirmed with biopsy within 2 weeks of the MR scan or, when not available, by means of imaging follow-up over 6 months.12 Duration of local NPC recurrence was calculated from the first day of the first treatment until the day of the first primary recurrence was observed. The univariate analysis, using Kaplan–Meier method and the log-rank test, was used to identify the individual factors predictive of local control for all NPC patients. A stepwise multivariate analysis using the Cox proportional hazards model was built to assess the effect of clinical and MRI covariates that were significantly associated with local control.
Clinical and radiologic covariates, such as sex, age at diagnosis, T stage, N stage, pathological subtype, maximal diameter of the primary tumour, uniformity of T2 signal intensity, uniformity of enhancement, invasion of the oropharynx, invasion of the nasal fossa, invasion of the parapharyngeal space, invasion of the sinus, invasion of the skull base, invasion of the orbit, invasion of the cranium and invasion of the infratemporal fossa were included in the uni- and multivariate analysis. All statistical calculations were performed using SPSS® statistical software, v. 16.0 (SPSS Inc., Chicago, IL).
RESULTS
Patients and lesions
Patients who had the following conditions were excluded from the study: distant metastasis at initial presentation (13 patients); nodal or distant metastasis at follow up without local recurrence (2 patients); radiation dose <60 Gy (9 patients); unable to complete chemotherapy (13 patients); and no suitable MRI (5 patients). The remaining 101 patients were included in this analysis of prognostic factors for local control.
101 patients, aged 23–79 years (median, 45 years) participated in the study; 78 patients were male (77.2%) and 23 were female (22.8%). The mean maximum tumour diameter was 31.1 mm (median, 30.0 mm; range, 10.0–70.0 mm). Histopathological examination of biopsy specimens showed WHO Type I in 20 patients (19.8%), WHO Type II in 5 patients (5.0%), and WHO Type III in 76 patients (75.2%) (Table 1). In this study, invasion of the skull base was found in 48 (47.5%) of 101 patients.
Table 1.
Univariate correlation of local recurrence risk with clinical and histopathological variables, estimated with the Kaplan–Meier method
| Variable | No. of patients | 3-year local control (%) | p-value (log rank) |
|---|---|---|---|
| Sex | 0.396 | ||
| Male | 78 | 78.2 | |
| Female | 23 | 87.0 | |
| Age (years) | 0.201 | ||
| ≤50 | 62 | 83.9 | |
| >50 | 39 | 74.4 | |
| T stage | 0.011 | ||
| T1 | 39 | 92.3 | |
| T2a | 7 | 100.0 | |
| T2b | 4 | 75.0 | |
| T3 | 33 | 75.8 | |
| T4 | 18 | 55.6 | |
| N stage | 0.079 | ||
| N1 | 22 | 68.2 | |
| N2 | 70 | 85.7 | |
| N3 | 9 | 66.7 | |
| Pathological type | <0.001 | ||
| WHOI | 20 | 45.0 | |
| WHOII | 5 | 60.0 | |
| WHOIII | 76 | 90.8 |
WHO, World Health Organization.
Association with local control
Local recurrence within 3 years was present in 20 patients and absent in 81. In patients without recurrent disease, the mean follow-up interval was 41.15 ± 4.23 months (range, 36–53 months). In patients with recurrent disease, the mean follow-up interval was 27.30 ± 9.64 months (range, 10–39 months).
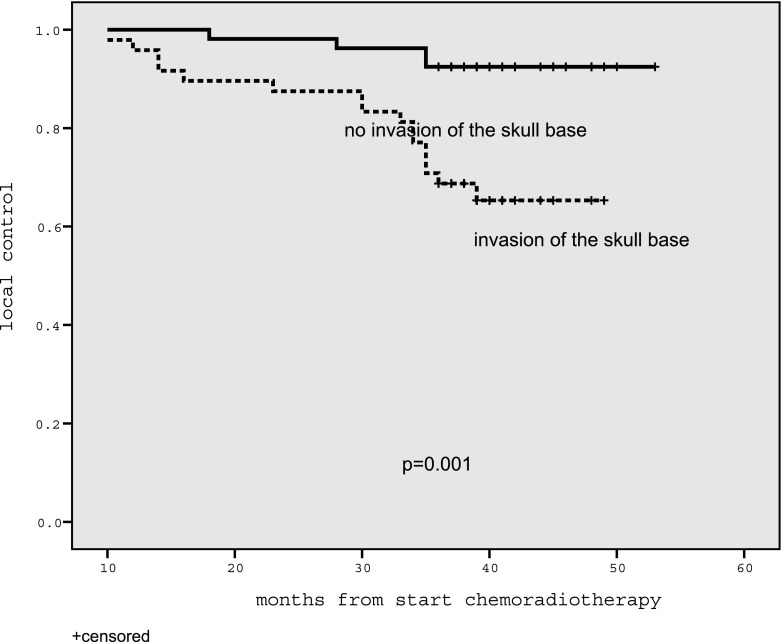
Univariate analysis showed that the clinical parameters of T stage and pathological subtype were significantly associated with local control (Table 1). Of the analysed MRI parameters, only invasion of the skull base was significantly associated with local control (Table 2, Figure 3).
Table 2.
Univariate correlation of local recurrence risk with MRI findings, estimated with the Kaplan–Meier method
| Variable | No. of patients | 3-year local control (%) | p-value (log rank) |
|---|---|---|---|
| Maximum diameter of primary tumour | 0.368 | ||
| 0–30.0 mm | 59 | 83.1 | |
| >30.0 mm | 42 | 76.2 | |
| Uniformity of T2 SI | 0.456 | ||
| No | 21 | 85.7 | |
| Yes | 80 | 78.8 | |
| Uniformity of enhancement | 0.968 | ||
| No | 15 | 80.0 | |
| Yes | 86 | 80.2 | |
| Invasion of the oropharynx | 0.768 | ||
| No | 78 | 75.9 | |
| Yes | 23 | 95.5 | |
| Invasion of the nasal fossa | 0.376 | ||
| No | 90 | 78.9 | |
| Yes | 11 | 90.9 | |
| Invasion of the parapharyngeal space | 0.940 | ||
| No | 80 | 80.0 | |
| Yes | 21 | 81.0 | |
| Invasion of the skull base | 0.001 | ||
| No | 53 | 92.5 | |
| Yes | 48 | 66.7 | |
| Invasion of the sinus | 0.817 | ||
| No | 84 | 79.8 | |
| Yes | 17 | 82.4 | |
| Invasion of the orbit | 0.177 | ||
| No | 97 | 81.4 | |
| Yes | 4 | 50.0 | |
| Invasion of the cranium | 0.150 | ||
| No | 87 | 82.2 | |
| Yes | 14 | 64.3 | |
| Invasion of the infratemporal fossa | 0.196 | ||
| No | 97 | 81.4 | |
| Yes | 4 | 50.0 |
SI, signal intensity.
Figure 3.
Graph showing that patients with nasopharyngeal carcinoma and invasion of the skull base had a poor prognosis (n = 48, 3-year local control rate 66.7%) compared with patients without skull base invasion (n = 53, 3-year local control rate 92.5%).
In the first step of the multivariate analysis, when the clinical covariates were entered, pathological subtype (p < 0.001), T stage (p = 0.005) and age (p = 0.009) were independent prognostic factors for local control. When the MRI covariates were entered, invasion of the skull base (p = 0.003) and pathological type (p < 0.001) were found to be significantly associated with local control (Table 3).
Table 3.
Final results of multivariate analysis of local recurrence
| Variable | Regression coefficient | Hazard ratio | 95% confidence interval | p-value |
|---|---|---|---|---|
| Invasion of the skull base | 1.651 | 5.211 | 1.723–15.757 | 0.003 |
| Histology (World Health Organization type) | <0.001 | |||
| Type I vs III | 2.154 | 8.618 | 3.210–23.135 | <0.001 |
| Type II vs III | 2.223 | 9.324 | 1.733–50.168 | 0.009 |
DISCUSSION
In our study, the most important independent prognostic factor for local recurrence among patients with locally advanced NPC was found to be MRI evidence of skull base invasion. T1 weighted MR images permit accurate assessment of whether there is a tumour extension into the adjacent skull base, as MRI shows tumour tissue in the skull base as a low-intensity area in the normally high-signal bone marrow. It is well established that MR is better than CT at identifying invasion of the skull base, and it has now become common practice to use MRI for pre-treatment evaluation of patients with NPC.10 Our results showed that abnormal MR signal intensity in the skull base was correlated with the risk of tumour recurrence. Univariate and multivariate analyses demonstrated that invasion of the skull base, as determined by MRI, was significantly associated with local control. This finding was in agreement with preliminary reports showing that CT evidence of skull base invasion was also associated with an increased risk of local tumour recurrence.13 Cheng et al14 demonstrated that MRI abnormalities in the skull base were also associated with a higher risk of local failure in advanced-stage NPC (after either concurrent chemoradiotherapy or post-radiation chemotherapy), especially when two or more bony skull base sites were involved.
The association that we observed between MRI evidence of skull base invasion and the risk of local disease recurrence reinforced the role of MRI as a non-invasive prognostic tool for NPC treated with radiation therapy and chemotherapy. Our study showed that patients with tumour invading the skull base had poor local control (only 66.7% after 3 years). In all patients with NPC, non-operative organ preservation strategies are considered the best initial treatment approach. The current practice recommends a radiation dose of 65–75 Gy (1.8–2.0 Gy per fraction), regardless of the primary tumour stage or the presence of other important prognostic factors. Although the dose–response relationship in NPC has not been universally accepted, in some institutions, patients with locally advanced tumours (such as those with skull-base invasion or extensive parapharyngeal involvement) are frequently treated with boost irradiation therapy. Nishioka et al15 reported that patients with MRI evidence of skull base invasion had no local recurrences if the patients received boost therapy, and this result supported that boost therapy might have contributed to the success of treatment in these patients. Further research is needed.
Primary tumour volume has been reported as a risk factor associated with local control.6,16 However, in our study, the maximal tumour diameter as determined by MRI was not significantly associated with local control in either the univariate or multivariate analyses. According to the response evaluation criteria in solid tumour guidelines, one-dimensional measurements of tumour size are consistent with three-dimensional measurements.17 Therefore, our data suggested that primary tumour size might not predict the risk of NPC recurrence. Sakata et al18 reported that deep infiltration of the primary tumour was a more important prognostic factor in NPC than tumour volume.
Lin et al19 reported that the local control failure of NPC was associated with nasal involvement. However, our results differed from those of that study. In our study, invasion of the nasal fossa as demonstrated by MRI was not associated with local control. The patients described by Lin et al19 were treated with conventional radiotherapy, whereas our patients were treated with both radiation therapy and chemotherapy, suggesting that the treatment approach may be responsible for the observed differences between the two studies.
An important clinical prognostic factor in our study was pathological type, as Subtypes I and II were associated with local recurrence. This observation was consistent with previous results.4,14
Our study had several limitations. First, this was a retrospective analysis, and only a small number of patients developed recurrent disease. Second, our time period for considering patients to be free of disease may be considered short at 3 years; however, >90% of NPC relapses occur in the first 3 years after initial treatment.20 Third, we must recognize the limitations of MRI. We can not rule out the possibility that abnormal signals in the skull base on MRI may be owing to a reactive change caused by the adjacent tumour; furthermore, slight erosions of the bone cortex may not be detectable by MRI. Fourth, the skull base is a difficult site to biopsy for pathological confirmation of disease, and we were unable to perform a pathological analysis because our entire patient population was treated with definitive chemoradiotherapy. To our knowledge, no previous report has correlated MRI findings with biopsy findings.
The results of our study confirmed that skull base invasion as shown by MRI may be helpful for predicting the outcome of primary chemoradiotherapy in NPC. Although our findings require prospective validation, these results suggest a potential role for MRI findings in predicting local control and prognosis in patients with NPC. Our findings may also have implications for treatment planning.
REFERENCES
- 1.Suárez C, Rodrigo JP, Rinaldo A, Langendijk JA, Shaha AR, Ferlito A. Current treatment options for recurrent nasopharyngeal cancer. Eur Arch Otorhinolaryngol 2010; 267: 1811–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corry J, Fisher R, Rischin D, Peters LJ. Relapse patterns in WHO 2/3 nasopharyngeal cancer: is there a difference between ethnic Asian vs. non-Asian patients? Int J Radiat Oncol Biol Phys 2006; 64: 63–71. [DOI] [PubMed] [Google Scholar]
- 3.Chang JT, See LC, Liao CT, Ng SH, Wang CH, Chen IH, et al. Locally recurrent nasopharyngeal carcinoma. Radiother Oncol 2000; 54: 135–42. [DOI] [PubMed] [Google Scholar]
- 4.Reddy SP, Raslan WF, Gooneratne S, Kathuria S, Marks JE. Prognostic significance of keratinization in nasopharyngeal carcinoma. Am J Otolaryngol 1995; 16: 103–8. [DOI] [PubMed] [Google Scholar]
- 5.Sham JS, Choy D. Prognostic value of paranasopharyngeal extension of nasopharyngeal carcinoma on local control and short-term survival. Head Neck 1991; 13: 298–310. [DOI] [PubMed] [Google Scholar]
- 6.Chua DT, Sham JS, Kwong DL, Tai KS, Wu PM, Lo M, et al. Volumetric analysis of tumor extent in nasopharyngeal carcinoma and correlation with treatment outcome. Int J Radiat Oncol Biol Phys 1997; 39: 711–19. [DOI] [PubMed] [Google Scholar]
- 7.Liu MT, Hsieh CY, Chang TH, Lin JP, Huang CC, Wang AY. Prognostic factors affecting the outcome of nasopharyngeal carcinoma. Jpn J Clin Oncol 2003; 33: 501–8. [DOI] [PubMed] [Google Scholar]
- 8.Cooper J, Flemming ID, Henson DE. American Joint Committee on Cancer manual for staging of cancer. 6th edn. Philadelphia, PA: JB Lippincott; 2002. [Google Scholar]
- 9.Sham JS, Wei WI, Kwan WH, Chan CW, Kwong WK, Choy D. Nasopharyngeal carcinoma. Pattern of tumor regression after radiotherapy. Cancer 1990; 65: 216–20. [DOI] [PubMed] [Google Scholar]
- 10.Chong VF, Fan YF. Skull base erosion in nasopharyngeal carcinoma: detection by CT and MRI. Clin Radiol 1996; 51: 625–31. [DOI] [PubMed] [Google Scholar]
- 11.Sakai O, Curtin HD, Romo LV, Som PM. Lymph node pathology. Benign proliferative, lymphoma, and metastatic disease. Radiol Clin North Am 2000; 38: 979–98. [DOI] [PubMed] [Google Scholar]
- 12.Comoretto M, Balestreri L, Borsatti E, Cimitan M, Franchin G, Lise M. Detection and restaging of residual and/or recurrent nasopharyngeal carcinoma after chemotherapy and radiation therapy: comparison of MR imaging and FDG PET/CT. Radiology 2008; 249: 203–11. 10.1148/radiol.2491071753 [DOI] [PubMed] [Google Scholar]
- 13.Teo P, Yu P, Lee WY, Kwan WH, Yu KH, Choi P, et al. Significant prognosticators after primary radiotherapy in 903 nondisseminated nasopharyngeal carcinoma evaluated by computer tomography. Int J Radiat Oncol Biol Phys 1996; 36: 291–304. [DOI] [PubMed] [Google Scholar]
- 14.Cheng SH, Tsai SY, Horng CF, Yen KL, Jian JJ, Chan KY, et al. A prognostic scoring system for locoregional control in nasopharyngeal carcinoma following conformal radiotherapy. Int J Radiat Oncol Biol Phys 2006; 66: 992–1003. 10.1016/j.ijrobp.2006.06.006 [DOI] [PubMed] [Google Scholar]
- 15.Nishioka T, Shirato H, Kagei K, Abe S, Hashimoto S, Ohmori K, et al. Skull-base invasion of nasopharyngeal carcinoma: magnetic resonance imaging findings and therapeutic implications. Int J Radiat Oncol Biol Phys 2000; 47: 395–400. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Lee JK. Prognostic value of tumor volume in nasopharyngeal carcinoma. Yonsei Med J 2005; 46: 221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 18.Sakata K, Hareyama M, Tamakawa M, Oouchi A, Sido M, Nagakura H, et al. Prognostic factors of nasopharynx tumors investigated by MR imaging and the value of MR imaging in the newly published TNM staging. Int J Radiat Oncol Biol Phys 1999; 43: 273–8. [DOI] [PubMed] [Google Scholar]
- 19.Lin ZX, Li DR, Chen ZJ, Zheng MZ, Shi YY, Lin BH, et al. What is the significance of nasal involvement in nasopharyngeal carcinoma? Int J Radiat Oncol Biol Phys 1999; 45: 907–14. [DOI] [PubMed] [Google Scholar]
- 20.Wang HY, Sun BY, Zhu ZH, Chang ET, To KF, Hwang JS, et al. Eight-signature classifier for prediction of nasopharyngeal [corrected] carcinoma survival. J Clin Oncol 2011; 29: 4516–25. [DOI] [PubMed] [Google Scholar]