Abstract
Transcranial current stimulation (tCS) is a promising noninvasive technique to elicit neuromodulation by passing weak electrical currents through scalp electrodes. While significant effort has been devoted towards designing stimulation protocols which “steer” current to regions of interest, previous work has been almost exclusively focused on the magnitude of the electric field, while ignoring the effects of direction. This is despite previous in vitro studies demonstrating that the angle between the field orientation and the cell axis of symmetry has significant effects on the resulting membrane polarization presumably underlying therapeutic effects. To that end, here we examine the impact of the desired electric field orientation on the optimal placement of electrodes for a given target region. Based on high-resolution head models derived from magnetic resonance scans of patients enrolled in a clinical trial examining the use of tCS in rehabilitation after stroke, we derive and employ an optimization algorithm which computes the montage maximizing directed current flow at the target. The results reveal a strong dependence of the optimal montage on the desired orientation; moreover, the magnitude of the induced electric field at the target region varies widely with the preferred direction. This suggests that identifying the desired electric field orientation at the region of interest is a crucial step in the development of rational electrical stimulation paradigms.
I. Introduction
Transcranial current stimulation (tCS) [1] is an emerging technique for neuromodulation, being investigated for a wide assortment of neurological disorders including, but not limited to: stroke rehabilitation, epilepsy, Parkinson's disease, and major depression. One may argue that clinical findings of therapeutic benefits have appeared faster than the neuroscientific explanations of the underlying action of mechanism. Indeed, the low cost, flexibility, and safety profile of tCS have led to a staggering number of completed and ongoing clinical trials. Despite the fact that the strength of the induced electric fields are weak (in the order of 1 V/m, which is roughly 100 times smaller than that produced during transcranial magnetic stimulation), it has been shown in vitro that tCS significantly alters spike timing and firing rate of neuronal populations, effects which are magnified by network dynamics [2]. At the level of a single cell, the membrane polarization presumably underpinning therapeutic benefits of tCS is dependent on the orientation of the external electric field in relation to the cell axis of symmetry [3]. Moreover, the pyramidal cells in the cortex are generally aligned perpendicularly to the cortical sheet, and thus the effects of stimulation on a patch of cortex stand to vary with the field direction.
Much work has been devoted to the computation of the so-called “forward model” in tCS: given an electrode configuration and applied current intensities, one aims to compute the electric field induced in the head during stimulation – see, for example, [4] – [7]. While such studies are indispensable to the determination of optimal tCS parameters, the analysis has been focused on the field magnitude. The issue of electric field orientation in rational tCS design has been raised previously in [8]. Nevertheless, it remains that clinical trials evaluating efficacy of tCS often employ generic montages in which the anode is placed over the presumed target, while the cathode is positioned at a distant, often extracephalic site. Here, we demonstrate with anatomical and functional magnetic resonance imagery (MRI/fMRI) data acquired from a clinical trial examining the use of tCS to aid rehabilitation after stroke that when selecting the optimized electrode montage, the desired field orientation has profound bearing on the resulting “dosage.”
Specifically, we employ a numerical optimization algorithm in conjunction with MRI based models of the head to compute the electrode montage which maximizes the electric field intensity in a preferred direction at a fixed target region. We demonstrate the strong dependence of the optimal solution on the chosen orientation, with the achieved target intensity exhibiting significant variation with the selected orientation. These results have implications on the clinical translation of tCS – namely, when designing the stimulation protocol, one should specify not only the physiological target, but also the desired orientation of the induced field across that target.
II. Methods
We obtained anatomical MRI scans from 3 stroke victims enrolled in a clinical trial to evaluate the efficacy of tCS to enhance rehabilitation, specifically improvement of symptoms of aphasia, after stroke. The MR images were first segmented automatically and then manually into one of 6 tissue categories: air, bone, skin, cerebrospinal fluid, gray matter, and white matter. The segmented models were then fitted with M = 74 high-density virtual electrodes (radius of 6mm) placed on the scalp according to the international 10/10 system. These electrode locations form the candidate location space from which a much smaller number (i.e., 4) of physical electrodes will be selected. Additionally, conductive gel was inserted into the model directly below each electrode to simulate actual stimulation practice. The resulting 8 tissue types were assigned an average conductivity value following [8], and the model was converted into a finite element (FE) mesh using proprietary software (Simpleware ScanIP, Exeter, UK). We designated electrode Iz as the reference, and “energized” each remaining electrode in succession, solving the induced electric field at all nodes in the head for all M − 1 bipolar configurations using the Abaqus software (Simulia, Providence, RI). These solutions form a linearly independent basis for the “beamforming” problem in tCS, where a multi-electrode montage is specified by an M − 1 length vector whose elements represent current intensities [8]. The net electric field follows as a linear combination of the columns of the “mixing matrix” A which has 3N rows (N is the number of finite element nodes in the brain) and M − 1 columns. Moreover, the element at row n, column m, of A represents the x-component of the electric field induced at node n by stimulating electrode m with unit current density. Similarly, An+N,m and An+2N,m denote the y- and z-components, respectively, of the electric field.
The optimization problem in tCS attempts to find the montage which maximizes some property of the induced electric field; one possible criterion is the field focality, or the concentration of the field around the target region. In this work, however, we focus on maximizing the field intensity at the target regardless of how the field behaves outside the region of interest. Given that the modeled electrodes are small (i.e., a 6mm radius), we restrict the current at each electrode to 1 mA, while allowing for a maximum of 2 mA total current delivered. The cost function is the electric field strength in a specified orientation, leading to the following optimization problem:
| (1) |
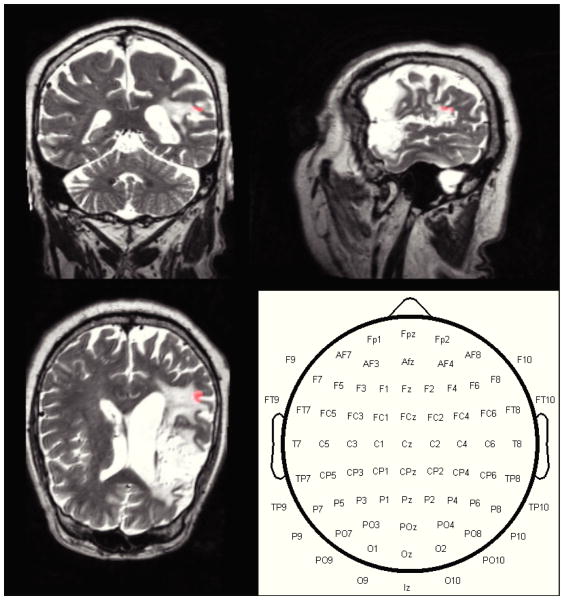
where u is a unit vector pointing in the desired orientation at the target, At is the 3-by-N forward model corresponding to the target area, s is the M − 1 length vector of current intensities defining the montage, Itotal is the total current delivered (i.e., 2 mA), while Imax is the maximum current intensity at each electrode (i.e., 1 mA). As intuition may suggest, the optimal solution always consists of 4 active electrodes each with unit current flow, with 2 electrodes acting as anodes and 2 as cathodes. In other words, both the injected and return currents are split evenly into two electrodes, with the position of these electrodes determining the achieved field intensity and orientation. Additionally, we also computed the electrode montage which maximizes current flow at the target regardless of orientation, by employing an outer optimization procedure (via gradient ascent) to scan for the preferred orientation which maximizes the directed current flow at the region of interest. The optimization was implemented in the MATLAB software (Mathworks, Natick, MA) and relied on the disciplined convex programming package “CVX” [9] to implement the constraints. Finally, for each patient, the target was determined from fMRI data acquired during an overt picture-naming recall task – please refer to [10] for details. The target region (Subject 2) and candidate electrode space are shown in Figure 1.
Fig. 1.
Anatomical MR images and functionally determined target region (highlighted in orange) of Subject 2. The target is in a perilesional area on the left hemisphere. The bottom right panel displays the candidate electrode space: the optimization procedure computes the positions of the anodes and cathodes such that the resulting stimulation maximizes directed current flow at the region of interest.
III. Results
For all subjects, we considered a total of 5 preferred directions: radial (defined as flowing into the head and directed towards the brain's centroid), left, left posterior, posterior, and right posterior. It is important to note that the montage maximizing a direction opposite to one of the above is simply a polarity reversed version of the corresponding configuration: for example, the montage which maximizes left-to-right current flow is equivalent to the montage maximizing right-to-left current flow, but with the polarity of all electrodes inverted (anodes become cathodes, cathodes become anodes). Additionally, we report the montage which maximizes current flow at the target regardless of orientation. This result serves as the upper bound of achievable current intensity at the target. For each preferred direction u, we solve the optimization problem of (1) for ŝ, yielding an M − 1 = 73 element vector which has four non-zero elements, corresponding to the active electrodes, with two being positively charged (anodes) and two negatively (cathodes). By then computing e = Aŝ, the electric field at the region of interest easily follows from the appropriate elements of e.
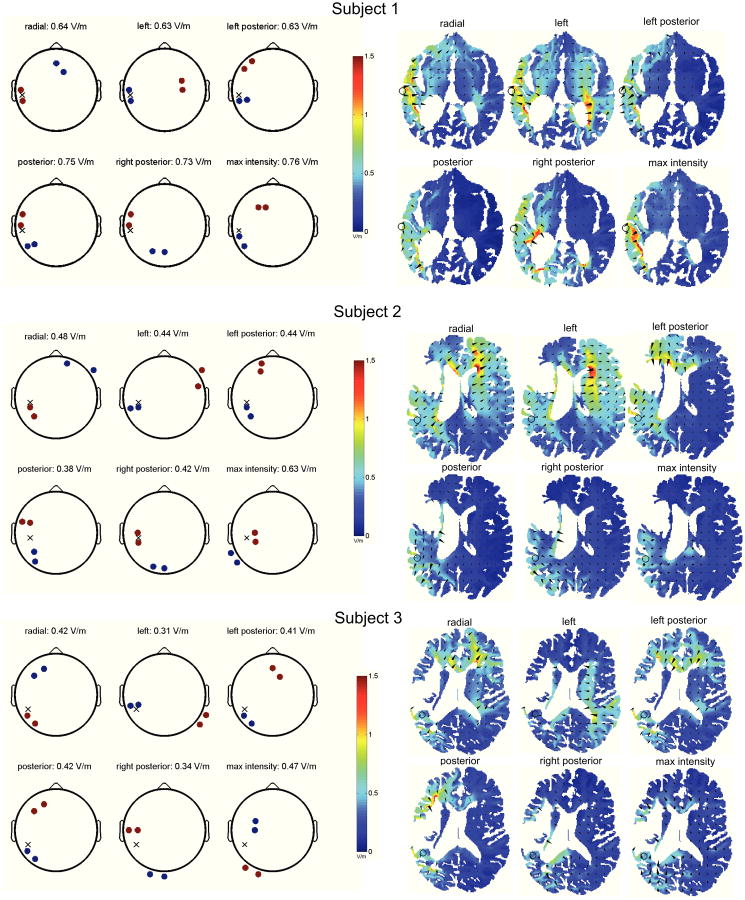
Figure 2 summarizes the results of the optimizations. The left column displays the optimal montages, while the corresponding electric fields (axial slices at the region of interest; color indicates field magnitude, while cones point in the direction of the induced field) are shown on the right. Consider first the optimal montages of subject 1 (top panel), whose target region is located on the brain-cerebrospinal fluid interface in the left hemisphere (temporal lobe).
Fig. 2.
Optimal montage depends on the desired electric field orientation at the target. The left column displays the montages maximizing directed current flow at the target, indicated on the “scalp” with a cross. Anodes (cathodes) are shown in red (blue), with 1 mA of current entering or leaving each electrode. Right column show the predicted electric fields at the axial slices containing the region of interest (indicated with a circle) – both magnitude (color) and direction of field (cones) are shown, and the flow of current from anodes to cathodes, passing through the target, is readily apparent.
The anodes are shown in red, cathodes in blue, and the scalp location whose coordinates minimize Euclidean distance to the target is indicated with a cross. When specifying radial current flow at the target, the optimal montage takes the form of anodes at electrodes T7 and TP7, with the cathodes positioned at AFz and F2. This is an intuitively satisfying configuration in which the injected current enters the head at the temporal lobe, passes through the target with a directed intensity of 0.64 V/m, and exits the brain at the frontal lobe. When desiring the applied current to impinge on the target in a right-to-left fashion, the optimal montage instead consists of cathodes at T7 and TP7, with the anodes located at the more distant FC4 and C4 sites. The achieved electric field has an intensity of 0.63 V/m in the left direction – it is important to understand that this is not equivalent to the electric field strength at the target, but rather the projection of the electric field vector on a unit vector oriented in a right-to-left manner. With this configuration, the injected current enters at the central electrodes on the right hemisphere, while passing through the target as it exits the brain at the temporal lobe. Note the strong polarization of the right lateral ventricle. The optimization of directed current flow in the remaining orientations follows the trend of positioning the anodes and cathodes such that the target is situated in between them along the axis of preferred direction. Moreover, the maximum achievable intensity is 0.76 V/m, as yielded by a montage consisting of anodes at F1 and F3, with the cathodes positioned at TP7 and P7.
The optimization analysis of Subjects 2 and 3 further validate the trends observed above. Note that the achieved current intensities are weaker than those of Subject 1, presumably owing to the fact that the targets are more medially located. Moreover, for Subject 2, the outer optimization which computes the montage maximizing current flow irrespective of its orientation yields a configuration whose achieved intensity (0.63 V/m) is significantly larger than those of the pre-defined orientations (< 0.48 V/m). Finally, it is evident that optimal stimulation does not necessarily follow from placing the anode directly over the target.
Discussion
Conceptually, the seminal study of [1] demonstrates that polarity is critical to achieving the desired neuromodulation: the effect of stimulation on motor excitability was found to be reversed when polarity was flipped. It should be pointed out that polarity is but a special case of orientation, and it would be interesting to perform such behavioral experiments across a larger parameter space than the two orientations examined therein. While the placement of electrodes in [1] was consistent with radial stimulation, the anode and cathode could also be positioned to attain tangential flow at the target, as illustrated in the appropriate montages of Figure 2.
For targets confined to the cortical sheet, the preferred orientation is seemingly straightforward to define: pyramidal neurons are oriented normally to the cortical surface, and thus the aim of the stimulation should be to direct current flow in this “radial” direction. In the case of a target in a sulcus, radial to the cortical sheet typically means tangential to the skull surface. The situation is less clear when attempting to stimulate subcortical regions: in the absence of a dominant cell orientation, one may indeed opt to select the montage which maximizes current magnitude.
On the technical side, it is worthwhile to point out that the maximization of field magnitude (a quadratic function of the vector of applied currents) is a non-convex problem. Here, we have mitigated this issue by instead optimizing the intensity in a specified direction, which follows as the projection of the electric field on a given unit vector, which is a (convex) linear programming problem. By then maximizing this projected field intensity with respect to the preferred direction (the “outer” optimization), we have effectively optimized for the field magnitude.
Ultimately, behavioral experiments and clinical trials will be required to assess the impact of varying current direction at the target. Both prospective and retrospective analyses will prove invaluable in elucidating the role of field orientation on the subsequent neuromodulatory effects.
Acknowledgments
The authors would like to thank Dennis Truong, Paul de Guzman, and Yu Huang for their help in manually segmenting the anatomical scans.
* This work was supported by an NIH-NINDS STTR to Soterix Medical.
Contributor Information
Jacek P. Dmochowski, Email: jdmochowski@ccny.cuny.edu.
Abhishek Datta, Email: adatta@soterixmedical.com.
Jessica Richardson, Email: j.d.richardson@sc.edu.
References
- 1.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology. 2010;527.3:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reato D, Rahman A, Bikson M, Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. Journal of Neuroscience. 2010;33(45):15067–15079. doi: 10.1523/JNEUROSCI.2059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in sub- and suprathreshold uniform electric field stimulation. Brain Stimul. 2009;2:215–228. doi: 10.1016/j.brs.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial DC stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulation. 2009;2(4):201–207. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clinican Neurophysiology. 2006;117:1623–1629. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Holdefer RN, Sadleir R, Russell MJ. Predicted current densities in the brain during transcranial electrical stimulation. Clinical Neurophysiology. 2006;117:1388–1397. doi: 10.1016/j.clinph.2006.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: a computer-based human model study. NeuroImage. 2007;35:1113–1124. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. Journal of Neural Engineering. 2011;8:046011. doi: 10.1088/1741-2560/8/4/046011. [DOI] [PubMed] [Google Scholar]
- 9.Grant M, Boyd S, Ye Y. Disciplined Convex Programming. In: Liberti L, Maculan N, editors. Chapter in Global Optimization: From Theory to Implementation. Springer; 2006. pp. 155–210. [Google Scholar]
- 10.Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41:1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]