Abstract
Objective: Homalium letestui Pellegr (Flacourtiaceae) is used in traditional medicine in parts of Nigeria for the treatment of malaria, ulcer, and inflammatory diseases and as an aphrodisiac. This investigation was aimed to evaluate the cytotoxic, immunomodulatory, and antileishmanial properties of stem extract and fractions of Homalium letestui (H. letestui).
Materials and Methods: Cytotoxic activity against HeLa cells was done using sulphorhodamine (SRB) method and DNA interaction activity using gel electrophoresis. Immunomodulatory activity of the extract in whole blood, neutrophils, and macrophages was also investigated using luminol/lucigenin-based chemiluminescence assay. The extract and fractions were similarly screened for antileishmanial activity against promastigotes of Leishmania major in vitro. The GCMS analysis of the most active fraction against HeLa cells was carried out.
Results: The stem extract exerted prominent cytotoxic activity with the dichloromethane fraction exhibiting the most pronounced effect (GI50 -5.12±1.45 µg/ml, LC50- 57.3±2.33 µg/ml, TGI -12.6±0.87 µg/ml). The crude extract and the fractions did not interact with DNA when investigated using electrophoresis. The extract significantly ((p<0.05 – 0.001) inhibited oxidative burst activity in whole blood (–27.90-66.90%), isolated polymorphonuclear cells (PMNs) (16.50-67.0%), and mononuclear cells (MNCs) (4.31-98.50%) when two different phagocytosis activators (serum opsonizing zymosan-A and PMA) were used. The extract also exhibited moderate antileishmanial activity against promastigotes of Leishmania major in vitro. GCMS analysis of active fraction revealed pharmacologically active compounds.
Conclusion: These results suggest that the stem extract/fractions of H. letestui possess cytotoxic, immunomodulatory, and antileishmanial activities.
Key Words: Antileishmanial, Antioxidant, Cytotoxic, Homalium letestui, Immunomodulatory
Introduction
Homalium letestui Pellegr (Flacourtiaceae) is a forest tree growing up to 25-30 meters and found in the rainforest of West Africa (Hutchinson & Daziel, 1963 ▶; Keay, 1989 ▶). The plant parts, particularly stem bark and root, are used in various decoctions traditionally by the Ibibios of the Niger Delta of Nigeria to treat stomach ulcer, malaria, and other inflammatory diseases as well as an aphrodisiac (Okokon et al., 2006 ▶). Reports of antiplasmodial (Okokon et al., 2006 ▶) and antidiabetic (Okokon et al., 2007 ▶) activities of the plant have been published. However, other members of the family (flacourtiaceae) and genus (Homalium) have been reported to possess various biological activities; anticancer activity has been reported on Casearia capitella (Ismail et al., 2012 ▶), Flacourtia indica has antioxidant activity (Madan et al., 2009; Tyagi et al., 2010 ▶), while Homalium deplanchi has antileishmanial, antitrypanosomal and antitrichomonal activities (Desrivot et al., 2007 ▶). Homalium panayanum has been reported to exert antibacterial activity against some Gram-positive and Gram-negative bacteria. (Chung et al., 2004 ▶) and Homalium cochinchinensis has antiviral activity (Ishikawa et al., 2004 ▶). Casearia sylvestris and Casearia lasiophylla reportedly have cytotoxic activity (Silva et al., 2008 ▶; Salvador et al., 2011 ▶) and Homalium africanum has antifilaricidal (Cho-Ngwa et al., 2010 ▶) activity. Cytotoxic, antioxidant, and antimicrobial activities were reported for Casearia grayi and Scolopia braunii (Mosaddik et al., 2004 ▶). Moreover, anthelmintic activity has been reported for Homalium zeylanicum (Gnananath et al., 2012 ▶). Information on the pharmacology and phytochemistry of H. letestui is scarce. The present study attempts to evaluate the anticancer, antileishmanial, and immunomodulatory activities of this plant in order to provide scientific basis for its use in traditional medicine.
Materials and Methods
Plants collection
The plant material H. letestui (stem) was collected in a forest in Uruan area, Akwa Ibom State, Nigeria in April, 2011. The plant was identified and authenticated by Dr. Margaret Bassey of Department of Botany and Ecological Studies, University of Uyo, Uyo, Nigeria (Voucher no. FPUU. 382).
Extraction
The stem was washed and shade-dried for two weeks. The dried plants’ materials were further chopped into small pieces and reduced to powder. The powdered material was macerated in 70% ethanol. The liquid filtrates were concentrated and evaporated to dryness in vacuo 40 C using rotary evaporator. The crude ethanolic extract (100 g) was further partitioned successively into 1 L each of n-Hexane, dichloromethane, ethyl acetate, and butanol to give the corresponding fractions of these solvents (Okokon et al., 2012 ▶).
Cellular antioxidant (Immunomodulatory) activity
The ethanolic crude extract was screened for cellular antioxidant activities in whole blood, neutrophils, and macrophages using chemiluminescence assay. Briefly, Luminol or lucigenin-enhanced chemiluminescence assay was performed as described by Helfand et al. (1982) ▶ and Haklar et al. (2001) ▶. Briefly, 25 µL diluted whole blood (1:50 dilution in sterile HBSS++) or 25 µL of PMNCs (1×106) or MNCs (5×106) cells were incubated with 25 µL of serially diluted plant extract with concentration ranges between 6.25 and 100 µg/mL. Control wells received HBSS++ and cells but no extract. Tests were performed in white 96 wells plates, which were incubated at 37 C for 30 min in the thermostated chamber of the luminometer. Opsonized zymosan-A or PMA 25 µL, followed by 25 µL luminol (7×105 M) or lucigenin (0.5 mM) along with HBSS++ was added to each well to obtain a 200 µL volume/well. The luminometer results were monitored as chemiluminescence RLU with peak and total integral values set with repeated scans at 30 s intervals and 1 s points measuring time.
Cytotoxic activity
The growth inhibitory and cytotoxic activities of the ethanolic extract and fractions were evaluated against HeLa cells (Cervix cancer cell) by using the sulforhodamine-B assay (Houghton et al., 2007 ▶). The cells (10000 cells/100 µL) in 96-well plate were incubated for 24 h at 37 °C in a humidified 5% CO2 incubator. The stock solutions of ethanolic extract, fractions were prepared in DMSO. Various dilutions of the ethanolic extracts and fractions (0.1, 1, 10, 100, and 250 µg/mL), were added (100 µL) in each well. After 48 h of incubation, 50 µL of cold TCA (50%) was added gently and left for 30 min at room temperature, followed by washing with distilled water and drying overnight. To each well, 100 µL of SRB solution (0.4% wt/vol in 1% acetic acid) was added and after 10 min, the unbound stain was removed by washing with acetic acid (1%), and air-dried at room temperature. The protein bound stain was solubilized with tris base (pH 10.2), and was shaken for 5 min. Absorbance was measured at 515 nm using a microplate reader. The absorbance of the appropriate blanks, including test substance blank, and control (without drug) was used to calculate the growth inhibition and cytotoxicity of the test compounds and represented as GI50, TGI, and LC50 (µg/mL) values.
DNA interaction study using gel electrophoresis
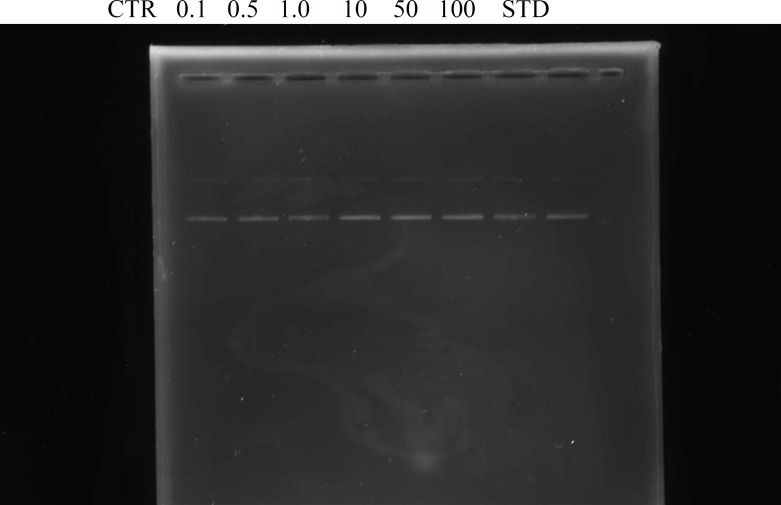
DNA interaction assay was performed according to the protocol of Tian and Hua (2005) ▶. The reaction was carried out in an Eppendorf tube at the total volume of 15 μl containing 0.5 μg of pBR322 DNA in 3 μl of 50 mM phosphate buffer (pH 7.4), and 5 μl of tested samples (DCM fraction) at concentrations 0.1, 0.5, 1.0, 10, 50, and 100 μg/ml and standard drug, paclitaxel, 20 µg/mL. Then, the mixture was incubated at 37 °C for 1 h. The mixture was subjected to 1% agarose gel electrophoresis. DNA bands (open circular, supercoiled, and linear) were stained with ethidium bromide and were analyzed qualitatively by scanning with Doc-IT computer program (VWR).
Antileishmanial activity
The antileishmanial activity of the extracts and fractions were evaluated against promastigotes of Leishmania major (DESTO) in culture using microplates. Leishmania major (L. major) promastigotes were grown in bulk, early in modified NNN biphasic medium, using normal physiological saline. Then, the promastigotes were cultured with RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum (FBS). The parasites L. major were harvested at log phase and centrifuged at 3000 rpm for 10 min.
They were washed three times with saline at same speed and time. Finally, the parasites were counted with the help of Neubauer chamber under the microscope and diluted with fresh culture medium to give a final density of 106 cells/ml. In a 96-well microtiter plate, 180 ml of the culture medium was added in different wells. The extracts and fractions were dissolved in PBS (Phospate buffered saline, pH 7.4 containing 0.5 % MeOH, 0.5 % DMSO) to make a stock concentration of 1000 mg/ml. Twenty µl of each extract/fraction concentration was added to the wells and serially diluted to get working concentrations ranging between 1.0 to 100 µg/ml. One-hundred ml of parasite culture (final density of 106 cells/ml) was added in all wells. Two rows were left, one for negative and other for positive control. Negative controls received the medium while the positive controls received pentamidine and amphotericin B as standard antileishmanial compounds. The plate was incubated between 21-22 °C for 72 h. The culture was examined microscopically for cell viability by counting the number of motile cells on an improved Neubauer counting chamber and IC50 values of compounds possessing antileishmanial activity were calculated (Atta-ur-Rahman, 2001 ▶).
GC-MS analysis of fractions
Quantitative and qualitative data were determined by GC and GC-MS, respectively. The fraction was injected onto a Shimadzu GC-17A system, equipped with an AOC-20i autosampler and a split/splitless injector. The column used was an DB-5 (Optima-5), 30 m, 0.25 mm i.d., 0.25 µm df, coated with 5% diphenyl-95% polydimethylsiloxane, operated with the following oven temperature programme: 50 °C, held for 1 min, rising at 3 °C/min to 250 °C, held for 5 min, rising at 2 °C/min to 280 °C, held for 3 min; injection temperature and volume, 250 °C and 1.0 µl, respectively; injection mode, split; split ratio, 30:1; carrier gas, nitrogen at 30 cm/s linear velocity and inlet pressure 99.8 KPa; detector temperature, 280 °C; hydrogen, flow rate, 50 ml/min; air flow rate, 400 ml/min; make-up (H2/air), flow rate, 50 ml/min; sampling rate, 40 ms. Data were acquired by means of GC solution software (Shimadzu).
Agilent 6890N GC was interfaced with a VG Analytical 70-250s double focusing mass spectrometer. Helium was used as the carrier gas. The MS operating conditions were: ionization voltage 70 eV, ion source 250 °C. The GC was fitted with a 30 m×0.32 mm fused capillary silica column coated with DB-5. The GC operating parameters were identical with those of GC analysis described above.
The identification of components present in the various active fractions of the plants’ extracts was based on direct comparison of the retention times and mass spectral data with those for standard compounds, and by computer matching with the Wiley and Nist Library, as well as by comparison of the fragmentation patterns of the mass spectra with those reported in the literatures (Adams, 2001 ▶; Setzer et al., 2007 ▶).
Statistical analysis
Data are reported as mean±SEM and were analyzed statistically using Tukey-kramer multiple comparison test and values of p<0.001 and 0.05 were considered significant.
Results
The results of cytotoxic activity of crude extract and fractions of Homalium letestui show prominent activity with the hexane fraction exerting highest activity than other fractions and crude extract (Table 1). The potency order was dichloromethane > ethylacetate > butanol > hexane > aqueous > crude extract.
Table 1.
Cytotoxic activity of crude extract and fractions of stem of Homalium letestui against HeLa cells
| Extract/Fraction | GI 50 (µg/ml) | LC 50 (µg/ml) | TGI (µg/ml) |
|---|---|---|---|
| Crude Extract | 116.0±1.45 | 240.3±3.46 | 204.6±1.45 |
| Hexane Fraction | 60.0±0.87 | - | 77.0±1.52 |
| DCM Fraction | 5.12±1.45 | 57.3±2.33 | 12.6±0.87 |
| Ethyl Acetate Fraction | 4.33±0.87 | 60.3±2.60 | 11.0±1.52 |
| Butanol | 16.0±2.31 | 68.2±4.50 | 9.66±1.20 |
| Aqueous Fraction | 64.6±0.87 | - | 81.3±1.66 |
| Doxorubucin | 0.61±0.03 µM | 7.80±0.80 µM | 3.60±0.30 µM |
Data are represented as mean±SEM of three independent experiments. Values in the table are concentrations of extract/fraction expressed as µg/ml, GI50=Concentration of the drug causing 50% growth inhibition of the cells, TGI = Concentration of the drug causing total growth inhibition of the cells, LC50 = Lethal concentration of the drug that killed 50% of the cells.
Cytotoxic activity against HeLa cells
Gel Electrophoresis
Gel electrophoresis results show that treatment of E. coli DNA with various concentrations of the hexane fraction of H. letestui did not produce any effect on the DNA. This effect was also observed with the standard drug used, paclitaxel (Figure 1).
Figure 1.
The effect of various concentrations of hexane fraction of H. letestui on DNA interaction using gel electrophoresis
Cellular antioxidant activity
Ethanolic stem extract of H. letestui was observed to produce significant (p<0.05 – 0.001) inhibitory effect on the oxidative burst activities of neutrophils and macrophages activated with Zymosan-A or PMA in a dose-dependent manner especially at the highest doses used. In the whole blood, the extract exhibited pro-oxidant activity at low doses (1-10 µg/ml) and antioxidant activity at the highest dose (100 µg/ml) with inhibitory effect of 59.6% (Table 2).
Table 2.
Cellular antioxidant activity of ethanolic stem extract of Homalium letestui
| Cell type | Dose (µg/Ml) | %Inhibition (Rlu) |
|---|---|---|
| Whole Blood | 1 | -7.80±2.77 |
| 10 | -2.70±0.82 | |
| 100 | 59.6 ±1.44b | |
| Neutrophils (Intracellular) | 0.5 | 0.00±0.00 |
| 5 | 13.50 ±3.87a | |
| 50 | 70.20 ± 3.52c | |
| Neutrophils (Extracellular) | 0.5 | 30.40± 1.79a |
| 5 | 51.10± 2.60b | |
| 50 | 65.70 ± 7.16c | |
| Macrophages | 0.5 | 31.30±4.39a |
| 5 | 56.60±3.58b | |
| 50 | 73.10±2.19c |
Data are represented as mean±SEM of three independent experiments. Significant at:
p< 0.05,
p< 0.01,
p< 0.001 when compared to control.
Antileishmanial activity
Crude extract and fractions of ethanolic stem extract of H. letestui exerted significant antileishmanial activity when tested against promastigotes of L. major. Ethyl acetate fraction exerted a higher activity than other fractions and crude extract though uncomparable to the standard drugs, pentamidine, and amphotericin B (Table 3).
Table 3.
Antileishmanial activity of Homalium letestui (ED50).
| Extract/Fraction | ED 50(µg/ml) |
|---|---|
| Crude Extract | 26.90±0.56 |
| Hexane Fraction | >100 |
| DCM Fraction | 63.06±1.90 |
| Ethyl Acetate Fraction | 21.50±0.16 |
| Butanol Fraction | >100 |
| Aqueous Fraction | >100 |
| Pentamidine | 5.09±0.04 |
| Amphotericin B | 0.29±0.05 |
Data are represented as mean±SEM (n=3).
GC-MS analysis
The results of GCMS analysis of dichloromethane fraction of stem extract of H. letestui revealed the presence of pharmacologically active compounds as shown on Table 4.
Table 4.
GC –MS Analysis of Dichloromethane Fraction of Homalium letestui
| S/No. | Name of Compound | Mol.Wt | Chemical Formula | RI | Concentration % |
|---|---|---|---|---|---|
| 1. | 2,4 Heptadien-6-ynal,(E,E) | 106 | C7H6O | 25 | 0.912 |
| 2. | 2-(4-formyphenyloxy)-acetic acid | 180 | C9H8O4 | 172 | 0.206 |
| 3. | 2-Coumaranone | 134 | C8H6O2 | 195 | 0.968 |
| 4. | Benzoic acid | 122 | C7H6O2 | 200 | 0.202 |
| 5. | 4-Hepten-3-one,5-methyl,(E)- | 126 | C8H14O2 | 207 | 8.876 |
| 6. | Salicyl alcohol | 124 | C7H8O2 | 234 | 3.286 |
| 7. | 4-Hepten-3-one,5-methyl,(E)- | 126 | C8H14O | 207 | 0.832 |
| 8. | Vanillin | 152 | C8H8O3 | 312 | 0.915 |
| 9. | 3,4,5-trimethoxy phenol | 184 | C9H12O4 | 456 | 5.761 |
| 10. | 2,4 Decadienal,(E,Z) | 152 | C10H16O | 428 | 0.872 |
| 11. | 4-(3-hydroxy-1-propenyl)-2-methoxy phenol | 180 | C10H12O3 | 527 | 2.017 |
| 12. | 4-hydroxy-3,5-dimethoxy Benzaldehyde | 182 | C9H10O4 | 477 | 0.866 |
| 13. | 5,6-dimethoxyphthaldehydic acid | 210 | C10H10O5 | 662 | 0.634 |
| 14. | Benzene(methylsulfinyl) methyl | 154 | C8H10O5 | 596 | 0.595 |
| 15. | 4-phenyl isocoumarin | 222 | C15H10O2 | 697 | 2.147 |
| 16. | 2,4-dinitrophenyl hydrazine butanal | 252 | C12H12N4O4 | 743 | 0.281 |
| 17. | 9H-Xanthen-9-one, 1,3-dihyroxy-4-methyl | 242 | C14H10O4 | 789 | 1.580 |
| 18. | Methanone,(2,4-dihydroxyphenyl) phenyl | 214 | C13H10O3 | 805 | 2.147 |
| 19. | 1,2,3,4-tetrahydro-5,8-dimethoxy-9,10-anthracenedione | 272 | C16H16O4 | 950 | 1.491 |
| 20. | Camphor | 327 | C19H21NO4 | 1153 | 5.589 |
| 21. | α-Terpineol | 154 | C10H18O | 1185 | 15.642 |
Discussion
Homalium letestui is used traditionally in the treatment of various ailments and diseases. The stem which has been reported to possess some pharmacological properties have been found in this study to exert pronounced cytotoxic activity against HeLa cells with the dichloromethane fraction having the highest activity. The cytotoxic mechanism of action was found to be unrelated to DNA interaction and is likely to involve interference with cell division processes. Anticancer and cytotoxic activities against cancer cell lines have been reported on Casearia sylvestris (Silva et al., 2008 ▶), Casearia lasiophylla (Salvador et al., 2011 ▶), Flacourtia indica (Pachute et al., 2011 ▶), and Casearia capitella (Ismail et al., 2012 ▶), all members of flacourtiaceae family and the activity has been ascribed to the presence of monoterpenes and sesquiterpenes in these plants as well as the presence of phenolic and polyphenolic compounds. However, the GCMS analysis revealed the presence of some pharmacologically active compounds such as anthracenedione, 2,4-Decadienal, (E,Z), vanillin, and 1,3-dihydroxy-4-methyl-9H-Xanthen-9-one which have been implicated in the anticancer activity of plants (Nappez et al.,1996 ▶; Murakami et al., 2007 ▶; Pedraza-Chaverri et al., 2008 ▶; Zhang et al. 2010 ▶; Mansour et al., 2010 ▶). These compounds and others are likely to be involved in the cytotoxic activity of this extract.
The stem extract was also observed to exhibit strong antioxidant activity in whole blood, neutrophils (extracellular and intracellular), and macrophages. This activity may have resulted from the presence of some polyphenolic compounds such as vanillin, 2-Coumaranone, 3, 4, 5-trimethoxy phenol and 4-phenyl isocoumarin, and 4-(3-hydroxy-1-propenyl)-2-methoxy phenol as well as monoterpene (α-Terpineol) as revealed by GCMS analysis. These compounds have been reported to possess antioxidant activity (Murakami et al., 2007 ▶; Raja et al., 2011 ▶; Soobrattee et al., 2005 ▶; Falah et al., 2008 ▶). Other members of the family such as Homalium brachybotrys, Flacourtia indica, Casearia grayi, and Scolopia braunii have been reported to possess antioxidant activities due to presence of polyphenolic compounds (Tyagi et al., 2010 ▶; Mosaddik et al., 2007 ▶; Mosaddik et al., 2004 ▶). The GCMS analysis also revealed the presence of some phenolic compounds such as xanthones which have been implicated for many biological activities such as antioxidant, antitumoral, anti-inflammatory, antiallergy, antibacterial, antifungal, and antiviral activities (Pedraza-Chaverri et al., 2008 ▶; Suksamrarn et al., 2006 ▶). These compounds present in this plant’s extract may be responsible for its antioxidant activity reported in the current study. The extract as well as the fractions especially dichloromethane fraction possess a significant cytotoxic activity against HeLa cells in culture. The significant antioxidant activity of this extract explains the strong cytotoxic activity of the stem extract. Generation of reactive oxygen species has been implicated in the pathogenesis of cancer and other diseases (Halliwel and Gutteridge, 1999 ▶). The activities of antioxidant counteract the redox state precipitated intracellularly and hence ensure cytotoxicity. This could possibly be one of the mechanisms of cytotoxic activity of this extract.
The stem extract also demonstrated antileishmanial activity. The extract was also observed to possess antileishmanial activity on L. major. Homalium deplanchi has also been reported to have antileishmanial, antitrypanosomal, and antitrichomonal activities (Desrivot et al., 2007 ▶) indicating a strong antiprotozoal activity. Antimicrobial activities are known to be promoted by proxidant state. In this study, lower doses of the extract have been observed to exhibit pro-oxidant activity. This activity has been reported to enhance antimicrobial activity (Anderson et al., 1981 ▶). Moreover, bioactive compounds such as xanthones which have been implicated in immune stimulation and antimicrobial activities have been reported to be present in this extract. Xanthones have been reported to possess antileishmanial activity (Mbwambo et al., 2006 ▶) and camphor, a well-known chemical with its pronounced antimicrobial potentials (Pattnaik et al., 1997 ▶) is also present in this extract. These compounds present in this plant may be responsible for its antileishmanial activity. This is the first report of antileishmanial activity of H. letestui.
From the results of this study, it can be concluded that the stem bark extract of H. letestui has cytotoxic activity against HeLa cells, immunomodulatory and antileishmanial activities which are due to the phytochemical constituents of the extract and fractions.
Acknowledgment
Dr. Jude Okokon is grateful to TWAS for financial support for postdoctoral fellowship and ICCBS for providing research facilities
Conflict of interest
There is no conflict of interest in this study.
References
- Adams RP. Identification of Essential oils by Gas Chromatography Quadrupole Mass Spectrometry. USA: Allured Publishing Corporation, Carol Stream; 2001. [Google Scholar]
- Anderson R, Gatner EMS, van Rensburg CE, Grabow G, Imkamp FH, Kok SK, van Rensburg J. In vitro and In vivo effect of dapsone on neutrophils and lymphocyte functions in normal individuals and patients with lepromatous leprosy. Antimicrob Agents Chemotherapy. 1981;19:495–503. doi: 10.1128/aac.19.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta-ur-Rahman , Choudhary MI, William JT. Bioassay techniques for drug development. Harward Academic Publisher; 1997. pp. 67–68. [Google Scholar]
- Cho-Ngwa F, Abongwa M, Ngemenya MN, Nyongbela KN. Selective activity of extracts of Margaritaria discoidea and Homalium africanum on Onchocerca ochengi. BMC Complement Altern Med. 2010;10:62. doi: 10.1186/1472-6882-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung PY, Chung LY, Ngeow YF, Goh SH, Imiyabir Z. Antimicrobial activities of Malaysian plant species. Pharm Biol. 2004;42:292–300. [Google Scholar]
- Desrivot J, Waikedre J, Cabalion P, Herrenknecht C, Bories C, Hocquemiller R, Fournet A. Antiparasitic activity of some New Caledonian medicinal plants. J Ethnopharmacol. 2007;112:7–12. doi: 10.1016/j.jep.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Ekabo OA, Farnsworth NR, Santisuk T, Reutrakul V. A phytochemical investigation of Homalium ceylanicum. J Nat Prod. 1993;56:699–707. doi: 10.1021/np50095a006. [DOI] [PubMed] [Google Scholar]
- Falah S, Katayama T, Suzuki T. Chemical constituents from Gmelina arborea bark and their antioxidant activity. J Wood Sci. 2008;54:483–489. doi: 10.3923/pjbs.2008.2007.2012. [DOI] [PubMed] [Google Scholar]
- Gnananath K, Kumar GP, Reddy CR, Kumar BN, Kumar RV. Evaluation of anthelmintic activity in the bark of Homalium zeylanicum. Int Res J Pharm. 2012;3:436–437. [Google Scholar]
- Haklar G, Ozveri ES, Yuksel M, Aktan A, Yalcin AS. Different kinds of reactive oxygen and nitrogen species were detected in colon and breast tumors. Cancer Lett. 2001;165:219–224. doi: 10.1016/s0304-3835(01)00421-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3rd ed. Oxford: Oxford University Press; 1999. [Google Scholar]
- Helfand S, Werkmeister J, Roder J. Chemiluminescence response of human natural killer cells. I. The relationship between target cell binding, chemiluminescence, and cytolysis. J Exp Med. 1982;156:492–505. doi: 10.1084/jem.156.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton P, Fang R, Techatanawat I, Steventon G, Hylands PJ, Lee CC. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods. 2007;42:377–387. doi: 10.1016/j.ymeth.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Hutchinson T, Daziel JM. Flora of West Tropical Africa. Vol. 2. London: Crown Agents for overseas government; 1963. [Google Scholar]
- Ishikawa T, Nishigaya K, Takami K, Uchikoshi H, Chen IS, Lih T. Isolation of salicin derivatives from Homaliumcochinchinensis and their antiviral activities. J Nat Prod. 2004;67:659–663. doi: 10.1021/np034052o. [DOI] [PubMed] [Google Scholar]
- Ismail M, Bagalkotkar G, Iqbal S, Adamu HA. Anticancer properties and phenolic contents of sequentially prepared extracts from different parts of selected medicinal plants indigenous to Malaysia. Molecules. 2012;17:5745–5756. doi: 10.3390/molecules17055745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay RWJ. In: Trees of Nigeria: A revised version of Nigerian trees. Keay RWJ, Onoche CFA, Stanfield DP, editors. (Vol 1 and 2) Oxford: Clarendon Press; 1989. [Google Scholar]
- Mansour OC, Evison BJ, Sleebs BE, Watson KG, Nudelman A, Rephaeli A., Buck DP, Collins JG, Bilardi RA, Phillips DR, Cutts SM. New Anthracenedione derivatives with improved biological activity by virtue of stable drug-DNA adduct formation. J Med Chem. 2010;53:6851–6866. doi: 10.1021/jm901894c. [DOI] [PubMed] [Google Scholar]
- Mbwambo ZH, Kapingu MC, Moshi MJ, Machumi F, Apers S, Cos P, Ferreira D, Jannie P, Marais JP, Dirk Berghe V, Maes L, Vlietinck A, Pieters L. Antiparasitic activity of some xanthones and biflavonoids from the root bark of Garcinia liwingstonei. J Nat Prod. 2006;69:369–372. doi: 10.1021/np050406v. [DOI] [PubMed] [Google Scholar]
- Madan S, Pannakal S, Ganapaty S, Singh GN, Kumar Y. Phenolic glucosides from Flacourtia indica. Nat Prod Commun. 2009;4:381–384. [PubMed] [Google Scholar]
- Mosaddik MA, Banbury L, Forster P, Booth R, Markham J, Leach D, Waterman PG. Screening of some Australian Flacourtiaceae species for in vitro antioxidant, cytotoxic and antimicrobial activity. Phytomedicine. 2004;11:461–466. doi: 10.1016/j.phymed.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Mosaddik A, Waterman PG. Three new 3-benzylbenzofuran-2-one derivatives from Homalium brachybotrys (Flacourtiaceae/Salicaceae) Nat Prod Res. 2007;21:1191–1198. doi: 10.1080/14786410601130679. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Hirata A, Ito S, Shoji M, Tanaka S, Yasui T, Machino M, Fujisawa S. Re-evaluation of Cyclooxygenase-2-inhibiting Activity of Vanillin and Guaiacol in Macrophages Stimulated with Lipopolysaccharide. Anticancer Res. 2007;27:801–808. [PubMed] [Google Scholar]
- Nappez C, Battu S, Beneytout JL. Trans, trans-2,4-decadienal: cytotoxicity and effect on glutathione level in human erythroleukemia (HEL) cells. Cancer Lett. 1996;99:115–119. doi: 10.1016/0304-3835(95)04045-5. [DOI] [PubMed] [Google Scholar]
- Okokon JE, Ita BN, Udokpoh AE. Antimalarial activity of Homaliumletestui. Phytotherapy Res. 2006;20:949–951. doi: 10.1002/ptr.1983. [DOI] [PubMed] [Google Scholar]
- Okokon JE, Antia BS, Ita BN. Antidiabetic effects of Homalium Letestui (Flacourtiaceae) in streptozotocin induced diabetic rats. Res J Med Plant. 2007;1:134–138. [Google Scholar]
- Okokon JE, Dar A, Choudhary MI. Chemical constituents and analgesic activity of Telfaria occidentalis. Phytopharmacology. 2012;3:359–366. [Google Scholar]
- Pachute AP, Tyagi S, Mishra A, Shakya A., Kumar D, Patel VK. Preliminary evaluation of anticancer activity of Flacourtia indica Merr. Bot Res Int. 2011;4:43–47. [Google Scholar]
- Pattnaik S, Subramanyam VR, Bapaji M, Kole CR. Antibacterial andantifungal activity of aromatic constituents of essential oils. Microbios. 1997;89:39–46. [PubMed] [Google Scholar]
- Pedraza-Chaverri J, Cardenas-Rodriguez N, Orozco- Ibarra M, Perez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem Toxicol. 2008;46:3227–3239. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Raja Rajeswari N, RamaLakshmi S, Muthuchelian K. GC-MS analysis of bioactive components from the ethanolic leaf extract of Canthium dicoccum (Gaertn.) Teijsm & Binn. J Chem Pharm Res. 2011;3:792–798. [Google Scholar]
- Salvador MJ, Carvalho J, Wisniewski A, Kassuya C, Santos EP, Riva D, Stefanello ME. Chemical composition and cytotoxic activity of the essential oil from the leaves of CaseariaLasiophylla. Brazilian J Pharmacognosy. 2011;21:864–868. [Google Scholar]
- Setzer WN, Stokes SL, Penton AF, Takaku S, Haber WA, Hansell E, Caffrey CR, McKerrow JH. Cruzain Inhibitory Activity of Leaf Essential Oils of Neotropical Lauraceae and Essential Oil Components. Nat Prod Commun. 2007;2:1203–1210. [Google Scholar]
- Silva SL, Chaar J, Figueiredo P, Yano T. Cytotoxic evaluation of essential oil from Casearia sylvestris Sw on human cancer cells and erythrocytes. Acta Amazonica. 2008;38:107–112. [Google Scholar]
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T, 2005 Mutat Res. Phenolics as potential antioxidant therapeutic agents: mechanism and actions;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Suksamrarn S, Komutiban O, Ratananukul P, Chimnoi N, Lartpornmatulee N, Suksamrarn A. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem Pharm Bull. 2006;54:301–305. doi: 10.1248/cpb.54.301. [DOI] [PubMed] [Google Scholar]
- Tian B, Hua Y. Concentration dependence of prooxidant and antioxidant effects of aloin and aloe-emodin on DNA. Food Chem. 2005;91:413–418. [Google Scholar]
- Tyag SN, Rakshit V, Singh A, Raghvendra P, Saxena A, Patel BD. In vitro antioxidant activity of methanolic and aqueous extract of Flacourtia indica Merr. Am-Euras J Sci Res. 2010;5:201–206. [Google Scholar]
- Zhang J, Tao L, Liang Y, Chen L, Mi Y, Zheng L, Wang F, She Z, Lin Y, To KK, Fu L. Anthracenedione Derivatives as Anticancer Agents Isolated from Secondary Metabolites of the Mangrove Endophytic Fungi. Mar Drugs. 2010;8:1469–1481. doi: 10.3390/md8041469. [DOI] [PMC free article] [PubMed] [Google Scholar]