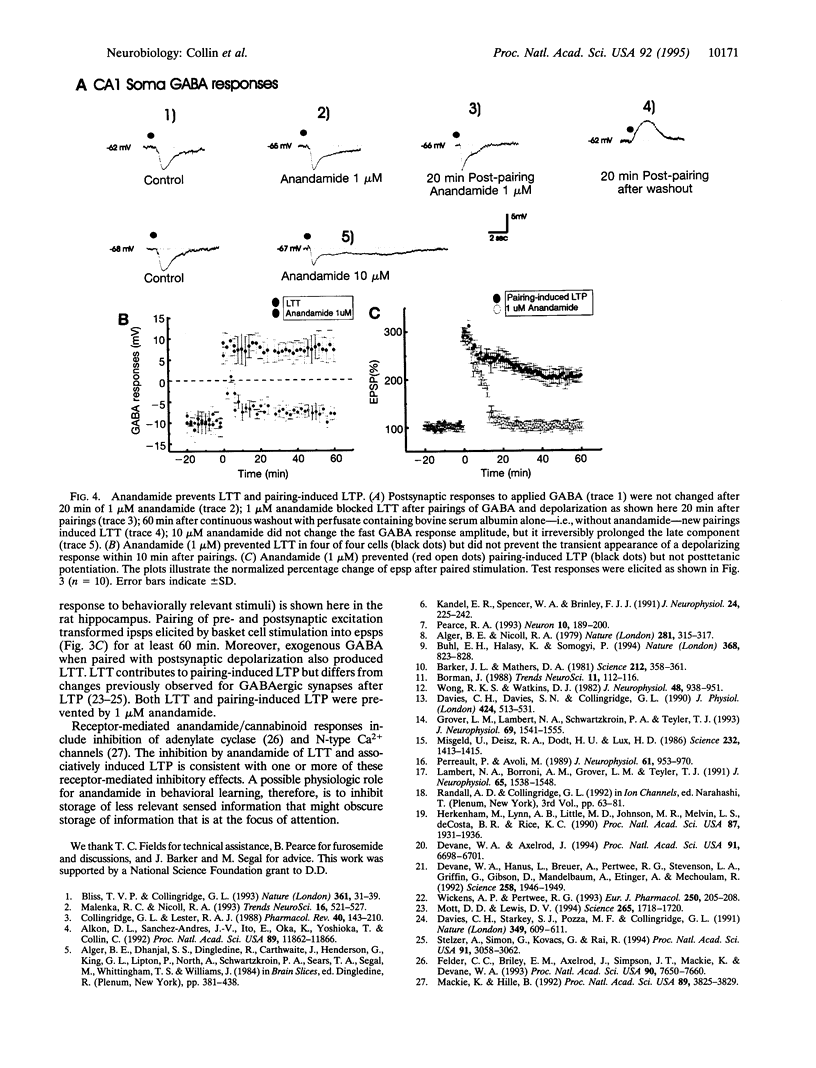
Abstract
Evidence is presented for a distinctive type of hippocampal synaptic modification [previously described for a molluscan gamma-aminobutyric acid (GABA) synapse after paired pre- and postsynaptic excitation]: transformation of GABA-mediated synaptic inhibition into synaptic excitation. This transformation persists with no further paired stimulation for 60 min or longer and is termed long-term transformation. Long-term transformation is shown to contribute to pairing-induced long-term potentiation but not to long-term potentiation induced by presynaptic stimulation alone. Further support for such mechanistic divergence is provided by pharmacologic effects on long-term transformation as well as these two forms of long-term potentiation by Cl- channel blockers, glutamate and GABA antagonists, as well as the endogenous cannabinoid ligand anandamide.
Full text
PDF
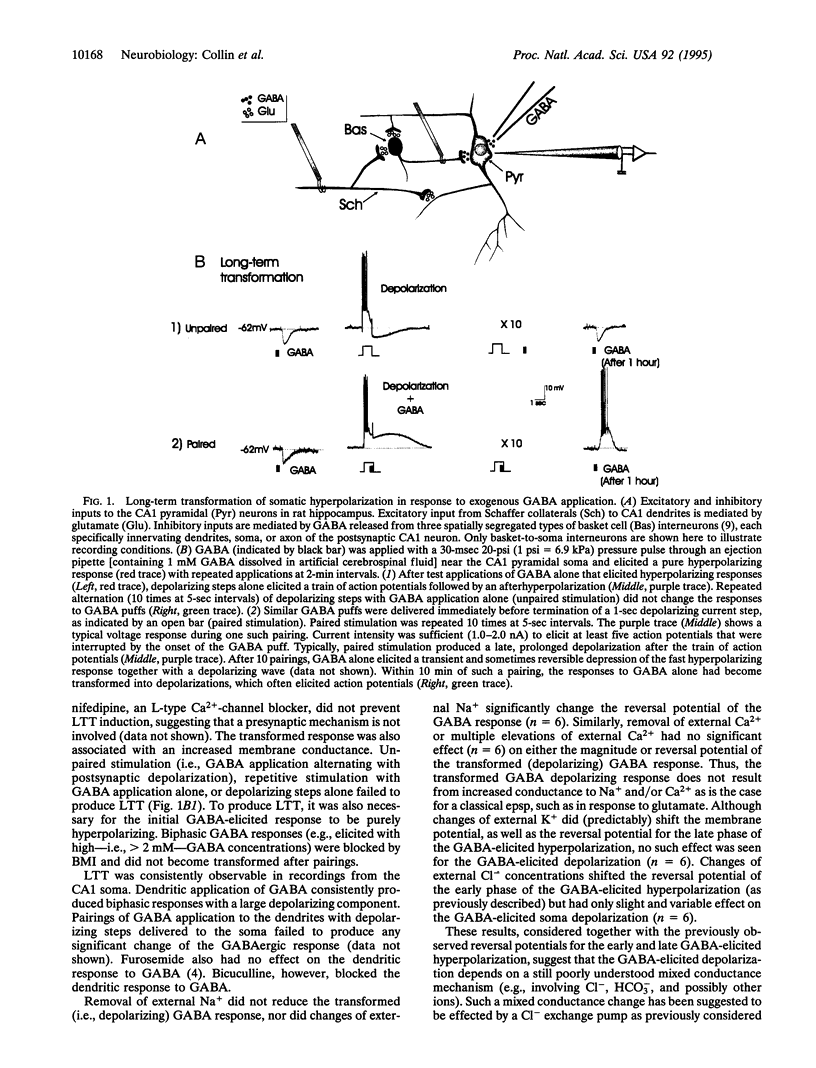
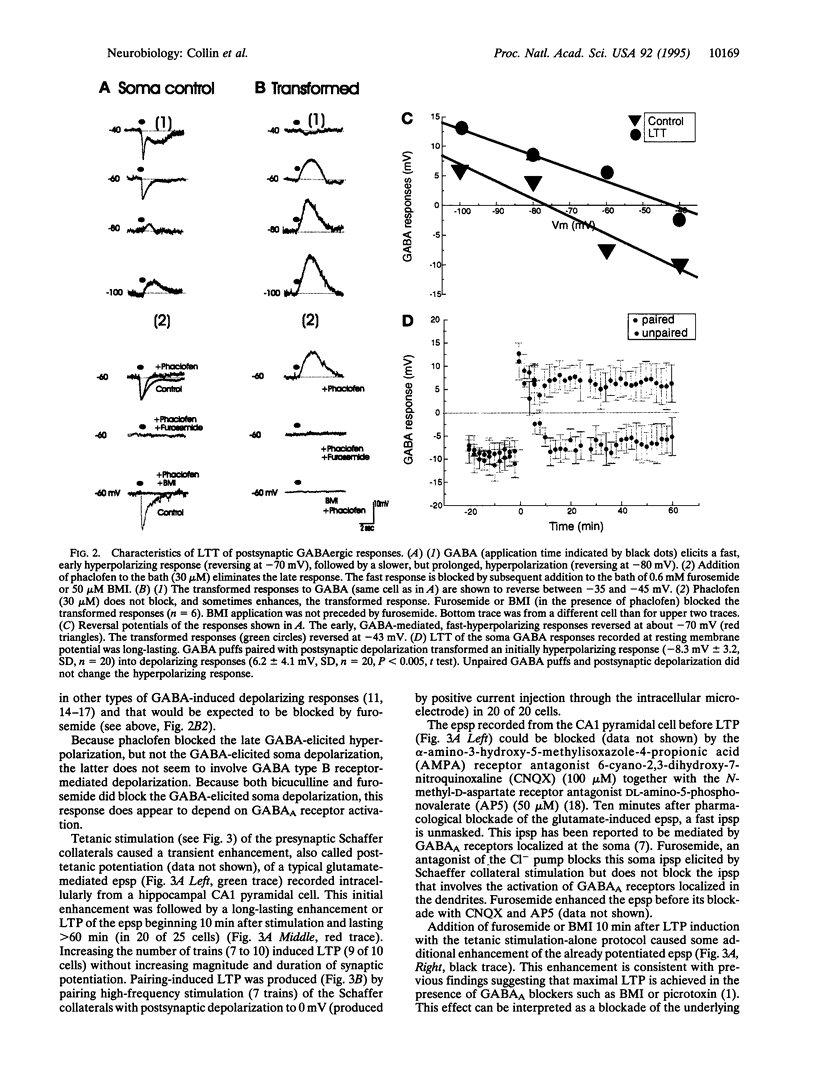
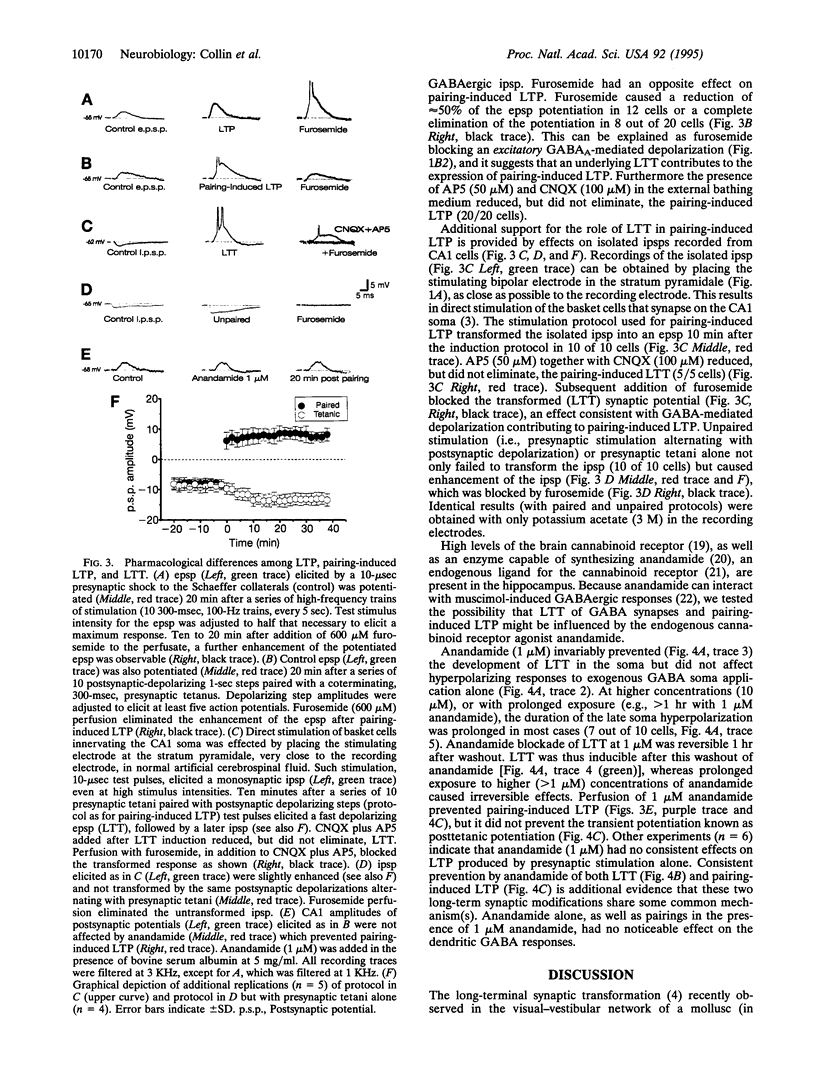
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. GABA-mediated biphasic inhibitory responses in hippocampus. Nature. 1979 Sep 27;281(5729):315–317. doi: 10.1038/281315a0. [DOI] [PubMed] [Google Scholar]
- Alkon D. L., Sánchez-Andrés J. V., Ito E., Oka K., Yoshioka T., Collin C. Long-term transformation of an inhibitory into an excitatory GABAergic synaptic response. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11862–11866. doi: 10.1073/pnas.89.24.11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Mathers D. A. GABA analogues activate channels of different duration on cultured mouse spinal neurons. Science. 1981 Apr 17;212(4492):358–361. doi: 10.1126/science.6259733. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bormann J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988 Mar;11(3):112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- Buhl E. H., Halasy K., Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994 Apr 28;368(6474):823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Davies S. N., Collingridge G. L. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990 May;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. H., Starkey S. J., Pozza M. F., Collingridge G. L. GABA autoreceptors regulate the induction of LTP. Nature. 1991 Feb 14;349(6310):609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- Devane W. A., Axelrod J. Enzymatic synthesis of anandamide, an endogenous ligand for the cannabinoid receptor, by brain membranes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6698–6701. doi: 10.1073/pnas.91.14.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992 Dec 18;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Briley E. M., Axelrod J., Simpson J. T., Mackie K., Devane W. A. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover L. M., Lambert N. A., Schwartzkroin P. A., Teyler T. J. Role of HCO3- ions in depolarizing GABAA receptor-mediated responses in pyramidal cells of rat hippocampus. J Neurophysiol. 1993 May;69(5):1541–1555. doi: 10.1152/jn.1993.69.5.1541. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Lynn A. B., Little M. D., Johnson M. R., Melvin L. S., de Costa B. R., Rice K. C. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A., BRINLEY F. J., Jr Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961 May;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- Lambert N. A., Borroni A. M., Grover L. M., Teyler T. J. Hyperpolarizing and depolarizing GABAA receptor-mediated dendritic inhibition in area CA1 of the rat hippocampus. J Neurophysiol. 1991 Nov;66(5):1538–1548. doi: 10.1152/jn.1991.66.5.1538. [DOI] [PubMed] [Google Scholar]
- Mackie K., Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C., Nicoll R. A. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993 Dec;16(12):521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Misgeld U., Deisz R. A., Dodt H. U., Lux H. D. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986 Jun 13;232(4756):1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- Pearce R. A. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993 Feb;10(2):189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Perreault P., Avoli M. Effects of low concentrations of 4-aminopyridine on CA1 pyramidal cells of the hippocampus. J Neurophysiol. 1989 May;61(5):953–970. doi: 10.1152/jn.1989.61.5.953. [DOI] [PubMed] [Google Scholar]
- Randall A. D., Collingridge G. L. Amino acid receptor-mediated synaptic currents in the CA1 region of the hippocampus. Ion Channels. 1992;3:63–81. doi: 10.1007/978-1-4615-3328-3_3. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Simon G., Kovacs G., Rai R. Synaptic disinhibition during maintenance of long-term potentiation in the CA1 hippocampal subfield. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3058–3062. doi: 10.1073/pnas.91.8.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens A. P., Pertwee R. G. delta 9-Tetrahydrocannabinol and anandamide enhance the ability of muscimol to induce catalepsy in the globus pallidus of rats. Eur J Pharmacol. 1993 Nov 30;250(1):205–208. doi: 10.1016/0014-2999(93)90646-y. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Watkins D. J. Cellular factors influencing GABA response in hippocampal pyramidal cells. J Neurophysiol. 1982 Oct;48(4):938–951. doi: 10.1152/jn.1982.48.4.938. [DOI] [PubMed] [Google Scholar]