Abstract
Aim:
Acetazolamide (AZA), a carbonic anhydrase (CA) inhibitor, has been found to alleviate inflammatory and neuropathic pain in rats. In the present study, we investigated the effects of AZA on thermal- and chemical-stimulated acute pain in mice and the possible mechanisms underlying the effects.
Methods:
Five acute pain models based on thermal and chemical stimuli were established to investigate the effects of AZA on different types of nociception in mice. The antinociceptive effects of methazolamide (another CA inhibitor) and diazepam (a positive allosteric modulator of GABAA receptor) were also examined. The drugs were administered either intraperitoneally (ip) or intrathecally.
Results:
AZA (50–200 mg/kg, ip) did not produce analgesia in two thermal-stimulated acute pain models, ie, mouse tail-flick and hot-plate tests. In contrast, AZA (50–200 mg/kg, ip) dose-dependently reduced paw licking time in both capsaicin and formalin tests in mice. A similar result was observed in a mouse acetic acid-induced writhing test. However, AZA (10 nmol/mouse, intrathecally) did not produce significant analgesia in the 3 chemical-stimulated acute pain models. In addition, methazolamide (50–200 mg/kg, ip) and diazepam (0.25–1.0 mg/kg, ip) did not produce significant analgesia in either thermal- or chemical-stimulated acute pain.
Conclusion:
AZA produces analgesia in chemical-stimulated, but not thermal-stimulated acute pain in mice. The attenuation of chemical-stimulated acute pain by AZA may not be due to enhancement of GABAA receptor-mediated inhibition via inhibiting CA activity but rather a peripheral ion channel-related mechanism.
Keywords: acetazolamide, methazolamide, diazepam, analgesia, tail-flick test, hot-plate test, capsaicin test, formalin test, acetic acid-induced writhing test, carbonic anhydrase, GABAA receptor
Introduction
Pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage1. This condition is classified as acute pain or chronic pain. Acute pain serves as a warning of disease or a threat to the body, including physical (thermal and mechanical stimuli) and chemical (capsaicin, formalin and acetic acid stimuli)-induced pain. Chronic pain is a type of disease and is often classified as chronic inflammatory pain or neuropathic pain.
Acetazolamide (AZA), a sulfonamide derivative, has various pharmacological activities. It is well known that AZA is a carbonic anhydrase (CA) inhibitor that has been shown to be effective in the treatment of glaucoma through the inhibition of HCO3– production2. AZA is a diuretic agent that can increase renal potassium excretion3 and alkalinize urine4 and it is a potent vasodilator of the cerebral vasculature as well5. Most recently, increasing interest has been focused on AZA as a prophylactic and therapeutic agent for acute mountain sickness6.
AZA has also been reported to relieve inflammation- and peripheral nerve injury-induced chronic pain. Injection with 3% carrageenan suspension into the left gastrocnemius muscle belly induced typical inflammatory pain in rats, resulting in thermal hyperalgesia and mechanical allodynia. However, 24 h after carrageenan injection in rats, intraperitoneal (ip) or intrathecal administration of AZA attenuated the inflammation-induced thermal hyperalgesia7. In a rat spinal nerve ligation (SNL) model, a typical neuropathic pain model, intrathecal administration of AZA not only reduced mechanical allodynia but also produced synergistic analgesia along with midazolam8. In inflammatory and neuropathic pain, a depolarizing efflux of HCO3– via GABAA instead of the normally occurring influx of Cl– can reduce the efficacy of GABAA receptor-mediated hyperpolarizing inhibition. AZA decreases the production of HCO3– by inhibiting CA, which further enhances the efficacy of GABAA receptor-mediated inhibition, eventually alleviating inflammatory and neuropathic pain7,8.
Thermal and chemical stimuli are the two main pathways that induce acute pain and have different neurobiological mechanisms. First, thermal-stimulated pain is transmitted by Aδ and C fibers, whereas chemical-stimulated pain is only transmitted by C fibers9. Second, the role of ion channels and receptors in thermal-stimulated acute pain is not completely the same as in chemical-stimulated acute pain10. However, whether AZA produces analgesia in acute pain remains unknown.
In the current study, using five models of acute pain based on thermal or chemical stimuli, we tried to answer the following questions: (1) does AZA have an analgesic effect on the two types of acute pain induced by thermal and chemical stimuli? and (2) does AZA attenuate acute pain by enhancing the efficacy of GABAA receptor-mediated inhibition via a similar mechanism as that of AZA's attenuation of inflammatory and neuropathic pain? To better understand the possible mechanism, the effects of methazolamide (MZA), another CA inhibitor, and diazepam, a positive allosteric modulator that activates GABAA receptors11, were investigated in acute pain and compared with the effects of AZA.
Materials and methods
Animals and drugs
Male and female KunMing mice weighing 18–22 g were obtained from Beijing Animal Center and housed at a temperature of 21±1 °C under a 12 h light/12 h dark cycle (lights on at 7:00 AM, lights off at 7:00 PM) and with food and water ad libitum. All animal experiments were conducted following the related regulations of the Institutional Review Committee for the Use of Animals.
AZA (Sigma, St Louis, MO, USA) and MZA (Sigma, St Louis, MO, USA) were separately dissolved in 1 mol/L NaOH, and the pH was adjusted to 7.4 with 1 mol/L HCl. The solutions were then diluted with isotonic (0.9% NaCl) saline to achieve 50, 100, and 200 mg/kg solutions for ip administration and a 10 nmol solution for intrathecal administration. Isotonic (0.9% NaCl) saline solution was used as a control.
Diazepam was administered intraperitoneally at 0.25, 0.5, or 1 mg/kg.
Tail-flick test
Male mice were used in this test. The method is an adaptation of that described by Delporte et al12. Lower and higher light intensities of 8 and 12, respectively, were used. A cut-off time of 16 s was chosen to avoid tissue damage. The tail-flick test was performed four times (at 30, 60, 90 and 120 min) post-drug administration. AZA (50, 100, or 200 mg/kg, ip), MZA (50, 100, or 200 mg/kg, ip), diazepam (0.25, 0.5, or 1 mg/kg, ip) or saline (10 mL/kg, ip) was administered. The results were expressed as the possible maximum analgesia percentage (PMAP) (%), which was calculated according to the formula [(T1–T0)/(T2–T0)]×100, where T0 is the baseline latency, T1 is the latency obtained after drug administration, and T2 is the cut-off time.
Hot-plate test
Female mice were used in this test. The procedure followed the protocol described by Jones et al13. Two different hot-plate temperatures, 52 °C and 55 °C, were used. A cut-off time of 60 s was chosen to avoid tissue damage. The hot-plate test was performed four times (at 30, 60, 90, and 120 min) post-drug administration. AZA (50, 100, or 200 mg/kg, ip), MZA (50, 100, or 200 mg/kg, ip), diazepam (0.25, 0.5, or 1 mg/kg, ip) or saline (10 mL/kg, ip) was administered. The results were expressed as PMAPs.
Capsaicin test
Male mice were used in this test. The method followed the procedure of Snatol et al14, with certain modifications. The mice were given saline (10 mL/kg, ip), AZA (50, 100, or 200 mg/kg, ip), MZA (50, 100, or 200 mg/kg, ip) or diazepam (0.25, 0.5, or 1 mg/kg, ip) 30 min before the right hind paw was injected with 10 μL capsaicin (3 μg/paw) prepared in 10% Tween-80 and 10% ethanol. Nociception was evaluated immediately after injection of capsaicin and quantified based on paw-licking time during a 5-min period. In addition, AZA (10 nmol/mouse, 5 μL) was administered by the intrathecal route. The method of intrathecal injection followed the procedure of Hylden et al15. In brief, the mice were administered AZA by intrathecal injection with a 50-μL microsyringe between lumbar segments 5 and 6, with the sudden appearance of sideways movements by the mice's tails as a sign of success. Ten minutes after AZA intrathecal administration, the mice received a capsaicin injection.
Formalin test
Male mice were used in this test. The formalin test was conducted as previously described by Couto et al16. The male mice were given saline (10 mL/kg, ip), AZA (50, 100, or 200 mg/kg, ip), MZA (50, 100, or 200 mg/kg, ip) or diazepam (0.25, 0.5, or 1 mg/kg, ip) 30 min before the right hind paw was injected with 20 μL 2% formalin solution. Nociception was evaluated immediately after the injection of formalin and quantified based on the total paw licking time in the early phase (phase 1: 0–5 min) and the late phase (phase 2: 15–30 min). In addition, AZA (10 nmol/mouse, 5 μL) was administered by the intrathecal route. Ten minutes after AZA intrathecal administration, the mice received a formalin injection.
Acetic acid-induced writhing test
Male mice were used in this test. This procedure was described by Chiba et al17. The male mice were given saline (10 mL/kg, ip), AZA (50, 100, or 200 mg/kg, ip), MZA (50, 100, or 200 mg/kg, ip) or diazepam (0.25, 0.5, or 1 mg/kg, ip) 30 min before the injection of acetic acid (0.6%, 20 mL/kg, ip). The number of writhing movements was counted for 15 min beginning 5 min after the injection of acetic acid. In addition, AZA (10 nmol/mouse, 5 μL) was administered by the intrathecal route. Ten minutes after AZA intrathecal administration, the mice received a acetic acid injection.
Locomotor activity test
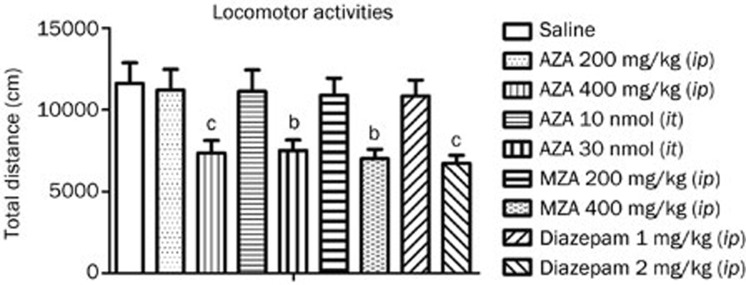
Before the analgesic properties of the drugs were tested, their effects on locomotor activities were evaluated. Mice were placed in individual locomotor activity cages without drug treatment for 30 min, and the locomotor activities of each mouse were recorded. The mice were grouped according to their basal locomotor activities. The mice were then injected with saline (10 mL/kg, ip) or AZA (200 or 400 mg/kg, ip), MZA (200 or 400 mg/kg, ip) or diazepam (1 or 2 mg/kg, ip) 30 min before they were placed in individual locomotor activity cages and assessed for 1 h. For intrathecal administration of AZA (10 and 30 nmol/mouse), locomotor activities were recorded beginning at 10 min after the intrathecal injection.
Statistical analysis
The data were expressed as the mean±SEM and analyzed with SPSS software (version 18.0). The data from the capsaicin, formalin and acetic acid-induced writhing tests (drugs with ip administration) and locomotor activity test were analyzed by one-way ANOVA, followed by Dunnett's t-test. The data from the tail-flick and hot-plate tests were analyzed by two-way ANOVA with repeated measurement, followed by Tukey's test. The data from the intrathecal administration of AZA were analyzed using a Student's t-test. P<0.05 was considered statistically significant.
Results
AZA failed to produce analgesia in thermal-stimulated acute pain but attenuated chemical-stimulated acute pain
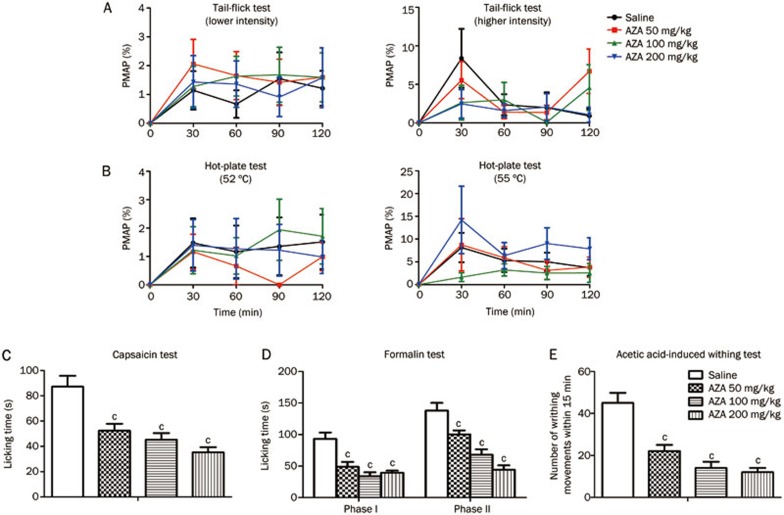
In the tail-flick test, AZA (50, 100, or 200 mg/kg, ip) failed to increase the latency of tail flicking from 0 to 120 min after treatment under lower and higher intensities, and the PMAPs were less than 10% (lower intensity, treatment: F3, 36=0.3546, P>0.05; time: F3, 36=3.142, P<0.05; treatment X time: F3, 36=0.1674, P>0.05; higher intensity, treatment: F3, 35=0.7601, P>0.05; time: F3, 35=4.142, P<0.05; treatment X time: F3, 35=1.157, P>0.05) (Figure 1A). The same result was obtained in the hot-plate test at 52 °C and 55 °C hot-plate temperatures (52 °C hot-plate, treatment: F3, 36=0.3689, P>0.05; time: F3, 36=3.000, P<0.05; treatment X time: F3, 36=0.3344, P>0.05; 55 °C hot-plate, treatment: F3, 34=1.811, P>0.05; time: F3, 34=5.421, P<0.05; treatment X time: F3, 34=0.6671, P>0.05) (Figure 1B). These results suggested that AZA produced no analgesia in thermal-stimulated acute pain. In the capsaicin test, compared with vehicle treatment, administration of AZA at a dose of 50, 100, or 200 mg/kg significantly reduced paw licking time (F3, 36=13.849, P<0.001) (Figure 1C). In the formalin test, similar to what was observed in the capsaicin test, AZA (50, 100, or 200 mg/kg, ip) dose-dependently reduced paw-licking time in both the first and the second phases (first phase: F3, 34=13.305, P<0.001; second phase: F3, 34=21.327, P<0.001) (Figure 1D). In the acetic acid-induced writhing test, compared with vehicle treatment, AZA (50, 100, or 200 mg/kg, ip) decreased the number of writhing movements in a dose-dependent manner (F3, 36=20.750, P<0.001) (Figure 1E). In addition, AZA at a dose of 200 mg/kg (ip) did not decrease locomotor activities (Figure 2), suggesting that the analgesic effect of AZA is not caused by its sedative effect. All of these results suggested that AZA has the potential to relieve chemical-stimulated acute pain, including cutaneous pain and visceral pain.
Figure 1.
AZA had no analgesia in thermal-stimulated acute pain, but attenuated chemical-stimulated acute pain. (A) mouse tail-flick test (n=9–10), (B) mouse hot-plate test (n=9–10). The basal responses of mice to thermal stimuli were tested before drug administration. (C) mouse capsaicin test (n=10), (D) mouse formalin test (n=8–10), (E) mouse acetic acid-induced writhing test (n=10). (A) and (B), no statistical significant difference was obtained. (C), (D), and (E), cP<0.01 vs control.
Figure 2.
Effects of AZA, MZA, and diazepam on locomotor activities. n=10/group. bP<0.05, cP<0.01 vs control.
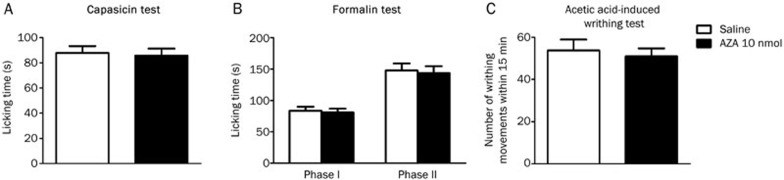
To determine the possibility of a peripheral or spinal mechanism, intrathecal administration of AZA was conducted. Compared with vehicle treatment, AZA (10 nmol/mouse, intrathecal injection) did not significantly reduce nociceptive responses in the capsaicin, formalin and acetic acid-induced writhing tests (Figure 3), whereas positive-control morphine (10 nmol/mouse, intrathecal injection) significantly reduced paw licking time in both the first and the second phases (data not shown). These results suggested that AZA attenuates chemical-induced acute pain by a peripheral but not spinal mechanism.
Figure 3.
AZA had no analgesia in chemical-stimulated acute pain by intrathecal injection. (A) mouse capsaicin test. (B) mouse formalin test. (C) mouse acetic acid-induced writhing test. n=10/group. No statistical significant difference was obtained.
MZA failed to attenuate thermal- and chemical-stimulated acute pain
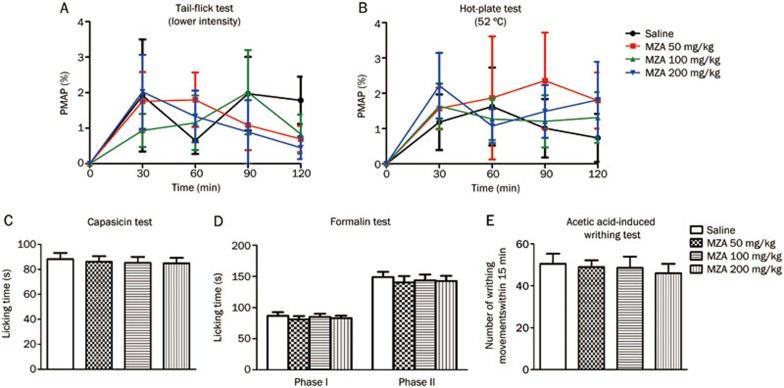
The effects of MZA, another CA inhibitor, were compared with AZA's effects. In contrast to AZA either inhibiting CA or affecting other ion channels, MZA merely inhibits CA and has no action on other ion channels. In the tail-flick test, MZA (50, 100, or 200 mg/kg, ip) did not increase the latency of tail flicking (treatment: F3, 36=0.1875, P>0.05; time: F3, 36=3.093, P<0.05; treatment X time: F3, 36=0.4715, P>0.05) (Figure 4A). The same result was observed in the hot-plate test (treatment: F3, 36=0.3807, P>0.05; time: F3, 36=3.027, P<0.05; treatment X time: F3, 36=0.2328, P>0.05) (Figure 4B). Moreover, MZA (50, 100, or 200 mg/kg, ip) had no significant analgesic effects in the capsaicin, formalin and acetic acid-induced writhing tests (capsaicin test, F3, 36=0.098, P>0.05; formalin test, first phase: F3, 36=2.956, P>0.05, second phase: F3, 36=1.335, P>0.05; acetic acid-induced writhing test, F3, 36=0.159, P>0.05) (Figure 4C–4E), which was not consistent with AZA's effects on chemical-stimulated acute pain. In addition, MZA at a dose of 200 mg/kg (ip) did not decrease locomotor activities (Figure 2). These results showed that MZA failed to produce analgesia in acute pain, suggesting that inhibition of CA might not play a role in AZA-related efficacy in the relief of chemical-stimulated acute pain.
Figure 4.
MZA did not produce analgesia in both thermal- and chemical-stimulated acute pain. (A) mouse tail-flick test, (B) mouse hot-plate test. The basal responses of mice to thermal stimuli were tested before drug administration. (C) mouse capsaicin test, (D) mouse formalin test, (E) mouse acetic acid-induced writhing test. n=10/group. No statistical significant difference was obtained.
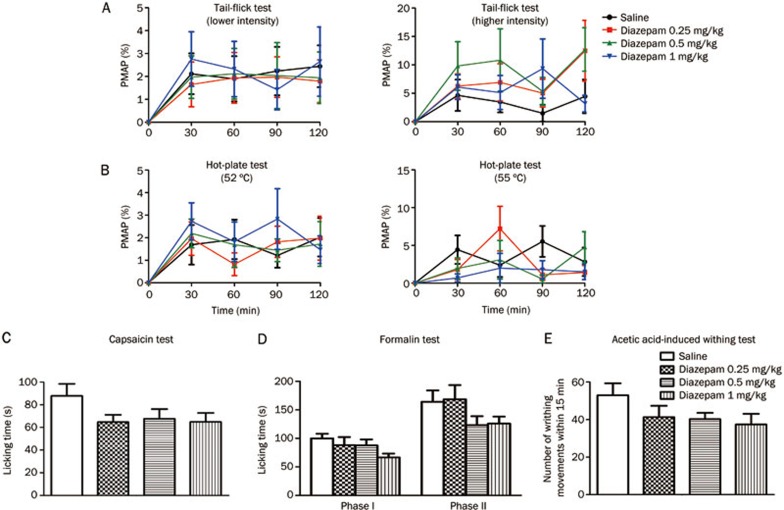
Diazepam failed to attenuate thermal- and chemical-stimulated acute pain
In the tail-flick test, diazepam (0.25, 0.5, or 1 mg/kg, ip) did not increase the latency of tail flicking (lower intensity, treatment: F3, 36=0.1294, P>0.05; time: F3, 36=3.710, P<0.05; treatment X time: F3, 36=0.1106, P>0.05; higher intensity, treatment: F3, 36=1.526, P>0.05; time: F3, 36=5.292, P<0.05; treatment X time: F3, 36=0.6898, P>0.05) (Figure 5A). The same result was observed in the hot-plate test (52 °C hot-plate, treatment: F3, 36=0.1783, P>0.05; time: F3, 36=7.213, P<0.05; treatment X time: F3, 36=0.5133, P>0.05; 55 °C hot-plate, treatment: F3, 36=1.126, P>0.05; time: F3, 36=3.267, P<0.05; treatment X time: F3, 36=1.479, P>0.05) (Figure 5B). Compared with vehicle treatment, diazepam (0.25, 0.5, or 1 mg/kg, ip) did not significantly reduce paw licking time in the capsaicin test (F3, 36=1.717, P>0.05) (Figure 5C) or in the formalin test (first phase: F3, 36=1.799, P>0.05; second phase: F3, 36=1.639, P>0.05) (Figure 5D). In the acetic acid-induced writhing test, compared with vehicle treatment, diazepam (0.25, 0.5, or 1 mg/kg, ip) also did not significantly decrease the number of writhing movements (F3, 36=1.532, P>0.05) (Figure 5E). However, morphine (5 mg/kg, sc), which served as a positive control in our experiment, produced significant analgesia in all five of these models (data not shown). In addition, diazepam at a dose of 1.0 mg/kg (ip) did not decrease locomotor activities (Figure 2). These results showed that diazepam failed to mimic AZA-produced analgesia in acute pain. Enhancement of GABAA receptor-mediated inhibition might not play a role in AZA's efficacy in the relief of chemical-stimulated acute pain.
Figure 5.
Diazepam did not produce analgesia in thermal- and chemical-stimulated acute pain. (A) mouse tail-flick test, (B) mouse hot-plate test. The basal responses of mice to thermal stimuli were tested before drug administration. (C) mouse capsaicin test, (D) mouse formalin test, (E) mouse acetic acid-induced writhing test. n=10/group. No statistical significant difference was obtained.
Discussion
In this study, we report that AZA attenuated chemical-induced, but not thermal-induced, acute pain in mice. The attenuation of chemical-stimulated acute pain by AZA may not be due to enhancement of GABAA receptor-mediated inhibition via inhibiting CA activity but might be due to a peripheral ion channel-related mechanism.
AZA is a potent inhibitor of CA18,19 and exerts antinociceptive effects on inflammatory and neuropathic pain by enhancing the efficacy of GABAA receptor-mediated inhibition by reducing the production of HCO3−7,8. We were interested in investigating whether AZA can also attenuate chemical-induced acute pain by enhancing GABAA receptor-mediated inhibition via inhibiting CA. However, our result showed that MZA, another CA inhibitor, did not attenuate thermal- or chemical-induced acute pain in our study. In contrast to AZA either inhibiting CA or affecting other ion channels, MZA merely inhibits CA and has no action on other ion channels. These results indicated that AZA's attenuation of chemical-stimulated acute pain might not be due to enhancement of GABAA receptor-mediated inhibition via inhibiting CA. Similar to MZA, diazepam, a positive allosteric modulator of the GABAA receptor, cannot attenuate thermal- and chemical-stimulated acute pain at a dose of 0.25, 0.5, or 1 mg/kg without reducing locomotor activities. Given that a reduction in locomotor activities after drug treatment may lead to a false-positive result in evaluating the analgesic effect, we selected the proper doses of diazepam, AZA and MZA that did not affect locomotor activities. In fact, a previous study reported that diazepam achieved analgesia in inflammatory and neuropathic pain models at a dose not producing sedation20. Thus, we thought that the lower dose that we utilized might not be the main cause of the lack of an analgesic effect for diazepam. Although diazepam can activate GABAA receptors as a positive allosteric modulator, this drug did not mimic AZA's analgesic effects in our study. In addition, Hansen et al observed that diazepam (1–5 mg/kg, ip) attenuated a capsaicin-induced nociceptive response in the secondary phase (6–30 min) but not in the first phase (0–5 min)21, which was consistent with our observation that diazepam did not relieve capsaicin-induced licking behavior at 0–5 min. Indeed, capsaicin could induce primary pain and secondary hypersensitivity, and the GABAA receptor might mediate the secondary hypersensitivity. In a formalin test, Kaneko et al observed that the GABAA receptor agonists isoguvacine and muscimol attenuated formalin-induced pain behaviors in rats22, but we did not observe that diazepam produced the same analgesia in mice. The contradiction might be due to the differences in animal species (rat vs mouse) and GABAA receptor agonists (isoguvacine or muscimol vs diazepam). Therefore, the mechanisms of AZA's attenuation of chemical-stimulated acute pain are different from that of AZA's attenuation of inflammatory and neuropathic pain. Our results indicated that AZA's relief of chemical-stimulated acute pain does not seem to be due to enhancement of GABAA receptor-mediated inhibition via inhibiting CA activity.
In addition to acting as a CA inhibitor, AZA is a non-selective inhibitor of aquaporin 4 (AQP4)23,24,25. The IC50 of AZA's inhibition of human AQP4-M23-mediated water transport is 0.9 μmol/L, and the reported maximum inhibition of AQP4-M23 by AZA is 85%23. AQP4 is mainly expressed in glial cells, which are activated in chronic pain but not in acute pain. Our unpublished data show that AQP4 deficiency failed to affect the responses of mice to the acute pain induced by thermal (tail-flick and hot-plate tests), chemical (formalin and capsaicin tests) and mechanical (von Frey hair test) stimuli. Taken together, the results indicate that the inhibition of AQP4 by AZA does not mediate the relief of chemical-stimulated acute pain by AZA.
Additionally, AZA has effects on certain ion channels, such as voltage-gated calcium channels and potassium channels. Voltage-gated calcium channels play a key role in neurotransmitter release from peripheral nociceptor terminals to generate pain10. The α1E subunit of the voltage-dependent calcium channel (R-type channel) was originally identified as a neuron-specific subunit. It was reported that in tail-flick and hot-plate tests, there were no obvious differences between wild-type and α1E knockout mice, whereas in a formalin test, nociceptive behavior was significantly attenuated in the α1E knockout mice26. It has also been reported that AZA can block α1E calcium channels7. In the periphery, the opening of calcium-activated potassium channels is antinociceptive in chemical-stimulated acute pain7, and AZA can activate these calcium-activated potassium channels26. Thus, AZA's relief of chemical-stimulated acute pain might be due to the drug's action on certain other ion channels. However, whether peripheral or spinal ion channels mediate AZA's analgesia is still unclear. We observed that intrathecal administration of AZA (10 nmol/mouse) failed to produce a significant analgesic effect in the capsaicin, formalin and acetic acid-induced writhing tests. Therefore, AZA's analgesic effects on chemical-stimulated acute pain might be due to the drug's action on certain peripheral ion channels, rather than on spinal ion channels.
In this study, we did not observe significant analgesia produced by AZA in thermal-stimulated acute pain. The possible explanations are that the ion channels and receptors involved in thermal- and chemical-stimulated acute pain are not completely the same. For example, 5-HT1 receptors, except for the 5-HT1A subtype, are involved in the spinally mediated antinociception induced by thermal noxious stimuli27. The presumptions mentioned above might be the reason that AZA failed to produce analgesia in thermal-stimulated acute pain. Further studies are needed to explain the differences between AZA's effects in thermal-stimulated and chemical-stimulated acute pain. Although there was no significant difference in the main effect of the treatment, suggesting that AZA has no analgesic effect on thermal-stimulated acute pain, the P values for time were all less than 0.05 in the tail-flick and hot-plate tests. The same results were found in the tail-flick and hot-plate tests using MZA and diazepam. The possible reason might be that fluctuations occurred in the data at each time point.
In conclusion, AZA exhibits analgesic effects on chemical-stimulated, but not thermal-stimulated, acute pain. The mechanism of AZA's attenuation of chemical-stimulated acute pain differs from that of AZA's relief of inflammatory and neuropathic pain. Herein, we supposed that the mechanism of AZA's relief of chemical-stimulated acute pain is the interaction of AZA with peripheral ion channels instead of enhancement of GABAA receptor-mediated inhibition via inhibiting CA activity. All of the above findings provide a new direction for examining the analgesic effects of AZA.
Author contribution
Jin LI and Ning WU designed the research; Ya-jie SUN, Ying CHEN, and Chong PANG performed the research; Ya-Jie SUN and Ning WU analyzed the data; Ya-jie SUN and Ning WU wrote the manuscript; and Jin LI revised the manuscript.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No 81171046).
References
- Moore ND. In search of an ideal analgesic for common acute pain. Acute Pain. 2009;11:129–37. [Google Scholar]
- Kaur IP, Smitha R, Aggarwal D, Kapil M. Acetazolamide: future perspective in topical glaucoma therapeutics. Int J Pharm. 2002;248:1–14. doi: 10.1016/s0378-5173(02)00438-6. [DOI] [PubMed] [Google Scholar]
- Velázquez H, Wright FS. Control by drugs of renal potassium handling. Annu Rev Pharmacol Toxicol. 1986;26:293–309. doi: 10.1146/annurev.pa.26.040186.001453. [DOI] [PubMed] [Google Scholar]
- Kassamali R, Sica DA. Acetazolamide a forgotten diuretic agent. Cardiol Rev. 2011;19:276–8. doi: 10.1097/CRD.0b013e31822b4939. [DOI] [PubMed] [Google Scholar]
- Aaslid R. Cerebral autoregulation and vasomotor reactivity. Front Neurol Neurosci. 2006;21:216–28. doi: 10.1159/000092434. [DOI] [PubMed] [Google Scholar]
- Leaf DE, Goldfarb DS. Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J Appl Physiol. 2007;102:1313–22. doi: 10.1152/japplphysiol.01572.2005. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Sluka KA. Acetazolamide, a carbonic anhydrase inhibitor, reverses inflammation-induced thermal hyperalgesia in rats. J Pharmacol Exp Ther. 2005;313:921–7. doi: 10.1124/jpet.104.082776. [DOI] [PubMed] [Google Scholar]
- Asiedu M, Ossipov MH, Kaila K, Price TJ. Acetazolamide and midazolam act synergistically to inhibit neuropathic pain. Pain. 2010;148:302–8. doi: 10.1016/j.pain.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voscopoulos C, Lema M. When does acute pain become chronic. Br J Anaesth. 2010;105:i69–i85. doi: 10.1093/bja/aeq323. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Amygdala-specific reduction of alpha1-GABAA receptors disrupts the anticonvulsant, locomotor, and sedative, but not anxiolytic, effects of benzodiazepines in mice. J Neurosci. 2010;30:7139–51. doi: 10.1523/JNEUROSCI.0693-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delporte C, Backhouse N, Inostroza V, Aquirre MC, Peredo N, Silva X, et al. Analgesic activity of Ugni molinae (murtilla) in mice models of acute pain. J Ethnopharmacol. 2007;112:162–5. doi: 10.1016/j.jep.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Jones CK, Peters SC, Shannon HE. Efficacy of duloxetine, a potent and balanced serotonergic and noradrenergic reuptake inhibitor, in inflammatory and acute pain models in rodents. J Pharmacol Exp Ther. 2005;312:726–32. doi: 10.1124/jpet.104.075960. [DOI] [PubMed] [Google Scholar]
- Santos AR, Calixto JB. Further evidence for the involvement of tachykinin receptor subtypes in formalin and capsaicin models of pain in mice. Neuropeptides. 1997;31:381–9. doi: 10.1016/s0143-4179(97)90075-5. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–6. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Couto VM, Vilela FC, Dias DF, Dos Santos MH, Soncini R, Nascimento CG, et al. Antinociceptive effect of extract of Emilia sonchifolia in mice. J Ethnopharmacol. 2011;134:348–53. doi: 10.1016/j.jep.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Chiba S, Nishiyama T, Yoshikawa M, Yamada Y. The antinociceptive effects of midazolam on three different types of nociception in mice. J Pharmacol Sci. 2009;109:71–7. doi: 10.1254/jphs.08094fp. [DOI] [PubMed] [Google Scholar]
- Temperini C, Cecchi A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Comparison of chlorthalidone, indapamide, trichloromethiazide, and furosemide X-ray crystal structures in adducts with isozyme II, when several water molecules make the difference. Bioorg Med Chem. 2009;17:1214–21. doi: 10.1016/j.bmc.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Yang MT, Chien WL, Lu DH, Liou HC, Fu WM. Acetazolamide impairs fear memory consolidation in rodents. Neuropharmacology. 2013;67:412–8. doi: 10.1016/j.neuropharm.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, et al. Reversal of pathological pain through specific spinal GABA receptor subtypes. Nature. 2008;451:330–4. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Hansen RR, Erichsen HK, Brown DT, Mirza NR, Munro G. Positive allosteric modulation of GABA-A receptors reduces capsaicin-induced primary and secondary hypersensitivity in rats. Neuropharmacology. 2012;63:1360–7. doi: 10.1016/j.neuropharm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Hammond DL. Role of spinal gamma-aminobutyric acidA receptors in formalin-induced nociception in the rat. J Pharmacol Exp Ther. 1997;282:928–38. [PubMed] [Google Scholar]
- Huber VJ, Tsujita M, Yamazaki M, Sakimura K, Nakada T. Identification of arylsulfonamides as aquaporin 4 inhibitors. Bioorg Med Chem Lett. 2007;17:1270–3. doi: 10.1016/j.bmcl.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Kim JE, Yeo SI, Ryu HJ, Kim MJ, Kim DS, Jo SM, et al. Astroglial loss and edema formation in the rat piriform cortex and hippocampus following pilocarpine-induced status epilepticus. J Comp Neurol. 2010;518:4612–28. doi: 10.1002/cne.22482. [DOI] [PubMed] [Google Scholar]
- Tanimura Y, Hiroaki Y, Fujiyoshi Y. Acetazolamide reversibly inhibits water conduction by aquaporin-4. J Struct Biol. 2009;166:16–21. doi: 10.1016/j.jsb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Pickkers P, Hughes AD, Russel FG, Thien T, Smits P. In vivo evidence for K (Ca) channel opening properties of acetazolamide in the human vasculature. Br J Pharmacol. 2001;132:443–50. doi: 10.1038/sj.bjp.0703825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeson R, Goodchild CS. Antinociceptive role of 5-HT1A receptors in rat spinal cord. Br J Anaesth. 2002;88:679–84. doi: 10.1093/bja/88.5.679. [DOI] [PubMed] [Google Scholar]