Abstract
Aim:
Highly reactive carbonyl methylglyoxal (MGO) is one of the metabolites excessively produced in diabetes. We have showed that prolonged exposure of vascular smooth muscle cells to MGO leads to instability of the mRNA encoding ATP-sensitive potassium (KATP) channel. In the present study we investigated the effects of MGO on the activity of KATP channels.
Methods:
Kir6.1/ SUR2B, Kir6.2/SUR2B or Kir6.2Δ36 (a truncated Kir6.2 isoform) alone was expressed in HEK293 cells. Whole-cell currents were recorded in the cells with an Axopatch 200B amplifier. Macroscopic currents and single-channel currents were recorded in giant inside-out patches and normal inside-out patches, respectively. Data were analyzed using Clampfit 9 software.
Results:
The basal activity of Kir6.1/SUR2B channels was low. The specific KATP channel opener pinacidil (10 μmol/L) could fully activate Kir6.1/SUR2B channels, which was inhibited by the specific KATP channel blocker glibenclamide (10 μmol/L). MGO (0.1-10 mmol/L) dose-dependently activated Kir6.1/SUR2B channels with an EC50 of 1.7 mmol/L. The activation of Kir6.1/SUR2B channels by MGO was reversible upon washout, and could be inhibited completely by glibenclamide. Kir6.2Δ36 channels expressed in HEK293 cells could open automatically, and the channel activity was enhanced in the presence of MGO (3 mmol/L). Single channel recordings showed that MGO (3 mmol/L) markedly increased the open probability of Kir6.1/SUR2B channels, leaving the channel conductance unaltered.
Conclusion:
Acute application of MGO activates KATP channels through direct, non-covalent and reversible interactions with the Kir6 subunits.
Keywords: methylglyoxal, reactive carbonyl species, ATP-sensitive potassium channel, Kir6.1, Kir6.2, Kir6.2Δ36, sulfonylurea receptor, diabetes, vascular dysfunction
Introduction
Diabetes affects approximately 25 million people in the United States1. Hyperglycemia, a major hallmark of diabetes, serves as a trigger for the development and progression of many diabetes-associated complications on a systemic level. One of the key consequences of hyperglycemia is alteration of the metabolic pathways, leading to the production and accumulation of metabolic byproducts. Among these metabolites is the highly reactive carbonyl methylglyoxal (MGO), which also plays a key role in mediating oxidative stress and carbonyl stress2. MGO is produced mainly by the transformation of triosephosphate intermediates during glycolysis and is also produced in limited quantities via protein catabolism and fatty acid oxidation mechanisms2. Patients with type I diabetes have been reported to have a 5–6-fold increase in plasma MGO levels, whereas in type II patients, a 2–3-fold increase has been observed3. Excess MGO, when present as a result of either impaired carbonyl clearance mechanisms, increased availability of precursor molecules or both, is observed in many pathological conditions, including diabetes. Increased levels of MGO can lead to structural and functional changes in the cell by attacking molecules such as transporters, ion channels, transcription factors and signal transduction components at both the mRNA and protein levels4,5,6,7.
Diabetic vascular complication is a severe event associated with many diabetic patients, and hyperglycemia is suggested to be a major contributor. Despite numerous studies hinting towards the importance of MGO in the regulation of the vascular functions in carbonyl stress8,9, the response pattern of key molecules in the vasculature to the presence of reactive carbonyl species is yet to be fully understood. The KATP channel is a major regulator of vascular tone and a critical pharmacological target for treating diabetes10,11,12,13. This channel couples the cellular metabolism to membrane excitability14. Activation of the KATP channel produces hyperpolarization in smooth muscle cells, which lowers the activity of voltage-dependent calcium channels (VDCC), leading to vasorelaxation. Kcnj8(encodes Kir6.1, a vascular KATP channel pore forming subunit) null mice show vasospasm in coronary arteries and sudden death15,16. In diabetic patients, the response of KATP channel to stimuli is impaired, resulting in defective vasodilation of the vascular rings17,18.
Because carbonyl stress has been suggested to play a critical role in the development of diabetes-associated vascular complications19, we previously tested if MGO, a major reactive carbonyl species, regulated the KATP channel. Interestingly, we found that prolonged exposure of vascular smooth muscle cells to MGO leads to mRNA instability of the KATP channel, which likely contributes to the dysfunction of the vasculature5. However, it is not yet known if acute MGO exposure may also exert an effect on the KATP channel gating. Therefore, in this current study, we investigated the effect of acute exposure of MGO on the KATP channel activity. We found that acute MGO treatment causes the activation of the vascular KATP channel in a receptor-independent mechanism, mediated through non-covalent interactions with the pore-forming Kir subunit of the KATP channel.
Materials and methods
Chemicals and reagents
All reagents and chemicals used in this study were purchased from Sigma-Aldrich unless stated otherwise. Reagents were freshly made and prepared in high-concentration stocks in double-distilled water or dimethyl sulfoxide (DMSO). MGO was freshly made and used within 4 h of preparation. The final concentration of DMSO in the solutions used for experiments was less than 0.1%, which did not have any detectable effect on the channel activity.
Cell culture and heterologous expression of KATP channels
HEK293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM/F12 with 10% fetal bovine serum and penicillin/streptomycin) at 37 °C in a humidified 5% CO2 atmosphere.
KATP channels were expressed in HEK293 cells as previously described20,21,22. Rat Kir6.1 (GenBank No D42145), mouse Kir6.2 (GenBank No D50581) and SUR2B (GenBank No D86038, mRNA isoform NM_011511) coding sequences were cloned into pcDNA3.1 (a eukaryotic expression vector). HEK293 cells cultured in 35-mm petri dishes were transfected with 1 μg Kir6.1 (or Kir6.2) and 3 μg SUR2B using Lipofectamine2000 (Invitrogen Inc, Carlsbad, CA, USA). Kir6.2Δ36 (a truncated Kir6.2 isoform, 3 μg) was transfected into HEK293 cells without SUR2B. Green fluorescent protein (GFP) cDNA (0.4 μg, pEGFP-N2, Clontech, Palo Alto, CA, USA) was included in the cDNA transfection mixture to facilitate the identification of positively transfected cells. One day after transfection, cells were disassociated with 0.25% trypsin, split and transferred to cover slips. Electrophysiology experiments were performed on the cells grown on the cover slips for 2 continuous days.
Electrophysiology
Patch clamp experiments were performed at room temperature as described previously23,24,25. In brief, 1.2-mm borosilicate glass capillaries were fire-polished to make patch pipettes of 2–5 MΩ resistance. Whole-cell currents were recorded in voltage clamps with a holding potential of 0 mV that was stepped to -80 mV every 3 s. The bath solution contained (in mmol/L): KCl 10, potassium gluconate 135, EGTA 5, glucose 5, and HEPES 10 (pH=7.4). The pipette was filled with a solution containing (in mmol/L): KCl 10, potassium gluconate 133, EGTA 5, glucose 5, K2ATP 1, NaADP 0.5, MgCl2 1, and HEPES 10 (pH=7.4). All solutions containing ATP and/or ADP were freshly made and used within 4 h to avoid nucleotide degradation. The recordings were obtained with an Axopatch 200B amplifier (Axon Instruments Inc, Foster City, CA, USA), low-pass filtered (2 kHz, Bessel 4-pole filter, -3 dB) and digitized (10 kHz, 16-bit resolution) with Clampex 9 (Axon Instruments Inc, USA). Macroscopic currents were recorded with giant inside-out patches and single-channel currents were recorded with normal inside-out patches with a constant single voltage of -80 or -60 mV. Symmetric high K+ (145 mmol/L in total) was used in both bath and pipette solutions to make the reverse potential of K+ close to 0 mV. K2ATP 1 mmol/L and NaADP 0.5 mmol/L was also included in the bath solution for maintaining the channel activity. For inside-out patch experiments, a higher sampling rate (20 kHz) was used to digitize the recorded currents. The data were analyzed using Clampfit 9 software (Axon Instruments Inc, USA).
Statistical analysis
Data are presented as the mean±SEM. Differences in the mean values were evaluated with Student's t- test or ANOVA and were accepted to be statistically significant when P<0.05.
Results
Acute MGO treatment leads to activation of the Kir6.1/SUR2B isoform of the KATP channel
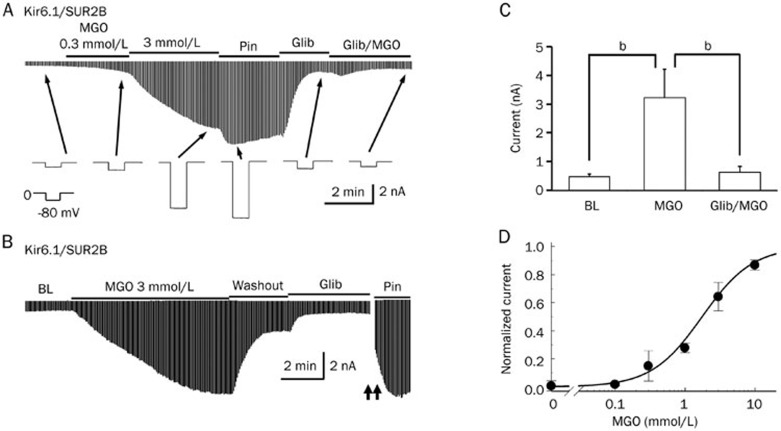
The Kir6.1/SUR2B isoform is the major isoform of the vascular KATP channel, although the presence of the Kir6.2 subunit has also been detected. To test if acute MGO treatment affected the activity of KATP channels, we expressed the Kir6.1/SUR2B channel in HEK293 cells and used whole cell voltage-clamp configuration to test the channel activity. Equal concentrations of K+ (145 mmol/L) were applied to both sides of the patch membranes. Membrane potential was held at 0 mV, and a -80 mV command potential was given to the cells every 3 s. The activity of the Kir6.1/SUR2B channel was low at the basal level (Figure 1A); however, it could be fully activated by the KATP channel-specific opener Pinacidil (Pin, 10 μmol/L) and inhibited by the channel-specific blocker Glibenclamide (Glib, 10 μmol/L) (Figure 1A).
Figure 1.
Acute MGO treatment led to activation of the Kir6.1/SUR2B channel. (A) The Kir6.1/SUR2B channel was expressed in HEK293 cells. Whole-cell currents were recorded from cells two days after transfection in a voltage-clamp configuration. MGO (0.3–3 mmol/L) treatment led to the activation of Kir6.1/SUR2B currents in a concentration-dependent manner. Pinacidil (Pin, 10 μmol/L), a KATP channel-specific opener, further opened the channel. The application of the KATP channel-specific inhibitor glibenclamide (Glib, 10 μmol/L) dramatically reduced the current. For quantitative analysis, the effect of MGO was normalized between the baseline current and the current activated by 10 μmol/L Pin. In the presence of Glib (10 μmol/L), the effect of the higher concentration of MGO (10 mmol/L) on channel activation was completely blocked. (B) The MGO-mediated channel activation was reversible. Following channel activation by MGO, washout with the bath solution returned the KATP channel currents to an almost baseline level. Glib caused a further reduction of the KATP channel currents. (C) Summary of the effect of MGO on the KATP channel currents in the presence and absence of Glib. bP<0.05. (D) Dose-response relationship between the concentration of MGO and the normalized current. Data were described by the Hill-equation with an EC50 of 1.7 mmol/L.
For quantitative analysis, the effect of MGO was normalized between the baseline current and the current activated by 10 μmol/L Pin as a percentage value. The Kir6.1/SUR2B channel was steadily activated in response to an increasing dosage of MGO. A 300 μmol/L MGO treatment increased the channel activity moderately (22.0%±10.6%, n=4) while a 3 mmol/L MGO treatment markedly increased the channel activity (57.4%±14.3%, n=4, Figure 1A). Interestingly, MGO-mediated channel activation was reversible: the application of the control bath solution caused a significant decrease (from 57.4%±14.3% to 29.3%±10.7%, P<0.05) in MGO induced channel activity (Figure 1B), thereby suggesting an involvement of non-covalent interactions between MGO and KATP channels. In the presence of Glib, an MGO concentration as high as 10 mmol/L was not able to induce an increase in the channel activity, suggesting that Glib and MGO target the same molecule (the KATP channel) (Figure 1C). The relationship between MGO dosage and KATP channel activities was described using the Hill equation with EC50 approximately 1.7 mmol/L (Figure 1D).
MGO activates the Kir6.1 and Kir6.2 subunit vascular KATP channel by targeting independently of the SUR subunit
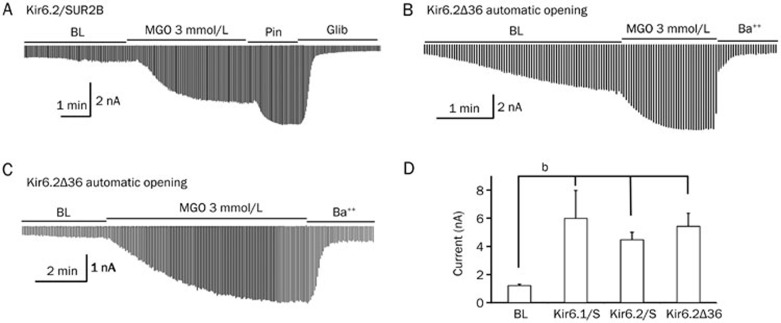
To determine whether the effect of MGO on the KATP channel activity was isoform-specific, we additionally tested the effect of MGO on the Kir6.2/SUR2B isoform of the KATP channel, another isoform found in vascular smooth muscle cells. In whole cell patch configuration, we found that the application of MGO (3 mmol/L) caused an increase in the Kir6.2/SUR2B activity (65.4%±7.9%, n=5, Figure 2A). This response was similar to what we observed in the Kir6.1/SUR2B recording (57.4%±14.3%, n=4). These data thus indicate that both the Kir6.1- and Kir6.2-containing channels can be targeted by MGO.
Figure 2.
MGO-mediated activation was dependent on the Kir but not the SUR subunit of the KATP channel. (A) MGO (3 mmol/L) activated the Kir6.2/SUR2B isoform of the vascular KATP channel to a similar extent as seen in the Kir6.1 isoform. (B) A truncated form of the Kir6.2 subunit (Kir6.2Δ36) channel, capable of expressing itself without the need for the SUR subunit, opened automatically, and the channel activity was further enhanced in the presence of MGO. (C) In the presence of 1.0 mmol/L ATP and 0.5 mmol/L ADP, the basal currents of the Kir6.2Δ36 channel remained small and the application of MGO (3 mmol/L) augmented the channel activity significantly. (D) Summary of the effect of MGO on the Kir6.1/SUR2B (Kir6.1/S), Kir6.2/SUR2B (Kir6.2/S) and Kir6.2Δ36 channels (note that unlike the Kir6.2 isoform, the truncated Kir6.1 channel was not able to express by itself); bP<0.05.
Naturally occurring KATP channels need both Kir and SUR subunits to be functional. However, previous studies have suggested that the truncation of several dozen amino acids in the C-terminus of the Kir6.2 subunit (Kir6.2Δ36) results in detectable currents, thereby eliminating the need for the SUR subunit to be present for the KATP channel to be functional26,27. This SUR-independent KATP channel functioning is isoform-specific, as the truncated Kir6.1 isoform cannot express by itself without the SUR subunit. To further investigate if the presence of Kir subunit alone was sufficient for MGO to exert its activating effect, we took advantage of the SUR-independent Kir6.2Δ36 channel. Because of the absence of the SUR subunit, the Kir6.2Δ36 channel was not sensitive to regulation by either Pin or Glib, but could still be blocked effectively by Ba++. In this condition, we quantified the effect of MGO against the Ba++ mediated channel blocking and expressed the changes in currents as fold changes. In the absence of ATP and ADP in the pipette solution, the Kir6.2Δ36 channels readily opened and the application of MGO led to a 3.87±1.81-fold increase in the channel currents (n=4, P<0.05, Figure 2B, 2D). In the presence of 1.0 mmol/L ATP and 0.5 mmol/L ADP, the basal currents of the Kir6.2Δ36 channel remained small and the application of MGO (3 mmol/L) was able to augment the channel activity by 3.35±0.6-fold (n=5, P<0.05, Figure 2C, 2D). Additionally, in both of the above experimental conditions, Ba++ treatment almost completely blocked the MGO-induced channel activity (Figure 2B, 2C). These data thus suggest that MGO is targeting the Kir rather than the SUR subunit.
Biophysical properties of MGO-mediated KATP channel activation
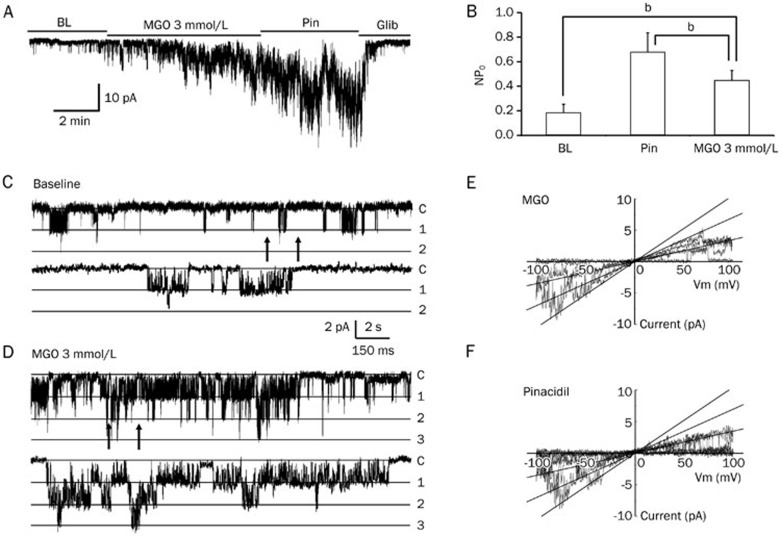
MGO-mediated activation of the KATP channel might be due to the direct modulation of the channel protein or involve the activation of other signaling pathways that regulate the KATP channel activity. To investigate these two possibilities, we studied the channel activity in giant inside-out patch configurations, in which the cytosolic components of the cell were excluded. In this condition, a 3 mmol/L MGO treatment led to a significant increase in the channel activity (52.7%±12.1%, n=6, P<0.05, Figure 3A, 3B), which was comparable to the results found in our whole-cell patch study.
Figure 3.
MGO affected the single-channel properties of the KATP channel. (A) In a giant inside-out patch configuration, 3 mmol/L MGO activated the Kir6.1/SUR2B currents in a manner similar to that seen in the whole cell configuration. This current could be further activated and inhibited by Pin and Glib, respectively. (B) Summary of the MGO treatment in the inside-out patch study. bP<0.05. (C) Single-channel level currents were recorded in inside-out patches with a holding potential of -80 mV. The lower trace was an expansion from the upper trace between the arrows. The presence of two active channels was evident at the baseline level. The NPO averaged 0.183 (n=4) in the baseline. (D) Treatment of the inside-out patch with 3 mmol/L MGO augmented the single-channel activity, as a result of the appearance of one more active channel. The NPO averaged 0.444 with MGO treatment. (E, F) Single-channel conductance was measured with a ramp protocol with voltage ranging from -100 mV to 100 mV. Three active channels were observed with both MGO alone (E) and Pin alone (F) treatments. The straight lines represent a slope conductance of 36 pS for all three channels.
Moreover, we performed single-channel patch experiments to study the effect of MGO treatment on channel open probability (PO) and channel conductance. In the single channel study, a few channel openings were observed and the PO had a basal level average of 0.183±0.069 (n=4, Figure 3C). With a 3 mmol/L MGO treatment, the PO of the Kir6.1/SUR2B channel increased to 0.444±0.084 (n=7, Figure 3D). Pin treatment further augmented the channel PO to 0.675±0.159 (n=6). In addition, we also tested the channel conductance using a ramp protocol with voltage ranging from -100 mV to 100 mV. Three active channels were observed with both MGO and Pin treatments (Figure 3E, 3F). The straight lines in Figure 3E and 3F represent a slope conductance of 36 pS for all three channels, indicating that the channel conductance remains unchanged with MGO treatment (Figure 3E, 3F). Taken together, these data indicate that the modulation of vascular KATP channels by MGO is mediated via an increase in the PO of the channel without changing the channel conductance or recruitment of other cellular signaling machinery.
Discussion
In this study, we found that acute MGO exposure led to the activation of KATP channels through direct targeting of the Kir6.x subunits. Furthermore, we found that this effect was independent of the SUR subunit and that MGO did not cause covalent modifications to the KATP channel. Single channel analysis revealed that MGO treatment augmented the KATP channel open probability without altering the channel conductance. In addition, MGO-mediated activation of the KATP channel did not require signal transduction components including receptors, secondary messenger molecules or cytosolic effectors.
In several instances, the concentration of MGO has been determined to be in the sub-millimolar range: in cultured mammalian cells, an approximate 300 μmol/L MGO concentration was detected28 while an approximate 400 μmol/L MGO concentration was reported in diabetic patients with poorly controlled hyperglycemia29,30. In other studies, the concentration of MGO was reported to be much lower31,32,33. It is possible that the reported concentrations of MGO represent the average concentration on a systemic level instead of the local concentration at a specific site. The levels of MGO in the body over a period of time could vary depending on the availability of the precursors and the efficiency of the carbonyl clearance system. During carbonyl stress, MGO levels in the body might follow a sudden burst pattern followed by a recession in the MGO spikes due to the carbonyl detoxification system “catching up” with the extent of carbonyl stress present. In our experimental condition, we found that the EC50 of MGO on the Kir6.x subunit activation appears to be 1.7 mmol/L. Our findings could thus be interpreted as the responses to an experimental condition that may represent the initial response of the vasculature to a rapid increase in the levels of MGO in a localized environment, as seen in pathological conditions.
Oxidative stress, characterized by the presence of excess reactive oxygen species (ROS) as a result of overproduction of ROS and impairment of cellular anti-oxidant machinery, has been long thought to be a major contributing factor for diabetes-associated vascular complications34. Several previous studies have suggested that important vascular tone regulators, including the vascular KATP channel, are targeted by ROS35,36,37. In agreement, we found that ROS modulates the vascular KATP channel by the covalent post-translational modification mechanism, S-glutathionylation21,38. In addition to oxidative stress, carbonyl stress has recently been proposed to be an underappreciated stress that may play a more dominant role in the progression of diabetes-associated vascular complications via the action of reactive carbonyl species (RCS). Among a variety of RCS, MGO is highly reactive. Excess MGO readily reacts with nucleophilic groups on proteins and nucleic acids, leading to cellular dysfunction and propagation of carbonyl stress39,40. Indeed, in our recent study, we found that a prolonged MGO treatment led to disruption of the KATP channel activity via mRNA instability, which was likely a contributing factor for the impairment of arterial function under carbonyl stress5. Interestingly, in the current study, we found that acute MGO treatment can activate the KATP channel. This differential response of the KATP channel to MGO treatment is dependent on the exposure time and works through different mechanisms of action5,21.
MGO is well known to interact with positively charged lysine residues of proteins to form advanced glycation adducts41. This type of modulation involves covalent interactions, which are not likely to be broken by a washout alone. Interestingly, in our present study, we found that MGO-mediated KATP channel activation could be rapidly reversed with washout, suggesting an activation mechanism not involving the formation of a covalent bond. We suspect that MGO can interact with the residues near the gating area, affecting the channel gating directly. It is also possible that MGO can target the phospholipid bilayer of the membrane associated with the KATP channel, affecting the channel gating. Future studies addressing these questions will provide valuable information regarding carbonyl stress-mediated KATP channel activation.
It has been shown that MGO contributes to the development of vascular complications of diabetes42, mainly through the formation of advanced glycation end products (AGEs) that interact with their cellular receptor, the receptor for advanced glycation end products (RAGEs)43. Interestingly, in this current study, this “classical” AGE/RAGE interaction does not appear to play a role in the MGO-mediated KATP channel activation, as our single channel preparation, which lacks all the signaling components, still yields similar results as our whole cell patch experiments.
In summary, our study demonstrated that acute MGO treatment led to reversible KATP channel activation in a signaling cascade-independent manner via interacting with the Kir6.x subunits, augmenting the channel-open probability. This finding may help us to better understand the response pattern of the vasculature to RCS and provide information necessary to design effective pharmacological treatments against carbonyl stress-mediated vascular dysfunction.
Author contribution
Yang YANG and Chun JIANG designed the experiments. Yang YANG, Anuhya S KONDURU, Ningren CUI, Lei YU, Timothy C TROWER, Yun SHI, and Weiwei SHI performed the experiments; Yang YANG, Anuhya S KONDURU, and Chun JIANG analyzed the data; Yang YANG, Anuhya S KONDURU, and Chun JIANG wrote the manuscript.
Acknowledgments
This study was supported by the NIH (1R211HD060959, 1R01NS073875) and the American Heart Association (09GNT2010037). Yang YANG was a Brains and Behavior fellow of Georgia State University.
References
- Department of Health and Human Services CfDCaP. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Centers for Disease Control and Prevention 2011;2011
- Desai KM, Chang T, Wang H, Banigesh A, Dhar A, Liu J, et al. Oxidative stress and aging: is methylglyoxal the hidden enemy. Can J Physiol Pharmacol. 2010;88:273–84. doi: 10.1139/Y10-001. [DOI] [PubMed] [Google Scholar]
- McLellan AC, Thornalley PJ, Benn J, Sonksen PH. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin Sci (Lond) 1994;87:21–9. doi: 10.1042/cs0870021. [DOI] [PubMed] [Google Scholar]
- Picklo MJ, Montine TJ, Amarnath V, Neely MD. Carbonyl toxicology and Alzheimer's disease. Toxicol Appl Pharmacol. 2002;184:187–97. doi: 10.1006/taap.2002.9506. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li S, Konduru AS, Zhang S, Trower TC, Shi W, et al. Prolonged exposure to methylglyoxal causes disruption of vascular KATP channel by mRNA instability. Am J Physiol Cell Physiol. 2012;303:1045–54. doi: 10.1152/ajpcell.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt MJ, Filipovic MR, Leffler A, de la Roche J, Kistner K, Fischer MJ, et al. Methylglyoxal activates nociceptors through transient receptor potential channel A1 (TRPA1): a possible mechanism of metabolic neuropathies. J Biol Chem. 2012;287:28291–306. doi: 10.1074/jbc.M111.328674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med. 2012;18:926–33. doi: 10.1038/nm.2750. [DOI] [PubMed] [Google Scholar]
- Brouwers O, Niessen PM, Haenen G, Miyata T, Brownlee M, Stehouwer CD, et al. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia. 2010;53:989–1000. doi: 10.1007/s00125-010-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukohda M, Okada M, Hara Y, Yamawaki H. Methylglyoxal accumulation in arterial walls causes vascular contractile dysfunction in spontaneously hypertensive rats. J Pharmacol Sci. 2012;120:26–35. doi: 10.1254/jphs.12088fp. [DOI] [PubMed] [Google Scholar]
- Bonev AD, Nelson MT. Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. J Gen Physiol. 1996;108:315–23. doi: 10.1085/jgp.108.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Yu L, Jin X, Yang Y, Cui N, Jiang C. Rosiglitazone inhibits vascular KATP channels and coronary vasodilation produced by isoprenaline. Br J Pharmacol. 2011;164:2064–72. doi: 10.1111/j.1476-5381.2011.01539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–6. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, et al. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. J Clin Invest. 2002;110:203–8. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, et al. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–72. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- Miura H, Wachtel RE, Loberiza FR, Jr, Saito T, Miura M, Nicolosi AC, et al. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res. 2003;92:151–8. doi: 10.1161/01.res.0000052671.53256.49. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gutterman DD. The coronary circulation in diabetes: influence of reactive oxygen species on K+ channel-mediated vasodilation. Vasc Pharmacol. 2002;38:43–9. doi: 10.1016/s1537-1891(02)00125-8. [DOI] [PubMed] [Google Scholar]
- Ellis EM. Reactive carbonyls and oxidative stress: potential for therapeutic intervention. Pharmacol Ther. 2007;115:13–24. doi: 10.1016/j.pharmthera.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Zhu G, Zhang Y, Xu H, Jiang C. Identification of endogenous outward currents in the human embryonic kidney (HEK 293) cell line. J Neurosci Methods. 1998;81:73–83. doi: 10.1016/s0165-0270(98)00019-3. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shi W, Cui N, Wu Z, Jiang C. Oxidative stress inhibits vascular KATP channels by S-glutathionylation. J Biol Chem. 2010;285:38641–8. doi: 10.1074/jbc.M110.162578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Cui N, Shi W, Jiang C. A short motif in Kir6.1 consisting of four phosphorylation repeats underlies the vascular KATP channel inhibition by protein kinase C. J Biol Chem. 2008;283:2488–94. doi: 10.1074/jbc.M708769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shi Y, Guo S, Zhang S, Cui N, Shi W, et al. PKA-dependent activation of the vascular smooth muscle isoform of KATP channels by vasoactive intestinal polypeptide and its effect on relaxation of the mesenteric resistance artery. Biochim Biophys Acta. 2008;1778:88–96. doi: 10.1016/j.bbamem.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Jin X, Yang Y, Cui N, Jiang C. Rosiglitazone inhibits vascular KATP channels and coronary vasodilation produced by isoproterenol. Br J Pharmacol. 2011;164:2064–72. doi: 10.1111/j.1476-5381.2011.01539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Yu L, Wu Y, Zhang S, Shi Z, Chen X, et al. S-Glutathionylation underscores the modulation of the heteromeric Kir4.1-Kir5.1 channel in oxidative stress. J Physiol. 2012;590(Pt 21):5335–48. doi: 10.1113/jphysiol.2012.236885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–83. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- Wu J, Xu H, Yang Z, Wang Y, Mao J, Jiang C. Protons activate homomeric Kir6.2 channels by selective suppression of the long and intermediate closures. J Membr Biol. 2002;190:105–16. doi: 10.1007/s00232-002-1029-1. [DOI] [PubMed] [Google Scholar]
- Chaplen FW, Fahl WE, Cameron DC. Evidence of high levels of methylglyoxal in cultured Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1998;95:5533–8. doi: 10.1073/pnas.95.10.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapolla A, Flamini R, Dalla Vedova A, Senesi A, Reitano R, Fedele D, et al. Glyoxal and methylglyoxal levels in diabetic patients: quantitative determination by a new GC/MS method. Clin Chem Lab Med. 2003;41:1166–73. doi: 10.1515/CCLM.2003.180. [DOI] [PubMed] [Google Scholar]
- Mukohda M, Yamawaki H, Okada M, Hara Y. Methylglyoxal enhances sodium nitroprusside-induced relaxation in rat aorta. J Pharmacol Sci. 2010;112:176–83. doi: 10.1254/jphs.09219fp. [DOI] [PubMed] [Google Scholar]
- Rabbani N, Thornalley PJ. Glyoxalase in diabetes, obesity and related disorders. Semin Cell Dev Biol. 2011;22:309–17. doi: 10.1016/j.semcdb.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Dobler D, Ahmed N, Song L, Eboigbodin KE, Thornalley PJ. Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification. Diabetes. 2006;55:1961–9. doi: 10.2337/db05-1634. [DOI] [PubMed] [Google Scholar]
- Beisswenger PJ, Howell SK, Touchette AD, Lal S, Szwergold BS. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes. 1999;48:198–202. doi: 10.2337/diabetes.48.1.198. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gutterman DD. Oxidative stress and potassium channel function. Clin Exp Pharmacol Physiol. 2002;29:305–11. doi: 10.1046/j.1440-1681.2002.03649.x. [DOI] [PubMed] [Google Scholar]
- Tang XD, Garcia ML, Heinemann SH, Hoshi T. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat Struct Mol Biol. 2004;11:171–8. doi: 10.1038/nsmb725. [DOI] [PubMed] [Google Scholar]
- Zha XM, Wang R, Collier DM, Snyder PM, Wemmie JA, Welsh MJ. Oxidant regulated inter-subunit disulfide bond formation between ASIC1a subunits. Proc Natl Acad Sci U S A. 2009;106:3573–8. doi: 10.1073/pnas.0813402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shi W, Chen X, Cui N, Konduru AS, Shi Y, et al. Molecular basis and structural insight of vascular K(ATP) channel gating by S-glutathionylation. J Biol Chem. 2011;286:9298–307. doi: 10.1074/jbc.M110.195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KO, Witz G, Witmer CM. Mutagenicity and toxicity studies of several alpha,beta-unsaturated aldehydes in the Salmonella typhimurium mutagenicity assay. Environ Mutagen. 1987;9:289–95. doi: 10.1002/em.2860090308. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, Ames BN. Naturally occurring carbonyl compounds are mutagens in Salmonella tester strain TA104. Mutat Res. 1985;148:25–34. doi: 10.1016/0027-5107(85)90204-0. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Argirov OK, Minhas HS, Cordeiro CA, Thornalley PJ. Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to Nepsilon-carboxymethyl-lysine- and Nepsilon-(1-carboxyethyl)lysine-modified albumin. Biochem J. 2002;364:1–14. doi: 10.1042/bj3640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers O, Niessen PM, Ferreira I, Miyata T, Scheffer PG, Teerlink T, et al. Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J Biol Chem. 2011;286:1374–80. doi: 10.1074/jbc.M110.144097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol (Noisy-le-grand) 1998;44:1013–23. [PubMed] [Google Scholar]