Abstract
OBJECTIVE
To determine the practice variance, prevalence, and economic burden of clinically diagnosed gastroesophageal reflux disease (GERD) in preterm infants.
METHODS
Applying a retrospective cohort study design, we analyzed data from 18 567 preterm infants of 22 to 36 weeks’ gestation and > 400 g birth weight from the NICUs of 33 freestanding children’s hospitals in the United States. GERD prevalence, comorbidities, and demographic factors were examined for their association with average length of stay (LOS) and hospitalization cost.
RESULTS
Overall, 10.3% of infants received a diagnosis of GERD (95% confidence interval [CI]: 9.8–10.7). There was a 13-fold variation in GERD rates across hospitals (P < .001). GERD diagnosis was significantly (P < .05) associated with bronchopulmonary dysplasia and necrotizing enterocolitis, as well as congenital anomalies and decreased birth weight. GERD diagnosis was associated with $70 489 (95% CI: 62 184–78 794) additional costs per discharge and 29.9 additional days in LOS (95% CI: 27.3–32.5).
CONCLUSIONS
One in 10 of these premature NICU infants were diagnosed with GERD, which is associated with substantially increased LOS and elevated costs. Better diagnostic and management strategies are needed to evaluate reflux-type symptoms in this vulnerable NICU population.
Keywords: economic burden, GERD, length of stay, neonate
Gastroesophageal reflux is frequent in thriving infants,1 and gastroesophageal reflux disease (GERD) is also common in infants with feeding problems.2–4 The distinction between gastroesophageal reflux and GERD in the premature infant in the NICU remains imprecise because of the heterogeneity and nonspecificity of aerodigestive and physical symptoms. Thus, physicians frequently clinically diagnose GERD in infants with symptoms of apnea, arching, and irritability; acute life-threatening events; chronic lung disease; congenital anomalies; and dysphagia.5,6
There is no clearly defined, accurate diagnostic method for GERD among premature infants, nor is there a consensus regarding therapeutic strategies. The prevalence and magnitude of symptoms that are clinically associated with GERD are unclear. Consequently, prolonged length of stay (LOS) and an increase in economic burden can be expected.7–9 These outcomes are likely to result in variable practices related to feeding methods and managing GERD. Although safe oral feeding competency before discharge is critical,10 reflux-type aerodigestive symptoms contribute to extended LOS resulting from delays in feeding skills.
The current study was undertaken to: (1) estimate the prevalence of GERD diagnosis among preterm infants in freestanding children’s hospital NICUs in the United States; (2) determine associated comorbidities and demographic factors; and (3) estimate the extent to which a GERD diagnosis is associated with additional resource utilization.
METHODS
Study Design and Data Source
The current study was a retrospective cohort study of premature infants ≤36 weeks’ gestational age (GA) admitted to 33 freestanding US children’s hospitals participating in the Pediatric Health Information System (PHIS) administrative database of the Child Health Corporation of America. Hospitals were selected that included detailed information for each patient hospitalization, including demographic characteristics, diagnoses, medications, and procedures. Data are accepted into PHIS only when classified errors occur in <2% of a hospital’s quarterly data.
The infants included in this study were admitted to participating NICUs from January 1, 2007, until December 31, 2010. We excluded term infants (≥37 weeks’ GA), infants at the limit of viability (<22 weeks’ GA or birth weight <400 g), infants with data for age missing at admission or if their age at admission was > 30 days, and infants who were missing the PHIS acuity score. In total, there were 18 567 unique admissions.
Patients were counted as having a GERD diagnosis if they had an International Classification of Diseases, Ninth Revision (ICD-9) code of 530.81. To characterize the severity of the preterm infants’ health problems at NICU admission, and presumably before the development of GERD, we created a set of variables based on ICD-9 codes. Up to 21 ICD-9 codes were reported for each infant. We ignored all codes that were recorded for <5 infants, because any effect that they might have on the development of GERD would be extremely uncommon. From the remaining codes, conditions were selected that were most likely to have started before the infant’s NICU admission. The selected codes were grouped into 25 diagnostic categories based on a consensus among 4 neonatologists and used as predictors of having a GERD diagnosis in the propensity score estimation. These predictors included birth trauma, major cardiac anomalies, congenital diaphragmatic hernia, craniofacial anomalies, obstetric risks or delivery complications, congenital dermatologic problems, in utero drug exposure, congenital endocrine problems, hematologic disorders, infant hypoxia, congenital immune problems, infant distress or malnutrition, infant or maternal exposure to infections, maternal risk factors, multiparous pregnancy, congenital musculoskeletal problems, congenital neurologic problems, congenital ophthalmologic problems, patent ductus arteriosus, congenital renal problems, congenital reproductive system problems, congenital airway and lung anomalies, congenital urinary problems, noncardiac vascular anomalies, and congenital gastrointestinal defects. Independent evaluations of the association of bronchopulmonary dysplasia (BPD) and necrotizing enterocolitis (NEC) with a GERD diagnosis were conducted. These illnesses, which often present later during the NICU hospitalization, are associated with increased mortality and high morbidity in preterm infants, and they independently affect cost and LOS.11–13
The cost of each admission was measured by using the hospital-specific ratio of cost to charge (RCC) estimate for the total cost of the stay (as calculated by PHIS). These cost estimates do not include physician charges.
The Nationwide Children’s Hospital institutional review board determined that this study was not human subjects’ research.
Statistical Analysis
All analyses were conducted by using R (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/) and Stata 11 (StataCorp, College Station, TX). We estimated the prevalence of diagnosed GERD in this population, and we used multivariate logistic regression methods to identify demographic and diagnostic covariates that were associated with the diagnosis of GERD. We included in the equations all of the variables that were significantly associated with a GERD diagnosis in the bivariate analyses. The regression included a random intercept for NICUs to account for variation across NICUs in diagnostic practices concerning GERD, as well as unmeasured patient factors associated with local populations that might have influenced the prevalence of a GERD diagnosis. We then calculated the average total charges, LOS, and total charges per hospital day for patients with and without GERD. Because GERD patients differ from non-GERD patients on many variables, the differences in total charges and LOS associated with the GERD diagnosis were re-estimated, adjusting for a propensity score estimated from the multivariate logistic regression equation for the occurrence of GERD (Stata function psmatch2). Patients with a GERD diagnosis were compared with kernel-weighted averages of patients without GERD (analyses according to Mahalanobis matching produced similar results).
RESULTS
Demographic Characteristics and Prevalence of Clinical GERD
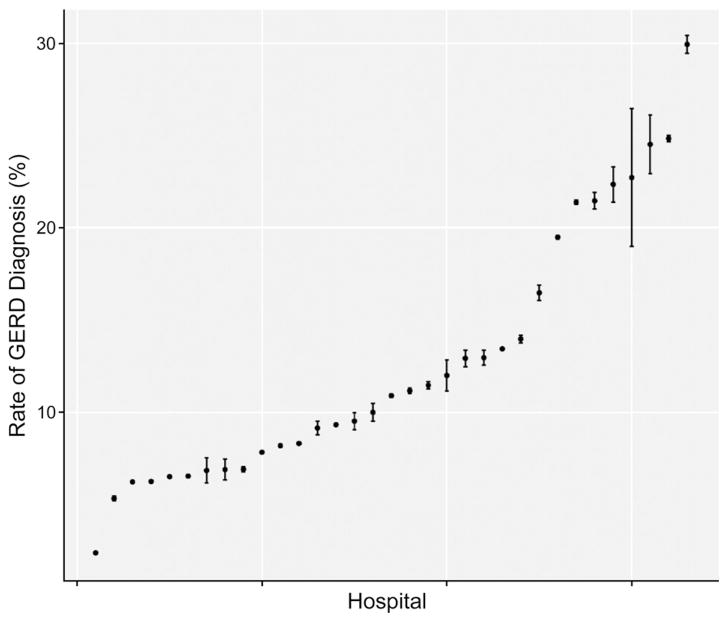
An ICD-9 code indicating a diagnosis of GERD was reported for 1907 (10.3%) preterm infants (95% confidence interval [CI]: 9.8–10.7). Rates of GERD diagnosis in preterm infants varied dramatically across NICUs (Fig 1), from 2.4% to 29.9% (χ2 [32]= 574.6, P = .000).
FIGURE 1.
Rates of diagnosis of GERD across participating PHIS hospitals. Each dot represents a data point with upper and lower 95% CIs.
Table 1 presents the associations between the GERD ICD-9 code and demographic characteristics of patients. GERD was diagnosed more commonly in patients admitted with an age > 7 days, in patients with lighter birth weights, in patients aged 28 to 33 weeks at birth, and in non-Hispanic patients. Table 2 presents the diagnoses of conditions that were likely to have characterized the infant on admission to the hospital and that were significantly associated with the GERD diagnosis. In addition, patients with GERD had a higher acuity score (mean: 23.3) than patients without the diagnosis (mean: 13.5; P = .000). Table 3 shows that BPD and NEC were independently associated with a diagnosis of GERD. GERD diagnosis was associated with stages I and II NEC (medically treated) and perforated NEC (stage III) requiring surgery.
TABLE 1.
Demographic Characteristics, Prevalence, and OR of GERD
| Variable | N (%)a | % GERDb | Bivariate Analysisc
|
Multiple Logistic Regressiond
|
||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | Adjusted OR (95% CI) | P | |||
| Female infant GA, wk | 8157 (43.9) | 10.0 | 0.95 (0.86–1.04) | .288 | 0.93 (0.84–1.03) | .156 |
| 34–36 | 1075 (5.8) | 12.7 | ||||
| 31–33 | 2157 (11.6) | 19.5 | 1.67 (1.36–2.07) | .000 | 1.70 (1.38–2.11) | .000 |
| 28–30 | 2723 (14.7) | 16.1 | 1.33 (1.08–1.64) | .007 | 1.41 (1.09–1.82) | .008 |
| 25–27 | 4584 (24.7) | 8.5 | 0.64 (0.52–0.79) | .000 | 0.82 (0.61–1.10) | .192 |
| 22–24 | 8028 (43.2) | 6.5 | 0.48 (0.39–0.59) | .000 | 0.71 (0.52–0.98) | .036 |
| Birth weight, g | ||||||
| >2500 | 3707 (20.0) | 6.1 | ||||
| 2001–2500 | 4333 (23.3) | 6.6 | 1.09 (0.91–1.30) | .361 | 1.06 (0.88–1.27) | .563 |
| 1501–2000 | 4105 (22.1) | 9.0 | 1.52 (1.28–1.81) | .000 | 1.29 (1.05–1.57) | .014 |
| 1001–1500 | 3157 (17.0) | 15.1 | 2.72 (2.31–3.22) | .000 | 1.55 (1.22–1.97) | .000 |
| 400–1000 | 3265 (17.6) | 16.7 | 3.08 (2.62–3.63) | .000 | 1.55 (1.16–2.06) | .003 |
| Race/ethnicity | ||||||
| White | 9016 (48.6) | 10.5 | ||||
| Black | 2363 (12.7) | 13.6 | 1.34 (1.17–1.53) | .000 | 1.06 (0.92–1.22) | .381 |
| Hispanic | 3396 (18.3) | 7.8 | 0.72 (0.62–0.83) | .000 | 0.67 (0.58–0.78) | .000 |
| Asian | 573 (3.1) | 13.1 | 1.28 (0.99–1.64) | .051 | 1.25 (0.96–1.60) | .092 |
| Other | 3219 (17.3) | 9.4 | 0.88 (0.77–1.01) | .066 | 0.85 (0.74–0.97) | .020 |
| Age at admission, d | ||||||
| 1 | 12 557 (67.6) | 10.1 | ||||
| 2–7 | 3857 (20.8) | 8.7 | 0.84 (0.74–0.96) | .008 | 0.97 (0.85–1.10) | .594 |
| >7 | 2153 (11.6) | 14.1 | 1.46 (1.28–1.67) | .000 | 1.15 (1.00–1.32) | .046 |
OR, odds ratio.
Number and percentage of infants with the condition.
Prevalence in that subgroup.
OR (95% CI) and P report the OR, CI, and P value for the association of the condition and GERD. For polytomous categorical variables such as birth weight, the OR compares a level of the variable against a reference level.
Adjusted OR (95% CI) and P report the OR adjusted for all covariates in Tables 1 and 2.
TABLE 2.
Association of GERD Diagnosis With Comorbidities Present at Admission
| Variable | N (%)a | % GERDb | Bivariate Analysisc
|
Multiple Logistic Regressiond
|
||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | Adjusted OR (95% CI) | P | |||
| Congenital cardiac defects | 2293 (12.3) | 15.1 | 1.67 (1.48–1.90) | .000 | 1.20 (1.04–1.38) | .010 |
| Congenital GI defects | 902 (4.9) | 19.3 | 2.20 (1.84–2.61) | .000 | 1.61 (1.33–1.95) | .000 |
| Congenital respiratory/airway | 1068 (5.8) | 25.4 | 3.30 (2.84–3.81) | .000 | 2.48 (2.11–2.90) | .000 |
| Craniofacial defects | 404 (2.2) | 23.0 | 2.70 (2.12–3.40) | .000 | 1.77 (1.35–2.30) | .000 |
| Diaphragmatic hernia | 121 (0.7) | 32.2 | 4.22 (2.85–6.15) | .000 | 2.59 (1.70–3.88) | .000 |
| Drug exposure | 930 (5.0) | 13.3 | 1.37 (1.12–1.66) | .002 | 1.43 (1.17–1.75) | .000 |
| Exposure to infections | 1381 (7.4) | 17.5 | 1.97 (1.70–2.28) | .000 | 1.67 (1.43–1.95) | .000 |
| Other genetic defects | 720 (3.9) | 15.0 | 1.57 (1.27–1.93) | .000 | 0.74 (0.58–0.94) | .014 |
| Patent ductus arteriosus | 4453 (24.0) | 16.4 | 2.17 (1.96–2.39) | .000 | 1.91 (1.72–2.13) | .000 |
| Total | 18 567 (100) | 10.3 | — | — | — | — |
GI, gastrointestinal.
Number and percentage of infants with the condition.
Prevalence in that subgroup.
OR (95% CI) and P report the OR, CI, and P value for the association of the condition and GERD. For polytomous categorical variables such as birth weight, the OR compares a level of the variable against a reference level.
Adjusted OR (95% CI) and P report the OR adjusted for all covariates in Tables 1 and 2.
Additional diagnostic variables (listed in the Methods) were examined in bivariate and multiple logistic analyses but were not included in this table because they were not significant in the multiple logistic regressions.
TABLE 3.
Association of GERD With BPD and NEC
| Variable | N (%) | % GERD | OR (95% CI) | P |
|---|---|---|---|---|
| BPD | 2278 (12.3) | 23.6 | 3.37 (3.01–3.77) | .000 |
| NEC | ||||
| No NEC | 17 175 (92.5) | 9.9 | ||
| NEC stage I | 46 (0.2) | 21.7 | 2.53 (1.19–4.92) | .010 |
| NEC stage II | 109 (0.6) | 21.1 | 2.44 (1.50–3.80) | .000 |
| NEC stage III | 196 (1.1) | 20.4 | 2.34 (1.62–3.28) | .000 |
| NEC NOS | 1041 (5.6) | 13.1 | 1.37 (1.13–1.65) | .001 |
| Total | 18 567 (100) | 10.3 | — | — |
NOS, no stage.
Resource Utilization: LOS and Total Charges
Table 4 presents the average RCC-adjusted cost per admission, LOS, and cost per hospital day for patients with or without ICD-9–diagnosed GERD. According to these estimates, GERD patients stayed in the hospital longer than other patients, and their NICU hospitalizations were more expensive. However, the daily cost of caring for a patient with GERD was slightly less than a patient without GERD.
TABLE 4.
LOS and Economic Burden Compared Between GERD Patients Versus Non-GERD Patients
| Cost Measure | Naive Estimates
|
Propensity Score–Adjusted Estimate: Average Effect of GERD on Patients With GERD | ||
|---|---|---|---|---|
| GERD (n = 1907)
|
Non-GERD (n = 16 660)
|
Mean (GERD) – Mean (Non-GERD) | ||
| Mean ± SD | Mean ± SD | |||
| Cost of visit, $ | 202 293 ± 180 905 | 85 452 ± 117 681 | 116 841 | 70 489 |
| LOS, d | 75.5 ± 55.8 | 31.5 ± 37.8 | 44.0 | 29.9 |
| Cost/d, $ | 2598 ± 947 | 2829 ± 3751 | −231 | −556 |
Differences between GERD and non-GERD patients on each measure were statistically significant according to unequal variance t tests (P < .001).
Differences in cost and cost per day were also statistically significant in log-transformed units.
We computed estimates of the additional cost of care and LOS associated with the GERD ICD-9 code, using propensity scores to match GERD and non-GERD patients. The estimated additional RCC-adjusted cost associated with GERD was $70 489 (95% CI: 62 184 to 78 794) per hospitalization. A patient with GERD had an estimated 29.9 additional days in LOS (95% CI: 27.3 to 32.5). In addition to the longer LOS, GERD patients had lower mortality than other patients. Mortality during the NICU stay was 7.9% for non-GERD patients but only 1.4% for GERD patients (P < .001). GERD patients cost $556 less per day than similar non-GERD patients (95% CI: −640 to −472).
DISCUSSION
Based on reflux-type aerodigestive symptoms, clinical suspicion and diagnosis of GERD are common across the neonatal age spectrum, although the literature has been unclear about the definition, prevalence, diagnostic, and management approaches among NICU premature infants. From the current study, we came to 5 conclusions. First, 1 of 10 patients from this large group of NICUs at freestanding children’s hospitals received a diagnosis of GERD according to ICD-9 code. Second, the GERD diagnosis was independently associated with several factors present at birth. The design of the study means that these associations are not necessarily causal. Third, the diagnosis of GERD was associated with substantially reduced mortality risk. The greater mortality in non-GERD patients may be related to extreme immaturity or illness, such that these infants never survived to experience GERD. At an earlier age or during more critical states, premature infants may have their airway and esophagus protected and/or bypassed by using ventilation methods and gavage tube–feeding strategies, or they may be on limited or no enteral feeding volumes. Thus, such infants may have less gastric volumes to cause distention-induced reflux. In contrast, GERD diagnosis was also associated with 2 important chronic morbidities, BPD and NEC. Fourth, a GERD diagnosis was associated with a difference of more than $70 000 in the cost of an NICU admission. This estimate was conservative because it excluded physician costs. Finally, admissions with a diagnosis of GERD cost more because the infants stayed ~30 days longer in the NICU.
The prolonged LOS in the GERD group may be attributable to severe comorbidities or the presence of reflux-type aerodigestive symptoms. The prolonged LOS for infants diagnosed with GERD is consistent with previous single-center studies that found infants who had GERD symptoms had longer LOS by 12 to 29 days.14–16 Notable among such symptoms are cardiorespiratory events, gagging, arching, and feeding problems.3,4,17 These symptoms may occur because premature infants have immature and maladaptive motility mechanisms and are fed a larger volume per kilogram of body weight.18 Therefore, it is likely that prolonged hospitalization of infants diagnosed with GERD may be related to delayed adaptation to esophageal provocation from gastroesophageal reflux events. In a single-center study in 1998, the difference in total hospital charges for premature infants with clinically significant GERD was nearly $50 000, which, if adjusted for inflation, is similar to the $70 000 difference reported in our study.14
GERD was actually diagnosed less in the 22- to 24-week GA infants than it was in the 25- to 28-week GA infants. It is likely that the former group, in which most deaths occur in the first month of life, did not survive long enough to be fed and display the resulting reflux-type symptoms. Moreover, the most premature infants are more likely to have other diagnoses that could be given higher priority by medical coders such as BPD, apnea of prematurity, intraventricular hemorrhage, or hydrocephalus, which could also explain our finding of an association between lower mortality and a diagnosis of GERD. Alternatively, these findings may be due to a prematurity-associated decrease in acid production, failure to recognize nonacid gastroesophageal reflux events, or simply a failure to diagnose GERD in tiny infants. If the latter is true, we may have underestimated the prevalence of GERD diagnosis.
Our finding of a 13-fold range across hospitals in rates of GERD diagnosis raises the question of whether all the infants identified in this study as having GERD had true pathologic GERD (Fig 1). This hospital variability and uncommon use of specific diagnostic procedures suggest that providers were using many different criteria to make the diagnosis, and this wide variation is consistent with that found regarding the diagnosis and treatment of GERD reported in previous studies.19,20 The 13-fold difference in GERD diagnosis rates across various centers may reflect differences in the culture of management of these problems rather than variation in the prevalence of GERD. It is therefore important to develop diagnostic criteria for GERD based on objective measurements or clinical risk scores.
We speculate that prolonged LOS may be related to persistent aerodigestive symptoms, growth problems, and feeding difficulties. Our clinical impression is that many infants spend extended periods in the NICU simply waiting for the resolution of problems associated with GERD and the developmental achievement of normal feeding milestones. Interventions that rapidly and effectively address GERD could significantly modify practice variability and lower LOS and related costs for both families and society. Alternatively, the use of acid-suppressive pharmacologic agents is not effective and is associated with complications, such as NEC, bacteremia, urinary tract infections, and pneumonia.21,22 These complications may also contribute to the increased LOS.
GERD may be frequently overdiagnosed and overtreated in preterm infants, and its real prevalence remains unknown.20 Such therapeutic applications are controversial, and long-term consequences of acid-suppressive therapies in the absence of pathologic GERD can be deleterious. A recent multicenter, prospective cohort investigation found an association between ranitidine use in infants with a birth weight <1500 g and increased mortality, infections, and NEC.22 Because ~10% of NICU patients received a diagnosis of GERD, there is a large population that could participate in randomized controlled trials to test novel diagnostic and therapeutic strategies. Further studies are needed to elucidate whether symptoms of reflux are part of a neonate’s normal functioning or if they are disease based; until then, the definition of true GERD and its treatment will remain controversial.
The study has several limitations. There was wide variation among centers, suggesting that the use of the GERD diagnosis is often subjective. Clearly, there needs to be a standardized method of diagnosing and managing GERD in the NICU. Unfortunately, there is no consensus on standardized testing for GERD or of its therapies in NICU infants.
Our estimate of the annual additional national NICU costs associated with a GERD diagnosis was based on data from freestanding children’s hospitals. Cost differences were likely confounded by interprovider practice differences, intrinsic patient differences, and center differences. Children in these hospitals may have been more severely ill than similar premature children cared for at other institutions. If so, this factor may have led us to overestimate the cost associated with GERD. However, because physician costs were not included in PHIS, cost estimates presented here were likely understated.
Finally, because the children’s hospitals in the PHIS database are usually not delivery centers, the patients in our cohort may have been referred for specialty care and thus may not be representative of all premature infants but rather representative of sicker premature infants and those with multisystemic problems. Despite these limitations, our investigation had multiple strengths. The use of the PHIS database provided us with a large, heterogeneous, multicenter sample of patients with GERD. To the best of our knowledge, our study is the first multicenter report on the prevalence and associated cost of a GERD diagnosis in preterm infants. This article provides insight into the providers’ perception of GERD and treatment options in NICUs.
CONCLUSIONS
There was a 10.3% prevalence of ICD-9–diagnosed GERD in this multicenter cohort of premature infants in the NICU. These infants required ~30 additional days of NICU care compared with similar infants without GERD, at an additional cost per admission of more than $70 000. This increased GERD-associated LOS may be related to the maturational changes in the development and adaptation to esophageal provocation during gastroesophageal reflux events. Such shortcomings underscore the need for better diagnostic methods and for treatment plans based on objective evidence. Interventions that rapidly and effectively address GERD should be sought to reduce LOS, with both its emotional cost to families and financial cost to society.
Acknowledgments
FUNDING: Supported in part by grant R01 NIH DK 068158 to Dr Jadcherla, grant R21 HS19524-01 from the Agency for Healthcare Research and Quality to Dr Gardner, and grant KL2RR025754 from the National Institutes of Health’s National Center for Research Resources to Dr Slaughter. Funded by the National Institutes of Health (NIH).
ABBREVIATIONS
- BPD
bronchopulmonary dysplasia
- CI
confidence interval
- GA
gestational age
- GERD
gastroesophageal reflux disease
- ICD-9
International Classification of Diseases, Ninth Revision
- LOS
length of stay
- NEC
necrotizing enterocolitis
- PHIS
Pediatric Health Information System
- RCC
ratio of cost to charge
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Drs Jadcherla and Kelleher provided the concept and Dr Jadcherla established the relevance and wrote the first draft; Drs Jadcherla, Slaughter, Kelleher, and Gardner were responsible for study design; Drs Jadcherla, Slaughter, Stenger, Klebanoff, and Kelleher provided analysis and interpretation; All authors were responsible for writing and approval of the final draft; Drs Jadcherla, Slaughter, Stenger, Klebanoff, and Gardner provided critical review and made revisions; Dr Gardner provided the PHIS concepts, data analysis, statistical analysis, figures and tables.
References
- 1.Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. North American Society for Pediatric Gastroenterology Hepatology and Nutrition; European Society for Pediatric Gastroenterology Hepatology and Nutrition. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J Pediatr Gastroenterol Nutr. 2009;49(4):498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 2.Jadcherla SR. Esophageal motility in the human neonate. Neoreviews. 2006;7(1):e7–e12. [Google Scholar]
- 3.Jadcherla SR, Gupta A, Fernandez S, et al. Spatiotemporal characteristics of acid refluxate and relationship to symptoms in premature and term infants with chronic lung disease. Am J Gastroenterol. 2008;103(3):720–728. doi: 10.1111/j.1572-0241.2007.01748.x. [DOI] [PubMed] [Google Scholar]
- 4.Jadcherla SR, Peng J, Chan CY, et al. Significance of gastroesophageal refluxate in relation to physical, chemical, and spatiotemporal characteristics in symptomatic intensive care unit neonates. Pediatr Res. 2011;70(2):192–198. doi: 10.1203/PDR.0b013e31821f704d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassall E. Over-prescription of acid-suppressing medications in infants: how it came about, why it’s wrong, and what to do about it. J Pediatr. 2012;160(2):193–198. doi: 10.1016/j.jpeds.2011.08.067. [DOI] [PubMed] [Google Scholar]
- 6.Poets CF, Brockmann PE. Myth: gastroesophageal reflux is a pathological entity in the preterm infant. Semin Fetal Neonatal Med. 2011;16(5):259–263. doi: 10.1016/j.siny.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Behrman RE, Butler AS. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 8.Jadcherla SR, Vijayapal AS, Leuthner S. Feeding abilities in neonates with congenital heart disease: a retrospective study. J Perinatol. 2009;29(2):112–118. doi: 10.1038/jp.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jadcherla SR, Wang M, Vijayapal AS, Leuthner SR. Impact of prematurity and comorbidities on feeding milestones in neonates: a retrospective study. J Perinatol. 2010;30(3):201–208. doi: 10.1038/jp.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics Committee on Fetus and Newborn. Hospital discharge of the high-risk neonate. Pediatrics. 2008;122(5):1119–1126. doi: 10.1542/peds.2008-2174. [DOI] [PubMed] [Google Scholar]
- 11.Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20(6):498–506. doi: 10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 12.Jobe AH. The new BPD. Neoreviews. 2006;7(10):e531–e545. [Google Scholar]
- 13.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 14.Ferlauto JJ, Walker MW, Martin MS. Clinically significant gastroesophageal reflux in the at-risk premature neonate: relation to cognitive scores, days in the NICU, and total hospital charges. J Perinatol. 1998;18(6 pt 1):455–459. [PubMed] [Google Scholar]
- 15.Frakaloss G, Burke G, Sanders MR. Impact of gastroesophageal reflux on growth and hospital stay in premature infants. J Pediatr Gastroenterol Nutr. 1998;26(2):146–150. doi: 10.1097/00005176-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Khalaf MN, Porat R, Brodsky NL, Bhandari V. Clinical correlations in infants in the neonatal intensive care unit with varying severity of gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2001;32(1):45–49. doi: 10.1097/00005176-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Jadcherla SR, Hogan WJ, Shaker R. Physiology and pathophysiology of glottic reflexes and pulmonary aspiration: from neonates to adults. Semin Respir Crit Care Med. 2010;31(5):554–560. doi: 10.1055/s-0030-1265896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadcherla SR, Chan CY, Moore R, Malkar M, Timan CJ, Valentine CJ. Impact of feeding strategies on the frequency and clearance of acid and nonacid gastroesophageal reflux events in dysphagic neonates. JPEN J Parenter Enteral Nutr. 2012;36(4):449–455. doi: 10.1177/0148607111415980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golski CA, Rome ES, Martin RJ, et al. Pediatric specialists’ beliefs about gastroesophageal reflux disease in premature infants. Pediatrics. 2010;125(1):96–104. doi: 10.1542/peds.2008-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malcolm WF, Gantz M, Martin RJ, Goldstein RF, Goldberg RN, Cotten CM National Institute of Child Health and Human Development Neonatal Research Network. Use of medications for gastroesophageal reflux at discharge among extremely low birth weight infants. Pediatrics. 2008;121(1):22–27. doi: 10.1542/peds.2007-0381. [DOI] [PubMed] [Google Scholar]
- 21.Guillet R, Stoll BJ, Cotten CM, et al. National Institute of Child Health and Human Development Neonatal Research Network. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006;117(2) doi: 10.1542/peds.2005-1543. Available at: www.pediatrics.org/cgi/content/full/117/2/e137. [DOI] [PubMed] [Google Scholar]
- 22.Terrin G, Passariello A, De Curtis M, et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics. 2012;129(1) doi: 10.1542/peds.2011-0796. Available at: www.pediatrics.org/cgi/content/full/129/1/e40. [DOI] [PubMed] [Google Scholar]