Abstract
The Chemical Weapons Convention prohibits the development, production, acquisition, stockpiling, retention, transfer or use of chemical weapons by Member States. Verification of compliance and investigations into allegations of use require accurate detection of chemical warfare agents (CWAs) and their degradation products. Detection of CWAs such as organophosphorus nerve agents in the environment relies mainly upon the analysis of soil. We now present a method for the detection of the nerve agent VX and its hydrolysis products by gas chromatography and liquid chromatography mass spectrometry of ethanol extracts of contaminated white mustard plants (Sinapis alba) which retained the compounds of interest for up to 45 days. VX is hydrolysed by the plants to ethyl methylphosphonic acid and then to methylphosphonic acid. The utility of white mustard as a nerve agent detector and remediator of nerve agent-polluted sites is discussed. The work described will help deter the employment of VX in conflict.
Keywords: chemical weapons, gas chromatography, liquid chromatography, mass spectrometry, Sinapis alba, VX
1. Introduction
The use of the organophosphorus (OP) nerve agent sarin in Syria [1–4] to gruesome effect has highlighted the need for improved detection methods for OP nerve agents. Chemical weapons are banned by the Chemical Weapons Convention (CWC) [5], whose importance was recognized by the award of the 2013 Nobel Peace Prize [6] to its implementing body, the Organisation for the Prohibition of Chemical Weapons (OPCW), based in The Hague. The analysis of OP nerve agents and their degradation products is vital for verification of compliance to the CWC, which now has 190 member states. It is also essential for supporting the UK National Security Strategy, which highlights international terrorism affecting the UK or its interests, including chemical terrorism, as a Tier One risk [7].
Detection of chemical warfare agents (CWAs) by OPCW designated laboratories supports the CWC. The Defence Science and Technology Laboratory (Dstl) at Porton Down has housed the UK Designated Laboratory since the CWC entered into force in 1997. Gas chromatography (GC) and liquid chromatography (LC), combined with mass spectrometry (MS), are established techniques for the detection and identification of OP nerve agents and related compounds, mostly in environmental samples [8]. Combining GC and LC-MS methods extends the range of analytes detectable in a single sample. Besides sarin (isopropyl methylphosphonofluoridate) and soman (pinacolyl methylphosphonofluoridate) [9], one of the most hazardous nerve agents is VX (O-ethyl S-2-diisopropylaminoethyl methylphosphonothiolate) [10]. These agents react with moisture to give respectively the alkyl methylphosphonic acids: isopropyl methylphosphonic acid (iPMPA), pinacolyl methylphosphonic acid (PMPA) and ethyl methylphosphonic acid (EMPA) [11–13] and more slowly, methylphosphonic acid (MPA) [14,15] (figure 1). Such products indicate the prior presence of the nerve agents. Sarin and soman have a greater volatility and reactivity to water than VX and persist for a shorter time in the environment [16,17]. The hydrolysis products iPMPA, PMPA, EMPA and MPA are water-soluble and are retained by soil according to its composition, which varies widely [18].
Figure 1.
The nerve agents sarin, soman and VX react with moisture in the environment to provide iPMPA, PMPA or EMPA. These acids react slowly with water with loss of isopropanol, pinacolyl alcohol, or ethanol to provide MPA. It was the discovery of sarin, iPMPA and MPA collectively by the United Nations investigating team, from analysis of soil samples collected after the 21 August 2013 attack on civilians in Ghouta in the Syrian Arab Republic, that confirmed that sarin-filled rockets had been deployed [2,4].
Vegetation absorbs nerve agents and their degradation products [19–22] (but note the paucity of information available). It acts as a time capsule whose interrogation can reveal nerve agent use. Recently, we communicated a novel method for the detection of VX and its hydrolysis products EMPA and MPA through GC- and LC-MS of ethanol extracts of white mustard plants (Sinapis alba) grown in contaminated loam, which localized the compounds of interest, and retained them in an extractable form longer than the soil [23,24]. This methodology could provide evidence of use of VX that would be unavailable from analysis of soil alone. Since then, we have extended the study to increase the power of detection (10 plants, rather than four, per experiment), doubled the time window available for detection (48 days instead of 28 days), examined VX absorption from different soils—sand, loam and clay—and studied whether the plants metabolize VX after uptake through the roots (versus absorbing VX and its degradation products from the soil). EMPA and MPA were spiked separately onto loam containing S. alba and the analytical results compared to those obtained from plants grown in VX spiked loam. The results presented should help attribute, and deter, aggressive nerve agent use.
2. Material and methods
(a). Spiking chemicals
VX and EMPA of 98% purity by NMR spectroscopy (1H, 13C and 31P nuclei) were synthesized at Dstl Porton Down [10,11]. MPA was purchased from Sigma-Aldrich Ltd. (Dorset, UK) and used as received. Caution! VX is highly toxic and should be handled only by trained personnel in an appropriate containment facility under the terms of the CWC.
(b). Plant cultivation and harvest
Sinapis alba is a pollution-tolerant plant [25] that originates from the Mediterranean floral region. Nowadays, it grows wild globally and in cultivation, reaching dimensions of 60×30 cm in sand, loam and heavy soils of acidic, basic and neutral pH [26]. It is grown for its edible leaves and seeds, and as a green manure due to its rapid growth and coverage. The seeds grow close to the ground and have no endosperm; the food reserve comprises the fleshy cotyledons, which emerge from the soil and become photosynthetic [27].
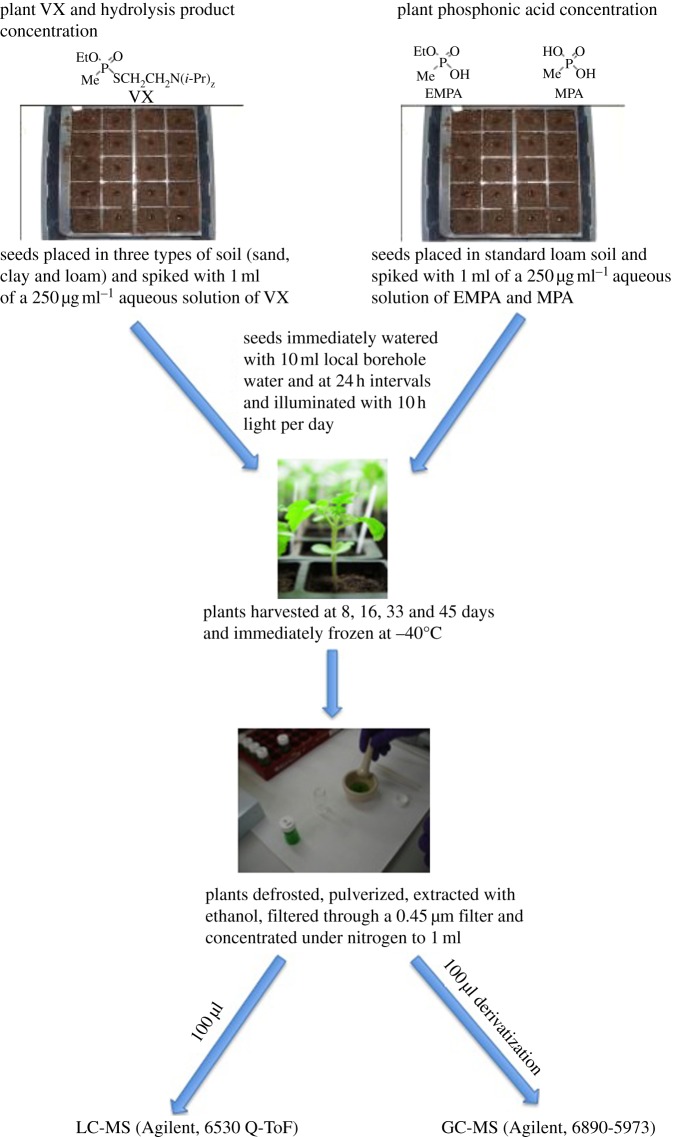
A standard seed tray (Grow It, 20 cell insert, Gardman, Lincs., UK) was filled to the top with one of three types of soil: clay (50% clay soil and 50% Levington Seed & Cutting Compost, The Scotts Miracle-Gro Company, Surrey, UK), loam (100% Levington Seed & Cutting Compost) or sandy soil (50% Kelkay Building Sand, East Yorkshire, UK, and 50% Levington Seed & Cutting Compost). A single divot was created in each compartment and one seed was placed into each divot. The seed was covered with soil and the latter was contaminated with 1 ml of a 250 μg ml−1 aqueous solution of VX, EMPA or MPA. This concentration was chosen for safety reasons and to allow rigour in measurement of sensitivity and detection limits. All three soil types were contaminated with VX while loam was contaminated separately with EMPA and MPA. The soil was watered with local borehole water (10 ml) immediately and then at 24 h intervals. The trays were placed under a lighting system (EvoLux Bright-Wing Mother Clone Lights, Growell, UK) that provided 38 400 lumens covering an area of 240×240 cm and on a timing system to provide 10 h of light every 24 h. Ten plants were harvested at each of four time intervals (8, 16, 33 and 45 days). The plants were cut at the soil surface ready for plant sample preparation.
(c). Plant sample preparation
The plant samples were pulverized using a pestle and mortar and extracted with ethanol (4 ml, 96% v/v, BDH AnalaR, VWR International Ltd., West Sussex, UK) and the pestle and mortar rinsed with ethanol (3×2 ml). The combined extracts were filtered using a 0.45 μm nylon filter (Whatman, Kent, UK) and concentrated under nitrogen gas to a volume of 1 ml. An aliquot (100 μl) of this solution was analysed by LC-MS (Q-ToF, Agilent), while another aliquot (100 μl) was dried at 35°C for 2 h using a SpeedVac (Thermo Scientific Ltd., UK). Derivatization was performed using N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide and 1% tert-butyldimethylchlorosilane (t-BDMCS, 50 μl; Sigma-Aldrich Ltd.), pyridine (5 μl; Pierce, silylation grade) and acetonitrile (45 μl; Thermo Scientific Ltd., silylation grade). The solution was heated at 100°C for 30 min (Regis Technologies Inc., IL, USA) and analysed by GC-MS.
(d). Instrumentation and analysis
GC-MS was performed on an Agilent Technologies 6890GC-5973 mass spectrometer (detection limit 1 pg μl−1 of octafluoronaphthalene) equipped with an autosampler. The GC was performed in splitless mode with the injection port kept at 250°C and 45.6 ml min−1 purge of helium. The initial temperature of the GC oven (90°C) was held for 0.7 min and ramped up 10°C min−1, to 300°C, where it was held for 2 min. The column used was an Agilent Technologies J&W DB-5MS (25 m in length, diameter of 0.2 mm, and a film thickness of 0.33 μm) in constant flow mode with a flow of 1.0 ml min−1 helium. The total run time was 23.70 min and the injection volume was 1 μl. External calibration standards of derivatized EMPA (10 μg ml−1) and MPA (10 μg ml−1) were used.
LC-MS was performed on an Agilent Technologies 6530 Q-ToF mass spectrometer (detection limit 1 pg on column of reserpine) equipped with an Agilent Technologies Infinity 1290 HPLC pump and autosampler. The mass spectrometer was equipped with an Agilent Jet Stream Electrospray Ionisation (AJS-ESI) source. The LC column was a Zorbax Eclipse Plus C18 Rapid Resolution HD (Agilent Technologies) of length 50 mm, internal diameter 2.1 mm and particle size 1.8 μm. A 1290 Infinity inline filter (0.3 μm) (Agilent Technologies) was fitted to the column inlet. Mobile phases were: A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile). The elution gradient was 5% B (0.0–3.5 min) to 50% B (3.5–4.0 min) to 100% B (4.0–5.0 min) at a flow rate of 1 ml min−1. AJS-ESI-MS conditions were: drying gas temperature (nitrogen) 300°C at 4 l min−1, nebulizer PSIG, sheath gas temperature (nitrogen) 300°C at 12 l min−1, capillary voltage 4000 V, nozzle voltage 1000 V, MS ToF fragmentor 175 V, skimmer 65 V, octopole 1 RF Vpp 750 V, scan range m/z 50–1700, and scan time 0.33 s. An injection volume of 10 μl and external calibration standards of 0.1, 0.5, 1.0 and 10.0 ng ml−1 aqueous VX were used, resulting in a calibration R value of 0.9980. For the quantitation of EMPA calibration standards of 0.1, 0.5, 1.0, 5.0 and 10.0 ng ml−1 aqueous EMPA were used, resulting in a calibration R value of 0.9949.
(e). Data analysis
Initial data analysis was performed using MassHunter (Agilent Technologies). Data were exported to Microsoft Excel for quantification and graphical representation. Quantification was based upon extracted ions; m/z 268.14946 for VX in positive mode and m/z 123.02165 for EMPA in negative mode by LC-MS, m/z 153 for EMPA and m/z 267 for MPA by GC-MS. Results for each time point represent the average of 10 plant analyses.
(f). Soil pH measurement
Ultra-high purity water (10 ml) was added to the soil matrix (10 g) and the mixture was vortexed for 2 min, and then left for 12 h. The pH was measured using Fisherbrand pH-Fix 0–14 pH strips (FB33003; Fisher Scientific Ltd., Loughborough, Leicestershire, UK). An aliquot of each aqueous soil extract was pipetted onto a pH strip. The pH of loam and sandy soils was 6.5, while the pH of the clay soil was 9.0. The pH of the sandy soil was similar to a literature value [28].
(g). Experiment flow diagram
An experiment flow diagram is shown in figure 2.
Figure 2.
Experiment flow diagram. (Online version in colour.)
3. Results and discussion
(a). Plant VX and ethyl methylphosphonic acid and methylphosphonic acid hydrolysis product detection
A nerve agent applied to soil enters a dynamic ecosystem and immediately begins to degrade or translocate. For sampling, it is important to determine the importance of such processes. Nerve agents that degrade may become less toxic or harmless, while those that move elsewhere may endanger the operator performing the sampling. Nerve agents and related compounds can leave soil by volatilization, but in the case of VX and its hydrolysis products, this is unlikely as the latter have very low vapour pressures [17,29,30]. The uptake of nerve agents and their degradation products depends on the type of soil containing the nerve agent. Soils are classified according to the proportion of mineral particles of different sizes present. Nerve agents absorb rapidly onto soil particles. Generally, the smaller the particles the longer a nerve agent persists due to absorption over a larger surface area. However, the relationship between particle size and persistence in soil is complex, because persistency depends on the organic content, clay content and pH [17,31–33]. Organic matter in soil varies from 1% to more than 50% and an increasing amount usually increases persistency. Most soil organic matter comprises humic compounds that have a high cation exchange capacity. These compounds have amino, carboxyl and hydroxyl groups that provide hydrogen bonding sites for nerve agents [34]. These characteristics provide the capacity for absorption and increased persistence. The smallest particles in soil (0.002 mm) are defined as clay and soils with greater than 40% clay particles are called clay soils. Such soils have a larger internal surface area available for absorption of nerve agents. The soil pH influences the nerve agent hydrolysis rate.
In the previous study [23], S. alba was grown in VX-spiked loam, and VX was found to increase in the plants up to day 9 and then fall by day 28. A similar trend for the degradation products EMPA and MPA was observed. The amount of VX in the soil remained constant until day 9, fell by day 16 and declined further by day 28 (agreeing with other studies of VX on soil [31–33] where VX hydrolysis, once started, is autocatalytic [35]). Thus, the VX amount in the white mustard increased while VX was available in the loam for uptake, but decreased as the VX amount in the loam diminished [23]. The plants grown on the VX-spiked loam contained EMPA (minor product) and MPA (major product) at days 5, 9, 16 and 28 in a distribution pattern mirroring that of VX in the same plants. However, while VX was absent at day 28, EMPA and MPA were present. Several interpretations were advanced: (i) S. alba absorbed VX and metabolized it to EMPA and MPA, (ii) it absorbed VX, EMPA and MPA present in the loam or (iii) both possibilities occurred. At that stage, it was impossible to determine a mechanism that accounted for the presence of all three analytes inside the plant. EMPA and MPA are difficult to detect in soils of high organic content, such as loam, owing to their absorption and retention. This was confirmed by a soil spiking study where EMPA and MPA were added to the loam and extracted by two techniques: (i) with ethanol or (ii) by basification of the sample, extraction with ultra-high purity water and derivatization. In both instances, it was not possible to detect EMPA and MPA, but this did not necessarily signify their absence from the loam, nor did it explain their presence in the plant.
The experiments showed that S. alba could act as a time capsule whose analysis could provide molecular evidence for the prior presence of VX. But for how long? Could the window for the extraction of such evidence be extended beyond day 28? Would it depend on the nature of the soil in which the plants were grown? If so, confirming nerve agent use under field conditions might be hit-and-miss and depend on the soil type, limiting detection to specific geographical regions. Using plants to indicate illegal production or use of nerve agents should apply to all parts of the world for maximum attribution and deterrence.
To answer these questions, and to build confidence in the plant concept for detecting nerve agent use, we extended our original study, where S. alba was grown in VX-spiked loam for 28 days, to experiments with the same species in sand, loam or clay, grown this time for 45 days. Ten plants were cultivated in each soil type spiked with 1 ml of a 250 μg ml−1 aqueous solution of VX. The seeds/seedlings were given 10 ml of water immediately and the same volume thereafter every 24 h, and each day were illuminated for 10 h. The plant stems were cut at the soil surface after 8, 16, 33 and 45 days, pulverized and extracted with ethanol (previously they were frozen and stored at −40°C until the experiment was complete [23]). The extracts were analysed by LC-MS and the amount of VX in the plants plotted against time (figure 3). Plants grown in all three soils (sand, loam and clay) absorbed the maximal amount of VX by day 8. Variation in VX absorbed was observed in plants raised in the different soils, with sand giving the greatest variation (possibly because containers housing seedlings in sandy soil retained water less than those containing loam or clay, and dried out more easily, affecting root hydration and VX uptake). The initial absorption of VX on day 8 appeared most efficient in sand, less efficient in loam (consistent with previous experiments) and least efficient in clay, following the order predicted by consideration of the soil structures and their chemical retention properties. The new data confirm that organic matter and clay content in soil influence the initial absorption of VX. Some variation in VX absorption by plants grown in each soil may also reflect different root development therein.
Figure 3.
(a) VX detected in S. alba grown in VX contaminated clay, loam and sand determined by LC-MS of ethanol extracts of pulverized plants. (b) EMPA detected in S. alba grown in different soils contaminated with VX from derivatization and GC-MS of the ethanol extracts. (c) MPA detected in plants grown in different soils contaminated with VX, revealed by derivatization and GC-MS of the plant extracts. (a–c) Data points in each graph are averages from analysis of 10 plants and error bars represent the data standard deviation. (Online version in colour.)
While the initial uptake of VX by plants grown in different soils showed a large variation, by days 16, 33 and 45 the uptake in sand, loam and clay converged and followed the same course. The initial uncertainty in the data is most probably due to natural variation between plants at the early time points and differences in extraction from a complex matrix. The VX in S. alba declined gradually from approximately 5 ng to almost zero nanogram per plant over the 45 day test (figure 3a). The similar rates of absorption of VX from clay, loam or sandy soil over the 45 days implies that leaching of VX from the soil upon daily watering is relatively unimportant (leaching would be expected to vary for the different soils, yet the VX uptake profiles by the plants after day 16 tracked one another). In a similar study examining the absorption by S. alba of a seed dressing of the insecticide phorate (EtO)2P(S)SCH2SEt, which bears some structural resemblance to VX, young and old leaves absorbed the insecticide continually from the soil to maintain insecticidal activity (phorate did not translocate from old to new leaves, old leaves lost their toxicity more slowly than young ones, and the plants quickly lost toxicity once transplanted, implying continuous absorption) [27].
In our experiments, GC-MS analysis of derivatized ethanol extracts of S. alba grown in VX-spiked soils revealed the plants to contain, in addition to VX, both EMPA and MPA (figure 3b,c). Considerable variation was again evident, making statistically significant conclusions on EMPA and MPA amounts versus soil composition difficult to draw. The only clear relationship was MPA content versus soil type on day 16–45, where the MPA amount in plants grown in clay rose from approximately 50 ng to approximately 180 ng per plant, but rose only slightly (approx. 50 ng to approx. 80 ng per plant) during the same time interval in plants grown in loam or soil. To explain these differences, further experiments were conducted to see whether plants grown separately in EMPA- or MPA-spiked soil absorbed these phosphonic acids. These experiments are described next.
(b). Plant phosphonic acid detection
Ten plants were grown in loam spiked with 1 ml of a 250 μg ml−1 aqueous solution of EMPA or MPA and cultivated, processed, extracted and analysed as before. No EMPA was detected in any of the plants grown in loam spiked with EMPA (data not shown), yet plants grown in MPA-contaminated loam absorbed MPA (figure 4). The profile of absorption—greatest by day 8, decaying to day 33—was similar to that observed for VX (figure 3a).
Figure 4.
MPA concentration in S. alba grown in MPA-contaminated loam determined by GC-MS of derivatized ethanol extracts of pulverized plants. Data points are averages from analysis of 10 plants and the error bars represent the data standard deviation. (Online version in colour.)
(c). Plant chemical distribution
Sinapis alba plants grown on VX-contaminated soil were found to contain VX, EMPA and MPA for at least 45 days. Plants grown on EMPA-contaminated soil did not contain measurable EMPA, while plants grown on MPA-contaminated soil, contained MPA. These data suggest that the EMPA detected in extracts from plants grown in soil containing VX originated from metabolism of VX (figure 5). The mechanism of metabolism is unknown: we were unable to find any reports hinting that S. alba or related species contain OP hydrolase enzymes (many plant species have cholinesterase in their roots and leaves, but this is expected, based on one study with diisopropyl phosphorofluoridate, (i-PrO)2P(O)F, to bind the OP nerve agent irreversibly [36], rather than act hydrolytically). Why VX and MPA are absorbed by the roots, but EMPA is not, is puzzling. Both VX and MPA have systemic action: they are absorbed, translocated, metabolized and/or stored by the plant. A positive correlation sometimes exists between the water solubility of a substance or the octanol–water partition coefficient (log Kow) and its systemic activity [37]. Unlike the leaves and stem, the roots do not have a waxy cuticle and therefore absorb hydrophilic compounds more easily than hydrophobic compounds. Translocation from the roots occurs through the flow of the plant nutrients in xylem tissue and lateral diffusion is limited. For compounds to move in plant sap, sufficient water solubility is required, at least after biotransformation. The roots are expected to be the major organ of absorption of the OP compounds studied—however, as the plants were grown from seed in contaminated loam, it is probable that some absorption by the seeds through the micropyle and hilum can occur, and throughout germination and early growth (contamination of the cotyledons as they emerge through soil). VX contains a tertiary amino group (pKa 9.4 at 25°C [38]) that is protonated in water at pH<8.0 [39] and therefore the cationic form is the one most likely to be absorbed and translocated by a plant in the loam and sandy soils (pH 6.5, 100% protonated VX) and the neutral form in the clay soil (pH 9.0, 72% protonated VX). EMPA and MPA contain the P−OH bond which ionizes in water [17] and therefore these molecules will exist in soil in their anionic form.
Figure 5.
Proposed metabolism (top) and uptake of chemicals in the soil (bottom) by S. alba grown from seed in VX contaminated soil. VX will be present in the loam and sand in 100% protonated form, but only 72% protonated in clay. (Online version in colour.)
Without further experimental data, it is difficult to explain why EMPA is not absorbed by S. alba and why MPA is. Helpful clues are difficult to glean from the literature; ethyl n-propylphosphonic acid EtO(n-Pr)P(O)OH (NIA 10637) retards plant growth, while compounds of structure RP(O)(OH)2, typified by the weedkiller glyphosate, HO2CCH2NHCH2P(O)(OH)2, are absorbed readily [40]. No correlation between the water solubilities and log Kow values of the OP compounds of interest and uptake by white mustard exists (table 1). Why EMPA is not absorbed, but iPMPA is absorbed (separate study [23]), remains mysterious. EMPA is less stable than iPMPA in soil; the half-lives are 8 days and more than 1900 years [17]. About 50% of EMPA applied to humic sand hydrolysed to MPA in 8 days [32]. iPMPA resists hydrolysis more than EMPA and persists for longer. Lack of detection of EMPA in S. alba grown in EMPA-spiked loam, but detection of iPMPA in the same species grown in iPMPA-spiked loam [23], is unlikely to reflect differences in the rates of hydrolysis (as both analytes persist in the soil for at least 8 days). Sinapis alba therefore appears to absorb selectively certain alkyl methylphosphonic acids, but not others.
Table 1.
Selected physical properties of VX, EMPA, MPA and iPMPA [17]. NA, not available; Y, yes; N, no.
| analyte | vapour pressure (mmHg) | water solubility (g l−1) | pKa (25°C) | log Kow | white mustardc uptake by |
|---|---|---|---|---|---|
| VX | 7.0×10−3 | 30 | 9.40a | +2.09 | Y |
| EMPA | 3.6×10−4 | 180 | 2.76 | −1.15 | N |
| MPA | 2.0×10−6 | >1000 | 2.38, 7.74b | −2.28 | Y |
| iPMPA | 3.4×10−3 | 48 | 1.98 | NA | Y |
Another factor affecting the distribution of chemicals in S. alba grown on VX-contaminated soil is the pH. VX hydrolyses more readily under basic conditions than under acidic conditions. The pH of the soils studied decreased in the order: clay (pH 9.0)>loam∼sand (pH 6.5). Hydrolysis of VX in clay soil is expected to occur faster than in loam or sandy soil, resulting in extra MPA in clay available for uptake. This explains why after day 16 more MPA was detected in plants grown in clay soil compared to those grown in loam or sand (figure 3c). The MPA that accumulated in plants grown in the VX-contaminated soils arose presumably from a combination of metabolic breakdown of VX and uptake of MPA from the soil after VX hydrolysis.
The fate of the VX side-chain was not determined; the side-chain detaches as the thiol HSCH2CH2N(i-Pr)2 during hydrolysis under aerobic conditions and oxidizes to the disulfide, which is stable in the environment [17,33]. Parallels can be drawn between our results and those from experiments with wheat (Triticum sativum) grown in hydroponic medium spiked with soman [19]. In these, a ‘steady state’, where the rate of uptake of soman was balanced by the rate of hydrolysis of soman to PMPA, was indicated (cf. VX uptake by S. alba grown in different soils). The PMPA degraded to MPA. In this case, this transformation was slow, possibly because of poor circulation of PMPA within the plant, or a lack of suitable enzyme activity. The fate of MPA in the plant was not established. The metabolism of nerve agents in plants evidently does not differ, at least in its early stages, to that in animals, including humans. The phosphonic degradation products are of low or negligible acute toxicity compared to the parent nerve agent.
(d). Sinapis alba cultivation in a fume cupboard
Growing S. alba or any other plant from seed in soil contaminated with highly toxic chemicals such as VX is not without challenge. The use of such chemicals necessitates that the plants are cultivated in a fume cupboard for safety reasons. The constant airflow—0.5 m s−1 in our experiments—and extra lighting system required increase transpiration from the plant and evaporation of water from the soil. Dehydration is most severe when sandy soils are used as these retain water less than loam or clay. Care must be taken to ensure the plants do not desiccate over the protracted course of the experiment. We observed that S. alba specimens inside the fume cupboard grew more slowly than those grown outside the fume cupboard, and had a lower biomass, consistent with the results of a study on the effect of windspeed on white mustard growth: measurements of white mustard plants grown in tunnels at wind speeds of 0.3, 2.2 and 6.0 m s−1 for 42 days were made, and the higher the wind speed, the poorer the growth [42]. No evidence of reduced photosynthesis with increasing wind speed was found; the wind stunted growth due to mechanical effects. In our experiments, the same effects operated. This should be accommodated when assessing S. alba as a chemical agent detector: in the wild a more fluid growth might improve analyte absorption and detection performance.
(e). Sinapis alba as a nerve agent detector
Sinapis alba belongs to the family Brassicaceae and is an alternative oilseed crop for dry and low rainfall climates. It is pest-resistant and has a short growing season. Heat and drought tolerance are superior compared with rapeseed (Brassica napus) or canola (Brassica rapa). It produces twice as many seeds as canola in regions receiving less than 30 cm of rainfall per year [43]. Sinapis alba has roots that penetrate soil deeply: over 50% of all moisture is absorbed from below a depth of 150 cm [43,44]. In studies of phorate uptake by white mustard, the depth of sowed seed did not affect insecticide uptake [27], but this may be different in the field. In arid areas, the roots grow less extensively near the soil surface and nerve agents deposited on the surface might become slightly less accessible. Despite this, there are many advantages of S. alba as a nerve agent detector: it (i) has a wide geographical range, (ii) grows in all soil types, (iii) out-competes many weed species, (iv) self-seeds and spreads and (v) tolerates different climates. We have shown that it can absorb VX and signature compounds associated with sarin from soil [23]. Evidence for the prior presence of VX in soil can be downloaded from the plant at least 45 days after application.
In investigations of allegations of use, such as those completed recently by the United Nations in the Syrian Arab Republic [4], evidence of a sarin attack in Ghouta on 21 August 2013 was obtained by analysis of soil samples by two OPCW designated laboratories. To the best of our knowledge, no vegetation was submitted for analysis. Guidance for personnel performing identification to support NATO operations recommends submitting samples, including vegetation, to a laboratory for analysis [45]. However, no body of work has accumulated so far to suggest that analysis of vegetation could provide evidence of chemical warfare. In this paper, we have demonstrated that S. alba can store evidence of nerve agent use that can be accessed later. Plants absorbing nerve agents will be toxic to insects and therefore pest-resistant, and uptake of the chemical agent shields it from weathering. With sufficient scientific knowledge the metabolites found in the plant at a certain time might indicate the date the chemical agent was used. Also, any change in plant physiology after nerve agent absorption might enable the use of the agent to be detected by remote sensors [46,47].
(f). Sinapis alba as a nerve agent remediator
The use of sarin within the Syrian Arab Republic has left a toxic environmental legacy. One solution to remediate the contaminated land might be to cultivate S. alba on it. The plant grows well in the Middle East [48] and achieves impressive coverage. In an outdoor trial, S. alba spaced by 15 cm produced the greatest yield of seed, and the projected population of plants this distance apart in 20 cm rows, was estimated at more than 300 000 per hectare [43]. The crop can be harvested and destroyed or returned to the soil to allow cultivation of a second crop. Each generation of plants will destroy a proportion of the nerve agent until it is fully depleted. It might also be possible to genetically engineer plants to hydrolyse nerve agents more efficiently [49,50].
4. Conclusion
S. alba grown in VX-spiked clay, loam or sandy soil absorbed VX via the roots. The different soils moderated the rate of initial uptake but not the longer term uptake: the duration the VX was detectable in the plant was the same regardless of the soil type. The plants metabolized the VX to EMPA and MPA. The MPA found in the plants increased when they were grown in VX-spiked clay. No EMPA was detected in the plants grown in EMPA-spiked loam. The MPA profile in plants grown in MPA-spiked loam matched the MPA profile in plants grown in VX-spiked loam. VX, EMPA and MPA were detected in the plants for up to 45 days after sowing the white mustard seeds. The data suggest that the EMPA found in the plants grown in soil contaminated with VX originated from metabolism of VX.
The method described for detecting nerve agent residues in the environment will help the OPCW and CWC member states confirm their presence in future. The localized sample and simple extraction procedure will increase the probability of discovering nerve agent use. The ability of plants such as S. alba to absorb nerve agents and sufficient marker compounds protects against the removal of evidence of use, as CWAs can leach from soil over time. It also suggests that green manures might be useful for remediating nerve agent-polluted sites.
Crown Copyright 2014. Published with permission of the Defence Science and Technology Laboratory on behalf of the Controller of Her Majesty's Stationery Office.
Acknowledgements
The authors thank Dr Dean Payne (Chief Scientist, Dstl Detection Department) and Prof. Vernon Gibson FRS (Chief Scientific Advisor to the UK Ministry of Defence) for their support and encouragement. M.J.B. and C.M.T. are grateful to the Royal Society Pairing Scheme for enabling this collaboration. The authors dedicate this paper to the memory of Dr David Howells, who led pioneering studies at Porton Down in the 1970s on the fate of soman in wheat plants.
Funding statement
The research was financed by the Dstl Detection Department capability development fund.
References
- 1.Carmichael H. 2013. Evidence of sarin use in Syria mounts, p. 8 Chemistry World, July 2013 [Google Scholar]
- 2.Sellström A, et al. 13 September 2013 UN Mission to Investigate Allegations of Chemical Weapons in the Syrian Arab Republic: Report on Allegations of the Use of Chemical Weapons in the Ghouta Area of Damascus on 21 August 2013. [Google Scholar]
- 3.Carmichael H. 2013. Dismantling Syria's chemical arsenal, p. 8 Chemistry World, December 2013 [Google Scholar]
- 4.Sellström A, et al. 2013. United Nations Mission to Investigate Allegations of the Use of Chemical Weapons in the Syrian Arab Republic. Final Report, 12 December 2013 [Google Scholar]
- 5. 1997 Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on their Destruction (Chemical Weapons Convention), Organisation for the Prohibition of Chemical Weapons (OPCW), The Hague (www.opcw.org/ ) [Google Scholar]
- 6.Üzümcü A. 2013. Working Together for a World Free of Chemical Weapons, and Beyond. Nobel Peace Prize Lecture OPCW (www.nobelprize.org) [Google Scholar]
- 7. A Strong Britain in an Age of Uncertainty: The National Security Strategy, presented to Parliament by the British Prime Minister by command of Her Majesty, UK Stationery Office, 18 October 2010. [Google Scholar]
- 8.Black RM, Read RW. 2007. Environmental and biomedical sample analysis in support of allegations of use of chemical warfare agents. Toxin Rev. 26, 275–298 (doi:10.1080/15569540701474328) [Google Scholar]
- 9.Timperley CM. 2000. Highly-toxic fluorine compounds. In Fluorine chemistry at the millennium: fascinated by fluorine (ed. Banks RE.), pp. 499–537, ch. 29 Oxford, UK: Elsevier [Google Scholar]
- 10.Bell AJ, Murrell J, Timperley CM, Watts P. 2001. Fragmentations and reactions of two isomeric O-alkyl S-(2-dialkylamino)ethyl methylphosphonothiolates studied by electrospray ionization/ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 12, 902–910 (doi:10.1016/S1044-0305(01)00274-4) [DOI] [PubMed] [Google Scholar]
- 11.Timperley CM, Bird M, Holden I, Black RM. 2001. Organophosphorus chemistry. Part 1. The synthesis of alkyl methylphosphonic acids. J. Chem. Soc. Perkin Trans. 1 26–30 (doi:10.1039/b007077g) [Google Scholar]
- 12.Barucki H, Black RM, Kinnear KI, Holden I, Read RW, Timperley CM. 2003. Solid-phase synthesis of some alkyl hydrogen methylphosphonates. Phosphorus, Sulfur, and Silicon 178, 2279–2286 (doi:10.1080/713744564) [Google Scholar]
- 13.Subramaniam R, Åstot C, Juhlin L, Nilsson C, Östin A. 2012. Determination of S-2-(N,N-diisopropylaminoethyl-) and S-2-(N,N-diethylaminoethyl) methylphosphonothiolate, nerve agent markers, in water samples using strong anion-exchange disk extraction, in vial trimethylsilylation, and gas chromatography-mass spectrometry analysis. J. Chromatogr. A 1229, 86–94 (doi:10.1016/j.chroma.2012.01.068) [DOI] [PubMed] [Google Scholar]
- 14.Black RM, Muir B. 2003. Derivatisation reactions in the chromatographic analysis of chemical warfare agents and their degradation products. J. Chromatogr. A. 1000, 253–281 (doi:10.1016/S0021-9673(03)00183-3) [DOI] [PubMed] [Google Scholar]
- 15.Riches J. 2013. Analysis of polar nerve agent hydrolysis products, p. 4–6 Chromatography Today, August/September 2013 [Google Scholar]
- 16.Kingery AF, Allen HE. 1995. The environmental fate of organophosphorus nerve agents: a review. Toxicol. Environ. Chem. 47, 155–184 (doi:10.1080/02772249509358137) [Google Scholar]
- 17.Munro NB, Talmage SS, Griffin GD, Waters LC, Watson AP, King JF, Hauschild V. 1999. The sources, fate, and toxicity of chemical warfare agent degradation products. Environ. Health Perspect. 107, 933–974 (doi:10.1289/ehp.99107933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt MWI, et al. 2011. Persistence of soil organic matter as an ecosystem property. Nature (London) 478, 49–56 (doi:10.1038/nature10386) [DOI] [PubMed] [Google Scholar]
- 19.Hambrook JL, Howells DJ, Utley D. 1971. Degradation of phosphonates. Breakdown of soman (O-pinacolyl-methylphosphonofluoridate) in wheat plants. Pestic. Sci. 2, 172–175 (doi:10.1002/ps.2780020410) [Google Scholar]
- 20.Howells DJ, Hambrook JL. 1972. The phytotoxicity of some methylphosphonofluoridates. Pestic. Sci. 3, 351–356 (doi:10.1002/ps.2780030312) [Google Scholar]
- 21.Howells DJ, Hambrook JL, Utley D, Woodage J. 1973. Degradation of phosphonates. II. The influence of the O-alkyl group on the breakdown of some O-alkyl methylphos-phonofluoridates in wheat plants. Pestic. Sci. 4, 239–245 (doi:10.1002/ps.2780040210) [Google Scholar]
- 22.Howells DJ, Hambrook JL, Allenby EA. 1976. Uptake of some volatile alkyl methylphosphonofluoridates from the vapour phase by wheat plants. Pestic. Sci. 7, 349–354 (doi:10.1002/ps.2780070405) [Google Scholar]
- 23.Gravett MR, Hopkins FB, Main MJ, Self AJ, Timperley CM, Webb AJ, Baker MJ. 2013. Detection of the organophosphorus nerve agent VX and its hydrolysis products in white mustard plants grown in contaminated soil. Anal. Methods 5, 50–53 (doi:10.1039/c2ay25883h) [Google Scholar]
- 24.Li Y. 2012. Plants detect nerve agents, p. 31 Chemistry World, December 2012 [Google Scholar]
- 25.Sverdrup LE, Krogh PH, Nielsen T, Kjaer C, Stenersen J. 2003. Toxicity of eight polycyclic aromatic compounds to red clover (Trifolium pratense), ryegrass (Lolium perenne), and mustard (Sinapis alba). Chemosphere 53, 993–1003 (doi:10.1016/S0045-6535(03)00584-8) [DOI] [PubMed] [Google Scholar]
- 26.Winter H. 2011. Sinapis. In Sinapis in wild crop relatives: genomic and breeding resources (ed. Kole C.), 275–288 Berlin, Germany: Springer [Google Scholar]
- 27.Bardner R. 1964. The uptake of phorate, a systemic insecticide, applied to a slurry to wheat and mustard seeds. Ann. Appl. Biol. 53, 445–458 (doi:10.1111/j.1744-7348.1964.tb07258.x) [Google Scholar]
- 28.Zuas O. 2008. Extraction of chemical warfare agent from soil: case study on O-ethyl S-2-(diisopropylamino)ethyl methylphosphonothiolate. MIPA 37, 53–60 [Google Scholar]
- 29.Brevett CAS, Sumpter KB, Pence J, Nickol RG, King BE, Giannaras CV, Dupont Durst H. 2009. Evaporation and degradation of VX on silica sand. J. Phys. Chem. C 113, 6622–6633 (doi:10.1021/jp8111099) [Google Scholar]
- 30.Love AH, Vance AL, Reynolds JG, Davisson ML. 2004. Investigating the affinities and persistence of VX nerve agent in environmental matrices. Chemosphere 57, 1257–1264 (doi:10.1016/j.chemosphere.2004.08.041) [DOI] [PubMed] [Google Scholar]
- 31.Kaaijk J, Frijlink C. 1977. Degradation of S-2-diisopropylaminoethyl O-ethyl methylphosphonothiolate in soil. Sulfur-containing products. Pestic. Sci. 8, 510–514 (doi:10.1002/ps.2780080513) [Google Scholar]
- 32.Verweij A, Boter HL. 1976. Degradation of S-2-diisopropylaminoethyl O-ethyl methylphosphonothioate in soil: phosphorus-containing products. Pestic. Sci. 7, 355–362 (doi:10.1002/ps.2780070406) [Google Scholar]
- 33.Montauban C, Bégos A, Bellier B. 2004. Extraction of nerve agent VX from soils. Anal. Chem. 76, 2791–2797 (doi:10.1021/ac035441q) [DOI] [PubMed] [Google Scholar]
- 34.Hayes MHB, Lundie PR, Stacey M. 1972. Interactions between organophosphorus compounds and soil materials. I. Adsorption of ethyl methylphosphonofluoridate by clay and organic matter preparations and by soils. Pestic. Sci. 3, 619–629 (doi:10.1002/ps.2780030514) [Google Scholar]
- 35.Yang YC, Szafraniec LL, Beaudry WT, Rohrbaugh DK, Procell LR, Samuel JB. 1996. Autocatalytic hydrolysis of V-type nerve agents. J. Org. Chem. 61, 8407–8413 (doi:10.1021/jo9614506) [Google Scholar]
- 36.Miura GA, Broomfield CA, Lawson MA, Worthley EG. 1982. Widespread occurrence of cholinesterase activity in plant leaves. Physiol. Plant 56, 28–32 (doi:10.1111/j.1399-3054.1982.tb04895.x) [Google Scholar]
- 37.Fest C, Schmidt KJ. 1973. The chemistry of organophosphorus pesticides Berlin, Germany: Springer-Verlag [Google Scholar]
- 38.van der Schans MJ, Lander BJ, van der Wiel H, Langenberg JP, Benschop HP. 2003. Toxicokinetics of the nerve agent (±)-VX in anesthetized and atropinized hairless guinea pigs and marmosets after intravenous and percutaneous administration. Toxicol. Appl. Pharmacol. 191, 48–62 (doi:10.1016/S0041-008X(03)00216-3) [DOI] [PubMed] [Google Scholar]
- 39.Creasy WR, McGarvey DJ, Brevett CAS. 2013. Speciation of VX in aqueous solution. J. Phys. Chem. C 117, 22677–22682 (doi:10.1021/jp409671y) [Google Scholar]
- 40.Eto M. 1974. Organophosphorus pesticides: organic and biological chemistry Cleveland, OH: CRC Press Inc [Google Scholar]
- 41.Crofts PC, Kosolapoff GM. 1953. Preparation and determination of apparent dissociation constants of some alkylphosphonic and dialkylphosphinic acids. J. Am. Chem. Soc. 75, 3379–3383 (doi:10.1021/ja01110a024) [Google Scholar]
- 42.Retuerto R, Woodward FI. 1992. Effects of windspeed on the growth and biomass allocation of white mustard Sinapis alba L. Oecologia 92, 113–123 (doi:10.1007/BF00317271) [DOI] [PubMed] [Google Scholar]
- 43.Hassan FU, Arif M. 2012. Response of white mustard (Sinapis alba L) to spacing under rainfed conditions. J. Anim. Plant Sci. 22, 137–141 [Google Scholar]
- 44.Hajzler M, Klimešová J, StŖeda T. 2011. Biomass production of white mustard (Sinapis alba L.) varieties in relation to the root system size. Tagung der Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs 62, 105–108 [Google Scholar]
- 45.Hancock JR, Dragon DC. 2005 Sample Preparation and Identification of Biological, Chemical and Mid-Spectrum Agents, A General Survey for the Revised NATO AC/225 (LG/7) AEP-IO Edition 6 Handbook, DRDC Suffield TM 2005–135, p. 14. [Google Scholar]
- 46.Lawrence RL, Wood SD, Sheley RL. 2006. Mapping invasive plants using hyperspectral imagery and Breiman Cutler classifications (RandomForest). Remote Sensing Environ. 100, 356–362 (doi:10.1016/j.rse.2005.10.014) [Google Scholar]
- 47.Motohka T, Nasahara KN, Oguma H, Tsuchida S. 2010. Applicability of green-red vegetation index for remote sensing of vegetation phenology. Remote Sensing 2, 2369–2387 (doi:10.3390/rs2102369) [Google Scholar]
- 48.Al-Qudah MA, Al-Jaber HI, Muhaidat R, Hussein EI, Hamid AAA, Al-Smadi ML, Abaza IF, Afifi FU, Abu-Orabi ST. 2011. Chemical composition and antimicrobial activity of the essential oil from Sinapis alba L. and Sinapis arvensis L. (Brassicaceae) growing wild in Jordan. RJPBCS 2, 1136–1144 [Google Scholar]
- 49.Milo R, Last RL. 2012. Achieving diversity in the face of constraints: lessons from metabolism. Science 336, 1663–1667 (doi:10.1126/science.1217665) [DOI] [PubMed] [Google Scholar]
- 50.Weng JK, Philippe RN, Noel JP. 2012. The rise of chemodiversity in plants. Science 336, 1667–1670 (doi:10.1126/science.1217411) [DOI] [PubMed] [Google Scholar]