Abstract
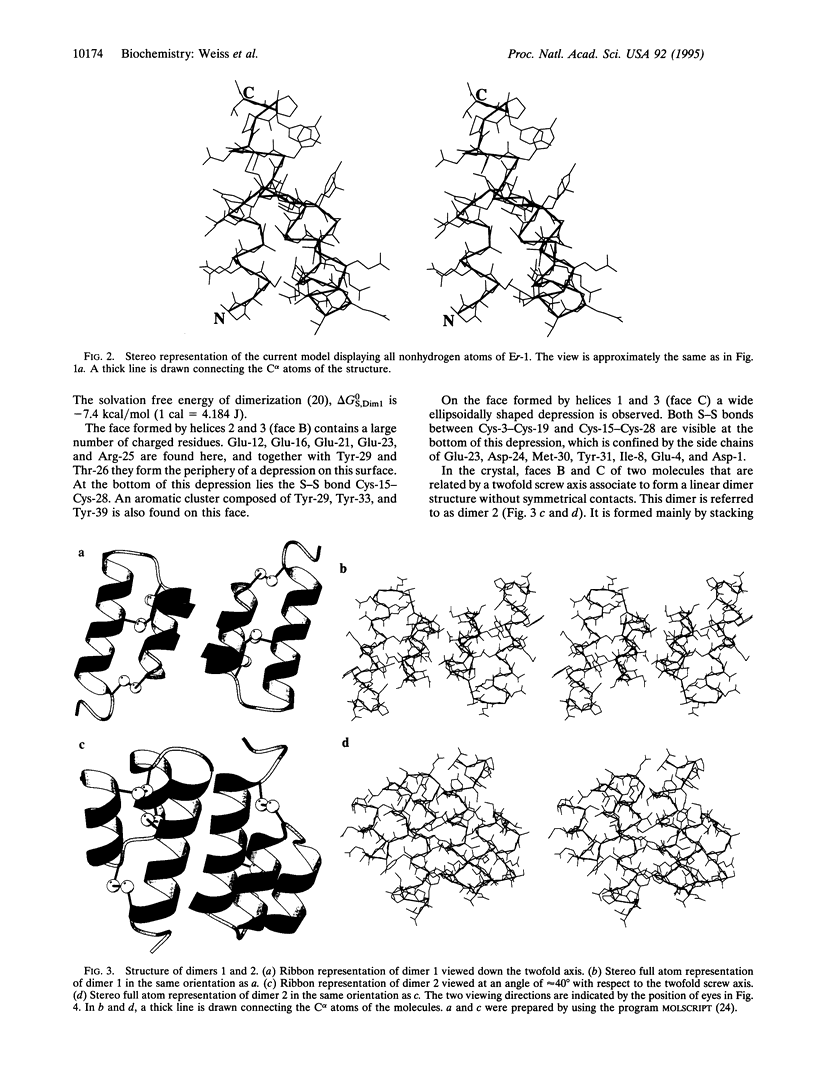
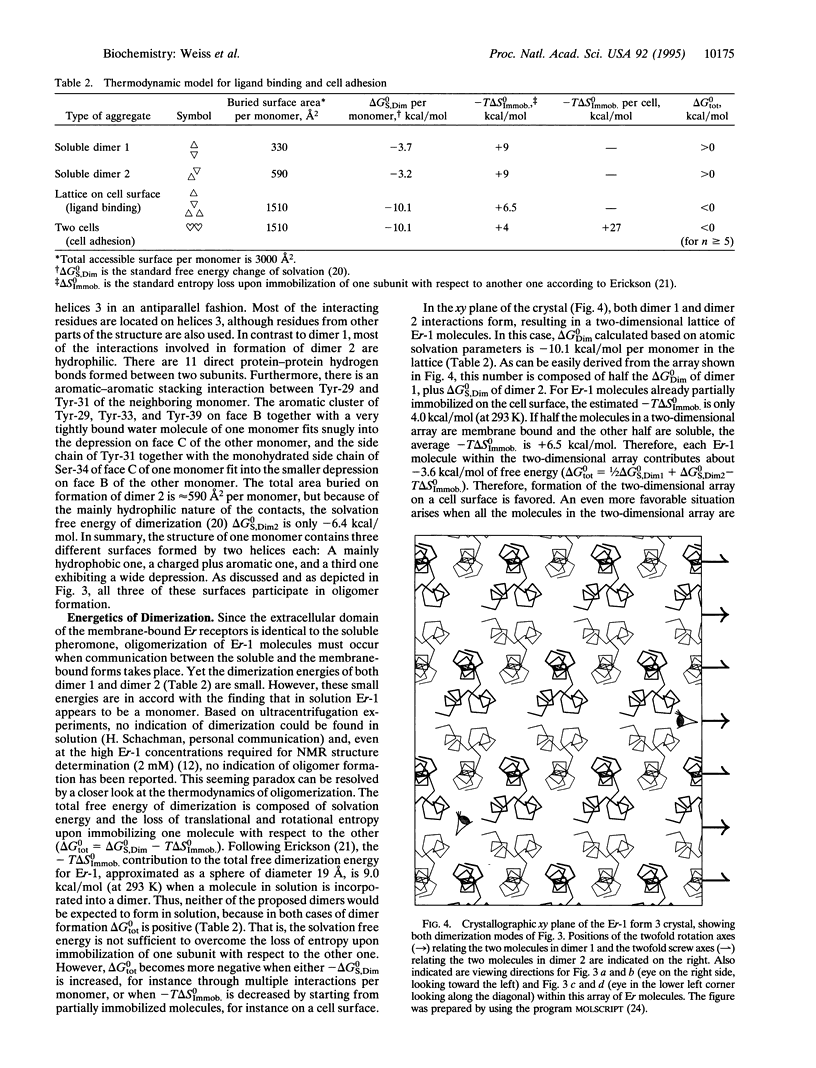
The crystal structure of the pheromone Er-1 from the unicellular eukaryotic organism Euplotes raikovi was determined at 1.6 A resolution and refined to a crystallographic R factor of 19.9%. In the tightly packed crystal, two extensive intermolecular helix-helix interactions arrange the Er-1 molecules into layers. Since the putative receptor of the pheromone is a membrane-bound protein, whose extracellular C-terminal domain is identical in amino acid sequence to the soluble pheromone, the interactions found in the crystal may mimic the pheromone-receptor interactions as they occur on a cell surface. Based on this, we propose a model for the interaction between soluble pheromone molecules and their receptors. In this model, strong pheromone-receptor binding emerges as a consequence of the cooperative utilization of several weak interactions. The model offers an explanation for the results of binding studies and may also explain the adhesion between cells that occurs during mating.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D., Raffioni S., Luporini P., Bradshaw R. A., Eisenberg D. Crystallization of the Euplotes raikovi mating pheromone Er-1. J Mol Biol. 1990 Nov 5;216(1):1–2. doi: 10.1016/S0022-2836(05)80055-5. [DOI] [PubMed] [Google Scholar]
- Beale G. Self and nonself recognition in the ciliate protozoan Euplotes. Trends Genet. 1990 May;6(5):137–139. doi: 10.1016/0168-9525(90)90132-p. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Mronga S., Bradshaw R. A., Ortenzi C., Luporini P., Wüthrich K. Nuclear magnetic resonance solution structure of the pheromone Er-10 from the ciliated protozoan Euplotes raikovi. J Mol Biol. 1993 Jun 5;231(3):800–816. doi: 10.1006/jmbi.1993.1327. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., McLachlan A. D. Solvation energy in protein folding and binding. Nature. 1986 Jan 16;319(6050):199–203. doi: 10.1038/319199a0. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Co-operativity in protein-protein association. The structure and stability of the actin filament. J Mol Biol. 1989 Apr 5;206(3):465–474. doi: 10.1016/0022-2836(89)90494-4. [DOI] [PubMed] [Google Scholar]
- Janin J., Miller S., Chothia C. Surface, subunit interfaces and interior of oligomeric proteins. J Mol Biol. 1988 Nov 5;204(1):155–164. doi: 10.1016/0022-2836(88)90606-7. [DOI] [PubMed] [Google Scholar]
- Luginbühl P., Ottiger M., Mronga S., Wüthrich K. Structure comparison of the pheromones Er-1, Er-10, and Er-2 from Euplotes raikovi. Protein Sci. 1994 Sep;3(9):1537–1546. doi: 10.1002/pro.5560030919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luporini P., Vallesi A., Miceli C., Bradshaw R. A. Chemical signaling in ciliates. J Eukaryot Microbiol. 1995 May-Jun;42(3):208–212. doi: 10.1111/j.1550-7408.1995.tb01567.x. [DOI] [PubMed] [Google Scholar]
- Luporini P., Vallesi A., Miceli C., Bradshaw R. A. Ciliate pheromones as early growth factors and cytokines. Ann N Y Acad Sci. 1994 Apr 15;712:195–205. doi: 10.1111/j.1749-6632.1994.tb33573.x. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Miceli C., La Terza A., Bradshaw R. A., Luporini P. Identification and structural characterization of a cDNA clone encoding a membrane-bound form of the polypeptide pheromone Er-1 in the ciliate protozoan Euplotes raikovi. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1988–1992. doi: 10.1073/pnas.89.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mronga S., Luginbühl P., Brown L. R., Ortenzi C., Luporini P., Bradshaw R. A., Wüthrich K. The NMR solution structure of the pheromone Er-1 from the ciliated protozoan Euplotes raikovi. Protein Sci. 1994 Sep;3(9):1527–1536. doi: 10.1002/pro.5560030918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottiger M., Szyperski T., Luginbühl P., Ortenzi C., Luporini P., Bradshaw R. A., Wüthrich K. The NMR solution structure of the pheromone Er-2 from the ciliated protozoan Euplotes raikovi. Protein Sci. 1994 Sep;3(9):1515–1526. doi: 10.1002/pro.5560030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffioni S., Luporini P., Bradshaw R. A. Purification, characterization, and amino acid sequence of the mating pheromone Er-10 of the ciliate Euplotes raikovi. Biochemistry. 1989 Jun 13;28(12):5250–5256. doi: 10.1021/bi00438a049. [DOI] [PubMed] [Google Scholar]
- Raffioni S., Luporini P., Chait B. T., Disper S. S., Bradshaw R. A. Primary structure of the mating pheromone Er-1 of the ciliate Euplotes raikovi. J Biol Chem. 1988 Dec 5;263(34):18152–18159. [PubMed] [Google Scholar]
- Raffioni S., Miceli C., Vallesi A., Chowdhury S. K., Chait B. T., Luporini P., Bradshaw R. A. Primary structure of Euplotes raikovi pheromones: comparison of five sequences of pheromones from cells with variable mating interactions. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2071–2075. doi: 10.1073/pnas.89.6.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. E., Raffioni S., Chaudhary T., Chait B. T., Luporini P., Bradshaw R. A. The disulfide bond pairing of the pheromones Er-1 and Er-2 of the ciliated protozoan Euplotes raikovi. Protein Sci. 1992 Jun;1(6):777–785. doi: 10.1002/pro.5560010609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot C. M., Thornton J. M. Beta-turns and their distortions: a proposed new nomenclature. Protein Eng. 1990 May;3(6):479–493. doi: 10.1093/protein/3.6.479. [DOI] [PubMed] [Google Scholar]





