Abstract
OBJECTIVE:
To assess blockade of matrix metalloproteinase (MMP)-2 and MMP-9, as well as the variation in FEV1, in patients with lymphangioleiomyomatosis (LAM) treated with doxycycline (a known MMP inhibitor) for 12 months.
METHODS:
An open-label, single-arm, interventional clinical trial in which LAM patients received doxycycline (100 mg/day) for 12 months. Patients underwent full pulmonary function testing, a six-minute walk test, and quality of life assessment, as well as blood and urine sampling for quantification of MMP-2, MMP-9, and VEGF-D levels-at baseline, as well as at 6 and 12 months after the initiation of doxycycline.
RESULTS:
Thirty-one LAM patients received doxycycline for 12 months. Although there was effective blockade of urinary MMP-9 and serum MMP-2 after treatment, there were no significant differences between pre and post-doxycycline serum levels of MMP-9 and VEGF-D. On the basis of their response to doxycycline (as determined by the variation in FEV1), the patients were divided into two groups: the doxycycline-responder (doxy-R) group (n = 13); and the doxycycline-nonresponder (doxy-NR) group (n = 18). The patients with mild spirometric abnormalities responded better to doxycycline. The most common side effects were mild epigastric pain, nausea, and diarrhea.
CONCLUSIONS:
In patients with LAM, doxycycline treatment results in effective MMP blockade, as well as in improved lung function and quality of life in those with less severe disease. However, these benefits do not seem to be related to the MMP blockade, raising the hypothesis that there is a different mechanism of action.
Keywords: Lymphangioleiomyomatosis, Doxycycline, Matrix metalloproteinases, Respiratory function tests
Abstract
OBJETIVO:
Avaliar o bloqueio da metaloproteinase da matriz (MMP)-2 e da MMP-9 e a variação do VEF1 em pacientes com linfangioleiomiomatose (LAM) após o uso de doxiciclina, um conhecido inibidor de MMP, durante 12 meses.
MÉTODOS:
Ensaio clínico aberto de braço único no qual as pacientes com diagnóstico de LAM receberam doxiciclina (100 mg/dia) durante 12 meses. Elas foram submetidas a prova de função pulmonar completa, teste de caminhada de seis minutos, avaliação da qualidade de vida e coleta de amostras séricas e urinárias para dosagem de MMP-2, MMP-9 e VEGF-D antes do início do tratamento com doxiciclina e após 6 e 12 meses de tratamento.
RESULTADOS:
Trinta e uma pacientes com LAM receberam doxiciclina durante 12 meses. Embora tenha havido um bloqueio efetivo da MMP-9 urinária e da MMP-2 sérica após o tratamento, os níveis séricos de MMP-9 e VEGF-D permaneceram estáveis. Com base na resposta à doxiciclina (determinada pela variação do VEF1), as pacientes foram divididas em dois grupos: respondedoras (doxi-R; n = 13) e não respondedoras (doxi NR; n = 18). As pacientes com alterações espirométricas leves apresentaram melhor resposta à doxiciclina. Os efeitos colaterais mais comuns foram epigastralgia, náusea e diarreia, todos de leve intensidade.
CONCLUSÕES:
Em pacientes com LAM, o tratamento com doxiciclina resulta em um bloqueio eficaz das MMP, além de melhorar a função pulmonar e a qualidade de vida daqueles com doença menos grave. No entanto, esses benefícios não parecem estar relacionados ao bloqueio das MMP, o que sugere um mecanismo de ação diferente.
Introduction
Lymphangioleiomyomatosis (LAM) is a rare disease that affects women of childbearing age. It can occur sporadically or in association with tuberous sclerosis complex (TSC), being characterized by lung cysts.( 1 ) The most common clinical manifestations of LAM are dyspnea and pneumothorax; other, less common, manifestations include hemoptysis, cough, and chylothorax.( 2 - 6 ) In patients with LAM, the most common abnormal pulmonary function test results are airflow obstruction and decreased DLCO.( 7 )
There is currently no curative treatment for LAM. Hormonal blockade is the most widely used treatment. However, there is a lack of evidence in this field. A retrospective study involving patients with LAM showed that the rates of decline in DLCO were significantly higher in those who were treated with progesterone.( 8 ) The results of small studies involving the use of gonadotropin-releasing hormone agonists have been controversial.( 9 , 10 )
A randomized clinical trial showed that sirolimus, an inhibitor of the mammalian target of rapamycin pathway, can be useful in treating patients with moderately severe LAM-related lung disease.( 11 )
Recently, there has been interest in the possible role of matrix metalloproteinases (MMPs) in the pathogenesis of cystic lung destruction in LAM, including the possibility that MMPs represent a therapeutic target.( 12 ) It is known that MMPs are functional components of the extracellular matrix. They degrade a variety of matrix substrates and play an important role in lung remodeling and lymphangiogenesis.( 13 , 14 ) In addition, MMPs are inhibited by tissue inhibitors of metalloproteinases (TIMPs), which are indispensable in the regulation of MMPs. An imbalance between MMPs and their inhibitors has been implicated in the pathogenesis of lung diseases such as asthma, COPD, and Langerhans cell histiocytosis.( 15 , 16 ) Protease imbalance has been described in LAM lesions.( 14 ) A study examining lung biopsy samples from patients with LAM found elastic fiber degradation in areas of smooth muscle cell proliferation.( 17 ) Likewise, immunohistochemical analysis of lung biopsy samples from patients with LAM revealed increased immunoreactivity of MMP-2 and MMP-9, although not of TIMP-1 and TIMP-2, in the LAM cells when compared with that of those in normal lung cells.( 18 ) One case report showed an improvement in pulmonary function test results and a reduction in urinary MMP levels in an LAM patient who had severely impaired lung function and was treated with doxycycline, an MMP inhibitor.( 19 ) In a previous open-label trial, our group found a significant decrease in serum levels of MMP-2 in a cohort of LAM patients treated with doxycycline for 6 months.( 20 )
The objective of the present study was to assess serum and urinary MMP-2 and MMP-9 levels in LAM patients treated with doxycycline for 12 months, as well as to assess the variation in FEV1 in those patients.
Methods
This was an open-label, single-arm, interventional clinical trial. All LAM patients receiving outpatient treatment at the University of São Paulo School of Medicine Hospital das Clínicas Outpatient Clinic for Interstitial Diseases, located in the city of São Paulo, Brazil, were invited to participate in the study. The diagnosis of LAM was made in accordance with the European Respiratory Society guidelines.( 21 , 22 ) The study was approved by the local research ethics committee, and all participants gave written informed consent. One LAM patient had undergone lung transplantation and therefore was not enrolled in the trial.
At baseline, the patients underwent full pulmonary function testing, which was followed by a six-minute walk test (6MWT) and health-related quality of life assessment. Urine and blood samples were collected in order to determine the levels of MMP-2, MMP-9, and VEGF-D. After this initial evaluation, the patients were started on doxycycline at a dose of 100 mg/day, which was maintained for at least 12 months. All of the abovementioned tests were repeated at 6 and 12 months after treatment initiation.
The primary endpoints were MMP-2 and MMP-9 blockade, defined as decreased MMP-2 and MMP-9 levels, and the variation in FEV1 after treatment with doxycycline for 12 months. The secondary endpoints were the variation in FVC, the variation in RV, and the variation in DLCO. We also evaluated the variation in the six-minute walk distance (6MWD), the variation in the minimum SpO2 (as assessed during the 6MWT), and the variation in serum VEGF-D levels.
Urinary and serum MMP-2 and MMP-9 levels, as well as serum VEGF-D levels, were measured by ELISA (R&D System, Inc., Minneapolis, MN, USA), in accordance with the manufacturer instructions. The procedure is described in detail in our previous study( 20 ) and in the appendix to this article (available online at http://www.jornaldepneumologia.com.br/english/artigo_detalhes.asp?id=1975). The detection limit of the assay was 15.6 pg/mL for MMP-9, 156.2 pg/mL for MMP-2, and 15.6 pg/mL for VEGF-D. Urine and blood samples were also collected from 10 age-matched healthy female nonsmokers.
All patients underwent spirometry, determination of lung volumes, and determination of DLCO with a whole-body plethysmograph (Elite D MedGraphics; Medical Graphics Co., Saint Paul, MN, USA). Pulmonary function testing was performed in accordance with the recommended guidelines for pulmonary function testing in Brazilian adults. The reference values for spirometry, lung volumes, and DLCO were those established for the Brazilian population.( 23 - 25 )
A symptom-limited 6MWT was performed in accordance with the recommended standards.( 26 ) A pulse oximeter (WristOx(r) 3100; Nonin Medical, Inc., Plymouth, MN , USA) was used in order to record SpO2 on room air every 2 s. The data obtained during the 6MWT were analyzed with the nVISION(r) software (Nonin Medical, Inc.) and exported to a Microsoft Excel spreadsheet. The parameters analyzed were the minimum SpO2 maintained for at least 10 s and the 6MWD, which was expressed in meters and in percentage of the predicted values for the Brazilian population.( 27 )
The Medical Outcomes Study 36-item Short-form Health Survey (SF-36) was used in order to assess quality of life, having previously been validated for use in the Brazilian population.( 28 , 29 )
The statistical analysis was performed with the Student's t-test or the Wilcoxon signed rank test for parametric variables and the Mann-Whitney test for nonparametric variables. Fisher's exact test was used for categorical variables. The ROC curve was used in order to identify the FEV1/FVC ratio that was most accurate in predicting the response to doxycycline treatment.
The data were analyzed with the Statistical Package for the Social Sciences, version 15.0 (SPSS Inc., Chicago, IL, USA), and differences were considered significant if p < 0.05.
(Brazilian Registry of Clinical Trials [ReBEC] identifier RBR-6g8yz9 [http://www.ensaiosclinicos.gov.brΑ)
Results
Between 2006 and 2009, 41 patients with LAM were enrolled to receive doxycycline (100 mg/day). Of those 41 patients, 10 were lost to follow-up: 5 withdrew from the trial; 2 had worsening of the symptoms; and 3 had drug-related adverse events.
A total of 31 patients completed 12 months of doxycycline treatment (Appendix, section 2, Table A1). Mean age was 43 ± 8 years. In 29 patients, the diagnosis of LAM was based on histopathological features, whereas, in 2, the diagnosis was based on typical clinical and radiological features. The median time from the onset of symptoms to the diagnosis of LAM was 9 months (range, 6-24 months).
Table 1. Pulmonary function test results, six-minute walk test results, biomarkers, and Medical Outcomes Study 36-item Short-form Health Survey scores at baseline and after 12 months of treatment with doxycycline in 31 patients with lymphangioleiomyomatosis. a .
| Variable | Result | p | |
| Baseline | After treatment | ||
| (n = 31) | (n = 31) | ||
| Pulmonary function test | |||
| FVC, L | 3.17 ± 0.60 | 3.19 ± 0.60 | 0.792 |
| FVC, % of predicted | 92 ± 14 | 93 ± 14 | 0.751 |
| FEV1, L | 2.24 ± 0.70 | 2.17 ± 0.70 | 0.034 |
| FEV1, % of predicted | 79 ± 23 | 77 ± 25 | 0.042 |
| FEV1/FVC ratio | 0.70 ± 0.20 | 0.67 ± 0.20 | 0.003 |
| TLC, L | 5.03 ± 0.80 | 5.09 ± 0.70 | 0.408 |
| TLC, % of predicted | 103 ± 14 | 105 ± 14 | 0.393 |
| RV, L | 1.82 ± 0.60 | 1.91 ± 0.70 | 0.263 |
| RV, % of predicted | 130 ± 47 | 137 ± 52 | 0.282 |
| RV/TLC ratio | 0.36 ± 0.08 | 0.37 ± 0.10 | 0.295 |
| DLCO, mL/min/mmHg | 17.0 ± 6.8 | 16.76 ± 6.0 | 0.713 |
| DLCO, % of predicted | 65 ± 25 | 64 ± 22 | 0.745 |
| Six-minute walk test b | |||
| Distance, m | 490 ± 109 | 515 ± 90 | 0.132 |
| Distance, % of predicted | 90 ± 20 | 95 ± 17 | 0.113 |
| Baseline SpO2, % c | 96 (95-98) | 96 (94-97) | 0.282 |
| Minimum SpO2, % c | 94 (87-95) | 93 (84-95) | 0.020 |
| Biomarkers c | |||
| Serum MMP-9, ng/mL | 933 (730-1,202) | 1,076 (809-1,367) | 0.140 |
| Urinary MMP-9, pg/mL | 10,487 (4,565-20,963) | 4,061 (712-9,985) | < 0.001 |
| Serum MMP-2, pg/mL | 0 (0-833) | 0 (0-179) | 0.005 |
| VEGF-D, pg/mL | 821 (407-2,113) | 913 (313-2,262) | 0.590 |
| SF-36 domains c | |||
| Physical functioning | 70 (60-85) | 70 (50-88) | 0.465 |
| Role-physical | 50 (50-50) | 75 (25-100) | 0.054 |
| Bodily pain | 78 (56-95) | 70 (58-90) | 1.000 |
| General health | 75 (48-80) | 75 (58-80) | 0.717 |
| Vitality | 65 (53-80) | 65 (55-80) | 0.611 |
| Role-emotional | 100 (33-100) | 100 (67-100) | 0.147 |
| Mental health | 62 (52-84) | 80 (58-84) | 0.006 |
| Social functioning | 88 (56-94) | 88 (63-100) | 0.165 |
Minimum SpO2: : minimum SpO2 sustained for 10 s;
matrix metalloproteinase;
SF-36: : Medical Outcomes Study 36-item Short-form Health Survey.
Values expressed as mean ± SD, except where otherwise indicated.
One patient did not perform the six-minute walk test after the 12-month treatment.
Values expressed as median (interquartile range).
In the 31 patients, baseline pulmonary function was characterized by mild obstruction and slightly reduced DLCO (Table 1). After 12 months of doxycycline treatment, there was a significant mean decrease in FEV1 (70 mL) but no significant variation in DLCO. The results of the 6MWT showed that there was an insignificant (25-m) increase in the mean 6MWD and a reduction in the minimum SpO2. There was an improvement in some of the SF-36 physical and mental domain scores (Table 1).
As shown in Figure 1A, there was a significant reduction in urinary MMP-9 levels (from 10,487 pg/mL at baseline to 4,061 pg/mL after 12 months of treatment with doxycycline; p < 0.001). Serum MMP-2 was also blocked. However, there were no significant differences between pre- and post-treatment serum MMP-9 levels (Figure 1B), and urinary MMP-2 levels were undetectable. All MMP-related data are shown in Table 1.
Figure 1. Urinary matrix metalloproteinase (MMP)-9 levels (in A), serum MMP-9 levels (in B), and serum VEGF-D levels (in C) in 31 lymphangioleiomyomatosis patients treated with doxycycline fo r 12 months in comparison with those in 10 healthy controls.

Blood and urine samples were collected from 10 healthy women (controls), whose mean age was 40 ± 4 years (p = 0.29 vs. the mean age of the LAM patients).
Median baseline serum and urinary MMP-9 levels were significantly higher in the LAM patients than in the healthy controls (933 vs. 89.6 ng/mL; p < 0.0001, and 10,487 vs. 200 pg/mL; p < 0.0001, respectively). Baseline serum and urinary MMP-2 levels were undetectable in the control group, with no significant differences between the controls and the LAM patients in terms of those levels.
Median baseline serum VEGF-D levels were significantly lower in the controls than in the LAM patients (185 vs. 821 pg/mL), in whom serum VEGF-D levels remained stable over the course of doxycycline treatment (Figure 1C).
Although all 31 patients showed a decrease in mean FEV1 after doxycycline treatment, a subset of those patients showed an increase in or a stabilization of FEV1. The 31 patients were divided into two groups on the basis of the variation in FEV1 (from baseline to doxycycline treatment month 12): the doxycycline-responder (doxy-R) group, which comprised 13 patients with increased or stable FEV1; and the doxycycline-nonresponder (doxy-NR) group, which comprised 18 patients with decreased FEV1 (Figure 2A).
Figure 2. In A, variation in FEV1 over the course of 12 months of treatment with doxycycline in the lymphangioleiomyomatosis patients who responded to the drug-the doxycycline-responder (doxy-R) group-and in those who did not-the doxycycline-nonresponder (doxy-NR) group. In B, median difference between FEV1 at baseline and FEV1 at 12 months of doxycycline treatment and between FVC at baseline and FVC at 12 months of doxycycline treatment in the doxy-R and doxy-NR groups. The doxy-R group comprised the lymphangioleiomyomatosis patients who responded to treatment with doxycycline, as evidenced by increased or stable FEV1 at doxycycline treatment month 12 in comparison with FEV1 at baseline. The doxy-NR group comprised the lymphangioleiomyomatosis patients who did not respond to treatment with doxycycline, as evidenced by decreased FEV1 at doxycycline treatment month 12 in comparison with FEV1 at baseline.
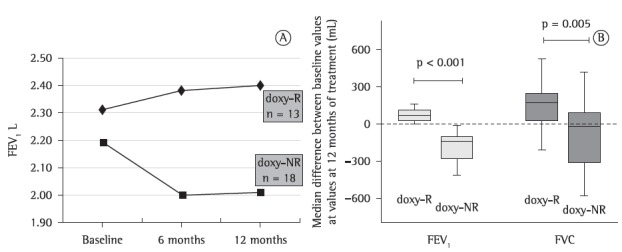
The functional parameters, SF-36 scores, MMP levels, and VEGF-D levels in the doxy-R and doxy-NR groups are shown in Tables 2 and 3. The patients in the doxy-NR group showed an obstructive pattern, with air trapping, whereas those in the doxy-R group did not. The median variation in FEV1 (in mL) from baseline to treatment month 12 was calculated. There was a median increase of 70 mL (range, 30-110 mL) in the doxy-R group and a median decrease of −140 mL (range, −260 to −110) in the doxy-NR group. The median variation in FVC in both groups followed the same trend as that of the variation in FEV1, with significant differences between the two groups (p = 0.005; Figure 2B). Functional impairment was greater in the doxy-NR group, as were MMP and VEGF-D levels (Tables 2 and 3).
Table 2. Pre- and post-doxycycline functional parameters, matrix metalloproteinase levels, VEGF-D levels, and Medical Outcomes Study 36-item Short-form Health Survey scores in the 13 lymphangioleiomyomatosis patients allocated to the doxycycline-responder groupa on the basis of their response to doxycycline (as determined by the variation in FEV1). b .
| Variable | Doxy-R group | p | ||
| Pre-doxycycline | Post-doxycycline | |||
| Pulmonary function test | ||||
| FVC, L | 3.0 ± 0.3 | 3.2 ± 0.3 | 0.023 | |
| FVC, % of predicted | 90 ± 13 | 95 ± 12 | 0.026 | |
| FEV1, L | 2.31 ± 0.40 | 2.40 ± 0.40 | 0.003 | |
| FEV1, % of predicted | 84 ± 14 | 86 ± 14 | 0.002 | |
| FEV1/FVC ratio | 0.77 ± 0.10 | 0.76 ± 0.10 | 0.173 | |
| TLC, L | 4.6 ± 0.5 | 4.8 ± 0.6 | 0.310 | |
| TLC, % of predicted | 98 ± 13 | 101 ± 15 | 0.327 | |
| RV, L | 1.6 ± 0.5 | 1.6 ± 0.6 | 0.956 | |
| RV, % of predicted | 117 ± 43 | 118 ± 47 | 0.940 | |
| RV/TLC ratio | 0.34 ± 0.08 | 0.33 ± 0.08 | 0.640 | |
| DLCO, mL/min/mmHg | 18.5 ± 6.0 | 18.0 ± 5.0 | 0.514 | |
| DLCO, % of predicted | 72 ± 20 | 70 ± 18 | 0.448 | |
| Six-minute walk test | ||||
| Distance, m | 515 ± 66 | 523 ± 52 | 0.633 | |
| Minimum SpO2, % c | 94 (93-95) | 94 (91-95) | 0.500 | |
| Biomarkers c | ||||
| Serum MMP-9, ng/mL | 837 (716-1,033) | 1,103 (773-1,237) | 0.328 | |
| Urinary MMP-9, pg/mL | 8,905 (5,607-21,829) | 6,735 (674-8,983) | 0.050 | |
| Serum MMP-2, pg/mL | 0 (0-182) | 0 (0-40) | 0.144 | |
| VEGF-D, pg/mL | 413 (277-2,113) | 328 (257-2,008) | 0.374 | |
| SF-36 domains c | ||||
| Physical functioning | 75 (65-85) | 80 (75-95) | 0.281 | |
| Role-physical | 50 (50-50) | 100 (50-100) | 0.020 | |
| Bodily pain | 57.5 (55-90) | 70 (55-77.5) | 1.000 | |
| General health | 80 (45-90) | 75 (70-85) | 0.412 | |
| Vitality | 70 (45-90) | 70 (70-85) | 0.428 | |
| Role-emotional | 33 (33-100) | 100 (33-100) | 0.158 | |
| Mental health | 68 (40-84) | 76 (52-88) | 0.051 | |
| Social functioning | 75.0 (50.0-88.5) | 87.5 (62.5-100) | 0.040 |
Doxy-R group: : doxycycline-responder group; minimum SpO2: minimum SpO2 sustained for 10 s;
: matrix metalloproteinase;
SF-36: : Medical Outcomes Study 36-item Short-form Health Survey.
Group of patients who responded to treatment with doxycycline, as evidenced by increased or stable FEV1 at doxycycline treatment month 12 in comparison with FEV1 at baseline.
Values expressed as mean ± SD, except where otherwise indicated.
Values expressed as median (interquartile range).
Table 3. Pre- and post-doxycycline functional parameters, matrix metalloproteinase levels, VEGF-D levels, and Medical Outcomes Study 36-item Short-form Health Survey scores in the 18 lymphangioleiomyomatosis patients allocated to the doxycycline-nonresponder groupa on the basis of their lack of response to doxycycline (as determined by the variation in FEV1). b .
| Variable | Doxy-NR group | p | ||
| Pre-doxycycline | Post-doxycycline | |||
| Pulmonary function test | ||||
| FVC, L | 3.2 ± 0.7 | 3.2 ± 0.7 | 0.114 | |
| FVC, % of predicted | 94 ± 15 | 91 ± 16 | 0.151 | |
| FEV1, L | 2.2 ± 0.9 | 2 ± 0.9 | < 0.001 | |
| FEV1, % of predicted | 75 ± 28 | 70 ± 28 | < 0.001 | |
| FEV1/FVC ratio | 0.65 ± 0.2 | 0.61 ± 0.2 | 0.009 | |
| TLC, L | 5.3 ± 0.8 | 5.3 ± 0.7 | 0.922 | |
| TLC, % of predicted | 107 ± 13 | 107 ± 12 | 0.844 | |
| RV, L | 2 ± 0.6 | 2.1 ± 0.7 | 0.129 | |
| RV, % of predicted | 140 ± 49 | 150 ± 54 | 0.155 | |
| RV/TLC ratio | 0.37 ± 0.08 | 0.4 ± 0.11 | 0.032 | |
| DLCO, mL/min/mmHg | 15.9 ± 7.5 | 15.8 ± 6.6 | 0.967 | |
| DLCO, % of predicted | 60 ± 27 | 60 ± 24 | 0.948 | |
| Six-minute walk test | ||||
| Distance, m | 472 ± 130 | 509 ± 112 | 0.119 | |
| Minimum SpO2, % c | 92.5 (83-95) | 92 (78-95) | 0.087 | |
| Biomarkers c | ||||
| Serum MMP-9, ng/mL | 1,011 (748-1,272) | 1,042 (809-1,307) | 0.756 | |
| Urinary MMP-9, pg/mL | 10,487 (4,526-15,653) | 3,970 (765-9,985) | 0.001 | |
| Serum MMP-2, pg/mL | 188 (0-905) | 0 (0-214) | 0.017 | |
| VEGF-D, pg/mL | 995 (535-2,000) | 1,124 (810-2,262) | 0.211 | |
| SF-36 domains c | ||||
| Physical functioning | 70 (51-84) | 62.5 (46-81) | 0.084 | |
| Role-physical | 50 (50-50) | 50 (25-100) | 0.668 | |
| Bodily pain | 79.0 (60.0-97.5) | 67.5 (57.5-90.0) | 0.821 | |
| General health | 70 (51-80) | 67.5 (50-80) | 0.905 | |
| Vitality | 63 (55-79) | 65 (55-80) | 0.954 | |
| Role-emotional | 100 (33-100) | 100 (67-100) | 0.426 | |
| Mental health | 66 (57-83) | 80 (63-84) | 0.053 | |
| Social functioning | 87.5 (75.0-97.0) | 87.5 (62.5-97.0) | 1.000 |
Doxy-NR group: : doxycycline-nonresponder group;
minimum SpO2: : minimum SpO2 sustained for 10 s;
: matrix metalloproteinase
SF-36: : Medical Outcomes Study 36-item Short-form Health Survey.
Group of patients who did not respond to treatment with doxycycline, as evidenced by decreased FEV1 at doxycycline treatment month 12 in comparison with FEV1 at baseline.
Values expressed as mean ± SD, except where otherwise indicated.
Values expressed as median (interquartile range).
After treatment with doxycycline, the patients in the doxy-R group showed an improvement in the SF-36 scores, the social functioning and role-physical domain scores being significantly higher. The patients in the doxy-NR group showed an improvement, although not significant, in the physical and mental domain scores. The SF-36 scores are shown in Tables 2 and 3.
In order to evaluate functional markers of response to doxycycline, we compared the doxy-R and doxy-NR groups before treatment initiation and found that the patients with less severe disease (lower TLC and milder obstruction) responded better to doxycycline (Appendix, section 2, Table A2). The ROC curve analysis showed that the FEV1/FVC ratio that was most accurate in predicting the response to treatment with doxycycline was 0.71 (area under the curve = 0.690; 95% CI: 0.50-0.88; p = 0.07; Appendix, section 2, Figure A1, panel A).
Of the 31 patients enrolled in the trial, 22 had previously received some type of hormonal blockade therapy. However, there was no significant difference between the doxy-R and doxy-NR groups in terms of the prevalence of previous hormonal blockade therapy (p = 0.696; Appendix, section 2, Table A3).
Twenty patients continued to receive doxycycline for a median time of 18 months after the end of the study period. Regarding the variation in FEV1 after the end of the study, the patients in the doxy-R group showed a slight reduction in FEV1, whereas those in the doxy-NR group showed a sizeable reduction, the variation in FEV1 after the end of the study having followed the same trend as that of the variation in FEV1 during the study period (Appendix, section 2, Figure A1, panel B).
The most common adverse events were epigastric pain (45%), nausea (19%), diarrhea (16%), and itching (6%). They were mild and self-limiting, specific treatment being rarely required. Of the 41 patients enrolled in the trial, 3 (7%) had adverse events requiring doxycycline discontinuation. Of those 3 patients, 1 had acute colitis, 1 had chronic diarrhea, and 1 had hemorrhoids.
Discussion
To the best of our knowledge, the present study is the first to demonstrate the effects of doxycycline, a known MMP inhibitor, on serum MMP levels, urinary MMP levels, and serum VEGF-D levels, as well as on pulmonary function test results and quality of life, in a cohort of LAM patients. The main findings of the present study include effective urinary MMP-9 and serum MMP-2 blockade induced by a 12-month treatment with doxycycline, without a significant influence on VEGF-D levels. We believe that treatment with doxycycline can be beneficial for patients with less severe functional impairment and that doxycycline is safe for patients with LAM.
One of the factors likely involved in cystic lung destruction in LAM is MMP overexpression. It is known that MMPs are enzymes that are capable of degrading collagen and elastin in the extracellular matrix of lung tissue.( 12 ) Chang et al. demonstrated the in vitro effect of doxycycline on TSC2-null cell adhesion, as well as on MMP-2 and MMP-9 expression, revealing a reduction in LAM cell proliferation, although high doses of doxycycline were required and there was no significant blockade of MMP expression.( 30 ) They also suggested that the antiproliferative effects of doxycycline are unlikely to be directly due to MMP inhibition, given that cell proliferation was not inhibited by ilomastat, a potent MMP inhibitor.( 30 ) However, Moir et al. demonstrated that doxycycline significantly reduced MMP-2 levels in LAM-derived cells and in TSC2-null mouse embryonic fibroblasts.( 31 )
Doxycycline has been described as an MMP blocker in previous studies of other diseases, being able to suppress cerebral MMP-9 expression and angiogenesis in a mouse model and to reduce MMP-2 and MMP-9 levels to prevent thoracic aortic aneurysms.( 32 , 33 )
In the present study, doxycycline was found to induce effective MMP blockade, a finding that corroborates our previous findings in a slightly larger cohort of patients.( 20 ) However, there was no association between MMP blockade and functional improvement. We also found a reduction in MMP levels in the doxy-R and doxy-NR groups, a finding that is suggestive of an MMP-independent mechanism of action for doxycycline. However, we tested only MMP-2 and MMP-9 and did not explore the possibility of involvement of other MMPs, such as MMP-1 and MMP-8, or the inhibition of TIMP activity, as previously described.( 34 , 35 )
Serum VEGF-D levels remained stable after doxycycline treatment (Figure 1A). Although vascular permeability and angiogenesis inhibition( 36 , 37 ) have been reported to be induced by doxycycline, the pathway seems to be MMP-independent. Although median baseline VEGF-D levels were found to be higher in the patients in the doxy-NR group than in those in the doxy-R group (995 ng/mL vs. 413 ng/mL), the difference was not significant (p = 0.325). These findings are concordant with those of previous studies that found no association between VEGF-D levels and lung function impairment.( 38 - 40 )
After 12 months of treatment with doxycycline, we found a mean decrease of 70 mL in FEV1 and a mean decrease of 0.24 mL/min/mmHg in DLCO. In comparison with the annual rates of decline in FEV1 reported by Taveira et al.( 8 ) and Johnson & Tattersfield,( 41 ) i.e., 75 mL and 118 mL, respectively, the rate of decline in FEV1 in the present study was slightly lower, although we found that doxycycline had no impact on pulmonary function decline. The rate of DLCO decline in our patients was also lower than were those reported in the two studies cited above (0.69 and 0.90 mL/min/mmHg, respectively). In the present study, the patients in the doxy-R group showed no decline in FEV1, having shown a slight but significant increase in FVC and FEV1 and stable SpO2 during the 6MWT. However, the doxy-NR group showed a decrease in FEV1 and in the FEV1/FVC ratio, as well as showing an increase in the RV/TLC ratio. By comparing the doxy-R and doxy-NR groups (Appendix, section 2, Table A2), we found that doxycycline can be beneficial for patients with mild spirometric abnormalities. This finding was supported by the ROC curve analysis, which showed that the FEV1/FVC ratio that was most accurate in predicting the response to doxycycline was 0.71. In addition, because the prevalence of hormonal blockade was similar between the doxy-R and doxy-NR groups, we hypothesized that hormonal therapy has no influence on the response to doxycycline.
Twenty patients continued to receive doxycycline after the end of the 12-month study period. An evaluation performed after the end of the study period revealed differences between the two groups in terms of the rate of FEV1 decline. This finding suggests that doxycycline is more effective in slowing functional impairment in patients with LAM that is less severe (Appendix, section 2, Figure A1, panel B).
The LAM patients investigated in the present study underwent health-related quality of life assessment before and after treatment with doxycycline, an assessment that was not performed in previous studies. We found an improvement in some of the physical and mental domain scores after treatment with doxycycline, a finding that was more prominent in the patients in the doxy-R group.
Doxycycline was found to be safe and well-tolerated. The most common side effects were those affecting the gastrointestinal tract and were self-limiting.
Our study has some limitations. Because the trial was not placebo-controlled, the fact that we included patients with less severe forms of LAM can be considered a bias, which could account for the beneficial effect of doxycycline in the doxy-R group patients, because those patients had less functional impairment at baseline. Another potential limitation is the fact that more than 10% of the patients enrolled in the trial were lost to follow-up. In addition, we did not assess MMPs other than MMP-2 and MMP-9, and we did not assess TIMPs. Furthermore, we tested doxycycline at only one dose level.
In summary, the present study showed that, in patients with LAM, doxycycline treatment resulted in effective MMP blockade. Doxycycline treatment also resulted in improved or stable lung function and in improved quality of life, particularly in the LAM patients with mild spirometric abnormalities. However, these benefits do not seem to be related to the blockade of MMPs, which raises the hypothesis of a different mechanism of action. Because the lack of a placebo arm limits the analysis of the clinical benefits of doxycycline, randomized placebo-controlled studies are needed in order to establish the actual role of doxycycline in the treatment of patients with LAM.
Acknowledgments
We wish to thank Dr. Milena Acencio for her contribution to the analysis of biomarkers.
Footnotes
Financial support: This study received financial support from the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council for Scientific and Technological Development).
Study carried out in the Department of Pulmonology, Instituto do Coração - InCor, Heart Institute - University of São Paulo School of Medicine Hospital das Clínicas, São Paulo, Brazil.
A versão completa em português deste artigo está disponível em www.jornaldepneumologia.com.br
Contributor Information
Suzana Pinheiro Pimenta, A. C. Camargo Hospital, São Paulo, Brazil. A. C. Camargo Hospital, São Paulo, Brazil.
Bruno Guedes Baldi, University of Sao Paulo, Medical School, São Paulo, Brazil. Pulmonary Division, Heart Institute (InCor), University of Sao Paulo Medical School, São Paulo, Brazil.
Ronaldo Adib Kairalla, University of Sao Paulo, Medical School, São Paulo, Brazil. Pulmonary Division, Heart Institute (InCor), University of Sao Paulo Medical School, São Paulo, Brazil.
Carlos Roberto Ribeiro Carvalho, University of Sao Paulo, Medical School, São Paulo, Brazil. Pulmonary Division, Heart Institute (InCor), University of Sao Paulo Medical School, São Paulo, Brazil.
References
- 1.Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc. 2000;75(6):591–594. doi: 10.4065/75.6.591. http://dx.doi.org/10.4065/75.6.591 [DOI] [PubMed] [Google Scholar]
- 2.Chu SC, Horiba K, Usuki J, Avila NA, Chen CC, Travis WD, et al. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest. 1999;115(4):1041–1052. doi: 10.1378/chest.115.4.1041. http://dx.doi.org/10.1378/chest.115.4.1041 [DOI] [PubMed] [Google Scholar]
- 3.Taveira-DaSilva AM, Steagall WK, Moss J. Lymphangioleiomyomatosis. Cancer Control. 2006;13(4):276–285. doi: 10.1177/107327480601300405. [DOI] [PubMed] [Google Scholar]
- 4.Johnson SR, Tattersfield AE. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax. 2000;55(12):1052–1057. doi: 10.1136/thorax.55.12.1052. http://dx.doi.org/10.1136/thorax.55.12.1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor JR, Ryu J, Colby TV, Raffin TA. Lymphangioleiomyomatosis. Clinical course in 32 patients. N Engl J Med. 1990;323(18):1254–1260. doi: 10.1056/NEJM199011013231807. [DOI] [PubMed] [Google Scholar]
- 6.Baldi BG, Pimenta SP, Kawassaki Ade M, Bernardi Fdel C, Dolhnikoff M, Carvalho CR. Pulmonary arterial involvement leading to alveolar hemorrhage in lymphangioleiomyomatosis. Clinics (Sao Paulo) 2011;66(7):1301–1303. doi: 10.1590/S1807-59322011000700031. http://dx.doi.org/10.1590/S1807-59322011000700031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu JH, Moss J, Beck GJ, Lee JC, Brown KK, Chapman JT, et al. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173(1):105–111. doi: 10.1164/rccm.200409-1298OC. http://dx.doi.org/10.1164/rccm.200409-1298OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Hathaway O, Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126(6):1867–1874. doi: 10.1378/chest.126.6.1867. http://dx.doi.org/10.1378/chest.126.6.1867 [DOI] [PubMed] [Google Scholar]
- 9.Baldi BG, Medeiros P, Junior, Pimenta SP, Lopes RI, Kairalla RA, Carvalho CR. Evolution of pulmonary function after treatment with goserelin in patients with lymphangioleiomyomatosis. J Bras Pneumol. 2011;37(3):375–379. doi: 10.1590/s1806-37132011000300015. http://dx.doi.org/10.1590/S1806-37132011000300015 [DOI] [PubMed] [Google Scholar]
- 10.Harari S, Cassandro R, Chiodini I, Taveira-DaSilva AM, Moss J. Effect of a gonadotrophin-releasing hormone analogue on lung function in lymphangioleiomyomatosis. Chest. 2008;133(2):448–454. doi: 10.1378/chest.07-2277. Erratum in: Chest. 2009;136(2):653. PMid:18071009 PMCid:2946894. http://dx.doi.org/10.1378/chest.07-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364(17):1595–1606. doi: 10.1056/NEJMoa1100391. http://dx.doi.org/10.1056/NEJMoa1100391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsui K, Takeda K, Yu ZX, Travis WD, Moss J, Ferrans VJ. Role for activation of matrix metalloproteinases in the pathogenesis of pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med. 2000;124(2):267–275. doi: 10.5858/2000-124-0267-RFAOMM. [DOI] [PubMed] [Google Scholar]
- 13.Odajima N, Betsuyaku T, Nasuhara Y, Inoue H, Seyama K, Nishimura M. Matrix metalloproteinases in blood from patients with LAM. Respir Med. 2009;103(1):124–129. doi: 10.1016/j.rmed.2008.07.017. http://dx.doi.org/10.1016/j.rmed.2008.07.017 [DOI] [PubMed] [Google Scholar]
- 14.Ji RC. Lymphatic endothelial cells, lymphangiogenesis, and extracellular matrix. Lymphat Res Biol. 2006;4(2):83–100. doi: 10.1089/lrb.2006.4.83. http://dx.doi.org/10.1089/lrb.2006.4.83 [DOI] [PubMed] [Google Scholar]
- 15.Vignola AM, Paganin F, Capieu L, Scichilone N, Bellia M, Maakel L, et al. Airway remodelling assessed by sputum and high-resolution computed tomography in asthma and COPD. Eur Respir J. 2004;24(6):910–917. doi: 10.1183/09031936.04.00032603. http://dx.doi.org/10.1183/09031936.04.00032603 [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T, Rush WL, Travis WD, Liotta LA. Stetler-Stevenson WG, Ferrans VJ. Immunohistochemical study of matrix metalloproteinases and their tissue inhibitors in pulmonary Langerhans' cell granulomatosis. Arch Pathol Lab Med. 1997;121(9):930–937. [PubMed] [Google Scholar]
- 17.Fukuda Y, Kawamoto M, Yamamoto A, Ishizaki M, Basset F, Masugi Y. Role of elastic fiber degradation in emphysema-like lesions of pulmonary lymphangiomyomatosis. Hum Pathol. 1990;21(12):1252–1261. doi: 10.1016/s0046-8177(06)80039-0. http://dx.doi.org/10.1016/S0046-8177(06)80039-0 [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Fleming MV, Stetler-Stevenson WG, Liotta LA, Moss J, Ferrans VJ, et al. Immunohistochemical study of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in pulmonary lymphangioleiomyomatosis (LAM) Hum Pathol. 1997;28(9):1071–1078. doi: 10.1016/s0046-8177(97)90061-7. http://dx.doi.org/10.1016/S0046-8177(97)90061-7 [DOI] [PubMed] [Google Scholar]
- 19.Moses MA, Harper J, Folkman J. Doxycycline treatment for lymphangioleiomyomatosis with urinary monitoring for MMPs. N Engl J Med. 2006;354(24):2621–2622. doi: 10.1056/NEJMc053410. http://dx.doi.org/10.1056/NEJMc053410 [DOI] [PubMed] [Google Scholar]
- 20.Pimenta SP, Baldi BG, Acencio MM, Kairalla RA, Carvalho CR. Doxycycline use in patients with lymphangioleiomyomatosis: safety and efficacy in metalloproteinase blockade. J Bras Pneumol. 2011;37(4):424–430. doi: 10.1590/s1806-37132011000400003. http://dx.doi.org/10.1590/S1806-37132011000400003 [DOI] [PubMed] [Google Scholar]
- 21.Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35(1):14–26. doi: 10.1183/09031936.00076209. http://dx.doi.org/10.1183/09031936.00076209 [DOI] [PubMed] [Google Scholar]
- 22.Rubin AS, Santana AN, Costa AN, Baldi BG, Pereira CA, Carvalho CR, et al. Diretrizes de doenças pulmonares intersticiais da Sociedade Brasileira de Pneumologia e Tisiologia. J Bras Pneumol. 2012;38(Suppl 2):S1–S133. [Google Scholar]
- 23.Pereira CA, Sato T, Rodrigues SC. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol. 2007;33(4):397–406. doi: 10.1590/s1806-37132007000400008. http://dx.doi.org/10.1590/S1806-37132007000400008 [DOI] [PubMed] [Google Scholar]
- 24.Neder JA, Andreoni S, Castelo-Filho A, Nery LE. Reference values for lung function tests. I. Static volumes. Braz J Med Biol Res. 1999;32(6):703–717. doi: 10.1590/s0100-879x1999000600006. [DOI] [PubMed] [Google Scholar]
- 25.Neder JA, Andreoni S, Peres C, Nery LE. Reference values for lung function tests. III. Carbon monoxide diffusing capacity (transfer factor) Braz J Med Biol Res. 1999;32(6):729–737. doi: 10.1590/s0100-879x1999000600008. http://dx.doi.org/10.1590/S0100-879X1999000600008 [DOI] [PubMed] [Google Scholar]
- 26.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 27.Soaresa MR, Pereira CA. Six-minute walk test: reference values for healthy adults in Brazil. J Bras Pneumol. 2011;37(5):576–583. doi: 10.1590/s1806-37132011000500003. http://dx.doi.org/10.1590/S1806-37132011000500003 [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann CS, Carvalho CR, Silveira KR, Yamaguti WP, Moderno EV, Salge JM, et al. Comparison of two questionnaires which measure the health-related quality of life of idiopathic pulmonary fibrosis patients. Braz J Med Biol Res. 2007;40(2):179–187. doi: 10.1590/s0100-879x2007000200004. http://dx.doi.org/10.1590/S0100-879X2006005000056 [DOI] [PubMed] [Google Scholar]
- 29.Ciconelli RM, Ferraz MB, Santos W, Meinão I, Quaresma MR. Tradução para língua portuguesa e validação do questionário genérico de avaliação de qualidade de vida SF-36 (Brasil SF-36) Rev Bras Reumatol. 1999;39:143–150. [Google Scholar]
- 30.Chang WY, Clements D, Johnson SR. Effect of doxycycline on proliferation, MMP production, and adhesion in LAM-related cells. Am J Physiol Lung Cell Mol Physiol. 2010;299(3):L393–L400. doi: 10.1152/ajplung.00437.2009. http://dx.doi.org/10.1152/ajplung.00437.2009 [DOI] [PubMed] [Google Scholar]
- 31.Moir LM, Ng HY, Poniris MH, Santa T, Burgess JK, Oliver BG, et al. Doxycycline inhibits matrix metalloproteinase-2 secretion from TSC2-null mouse embryonic fibroblasts and lymphangioleiomyomatosis cells. Br J Pharmacol. 2011;164(1):83–92. doi: 10.1111/j.1476-5381.2011.01344.x. http://dx.doi.org/10.1111/j.1476-5381.2011.01344.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang GY, Young WL. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke. 2004;35(7):1715–1719. doi: 10.1161/01.STR.0000129334.05181.b6. http://dx.doi.org/10.1161/01.STR.0000129334.05181.b6 [DOI] [PubMed] [Google Scholar]
- 33.Chung AW, Yang HH, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res. 2008;102(8):e73–e85. doi: 10.1161/CIRCRESAHA.108.174367. http://dx.doi.org/10.1161/CIRCRESAHA.108.174367 [DOI] [PubMed] [Google Scholar]
- 34.Steagall WK, Glasgow CG, Hathaway OM, Avila NA, Taveira-Dasilva AM, Rabel A, et al. Genetic and morphologic determinants of pneumothorax in lymphangioleiomyomatosis. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L800–L808. doi: 10.1152/ajplung.00176.2007. http://dx.doi.org/10.1152/ajplung.00176.2007 [DOI] [PubMed] [Google Scholar]
- 35.Yao JS, Shen F, Young WL, Yang GY. Comparison of doxycycline and minocycline in the inhibition of VEGF-induced smooth muscle cell migration. Neurochem Int. 2007;50(3):524–530. doi: 10.1016/j.neuint.2006.10.008. http://dx.doi.org/10.1016/j.neuint.2006.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fainaru O, Adini I, Benny O, Bazinet L, Pravda E, D'Amato R, et al. Doxycycline induces membrane expression of VE-cadherin on endothelial cells and prevents vascular hyperpermeability. FASEB J. 2008;22(10):3728–3735. doi: 10.1096/fj.08-110494. http://dx.doi.org/10.1096/fj.08-110494 [DOI] [PubMed] [Google Scholar]
- 37.Gilbertson-Beadling S, Powers EA, Stamp-Cole M, Scott PS, Wallace TL, Copeland J, et al. The tetracycline analogs minocycline and doxycycline inhibit angiogenesis in vitro by a non-metalloproteinase-dependent mechanism. Cancer Chemother Pharmacol. 1995;36(5):418–424. doi: 10.1007/BF00686191. http://dx.doi.org/10.1007/BF00686191 [DOI] [PubMed] [Google Scholar]
- 38.Glasgow CG, Avila NA, Lin JP, Stylianou MP, Moss J. Serum vascular endothelial growth factor-D levels in patients with lymphangioleiomyomatosis reflect lymphatic involvement. Chest. 2009;135(5):1293–1300. doi: 10.1378/chest.08-1160. http://dx.doi.org/10.1378/chest.08-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young LR, Vandyke R, Gulleman PM, Inoue Y, Brown KK, Schmidt LS, et al. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest. 2010;138(3):674–681. doi: 10.1378/chest.10-0573. http://dx.doi.org/10.1378/chest.10-0573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang WY, Cane JL, Blakey JD, Kumaran M, Pointon KS, Johnson SR. Clinical utility of diagnostic guidelines and putative biomarkers in lymphangioleiomyomatosis. Respir Res. 2012;13(34) doi: 10.1186/1465-9921-13-34. http://dx.doi.org/10.1186/1465-9921-13-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson SR, Tattersfield AE. Decline in lung function in lymphangioleiomyomatosis: relation to menopause and progesterone treatment. Am J Respir Crit Care Med. 1999;160(2):628–633. doi: 10.1164/ajrccm.160.2.9901027. [DOI] [PubMed] [Google Scholar]


