Abstract
Lung volume recruitment involves deep inflation techniques to achieve maximum insufflation capacity in patients with respiratory muscle weakness, in order to increase peak cough flow, thus helping to maintain airway patency and improve ventilation. One of these techniques is air stacking, in which a manual resuscitator is used in order to inflate the lungs. Although intrathoracic pressures can rise considerably, there have been no reports of respiratory complications due to air stacking. However, reaching maximum insufflation capacity is not recommended in patients with known structural abnormalities of the lungs or chronic obstructive airway disease. We report the case of a 72-year-old woman who had poliomyelitis as a child, developed torsion scoliosis and post-polio syndrome, and had periodic but infrequent asthma attacks. After performing air stacking for 3 years, the patient suddenly developed a pneumothorax, indicating that this technique should be used with caution or not at all in patients with a known pulmonary pathology
Keywords: Barotrauma, Pneumothorax, Insufflation
Abstract
O recrutamento do volume pulmonar envolve técnicas de insuflações pulmonares profundas para se atingir a capacidade de insuflação máxima em pacientes com fraqueza da musculatura respiratória, a fim de aumentar o pico de fluxo da tosse e assim auxiliar a manutenção da patência de vias aéreas e melhorar a ventilação. Uma dessas técnicas é o empilhamento de ar, na qual se utiliza um ressuscitador manual para insuflar os pulmões. Embora as pressões intratorácicas possam aumentar consideravelmente, não há relatos de complicações por empilhamento de ar. Entretanto, atingir a capacidade de insuflação máxima não é recomendado em pacientes com anormalidades na estrutura pulmonar ou doença obstrutiva crônica das vias aéreas. Relatamos o caso de uma paciente de 72 anos que teve poliomielite quando criança, desenvolveu escoliose de torção e síndrome pós-pólio e tinha exacerbações de asma periódicas, mas infrequentes. Após realizar empilhamento de ar por 3 anos, a paciente subitamente desenvolveu pneumotórax, mostrando que essa técnica deve ser utilizada com cuidado ou não ser utilizada por pacientes com patologia pulmonar conhecida
Introduction
Lung volume recruitment (LVR) is a dynamic physiologic process used to reopen collapsed aveoli; augment voice volume and duration; prevent (micro)atelectasis; preserve or improve respiratory compliance; and augment cough flows to facilitate continued use of noninvasive ventilation (NIV). When deeply insufflated air volumes are followed by coughing, cough flows can be greatly increased, facilitating the expulsion of bronchial secretions. In patients with decreased respiratory muscle function due to neuromuscular disease or chest wall deformity, air stacking has been recommended as a method for deep lung insufflation (Chart 1). With this technique, a manual resuscitator is used to deliver volumes of air that the glottis holds to volumes greater than VC until the maximum insufflation capacity (MIC) is reached.( 1 ) The intrathoracic pressures are thereby elevated. Despite sometimes achieving transpulmonary pressures of 80 cmH2O,( 2 , 3 ) the technique is considered to be safe, because complications have been seldom reported.( 4 , 5 ) We report the case of a chronically ventilator dependent patient who presented with severe respiratory distress almost a day after she experienced acute chest pain and dyspnea while stacking air. We discuss the findings and the possible consequences of management, as well as suggest future recommendations.
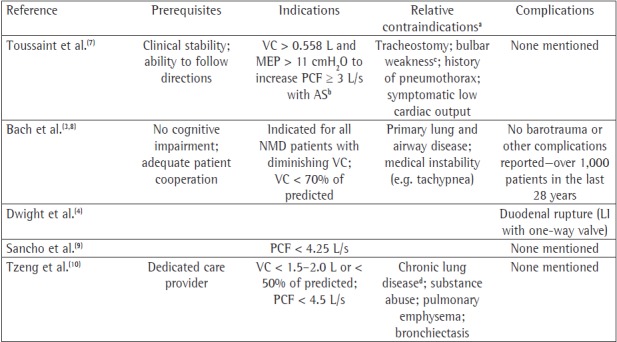
Chart 1. Recommendations and complications for air stacking/deep passive lung insufflation. PCF: peak cough flow; AS: air stacking; LI: lung insufflation; NMD: neuromuscular disease. aExclusion criteria in the studies. bVC and MEP of at least 0.56 L and 11 cmH2O, respectively, are mandatory to elevate PCF > 3 L/s using AS. cAS cumbersome or impossible; passive lung insufflation remains feasible, according to Bach et al.(8) dDefined as chronic abnormalities in chest X-ray and SpO2 < 95% or FEV1/FVC ratio two standard deviations less than normal.
Case report
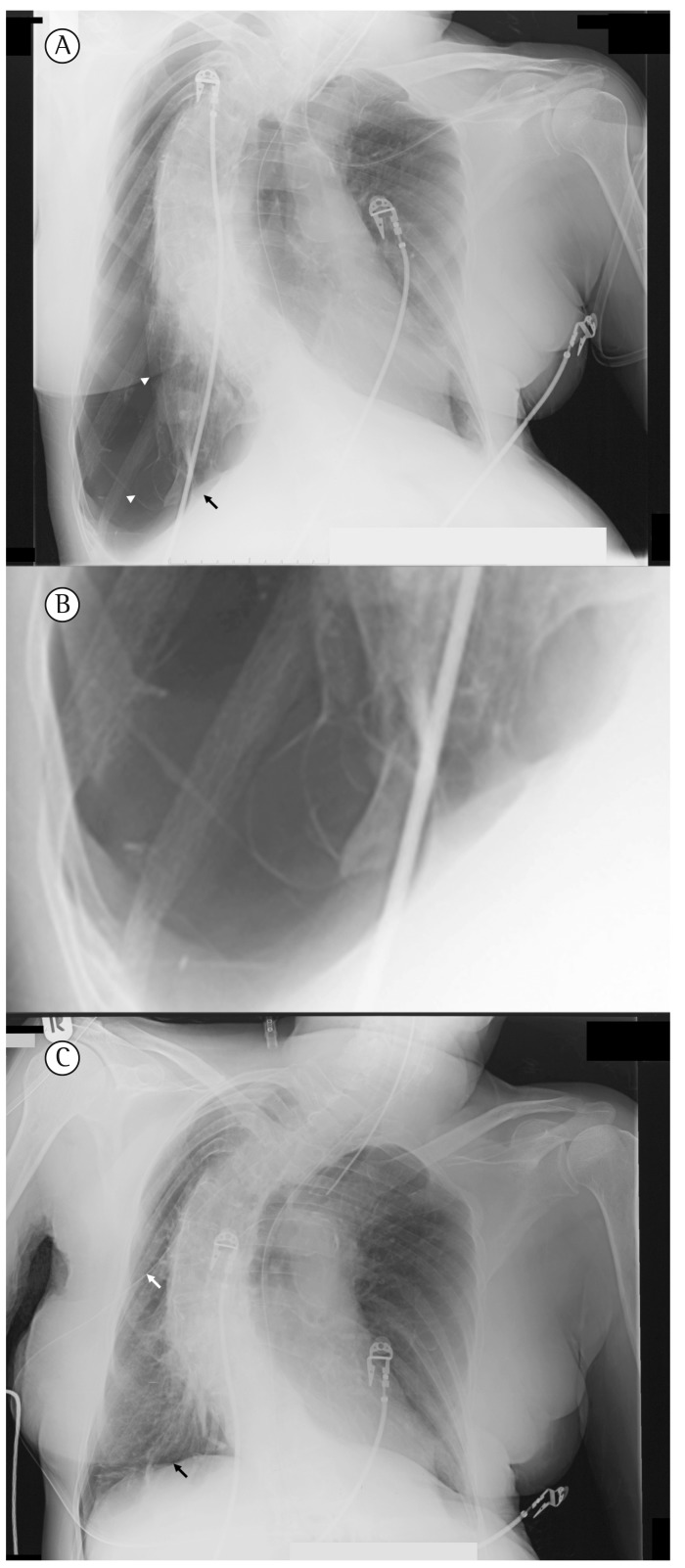
A 72-year-old woman who had poliomyelitis as a child developed scoliosis and post-polio syndrome, which led to chronic respiratory failure at age 54. She had been on nocturnal ventilatory assistance for 15 years, resulting in normal blood gases during the day. She had a history of bronchial asthma and a 20 pack-year smoking history, having quit smoking 30 years prior. Because of ineffective peak cough flows registered in 2005, she was taught how to stack air. Thus, she gradually improved her assisted as well as her spontaneous peak cough flows (PCF, Table 1). After having used air stacking uneventfully for nearly 3 years, the patient suddenly experienced a sharp pain during deep inflation one morning in June of 2008. The pain, which was located in her back and on her right side, was accompanied by acute dyspnea. She began ventilation earlier that evening to alleviate dyspnea. The following morning, she was referred to the emergency room and presented with worsening respiratory distress. She required emergent intubation and invasive ventilation before a diagnostic work-up could be initiated. Subsequently, blood gas analysis results were as follows: pH, 6.99; PaCO2, 127.5 mmHg; bicarbonate, 29.0 mmol/L; base excess, −8.4 mmol/L; PaO2, 101.3 mmHg; and SaO2, 92%. A chest X-ray revealed right-sided pneumothorax (Figure 1). Immediately after chest tube drainage her respiratory distress resolved and she was successfully extubated. In order to minimize pleural air leakage during positive pressure ventilation, we considered to temporarily discontinue nocturnal NIV, but she could not tolerate sleeping without NIV. Four days later, the lung had completely expanded and talcum pleurodesis was performed. After healing of the pleurodesis (16 days later) the patient was oxygen dependent during spontaneous breathing and she was discharged home on long-term oxygen therapy for 15 hours daily. During nocturnal NIV the use of oxygen was not indicated. The patient discontinued air stacking.
Table 1. Lung volume recruitment and pulmonary function during admissions due to asthma exacerbation.
| Variables, 1991-2009 | 1991 | 1994 | 2006 | 2008 | April 1, 2009 | ||
| (3 mo. after the start of NIV) | (1.5 mo. before the incident) | ||||||
| Pulmonary function | |||||||
| VC, L (%) | 1.00 (37) | 1.32 (49) | 1.2 (51) | ||||
| FEV1, L (%) | 0.70 (30) | 0.92 (41) | 0.6 (34) | ||||
| Albuterol | 0.80 (34) | 0.7 (38) | |||||
| FEV1/VC, % | 70 | 70 | 51 | ||||
| Albuterol, % | 78 | 58 | |||||
| TLC, L (%) | 3.00 (65) | 3.38 (74) | 3.47 (77) | ||||
| ITGV, L (%) | 2.30 (90) | 2.53 (99) | |||||
| Raw, kPa.L-1.s (n) | 0.56 (0.3) | 0.57 (0.3) | |||||
| Blood gas analysis | arterial | arterial | capillary | capillary | capillary | ||
| pH | 7.37 | 7.39 | 7.40 | 7.42 | 7.44 | ||
| PCO2, mmHg | 52 | 48.0 | 45 | 44 | 51 | ||
| Bic, mmol/L | 31 | 30 | 27.9 | 28.0 | 31.6 | ||
| PO2, mmHg | 52 | 61 | 61 | 61 | 59 | ||
| SO2, % | 85 | 90 | 91 | 91 | 90 | ||
| SpO2, % | 95 | 94 | 93 | ||||
| Variables, 2004-2009LVR-related measurements | 2004 | 2005 | 2006 | 2007 | 2009 | ||
| (prior to the start of AS) | (3 mo. after the start of AS) | ||||||
| PCF unassisted, L/s | 2.20 | 2.25 | 2.50 | 3.08 | 2.83 | -- | |
| PCF after MIC, L/s | 2.75 | 3.50 | 4.08 | 4.00 | -- | ||
| RR, min-1 | 16 | 18 | 22 | 16 | 20-24 | ||
: months
: noninvasive ventilation; incident: acquisition of pneumothorax
: intrathoracic gas volume
: airway resistance
: bicarbonate
: carbon dioxide tension
: oxygen tension
: oxygen saturation
: lung volume recruitment
: peak cough flow
: maximum insufflation capacity
Figure 1. In A, a chest X-ray, taken at admission, shows right-sided pneumothorax. Notice the total collapse of the lung (upper white arrowhead), diaphragmatic bullae (lower white arrowhead), and widened intercostal spaces. The black arrow shows the depressed right hemidiaphragm. In B, a detail of the right lower lobe reveals inflated bullae in the collapsed lung. In C, a chest X-ray, taken an hour after the first one (A) and immediately after chest tube drainage (white arrow) shows the normal position of the hemidiaphragm (black arrow).
Nine months later (April 1st, 2009), the patient visited the outpatient clinic. She was slightly hypercapnic, without wheezing, and used O2 1 L/min during spontaneous respiration (Table 1). The patient did not report having experienced chest pain, backache, or sudden dyspnea.
Discussion
To our knowledge, this is the first report of pulmonary barotrauma/volutrauma caused by LVR using air stacking. Although it seems prudent to avoid LVR when patients have a history of recent pneumothorax, pulmonary emphysema, or bronchiectasis, barotrauma and volutrauma have seldom been reported as complications of air stacking. We identified only one report of intestinal barotrauma as a complication of passive lung insufflation via a manual resuscitator with a one-way valve in a nine-month-old infant with neuromuscular weakness.( 4 ) Recently, Suri et al. reported two cases of pneumothorax (possibly resulting from volutrauma) associated with daily use of mechanical insufflation-exsufflation via a cough-assist device and NIV. Although one of those patients resumed using the cough-assist device without recurrence of pneumothorax, the second patient died of acute-on-chronic congestive heart failure after re-expansion of the collapsed lung.( 5 )
Our patient used neither a one-way valve for air stacking nor a cough-assist device for maximum lung insufflation. Her symptoms of pain and dyspnea occurring during air stacking suggest a causal connection. A pneumothorax suggests a pleural (i.e., peripheral alveolar) lesion, a bleb, or an emphysematous bulla (Figure 1). It remains unknown how often milder forms of alveolar damage, such as pulmonary interstitial emphysema or subclinical pneumothorax, occur as a consequence of deep insufflation of the lungs. Although rare, the clinical course in our patient clearly shows that potentially life-threatening complications can develop. However, we still feel that, after having weighed the pros and cons at that time (in 2005), there were no reasons to withhold air stacking for deep lung insufflation in this patient. In fact, the patient met several of the criteria for being trained in the use of and using this LVR technique (Chart 1), which improved her PCF and might have prevented respiratory deterioration for a period of 3 years. A PCF > 2.7 L/s is considered minimal for airway secretion clearance, whereas a PCF > 4.5 L/s is needed in order to achieve such clearance during respiratory tract infections. In retrospect, this complication might have been aggravated by her stable bronchial asthma, which occasionally caused airflow limitation. However, even though the patient had asthma attacks, they were infrequent (only four hospitalizations in 18 years), and she did not meet the criteria for a diagnosis of COPD( 6 ); nor was she suffering from chronic asthma. In addition, since a pneumothorax would have been expected to induce a hypercapnic exacerbation in the presence of COPD, the fact that, following chest tube drainage, she immediately could be extubated and maintained spontaneous respiration, argues against concomitant airflow limitation. Furthermore, her DLCO was normal (after correction for her restricted alveolar ventilation-92% of the predicted value), which excludes parenchymatous pulmonary disease. Moreover, her former smoking habit and the use of NIV might have put her at risk for developing a pneumothorax, as may have her severe scoliosis. Presumably, the use of nocturnal NIV following the incident and the delay in seeking medical attention might have contributed to the severity of the clinical condition at presentation. Despite the use of nocturnal NIV oxygen was needed after pleurodesis to keep her sufficiently oxygenated during spontaneous breathing. The daytime hypercapnia noted nine months after discharge might have been caused by progressive restriction due to pleurodesis or the use of oxygen.
Although LVR is considered safe for individuals with normal lungs and the risk-benefit ratio of LVR, after thousands of patient-hours of use, is still favorable, this event raises concern as to whether the indiscriminate use of LVR is advisable in all patients with restrictive respiratory disease, especially in older patients, who might have an undiagnosed pulmonary pathology resulting in structural abnormalities. In addition, patients should be advised of the warning symptoms of possible complications of LVR in order to avoid a delay in seeking medical attention and to ensure immediate access to medical care.
We therefore recommend that patients with restrictive respiratory disorders who develop chest pain or backache with acute dyspnea during or following LVR be referred for urgent diagnostic evaluation to exclude pulmonary barotrauma. In certain cases, and depending on the medical history, diagnostic imaging of the chest should be considered prior to LVR, especially in elderly individuals and in those with known risk factors for a parenchymatous pulmonary pathology or with a history of pulmonary disease.
Acknowledgments
We would like to express our gratitude to Professor João Carlos Winck, MD, PhD, of Porto, Portugal, for his kind assistance in translating the abstract to Portuguese.
Footnotes
Patient management carried out at the Beatrix Hospital, Gorinchem, The Netherlands
A versão completa em português deste artigo está disponível em www.jornaldepneumologia.com.br
Financial support: None.
Contributor Information
Erik J.A. Westermann, University Medical Center Utrecht, Department of Internal Medicine and Dermatology, Utrecht, The Netherlands. Centre for Home Mechanical Ventilation Utrecht, Department of Internal Medicine and Dermatology, University Medical Center Utrecht, Utrecht, The Netherlands.
Maurice Jans, Beatrix Hospital, Department of Pulmonology, Gorinchem, The Netherlands. Department of Pulmonology, Rivas Zorggroep, Beatrix Hospital, Gorinchem, The Netherlands.
Michael A. Gaytant, University Medical Center Utrecht, Department of Internal Medicine and Dermatology, Utrecht, The Netherlands. Centre for Home Mechanical Ventilation Utrecht, Department of Internal Medicine and Dermatology, University Medical Center Utrecht, Utrecht, The Netherlands.
John R. Bach, University of Medicine and Dentistry of New Jersey, New Jersey Medical School, Newark, NJ, USA. Department of Physical Medicine and Rehabilitation, University Hospital, University of Medicine and Dentistry of New Jersey, New Jersey Medical School, Newark, NJ, USA.
Mike J. Kampelmacher, University Medical Center Utrecht, Department of Internal Medicine and Dermatology, Utrecht, The Netherlands. Centre for Home Mechanical Ventilation Utrecht, Department of Internal Medicine and Dermatology, University Medical Center Utrecht, Utrecht, The Netherlands.
References
- 1.Kang SW, Bach JR. Maximum insufflation capacity. Chest. 2000;118(1):61–65. doi: 10.1378/chest.118.1.61. http://dx.doi.org/10.1378/chest.118.1.61 [DOI] [PubMed] [Google Scholar]
- 2.Bach JR, Kang SW. Disorders of ventilation: weakness, stiffness, and mobilization. Chest. 2000;117(2):301–303. doi: 10.1378/chest.117.2.301. http://dx.doi.org/10.1378/chest.117.2.301 [DOI] [PubMed] [Google Scholar]
- 3.Bach JR, Bianchi C, Vidigal-Lopes M, Turi S, Felisari G. Lung inflation by glossopharyngeal breathing and "air stacking" in Duchenne muscular dystrophy. Am J Phys Med Rehabil. 2007;86(4):295–300. doi: 10.1097/PHM.0b013e318038d1ce. http://dx.doi.org/10.1097/PHM.0b013e318038d1ce [DOI] [PubMed] [Google Scholar]
- 4.Dwight P, Poenaru D. Duodenal perforation associated with breath stacking and annular pancreas. J Pediatr Surg. 2004;39(10):1593–1594. doi: 10.1016/j.jpedsurg.2004.06.029. http://dx.doi.org/10.1016/j.jpedsurg.2004.06.029 [DOI] [PubMed] [Google Scholar]
- 5.Suri P, Burns SP, Bach JR. Pneumothorax associated with mechanical insufflation-exsufflation and related factors. Am J Phys Med Rehabil. 2008;87(11):951–955. doi: 10.1097/PHM.0b013e31817c181e. http://dx.doi.org/10.1097/PHM.0b013e31817c181e [DOI] [PubMed] [Google Scholar]
- 6.Celli BR, MacNee W, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. Erratum in: Eur Respir J. 2006;27(1):242. http://dx.doi.org/10.1183/09031936.04.00014304 [DOI] [PubMed] [Google Scholar]
- 7.Toussaint M, Boitano LJ, Gathot V, Steens M, Soudon P. Limits of effective cough-augmentation techniques in patients with neuromuscular disease. Respir Care. 2009;54(3):359–66. [PubMed] [Google Scholar]
- 8.Bach JR, Mahajan K, Lipa B, Saporito L, Goncalves M, Komaroff E. Lung insufflation capacity in neuromuscular disease. Am J Phys Med Rehabil. 2008;87(9):720–725. doi: 10.1097/PHM.0b013e31817fb26f. http://dx.doi.org/10.1097/PHM.0b013e31817fb26f [DOI] [PubMed] [Google Scholar]
- 9.Sancho J, Servera E, Díaz J, Marín J. Predictors of ineffective cough during a chest infection in patients with stable amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 2007;175(12):1266–1271. doi: 10.1164/rccm.200612-1841OC. http://dx.doi.org/10.1164/rccm.200612-1841OC [DOI] [PubMed] [Google Scholar]
- 10.Tzeng AC, Bach JR. Prevention of pulmonary morbidity for patients with neuromuscular disease. Chest. 2000;118(5):1390–1396. doi: 10.1378/chest.118.5.1390. http://dx.doi.org/10.1378/chest.118.5.1390 [DOI] [PubMed] [Google Scholar]