In recent years, there has been an increasing interest in the use of ultrasound for the evaluation of chest diseases, especially for the study of bedridden, critically ill patients. In fact, the ultrasound method presents various advantages: it uses no radiation; it is inexpensive; it can be used at the bedside; it is noninvasive; and it can be repeated as necessary. In addition, ultrasound is starting to be a method used by professionals, other than radiologists, who have specific clinical questions,(1) having become an important tool for the pulmonary physician. In this context, the utility of ultrasound for the diagnosis and management of pleural effusion is well documented.
In the present issue of the Brazilian Journal of Pulmonology, Perazzo et al.(2 ) present a randomized controlled trial aimed at assessing whether ultrasound-assisted thoracentesis, in contrast with a blinded method, would reduce the rate of pneumothorax. The authors also aimed to assess whether ultrasound improves the efficacy of the procedure (in terms of the number of successful fluid removal procedures and the amount of fluid removed). It is of note that, in that study, experienced operators performed both methods, following a standardized protocol, in order to focus attention on the influence of using ultrasound or not, and removed other factors that could be responsible for complications. For these purposes, 160 inpatients and outpatients with pleural effusion requiring pleural puncture were randomized into two groups. In the study group (comprising 80 patients), thoracentesis was performed with the use of ultrasound, whereas it was performed without ultrasound in the control group (also comprising 80 patients). In comparing the study and control groups, the authors observed that the former had a significantly lower pneumothorax rate (1.25% vs. 12.5%; p = 0.009; OR = 0.09), a higher number of patients with successful drainage (79/80 vs. 72/80), and a higher amount of fluid drained (mean ± SD: 960 ± 500 mL vs. 770 ± 480 mL). They concluded that the use of ultrasound during thoracentesis reduced the number of cases of pneumothorax and increased the efficiency of the procedure.
The findings of Perazzo et al.(2) corroborate data already described in the literature-thoracentesis involving the use of ultrasound is safer than is the blinded approach. Nevertheless, the study is interesting because it confirms the idea that ultrasound can provide advantages even to more experienced operators. In addition, the study is a randomized controlled trial, which increases the power of their findings.
Despite its utility, ultrasound presents some limitations. Soft tissue edema, subcutaneous emphysema, or obesity can reduce the quality of the images. We also think that physicians need adequate training in order to avoid misreading ultrasound images and, consequently, to avoid mistakes.
Ultrasound and diagnosis of pleural effusion
The first step in the evaluation of patients with suspected pleural effusion is to confirm the diagnosis, especially in the case of a white hemithorax on chest X-rays. Ultrasound is a useful method for these purposes because it allows the distinction between effusion and lung consolidations(3) and has a higher accuracy in detecting pleural effusion in comparison with bedside chest X-rays (93% vs. 47%).(4 ) In fact, chest X-rays can detect the presence of pleural effusion in patients in the orthostatic position only if the volume of the effusion is at least 200 mL,(5) and the sensitivity of this method decreases in the supine position, whereas ultrasound can detect effusions as small as 20 mL.(6)
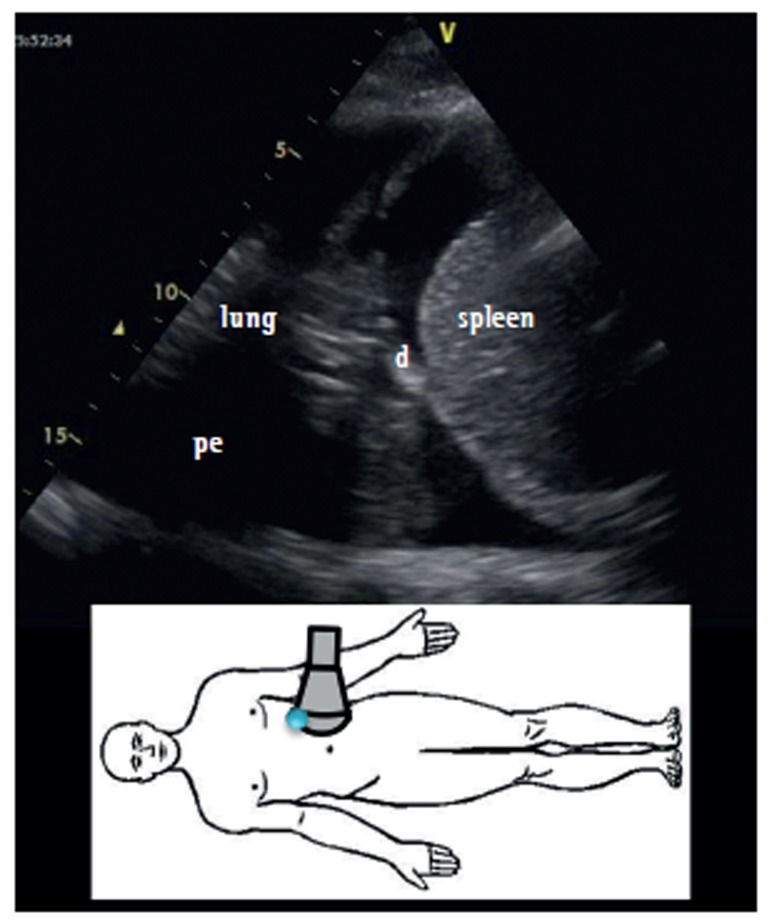
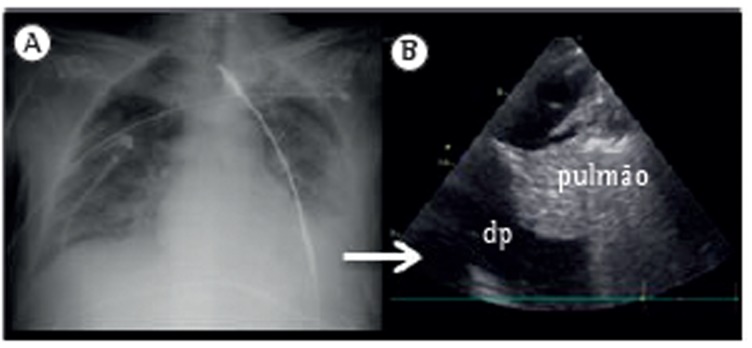
The ultrasound evaluation of a patient in a sitting position is better because it allows a more precise quantification of pleural effusion. In this position, the free fluid will collect in the dependent space, whereas it will be found in a posterior location with the patient in the supine position. In addition, ultrasound allows the identification of adjacent structures: chest wall, hemidiaphragm (over the liver or spleen), and visceral pleural surface. This is important, especially in the case of an invasive procedure, in order to avoid organ injury (Figure 1).
Figure 1. Ultrasound identification of pleural effusion at a specific site (lower image). pe: pleural effusion; d: diaphragm; and c: chest wall.
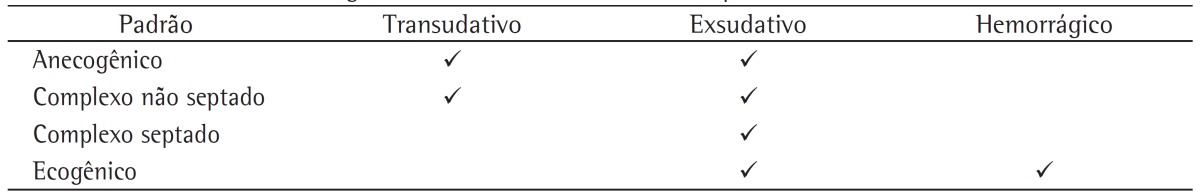
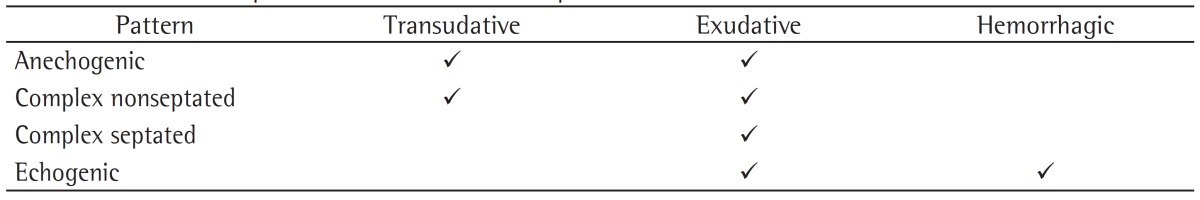
A second step is the distinction between transudative and exudative pleural effusions. The aspect of pleural effusion on ultrasound can suggest the nature of the fluid, although a definitive diagnosis requires a thoracentesis in order to allow physical, chemical, and microbiological studies. According to the characteristics of the pleural effusion on ultrasound, it can appear as anechoic (black), complex nonseptated (black with white strands), complex septated (black with white septa), or homogeneously echogenic (white).(7) In general, the presence of complex pleural effusion suggests exudative effusion, whereas an anechogenic effusion might be transudative. However, in contrast to what we expect, transudative effusion can also appear as complex nonseptated effusion(8); this is due to the fact that transudates are not pure water, having various components (i.e., cells, proteins, and lipids), and exudative effusions can also appear as anechogenic effusion. Homogeneous echogenic effusions are the result of hemorrhagic effusions or empyema (Table 1).
Table 1. Ultrasound patterns and the nature of pleural effusion.
In some cases, ultrasound images other than those of the effusion can help assess the nature of the pleural effusion. For example, the presence of thickened pleura or of a pulmonary consolidation with dynamic air bronchogram (suggestive of an infectious origin) is usually indicative of an exudate. The presence of a diffuse sign of lung congestion (B lines) suggests transudative effusion during heart failure.
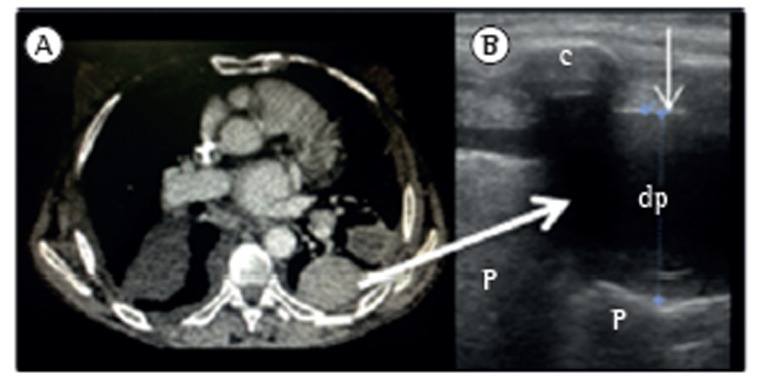
Laing & Filly(9) reported that nearly 20% of the anechogenic images of the pleura revealed a solid lesion, not the presence of fluid. Therefore, especially in cases of small or loculated pleural effusion (Figure 2), or when thoracentesis is requested, it is important to focus on the differential diagnosis. One aspect that can facilitate the diagnosis is that pleural effusions are associated with a typical movement of the adjacent structure that determines a change in the shape of the effusion-the movement of the collapsed lung into the effusion or that of particles inside the fluid. The use of the M mode can help in the visualization of the sinusoidal movement of the collapsed lung in the fluid (sinusoid sign).(10) However, very dense or loculated pleural effusions might present no variation in the shape.
Figure 2. CT (in A) and ultrasound (in B) revealing loculated pleural effusion. pe: pleural effusion; L:lung; and r: rib. The thin arrow indicates the parietal pleural line.
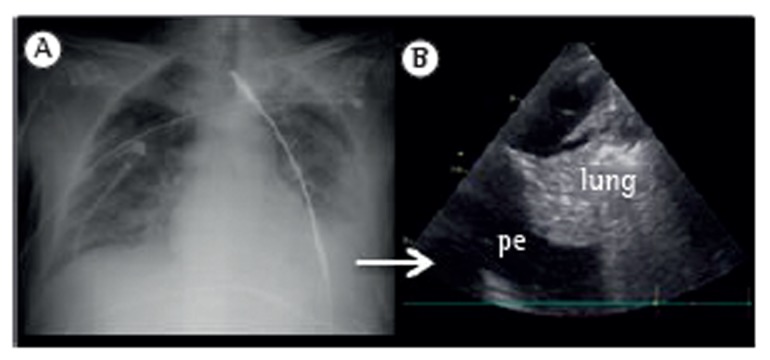
Although various ultrasound methods have been described for the quantification of the volume of pleural effusions,(11) they all require several measurements. We believe that knowledge of the exact amount of fluid has limited usefulness in clinical practice. Therefore, we prefer a qualitative approach, which is summarized in Table 2. In addition, ultrasound can help estimate the effect of pleural effusion on the lung parenchyma by enabling the visualization of different degrees of collapse. This information, combined with clinical judgment, can help physicians in the decision-making process regarding thoracentesis (Figure 3).
Table 2. Ultrasound quantification of pleural effusion.
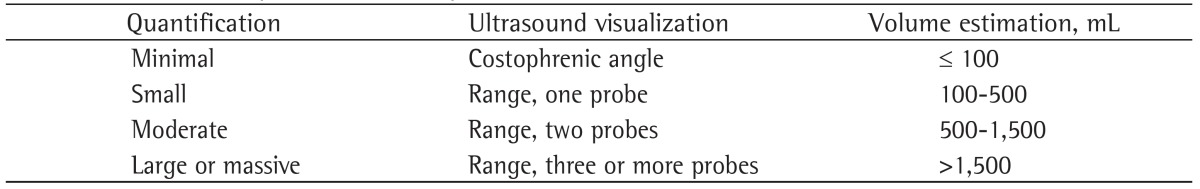
Figure 3. X-ray (in A) and ultrasound (in B) revealing pleural effusion and lung collapse. pe: pleural effusion.
Ultrasound and thoracentesis
The use of ultrasound in thoracentesis reduces the rate of complications (i.e., pneumothorax) and increases the successfulness of fluid removal when compared with traditional methods.(12) Ultrasound is especially useful when the pleural effusion is small or loculated.
Ultrasound allows the identification of the best site to perform the puncture and the measurement of the depth of the adjacent organs in order to avoid organ injury. For experts, ultrasound allows the study of the intercostal spaces prior to needle insertion, in order to identify aberrantly positioned intercostal vessels, thus avoiding vascular injury.
On ultrasound images, the appearance of pleural effusion can also provide clues to the necessary intervention: for example, a complex septated effusion could require the use of a larger catheter. There are two different techniques employed in thoracentesis with the use of ultrasound: the landmark-based method, in which ultrasound is used in order to identify the best site of the puncture; and the ultrasound-guided method, in which the procedure is closely monitored in real time by continuous visualization of the needle. This second method requires the involvement of a professional who is more experienced in the use of ultrasound.
Ultrasound and pneumothorax
The use of ultrasound reduces the risk of pneumothorax following thoracocentesis from 18% to 3%.(13). As shown in one retrospective study,(13) that is especially true when the ultrasound-guided method is used, the rates of pneumothorax being significantly lower than when the landmark-based method is used (4% vs. 10%). In addition, Weingardt et al.(14) demonstrated that ultrasound can be an effective rescue method in 88% of cases in which blind thoracocentesis is unsuccessful. The authors noted that, in 69% of those cases, the site of puncture chosen in the blind approach was below the diaphragm. Interestingly, ultrasound-guided thoracentesis resulted safe for use in mechanically ventilated patients as well.(15)
Ultrasound is also a more useful method to detect pneumothorax after thoracentesis than are chest X-rays using a supine anterior approach. The sensitivity of these two methods is 78.6% and 39.8%, respectively, whereas their specificity is 98.4% and 99.3%, respectively.(16)
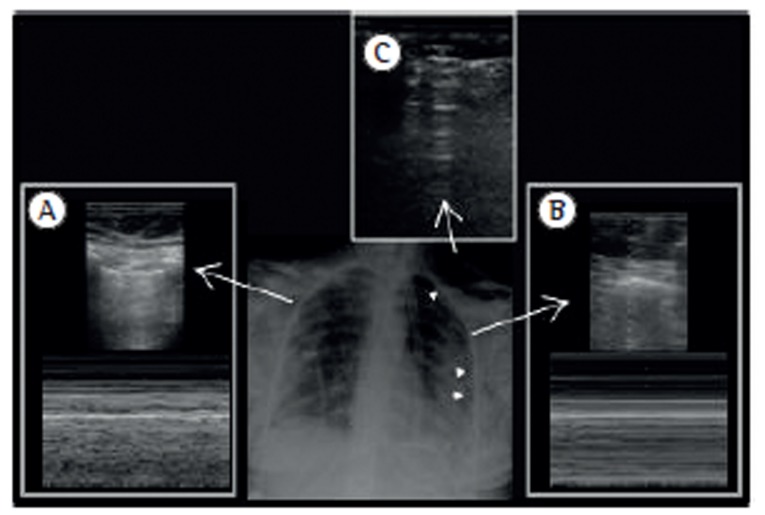
As shown in Figure 4, the major ultrasonographic signs for the diagnosis of pneumothorax are the absence of lung sliding-movement of the pleura during respiratory excursion-which is more evident using the M mode with the stratosphere sign; the absence of B lines (negative predictive value: 100%); and the presence of the lung point (positive predictive value: 100%) in the absence of massive pneumothorax.
Figure 4. Ultrasound signs of pneumothorax. In A, normal lung, showing the seashore sign in M mode. In B, pneumothorax, showing the stratosphere sign in M mode. In C, subcutaneous emphysema. Arrowheads indicate the pneumothorax.
In summary, ultrasound represents a highly useful tool for the evaluation of patients with pleural effusion during the diagnostic phase and in combination with invasive procedures.
Footnotes
Dr. Prina is a research fellow supported by the long-term research fellowship program of the European Respiratory Society.
Contributor Information
Elena Prina, Department of Pulmonology, Heart Institute, University of São Paulo School of Medicine Hospital das Clínicas, São Paulo, Brazil, Institut Clínic del Tórax (ICT), Servei de Pneumologia, Hospital Clínic de Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Ciber de Enfermedades Respiratorias, Barcelona, Spain.
Antoni Torres, Institut Clínic del Tórax (ICT), Servei de Pneumologia, Hospital Clínic de Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Ciber de Enfermedades Respiratorias, Barcelona, Spain.
Carlos Roberto Ribeiro Carvalho, Heart Institute (InCor), Hospital das Clínicas, University of São Paulo School of Medicine, São Paulo, Brazil.
References
- 1.Koenig SJ, Narasimhan M, Mayo PH. Thoracic ultrasonography for the pulmonary specialist. Chest. 2011;140(5):1332–1341. doi: 10.1378/chest.11-0348. http://dx.doi.org/10.1378/chest.11-0348 [DOI] [PubMed] [Google Scholar]
- 2.Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6–12. doi: 10.1590/S1806-37132014000100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu CJ, Yang PC, Wu HD, Chang DB, Kuo SH, Luh KT. Ultrasound study in unilateral hemithorax opacification. Image comparison with computed tomography. Am Rev Respir Dis. 1993;147(2):430–434. doi: 10.1164/ajrccm/147.2.430. http://dx.doi.org/10.1164/ajrccm/147.2.430 [DOI] [PubMed] [Google Scholar]
- 4.Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9–15. doi: 10.1097/00000542-200401000-00006. http://dx.doi.org/10.1097/00000542-200401000-00006 [DOI] [PubMed] [Google Scholar]
- 5.Blackmore CC, Black WC, Dallas RV, Crow HC. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3(2):103–109. doi: 10.1016/s1076-6332(05)80373-3. http://dx.doi.org/10.1016/S1076-6332(05)80373-3 [DOI] [PubMed] [Google Scholar]
- 6.Rahman NM, Singanayagam A, Davies HE, Wrightson JM, Mishra EK, Lee YC, et al. Diagnostic accuracy, safety and utilisation of respiratory physician-delivered thoracic ultrasound. Thorax. 2010;65(5):449–453. doi: 10.1136/thx.2009.128496. http://dx.doi.org/10.1136/thx.2009.128496 [DOI] [PubMed] [Google Scholar]
- 7.Lomas DJ, Padley SG, Flower CD. The sonographic appearances of pleural fluid. Br J Radiol. 1993;66(787):619–624. doi: 10.1259/0007-1285-66-787-619. http://dx.doi.org/10.1259/0007-1285-66-787-619 [DOI] [PubMed] [Google Scholar]
- 8.Chen HJ, Tu CY, Ling SJ, Chen W, Chiu KL, Hsia TC, et al. Sonographic appearances in transudative pleural effusions: not always an anechoic pattern. Ultrasound Med Biol. 2008;34(3):362–369. doi: 10.1016/j.ultrasmedbio.2007.09.009. http://dx.doi.org/10.1016/j.ultrasmedbio.2007.09.009 [DOI] [PubMed] [Google Scholar]
- 9.Laing FC, Filly RA. Problems in the application of ultrasonography for the evaluation of pleural opacities. Radiology. 1978;126(1):211–214. doi: 10.1148/126.1.211. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1–1. doi: 10.1186/2110-5820-4-1. http://dx.doi.org/10.1186/2110-5820-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remérand F, Dellamonica J, Mao Z, Ferrari F, Bouhemad B, Jianxin Y, et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656–664. doi: 10.1007/s00134-010-1769-9. http://dx.doi.org/10.1007/s00134-010-1769-9 [DOI] [PubMed] [Google Scholar]
- 12.Diacon AH, Brutsche MH, Solèr M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436–441. doi: 10.1378/chest.123.2.436. http://dx.doi.org/10.1378/chest.123.2.436 [DOI] [PubMed] [Google Scholar]
- 13.Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442–446. doi: 10.1002/jcu.20163. http://dx.doi.org/10.1002/jcu.20163 [DOI] [PubMed] [Google Scholar]
- 14.Weingardt JP, Guico RR, Nemcek AA Jr, Li YP, Chiu ST. Ultrasound findings following failed, clinically directed thoracenteses. J Clin Ultrasound. 1994;22(7):419–426. doi: 10.1002/jcu.1870220702. http://dx.doi.org/10.1002/jcu.1870220702 [DOI] [PubMed] [Google Scholar]
- 15.Lichtenstein D, Hulot JS, Rabiller A, Tostivint I, Mezière G. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med. 1999;25(9):955–958. doi: 10.1007/s001340050988. http://dx.doi.org/10.1007/s001340050988 [DOI] [PubMed] [Google Scholar]
- 16.Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17(5):R208–R208. doi: 10.1186/cc13016. http://dx.doi.org/10.1186/cc13016 [DOI] [PMC free article] [PubMed] [Google Scholar]