Abstract
Purpose
To compare the in vitro effect of rose bengal and riboflavin as photosensitizing agents for photodynamic therapy (PDT) on fungal isolates that are common causes of fungal keratitis
Design
Experimental study
Methods
Three isolates (Fusarium solani, Aspergillus fumigatus, Candida albicans) recovered from patients with confirmed fungal keratitis were used in the experiments. Isolates were grown on Sabouraud-Dextrose agar, swabbed and prepared in suspension, and one milliliter aliquots were inoculated onto test plates in triplicate. Test plates were separated into 5 groups: Group 1 - no treatment, Group 2 - 0.1% rose bengal alone, Group 3 - 518 nm irradiation alone, Group 4 - riboflavin PDT (riboflavin + 375 nm irradiation), and Group 5 - rose bengal PDT (rose bengal + 518 nm irradiation). Irradiation was performed over a circular area using either a green LED array (peak wavelength: 518 nm) or a UV-A LED array (peak wavelength: 375 nm). Test plates were irradiated with an energy density of 5.4 J/cm2. Later, plates were placed in a 30° C incubator and observed for growth.
Results
Rose bengal-mediated PDT successfully inhibited the growth of all three fungal isolates in the irradiated area. All other groups exhibited unrestricted growth throughout the plate.
Conclusions
Rose bengal-mediated PDT successfully inhibited the growth of three types of fungi. No other experimental groups, including riboflavin-mediated PDT, had any inhibitory effect on the isolates. The results might be useful for the treatment of patients suffering from corneal infection.
INTRODUCTION
Fungal keratitis is a potentially blinding disease. Its incidence has been reported to be between 1-44% of all microbial keratitis cases depending on the geographic location.1-3 It is more common in tropical and subtropical geographic locations Fusarium is the most common isolate found globally in patients with fungal keratitis, followed by Aspergillus in tropical climates and Candida in temperate areas.4 No standard treatment has been established for these infections and treatment remains limited by scarcity of effective antifungal agents and poor penetration of existing medications. Resolution of the infections thus tends to be slow, usually lasting several months, with many cases requiring a therapeutic penetrating keratoplasty.5
Photodynamic therapy (PDT) involves the activation of a photosensitizing agent by light ranging from ultraviolet-A (UV-A) to near infrared wavelengths. The photosensitizer reaches an excited state that undergoes a reaction with ambient oxygen to create a reactive oxygen species (ROS). These ROS then react with intracellular components and produces cell inactivation and death.6 In the field of ophthalmology, PDT has been used for numerous applications including choroidal neovascularization in age-related macular degeneration, corneal neovascularization, and experimentally, using photosensitizer dihematoporphyrin ether (DHE, Photofin II) for tumor treatment (Chan YC, et al., IOVS 1990;31:Abstract 421), acanthaomoeba keratitis, and to prevent lens epithelial cell proliferation (Takesue Y, et al., SPIE 1993;Proc 1877), and corneal neovascularization.7-9
Recently, PDT has been proposed as an alternative approach for localized corneal infections. The studies regarding PDT for keratitis thus far have followed the collagen crosslinking (CXL) protocol using Riboflavin and UV-A irradiation for patients having keratoconus.10 In vitro studies of CXL have found this treatment to be effective against certain common bacteria strains but ineffective against Candida, Fusarium, and Acanthamoeba.11-17
Rose bengal is a dye routinely used in ophthalmology clinics to stain for defects and degeneration of the ocular surface epithelium.18 Recent studies have attempted to use rose bengal and green light for CXL and in other medical fields this technology has been used for PDT on Candida albicans in biofilms.19, 20 Therefore, our study was designed to assess in vitro efficacy of PDT using rose bengal and riboflavin as photosensitizing agents on 3 types of fungi: Fusarium solani, Aspergillus fumigatus, and Candida albicans.
MATERIALS AND METHODS
Preparation of Fungal isolates
All fungal isolates (Fusarium solani, Aspergillus fumigatus, Candida albicans) were isolated from the corneal scrapings of patients diagnosed with a fungal keratitis at Bascom Palmer Eye Institute, Miami, FL. All isolates were confirmed using traditional and phenotypic microbiology techniques. Spore inoculum was prepared as previous described by Aberkane et al with modification;21 spore suspensions were prepared by gently scraping 3 day old colonies grown on Sabouraud-Dextrose Emmons (Sab-Dex) agar plates (W20, Hardy Diagnostics, Santa Maria, CA) at 30°C in a non-CO2 incubator. Colonies were covered with 5 mL of sterile water, and spores collected by rubbing with a sterile cotton swab and transferring the suspension to a sterile 15 mL conical tube. Spore concentration and presence of hyphae or clumps were checked with an initial hematocytometer screen (Neubauer chamber). Suspensions were vortexed, and diluted, and placed in racks for 5 minutes to let hyphae and clumps settle. 10 microliters of the supernatant were loaded into a clean hemacytometer and all of the spores in each of the four 0.1 mm2 corner squares were counted. Spores touching the top, bottom, left and or right borders were not counted. Spore counts were determined by the equation cfu/mL = (n) × 104, where n = the average cell count per square of the four corner squares counted. Isolates were suspended in a sterile saline solution and the concentration adjusted to 103 cfu/mL with each photosensitizing agent. Sterile water was used to dilute the organisms to 103 in the control run.
The final concentrations used in the experiments for each of the organisms were: Fusarium solani - 5.7 × 103 cfu/mL, Aspergillus fumigatus - 5.4 × 103 cfu/mL, Candida albicans – 3.8 × 103 cfu/mL.
Light source & Irradiation
A custom-built LED source was fabricated with two irradiation heads: Green (518 nm) and UV-A (375 nm). (Figure 1) Each irradiation head was assembled using an array of twenty-four LEDs. The green LED source (L1-0-G5TH45-1, LEDSupply, Randolph, VT, USA) had a 518 nm peak irradiance (I40%: 500-541 nm) and produced 2.2mW/cm2 over a surface of 28.3 cm2. The UV-A source used LEDs with a peak wavelength of 375 nm (I40%: 370-383 nm) producing an irradiance of 2.91 mW/cm2 on a surface of 13.8 cm2. The spectra were measured using a spectrometer (SM442, Spectral Products, Putnam, CT, USA) and the irradiances were measured with an optical power meter (Model 1916C, Newport, Irvine, CA, USA).
Figure 1.
Ophthalmic Biophysics Center Photodynamic Therapy Irradiating System (Left) Image of the Irradiating System including the UV-A LED array (arrow) and green LED array (asterisk) heads as well as the camera (triangle) to take images for analysis. (Top Right) Green irradiating head turned on. The agar plate lies on a rising stage which is moved to place the light at 1 cm. The clear tubing is connected to the vacuum for heat dissipation. (Bottom Right) Diagram of the LED array used to make the irradiation head.
Preparation of the Photosensitizing Agents
The 0.1% rose bengal solution was produced by dissolving 100 mg of rose bengal (198250, Sigma-Aldrich, St. Louis, MO, USA) in 100 mL of sterile water. Similarly, the 0.1% riboflavin solution was made by dissolving 100 mg of riboflavin (R7774, Sigma-Aldrich, St. Louis, MO, USA) in 100 mL of sterile water. Solutions were made at room temperature immediately before experimentation and kept in the dark until irradiation to ensure that photobleaching of the solutions did not occur.
Experimental Protocol
The plates were divided into five groups according to the treatment group: Group 1 - no treatment, Group 2 - 0.1% rose bengal only, Group 3 - 518 nm irradiation only, Group 4 – riboflavin PDT (0.1% riboflavin + 375 nm irradiation), and Group 5 – rose bengal PDT (RB PDT) (0.1% rose bengal + 518 nm irradiation). Separate groups testing 0.1% riboflavin and 375 nm against the fungi were not included in the experimental design.
Groups 1 and 2 received no irradiation. For the irradiated experiments, the Ophthalmic Biophysics Center’s (OBC) irradiating source prototypes were used; Groups 3 and 5 were irradiated with the 518 nm source and Group 4 was irradiated using the 375 nm irradiating source. The study was conducted in triplicate under aseptic conditions. One milliliter of each fungal suspension was inoculated onto a 100 mm diameter Sabouraud-Dextrose agar plate and allowed to diffuse evenly over the entire plate. The agar plates were placed at 1 cm from the irradiator heads (Figure 1) and subject to an energy density of 5.4 J/cm2 (30 minutes of irradiation for the 375 nm source and 40 minutes for the 518 nm sources using the irradiator prototypes), corresponding to the value defined by the Dresden protocol for clinical cornea crosslinking.10 Using a digital 2K thermocouple (DM6802A+, MN Measurement Instruments LLC, St Paul, MN, USA), the temperature of the plate was measured for each sources. The rise in temperature after a full 5.4 J/cm2 exposure was of only 9° C. After the irradiation treatment the last step of the protocol was to seal the plates and to put them into an incubator (ThermoFisher Scientific, Waltham, MA, USA) at 30°C.
Labview Program for Fungal Viability Assessment
Images of the agar plates were taken at each 24 hour checkpoint with a digital camera (Nikon D7000, Nikon Inc., USA) and processed using a custom-made program written in LabVIEW 6 (National Instruments, Austin, TX, USA) to determine the percent growth of the fungus on the irradiated area of each plate. Each image has a resolution of 13.5 (±0.5) pixels/mm and was calibrated using the standard dimensions of the 100 mm diameter agar plate. Following calibration, the irradiation zone of each image was extracted and segmented by application of a threshold based on hue, saturation, and luminance. Inner diameters of fifty millimeters for the 518 nm and forty-two millimeters for the 375 nm for UV-A of each plate were analyzed because they correspond to the irradiation zone of the OBC sources. The total area of the growth inhibition was then divided by the area of the irradiation zone to determine the percent inhibition.
Statistics
A one-tailed 2-sample z-test for proportions was performed to compare the percent growth of each experimental group with respect to the control for each organism. Statistical significance was set at p<0.05.
RESULTS
For F. solani, the growth of the organisms was visible beginning at Day 2. At day 3 they reached the peak of growth. Group 5 was the only group exhibiting statistically significant inhibition (p<0.049). Beginning at Day 3, the organism started growing from the area outside of the irradiation zone into the region of interest for Group 5. However for all other groups, there was uniform growth throughout the entire surface of the agar plates. The findings for Day 0 and Day 3 are shown in Figure 2.
Figure 2.

Assessment of rose bengal vs. riboflavin photodynamic therapy for the inhibition of Fusarium solani keratitis isolates. (Top), (Bottom). Results shown at Day 0 and Day 3. Maximum confluency is seen at Day 3 with only the rose bengal-mediated PDT group (Group 5) showing growth inhibition. (First Column) Group 1, (Second Column) Group 2, (Third Column) Group 3, (Fourth Column) Group 4, (Fifth Column) Group 5.
Similarly, A. fumigatus organisms were visible beginning at Day 2. At Day 3 they reached the peak of growth. Group 5 was the only group exhibiting statistically significant inhibition (p<0.049). Beginning at Day 3, the organism started growing from the area outside of the irradiation zone into the region of interest for Group 5. For all other groups, there was uniform growth throughout the entire surface of the agar plates. The findings for Day 0 and Day 3 are shown in Figure 3.
Figure 3.
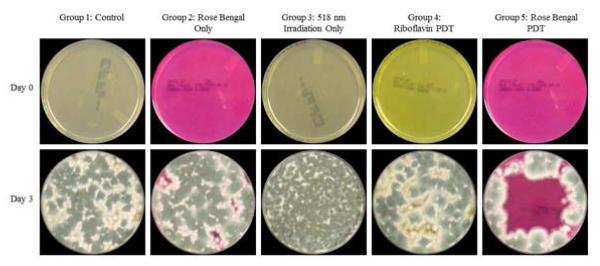
Assessment of rose bengal vs. riboflavin photodynamic therapy for the inhibition of Aspergillus fumigatus keratitis isolates. (Top), (Bottom). Results shown at Day 0 and Day 3. Maximum confluency is seen at Day 3 with only the rose bengal-mediated PDT group (Group 5) showing growth inhibition. (First Column) Group 1, (Second Column) Group 2, (Third Column) Group 3, (Fourth Column) Group 4, (Fifth Column) Group 5.
C. albicans also began growing Day 2. At Day 3 it reached the peak of growth. Group 5 was the only group exhibiting statistically significant inhibition (p<0.025). For all groups other than Group 5, there was uniform growth throughout the entire surface of the agar plates. For the rose bengal-mediated PDT group there was no growth from the outside area into the irradiation zone. The findings for Day 0 and Day 3 are shown in Figure 4.
Figure 4.
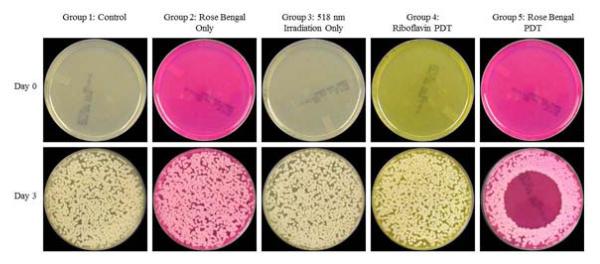
Assessment of rose bengal vs. riboflavin photodynamic therapy for the inhibition of Candida albicans keratitis isolates. (Top), (Bottom). Results shown at Day 0 and Day 3. Maximum confluency is seen at Day 3 with only the rose bengal-mediated PDT group (Group 5) showing growth inhibition. (First Column) Group 1, (Second Column) Group 2, (Third Column) Group 3, (Fourth Column) Group 4, (Fifth Column) Group 5.
The average percent inhibition of growth for each of the organisms according to the applied treatment (Group 1 to 5) is shown in Table 1. Similar findings were seen in each of the three replicates for all experimental conditions, as can be observed in the representative photographs in Figures 2-4.
Table 1.
Growth Inhibition Percentage of Fungal Keratitis Isolates after Photodynamic Therapy
| Day 3 |
|||||
|---|---|---|---|---|---|
| Organisms | Rose bengal + 518 nm irradiation |
Riboflavin + 375 nm irradiation |
Rose bengal only |
518 nm irradiation only |
Control |
|
F. solani (Inhibited % ± SD) |
78.2±2.1 | 0 | 7.3±1.1 | 6.8±9.3 | 9.8±4.7 |
|
A. fumigatus (Inhibited % ± SD) |
79.8±9 | 3.5±0.8 | 6.6±0.9 | 0.6±0.2 | 0 |
|
C. albicans (Inhibited % ± SD) |
95.6±3 | 35.3±10 | 42.2±3.7 | 35.6±6.7 | 24.2±2.6 |
Growth inhibition expressed in % (mean ± standard deviation) measured by the Labview program on triplicate agar plates of the three fungal isolates. The treatment groups were rose bengal-mediated photodynamic therapy (PDT) (rose bengal + 518 nm irradiation), riboflavin-mediated PDT (Riboflavin + 375 nm irradiation), rose bengal only, 518 nm irradiation only, and the control group (fungus only).
DISCUSSION
In this study, rose bengal and green light PDT was the only effective intervention to inhibit the growth of all the fungal isolates tested in vitro: C. albicans, F. solani and A. fumigatus. In the rose bengal-mediated PDT group, there was a minimum zone of growth inhibition for all the fungal isolates of 78% on the Sab-Dex agar plates. There was no significant inhibition in any other groups (Table 1). This finding suggests that growth inhibition is caused by the photoactivation of rose bengal by 518 nm irradiation; not by any chemotherapeutic effect of the rose bengal dye or phototoxicity of the 518 nm irradiation. Our results also showed that riboflavin-mediated PDT is not an effective treatment for these fungal species. To verify that an anti- synergetic effect was not at work, separate groups testing of the effect of riboflavin alone and the use of 375 nm irradiation alone were subsequently performed. These groups showed no inhibition of the fungal isolates.
Past studies have shown the successful use of PDT on organisms including viruses, Gram (+), Gram (-) and drug-resistant bacteria, molds, and yeasts.22-28 Rose bengal is one of the photosensitizing agents used to conduct PDT for these microorganisms.20, 29-32 Costa et al studied the effect of rose bengal- and erythrosine-mediated PDT on C. albicans for treatment of oral infections. They conducted PDT on clinical and standard strains of planktonic C. albicans cultures and showed a 1.97 log10 reduction in concentration using erythrosin B. They also tested the effect of PDT on biofilms and achieved a 0.15 log10 decrease with rose bengal. Demidova and Hamblin studied the effect of rose bengal, toluidine blue O, and a poly-L-lysine chlorine(e6) conjugate on E. coli, S. aureus, and C. albicans. They showed that for rose bengal, the order of susceptibility of the organisms to PDT was S. aureus, E. coli, and followed by C. albicans. When concentrations of 107 and 106 organisms/mL were used, 4 log10 and 6 log10 of killing were achieved respectively.32 Similarly, in our study we found the growth of C. albicans inhibited after rose bengal PDT. We also observed the same effect for F. solani and A. fumigatus which has not been reported in the literature thus far.
In vitro and clinical studies of therapeutic UV-A CXL have found this treatment to be effective against both Gram (+) and Gram (-) bacteria such as Staphylococcus aureus, Staphylococcus epidermidis and Pseudomonas aeruginosa but ineffective when used without medication against Candida, Fusarium and Acanthamoeba.11-15, 33 Martins et al showed that riboflavin activated by UV-A is an effective inhibitor of numerous bacterial strains but did not show effective inhibition for Candida albicans, even when increasing the concentration of riboflavin was increased from 0.1% to 0.5%. Kashiwabuchi et al further demonstrated that riboflavin + UV-A had no effect on F. solani or C. albicans. In their experiments, the control group, riboflavin only group, UV-A only group, and riboflavin + UV-A group all had the same amount of viability after treatment.11, 12 Our results are consistent with those of previous studies showing that the combined application of riboflavin and UV-A irradiation was ineffective in inhibiting the growth of fungi.
Safety to the cornea and limbal cellular components area are an important aspect to study with these procedures. Recently, Cherfan et al have reported that rose bengal and green light can be used to create crosslinks between the stromal lamellae and increase corneal stiffness in corneas of rabbit eyes with a uniaxial stretcher and by Brillouin microscopy.19 They showed that there was no detrimental effect to the keratocytes which is commonly seen with riboflavin UV-A CXL.
There are two basic mechanisms associated with photodynamic inactivation (PDI) that have been proposed to account for the lethal damage caused to microorganisms by PDT: damage to the DNA and damage to the cytoplasmic membrane, allowing leakage of cellular contents or inactivation of membrane transport systems and enzymes.34 We do not know at present why we see significant results with rose bengal and 518 nm irradiation but none with riboflavin and UVA for fungal isolates. We presume that this difference is related to the variation in the absorption of rose bengal versus riboflavin by the various fungi but this needs to be further investigated. Despite the advantage that the cornea is easily accessible by topical drops and light exposure, little work has been reported so far regarding PDT for corneal infections. The results of this study demonstrate that in an in vitro environment rose bengal-mediated PDT with 518 nm irradiation is extremely effective against the clinical fungal isolates tested. This suggests that this may be a possible option to treat fungal corneal infections in patients, though further experiments need to be performed to assess the efficacy and safety. In past studies, the observed phototoxicity was sensitizer, light and oxygen-dependent and was therefore considered a photodynamic effect. Therefore, experiments need to be conducted to find the ideal parameters for the rose bengal-mediated PDT of fungal isolates. For this purpose, LEDs that are ten times more powerful have been selected to fabricate a new irradiator. Furthermore, since results obtained in vitro do not always correlate with clinical efficacy, further in vivo studies on animal models are under way to test the efficacy of this treatment for fungal keratitis, so as to evolve an optimal and safe protocol for PDT in patients.
ACKNOWLEDGEMENTS
Funding/Support: Florida Lions Eye Bank; Drs. KR Olsen and ME Hildebrandt, NIH P30EY14801 (Center Grant); an unrestricted grant from Research to Prevent Blindness and the Henri and Flore Lesieur Foundation (JMP)
Financial Disclosures: A.A, D.M., F.C., M.T., M.C.A., K.A., G.A: none, J-M.P.: NIH, DoD, Croma GmbH, InnFocus LLC, EyeGatePharma, Australian Government Cooperative Research Centre scheme (Vision CRC, Sydney), S.H.Y: Alcon, Allegan, Optimedia, Carl Zeiss Meditec, Transcend Medical, Bausch & Lomb,
Contributions of Authors: Design of the study (A.A., D.M., G.A., S.H.Y., J-M.P.); management and conduct of the study and collection of the data (A.A., D.M., F.C., M.T., M.C.A, K.A.); analysis of data (A.A, D.M., F.C., M.T., M.C.A, K.A., G.A., S.H.Y., J-M.P.); preparation of the manuscript (A.A., F.C., M.T., J-M.P.); and review and approval of the manuscript (A.A, D.M., F.C., M.T., M.C.A, K.A., G.A., S.H.Y., J-M.P.).
The authors would like to acknowledge the technical and scientific contributions of the following members of the Ophthalmic Biophysics Center (Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, FL, USA) team: Cornelis Rowaan, Izuru Nose, and William Lee for the fabrication of the UV-A irradiator, Cornelis Rowaan for his help fabricating the 518 nm irradiator, and Esteban Perez and Francisco Halili Jr for their assistance in the preparation and conduction of the experiments.
Biography
 Alejandro Arboleda is a Graduate Research Assistant at the Ophthalmic Biophysics Center of Bascom Palmer Eye Institute. He is a BS/MS Biomedical Engineering Candidate, graduating May 2014, at the University of Miami. During his undergraduate education he received the Florida-Georgia Louis Stokes Alliance for Minority Participation Scholarship. During his period at the OBC he has focused on research involving cataract surgery, drug delivery for corneal crosslinking, and photodynamic therapy for infectious keratitis.
Alejandro Arboleda is a Graduate Research Assistant at the Ophthalmic Biophysics Center of Bascom Palmer Eye Institute. He is a BS/MS Biomedical Engineering Candidate, graduating May 2014, at the University of Miami. During his undergraduate education he received the Florida-Georgia Louis Stokes Alliance for Minority Participation Scholarship. During his period at the OBC he has focused on research involving cataract surgery, drug delivery for corneal crosslinking, and photodynamic therapy for infectious keratitis.
 Jean-Marie Parel, Ph.D. is the Henri and Flore Lesieur Chair in Ophthalmology and Director of the Ophthalmic Biophysics Center at the Bascom Palmer Eye Institute. Over the past 40 years, he has been involved in the advancement and translation of innovative technology to improve patient care. During this time, he has pioneered research projects focusing on Phaco-Ersatz, Coulomb-Controlled Iontophoresis, automated vitreoretinal surgery instrumentation, keratoprosthesis, glaucoma implants, controlled drug-release implants, photodynamic therapy, and SD-OCT imaging systems.
Jean-Marie Parel, Ph.D. is the Henri and Flore Lesieur Chair in Ophthalmology and Director of the Ophthalmic Biophysics Center at the Bascom Palmer Eye Institute. Over the past 40 years, he has been involved in the advancement and translation of innovative technology to improve patient care. During this time, he has pioneered research projects focusing on Phaco-Ersatz, Coulomb-Controlled Iontophoresis, automated vitreoretinal surgery instrumentation, keratoprosthesis, glaucoma implants, controlled drug-release implants, photodynamic therapy, and SD-OCT imaging systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gower EW, Keay LJ, Oechsler RA, et al. Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology. 2010;117(12):2263–2267. doi: 10.1016/j.ophtha.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 2.Garg P. Fungal, Mycobacterial, and Nocardia infections and the eye: an update. Eye (Lond) 2012;26(2):245–251. doi: 10.1038/eye.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea. 2002;21(6):555–559. doi: 10.1097/00003226-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Galarreta DJ, Tuft SJ, Ramsay A, Dart JK. Fungal keratitis in London: microbiological and clinical evaluation. Cornea. 2007;26(9):1082–1086. doi: 10.1097/ICO.0b013e318142bff3. [DOI] [PubMed] [Google Scholar]
- 5.Chang HY, Chodosh J. Diagnostic and therapeutic considerations in fungal keratitis. Int Ophthalmol Clin. 2011;51(4):33–42. doi: 10.1097/IIO.0b013e31822d64dc. [DOI] [PubMed] [Google Scholar]
- 6.Dai T, Fuchs BB, Coleman JJ, et al. Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front Microbiol. 2012;3:120. doi: 10.3389/fmicb.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Bergh H. Photodynamic therapy of age-related mcular degeneration: history and principles. Sem Ophthalmol. 2001;16(4):181–200. doi: 10.1076/soph.16.4.181.10299. [DOI] [PubMed] [Google Scholar]
- 8.Sheppard JD, Epstein RJ, Lattanzio FA, Marcantonio D, Williams PB. Argon Laser Photodynamic Therapy of Human Corneal Neovascularization After Intravenous Administration of Dihematoporphyrin Ether. Am J Ophthalmol. 2006;141(3):524–529. doi: 10.1016/j.ajo.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Lingua RW. Photodyamic therapy fo ocular tumors. J Photochem Photobiol. 1991;9:119–122. doi: 10.1016/1011-1344(91)80010-f. [DOI] [PubMed] [Google Scholar]
- 10.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a–induced collagen crosslinking for the treatment of keratoconus. American Journal of Ophthalmology. 2003;135(5):620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 11.Martins SA, Combs JC, Noguera G, et al. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci Aug. 2008;49(8):3402–3408. doi: 10.1167/iovs.07-1592. [DOI] [PubMed] [Google Scholar]
- 12.Kashiwabuchi RT, Carvalho FR, Khan YA, Hirai F, Campos MS, McDonnell PJ. Assessment of fungal viability after long-wave ultraviolet light irradiation combined with riboflavin administration. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):521–527. doi: 10.1007/s00417-012-2209-z. [DOI] [PubMed] [Google Scholar]
- 13.del Buey MA, Cristobal JA, Casas P, et al. Evaluation of in vitro efficacy of combined riboflavin and ultraviolet a for Acanthamoeba isolates. Am J Ophthalmol. 2012;153(3):399–404. doi: 10.1016/j.ajo.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Makdoumi K, Mortensen J, Sorkhabi O, Malmvall BE, Crafoord S. UVA-riboflavin photochemical therapy of bacterial keratitis: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2012;250(1):95–102. doi: 10.1007/s00417-011-1754-1. [DOI] [PubMed] [Google Scholar]
- 15.Price MO, Tenkman LR, Schrier A, Fairchild KM, Trokel SL, Price FW., Jr. Photoactivated riboflavin treatment of infectious keratitis using collagen cross-linking technology. J Refract Surg. 2012;28(10):706–713. doi: 10.3928/1081597X-20120921-06. [DOI] [PubMed] [Google Scholar]
- 16.Khan YA, Kashiwabuchi RT, Martins SA, et al. Riboflavin and Ultraviolet Light A Therapy as an Adjuvant Treatment for Medically Refractive Acanthamoeba Keratitis. Ophthalmology. 2011;118(2):324–331. doi: 10.1016/j.ophtha.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 17.St Denis TG, Dai T, Izikson L, et al. All you need is light: antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence. 2011;2(6):509–520. doi: 10.4161/viru.2.6.17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feenstra RP, Tseng SG. What is Actually Stained by Rose Bengal? Arch Ophthalmol. 1992;110(7):984–993. doi: 10.1001/archopht.1992.01080190090035. [DOI] [PubMed] [Google Scholar]
- 19.Cherfan D, Eri Verter E, Melki S, et al. Collagen Cross-Linking Using Rose Bengal and Green Light to Increase Corneal Stiffness. Invest Ophthalmol Vis Sci. 2013;54(5):3426–3433. doi: 10.1167/iovs.12-11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa ACBP, Rasteiro VMC, Pereira CA, Rossoni RD, Junqueira JC, Jorge AOC. The effects of rose bengal- and erythrosine-mediated photodynamic therapy on Candida albicans. Mycoses. 2012;55(1):56–63. doi: 10.1111/j.1439-0507.2011.02042.x. [DOI] [PubMed] [Google Scholar]
- 21.Aberkane A, Cuenca-Estrella M, Gomez-Lopez A, et al. Comparative evaluation of two different methods of inoculum preparation for antifungal susceptibility testing of filamentous fungi. Journal of Antimicrob Chemother. 2002;50(5) doi: 10.1093/jac/dkf187. [DOI] [PubMed] [Google Scholar]
- 22.Martin JP, Logsdon N. The role of oxygen radicals in dye-mediated photodynamic effects in Escherichia coli B. J Biol Chem. 1987;262(15):7213–7219. [PubMed] [Google Scholar]
- 23.Wainwright M, Phoenix DA, Laycock SL, Wareing DR, Wright PA. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol Lett. 1998;160(2):177–181. doi: 10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- 24.Wilson M, Yianni C. Killing of methicillin-resistant Staphylococcus aureus by low-power laser light. J Med Microbiol. 1995;42(1):62–66. doi: 10.1099/00222615-42-1-62. [DOI] [PubMed] [Google Scholar]
- 25.Santus R, Grellier P, Schrevel J, Maziere JC, Stoltz JF. Photodecontamination of blood components: advantages and drawbacks. Clin Hemorheol Microcirc. 1998;18(4):299–308. [PubMed] [Google Scholar]
- 26.Lenard J, Rabson A, Vanderoef R. Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: inhibition of fusion and syncytia formation. Proc Natl Acad Sci U S A. 1993;90(1):158–162. doi: 10.1073/pnas.90.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paardekooper M, Van Gompel AE, Van Steveninck J, Van den Broek PJ. The effect of photodynamic treatment of yeast with the sensitizer chloroaluminum phthalocyanine on various cellular parameters. Photochem Photobiol. 1995;62(3):561–567. doi: 10.1111/j.1751-1097.1995.tb02385.x. [DOI] [PubMed] [Google Scholar]
- 28.Friedberg JS, Skema C, Baum ED, et al. In vitro effects of photodynamic therapy on Aspergillus fumigatus. J Antimicrob Chemother. 2001;48(1):105–107. doi: 10.1093/jac/48.1.105. [DOI] [PubMed] [Google Scholar]
- 29.Banks JG, Board RG, Carter J, Dodge AD. The cytotoxic and photodynamic inactivation of micro-organisms by Rose Bengal. J Appl Bacteriol. 1985;58(4):391–400. doi: 10.1111/j.1365-2672.1985.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 30.Lazarova G, Tashiro H. Protective effect of amphotericin B against lethal photodynamic treatment in yeast. Microbios. 1995;82(332):187–196. [PubMed] [Google Scholar]
- 31.Lazarova G. Effect of glutathione on rose bengal photosensitized yeast damage. Microbios. 1993;75(302):39–43. [PubMed] [Google Scholar]
- 32.Demidova TN, Hamblin MR. Effect of Cell-Photosensitizer Binding and Cell Density on Microbial Photoinactivation. Antimicrob. Agents Chemother. 2005;49(6) doi: 10.1128/AAC.49.6.2329-2335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makdoumi K, Backman A, Mortensen J, Crafoord S. Evaluation of antibacterial efficacy of photo-activated riboflavin using ultraviolet light (UVA) Graefes Arch Clin Exp Ophthalmol. 2010;248(2):207–212. doi: 10.1007/s00417-009-1231-2. [DOI] [PubMed] [Google Scholar]
- 34.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3(5):436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]



