Abstract
Purpose
The normal gliding environment in the carpal tunnel is complex. The median nerve and flexor tendons are surrounded by a multilayered subsynovial tissue. To date, observations of the relative motions of the flexor tendon, median nerve and multilayered subsynovial tissue have been through a surgically released open carpal tunnel. The purpose of this study was to compare the motions of these tissues in an intact and open carpal tunnel.
Method
We measured the relative motion of the middle finger flexor digitorum superficialis (FDS) tendon, its surrounding subsynovial connective tissue (SSCT) and the median nerve in eight human cadavers. The flexor retinaculum was used as a fixed reference point. The motions were compared for simulated isolated middle finger and simulated fist motion as measured fluoroscopically in the closed carpal tunnel and directly in the open carpal tunnel.
Results
While the simulated isolated finger motion produced significantly less SSCT and median nerve motion (p<0.05), there was no difference in FDS, SSCT or nerve motion when comparing the fluoroscopic measurements in the closed carpal tunnel with the direct visual measurements in the open carpal tunnel.
Conclusion
Relative motion of the flexor tendons, SSCT, and median nerve within carpal tunnel follows a certain pattern, which may indicate the physiological state of the SSCT. This relative motion pattern was not affected by flexor retinaculum release.
Keywords: Carpal Tunnel, Fluoroscopy, Nerve, Subsynovial Connective Tissue, Tendon
INTRODUCTION
Carpal tunnel syndrome (CTS) is the most frequently encountered peripheral compression neuropathy (1–4). The subsynovial connective tissue (SSCT) in the carpal tunnel is highly specialized for tendon gliding and tendon nutrition (5,6). The most characteristic pathological factor noted in patients with CTS is non-inflammatory fibrosis and thickening of the SSCT. Lluch and others have hypothesized that activity-related damage to the SSCT and the resulting non-inflammatory fibrosis may be the cause of CTS and not just an associated finding (7–12). Shearing injury to the SSCT is one possible mechanism for such damage.
To begin to test the hypothesis that shearing injury may occur in the SSCT, it is necessary to know its normal motion. While the motions of the median nerve and flexor tendons in the carpal tunnel have been studied (13–18), and SSCT motion has been studied when the flexor retinaculum (commonly referred to as the transverse carpal ligament) has been incised (19), less is known about SSCT function when the flexor retinaculum is intact. To obtain this baseline data, we measured the relative motion of the tendon, nerve and SSCT in normal human cadaver specimens before and after flexor retinaculum division.
METHODS
Eight fresh frozen human upper extremities (1 bilateral and 6 unilateral), amputated approximately 15 cm proximal to the wrist joint, were obtained from 3 female and 4 male cadavers (mean age of death 74.8 years). The cadavers had been donated to our medical institution, so local medical records were available for each. After IRB review and exemption, these records were accessed. Cadaver specimens were excluded if there was a notation in the medical history documenting a history of carpal tunnel syndrome or other peripheral nerve disease, as well as potentially associated conditions, including diabetes or glucose intolerance, thyroid disease, rheumatoid arthritis, osteoarthritis, gout, hemodialysis, BMI >30, sarcoidosis, amyloidosis and traumatic injuries to the ipsilateral arm.
The cadaver upper extremities were thawed at room temperature immediately prior to testing. Two screws were inserted into the index metacarpal bone at the radial side of the hand and two screws were inserted into the radial side of the distal radius. A custom made external fixator with a universal joint was attached to each pair of screws and the wrist was immobilized in the anatomical neutral position. A skin incision was made longitudinally to expose the middle finger flexor digitorum superficialis (FDS) tendon from the muscle tendon junction to the proximal end of the finger flexor sheath, with the flexor retinaculum and ulnar bursa intact. The FDS tendon excursion of the middle finger was determined by passive full MCP, PIP and DIP joint flexion/extension with a 5 Newton dead weight was attached to the proximal tendon end in two motion patterns, i.e. middle finger motion alone and all fingers motion together. This tendon excursion measurement would be used to pre set up the motor to perform the finger full flexion and extension motion. As the middle finger has the longest tendon excursion among fingers, the other fingers would also reach the full flexion and might be over stretched. However, we only focused and measured the middle finger FDS tendon excursion and its relative SSCT motion. A small window (5 mm diameter) was made in the flexor retinaculum, visceral synovium and subsynovial connective tissue to expose the middle finger FDS tendon. With the fingers fully flexed, a small metal marker (1mm in diameter) was inserted into the tendon and median nerve. Then, the fingers were fully extended and another small metal marker was glued on the visceral synovium surface by cyanoacrylate adhesive (401 Prism, Loctite Co. Rocky Hill, CT). A fourth metal marker was inserted into the flexor retinaculum to serve as a reference point. The carpal tunnel was otherwise left undisturbed. The hand was mounted in a custom fixture by clamping the proximal ends of the radius and ulna bones. The proximal ends of the finger FDS tendons were fixed with sutures and connected to an electric motor by one of two methods: the middle finger alone (simulated isolated middle finger motion), or all fingers together (simulated fist). A 0.1 N weight was attached to the fingertips to maintain extension. The motion of the four markers was recorded by anteroposterior view fluoroscopy (BV 25, Scopofix MDPM, Philips) (Figure 1). After testing, the flexor retinaculum was incised and the relative motion of the four makers was measured directly and captured on a digital video recording. The flexion movement of the fingers was monitored from full extension to maximum flexion. A millimeter ruler was included in the camera field, so that the data measured with the camcorder could be converted into a distance figure. The data was digitized with the use of Analyze Software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) to determine the motion characteristics of the four markers.
Figure 1.
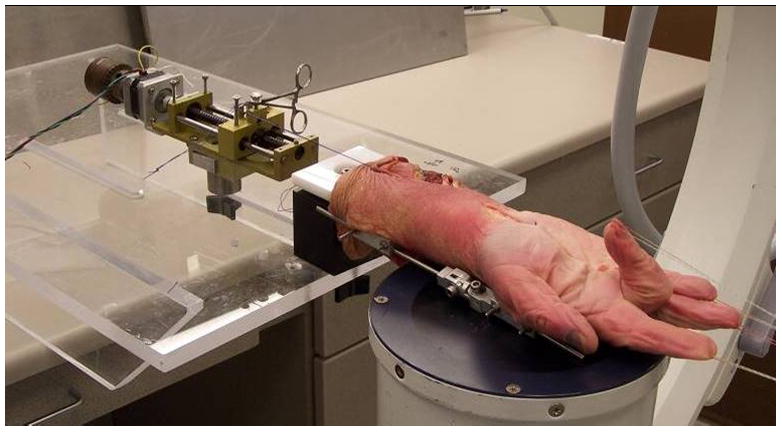
Test set up for specimen during middle finger flexion.
The marker motion was analyzed for the two motion styles (single digit or fist) and the two conditions (intact or incised flexor retinaculum, simulating a closed or open carpal tunnel, respectively). All data were expressed as the mean ± standard error of the mean (SEM). The SSCT and median nerve motion during middle finger motion and fist motion were compared using the Wilcoxon signed rank test.
RESULTS
The mean tendon motion (± SEM) for isolated middle finger motion with a closed carpal tunnel was 29.5 ± 2.8 mm. The mean tendon motion for isolated middle finger motion with an open carpal tunnel was 30.1 ± 2.1 mm. The mean tendon motion for fist motion with a closed carpal tunnel was 29.9 ±2.8 mm. The mean tendon motion for fist motion with an open carpal tunnel was 30.6 ± 2.7 mm. None of the differences in tendon motion were significant.
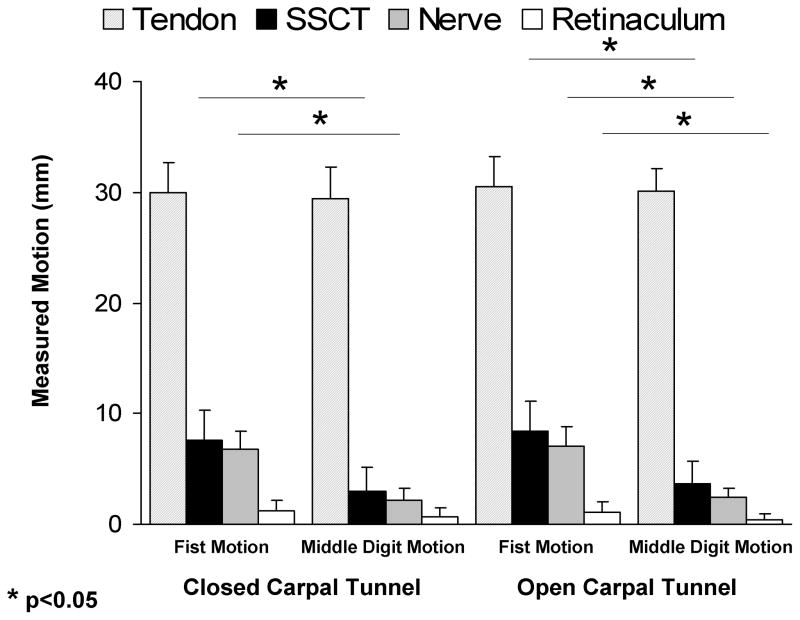
The mean SSCT motion for isolated middle finger motion with a closed carpal tunnel was 3.5 ± 2.8 mm. The mean SSCT motion for isolated middle finger motion with an open carpal tunnel was 4.1 ± 2.6 mm. The mean SSCT motion for fist motion with a closed carpal tunnel was 7.7 ± 2.6 mm. The mean SSCT motion for fist motion with an open carpal tunnel was 9.5 ±2.9 mm (Figure 2).
Figure 2.
Motion of FDS, SSCT, median nerve and flexor retinaculum.
The mean median nerve motion for isolated middle finger motion with a closed carpal tunnel was 2.2 ± 1.0 mm. The mean median nerve motion for isolated middle finger motion with an open carpal tunnel was 2.5 ± 0.7 mm. The mean median nerve motion for fist motion with a closed carpal tunnel was 6.8 ± 1.7 mm. The mean median nerve motion for fist motion with an open carpal tunnel was 7.0 ± 1.8 mm (Figure 2).
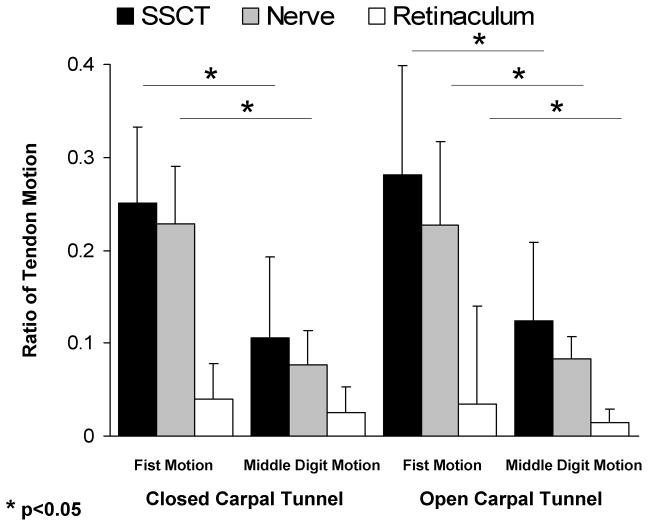
Comparison between simultaneous (fist) and single digit motion showed a statistically significant difference in the SSCT and median nerve motions for both conditions (open and closed carpal tunnel) (p<0.05) (Figure 2). There was no significant difference in the SSCT and median nerve motions when comparing isolated middle finger motion or fist motion across open or closed carpal tunnel conditions. The ratio of SSCT and nerve motion to tendon motion was least (i.e., there was the greatest difference in relative motion) for the isolated middle finger motion condition (Figure 3). This difference was statistically significant. Again the effect of open versus closed carpal tunnel condition was not significant.
Figure 3.
Relative motion of SSCT, median nerve and flexor retinaculum compared to that of FDS.
There was a small amount of motion (< 1 mm) of the flexor retinaculum in all conditions, but this motion was only significantly different in conditions in which the flexor retinaculum was cut (Figures 2 and 3).
DISCUSSION
In the normal human carpal tunnel, the tendons are surrounded by a multilayered SSCT (5,6). Normally this SSCT allows differential gliding of the tendons, as when one finger flexes and an adjacent finger extends or remain still, as may occur in many tasks. Such activity imposes a shear strain on the SSCT. Recent studies suggest that there is evidence of damage to the SSCT in patients with CTS (8,10,11,19–23). If that is so, the damage may be reflected in abnormal SSCT motion. Ettema et al. (24,25) have reported on the measurement of SSCT motion in patients with CTS and compared that with SSCT motion in cadaver specimens in which the flexor retinaculum was divided. They showed that there was a qualitative difference in the extent and pattern of SSCT motion when comparing these two groups. We have here measured the amplitude of motion in the normal SSCT, as Ettema et al. did and have shown that fluoroscopic measurements in the closed carpal tunnel are comparable to those measured directly in the open carpal tunnel. This finding is important, as it suggests that the SSCT motion could be analyzed non-invasively. Such an assessment could serve as a useful baseline for the study of SSCT mechanics in patients with carpal tunnel syndrome. Indeed, there is some human cadaver data suggesting that ultrasound may be useful in measuring SSCT motion (24,25).
Recently, Zhao et al. (26) measured the gliding resistance of the flexor tendons within carpal tunnel. They demonstrated that peak gliding resistance occurred at the end of tendon flexion, due to SSCT stretching the most at that point. In that study, the isolated III FDS tendon motion had a higher peak gliding resistance. In the current study, isolated III FDS tendon motion resulted in the least SSCT motion. The findings of these two studies are compatible- if the SSCT moves less for a given tendon excursion, then the relative SSCT-tendon motion differential will be more. This in turn will translate into greater tendon-SSCT shear strain and higher shear force, as noted by Zhao et al. (26).
Other studies have also looked at the effect of tendon motion after carpal tunnel release (14,27). While the findings of Brown and Peimer (14) differ from ours, the conditions were different, in that we only monitored middle finger FDS motion after release of the flexor retinaculum, with the fingers moving and the wrist fixed, while Brown and Peimer looked at the effect of wrist motion with the fingers fixed. The motion of the median nerve in carpal tunnel syndrome has been studied by others (13,15–18,27,28). These data suggest that the nerve motion is restricted in patients with carpal tunnel syndrome. Our findings of tendon and median nerve motion are comparable to those of Ugbolue et al. (27). Although the total tendon and nerve excursion recorded by Ugbuloe et al. was smaller than what we recorded, probably due to the fact that they used PIP motion while we used full finger motion, their ratio of tendon to nerve motion was about 4.5 to 1, comparable to our finding of 4.3 to 1. In contrast, the excursion ratio of FDS III tendon and median nerve reported by Bay, et al. was greater than our finding or that of Ugbolue et al., but this might due to differences in loading applied to the FDS III tendon in the two studies (13,27).
The strengths of this study are that it validated an indirect measure of tendon, SSCT and median nerve motion in a human cadaver model. It has also demonstrated the relative motion of these structures in situ, and has shown that opening the carpal tunnel to directly observe their motion does not alter that motion, provided that the wrist is fixed in the neutral position.
The weaknesses of this study relate to the fact that it is a cadaver study. The results need to be verified in vivo. However, there is already some data on the relative motion of the FDS and SSCT in patients with carpal tunnel syndrome (19), which has been correlated with cadaver motion. Thus, we believe it is likely that the measurements observed here will be comparable to in vivo motion. Secondly, the motion of the tendon, SSCT, and nerve was calculated and reported only in two-dimensional motion, i.e. the longitude motion which is the major motion of the tendon, SSCT and nerve.
In conclusion, we have demonstrated that fluoroscopic imaging of FDS, SSCT and median nerve motion in the intact carpal tunnel is comparable to direct imaging with the carpal tunnel open, provided that the wrist is fixed in the neutral position.
References
- 1.Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 2.Bland JD, Rudolfer SM. Clinical surveillance of carpal tunnel syndrome in two areas of the united kingdom, 1991–2001. J Neurol Neurosurg Psychiatry. 2003;74:1674–1679. doi: 10.1136/jnnp.74.12.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77:263–265. doi: 10.1136/jnnp.2005.066696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mondelli M, Giannini F, Giacchi M. Carpal tunnel syndrome incidence in a general population. Neurology. 2002;58:289–294. doi: 10.1212/wnl.58.2.289. [DOI] [PubMed] [Google Scholar]
- 5.Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg (Am) 2004;86:1458–1466. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Guimberteau JC. The sliding system. Vascularized flexor tendon transfers. France: Aquitaine Domaine Forestier; 2001. New ideas in hand flexor tendon surgery; pp. 68–73. [Google Scholar]
- 7.Bland JD. Carpal tunnel syndrome. Curr Opin Neurol. 2005;18:581–585. doi: 10.1097/01.wco.0000173142.58068.5a. [DOI] [PubMed] [Google Scholar]
- 8.Ettema AM, Amadio PC, Zhao C, Wold LE, O’Byrne MM, Moran SL, et al. Changes in the functional structure of the tenosynovium in idiopathic carpal tunnel syndrome: A scanning electron microscope study. Plast Reconstr Surg. 2006;118:1413–1422. doi: 10.1097/01.prs.0000239593.55293.c7. [DOI] [PubMed] [Google Scholar]
- 9.Hirata H, Tsujii M, Yoshida T, Imanaka-Yoshida K, Morita A, Okuyama N, et al. Mmp-2 expression is associated with rapidly proliferative arteriosclerosis in the flexor tenosynovium and pain severity in carpal tunnel syndrome. J Pathology. 2005;205:443–450. doi: 10.1002/path.1709. [DOI] [PubMed] [Google Scholar]
- 10.Lluch AL. Thickening of the synovium of the digital flexor tendons: Cause or consequence of the carpal tunnel syndrome? J Hand Surg (Br) 1992;17:209–212. doi: 10.1016/0266-7681(92)90091-f. [DOI] [PubMed] [Google Scholar]
- 11.Oh J, Zhao C, Amadio PC, An KN, Zobitz ME, Wold LE. Vascular pathologic changes in the flexor tenosynovium (subsynovial connective tissue) in idiopathic carpal tunnel syndrome. J Orthop Res. 2004;22:1310–1315. doi: 10.1016/j.orthres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Tucci MA, Barbieri RA, Freeland AE. Biochemical and histological analysis of the flexor tenosynovium in patients with carpal tunnel syndrome. Biomed Sci Instrum. 1997;33:246–251. [PubMed] [Google Scholar]
- 13.Bay BK, Sharkey NA, Szabo RM. Displacement and strain of the median nerve at the wrist. J Hand Surg (Am) 1997;22:621–627. doi: 10.1016/S0363-5023(97)80118-9. [DOI] [PubMed] [Google Scholar]
- 14.Brown RK, Peimer CA. Changes in digital flexor tendon mechanics after endoscopic and open carpal tunnel releases in cadaver wrists. J Hand Surg (Am) 2000;25:112–119. doi: 10.1053/jhsu.2000.jhsu025a0112. [DOI] [PubMed] [Google Scholar]
- 15.LaBan MM, Friedman NA, Zemenick GA. “tethered” median nerve stress test in chronic carpal tunnel syndrome. Arch Phys Med Rehabil. 1986;67:803–804. [PubMed] [Google Scholar]
- 16.Nakamichi K, Tachibana S. Restricted motion of the median nerve in carpal tunnel syndrome. J Hand Surg (Br) 1995;20:460–464. doi: 10.1016/s0266-7681(05)80153-6. [DOI] [PubMed] [Google Scholar]
- 17.Rath T, Millesi H. The gliding tissue of the median nerve in the carpal tunnel. Handchir Mikrochir Plast Chir. 1990;22:203–205. [PubMed] [Google Scholar]
- 18.Valls-Sole J, Alvarez R, Nunez M. Limited longitudinal sliding of the median nerve in patients with carpal tunnel syndrome. Muscle Nerve. 1995;18:761–767. doi: 10.1002/mus.880180713. [DOI] [PubMed] [Google Scholar]
- 19.Ettema AM, Zhao C, Amadio PC, O’Byrne MM, An KN. Gliding characteristics of flexor tendon and tenosynovium in carpal tunnel syndrome: A pilot study. Clin Anat. 2007;20:292–299. doi: 10.1002/ca.20379. [DOI] [PubMed] [Google Scholar]
- 20.Kerr CD, Sybert DR, Albarracin NS. An analysis of the flexor synovium in idiopathic carpal tunnel syndrome: Report of 625 cases. J Hand Surg (Am) 1992;17:1028–1030. doi: 10.1016/s0363-5023(09)91053-x. [DOI] [PubMed] [Google Scholar]
- 21.Neal NC, McManners J, Stirling GA. Pathology of the flexor tendon sheath in the spontaneous carpal tunnel syndrome. J Hand Surg (Br) 1987;12:229–232. doi: 10.1016/0266-7681_87_90020-9. [DOI] [PubMed] [Google Scholar]
- 22.Oh J, Zhao C, Zobitz ME, Wold LE, An KN, Amadio PC. Morphological changes of collagen fibrils in the subsynovial connective tissue in carpal tunnel syndrome. J Bone Joint Surg (Am) 2006;88:824–831. doi: 10.2106/JBJS.E.00377. [DOI] [PubMed] [Google Scholar]
- 23.Pickering SA, Stevens A, Davis TR. Work practices and histopathological changes in the tenosynovium in carpal tunnel syndrome in men. J Hand Surg (Br) 2004;29:325–328. doi: 10.1016/j.jhsb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Oh S, Belohlavek M, Zhao C, Osamura N, Zobitz ME, An KN, et al. Detection of differential gliding characteristics of the flexor digitorum superficialis tendon and subsynovial connective tissue using color doppler sonographic imaging. J Ultrasound Med. 2007;26:149–155. doi: 10.7863/jum.2007.26.2.149. [DOI] [PubMed] [Google Scholar]
- 25.Ettema AM, Belohlavek M, Zhao C, Oh SH, Amadio PC, An KN, et al. High-resolution ultrasound analysis of subsynovial connective tissue in human cadaver carpal tunnel. J Orthop Res. 2006;24:2011–2020. doi: 10.1002/jor.20252. [DOI] [PubMed] [Google Scholar]
- 26.Zhao C, Ettema AM, Osamura N, Berglund LJ, An KN, Amadio PC. Gliding characteristics between flexor tendons and surrounding tissues in the carpal tunnel: A biomechanical cadaver study. J Orthop Res. 2007;25:185–190. doi: 10.1002/jor.20321. [DOI] [PubMed] [Google Scholar]
- 27.Ugbolue UC, Hsu WH, Goitz RJ, Li ZM. Tendon and nerve displacement at the wrist during finger movements. Clin Biomech. 2005;20:50–56. doi: 10.1016/j.clinbiomech.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Erel E, Dilley A, Greening J, Morris V, Cohen B, Lynn B. Longitudinal sliding of the median nerve in patients with carpal tunnel syndrome. J Hand Surg (Br) 2003;28:439–443. doi: 10.1016/s0266-7681(03)00107-4. [DOI] [PubMed] [Google Scholar]