Abstract
Post-herpetic neuralgia (PHN) is neuropathic pain persisting after an acute episode of herpes zoster, and is associated with severe pain and sensory abnormalities that adversely affect the patient’s quality of life and increase health care costs. Up to 83% of patients with PHN describe localized neuropathic pain, defined as “a type of neuropathic pain characterized by consistent and circumscribed area(s) of maximum pain”. Topical treatments have been suggested as a first-line treatment for localized neuropathic pain. Use of 5% lidocaine medicated plaster could reduce abnormal nervous peripheral discharge and via the plaster could have a “protective” function in the affected area. It has been suggested that use of this plaster could reduce pain as well as the size of the painful area. To evaluate this possible outcome, we retrospectively reviewed eight patients with PHN, treated using 5% lidocaine medicated plaster. During a follow-up period of 3 months, we observed good pain relief, which was associated with a 46% reduction in size of the painful area after one month (from 236.38±140.34 cm2 to 128.80±95.7 cm2) and a 66% reduction after 3 months (81.38±59.19 cm2). Our study cohort was composed mainly of elderly patients taking multiple drugs to treat comorbidities, who have a high risk of drug–drug interactions. Such patients benefit greatly from topical treatment of PHN. Our observations confirm the effectiveness of lidocaine plasters in the treatment of PHN, indicating that 5% lidocaine medicated plaster could reduce the size of the painful area. This last observation has to be confirmed and the mechanisms clarified in appropriate larger randomized controlled trials.
Keywords: localized neuropathic pain, topical treatment, chronic pain, drug–drug interactions, patient’s outcome
Introduction
Neuropathic pain, defined as “pain arising as a direct consequence of a lesion or a disease affecting the somatosensory system”,1 is a disorder that can considerably affect quality of life for patients. Even though up to 5% of the general population could be involved, treatment of neuropathic pain continues to be challenging due to the inadequate effectiveness of systemic therapies and their frequent central side effects.2
In the wide area of peripheral neuropathic pain, the identification of specific neuropathic pain syndromes that affect only a circumscribed area of the body may drive a clinical decision to use targeted localized treatment(s). Recently, Mick et al proposed defining all peripheral neuropathic pain “characterized by consistent and circumscribed area(s) of maximum pain associated with negative or positive sensory signs”3 as localized neuropathic pain (LNP). According to the relevant guidelines,4–6 5% lidocaine medicated plaster could be considered as a first-line treatment option for LNP due to its good efficacy and safety profile, especially in elderly patients where comorbidities are quite often present and polypharmacy is poorly tolerated.
Up to 83% of patients with post-herpetic neuralgia (PHN) complain of an LNP syndrome,3 for which 5% lidocaine medicated plaster showed better effectiveness versus placebo7,8 and substantially better pain relief than pregabalin,9,10 with fewer systemic side effects even in long-term treatment.11–13
Several different mechanisms sustain PHN,14 including peripheral inflammation, nerve damage and denervation, and peripheral and central sensitization. Lidocaine acts by blocking abnormally functioning (sensitized) Nav 1.7 and Nav 1.8 sodium channels in dermal nociceptors,15 thereby reducing ectopic discharges, which raise the peripheral ectopic discharge threshold.15 Topical 5% lidocaine plaster does not affect A-beta fibers, so does not cause any paresthesia and/or numbness.16 Finally, a passive protective action of the plaster itself has been reported.8–10
In a cohort of patients with LNP due to nerve trauma,17 5% lidocaine medicated plaster showed optimal efficacy in reducing pain and the size of the painful area. To our knowledge, no other structured trial has investigated this possible “new” endpoint in PHN patients. In our first eight PHN patients treated with Versatis 5% plaster (Grunenthal, Germany) (the drug was approved in Italy in May 2013), we investigated whether it could be helpful both in reducing pain and allodinic (static and/or dynamic) areas. Allodynia is a phenomenon in which normally nonpainful stimuli are perceived as painful, and can be a clinical sign of peripheral and central sensitization. The two components can be differentiated using different types of stimuli, ie, peripheral sensitization can be evoked by static mechanical stimulation (static mechanical allodynia) whereas allodynia to dynamic mechanical stimulation is the result of central sensitization. While the static component is only found in the injured area, the dynamic component also extends into the halo of undamaged tissue surrounding the injury.18
Case series
Consistent with the literature and following approval in Italy, 5% lidocaine medicated plaster was used in a group of PHN patients with untreated LNP, LNP that was inadequately controlled, or side effects from systemic drugs. Following our hospital administrative rules, all patients signed an informed consent allowing anonymous use of their clinical data for research purposes. In accordance with our clinical practice, the patients were screened at the first visit and then followed up after 15, 30, and 90 days. Patients could telephone and ask for another consultation if they experienced side effects or inadequate analgesia.
At all visits, we recorded: daily pain intensity (mean, least, and worst, using the Numeric Rating Scale); paresthesia and dynamic allodynia (scale 0 [none] to 5 [worst imaginable]); and static allodynia with the Von Frey hair test (caliber from 0 [worst allodynia] to 18 [normal sensation] of Von Frey monofilaments perceived as painful – less is the caliber perceived as painful, greater is the static allodynia). Percentage pain relief was also recorded. We used a well established Von Frey hair set (Touch Test™ sensory evaluators; North Coast Medical, Inc. Morgan Hill, CA, USA), with a 5.07 caliper corresponding to 10 g applied for 1.5 seconds, which delivers a clear mechanical suprathreshold nonpainful stimulus. The area of statical tactile allodynic area was measured in cm2.
During their first visit, patients were instructed regarding how to apply the 5% lidocaine medicated plaster to the most painful areas of intact skin; they were allowed to use a maximum of three plasters for 12 hours a day. Depending on the clinical results seen during follow-up, we reduced their systemic analgesic medication if good pain relief (of at least 30%) was achieved. The data reported here are the results of a 3-month follow up.
Eight patients with PHN LNP (five men and three women) were observed from May to September 2013. Their mean age was 77.75±7.10 (range 69–89) years and mean Body Mass Index (BMI) was 28.1±5.5 kg/m2. The acute episode of herpes zoster had occurred at a mean of 3.7±3.6 years earlier. The rash affected the trunk in all cases, except for one patient whose left hand was affected. All patients were on polypharmacy, taking a mean of 4±2 nonanalgesic drugs for comorbid conditions.
All patients reported having tried at least one systemic drug for pain relief (antidepressant, anticonvulsant, and/or opioids) with inadequate pain control (five patients) or good pain control but severe side effects (three patients). One patient reported an accidental fall with a rib fracture due to dizziness on anticonvulsant treatment.
The patients reported good pain relief (45.00%±19.75%) as early as 2 weeks after the start of topical therapy, with further improvement as treatment continued (52.00%±23.87% by month 1 and 60.00%±18.70% by month 3), and reduction/cessation of systemic treatment in six patients (Table 1). Paresthesia and dynamic allodynia decreased significantly at 3 months from 2.88±1.64 to 1.20±1.10 and from 3.75±0.71 to 1.80±1.64, respectively. We observed a similarly consistent reduction in static allodynia from 8.63±3.58 to 14.00±0.82 over 3 months.
Table 1.
Evolution of systemic therapy for each patient before and after introduction of topical lidocaine
| Patient | Baseline oral therapy | Final oral therapy |
|---|---|---|
| 1 | Tapentadol 100 mg ×2 | Tapentadol 100 mg |
| Pregabalin 175 mg ×2 | Pregabalin 100 mg + 75 mg | |
| Duloxetine 60 mg | Duloxetine 60 mg | |
| 2 | Tramadol/paracetamol 37.5/325 mg ×2 and tramadol/paracetamol 37.5/325 mg as needed | Tramadol/paracetamol 37.5/325 mg only as needed |
| 3 | Tapentadol 150 mg ×2 | Tapentadol 150 mg ×2 |
| 4 | Clonazepam 0.5 mg | Clonazepam 0.5 mg |
| 5 | Pregabalin 75 mg ×2 | Paracetamol 1 g only as needed |
| 6 | Tapentadol 50 mg ×2 | Nothing |
| 7 | Pregabalin 75 mg and tramadol/paracetamol 37.5/325 mg as needed | Nothing |
| 8 | Paracetamol 1 g ×2 and paracetamol 1 g as needed | Paracetamol 1 g only as needed |
The allodynic (static or dynamic) area was measured in five of eight patients with thoracic PHN (Figure 1A). The area became progressively smaller from a baseline measurement of 236.38±140.34 cm2 to 128.80±95.7 cm2 after one month (46% reduction, P=0.129) and to 81.38±59.19 cm2 after 3 months (66% reduction, P=0.02). With the reduction in size of the area, patients were able to apply fewer and smaller plasters. A more pronounced reduction was observed in the height (50%) than in the length (33%) of the affected area (Figure 1B).
Figure 1.
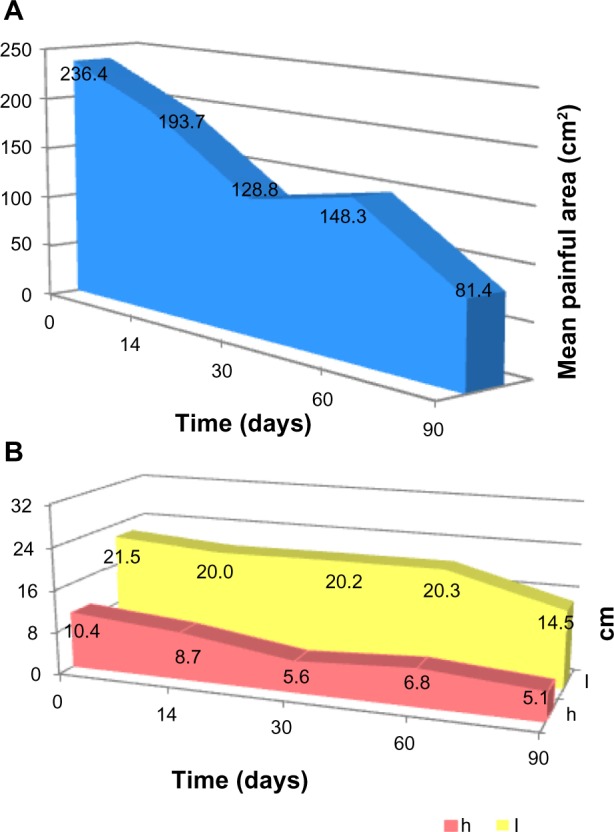
(A) Reduction of the whole allodynic area and (B) reduction of the height (h) and length (l) of the same area.
Discussion
Neuropathic pain continues to be a challenging clinical problem.2 People with neuropathic pain are often elderly and may have several comorbidities and a high risk of drug–drug interactions, which presents a serious limitation to therapy.19 To achieve good pain relief, it is often necessary to give a combination of two or more drugs,20 which increases the risk of drug–drug interactions and side effects.
The mainstay of treatment for neuropathic pain is still adequate personalized therapy based on an understanding of the pain pathophysiology and the patient’s clinical features.21 The majority of patients evaluated in our case series complained of the limited effectiveness of pain treatments and/or side effects from systemic treatment for PHN, and had had their pain for several months or years.
When patient refers a LNP, correctly diagnosed according to validated diagnostic algorithm,22 more recent guidelines4,5 suggest topical products as the first-line therapeutic option since they are better tolerated, have no or few systemic effects and drug–drug interactions, and have better patient compliance.9,10,23,24 Our case series reflects all these common clinical aspects.
The main mechanism of the therapeutic action of lidocaine is blockade of voltage-gated sodium channels.15,25 However, topical lidocaine may have other peripheral actions through a desensitizing effect of TRPA1 channels, contributing to its “nonanesthetic analgesic” effects.26 Thus, the reduction in peripheral sensitization could be attributed to both blockade of pathological sodium channel expression and desensitization of TRPA1 channels.
A characteristic feature of PHN is the presence of an area of primary hyperalgesia (static mechanical and thermal allodynia within the damaged area) and secondary hyperalgesia (dynamic mechanical allodynia that surrounds the first area).27 The static mechanical allodynia is mainly mediated by sensitized peripheral nociceptors, while the dynamic component is probably a consequence of altered processing of large diameter primary afferent inputs in the central nervous system. These alterations are at least partially maintained by the barrage of nociceptor activity from both normal and abnormal inputs.27 The importance of ongoing activity to maintain the secondary hyperalgesia has not been completely elucidated, even though in patients with neuropathic pain, secondary hyperalgesia and allodynia have been seen to be critically dependent on continuous afferent input.18
Hence, 5% lidocaine medicated plaster could act by adding blockade of pathological sodium and TRPV1 channels to the reduction of mechanical noxious/non-noxious inputs via the protective action of the plaster. This reduction of peripheral inputs could contribute to reducing both primary and secondary sensitization, and consequently the size of the painful area,28,29 as also suggested by a healthy volunteer study.30
In our study, the mean size of the painful area was 236.38±140.34 cm2, which is in agreement with data in the literature.31 Regarding the reduction of the static allodynic area, we noticed that there is a much greater reduction of the height (number of metameres involved) rather than of the length (area of the same nerve involved) of the painful area. In PHN, the secondary hyperalgesic area is mainly distributed above and below the primary painful area in the dermatome where an overlapping of innervation is anatomically present. It could be arguable that if neural plasticity can be induced by treatment, it may be more evident in peripheral areas of secondary hyperalgesia.18
Hence this treatment could have another important clinical effect: the reduction of the allodynic area, as also suggested in a study about LNP with recent onset.17 In fact, a more than 50% reduction in the size of the painful area was recorded, even though the patients had been complaining of PHN for several months or years.
This study has several limitations. Due to the small number of patients, it was not possible to evaluate if the reduction in size of the painful area was related to the time of onset of PHN or to the more pronounced reduction of static rather than dynamic allodynia. Nevertheless, our case series highlights a possible role of chronic topical treatment not only in treating LNP but also in reducing the size of the painful area. These data have to be confirmed by appropriately designed controlled trials investigating this endpoint as well as the mechanisms for the activity of 5% lidocaine medicated plaster, ie, whether it acts more on primary rather than secondary hyperalgesia, and whether the time of onset of PHN/LNP could be a predictive factor of efficacy.
Footnotes
Disclosure
RC has been a speaker for and received research support from Grünenthal. MA has been a speaker for Grünenthal. No funding or sponsorship was involved in this study.
References
- 1.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(Suppl 10):S22–S32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Mick G, Baron R, Finnerup NB, et al. What is localized neuropathic pain? A first proposal to characterize and define a widely used term. Pain Manag. 2012;2(1):71–77. doi: 10.2217/pmt.11.77. [DOI] [PubMed] [Google Scholar]
- 4.Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–e88. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(Suppl 3):S3–S14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meier T, Wasner G, Faust M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain. 2003;106(1–2):151–158. doi: 10.1016/s0304-3959(03)00317-8. [DOI] [PubMed] [Google Scholar]
- 8.Binder A, Bruxelle J, Rogers P, et al. Topical 5% lidocaine (lignocaine) medicated plaster treatment for post-herpetic neuralgia: results of a double-blind, placebo-controlled, multinational efficacy and safety trial. Clin Drug Investig. 2009;29(6):393–408. doi: 10.2165/00044011-200929060-00003. [DOI] [PubMed] [Google Scholar]
- 9.Rehm S, Binder A, Baron R. Post-herpetic neuralgia: 5% lidocaine medicated plaster, pregabalin, or a combination of both? A randomized, open, clinical effectiveness study. Curr Med Res Opin. 2010;26(7):1607–1619. doi: 10.1185/03007995.2010.483675. [DOI] [PubMed] [Google Scholar]
- 10.Baron R, Mayoral V, Leijon G, et al. 5% lidocaine medicated plaster versus pregabalin in post-herpetic neuralgia and diabetic polyneuropathy: an open-label, non-inferiority two-stage RCT study. Curr Med Res Opin. 2009;25(7):1663–1676. doi: 10.1185/03007990903047880. [DOI] [PubMed] [Google Scholar]
- 11.Hans G, Sabatowski R, Binder A, et al. Efficacy and tolerability of a 5% lidocaine medicated plaster for the topical treatment of post-herpetic neuralgia: results of a long-term study. Curr Med Res Opin. 2009;25(5):1295–1305. doi: 10.1185/03007990902901368. [DOI] [PubMed] [Google Scholar]
- 12.Pickering G, Pereira B, Clère F, et al. Cognitive function in older patients with postherpetic neuralgia. Pain Pract. 2014;14(1):E1–E7. doi: 10.1111/papr.12079. [DOI] [PubMed] [Google Scholar]
- 13.Sabatowski R, Hans G, Tacken I, et al. Safety and efficacy outcomes of long-term treatment up to 4 years with 5% lidocaine medicated plaster in patients with post-herpetic neuralgia. Curr Med Res Opin. 2012;28(8):1337–1346. doi: 10.1185/03007995.2012.707977. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RW, Wasner G, Saddier P, Baron R. Postherpetic neuralgia: epidemiology, pathophysiology and management. Expert Rev Neurother. 2007;7(11):1581–1595. doi: 10.1586/14737175.7.11.1581. [DOI] [PubMed] [Google Scholar]
- 15.Liu M, Wood JN. The roles of sodium channels in nociception: implications for mechanisms of neuropathic pain. Pain Med. 2011;12(Suppl 3):S93–S99. doi: 10.1111/j.1526-4637.2011.01158.x. [DOI] [PubMed] [Google Scholar]
- 16.Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. J Clin Pharmacol. 2003;43(2):111–117. doi: 10.1177/0091270002239817. [DOI] [PubMed] [Google Scholar]
- 17.Correa-Illanes G, Roa R, Piñeros JL, Calderón W. Use of 5% lidocaine medicated plaster to treat localized neuropathic pain secondary to traumatic injury of peripheral nerves. Local Reg Anesth. 2012;5:47–53. doi: 10.2147/LRA.S31868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koltzenburg M, Lundberg LE, Torebjörk HE. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain. 1992;51(2):207–219. doi: 10.1016/0304-3959(92)90262-A. [DOI] [PubMed] [Google Scholar]
- 19.Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009;22(5):467–474. doi: 10.1097/WCO.0b013e3283311e13. [DOI] [PubMed] [Google Scholar]
- 20.Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012;7:CD008943. doi: 10.1002/14651858.CD008943.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allegri M, Clark MR, De Andrés J, Jensen TS. Acute and chronic pain: where we are and where we have to go. Minerva Anestesiol. 2012;78(2):222–235. [PubMed] [Google Scholar]
- 22.Casale R, Mattia C. Building a diagnostic algorithm on localized neuropathic pain (LNP) and targeted topical treatment: focus on 5% lidocaine medicated plaster. Ther Clin Risk Manag. 2014;10:259–268. doi: 10.2147/TCRM.S58844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brix Finnerup N, Hein Sindrup S, Staehelin Jensen T. Management of painful neuropathies. Handb Clin Neurol. 2013;115:279–290. doi: 10.1016/B978-0-444-52902-2.00017-5. [DOI] [PubMed] [Google Scholar]
- 24.Clère F, Delorme-Morin C, George B, et al. 5% lidocaine medicated plaster in elderly patients with postherpetic neuralgia: results of a compassionate use programme in France. Drugs Aging. 2011;28(9):693–702. doi: 10.2165/11595600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Sheets PL, Jarecki BW, Cummins TR. Lidocaine reduces the transition to slow inactivation in Na(v)1.7 voltage-gated sodium channels. Br J Pharmacol. 2011;164(2b):719–730. doi: 10.1111/j.1476-5381.2011.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leffler A, Lattrell A, Kronewald S, Niedermirtl F, Nau C. Activation of TRPA1 by membrane permeable local anesthetics. Mol Pain. 2011;7:62. doi: 10.1186/1744-8069-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treede RD, Davis KD, Campbell JN, Raja SN. The plasticity of cutaneous hyperalgesia during sympathetic ganglion blockade in patients with neuropathic pain. Brain. 1992;115(Pt 2):607–621. doi: 10.1093/brain/115.2.607. [DOI] [PubMed] [Google Scholar]
- 28.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 29.Baron R, Hans G, Dickenson AH. Peripheral input and its importance for central sensitization. Ann Neurol. 2013;74(5):630–636. doi: 10.1002/ana.24017. [DOI] [PubMed] [Google Scholar]
- 30.Gustorff B, Hauer D, Thaler J, Seis A, Draxler J. Antihyperalgesic efficacy of 5% lidocaine medicated plaster in capsaicin and sunburn pain models – two randomized, double-blinded, placebo-controlled crossover trials in healthy volunteers. Expert Opin Pharmacother. 2011;12(18):2781–2790. doi: 10.1517/14656566.2011.601868. [DOI] [PubMed] [Google Scholar]
- 31.Rowbotham MC, Davies PS, Verkempinck C, Galer BS. Lidocaine patch: double-blind controlled study of a new treatment method for post-herpetic neuralgia. Pain. 1996;65(1):39–44. doi: 10.1016/0304-3959(95)00146-8. [DOI] [PubMed] [Google Scholar]


