Abstract
A thorough study was done to test the reactivity of the Folin-Ciocalteu (F-C) reagent towards various compound classes. Over 80 compounds were tested. Compound classes included phenols, thiols, vitamins, amino acids, proteins, nucleotide bases, unsaturated fatty acids, carbohydrates, organic acids, inorganic ions, metal complexes, aldehydes and ketones. All phenols, proteins and thiols tested were reactive towards the reagent. Many vitamin derivatives were also reactive, as were the inorganic ions Fe+2, Mn+2, I− and SO3−2. Other compounds showing reactivity included the nucleotide base guanine and the trioses glyceraldehyde and dihydroxyacetone. Copper complexation enhanced the reactivity of salicylate derivatives towards the reagent while zinc complexation did not. Several amino acids and sugars which were reported to be reactive towards the F-C reagent in earlier studies were found not to be reactive in our study, at least in the concentrations used.
Reaction kinetics of each compound with the F-C reagent were also measured. Most compounds tested showed a biphasic kinetic pattern with half lives under one minute. Trolox and ascorbic acid displayed a rapid monophasic pattern in which the reaction reached endpoint within one minute.
In summary, our study has shown that the F-C reagent is significantly reactive towards other compounds besides phenols. As other investigators have suggested, the F-C assay should be seen as a measure of total antioxidant capacity rather than phenolic content. Since phenolics are the most abundant antioxidants in most plants, it gives a rough approximation of total phenolic content in most cases.
Keywords: Folin-Ciocalteu Assay, phenolics, thiols, antioxidants, antioxidant assays
INTRODUCTION
The Folin-Ciocalteu (F-C) Assay was developed in 1927 for the measurement of tyrosine (1).The reagent consists of a mixture of sodium molybdate, sodium tungstate and other reagents. Upon reaction with phenols, it produces a blue color which absorbs at 765 nm. It is believed that the blue color is due to a complexed Mo(V) species (2). The assay has been used for many years by the food and agricultural industries for determining phenolic content of plant products (3). A modification of this assay, called the Folin-Lowry Assay, is used for quantitation of proteins (4).
Folin and Ciocalteu noted during the development of the assay that tryptophan, which is non-phenolic, is reactive towards the reagent and contributes towards protein absorbance in the Folin-Lowry Assay (1). Over the years, there have been numerous reports that the F-C reagent reacts with other antioxidants besides phenols (3). Possible contributors mentioned include proteins, carbohydrates, amino acids, nucleotides, thiols, unsaturated fatty acids, vitamins, amines, aldehydes and ketones.
Ikawa and co-workers (5) performed an exhaustive study to determine the scope of reactivity of the F-C reagent towards nitrogenous compounds. They tested over 60 nitrogen containing compounds representing several chemical classes. Their findings indicated that several of these classes showed considerable reactivity towards the F-C reagent. Classes showing reactivity included hydrazines, hydroxylamines, guanidines, tertiary amines, aromatic amines, pyrroles and indoles. Since some compounds in these classes are present in plants, one might think that measurement of phenols by the F-C method could possibly give too high of estimate of phenolic content. One study showed only a moderate correlation (r=0.64) between phenolic content of olive oils determined by F-C and HPLC methods (6).
An analogous study to determine the reactivity of various classes of non-nitrogenous compounds towards the F-C reagent has not yet been reported. Non-phenolic, non-nitrogenous antioxidants are common in plants. These include vitamins and thiols. In all probability, these compounds are also reactive towards the F-C reagent. Therefore, we believe that a study of this type would be of interest to researchers.
MATERIALS AND METHODS
The thiol derivative 2(RS)-n-propylthiazolidine-4(R)-carboxylic acid (PTCA) was synthesized in our laboratory by a method formerly reported in the literature (7). The metal complexes Cu(II)(DIPS)4 (8), Zn(II)(DTBS)2(8), and Cu(II)2(DTBS)4(9) were synthesized by methods reported in the literature. Cu(II)2(DBS)4 and Zn(II)(DTBS)2 were synthesized in our laboratory by a procedure listed below. All other materials were purchased either from Fisher Scientific Company, Houston, TX or Sigma-Aldrich Company, St. Louis, MO.
Synthesis of Cu or Zn chelates of 3, 5-dibromosalicylate (DBS) and 3, 5-ditertiarybutylsalicylate (DTBS)
The metal chelates were synthesized from commercially available substituted salicylic acids (Aldrich) and copper(II) chlorides or zinc(II) chlorides (Aldrich) using methods that have been developed (8,9). Solutions of the sodium salt of each substituted salicylic acid were prepared by dissolving respective substituted salicylic acid in deionized H2O by adding equivalent moles of NaOH. This solution was filtered to remove any insoluble impurity, vigorously stirred with a magnetic stirrer, and the pH adjusted to the range of 9–11 using 10% sodium hydroxide and/or 10% HCl. For copper complexes the molar ratio of the respective salicylate salt to copper(II) chlorides was 2:1, and for zinc chelates the molar ratio the respective salicylate salt to zinc(II) chlorides was 1:1. A filtered deionized water solution of 1:1 or 2:1 equivalents of the metal chloride were added drop wise with a separatory funnel to a vigorously stirred solution of respective sodium salicylate. Vigorous stirring was employed to provide shearing and avoid entrapment of any of the starting materials in the precipitating product of this reaction. Following the completion of this addition the precipitate were collected by filtration, washed with deionized water, and dried overnight at room temperature in the sintered glass filter funnel attached to laboratory vacuum (15 mm Hg). The metal complexes were characterized by melting point or decomposition range, elemental analysis and Fourier Transformer Infrared Spectroscopy (Nicolet FT-IR spectrometer). The elemental analyses for the two new compounds, Cu(II)2(DBS)4 and Zn(II)(DTBS)2 were within acceptable range.
Measurement of Reactivity of Compounds Towards the Folin-Ciocalteu Reagent (Endpoint Assay)
A slightly modified version of the method of Singleton et al. was used. (10). A 50 milligram quantity of each test compound was dissolved in either water or ethanol, dependent upon solubility. For compounds suspected of having high reactivity, the following dilutions were made: 1:10, 1:20, 1:30, 1:50, 1:100. For compounds suspected of having low reactivity the dilutions made were 1:5, 2:5, 3:5 and 4:5. Cuvettes were prepared such that there were three replicates for each of the dilutions mentioned above. To each cuvette was added 1.58 mL water. 0.1 mL F-C reagent and 20 uL of the proper dilution of test compound. Cuvettes were stirred and allowed to stand 5 minutes. After this, 0.3 mL of a 20% aqueous sodium carbonate solution was added to each cuvette. Cuvettes were again stirred and incubated at 45°C for 30 minutes in a dry bath. Absorbances were read at 765 nm using a Bio-Rad Smart Spec 3000 spectrophotometer. Graphs of absorbance versus concentration were prepared using Sigma Plot software. Activity of compounds is expressed in terms of Gallic Acid Equivalents (GAE). GAE is defined as slope of test compound standard curve / slope of gallic acid standard curve.
Measurement of Reaction Kinetics
A single cuvette was prepared containing 1.58 mL water. 0.1 mL F-C reagent and 20 uL of the highest dilution of test compound used in the endpoint assay. Cuvettes were stirred and allowed to stand 5 minutes. This was used as the blank. After this, 0.3 mL of a 20% aqueous sodium carbonate solution was added to the cuvette and kinetics measurements were made using a Bio-Rad Smart Spec 3000 spectrophotometer. Readings were taken at 30 second intervals over a one hour period at room temperature. Absorbance versus time was plotted using Sigma Plot Software.
RESULTS AND DISCUSSION
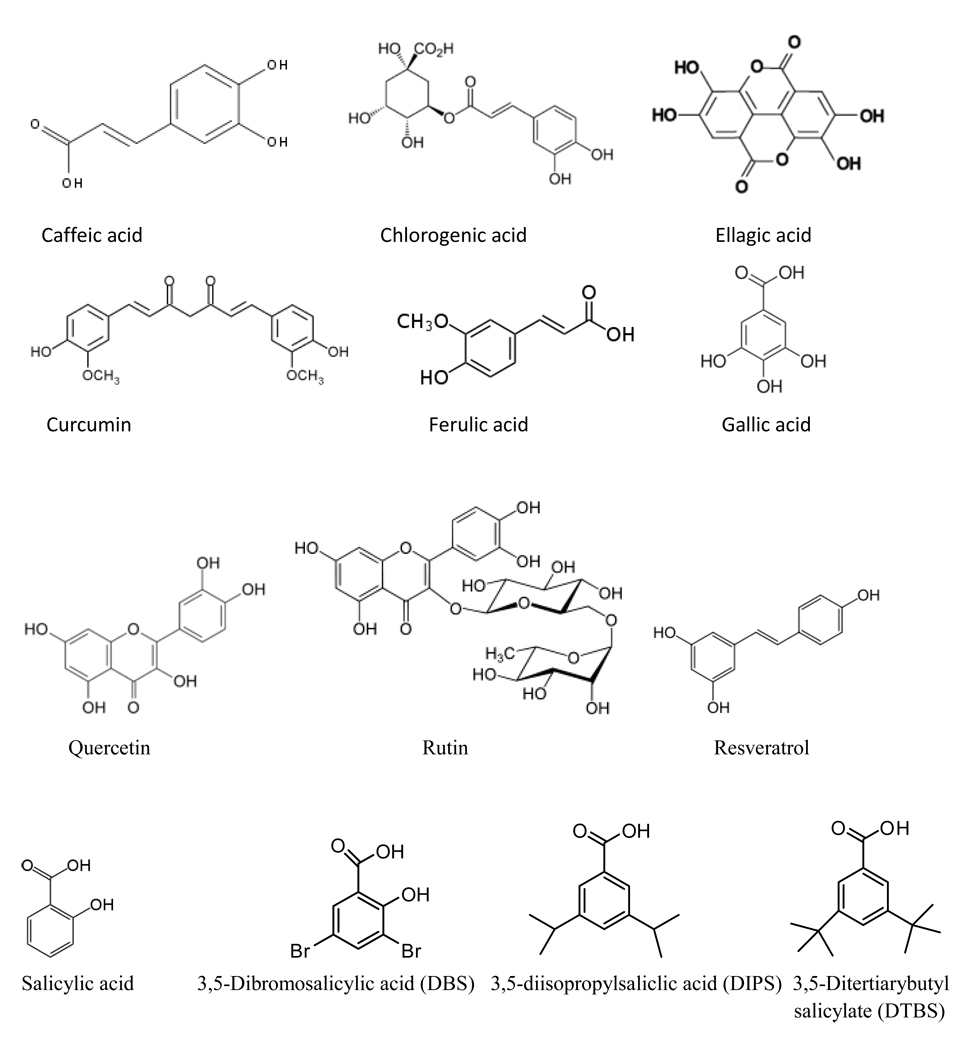
Compounds tested in this study were chosen because of earlier reports that these or similar compounds showed reactivity towards the F-C reagent (3). We tested several phenolic compounds to serve as a basis of comparison to the other compounds tested in this study. All phenolics tested were significantly active and the GAE values measured for compounds formerly reported in the literature were consistent with the earlier reported values (11). The chemical structures of the phenolic compounds used in this study are shown in Figures 1 and 2. Table 1 shows the results of the Endpoint Assay for all compounds tested in this study. The data show that all thiol derivatives and proteins tested, as well as many of the vitamin derivatives, were also reactive towards the reagent.
Figure 1.
Chemical Structures of Phenolic Compounds Used in This Study
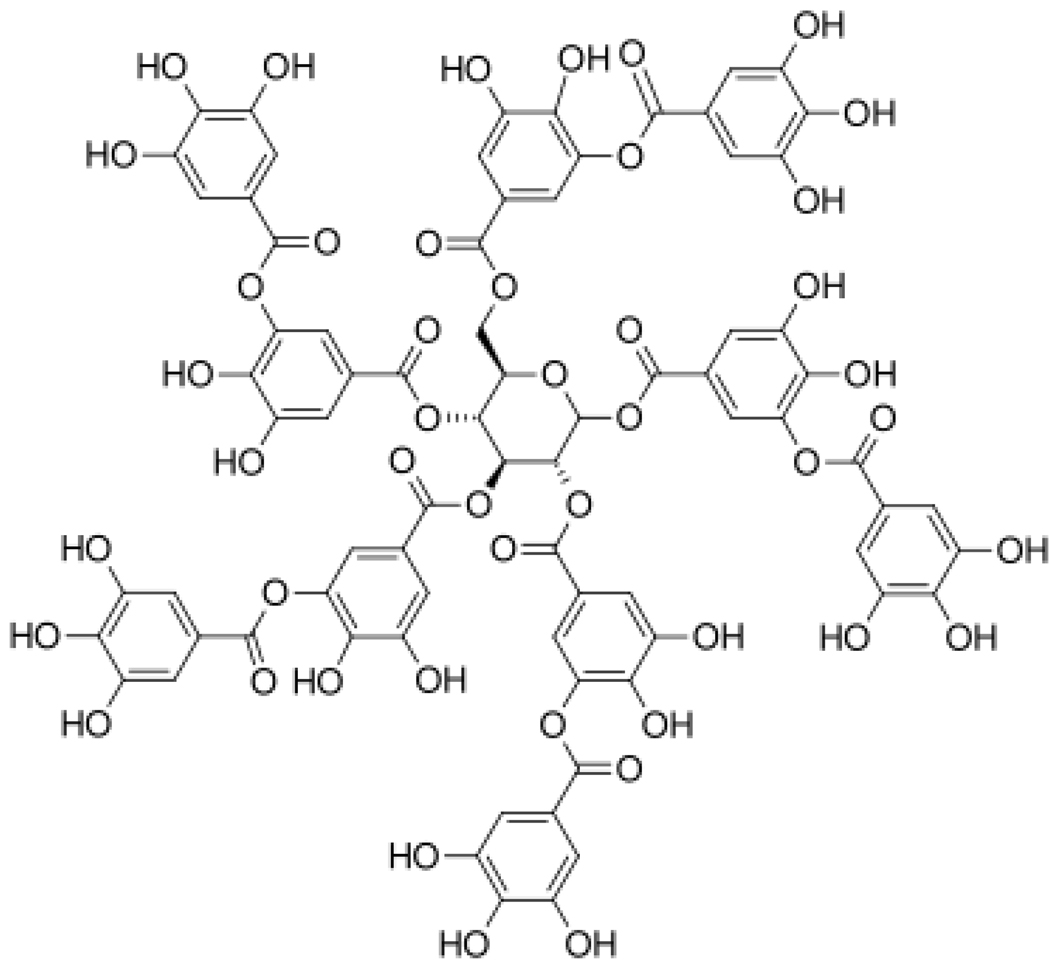
Figure 2.
Chemical Structure of Tannic Acid
Table 1.
Reactivity of Various Substances with Folin-Ciocalteu Phenol Reagent
| Compound | Molar Mass (g/mol) | GAE (mass) | GAE (molar) |
|---|---|---|---|
| Phenolic compounds | |||
| caffeic acid | 180.16 | 1.00 | 0.958 |
| chlorogenic acid | 354.31 | 0.722 | 1.36 |
| curcumin | 368.40 | 0.722 | 1.41 |
| ellagic acid | 302.19 | 1.32 | 2.12 |
| ferulic acid | 194.18 | 1.05 | 1.08 |
| gallic acid | 188.14 | 1.00 | 1.00 |
| quercetin | 338.00 | 1.16 | 2.08 |
| resveratrol | 228.25 | 1.01 | 1.23 |
| rutin | 610.52 | 0.568 | 1.53 |
| salicylic acid | 138.12 | 0.357 | 0.262 |
| tannic acid | 1701.00 | 0.878 | 9.04 |
| Thiol derivatives | |||
| amifostine | 214.22 | 0.378 | 0.430 |
| captopril | 217.29 | 0.323 | 0.373 |
| cysteamine HCl | 113.61 | 0.304 | 0.184 |
| glutathione | 307.30 | 0.161 | 0.263 |
| MPG | 163.20 | 0.342 | 0.297 |
| N-acetylcysteine | 163.20 | 0.395 | 0.378 |
| penicillamine | 149.21 | 0.333 | 0.264 |
| PTCA | 175.25 | 0.180 | 0.141 |
| RibCys | 253.23 | 0.202 | 0.271 |
| WR-1065 | 134.24 | 0.375 | 0.268 |
| Vitamin derivatives | |||
| ascorbic acid | 176.12 | 0.662 | 0.620 |
| biotin | 244.31 | 0.000 | 0.000 |
| folic acid | 441.40 | 0.071 | 0.167 |
| folinic acid | 473.44 | 0.069 | 0.174 |
| menadione | 172.18 | 0.000 | 0.000 |
| NADH | 709.40 | 0.054 | 0.204 |
| nicotinic acid | 123.11 | 0.000 | 0.000 |
| pyridoxine | 205.64 | 0.211 | 0.231 |
| retinoic acid | 300.42 | 0.404 | 0.645 |
| riboflavin | 376.36 | 0.000 | 0.000 |
| thiamine | 337.28 | 0.183 | 0.328 |
| trolox | 250.29 | 0.395 | 0.525 |
| Amino acids | |||
| alanine | 89.09 | 0.000 | 0.000 |
| arginine | 174.20 | 0.000 | 0.000 |
| cysteine | 121.16 | 0.281 | 0.181 |
| glycine | 75.07 | 0.000 | 0.000 |
| histidine | 155.16 | 0.000 | 0.000 |
| hydroxyproline | 131.13 | 0.000 | 0.000 |
| lysine | 146.19 | 0.000 | 0.000 |
| methionine | 149.21 | 0.000 | 0.000 |
| tryptophan | 204.22 | 0.413 | 0.448 |
| tyrosine | 181.19 | 0.397 | 0.382 |
| Nucleotide Bases | |||
| adenine | 135.13 | 0.0223 | 0.0160 |
| guanine | 151.13 | 0.340 | 0.273 |
| cytosine | 111.10 | 0.000 | 0.000 |
| thymine | 126.11 | 0.000 | 0.000 |
| Unsaturated fatty acids | |||
| arachidonic acid | 304.50 | 0.0045 | 0.0160 |
| linoleic acid | 280.45 | 0.000 | 0.000 |
| linolenic acid | 278.42 | 0.000 | 0.000 |
| Carbohydrates and proteins | |||
| Dihydroxyacetone | 90.08 | 0.0444 | 0.0212 |
| D-deoxyribose | 164.16 | 0.000 | 0.000 |
| D-fructose | 180.16 | 0.000 | 0.000 |
| D-glucose | 180.16 | 0.000 | 0.000 |
| D-glyceraldehyde | 90.08 | 0.0243 | 0.0120 |
| D-lactose | 342.30 | 0.000 | 0.000 |
| D-maltose | 342.30 | 0.000 | 0.000 |
| Methylcellulose | ~440 kd | 0.000 | 0.000 |
| potato starch | ~1000 kd | 0.000 | 0.000 |
| D-sucrose | 342.30 | 0.000 | 0.000 |
| egg albumin | ~65 kd | 0.0163 | 5.63 |
| bovine serum albumin | 69.3 kd | 0.0282 | 10.39 |
| Aldehydes, ketones and carboxylic acids | |||
| cinnamic acid | 148.17 | 0.000 | 0.000 |
| citric acid | 192.12 | 0.000 | 0.000 |
| oxalic acid | 126.07 | 0.000 | 0.000 |
| quinic acid | 197.17 | 0.000 | 0.000 |
| sodium tartrate | 196.07 | 0.000 | 0.000 |
| alpha ionone | 192.30 | 0.0043 | 0.0044 |
| 2,3-butanedione | 86.09 | 0.180 | 0.00824 |
| cinnamaldehyde | 132.16 | 0.000 | 0.000 |
| citronellal | 154.25 | 0.000 | 0.000 |
| Inorganic salts | |||
| iron (II) chloride | 126.73 | 0.149 | 0.100 |
| manganese (II) chloride | 125.84 | 0.0432 | 0.0289 |
| sodium nitrite | 85.01 | 0.000 | 0.000 |
| sodium sulfite | 126.04 | 0.0506 | 0.0339 |
| potassium iodide | 166.00 | 0.0224 | 0.0198 |
| Miscellaneous compounds | |||
| caffeine | 194.19 | 0.000 | 0.000 |
| cystamine * | 225.20 | 0.000 | 0.000 |
| glutathione disulfide | 612.60 | 0.000 | 0.000 |
| menthol | 156.27 | 0.000 | 0.000 |
| Substituted salicylic acids | |||
| 3,5-diisopropylsalicylic acid (DIPS) | 222.28 | 0.241 | 0.285 |
| 3,5-dibromosalicylic acid (DBS) | 295.93 | 0.135 | 0.213 |
| 3,5-ditert-butylsalicylic acid (DTBS) | 250.34 | 0.0552 | 0.0735 |
| Metal complexes | |||
| Zn(II)(DIPS)2 | 543.92 | 0.0875 | 0.253 |
| Zn(II)(DTBS)2 | 600.08 | 0.0333 | 0.106 |
| Cu(II)2(DIPS)4 | 1084.25 | 0.0713 | 0.411 |
| Cu(II)2(D BS)4 | 1378.78 | 0.0883 | 0.647 |
| Cu(II)2(DTBS)4 | 1196.47 | 0.0198 | 0.126 |
Cystamine is the disulfide of cysteamine
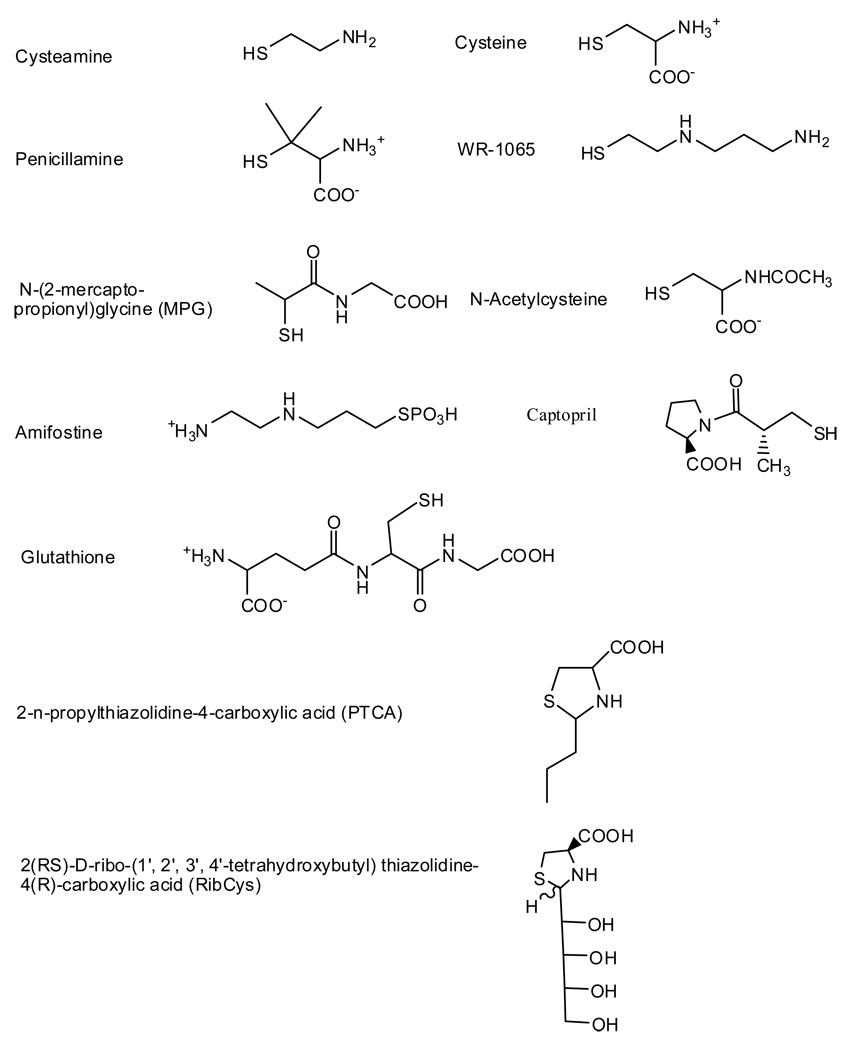
The chemical structures of the thiol derivatives used in the study are shown in Figure 3. Amifostine, captopril, MPG, N-acetylcysteine and penicillamine are currently being used as drugs. Glutathione, PTCA and RibCys are compounds of medicinal interest (7,12,13,14). All thiol derivatives tested had significant activity. In all cases, thiols showed less reactivity than phenolics. Of the thiols tested, amifostine showed the most reactivity while PTCA showed the least. The disulfides tested, glutathione disulfide and cystamine, showed no reactivity under the conditions of the assay. This is in contrast to the 2,2’-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS)assay, where the disulfides show slight reactivity (15).
Figure 3.
Chemical Structures of Thiol Derivatives Used in This Study
Of the vitamin derivatives tested, ascorbic acid, folic acid, folinic acid, NADH, pyridoxine, retinoic acid (Vitamin A derivative), thiamine and Trolox (Vitamin E derivative) had significant activity. Ascorbic and retinoic acids had the greatest reactivity. Since these vitamin derivatives are present in many plants, it would be expected that they would contribute towards the estimation of phenols by the F-C method.
Of the nucleotide bases, adenine showed very slight reactivity while guanine showed considerable reactivity. Thymine and cytosine were unreactive. Of the fatty acids, arachidonic acid had slight reactivity, while the other fatty acids tested showed none. Of the carbohydrates tested, only the trioses glyceraldehyde and dihydroxyacetone showed significant reactivity. The inorganic ions Fe+2, Mn+2, I− and SO3−2 also showed reactivity towards the reagent. Of the aldehydes, ketones and carboxylic acids tested, only butanedione and alpha ionone had significant activity.
Of the amino acids tested, only tyrosine, tryptophan and cysteine had significant reactivity towards the F-C reagent. This would imply that cysteine also makes a major contribution towards the absorbance of proteins in the Folin-Lowry assay. It is also interesting to note that bovine serum albumin was almost twice as reactive towards the reagent as egg albumin, on a mole basis. This would imply that proteins may vary considerably in reactivity towards the F-C reagent used in the Folin-Lowry Assay, based on their relative content of tryptophan, tyrosine and cysteine.
Also, our study found no reactivity, at the concentrations used, for the following compounds which were earlier reported to be reactive towards the F-C reagent: oleic acid, fructose, glycine, histidine, cytosine, sucrose, potassium nitrite and organic acids (3). We also tested several substituted salicylic acid derivatives and their metal complexes. These compounds are of interest because they have been shown to have desirable cytoprotective properties in cellular and animal models (8, 9, 16). It is believed that this cytoprotection is due to their ability to scavenge reactive oxygen species (16).
We are currently studying these compounds in our laboratory. We felt that they would be of relevance to this paper because they are phenolic in nature. Metal ions are found in plants and may complex with phenolics present. Metal ions have been shown to alter the redox properties of phenols (17). Therefore, the effects of metal complexation may be relevant to determination of phenols by this method.
Of the three substituted salicylic acids tested, the 3,5-di-isopropyl (DIPS) and 3,5-dibromo (DBS) salicylic acids had similar reactivity to unsubstituted salicylic acid. However, the 3,5-ditert-butyl (DTBS) derivative was less reactive than salicylic acid. Copper complexation enhanced reactivity of all three substituted salicylic acids, while zinc complexation appeared to have little effect. It is believed that this is because copper (II) is able to serve as a redox carrier, while zinc (II) is not (9, 17, 18). Quercetin has been shown to form a complex with Cu(II) which is more redox-active than uncomplexed quercetin. This observation would support the hypothesis that the copper(II) is serving as a redox carrier (17).
One could propose the following mechanism:
-
reduced phenol Cu(II) complex → oxidized phenol Cu(I) complex
oxidized phenol Cu(I) complex + oxidized F-C reagent → oxidized phenol Cu(II) complex + reduced F-C reagent
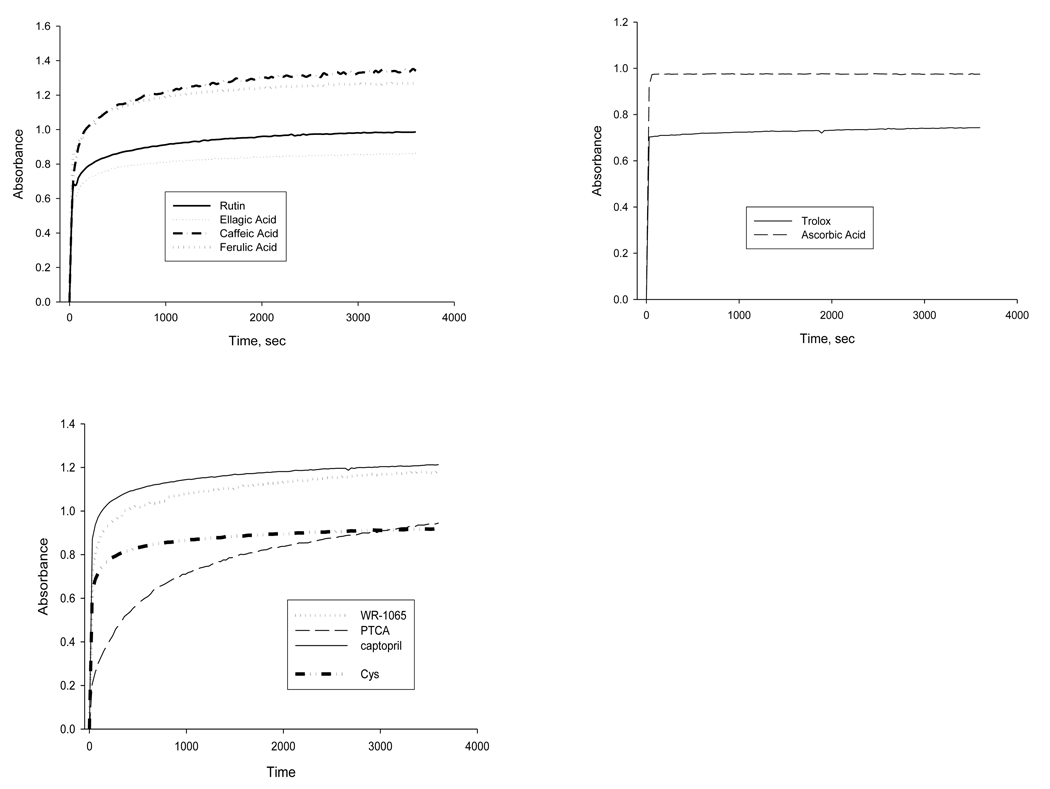
Figure 4 shows the kinetic profiles of several compounds used in this study. Kinetic measurements were made for each compound in this study which showed appreciable reactivity towards the F-C reagent. However, because of similar kinetic patterns observed between many compounds, not all are shown in Figure 4. Compounds not shown in Figure 4 displayed similar kinetic profiles to those shown. Most compounds tested displayed what appeared to be a biphasic kinetic pattern with a very fast and a very slow step. Most compounds had half lives of less than one minute and reached endpoint within one hour. Because of the very rapid initial rates, reaction order or rate constants could not be determined by methods we used.
Figure 4.
Reaction Kinetics of Selected Compounds towards the FC Reagent
The phenolics showed slight variability, with caffeic acid and curcumin showing the slowest kinetics. The thiols tested showed a similar pattern to the phenolics. The only exception was the thiazolidine derivative PTCA which showed considerably slower kinetics. The low GAE value of PTCA compared to those of the other thiol derivatives may be because the reaction of this compound with the F-C reagent had not reached endpoint under the conditions used. Of the compounds tested, Trolox and ascorbic acid showed the fastest kinetics, reaching endpoint in less than one minute. In an earlier study, we noted that Trolox and ascorbic acid displayed a similar kinetic pattern in their reactivity towards the ABTS radical cation (15).
This study showed that many nonphenolic compounds do indeed show considerable reactivity towards the F-C reagent. Therefore, our findings confirm the observations of other investigators that the assay should not be viewed as a measure of total phenolic content, but rather a measure of overall antioxidant capacity, similar to the ABTS assay (3, 19). In fact, studies have shown a strong correlation between the F-C and ABTS assays (19).
The F-C assay is normally described as an antioxidant capacity (AOX) method which involves a single electron transfer (SET). It is believed that a single electron is transferred from the substrate, presumably a phenol, to the complexed Mo(VI) in the reagent. In this process, Mo(VI) is reduced to Mo(V) (3). If this mechanism is correct, one might expect the initial product formed from a phenolic substrate to be a semiquinone. Semiquinones have been identified as the initial product formed upon reaction of phenols with the ABTS radical cation (18). The mechanism of the reaction of the F-C reagent with phenols and other compounds needs to be further explored.
The F-C assay is simple to perform, straightforward and yields consistent results. The similarities between the F-C and the ABTS assay suggest that the F-C assay, like the ABTS assay, may be useful for assaying compounds of biomedical interest for antioxidant activity. The F-C method has the advantage of not requiring an overnight incubation time for preparation of reagents.
In most plants, phenolics are the most abundant antioxidants present (19). Therefore, the F-C assay gives a good “ballpark” estimation of total phenolic content for most plants. Correction factors for Vitamin C have been developed. These involve both the use of specific assays for Vitamin C and extraction methods which remove most Vitamin C (20). More precise assays specific for phenols need to be explored.
ACKNOWLEDGEMENTS
Funding for the project was obtained from Arkansas Space Grant Consortium and NIH Grant Number P20 RR-16460 from the IDeA Networks of Biomedical Research Excellence (INBRE) Program of the National Center for Research Resources.
LITERATURE CITED
- 1.Folin O. Tyrosine and tryptophan determination in proteins. J. Biol Chem. 1927;73:649–673. [Google Scholar]
- 2.Singleton V, Rossi J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 3.Prior R, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 4.Lowry O, Rosebrough J, Farr A, Randall R. Protein measurement with the Folin-Phenol reagents. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 5.Ikawa M, Schafer T, Dollard C, Sasner J. Utilization of Folin-Ciocalteu reagent for the detection of certain nitrogen compounds. J. Agric. Food Chem. 2003;51:1811–1815. doi: 10.1021/jf021099r. [DOI] [PubMed] [Google Scholar]
- 6.Andjelkovik M, Van Camp J, Pedra M, Renders K, Socaciu, Verhe’ R. Correlations of the phenolic compounds and the phenolic content in some Spanish and French olive oils. J.Agric. Food Chem. 2008;56:5181–5187. doi: 10.1021/jf0724419. [DOI] [PubMed] [Google Scholar]
- 7.Nagasawa HT, Goon DJW, Muldoon WP, Zera RT. 2-Substituted thiazolidine-4-carboxylic acids as prodrugs of L-cysteine. Protection of mice against acetaminophen toxicity. J. Med. Chem. 1984;27:591–596. doi: 10.1021/jm00371a006. [DOI] [PubMed] [Google Scholar]
- 8.Sorenson JRJ, Soderberg LSF, Chang LW, Willingham WM, Baker ML, Barnett JB, Salari H, Bond K. Copper, iron, manganese, and zinc 3,5- diisopropylsalicylate chelates increase survival of gamma irradiated mice. European J. Med. Chem. 1993;28:221–229. [Google Scholar]
- 9.Wangila GW, Nagothu KK, Steward R, III, Bhatt R, Iyere PA, Willingham WM, Sorenson JRJ, Shah SV, Portilla D. “Prevention of cisplatin-induced kidney epithelial cell apoptosis with a Cu superoxide dismutase-mimetic [CopperII2(3, 5-ditertiarybutylsalicylate)4(ethanol)4]”. Toxicology in Vitro. 2006;20:1300–1312. doi: 10.1016/j.tiv.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Singleton V, Orthofer R, Lamuela-Reventos R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods in Enzymology. 1999;299:152–178. [Google Scholar]
- 11.Apak R, Güçlü K, Demirata B, Özyürek M, Çelic S, Bektaşoğlu B, Berker I, Özyur D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12:1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: The first selective-target and broad spectrum radioprotector. Oncologist. 2007;12:738–747. doi: 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JC, Francetic DJ. Mechanism of chemoprotection by RibCys: A thiazolidine prodrug of L-cysteine. Med. Chem. Res. 1991;1:213–219. [Google Scholar]
- 14.Atkinson MC. The use of N-acetylcysteine in intensive care. Crit Care Resusc. 2002;4:21–27. [PubMed] [Google Scholar]
- 15.Walker RB, Everette JD. Comparative reaction rates of various antioxidants with ABTS radical cation. J. Agric. Food Chem. 2009;57:1156–1161. doi: 10.1021/jf8026765. [DOI] [PubMed] [Google Scholar]
- 16.Sorenson JRJ. Cu, Fe and Mn chelates offer a medicinal chemistry approach to overcoming radiation injury. Current Medicinal Chemistry. 2002;9:639–662. doi: 10.2174/0929867023370725. [DOI] [PubMed] [Google Scholar]
- 17.El Hajji H, Nkhili E, Tomao V, Dangles O. Interactions of quercetin with iron and copper ions: Complexation and autoxidation. Free Radical Res. 2006;40:303–320. doi: 10.1080/10715760500484351. [DOI] [PubMed] [Google Scholar]
- 18.Huang D, Ou B, Prior RL. The chemistry behind antioxidant assays. J. Agric. Food. Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 19.Dudonne’ S, Vitrac X, Coutiere P, Woillez M, Merillon J. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD and ORAC assays. J. Agric. Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 20.Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nature Protocols. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]