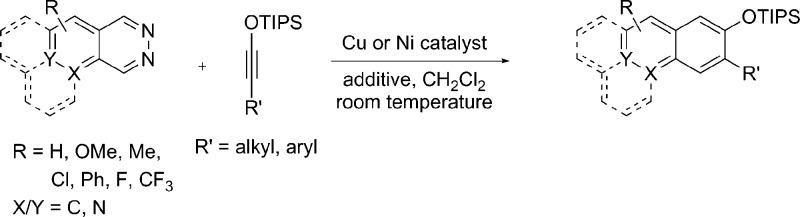
Abstract
Copper(I) and nickel(0) complexes catalyze the formal [4 + 2] cycloaddition reactions of 1,2-diazines and siloxyalkynes, a reaction hitherto best catalyzed by silver salts. These catalysts based on earth abundant metals are not only competent, but the copper catalyst, in particular, promotes cycloadditions of pyrido[2,3-d]pyridazine and pyrido[3,4-d]pyridazine, enabling a new synthesis of quinoline and isoquinoline derivatives, as well as the formal [2 + 2] cycloaddition reaction of cyclohexenone with a siloxyalkyne.
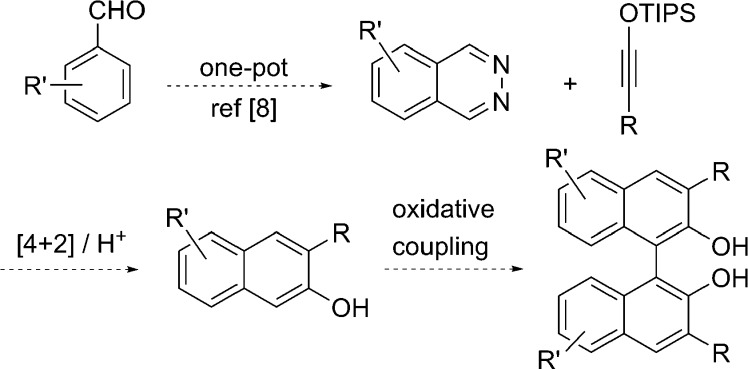
The axially chiral 1,1′-binaphthalene-2,2′-diol (binol) has impacted asymmetric catalysis profoundly.1 Used widely as a chiral ligand and as a precursor to chiral ligands and catalysts, binol is best prepared by the oxidative dimerization of 2-naphthol.1,2 Binol derivatives, particularly those with substituents at the 3,3′-positions, allow fine-tuning of steric and electronic properties as well as sculpting of the scaffold’s chiral environment, thereby greatly enhancing the utility of this scaffold.3−5 Such 3,3′-disubstituted binols are nearly always made through a multistep sequence starting from a preformed binol, since the direct oxidative dimerization of 3-substituted naphthols is impractical given the limited availability of such naphthol precursors.6 Recently, we reported a general route to 3-substituted naphthol silyl ethers via a silver salt-catalyzed formal inverse electron-demand Diels–Alder (IEDDA) reaction between phthalazines and siloxyalkynes.7 Since phthalazines are prepared by a one-pot procedure from aromatic aldehydes,8 this cycloaddition methodology provides direct access to a variety of 2-hydroxynaphthalene derivatives,9 potential precursors to modified binol ligands (Scheme 1).
Scheme 1. Route to 3-Substituted 2-Naphthols.
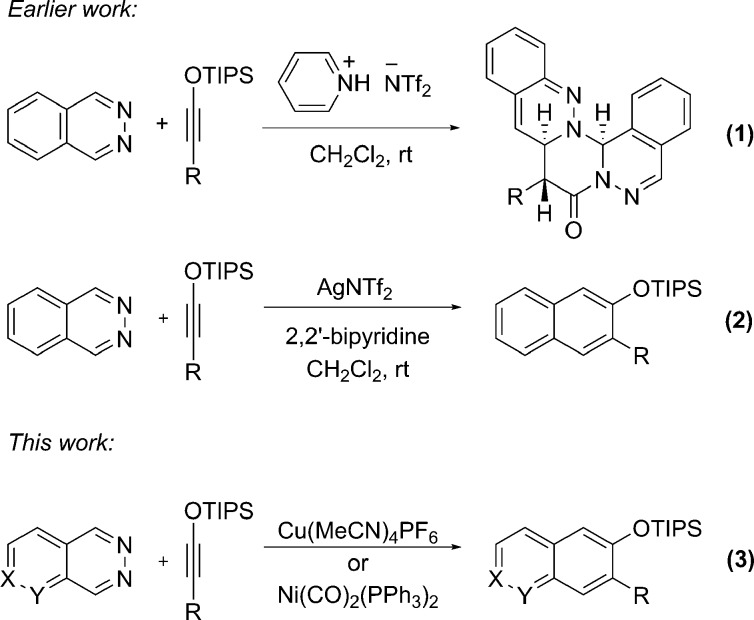
Nearly all reported IEDDA reactions of heterocyclic azadienes are thermal processes, typically requiring harsh conditions.10 Our interest in hydrogen-bonding catalysis11 prompted us to explore pyridinium salts designed to form two hydrogen bonds to the phthalazine nitrogen atoms as catalysts for the IEDDA reaction between phthalazine and electron-rich alkynes.12 The reaction with siloxyalkynes,13 while promoted by dual hydrogen bond donors, was catalyzed even by simple pyridinium salts and afforded, rather than the anticipated 2-naphthol derivative, an intriguing tetra-azapentacyclic compound, arising from a formal [2 + 2 + 2] cycloaddition between two phthalazines and a siloxyalkyne (Scheme 2, eq 1).14 Inspired by the ample precedent with normal electron demand Diels–Alder reactions,15 we evaluated various metals for promoting the desired IEDDA reaction and discovered that Ag(I) salts, especially when paired with bidentate N-donor ligands such as 2,2′-bipyridine and 1,10-phenanthroline, catalyzed the desired process to afford 3-substituted 2-naphthol silyl ethers in good yields (Scheme 2, eq 2).7,16 To make this strategy more versatile and practical, we have examined complexes of earth abundant metals as catalysts for the IEDDA reaction and report here that Cu(I) and Ni(0) complexes can supplant Ag(I) salts for these cycloadditions (Scheme 2, eq 3).17 Significantly, the copper salt catalyzes not only the cycloaddition of a broader range of substrates but also the [2 + 2] cycloaddition reaction between a siloxyalkyne and cyclohexenone, a transformation previously catalyzed by silver salts.13c
Scheme 2. Cycloaddition Reactions of 1,2-Diazines and Siloxyalkynes.
The contrasting reactivity displayed by pyridinium salts and silver salts combined with a desire to identify non-precious metals for catalysis motivated us to examine a broader range of metal complexes for the IEDDA reaction of phthalazine (1) and 1-siloxyhexyne (2) (Table 1). Commonly employed Lewis acids, such as ZnBr2, TiCl4, Yb(OTf)3, Sc(OTf)3, Bi(OTf)3, Co(BF4)2, La(OTf)3, In(OTf)3, and BF3·OEt2, all failed to catalyze the reaction.18 Interestingly, while NiCl2 was also ineffective, the corresponding Ni(0) complexes, Ni(PPh3)4 and Ni(cod)2, afforded the desired cycloadduct 3 in 21% and 43% yields, respectively (entries 1 and 2). This result suggests that the metal is not activating the diazine simply through Lewis acid/base interaction, since Ni(0) complexes are expected to be weakly Lewis acidic, certainly compared to NiCl2. We were pleased to find that Ni(CO)2(PPh3)2, at 10 mol % catalyst loading, nicely catalyzed the cycloaddition at room temperature, affording the product in 80% yield (entry 3). Further addition of PPh3 or P(OMe)3 to the Ni(0) complex gave the product in diminished yields (entries 4 and 5). Isoelectronic with Ag(I) and Ni(0), Cu(I) salts were expected to also catalyze the cycloaddition reactions. Indeed, CuCl when used in conjunction with 2,2′-bipyridine did catalyze the reaction, albeit poorly (5% yield). The efficacy of Cu(I) salts improved with decreasing nucleophilicity of the counterion, as evidenced by increasing yields on going from CuI to CuCl to Cu(OTf). Even better results were obtained with highly dissociated counterions, such as PF6– and BF4– (entries 7–10). The best result was obtained using 10 mol % of tetrakis(acetonitrile)copper(I) hexafluorophosphate with 2,2′-bipyridine as the ligand, which provided naphthalene 3 in 80% isolated yield after 4 h reaction time (entry 11). Several other ligands were examined, but they did not better the yields (entries 12–15). Reactions proceeded in lower yields in acetonitrile and 1,4-dioxane, and no product was formed in chloroform or in protic solvents, such as methanol and water. The interchangeable use of silver, copper, or nickel complexes for the catalysis of these formal cycloadditions is noteworthy.17,19
Table 1. Reaction Development and Optimizationa.
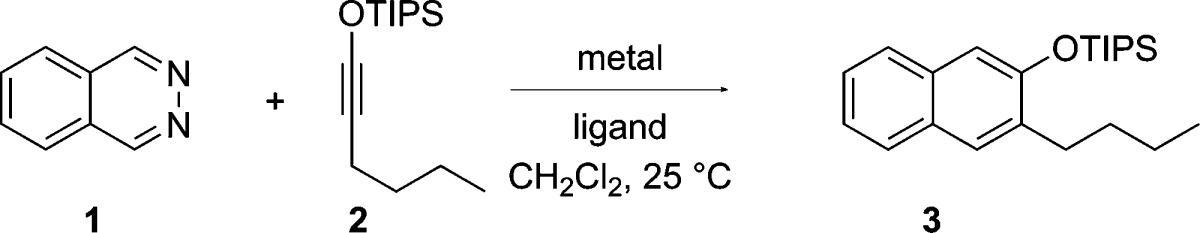
| entry | catalyst (mol %) | ligand (mol %) | time (h) | yieldb (%) |
|---|---|---|---|---|
| 1 | Ni(PPh3)4 (10) | 24 | 21 | |
| 2 | Ni(cod)2 (10) | 24 | 43 | |
| 3 | Ni(CO)2(PPh3)2 (10) | 24 | 81 (80)c | |
| 4 | Ni(CO)2(PPh3)2 (10) | PPh3 (10) | 24 | 67 |
| 5 | Ni(CO)2(PPh3)2 (10) | P(OMe)3 (10) | 24 | 35 |
| 6d | CuOTf (5) | bpy (5) | 24 | 34 |
| 7d | Cu(MeCN)4OTf (5) | bpy (5) | 24 | 38 |
| 8d | Cu(MeCN)4PF6 (5) | bpy (5) | 24 | 49 |
| 9 | Cu(MeCN)4PF6 (10) | bpy (10) | 4 | 79 |
| 10 | Cu(MeCN)4BF4 (10) | bpy (10) | 4 | 67 |
| 11 | Cu(MeCN)4PF6 (10) | bpy (7.5) | 4 | 83 (80)c |
| 12 | Cu(MeCN)4PF6 (10) | 1,10-phenanthroline (10) | 24 | 32 |
| 13 | Cu(MeCN)4PF6 (10) | pyr (20) | 16 | 72 |
| 14 | Cu(MeCN)4PF6 (10) | 4,4′-di-tert-butyl-2,2′-dipyridine (10) | 24 | 60 |
| 15 | Cu(MeCN)4PF6 (10) | 2,2′:6′,2″-terpyridine (10) | 24 | 28 |
All reactions were carried out using 0.5 mmol of 1 and 1.0 mmol of 2 in 1 mL of CH2Cl2.
Yields were determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard.
Isolated yield.
Reaction was carried out using 1.5 equiv of 2 per equivalent of 1 in CH2Cl2 (0.5 mmol of 1 in 1.0 mL of CH2Cl2).
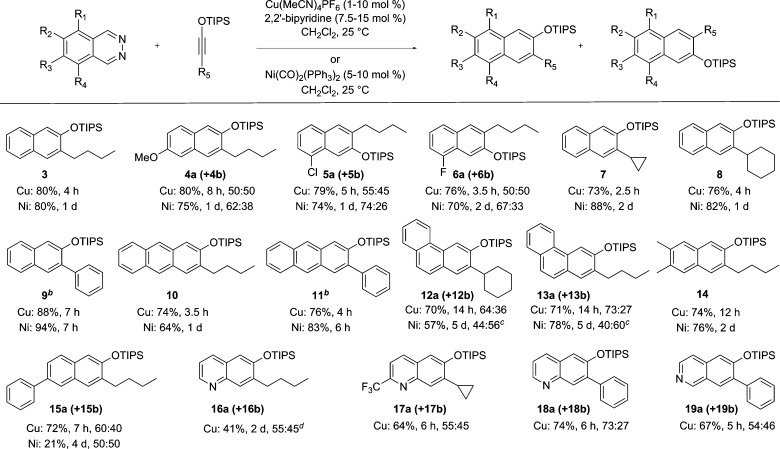
Having defined effective conditions for Cu(I)- and Ni(0)-catalyzed cycloaddition of the parent system, we next evaluated the substrate scope for the new catalyst systems (Figure 1). The two catalysts were expected to behave differently, since Ni(CO)2(PPh3)2 is hindered, having a sterically crowded neutral Ni(0) center, whereas Cu(I), as a soft Lewis acid, was expected to coordinate more strongly.20 Indeed, as noted above, while both provided silyl ether 3 in the same yield, the reaction was noticeably faster with the Cu(I) catalyst. Additionally, the two complexes displayed differing regioselectivities with unsymmetrical phthalazines. Whereas Cu(I) gave 1:1 mixture of the two regioisomeric products of 6-methoxyphthalazine, the Ni(0) catalyst gave 4 in a 1.5:1 preference over its regioisomer (not shown). A similar preference was observed with 5-chlorophthalazine and 5-fluorophthalazine, which produced naphthalenes 5 and 6, respectively, as the major products. In these instances, the slower reaction was also the more regioselective one. Other alkyl- and arylsiloxyalkynes were examined, and all gave the naphthalene products (7–9) in good yields. Benzo-fused derivatives of phthalazine enabled the synthesis of anthracene and phenanthrene derivatives. Thus, benzo[g]phthalazine gave anthracene products 10 and 11, whereas benzo[f]phthalazine gave phenanthrenes 12 and 13.
Figure 1.
Investigation of Cu(I)/Ni(0) catalyzed formal [4 + 2] cycloaddition reaction of 1,2-diazines and siloxyalkynes. (a) Unless otherwise stated, reactions were carried out with 10 mol % of copper(I) or nickel(0) catalyst with with 2.0 equiv of siloxyalkyne. Yields are for the chromatographically purified products. The major regioisomeric product is shown. (b) Carried out using 1 mol % of Cu(MeCN)4PF6 or 5 mol % of Ni(CO)2(PPh3)2 (1.5 equiv of siloxyalkyne). (c) Note that Ni(0) catalyst affords the other regioisomer (not shown) as the major product. (d) Reaction was carried out in refluxing DCE.
We had recognized early on that extending the IEDDA reactions to pyridopyridazines would open up a new route to quinolines and isoquinolines but had found the silver catalyst to be ineffective with most such substrates.21 We are delighted to note that this limitation can be overcome with the Cu catalyst. The reaction of pyrido[2,3-d]pyridazine and 1-siloxyhexyne (2), under standard Cu-catalysis conditions, gave small amounts of the quinoline product. When the same reaction was carried out in refluxing DCE, siloxyquinoline 16 was isolated in 41% yield, as a mixture of regioisomers.22 The related trifluoromethyl-substituted pyrido[2,3-d]pyridazine reacted smoothly at room temperature, thus affording the corresponding product mixture 17 in 64% yield.23 As observed with phthalazine (1), the reaction with the phenyl-substituted siloxyalkyne was more facile, giving the regioisomers of siloxyquinoline 18 in 74% yield. Similar success was enjoyed in the reaction between pyrido[3,4-d]pyridazine and phenylsiloxyalkyne, which gave the regioisomeric isoquinoline products 19 in 67% yield.
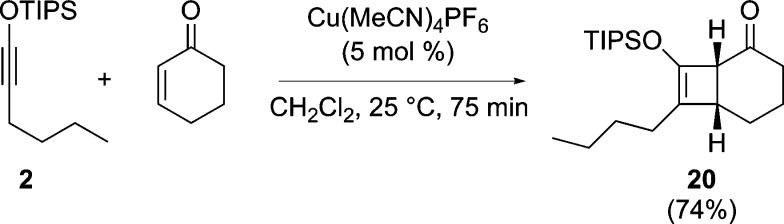
The fruitful switch from a silver catalyst to a nickel or copper catalyst spurred a brief look at other reactions that might be amenable to such a change. Among the reactions of siloxyalkynes, we examined its formal [2 + 2]-cycloaddition with cyclohexenone, a transformation reported by Kozmin et al. to be catalyzed by AgNTf2.13c Treatment of a solution of the two reactants with 5 mol % of Cu(MeCN)4PF6 promoted its clean conversion to the [2 + 2]-cycloadduct (20), isolated in 74% yield (Scheme 3).24
Scheme 3. Cu(I) Catalysis of a formal [2 + 2] Cycloaddition Reaction of Siloxyalkynes.
In conclusion, we have demonstrated that Cu(I) and Ni(0) complexes catalyze the formal [4 + 2] cycloaddition reactions of 1,2-diazines and siloxyalkynes to give siloxy derivatives of naphthalene, anthracene, and phenenathrene. The copper catalyst was also effective in promoting the corresponding cycloaddition to generate quinoline and isoquinoline derivatives, as well as for the [2 + 2]-cycloaddition of siloxyalkyne and cyclohexenone. In more general terms, this study demonstrates the feasibility of switching from a precious metal to more economical, isoelectronic metals for catalyzing reactions.
Acknowledgments
We thank the National Institutes of Health for supporting this work (P50GM086145 and GM 069990).
Supporting Information Available
Experimental procedures, characterization, and copies of 1H and 13C NMR spectra for all new compounds being reported in the text. This material is available free of charge via the Internet at http://pubs.acs.org
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Brunel J. M. Chem. Rev. 2005, 105, 857. [DOI] [PubMed] [Google Scholar]; b Wang H. Chirality 2010, 22, 827. [DOI] [PubMed] [Google Scholar]
- For selected examples of oxidative dimerization of naphthols, see:; a Guo Q.-X.; Wu Z.-J; Luo Z.-B.; Liu Q.-Z.; Ye J.-L.; Luo S.-W; Cun L.-F.; Gong L.-Z. J. Am. Chem. Soc. 2007, 129, 13927. [DOI] [PubMed] [Google Scholar]; b Egami H.; Matsumoto K.; Oguma T.; Kunisu T.; Katsuki T. J. Am. Chem. Soc. 2010, 132, 13633. [DOI] [PubMed] [Google Scholar]
- Reviews:; a Chen Y.; Yekta S.; Yudin A. K. Chem. Rev. 2003, 103, 3155. [DOI] [PubMed] [Google Scholar]; b Berthod M.; Mignani G.; Woodward G.; Lemaire M. Chem. Rev. 2005, 105, 1801. [DOI] [PubMed] [Google Scholar]; c Shibasaki M.; Matsunaga S. Chem. Soc. Rev. 2006, 35, 269. [DOI] [PubMed] [Google Scholar]; d Pereira M. M.; Calvete M. J. F.; Carrilho R. M. B.; Abreu A. R. Chem. Soc. Rev. 2013, 42, 6990. [DOI] [PubMed] [Google Scholar]
- For selected examples of binol derivatives in catalysis, see:; a Bao J.; Wulff W. D.; Dominy J. B.; Fumo M. J.; Grant E. B.; Rob A. C.; Whitcomb M. C.; Yeung S.-M.; Ostrander R. L.; Rheingold A. L. J. Am. Chem. Soc. 1996, 118, 3392. [Google Scholar]; b Knöpfel T. F.; Aschwanden P.; Ichikawa T.; Watanabe T.; Carreira E. M. Angew. Chem., Int. Ed. 2004, 43, 5971. [DOI] [PubMed] [Google Scholar]; c Unni A. K.; Takenaka N.; Yamamoto H.; Rawal V. H. J. Am. Chem. Soc. 2005, 127, 1336. [DOI] [PubMed] [Google Scholar]; d Li G.-Q.; Gao H.; Keene C.; Devonas M.; Ess D. H.; Kürti L. J. Am. Chem. Soc. 2013, 135, 7414. [DOI] [PubMed] [Google Scholar]
- A particularly important class of binol derivatives are chiral phosphoric acids:; a Connon S. J. Angew. Chem., Int. Ed. 2006, 45, 3909. [DOI] [PubMed] [Google Scholar]; b Terada M. Synthesis 2010, 1929. [Google Scholar]; For pioneering applications, see:; c Akiyama T.; Itoh J.; Yokota K.; Fuchibe K. Angew. Chem., Int. Ed. 2004, 43, 1566. [DOI] [PubMed] [Google Scholar]; d Uraguchi D.; Terada M. J. Am. Chem. Soc. 2004, 126, 5356. [DOI] [PubMed] [Google Scholar]
- a Simonsen K. B.; Gothelf K. V.; Jørgensen K. A. J. Org. Chem. 1998, 63, 7536. [DOI] [PubMed] [Google Scholar]; b Maruoka K.; Itoh T.; Araki Y.; Shirasaka T.; Yamamoto H. Bull. Chem. Soc. Jpn. 1998, 61, 2975. [Google Scholar]; c Huang W.-S.; Pu L. Tetrahedron Lett. 2000, 41, 145. [Google Scholar]; d Romanov-Michailidis F.; Guénée L.; Alexakis A. Angew. Chem., Int. Ed. 2013, 52, 9266. [DOI] [PubMed] [Google Scholar]
- Türkmen Y. E.; Montavon T. J.; Kozmin S. A.; Rawal V. H. J. Am. Chem. Soc. 2012, 134, 9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. N.; Wegner H. A. Org. Lett. 2012, 14, 3268. [DOI] [PubMed] [Google Scholar]
- For reviews on the synthesis of substituted naphthalenes, see:; a Katritzky A. R.; Li J.; Xie L. Tetrahedron 1999, 55, 8263. [Google Scholar]; b Rousseau A. L.; de Koning C. B.; van Otterlo W. A. L. Tetrahedron 2003, 59, 7. [Google Scholar]; For recent examples for the synthesis of 2-naphthols, see:; c Juteau H.; Gareau Y.; Lachance H. Tetrahedron Lett. 2005, 46, 4547. [Google Scholar]; d Zhang X.; Sarkar S.; Larock R. C. J. Org. Chem. 2006, 71, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Dai Y.; Feng X.; Liu H.; Jiang H.; Bao M. J. Org. Chem. 2011, 76, 10068. [DOI] [PubMed] [Google Scholar]; f Xia Y.; Qu P.; Liu Z.; Ge R.; Xiao Q.; Zhang Y.; Wang J. Angew. Chem., Int. Ed. 2013, 52, 2543. [DOI] [PubMed] [Google Scholar]; g He Y.; Zhang X.; Shen N.; Fan X. J. Org. Chem. 2013, 78, 10178. [DOI] [PubMed] [Google Scholar]; h Chang M.-Y.; Chan C.-K.; Lin S.-Y. Tetrahedron 2013, 69, 1532. [Google Scholar]
- For reviews on Diels–Alder reactions of azadienes, see:; a Boger D. L. Tetrahedron 1983, 39, 2869. [Google Scholar]; b Boger D. L. Chem. Rev. 1986, 86, 781. [Google Scholar]; c Foster R. A. A.; Willis M. C. Chem. Soc. Rev. 2013, 42, 63. [DOI] [PubMed] [Google Scholar]; For selected examples of Diels–Alder reactions of 1,2-diazines, see:; d Higashino T.; Miyashita A.; Iwamoto K.; Taido N.; Oishi E. Chem. Pharm. Bull. 1990, 38, 3268. [Google Scholar]; e Coleman R. S.; Boger D. L. J. Am. Chem. Soc. 1987, 109, 2717. [Google Scholar]; f Rastogi S. K.; Medellin D. C.; Kornienko A. Org. Biomol. Chem. 2014, 12, 410. [DOI] [PubMed] [Google Scholar]
- a Huang Y.; Unni A. K.; Thadani A. N.; Rawal V. H. Nature 2003, 424, 146. [DOI] [PubMed] [Google Scholar]; b Malerich J. P.; Hagihara K.; Rawal V. H. J. Am. Chem. Soc. 2008, 130, 14416. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Türkmen Y. E.; Rawal V. H. J. Org. Chem. 2013, 78, 8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For bidentate Lewis acid activation of 1,2-diazines, see:; a Kessler S. N.; Wegner H. A. Org. Lett. 2010, 12, 4062. [DOI] [PubMed] [Google Scholar]; b Kessler S. N.; Neuburger M.; Wegner H. A. Eur. J. Org. Chem. 2011, 3238. [Google Scholar]; c Kessler S. N.; Neuburger M.; Wegner H. A. J. Am. Chem. Soc. 2012, 134, 17885. [DOI] [PubMed] [Google Scholar]; d Kessler S. N.; Wegner H. A. Synlett 2012, 5, 699. [Google Scholar]; e Schweighauser L.; Bodoky I.; Kessler S. N.; Häussinger D.; Wegner H. A. Synthesis 2012, 44, 2195. [Google Scholar]; f Bader S. L.; Kessler S. N.; Zampese J. A.; Wegner H. A. Monatsh. Chem. 2013, 144, 531. [Google Scholar]
- For other related reactions of siloxyalkynes, see:; a Lal G. S.; Kowalski C. J. J. Am. Chem. Soc. 1988, 110, 3693. [Google Scholar]; b Brisbois R. G.; Kowalczyk J. L.; Miller R. F.; Danheiser R. L. J. Am. Chem. Soc. 1990, 112, 3093. [Google Scholar]; c Sweis R. F.; Schramm M. P.; Kozmin S. A. J. Am. Chem. Soc. 2004, 126, 7442. [DOI] [PubMed] [Google Scholar]; d Zhang L.; Kozmin S. A. J. Am. Chem. Soc. 2004, 126, 10204. [DOI] [PubMed] [Google Scholar]; e Zhang L.; Kozmin S. A. J. Am. Chem. Soc. 2004, 126, 11806. [DOI] [PubMed] [Google Scholar]; f Austin W. F.; Zhang Y.; Danheiser R. L. Org. Lett. 2005, 7, 3905. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Qi X.; Ready J. M. Angew. Chem., Int. Ed. 2008, 47, 7068. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Zhao W.; Wang Z.; Sun J. Angew. Chem., Int. Ed. 2012, 51, 6209. [DOI] [PubMed] [Google Scholar]; i Cabrera-Pardo J. R.; Chai D. I.; Liu S.; Mrksich M.; Kozmin S. A. Nat. Chem. 2013, 5, 423. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Zhao W.; Li Z.; Sun J. J. Am. Chem. Soc. 2013, 135, 4680. [DOI] [PubMed] [Google Scholar]; k Cabrera-Pardo J. R.; Chai D. I.; Kozmin S. A. Adv. Synth. Catal. 2013, 355, 2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montavon T. J.; Türkmen Y. E.; Shamsi N. A.; Miller C.; Sumaria C. S.; Rawal V. H.; Kozmin S. A. Angew. Chem., Int. Ed. 2013, 52, 13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. A.; Johnson J. S.; Jacobsen E. N.; Pfaltz A.; Yamamoto H.. Comprehensive Asymmetric Catalysis; Springer: New York, 1999; Vol. 3, p 1177.and references cited therein. [Google Scholar]
- For a study of ternary Ag(I) complexes of 1,2-diazines and chelating heteroarenes, which found two silver atoms bound to a bridging pyridazine unit, see:Türkmen Y. E.; Sen S.; Rawal V. H. CrystEngComm 2013, 15, 4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock R. M., Ed. Catalysis without Precious Metals; Wiley-VCH: Weinheim, 2010. [Google Scholar]
- See the Supporting Information for a full list of catalysts evaluated.
- For an example of silver and copper complexes giving two different products from the same precursor, see:Fang Y.; Wang C.; Su S.; Yu H.; Huang Y. Org. Biomol. Chem. 2014, 12, 1061. [DOI] [PubMed] [Google Scholar]
- Ho T.-L. Chem. Rev. 1975, 75, 1. [Google Scholar]
- Other potential azadiene precursors to heterocycles were evaluated. See the Supporting Information for details.
- The Ag(I) and Ni(0) catalysts did not promote this reaction.
- Interestingly, the reaction of the trifluoromethyl-substituted pyrido[2,3-d]pyridazine, wherein the adjacent nitrogen atom is expected to be less basic, can also be catalyzed by 5 mol% AgNTf2 to give the cycloadduct in 71% yield. See the Supporting Information for a detailed procedure.
- This reaction was not catalyzed by Ni(CO)2(PPh3)2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.