Abstract
Objective
Opioid pharmacotherapy is now the leading treatment for chronic pain, a problem that affects nearly one-third of the United States population. Given the dramatic rise in prescription opioid misuse and opioid-related mortality, novel behavioral interventions are needed. The purpose of this study was to conduct an early stage randomized controlled trial of Mindfulness-Oriented Recovery Enhancement (MORE), a multimodal intervention designed to simultaneously target mechanisms underpinning chronic pain and opioid misuse.
Method
Chronic pain patients (N=115; mean age = 48±14; 68% female) were randomized to 8 weeks of MORE or a Support Group (SG). Outcomes were measured at pre- and post-treatment, and at 3-month follow-up. The Brief Pain Inventory assessed changes in pain severity and interference. Changes in opioid use disorder status were measured by the Current Opioid Misuse Measure. Desire for opioids, stress, nonreactivity, reinterpretation of pain sensations, and reappraisal were also evaluated.
Results
MORE participants reported significantly greater reductions in pain severity (p = .038) and interference (p = .003) than SG participants, which were maintained by 3-month follow-up and mediated by increased nonreactivity and reinterpretation of pain sensations. Compared with SG participants, participants in MORE evidenced significantly less stress arousal (p = .034) and desire for opioids (p = .027), and were significantly more likely to no longer meet criteria for opioid use disorder immediately following treatment (p = .05); however, these effects were not sustained at follow-up.
Conclusions
Findings demonstrate preliminary feasibility and efficacy of MORE as a treatment for co-occurring prescription opioid misuse and chronic pain.
Keywords: addiction, chronic pain, mindfulness, opioid, cognitive reappraisal
Prescription opioid misuse is an emerging public health concern with significant health and psychological risks. Though opioid analgesic therapy for chronic pain is often efficacious, and most patients take medicine as prescribed, some individuals exhibit addictive tendencies toward opioids (Fishbain, Cole, Lewis, Rosomoff, & Rosomoff, 2007). Opioid addiction among chronic pain patients involves cognitive, affective, and behavioral dysregulation that, when coupled with persistent or worsening pain, may result in significant functional impairment and suffering (Højsted, Nielsen, Guldstrand, Frich, & Sjøgren, 2010). Opioid addiction may be presaged by the occurrence of opioid misuse behaviors, such as dose escalation or use of prescribed opioids to self-medicate negative emotions and stress (Butler et al., 2007); these medication-misusing behaviors are common, with more than one-in-ten chronic pain patients exhibiting signs of opioid misuse (Fishbain et al., 2007). Although opioid agents with lower addiction liability like buprenorphine can effectively substitute for unauthorized opioid use (Ling et al., 2002), extant treatments for opioid addiction are typically ineffective in the absence of ongoing maintenance pharmacotherapy (Weiss et al., 2011). Further, persons seeking treatment for chronic pain respond especially poorly to motivational and behavioral addiction treatments (Larson et al., 2007). Current best practices for persons with chronic pain who are at risk for prescription opioid misuse and addiction (e.g., Jamison, Serraillier, & Michner, 2011; Oliver et al., 2012) include frequent opioid adherence monitoring, opioid treatment agreements and compliance training, and cognitive-behavioral substance misuse counseling (Jamison et al., 2010). Yet, new interventions are needed to effectively address the maladaptive cognitive-affective processes and appetitive responses elicited by pain, stress, and drug-related cues that undergird the risk chain from chronic pain to opioid misuse and addiction.
This risk chain initiates from prolonged use of opioids, which produces physical dependence via neuroadaptations resulting in tolerance, withdrawal, and, in some cases, opioid-induced hyperalgesia (Chu, Angst, & Clark, 2008). Heightened pain sensitivity, when coupled with tolerance to the analgesic effects of opioids, can result in increased opioid craving (Ren, Shi, Epstein, Wang, & Lu, 2009) and consumption (Martell et al., 2007), and can elicit negative emotions that feed back to magnify pain perception. This process may result in appraisal of pain sensations as threatening and perseveration on the affective components of pain (Garland, 2012). Consequently, opioids are often misused to self-medicate (Khantzian, 1997; Kirsh, Jass, Bennett, Hagen, & Passik, 2007) the negative affective states and autonomic arousal that cause, co-occur with, or result from pain (Jänig, 1995; Martenson, Cetas, & Heinricher, 2009). As with pain, stress and negative affect can become internal cues associated with past opioid use episodes that elicit the habit of opioid use, particularly among opioid misusers who take opioids to cope with emotional distress. Concomitantly, the habitual drive to engage in prescription opioid misuse (including unauthorized dose escalation) involves implicit neurocognitive operations that promote craving and aberrant drug taking (Goldstein et al., 2009; Stacy & Wiers, 2010) by biasing attention towards opioid-related cues (e.g., the sight of a pill bottle) (Garland, Froeliger, Passik, & Howard, 2012). Theory suggests that addiction occurs when the appetitive motivation to obtain natural rewards is re-organized around seeking drug-induced reward and the desire to alleviate dysphoria stemming from withdrawal and aversive experiences (e.g., pain and stress) (Alcaro & Panksepp, 2011; Koob & Le Moal, 2008). In that regard, decreased responsiveness to natural reinforcers has been observed among opiate dependent individuals and is robustly predictive of future opiate consumption (Lubman et al., 2009; Lubman, Allen, Peters, & Deakin, 2007, 2008).
Hence, the problem of co-occurring chronic pain and prescription opioid misuse may involve a cycle of behavioral escalation where nociception and stress amplify pain and provoke recurrent self-medication with opioids, which in turn biases attention towards opioid-related cues that come to elicit the habit of opioid use despite ever diminishing analgesia and increasing dysphoria (Garland, Froeliger, Zeidan, Partin, & Howard, 2013). Despite such risks, opioids remain medically necessary for many individuals experiencing prolonged and intractable pain. Thus, therapeutic interventions are needed to target comorbid pain and opioid misuse. Though cognitive-behavioral therapy (CBT) has been shown to produce therapeutic reductions in pain (Williams, Eccleston, & Morley, 2012) and opioid misuse (Jamison et al., 2010) in isolation, there is scant research on psychological treatments that simultaneously address symptoms of co-occurring chronic pain and opioid misuse. To that end, we conducted an early-stage randomized controlled trial of Mindfulness-Oriented Recovery Enhancement (MORE) (Garland, 2013), a novel multimodal intervention that integrates mindfulness training, cognitive reappraisal skills, and positive emotion regulation into a therapeutic approach designed to modify attentional biases, habit behavior, affective dysregulation, and autonomic stress responses underlying the feedback loop between chronic pain, craving, and opioid misuse behaviors.
Each of these three intervention components has been shown to be beneficial in isolation. Mindfulness training leads to reductions in pain (Gaylord et al., 2011; Kabat-Zinn et al., 1992; Rosenzweig et al., 2010; Zeidan et al., 2011) that are mediated by increased nonreactivity to aversive mental experiences and reinterpretation of affectively-laden pain sensations as innocuous sensory signals (Garland, Gaylord, Palsson, Faurot, Mann, & Whitehead, 2012). Moreover, mindfulness training produces salutary effects on emotional distress (Grossman, Niemann, Schmidt, & Walach, 2004; Hofmann, Sawyer, Witt, & Oh, 2010) and addiction-related factors (Bowen et al., 2009; Garland, Froeliger, & Howard, 2013), including attentional bias and autonomic cue-reactivity (Garland, Gaylord, Boettiger, & Howard, 2010). Similarly, cognitive reappraisal has been shown to significantly decrease negative emotions and downregulate stress physiology (Ochsner & Gross, 2005), as well as reduce substance craving (Kober et al., 2011). In complementary fashion, positive emotion regulatory strategies (e.g., savoring pleasant events) may upregulate positive affect, reduce anhedonia, and promote psychological resilience (Garland, Fredrickson, Kring, Johnson, Meyer, & Penn, 2010). MORE, which was originally tested as a treatment for alcohol dependence (Garland, Gaylord et al., 2010), capitalizes on the synergy of these three treatment components by integrating them into a multimodal intervention.
The aim of this study was to evaluate the feasibility of developing a clinical trial comparing acute (pre-post) and longer-term (3-month follow-up) efficacy of MORE with that of a conventional support group (SG) in reducing chronic pain and prescription opioid misuse. The study employed an active control condition which attempted to control for non-specific therapeutic factors such as social interaction and support. We hypothesized that participation in MORE would be associated with significantly greater reductions in pain severity, pain interference, stress symptoms, and desire for opioids than would participation in a SG. We also hypothesized that compared to SG participants, a significantly greater proportion of individuals completing MORE who met clinical criteria for prescription opioid use disorder before treatment would no longer meet opioid use disorder criteria following treatment. Insofar as clinicians in the field must often make dichotomous diagnostic judgments in practice and may need to ascertain the extent to which a disorder can be rendered subclinical following treatment (cf., Eftekhari et al., 2013), we were interested in whether MORE could reduce symptoms below the clinical threshold for opioid use disorder. Lastly, although MORE was designed to target a wide array of cognitive, affective, and autonomic mechanisms as detailed above, in the present study we tested the effects of the intervention on a focused set of mediators selected for their direct relevance to primary study outcomes. Because emotional reactivity and maladaptive appraisals of pain and stress undergird the risk chain linking chronic pain and opioid misuse, we hypothesized that the therapeutic effects of MORE on pain severity and opioid use disorder status would be associated with increased nonreactivity, cognitive reappraisal, and reinterpretation of pain sensations as innocuous sensory information.
METHODS
Participants
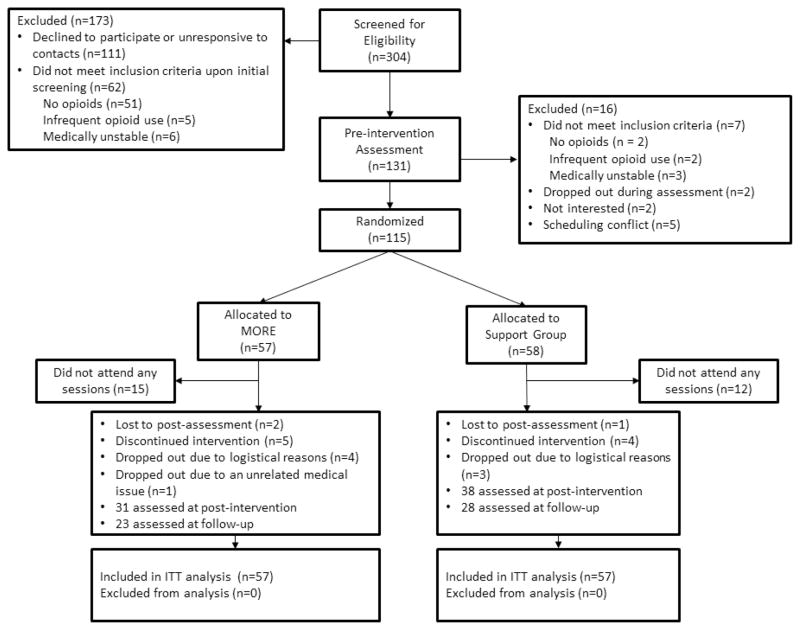
Participants met study inclusion criteria if they reported recurrent pain (i.e., pain on more days than not) stemming from chronic benign (i.e., non-cancer-related) pain conditions, arthritis or fibromyalgia and had been prescribed and taken opioids for analgesia daily or nearly every day (≥5 days/week) for at least the past 90 days (Chou et al., 2009). Participants were recruited between 2011 – 2012 from primary care clinics, pain clinics, and neurology clinics in Tallahassee, FL through posted flyers, as well as from online classified ads. Advertisements were focused on recruiting participants who suffered from, and were prescribed medicine for, chronic pain for a study focused on improving ways to address problems with chronic pain and prescription pain medication. Participants were assessed for comorbid psychiatric disorders with the Mini-International Neuropsychiatric Interview 6.0 (Sheehan et al., 1998) and excluded if they were actively suicidal or psychotic. Over the course of 1.5 years, 304 patients were recruited, 115 of whom met study criteria and were randomly assigned to treatment. From this pool, 88 patients began treatment, 70 completed treatment, and 52 completed 3-month follow-up measures. See Figure 1 for the study flow diagram.
Figure 1.
Flow diagram of the progress through the study.
Procedures
Following a preliminary phone screening for eligibility, potential participants were screened in the first author’s lab. Individuals who met eligibility criteria and agreed to participate in the study completed a pre-intervention assessment where they reported demographic and clinical information on questionnaires. Following this assessment, participants were randomly allocated to MORE or to the SG (which served as the active control condition). Order of randomization was computer-generated using simple randomization in blocks of varying sizes (6 – 8) to preserve unpredictability of allocation, and the allocation list was stored in a protected file inaccessible to project staff involved in study enrollment and assignment. Assessments were conducted by project staff blind to each respondent’s group assignment, which was concealed throughout the study. After participants had completed the 8-week MORE or SG intervention, they returned to the lab to complete a post-intervention assessment including the same questionnaires administered at pre-treatment. Informed consent and study procedures were conducted in compliance with the Florida State University Human Subjects Committee.
MORE intervention
MORE unites complementary aspects of mindfulness training, third-wave cognitive-behavioral therapy (CBT), and principles from positive psychology into an integrative intervention strategy (Garland, 2013). Techniques drawn from these therapeutic approaches were integrated into a manualized 8-session group intervention designed to address the multiplicity of pathogenic factors involved in chronic pain and long-term opioid use. MORE sessions involved mindfulness training to target automatic habit behavior and foster nonreactivity; positive reappraisal training to regulate negative emotions and foster a sense of meaningfulness in life; and training in savoring pleasant events and emotions to ameliorate deficits in natural reward processing and positive affectivity. Sessions were held in groups of 8 to 12 individuals, were 2 hours in length, and were administered by a Masters-level clinical social worker who had practiced mindfulness for over a decade and had clinical experience offering mindfulness training to persons with psychiatric disorders. This individual was supervised by the first author (the developer of MORE and an experienced, licensed psychotherapist). The first author reviewed video/audio-recordings of the sessions on the following day to monitor therapist adherence to the MORE treatment manual via a fidelity checklist that tapped therapist behaviors unique to MORE, essential to MORE, or compatible with MORE but neither necessary nor unique to it, and behaviors that are proscribed. All sessions were reviewed. Any deviations noted were communicated weekly prior to the next session during clinical supervision and corrected by the therapist in successive MORE group sessions. Minor deviations (e.g., omission of non-essential session content, abbreviated meditation debriefings) were observed infrequently, and adherence improved over time; no major deviations (e.g., proscribed behaviors) were noted.
Per the MORE treatment manual (Garland, 2013), sessions offered instruction in applying mindfulness and related skills to the following topics: discriminating between nociception, pain, and suffering; gaining awareness of automaticity and coping habits in chronic pain; disrupting the link between negative emotions, catastrophizing, and pain experience through reappraisal; refocusing attention from pain and life stressors to savor pleasant experiences; regulating opioid craving through mindful attention and awareness; decreasing opioid craving through mindful stress reduction; promoting acceptance instead of suppression of experience; and developing a mindful recovery plan. Mindfulness training involved mindful breathing and body scan techniques, with an emphasis on developing metacognitive awareness and shifting attention from affective to sensory processing of pain and craving sensations. MORE participants were asked to engage in daily 15-minute mindfulness practice sessions at home guided by a CD developed by the first author. In addition, participants were asked to engage in 3 minutes of mindful breathing prior to taking their opioid medication. This exercise was intended to increase awareness of opioid craving, clarify whether opioid use was driven by appetitive motivations (i.e., urges) versus a legitimate need for pain relief, prevent unnecessary opioid dosing by providing a non-pharmacologic means of pain management, and to synergistically increase the analgesic efficacy of opioid medication. Participants were requested to log how many minutes/day they engaged in homework practice, and daily logs were examined by the clinician and discussed to facilitate therapy.
Support group intervention
The active control condition in this study consisted of 8 weekly, 2-hour conventional SG sessions comprised of 8 to 12 participants, in which a Master’s level clinical social worker (different from the MORE facilitator) led discussion on topics pertinent to chronic pain and long-term opioid use that were selected to roughly match corresponding themes in the MORE intervention: the physical and psychological dimensions of pain experience; ways of coping with chronic pain; ways of coping with negative emotions; the impact of life events on pain; the stigma and experience of opioid craving; the relation between stress and craving; acceptance versus denial; and plans for the future. This SG format was derived from the active, evidence-based treatment condition outlined in the Matrix Model intensive outpatient treatment manual (Rawson & McCann, 2006). SG participants were guided to disclose feelings and thoughts about group topics, as well as to provide advice and emotional support for their peers. The clinician facilitated discussion using client-centered, reflective listening techniques (Rogers, 2003) but did not prescribe any specific recommendations for change. This intervention, which typifies a commonly-available form of conventional group therapy for chronic pain, was found in prior RCTs to have equivalent perceived credibility to mindfulness-based interventions and to significantly reduce psychological distress among persons suffering from chronic pain (Gaylord et al., 2011) and addiction (Garland, Gaylord, et al., 2010).
The first author reviewed video/audio-recordings of the sessions on the following day to monitor therapist adherence to the SG treatment manual via a fidelity checklist similar to that used in the MORE intervention. As above, all sessions were reviewed, and any deviations noted were communicated weekly prior to the next session during clinical supervision and corrected by the therapist in successive SG sessions. Minor deviations (e.g., use of superficial reflection versus deep empathic responding, participants monopolizing discussion time) were uncommon, and adherence improved over time; no major deviations (e.g., proscribed behaviors) were noted. SG participants were asked to engage in 15 minutes of journaling a day on chronic pain-related themes at home. Participants were requested to record how many minutes/day they engaged in journaling, and journals were examined by the clinician and discussed to facilitate therapy.
Measures
Pain severity
Pain severity was measured with the four-item pain severity subscale from the Brief Pain Inventory (BPI; α = .87) a well-validated measure that has been widely used to tap acute and chronic pain (Cleeland, 1994). Participants reported their worst pain during the past week, least pain during the past week, average pain, and current pain. Response options ranged from 0 (no pain) to 10 (pain as bad as I can imagine). An overall pain severity score was computed by taking the mean of the four items.
Pain interference
Pain-related functional interference was assessed with the pain interference subscale of the BPI (α = .88). Subjects rated on a 0 (does not interfere) to 10 (completely interferes) scale the extent to which pain had interfered with each of seven domains of normal functioning in the past week, including: general activity, mood, walking ability, normal work, relations with other people, sleep and enjoyment of life. An overall pain interference score was computed by taking the mean of the seven items.
Desire for opioids
A single item “How much do you want your opioids right now?” anchored on a 10-point scale (1 = not at all, 10 = extremely) was used to assess current desire for opioids. We used this item as an indirect proxy for craving, due to the possibility that asking directly about craving in this sample might elicit defensive responding or denial. Single item measures of craving have been shown to distinguish high- from low-risk opioid using chronic pain patients and predict opioid misuse (Wasan et al., 2009; Weiss et al., 2010).
Self-reported opioid misuse
The Current Opioid Misuse Measure (COMM; α = .83) (Butler et al., 2007) was used to assess prescription opioid misuse. Participants responded to 17 items rated on a Likert scale (0 = never, 4 = very often) regarding how often in the past 30 days they had engaged in behaviors linked with opioid misuse or took opioid medication in excessive doses or ways other than how it was prescribed. The original COMM validation study conducted with patients treated in specialty pain management clinics found that a score of ≥9 was suggestive of prescription opioid misuse. However, according to a study of a broad sample of chronic pain patients from a variety of primary care settings who took prescription opioids but not necessarily on a daily basis, receiver–operator characteristic curve analyses revealed that a score of 13 or higher on the COMM had maximum sensitivity and specificity to identify prescription opioid use disorder among chronic pain patients in primary care settings (Meltzer et al., 2011). We chose this more conservative COMM threshold value to minimize false positives and because, similar to Meltzer et al. (2011), our sample was broad and not confined to patients from specialty pain clinics.
Nonreactivity
Nonreactivity to distressing thoughts and emotions was measured with the 7-item nonreactivity subscale (α = .82) of the Five Facet Mindfulness Questionnaire (Baer, Smith, Hopkins, Krietemeyer, & Toney, 2006). This subscale, comprised of items such as “When I have distressing thoughts or images, I ‘step back’ and I am aware of the thought or image without getting taken over by it,” appears to tap metacognitive decentering or disengagement from aversive experiences, and has been shown to mediate the effects of mindfulness training on decreased pain (Garland et al., 2012).
Reinterpretation of Pain Sensations
Cognitive coping with pain by reinterpreting painful sensations as innocuous sensory experiences was assessed via the reinterpreting pain sensations subscale of the CSQ (Rosenstiel & Keefe, 1983). This subscale has good internal consistency (α= 0.88), and is comprised of 6 items including “I don’t think of it as pain but rather as a dull or warm feeling,” and “I just think of it as another sensation such as numbness.” Participants were asked to report how much they generally engaged in this form of coping when they felt pain. Responses are rated on a scale ranging from 0 (never) to 6 (always); a reinterpretation of pain sensations total score can be obtained by adding up the four items (range: from 0 to 36). Scores on this scale are meaningfully related to measures of pain and adjustment to pain (Rosenstiel & Keefe, 1983), and have been shown to mediate the therapeutic effects of mindfulness training on chronic pain (Garland et al., 2012).
Reappraisal
Reappraisal was measured with the 4-item positive reappraisal subscale of the Cognitive Emotion Regulation Questionnaire (CERQ) (Garnefski & Kraaij, 2007), an internally-consistent subscale (α = .85) which asks the respondent how often they “think I can become a stronger person as a result of what has happened” or “look for positive sides to the matter” to cope with stressful events. Responses are rated on a scale ranging from 1 (almost never) to 5 (almost always); a reappraisal total score can be obtained by summing the four items (range: from 4 to 20). In prior research, scores on this reappraisal scale were prospectively predictive of lower levels of future affective symptoms (Garnefski & Kraaij, 2007), and changes in CERQ reappraisal scores mediated the stress-reductive effects of mindfulness (Garland, Gaylord, & Fredrickson, 2011).
Affective and Somatic Symptoms of Stress
The 56-item Calgary Symptoms of Stress Inventory (C-SOSI; Carlson & Thomas, 2007) was used to assess affective and somatic symptoms of stress. This scale, comprised of 8, internally consistent subscales with adequate convergent and discriminant validity (Carlson & Thomas, 2007), taps depression (α = .89), sympathetic arousal (α = .75), anger (α = .91), and cognitive disorganization (α = .84), as well as muscle tension (α = .84), cardiopulmonary (α = .91), neurological (α = .84), and upper respiratory stress symptoms (α = .80).
Treatment credibility
We assessed participants’ perceptions of the credibility of the treatment to which they were allocated to using three items based on the measure by Borkovec and Nau (1972). The measure was administered at the end of Session 3 of each intervention condition, and a total perceived credibility score was computed (α= .86).
Analyses
We conducted per-protocol (PP) analyses as primary analyses and used an additional intention-to-treat (ITT) approach for sensitivity analyses. The PP sample consisted of participants who attended at least 5 of the 8 MORE or SG sessions and who completed post-treatment assessments. Hypothesis testing in the PP sample was conducted via an Analysis of Covariance (ANCOVA) strategy (Frison & Pocock, 1992) for continuous outcomes (i.e., pain severity, pain interference, desire for opioids, and symptoms of stress) and chi-square analysis for differences in proportions in categorical outcomes (i.e., opioid use disorder status). In the case of continuous outcomes, we controlled for pre-treatment differences using the pre-randomization measures as covariates. In accordance with the classical ANCOVA approach endorsed by Frison and Pocock (1992) for analyzing clinical trial outcomes, covarying baseline values performs statistical matching on the prerandomization scores and ensures that comparisons of postrandomization values by treatment group are independent of baseline differences. In ANCOVA models, post-treatment values of outcome variables were regressed on intervention group (MORE vs. SG) after covarying pre-treatment values. A similar set of ANCOVA models was conducted by regressing 3-month follow-up values on intervention group after covarying pre-treatment values. For our categorical outcome, we used a chi-square analysis to determine whether there was a significant difference in the proportion of participants who met opioid use disorder criteria at baseline but no longer met clinical criteria for opioid use disorder at post-treatment and 3-month follow-up. Because of missing data, the N for PP analyses for pre-post treatment data ranged from 65 to 67 and for follow-up data ranged from 50 to 51, depending on the variable.
ITT analyses were conducted on the entire randomized sample (N = 115). Of the 115 participants who were assessed at pre-intervention and randomized to intervention conditions, 88 (76.5%) attended one or more sessions, and 72 (62.6% of the randomly allocated sample, 81.2% of those who attended one or more sessions) completed the treatments. Three participants were lost to the post-treatment assessment. The majority (93.0%) of non-starters cited their inability to meet the time commitment required by study involvement as a reason for leaving the trial prior to the beginning of treatment. Non-significant t-tests and chi-square statistics indicated there were no significant differences between participants who dropped out vs. those who completed the study across demographic or clinical variables, including major depressive disorder and other psychiatric diagnoses. Similarly, there were no significant differences in numbers of participants who met criteria for opioid use disorder at baseline and then dropped out of MORE and SG.
To analyze patterns of missing data, we performed Little’s MCAR test (Little, 1988). The pattern of missing data was consistent with being missing completely at random; thus, maximum likelihood estimation was employed to handle missing data. To reduce potential bias resulting from listwise deletion or last-observation carried forward techniques, maximum likelihood estimation procedures estimate the variance-covariance matrix for all available data, including data from cases assessed at only one time point (e.g., treatment non-completers or non-starters). For the ITT sample, hypothesis testing was conducted using an ANCOVA strategy with maximum likelihood estimation conducted within the Analysis of Moment Structures 17.0 (AMOS 17.0) software package, a method that has been employed previously to assess clinical trial outcomes in biomedical (Hurwitz et al., 2007) and psychotherapy (Durham, Chambers, MacDonald, Power, & Major, 2003) studies. Additional ITT sensitivity analyses were conducted controlling for age, gender, education, income level, and baseline self-reported opioid misuse. For effect size estimates, we report Cohen’s d, corresponding to the magnitude of differences among the treatment groups after controlling for baseline levels in the variable of interest. Study sample size was determined a priori based on a power analysis conducted with G-Power software using medium-large effect size estimates derived from earlier trials demonstrating the effects of MORE on clinical outcomes in alcohol dependent individuals (Garland, Gaylord et al., 2010) and effects of mindfulness training in chronic pain patients (Gaylord et al., 2011) .
RESULTS
Participant characteristics
More than two-thirds (68%) of participants were female (mean age = 48, SD = 14), and the majority came from lower or middle socioeconomic strata. The most common current chronic pain diagnosis reported by participants across both intervention conditions was lumbago (56.5%), followed by fibromyalgia (20.0%), arthritis (7.0%), cervicalgia (6.0%), or “other” pain conditions (10.5%). The most prevalent comorbid current psychiatric condition was major depressive disorder (68.3%), followed by generalized anxiety disorder (30.7%), alcohol use disorder (12.8%), post-traumatic stress disorder (11.9%), and substance use disorder (9.0%). Other less common comorbid conditions included OCD and social phobia. Using the established cut point on the COMM, 72.2% of the total randomized sample met criteria for prescription opioid use disorder.
There were no significant differences between MORE and SG participants at baseline on any demographic, clinical, or outcome variable for the PP or ITT sample (Table 1). MORE participants had non-significantly higher incomes than SG participants.
Table 1.
Demographic and Clinical Characteristics of the Randomized Chronic Pain Sample (N = 115).
| Measure | MORE (n = 57) | Support Group (n = 58) |
|---|---|---|
| Female, N (%) | 40 (70%) | 38 (66%) |
| Age | 49.3 ± 13.68 | 47.4 ± 13.56 |
| Work status, full time, N (%) | 15 (26%) | 14 (24%) |
| Race | ||
| Not respond | 7 (12%) | 4 (7%) |
| American Indian | 2 (4%) | 2 (3%) |
| African American | 10 (18%) | 11 (19%) |
| Caucasian | 36 (63%) | 39 (67%) |
| Other | 2 (4%) | 2 (3%) |
| Income level | ||
| Not respond | 16 (28%) | 18 (31%) |
| Below $20,000 | 12 (21%) | 15 (26%) |
| $20,000–$39,999 | 11 (19%) | 16 (28%) |
| $40,000–$59,999 | 7 (12%) | 3 (5%) |
| $60,000–$79,999 | 6 (11%) | 3 (5%) |
| Over $80,000 | 5 (9%) | 3 (5%) |
| Education, some college, N (%) | 40 (70%) | 41 (71%) |
| Primary pain condition, N (%) | ||
| Lumbago | 30 (53%) | 35 (60%) |
| Fibromyalgia | 11 (19%) | 12 (21%) |
| Arthritis | 4 (7%) | 4 (7%) |
| Cervicalgia | 5 (9%) | 2 (3%) |
| Other | 7 (12%) | 5 (9%) |
| Opioid use disorder status, N% | 41 (72%) | 42 (72%) |
Note. There were no significant between-groups differences on any of these variables. Positive opioid use disorder status was determined by exceeding a cut-point of 13 on the Current Opioid Misuse Measure.
MORE = Mindfulness-Oriented Recovery Enhancement; SG = Support Group.
Pre-post treatment analyses
Pain severity and functional interference
Table 2 displays the baseline, post-treatment, and follow-up means for MORE and SG participants. For the primary analyses (per-protocol analyses), the ANCOVA comparing the BPI pain severity score at post-treatment for MORE and SG participants and including pre-treatment pain severity as a covariate revealed a statistically significant effect of treatment condition, β = .77, SE = .36, 95% CI [.05, 1.49], p = .038, d = .50, with MORE participants showing significantly lower levels of pain severity at post-treatment than SG participants. The 10% baseline adjusted mean pain reduction by post-treatment in the MORE group met the threshold for minimally clinically significant change (Dworkin et al., 2008). Similarly, the ANCOVA comparing the BPI pain functional interference score at post-treatment for MORE and SG participants and including pre-treatment functional interference as a covariate revealed a significant effect of treatment condition, with MORE participants showing significantly lower levels of functional interference at post-treatment than SG participants, β = 1.24, SE = .40, 95% CI [.44, 2.03], p = .003, d = .78.
Table 2.
Mean (SD) Value of Outcome and Mediation Variables at Baseline (T1), Post-treatment (T2), and 3-Month Follow-Up (T3) by Treatment Condition (MORE vs. Support Group)
| Variable | Time 1 | Time 2 | Time 3 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| MORE | SG | MORE | SG | MORE | SG | |
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Primary Outcomes | ||||||
| Pain severity | 5.44 (1.40)a | 5.49 (1.54)a | 4.86 (1.38)b | 5.71 (1.58)a | 4.77 (1.95)b | 6.10 (1.48)a |
| Pain interference | 5.87 (2.15)a | 6.37 (2.02)a | 5.22 (1.88)b | 6.90 (1.50)a | 4.60 (2.66)b | 6.75 (1.86)a |
| Opioid craving | 4.36 (3.00)a | 5.03 (3.44)a | 2.60 (1.61)b | 4.32 (3.35)a | 3.82 (3.15)a | 4.45 (2.91)a |
| Opioid misuse | 17.19 (7.90)a | 18.62 (11.24)a | 11.27 (7.67)b | 15.68 (9.56)b | 13.90 (6.91)a | 16.44 (8.28)a |
| Secondary Outcomes | ||||||
| Sympathetic arousal sxs | 17.84 (5.58)a | 18.76 (6.64)a | 14.65 (7.52)b | 18.89 (5.96)a | --- | --- |
| Depression sxs | 10.63 (6.82)a | 11.50 (7.37)a | 8.20 (7.09)a | 10.76 (6.44)a | --- | --- |
| Anger sxs | 10.36 (6.48)a | 9.00 (6.11)a | 9.91 (6.39)a | 9.10 (5.47)a | --- | --- |
| Cognitive sxs | 7.57 (4.59)a | 8.94 (5.38)a | 5.80 (3.54)a | 7.55 (5.19)a | --- | --- |
| Muscle tension sxs | 17.87 (6.56)a | 20.24 (7.30)a | 16.50 (7.42)a | 18.26 (6.86)a | --- | --- |
| Cardiopulmonary sxs | 5.45 (4.42)a | 8.46 (6.62)a | 4.00 (4.06)a | 7.68 (6.24)a | --- | --- |
| Neurological sxs | 6.26 (5.07)a | 8.08 (5.80)a | 3.90 (3.58)b | 6.68 (5.20)a | --- | --- |
| Upper respiratory sxs | 5.43 (5.08)a | 5.96 (4.72)a | 5.20 (5.11)a | 7.47 (4.70)a | --- | --- |
| Potential Mediators | ||||||
| Nonreactivity | 21.23 (4.58)a | 22.16 (5.70)a | 23.34 (3.55)b | 22.18 (4.17)a | --- | --- |
| Reappraisal | 13.09 (3.82)a | 13.22 (4.25)a | 16.31 (6.89)b | 13.47 (4.17)a | --- | --- |
| Reinterpretation of pain sensations | 8.01 (6.85)a | 9.29 (8.52)a | 12.40 (7.81)b | 8.28 (8.21)a | --- | --- |
Note. Per row, variables that do not share the same subscript significantly differ at p < .05.
MORE = Mindfulness-Oriented Recovery Enhancement; SG = Support Group. Sxs = symptoms. Because of missing data, ns ranged from 113 to 115 at Time 1, 65 to 67 at Time 2, and 50 to 51 for Time 3, depending on the variable.
Affective and somatic symptoms of stress
In per-protocol analyses, the ANCOVA comparing the sympathetic arousal score at post-treatment for MORE and SG participants controlling for pre-treatment sympathetic arousal revealed a statistically significant effect of treatment condition, with MORE participants reporting significantly lower levels of sympathetic arousal at post-treatment than SG participants, β = 3.02, SE = 1.40, 95% CI [.24, 5.81], p = .034, d = .45. Similarly, controlling for pre-treatment values, MORE participants endorsed lower levels of self-reported neurological symptoms of stress at post-treatment than SG patients, β = 1.84, SE = .94, 95% CI [−.04, 3.73], p = .055, d = .41. No other between-groups differences were observed on the remaining subscales of the C-SOSI.
Desire for opioids
In per-protocol analyses, the ANCOVA comparing the desire for opioids score at post-treatment for MORE and SG participants and including pre-treatment desire for opioids as a covariate revealed a statistically significant effect of treatment condition, with MORE participants showing significantly less desire for opioids at post-treatment than SG patients, β = 1.39, SE = .62, 95% CI [.15, 2.63], p = .027, d = .50. MORE patients continued to exhibit significantly less desire for opioids at post-treatment even after controlling for pre-post change in pain severity, β = 1.31, SE = .63, 95% CI [.05, 2.57], p = .043, d = .46, suggesting that this decrease in desire for opioids was partially independent from the overall pain reduction resulting from participation in MORE. Indeed, change in desire for opioids was not significantly correlated with change in pain severity, r = .07, p = .60 or pain interference, r = .19, p = .11.
Opioid use disorder
Though inspection of opioid misuse means revealed that participants in both MORE and SG exhibited decreased self-reported opioid misuse scores over the course of treatment, we wanted to know whether significantly fewer participants continued to meet criteria for disordered opioid use following treatment with MORE than with the SG. Using a validated cut-point on the COMM (Meltzer et al., 2011), we identified individuals who met criteria for opioid use disorder at baseline. Chi-square analysis revealed that relative to those in the SG, a significantly greater proportion of individuals meeting opioid use disorder criteria at baseline who participated in MORE no longer met opioid use disorder criteria following treatment. There was a 63% reduction in opioid use disorders in the MORE group, compared to a 32% reduction in the SG, χ2 = 3.74, p = .05.
Sensitivity analyses
Results of sensitivity analyses (ITT analyses) supported the PP findings on acute effects of treatment. ANCOVAs conducted with maximum likelihood estimation of missing data revealed a significant main effect for treatment condition for pain severity (β = .74, SE = .35, 95% CI [.05, 1.43], p = .034, d = .54), functional interference (β = 1.21, SE = .36, 95% CI [.50, 1.92], p < .001, d = .75), sympathetic arousal (β = 3.02, SE = 1.34, 95% CI [.40, 5.62], p = .02, d = .54), neurological stress symptoms (β = 1.84, SE = .90, 95% CI [.08, 3.60], p = .04, d = .47), and desire for opioids (β = 1.39, SE = .59, 95% CI [.22, 2.56], p = .02, d = .57), with outcomes favoring the MORE group. These main effects for treatment remained significant even after controlling for age, gender, education, income level, and baseline opioid use disorder status. Similarly, in an ITT analysis controlling for the same set of covariates, participation in MORE was associated with significantly lower rates of opioid use disorder at post-treatment.
Follow-up analyses
PP and ITT ANCOVAs of data at the 3-month follow-up revealed a statistically significant effect of treatment condition, with MORE patients showing significantly lower levels of pain severity (PP results: β = .92, SE = .44, 95% CI [.03, 1.80], p = .04, d = .56; ITT results: β = 1.06, SE = .43, 95% CI [.21, 1.91], p = .014, d = .63) and significantly less pain interference (PP results: β = 1.82, SE = .62, 95% CI [.57, 3.06], p = .005, d = .78; ITT results: β = 1.82, SE = .58, 95% CI [.67, 2.97], p = .002; d = .84) at follow-up than SG patients. The 22% baseline adjusted mean interference reduction by follow-up in the MORE group met threshold for minimally clinically significant change (Dworkin et al., 2008). However, there was no significant effect of treatment condition on desire for opioids or opioid use disorder status at 3-month follow-up.
Therapeutic mechanisms
Statistically significant changes from pre-post treatment were observed in all three potential mediating variables (see Table 2). To explore the therapeutic mechanisms of MORE on pain severity, we conducted a multivariate path analysis on the ITT sample (see Fig 2). Path analysis revealed that residualized change (pre-post) in reinterpretation of pain sensations and residualized change (pre-post) in nonreactivity significantly predicted follow-up levels of pain severity, controlling for baseline levels of pain severity, baseline opioid use disorder status, age, gender, education, and income level. Change in reappraisal did not predict pain severity. Model fit statistics were adequate (χ2/df = 1.42; IFI = .92; RMSEA = .06, 95% CI = .00, .11), and the indirect effects of reinterpretation of pain sensations (Sobel-Goodman test = 2.17, SE = .18, p = .03), and nonreactivity (Sobel-Goodman test = 1.97, SE = .18, p = .049) were both significant.
Figure 2.
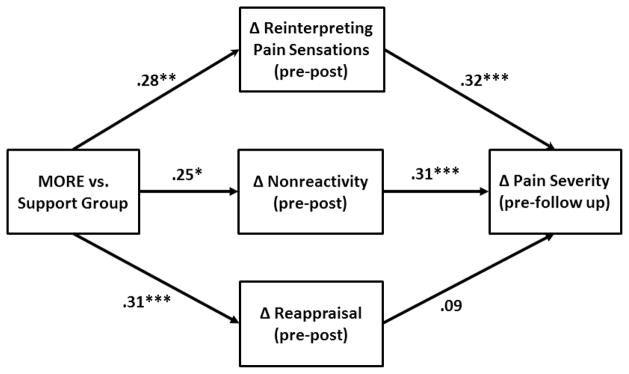
Multivariate path model of therapeutic mechanisms of Mindfulness-Oriented Recovery Enhancement on pain severity (N = 115). Model parameters reflect adjustment for the following covariates: age, gender, education, income level, and baseline opioid use disorder status.
To elucidate correlates of change in opioid use disorder status, we evaluated three logistic regression models with treatment group and residualized change (pre-post) in each potential mediating variable as predictors of post-treatment opioid use status, and baseline opioid use disorder status as a covariate. Residualized change in nonreactivity significantly predicted opioid use disorder status at post-treatment (β = −.24, SE = .13, p = .03, OR = .73, 95% CI = .63, .98), such that greater increases in nonreactivity were associated with reduced odds of meeting opioid use disorder criteria following treatment. In contrast, changes in reinterpreting pain sensations and reappraisal did not predict opioid use disorder status.
Additionally, because unauthorized opioid use and increased wanting for opioids can arise as a result of undertreated pain (i.e., pseudoaddiction; Jamison et al., 2011), we conducted an exploratory multivariate path analysis on the ITT sample to examine whether reduced desire for opioids mediated the relation between treatment-related decreases in pain severity on residualized change in opioid misuse at post-treatment. Results from this path model (χ2/df = 1.48; IFI = .92; RMSEA = .07, 95% CI = .00, .12) indicated that desire for opioids was not significantly associated with reductions in pain and opioid misuse at post-treatment.
Lastly, we examined whether participation in MORE modulated the strength of the relationship between desire for opioids and self-reports of opioid misuse. We found that prior to treatment, desire for opioids was significantly associated with opioid misuse among participants randomly assigned to the SG (r = .32, p = .02) and MORE treatments (r = .53, p < .001). At post-treatment, desire for opioids was significantly associated with opioid misuse among SG participants (r = .51, p = .001) but was uncorrelated with opioid misuse among MORE participants (r = −.00, p = .99). Using Fisher’s r-to-z transformation, we found that these two correlation coefficients were significantly different, z = 2.17, p = .01, suggesting that MORE significantly decreased the strength of the relationship between desire for opioids and self-reported opioid misuse.
Treatment credibility
Analysis of the credibility scale revealed no significant difference in credibility between treatment groups, F(1,61) = 3.15, p = .08 (MORE M = 21.37, SD = 4.03; SG M = 19.31, SD = 4.87). Perceived credibility was not significantly correlated with any treatment outcomes.
Adherence to at-home practice
Adherence to at-home practice exercises during active treatment was assessed via daily homework logs: 73.1% of the PP sample complied and recorded their homework practice on these logs. There was no significant between-groups difference in duration of weekly homework practice, F(1,48) = 2.13, p = .15; the average number of minutes practiced a week by MORE group participants was 166.9 (SD = 93.4), whereas the average number of minutes practiced a week by support group participants was 114.7 (SD = 149.6).
DISCUSSION
Study results indicate that MORE significantly reduced symptoms associated with chronic pain and prescription opioid misuse among a sample of patients receiving long-term opioid analgesic pharmacotherapy. Specifically, MORE led to greater post-treatment reductions in pain severity and pain-related functional interference than did participation in a SG, which were maintained for 3 months following the end of treatment. MORE also decreased symptoms of self-reported sympathetic stress arousal by post-treatment. In addition, by the post-treatment assessment point MORE led to short-term reductions in desire for opioid medication and ameliorated disordered opioid use in nearly two-thirds of patients who completed treatment.
The observed therapeutic effect of MORE on pain severity and interference was modest yet clinically significant at 3-month follow-up according to benchmarks for chronic pain treatment RCTs established by Dworkin et al. (2008). This finding is notable given that many participants were taking prescription opioids in large doses, and that many participants reported a discrete site of pain and symptoms indicative of a specific anatomical abnormality or degeneration suggestive of organic etiology (e.g., arthritis, degenerative joint disease). Thus, MORE may have not been merely treating a psychological overlay, but also modulating the impact of chronic injury or disease. Our prior path analytic research indicated that mindfulness training may alleviate chronic pain by enhancing nonreactivity toward distressing thoughts and emotions, as well as by promoting a shift from affective to sensory processing of pain sensations (Garland et al., 2012). Congruent with this prior research, in the present trial we observed significant increases in nonreactivity and reinterpretation of pain sensations that statistically mediated the pain-reductive effects of MORE; replication of these findings across two distinct samples suggests that these factors may indeed be important therapeutic mechanisms of action underpinning the effect of mindfulness on chronic pain. A recent study also demonstrated that MORE led to significant reductions in pain attentional bias coupled with improved perceived control over pain (Garland & Howard, 2013). Additional research is needed to elucidate the pathways by which mindfulness modulates chronic pain symptoms and pain-related impairment.
Immediately following treatment (i.e., post-treatment assessment), participants receiving MORE reported significant reductions in their desire for opioids; importantly, this therapeutic effect was statistically independent from pain reduction. Given that a subjective “wanting” for opioids is characteristic of dopaminergically-mediated craving responses (Robinson & Berridge, 2008) this reduction in desire for opioid medication may be reflective of a decrease in craving. In that regard, Mindfulness-Based Relapse Prevention has been shown to reduce substance craving (Bowen et al., 2009). Notably, participation in MORE was associated with decreased correlation strength between desire for opioids and self-reported opioid misuse, suggesting that MORE may have decoupled craving responses from addictive behaviors. This interpretation is consistent with models suggesting that mindfulness facilitates awareness, acceptance, and nonjudgment of craving without engaging in addictive responses (Garland, Froeliger, & Howard, 2013; Witkiewitz, Bowen, Douglas, Tsu, 2013). Nonetheless, given the multidimensionality of craving, our ability to measure this construct may have been constrained by our use of a single question item. Effects on desire for opioids waned by the 3-month follow-up, suggesting that booster sessions might be necessary to sustain this effect over time. After the conclusion of the MORE intervention, participants may have practiced mindfulness skills less frequently (e.g., the 3 minute mindful breathing exercise prior to taking opioids), resulting in a loss of this treatment benefit. Unfortunately, we did not collect measures of mindfulness practice during the follow-up period to verify this hypothesis. Future studies should carefully measure adherence to home practice during the follow-up period.
Though participation in both study interventions was associated with decreased self-reported opioid misuse, MORE led to a significantly greater reduction in the proportion of patients who continued to meet criteria for prescription opioid use disorder by the post-treatment assessment point. MORE also led to increased nonreactivity which was associated with reduced odds of meeting opioid use disorder criteria following treatment. These findings may be of direct relevance to clinicians who must make dichotomous judgments in practice - e.g., whether or not a treatment can promote remission from disordered opioid use or a high risk patient can be continued on opioid pharmacotherapy. Though promising, these results are derived from a self-report instrument (COMM) used to determine the presence of opioid use disorder. Future studies should employ structured diagnostic evaluations both pre- and post-treatment to determine whether participation in MORE is associated with greater occurrence of remission from prescription opioid use disorder.
The primary limitation of the present study was our inability to quantify differences in opioid dosing. While we asked open-ended questions about opioid dosing, missing data and the extreme variability in quality of responses made it impossible to quantify this variable for use as a covariate in the current study. Theoretically randomization equated these two groups with regard to opioid dosing – this is likely because there were no other significant between-groups differences observed at baseline. For a pilot study of this nature, focusing on reduced opioid dosing as a study outcome was not appropriate because it may not have been medically warranted, it could have made recruitment difficult, and the prescribing physicians were not members of the investigative team. We are currently implementing a larger and longer-term RCT that will assess opioid dosing in a fine-grained manner prior to, during, and following treatment with MORE to examine changes in dose over time. Among other study aims, we will specifically assess the extent to which chronic pain patients participating in MORE are able to reduce their opioid doses over time relative to chronic pain patients in an active control support group condition similar to the one used in this study. In addition, though we qualitatively monitored therapist adherence continuously throughout the trial, this study was also limited by lack of quantitative tracking of non-adherence and assessment of the relationship between treatment fidelity and outcome. The study was also limited by the use of different therapists for the MORE and SG interventions, which, despite the fact they had comparable levels of clinical experience with the study population, may have resulted in therapist effects that could have confounded study outcomes. Also, there were substantial numbers of attriters and non-starters in the study, although the attrition rate was comparable to other intervention studies of individuals with prescription opioid use disorders (Weiss et al., 2011). The time demands required by study participation, the transient nature of the study sample, and the possibility that prior to the study interventions some participants might have experienced an unreported change in medical status or engaged in medical procedures requiring convalescence may have precluded full participation in the trial.
Finally, although MORE is founded on a conceptual framework that incorporates a number of interconnected and complex mechanisms (for reviews, see Garland, Boettiger, & Howard, 2011; Garland, Froeliger, & Howard, 2013; Garland et al., 2013), the present study was limited in its measurement of a circumscribed subset of these manifold treatment targets. Prior research identified significant effects MORE on attentional bias for substance-related cues and autonomic recovery from stress-primed cue-exposure (Garland, Gaylord et al., 2010), and ongoing research in our lab is currently assessing the effects of MORE on habit responses, natural reward processing, and opioid cue-reactivity (Garland, Froeliger, & Howard, under review). Taken together, these studies are beginning to outline the multiple pathways by which MORE may exert salutary effects. Nonetheless, additional research is needed. Future investigations should carefully assess the reasons for non-starting and attrition, use multiple therapists in each treatment condition, carefully monitor opioid dosing (including use of urine toxicology screens), quantitatively evaluate treatment fidelity, and employ a wider set of self-report measures coupled with psychophysiological and neuroimaging paradigms to comprehensively probe the therapeutic mechanisms of MORE and predictors of treatment response.
In conclusion, study results indicate that among patients suffering from chronic pain MORE reduces pain severity and functional interference for up to 3 months following treatment, and decreases sympathetic stress arousal, desire for opioids, and disordered opioid use at the end of treatment. These outcomes appear to be linked with key therapeutic mechanisms, including mindful disengagement from negative appraisals and re-orienting of attention onto interoceptive data with less affective bias. By strengthening these and other cognitive-affective processes, MORE may facilitate the generation of adaptive reappraisals and modulate intervention targets implicated in distress intolerance and addictive behavior. Findings from this early stage RCT demonstrate preliminary feasibility and efficacy of MORE as a treatment for co-occurring prescription opioid misuse and chronic pain, a vexing problem of increasing medical and social significance.
Acknowledgments
This trial was funded by grant R03DA032517 from the National Institute on Drug Abuse, and a grant from the Fahs Beck Fund for Research and Experimentation, both awarded to ELG. ELG was also supported by grant R34DA037005 from the National Institute on Drug Abuse during the preparation of this manuscript. We are grateful to the patients who participated in the trial.
Contributor Information
Eric L. Garland, University of Utah
Eron G. Manusov, Duke Southern Regional Area Health Education Center
Brett Froeliger, Medical University of South Carolina.
Amber Kelly, Smith College.
Jaclyn M. Williams, Florida State University
Matthew O. Howard, University of North Carolina at Chapel Hill
References
- Alcaro A, Panksepp J. The SEEKING mind: Primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neuroscience & Biobehavioral Reviews. 2011;35(9):1805–1820. doi: 10.1016/j.neubiorev.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Nau SD. Credibility of analogue therapy rationales. Journal of Behavior Therapy and Experimental Psychiatry. 1972;3(4):257–260. [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, Marlatt A. Mindfulness-based relapse prevention for substance use disorders: A pilot efficacy trial. Substance Abuse. 2009;30(4):295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130(1–2):144–156. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Thomas BC. Development of the Calgary Symptoms of Stress Inventory (C-SOSI) International Journal of Behavioral Medicine. 2007;14(4):249–256. doi: 10.1007/BF03003000. [DOI] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Gasternak GW, Portenoy RK, Rich BA, Roberts TG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. The Journal of Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clinical Journal of Pain. 2008;24(6):479–496. doi: 10.1097/AJP.0b013e31816b2f43. [DOI] [PubMed] [Google Scholar]
- Cleeland CS. Brief Pain Inventory–Short Form (BPI–SF) Houston, TX: 1994. [Google Scholar]
- Durham R, Chambers JA, MacDonald RR, Power KG, Major K. Does cognitive-behavioural therapy influence the long-term outcome of generalized anxiety disorder? An 8–14 year follow-up of two clinical trials. Psychological Medicine. 2003;33(3):499–509. doi: 10.1017/s0033291702007079. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkold C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. The Journal of Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Eftekhari A, Ruzek JI, Crowley JJ, Rosen CS, Greenbaum MA, Karlin BE. Effectiveness of national implementation of prolonged exposure therapy in Veterans Affairs care. JAMA Psychiatry. 2013;70(9):949–955. doi: 10.1001/jamapsychiatry.2013.36. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Medicine. 2007;9(4):444–459. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- Frison L, Pocock SJ. Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Statistics in Medicine. 1992;11(13):1685–1704. doi: 10.1002/sim.4780111304. [DOI] [PubMed] [Google Scholar]
- Garland EL. Pain processing in the human nervous system: A selective review of nociceptive and biobehavioral pathways. Primary Care: Clinics in Office Practice. 2012;39(3):561–571. doi: 10.1016/j.pop.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL. Mindfulness-Oriented Recovery Enhancement for addiction, stress, and pain. NASW Press; Washington, DC: 2013. [Google Scholar]
- Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: Results from a randomized controlled pilot trial. Journal of Psychoactive Drugs. 2010;42(2):177–192. doi: 10.1080/02791072.2010.10400690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Fredrickson BL, Kring AM, Johnson DP, Meyer PS, Penn DL. Upward spirals of positive emotions counter downward spirals of negativity: Insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clinical Psychology Review. 2010;30:849–864. doi: 10.1016/j.cpr.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Boettiger CA, Howard MO. Targeting cognitive-affective risk mechanisms in stress-precipitated alcohol dependence: An integrated, biopsychosocial model of allostasis, automaticity, and addiction. Medical Hypotheses. 2011;76:745–754. doi: 10.1016/j.mehy.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Gaylord SA, Fredrickson BL. Positive reappraisal coping mediates the stress-reductive effect of mindfulness: An upward spiral process. Mindfulness. 2011;2(1):59–67. [Google Scholar]
- Garland EL, Gaylord SA, Palsson O, Faurot K, Mann JD, Whitehead B. Therapeutic mechanisms of a mindfulness-based treatment for irritable bowel syndrome: Effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. Journal of Behavioral Medicine. 2012;35(6):591–602. doi: 10.1007/s10865-011-9391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Howard MO. Mindfulness-Oriented Recovery Enhancement reduces pain attentional bias in chronic pain patients. Psychotherapy and Psychosomatics. 2013;82:311–318. doi: 10.1159/000348868. [DOI] [PubMed] [Google Scholar]
- Garland EL, Froeliger BE, Passik SD, Howard MO. Attentional bias for prescription opioid cues among opioid-dependent chronic pain patients. Journal of Behavioral Medicine. 2013;36(6):611–620. doi: 10.1007/s10865-012-9455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger BE, Howard MO. Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Frontiers in Psychiatry. 2013;4:173. doi: 10.3389/fpsyt.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Zeidan F, Partin K, Howard MO. The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neuroscience & Biobehavioral Reviews. 2013;37:2597–2607. doi: 10.1016/j.neubiorev.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard MO. Mindfulness-Oriented Recovery Enhancement reduces opioid craving by restructuring reward responsiveness. (under review) [Google Scholar]
- Garnefski N, Kraaij V. The cognitive emotion regulation questionnaire: Psychometric features and prospective relationships with depression and anxiety in adults. European Journal of Psychological Assessment. 2007;23(9):141–149. [Google Scholar]
- Gaylord SA, Palsson OS, Garland EL, Faurot KR, Coble RS, Mann JD, Frey W, Whitehead WE. Mindfulness training reduces the severity of irritable bowel syndrome in women: Results of a randomized controlled trial. The American Journal of Gastroenterology. 2011;106(9):1678–1688. doi: 10.1038/ajg.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cognitive in Sciences. 2009;13(9):372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. Journal of Psychosomatic Research. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Højsted J, Nielsen PR, Guldstrand SK, Frich L, Sjøgren P. Classification and identification of opioid addiction in chronic pain patients. European Journal of Pain. 2010;14(10):1014–1020. doi: 10.1016/j.ejpain.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Hurwitz BE, Klaus JR, Llabre MM, Gonzalez A, Lawrence PJ, Maher KJ, Greeson JM, Baum MK, Shor-Posner G, Skyler JS, Schneiderman N. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Archives of Internal Medicine. 2007;167(2):148–154. doi: 10.1001/archinte.167.2.148. [DOI] [PubMed] [Google Scholar]
- Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: A randomized trial. Pain. 2010;150(3):390–400. doi: 10.1016/j.pain.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison RN, Serrailier, Michna E. Assessment and treatment of abuse risk in opioid prescribing for chronic pain. Pain Research and Treatment. 2011;2011:Article ID 941808. doi: 10.1155/2011/941808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W. The sympathetic nervous system in pain. European Journal of Anaesthesiology Supplement. 1995;10:53–60. [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice on mindfulness meditation: Theoretical considerations and preliminary results. General Hospital Psychiatry. 1982;4(1):33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kelley A, Garland EL. Treatment of depression and coping with chronic pain through Mindfulness-Oriented Recovery Enhancement. In: LeCrory CW, editor. Case Studies in Social Work Practice. Wiley; New York: (in press) [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4(5):231–44. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kirsh KL, Jass C, Bennett DS, Hagen JE, Passik SD. Initial development of a survey tool to detect issues of chemical coping in chronic pain patients. Palliative & Supportive Care. 2007;5(3):219–226. doi: 10.1017/s1478951507000387. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of the Sciences USA. 2011;107(33):14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1507):3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson M, Paasche-Orlow M, Cheng D, Lloyd-Travaglini C, Saitz R, Samet J. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction. 2007;102(5):752–760. doi: 10.1111/j.1360-0443.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- Ling W, Charuvastra C, Collins JF, Batki S, Brown LS, Kintaudi P, Wesson DR, McNicholas L, Tusel DJ, Malkerneker U, Renner JA, Santos E, Casadonte P, Fye C, Stine S, Wang RI, Segal D. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction. 2002;93(4):475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- Little RJA. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988:1198–1202. [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence of the motivational salience of drug cues in opiate addiction. Psychological Medicine. 2007;37(8):1203–1209. doi: 10.1017/S0033291707009932. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. Journal of Psychopharmacology. 2008;22(8):836–842. doi: 10.1177/0269881107083846. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Archives of General Psychiatry. 2009;66(2):205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- Martell BA, O’Connor PG, Kerns RD, Becker WC, Morales KH, Kosten TR, Fiellin DA. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Annals of Internal Medicine. 2007;146(2):116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain. 2009;142(3):236–244. doi: 10.1016/j.pain.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer EC, Rybin D, Saitz R, Samet JH, Schwartz SL, Butler SF, Liebschutz JM. Identifying prescription opioid use disorder in primary care: diagnostic characteristics of the Current Opioid Misuse Measure (COMM) Pain. 2011;152(2):397–402. doi: 10.1016/j.pain.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Oliver J, Coggins C, Compton P, Hagan S, Matteliano D, Stanton M, St Marie B, Strobbe S, Turner HN. American Society for Pain Management Nursing position statement: Pain management in patients with substance use disorders. Pain Management Nursing. 2012;13(5):169–183. doi: 10.1016/j.pmn.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson R, McCann MJ. DHHS Publication No (SMA) 2006. Counselor’s treatment manual: Matrix intensive outpatient treatment for people with stimulant use disorders. [Google Scholar]
- Ren ZY, Shi J, Epstein DH, Wang J, Lu L. Abnormal pain response in pain-sensitive opiate addicts after prolonged abstinence predicts increased drug craving. Psychopharmacology. 2009;204(3):423–429. doi: 10.1007/s00213-009-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C. Client-centered therapy: Its current practice, implications, and theory. New York: Constable; 2003. [Google Scholar]
- Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain. 1983;17(1):33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D. Mindfulness-based stress reduction for chronic pain conditions: variation in treatment outcomes and role of home meditation practice. Journal of Psychosomatic Research. 2010;68(1):29–36. doi: 10.1016/j.jpsychores.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Stacy AW, Wiers RW. Implicit cognition and addiction: A tool for explaining paradoxical behavior. Annual Review of Clinical Psychology. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan AD, Butler SF, Budman SH, Fernandez K, Weiss RD, Greenfield SF, Jamison RN. Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? Clinical Journal of Pain. 2009;25(3):193–198. doi: 10.1097/AJP.0b013e318193a6c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL, Hasson AL, Huang Z, Jacobs P, Kosinski AS, Lindblad R, McCance-Latz EF, Provost SE, Selzer J, Somoza EC, Sonne SC, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Archives of General Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss Roger D, Potter JS, Provost SE, Huang Z, Jacobs P, Hasson A, Ling W. A multi-site, two-phase, prescription opioid addiction treatment study (POATS): Rationale, design, and methodology. Contemporary Clinical Trials. 2010;31(2):189–199. doi: 10.1016/j.cct.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Systematic Reviews. 2012:11. doi: 10.1002/14651858.CD007407.pub3. Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/14651858/CD007407.pub3/pdf/standard. [DOI] [PMC free article] [PubMed]
- Witkiewitz K, Bowen S, Douglas H, Hsu SH. Mindfulness-based relapse prevention for substance craving. Addictive Behaviors. 2013;38(2):1563–1571. doi: 10.1016/j.addbeh.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. The Journal of Neuroscience. 2011;31(14):5540–5548. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]