Abstract
Malaria is a disease caused by Plasmodium parasites and is responsible for high mortality in humans. This disease is caused by four different species of Plasmodium though the main source of mortality is Plasmodium falciparum. Humans have a number of genetic adaptations that act to combat Plasmodium. One adaptation is a deletion in the SLC4A1 gene that leads to Southeast Asian ovalocytosis (SAO). There is evidence that SAO erythrocytes are resistant to multiple Plasmodium species. Here we analyze SLC4A1 in 23 primates and mammals to test for differential selective pressures among different primate lineages. Because primates are infected with both human Plasmodium parasites and their relatives, this analysis can be used to test which human Plasmodium parasite is the likely target of SAO. A significantly different pattern of molecular evolution was found in humans and African apes, species that are infected by P. falciparum and its relatives. This effect was restricted to the cytosolic domain of the SLC4A1 gene. The evidence is consistent with a different selective regime operating on this gene domain in humans and African apes, when compared to other primates and mammals. Alternatively, this pattern is consistent with a relaxation of selection or weak adaptive evolution operating on a small number of amino acids. The adaptive interpretation of the results is consistent with the SAO allele of the SLC4A1 gene interacting with P. falciparum in humans, rather than other Plasmodium parasites. However, additional investigation of the relationship between SLC4A1 variants and Plasmodium in humans and African apes is required to test whether the different selective regime in humans and African apes is due to natural selection or relaxed constraint.
Keywords: Malaria, Evolution, Adaptation, African ape, Hominoid, Plasmodium
1. Introduction
Malaria is a parasitic disease responsible for approximately 1 million human deaths annually (WHO, 2008). This disease is caused by four different species of Plasmodium parasite: P. falciparum, P. vivax, P. malariae, and P. ovale. The main source of human mortality is the P. falciparum parasite, though P. vivax infections are also responsible for considerable levels of morbidity (Mendis et al., 2001). A number of genetic traits have evolved by natural selection to mitigate this strong selective agent in humans (Kwiatkowski, 2005). One malarial adaptation is apparently related to Southeast Asian Ovalocytosis (SAO) (Baer et al., 1976). SAO is a condition caused by a mutation in the solute carrier, anion exchanger, member 1 gene, SLC4A1, which encodes the band 3 protein, a major component of erythrocytes (Liu et al., 1990). Band 3 has two main functional domains (Fig. 1). The N-terminal intracellular region plays a role in the structure of the erythrocyte (Jay, 1996) and the C-terminal transmembrane region plays a role in anion exchange (Tanner, 1993). The SAO-causing mutation is a 27 base pair deletion in SLC4A1 that removes amino acids 400–408, a region at the junction of the functional domains of the protein. This deletion causes an increased rigidity of erythrocytes (Jarolim et al., 1991; Mohandas et al., 1992). The prevalence of the SAO allele can be as high as 35% in malarial areas (Mgone et al., 1996), suggesting that there is an extremely high selective benefit for the heterozygote carriers of the deletion allele, given that homozygosity is apparently lethal (Liu et al., 1994). The evidence is clear that SAO chiefly protects individuals against severe cerebral malaria (Allen et al., 1999; Genton et al., 1995), a form of the disease caused almost exclusively by P. falciparum. Although it was originally thought that SAO worked via a reduction in cytoadherence (Allen et al., 1999), Cortés et al. (2005) suggested that the protective mechanism of SAO is actually an increased cytoadherence of infected erythrocytes through the CD36 protein.
Fig. 1.
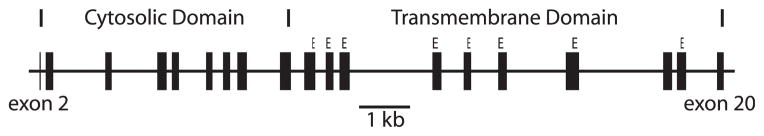
Schematic organization of the human SLC4A1 gene, showing all translated exons (2–20). The cytosolic and transmembrane domains are shown. The external residues of the transmembrane are indicated with the letter “E.” To enhance legibility, these “E” notations approximate the codon lengths of the external residues but are not exact.
Aside from a link between SAO and malaria, there is also evidence that band 3 has a more general role in erythrocyte invasion by Plasmodium. Though the major erythrocyte receptor for P. falciparum invasion is glycophorin A (GYPA) (Pasvol et al., 1982; Sim et al., 1994), the parasite is not solely dependent on this protein (Okoyeh et al., 1999). Indeed, P. falciparum uses a suite of surface receptors for erythrocyte invasion, some of which are yet to be fully characterized (reviewed in Baum et al., 2005). The band 3 protein has been forwarded as an alternate receptor for Plasmodium that is sialic acid-independent (Goel et al., 2003; Kariuki et al., 2005; Li et al., 2009, 2008, 2004; Okoye and Bennett, 1985). Finally, aside from its role in the erythrocyte, a shorter isoform of SLC4A1 is expressed in the kidney where it plays a role in acid secretion; mutations in the gene can lead to distal renal tubular acidosis (reviewed in Williamson and Toye, 2008).
While SAO clearly targets P. falciparum-caused severe malaria (Allen et al., 1999; Genton et al., 1995), there is some evidence that the SAO allele may protect against other Plasmodium parasites. SAO erythrocytes were shown to be relatively resistant to parasitemia or invasion by multiple Plasmodium parasites (Cattani et al., 1987; Foo et al., 1992; Hadley et al., 1983; Kidson et al., 1981; Serjeantson et al., 1977). However, these findings may have been artifactual due to methodological concerns in the handling of SAO cells (Bruce et al., 1999; Dluzewski et al., 1992). It also is possible that SAO may only be effective against erythrocyte invasion in particular strains of P. falciparum (Cortés et al., 2004), suggesting the efficacy of SAO is restricted even within this species. However, Allen et al. (1999) found that SAO did not effect erythrocyte invasion by P. vivax or P. falciparum, but instead caused resistance to cerebral malaria. Though cerebral malaria is most closely associated with P. falciparum, P. vivax can cause cerebral and other forms of severe malaria (Kochar et al., 2005; Rogerson and Carter, 2008). Given this, it is possible that SAO may be an adaptation to cerebral malaria, which can be caused by different Plasmodium species. A general relationship between SLC4A1 and Plasmodium is supported by the conservation of the extracellular portion of the Plasmodium PfSPP protein, which may interact with band 3 (Li et al., 2009).
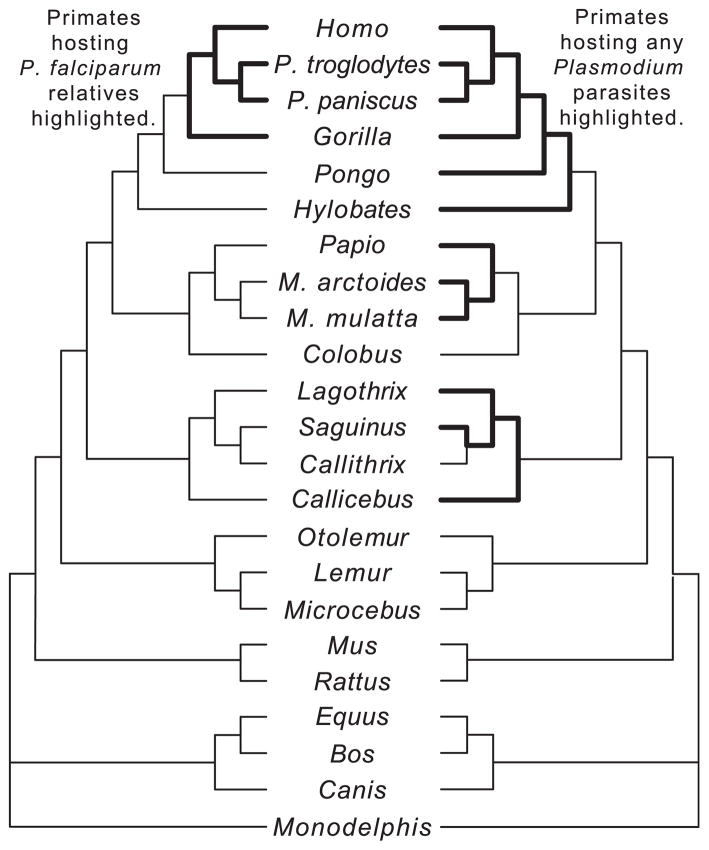
Here we examine the pattern of molecular evolution at SLC4A1 in humans, primates, and other mammals to determine whether this gene is evolving adaptively in primates that harbor P. falciparum related parasites or primates that harbor Plasmodium parasites generally. A pattern of differential selection at the SLC4A1 gene in primates with particular Plasmodium infections will help to ascertain which malarial parasites are being targeted by this locus. Primates are useful models for testing between these hypotheses because they are infected with many different Plasmodium species (Table 1). Critically, it is now well established that wild African apes (chimpanzees, bonobos, and gorillas) are naturally infected with the human parasite P. falciparum and a series of its close phylogenetic relatives (Krief et al., 2010; Liu et al., 2010; Ollomo et al., 2009; Prugnolle et al., 2010; Rich et al., 2009). Phylogenetic relatives of the other human parasites (P. vivax, P. malariae, and P. ovale), on the other hand, infect a larger and more diverse set of primates (Hayakawa et al., 2008; Perkins and Schall, 2002). For this study, it is especially noteworthy that the P. falciparum group infects a relatively small set of primates and these parasites are only distantly related to the other human Plasmodium parasites (Hayakawa et al., 2008).
Table 1.
Species examined, sample information, accession numbers, and Plasmodium infection status. (See below-mentioned references for further information.)
| Host Group | Host Species | Common Name | Coriell Sample ID | Accession Number | Genome Project | Genome Sequence Coordinates | Infections from P. falciparum and similar spp. | Other infecting Plasmodium spp. |
|---|---|---|---|---|---|---|---|---|
| Humans | Homo sapiens | Human | NG_007498.1 † | P. falciparum | P. vivax, P. malariae, P. ovale | |||
|
| ||||||||
| African Apes | Pan paniscus | Bonobo | NG05253 | HM065568 | P. falciparum (Krief et al. 2010) | Spp. related to P. malariae (Krief et al. 2010) | ||
| Pan troglodytes | Chimpanzee | NG06939 | HM065581 | panTro2 | Chr 17:13296154-13309268 | P. reichenowi, P. gaboni, unnamed parasite spp. (Krief et al. 2010; Ollomo et al. 2009; Rich et al. 2009; Prugnolle et al. 2010) | P. rodhaini, P. schwetzi, unnamed parasite spp. (similar to P. malariae, P. vivax and/or P. ovale, Coatney 1971, Duval et al. 2009, Krief et al. 2010, Hayakawa et al 2009) | |
| Gorilla gorilla | Gorilla | NG05251 | HM065569 | P. falciparum, unnamed parasite spp. (Prugnolle et al. 2010) | P. rodhaini, P. schwetzi (similar to P. malariae, P. vivax and/or P. ovale, Coatney 1971) | |||
|
| ||||||||
| Asian Apes | Pongo pygmaeus | Orangutan | NA04272 | HM065570 | none | P. silvaticum, P. pitheci (similar to P. vivax, Wolfe 1999) | ||
| Hylobates gabriellae | Yellow Cheeked Gibbon | PR00381 | HM065571 | none | P. hylobati (similar to P. vivax, Perkins and Schall 2002, Hayakawa et al. 2008) | |||
|
|
|
|||||||
| Old World Monkeys | Macaca arctoides | Stump Tailed Macaque | GM03443 | HM065572 | none | Multiple spp. (similar to P. vivax, Perkins and Schall 2002, Hayakawa et al. 2008) | ||
| Macaca mulatta | Rhesus Macaque | NG07109 | HM065580 | rheMac2 | Chr 16:54355363-54341551 | none | Multiple spp. (similar to P. vivax, Perkins and Schall 2002, Hayakawa et al. 2008) | |
| Colobus guereza | Black and White Colobus | PR00980 | HM065573 | none | none | |||
| Papio anubis | Baboon | PR00036 | HM065574 | none | Hepatocystis (silimar to P. ovale, Perkins and Schall 2002) | |||
|
| ||||||||
| New World Monkeys | Callithrix jacchus | Marmoset | calJac1 | Contig103:955511- 939806 | none | none | ||
| Callicebus moloch | Dusky Titi Monkey | NG06115 | HM065575 | none | P. brasilanum (similar to P. malariae, Escalante et al. 1995) | |||
| Lagothrix lagothricha | Wooly Monkey | NG05356 | HM065576 | none | ” | |||
| Saguinus labiatus | White Lipped Tamarin | NG05308 | HM065577 | none | ” | |||
|
| ||||||||
| Strepsirhines | Otolemur garnettii | Small Eared Galago | PR00049 | HM065578 | none | none | ||
| Lemur catta | Ring Tailed Lemur | ID#6351* | HM065579 | none | none | |||
| Microcebus murinus | Gray Mouse Lemur | ensembl | Genescaffold1055:63426-52166 | none | none | |||
|
| ||||||||
| Rodents | Mus musculus | Mouse | mm9 | Chr 11:102222709 102211601 | none | Murinae infected with Plasdmodium that is outgroup to a a larger P. vivax, P. malariae, P. ovale, Hepatocystis etc. group, Perkins and Schall 2002, Hayakawa et al. 2008) | ||
| Rattus norvegicus | Rat | rn4 | Chr 10:91467178-91456310 | none | ” | |||
|
| ||||||||
| Other Mammals | Bos taurus | Cow | bosTau4 | Chr 19:45496100-45485259 | none | none | ||
| Canis familiaris | Dog | canFam2 | Chr 9:22463842-22475102 | none | none | |||
| Equus caballus | Horse | equCab2 | Chr 11:19271365-19283645 | none | none | |||
| Monodelphis domestica | Opossum | monDom5 | Chr 2:204285378-204264654 | none | none | |||
To address the conflicting evidence as to whether SAO is an adaptation to P. falciparum specifically or to Plasmodium generally, we probe the relationship between band 3 and Plasmodium by analyzing the coding region of SLC4A1 in 23 mammals. The following hypotheses are tested. First, we test whether SLC4A1 has been evolving adaptively across all 23 species. Second, we test whether patterns of molecular evolution at SLC4A1 differ between primate species harboring P. falciparum related parasites (humans and African apes) and those harboring other Plasmodium parasites (Table 1; Fig. 2). Third, we test whether specific structural and functional domains of SLC4A1 are evolving differently from one another.
Fig. 2.
Phylogeny of the species examined in the present study. To the left of the species names, the phylogenetic tree highlights the lineages inferred to have harbored P. falciparum and related parasites (see Table 1). To the right of the species names, the phylogenetic tree highlights the lineages inferred to have harbored any species of Plasmodium, except for rodents (see Section 2 and Table 1). These bold branches are those used by PAML to enable the branch tests and branch site tests as described in the Methods.
2. Methods
2.1. Samples
The primate samples outlined in Table 1 were obtained from the Coriell Institute and the Duke Lemur Center. These were targeted for PCR and sequencing of the entire coding region of the SLC4A1 gene as described in the following sections. These sequences were added to existing DNA sequences from other primates and non-primate mammals from the UCSC Genome Center (Kent et al., 2002), Genbank (Benson et al., 2009), and Ensembl (Hubbard et al., 2009) (Table 1).
2.2. PCR and sequencing
The region targeted for sequencing in these primates was the region encompassed by the human SLC4A1 gene (NG_007498.1). The entire coding region was sequenced, including all translated exons and intervening introns. In general, large overlapping regions of between 1 and 5 kb were targeted by PCR, amplicons were gel purified, these amplicons were subsequently cloned, clones were screened for the presence of the desired insert, and positive clones were sequenced in both directions. Occasionally, small fragments were sequenced directly from purified PCR products. PCRs were done using the Eppendorf Triplemaster and 5 Prime PCR Extender kits using a large number of primer sets and PCR parameters that were often species specific and fragment specific (protocol details and primer sequences available upon request). Both kits utilize a proofreading enzyme. Gel purification was done using the Eppendorf Perfectprep Gel Cleanup kit and TOPO XL Gel Purification kit. PCR purification was done using Millipore Montage Columns. Amplicons were cloned via Invitrogen TA and XL cloning kits. Plasmid DNA from multiple colonies was miniprepped using the Eppendorf FastPlasmid kit and screened for the insert via clone-test PCR and/or EcoRI restriction digestion. Plasmids were sequenced with the M13R and T7 sequencing primers, as well as PCR primers. DNA sequencing was performed by the Dana-Farber/Harvard Cancer Center (DF/HCC) High-Throughput DNA Sequencing Facility, Macrogen Sequencing Services, and Genewiz. Clones positive for the desired insert were sequenced in both directions for the entire sequence using multiple primers (sequencing primer sequences available upon request).
2.3. Sequence alignment and analysis
The codon sequences were aligned by eye to the human exonic sequences. This approach was satisfactory due to the relatively constrained exonic structure across the mammals studied. However, a limited number of exons were aligned using backtranslation from amino acid sequences (Bininda-Emonds, 2005). Codon sequences were analyzed for the action of positive natural selection by maximum likelihood using the codeml program of the PAML package (v. 4) (Yang, 2007). In each test, the F3x4 codon model was used, κ was estimated, and a user tree was supplied. The user tree (Fig. 2) was based on a generally accepted mammalian and primate phylogeny (Goodman et al., 1998; Murphy et al., 2004; Wildman et al., 2009). This was used to generate likelihood values under a number of different models. The convergence of the likelihood values was assessed by running each analysis multiple times. These likelihoods were compared using a series of nested likelihood ratio tests (LRTs) (Yang, 2006). In a LRT, a significant difference between models is assessed by the test statistic 2ΔlnL, which follows a χ2 distribution with degrees of freedom equal to the difference between the numbers of parameters in the competing models. When a significant difference is detected, the model with the higher likelihood is favored. If a significant difference is not detected, the simpler model is preferred, i.e. the one with fewer parameters. Three types of analyses (Yang, 2006) were conducted to determine that pattern of adaptive molecular evolution at the SLC4A1 gene, ‘branch tests’, ‘site tests’, and ‘branch site tests’.
In the branch tests, a LRT is used to determine whether sequences across the entire tree were evolving neutrally by comparing the likelihood of a model where dn/ds (or ω) is fixed at 1 (the neutral model) against a model where the most likely value for ω is estimated. Subsequently, a model where ω was estimated was compared to two alternative models (Fig. 2). In both models, ω values are estimated for two sets of branches. In the P. falciparum model, the two sets of branches are (1) those primates infected with P. falciparum and P. falciparum-related parasites and (2) the remaining branches. In the Plasmodium model, the two sets of branches are (1) those primates infected with any Plasmodium species and (2) the remaining branches (not infected with any malarial parasite). In these models, we did not include stem branches among those being infected because some of these branches are exceptionally long in the primate phylogeny. It is not clear when along these long lineages that Plasmodium transferred into them. Therefore, it is more conservative not to include these lineages. Also, for the Plasmodium model, we infer three origins of Plasmodium infection in primates, one each in apes, Old World monkeys, and New World monkeys. An alternative would be to infer that primates are ubiquitously infected with Plasmodium, with infection being lost in Callithrix, Colobus, and lemurs and lorises. However, there is no evidence to suggest that Plasmodium is common in all of the main primate groups. This is especially true in lemurs, where infections have only been detected in Eulemur (Ellegren, 2008) and in a single specimen of Propithecus, despite sampling 55 samples from 6 lemur genera (Duval et al., 2010). Further information on the Plasmodium infections of the species sampled is presented in Table 1. In this analysis, Papio was included due to its infection with Hepatocystis, a relative of Plasmodium and known selective force (Tung et al., 2009). While rodents of the subfamily Murinae are infected by Plasmodium, Mus musculus and Rattus norvegicus were not included because these two species themselves are not infected.
Because multiple branch tests are being done (two alternative models vs. a null model) using the same data, a correction for multiple tests is necessary (Anisimova and Yang, 2007). There are a number of correction methods, which all essentially adjust the critical value (α) to reduce the type I error rate. Anisimova and Yang (2007) examined a number of such tests regarding the branch tests used here and determined that many methods were able to control error rates. Here, we used the Bonferroni correction, which is very conservative. The Bonferroni correction adjusts α by the number of tests. In the present case we are using two tests, so the adjusted α value is 0.025. These branch tests were conducted on four different functionally relevant partitions of exons. These are the entire gene (911 AA), the cytosolic domain (403 AA in humans), the transmembrane domain (508 AA), and the external sites, a subset of transmembrane amino acids that occur on the cell surface (122 AA) (from Bruce, 2006). The inferred nine amino acid insertion found in African apes was not included in the selection tests. In all cases, the number of the codon given corresponds to that from the human SLC4A1 gene. Here, the tests of the different partitions are a refinement used to determine which regions of the gene are under selection. In this regard, the tests across these different partitions is considered a test of the robustness of the findings are do not require a correction for multiple tests.
Site tests determine if particular amino acid sites have experienced positive selection across the entire phylogeny (Yang, 2006). In these cases, three site models are compared. One is a neutral model with one ω estimated for all sites, the second is a nearly neutral model (with two ω classes, one evolving neutrally and a second evolving under purifying selection), and a third is a model with three ω classes (one neutral (ω = 1), one purifying (ω < 1), and one for sites evolving under positive selection (ω > 1)). (These are models M0, M1, and M2 of PAML.) A related comparison fits 10 site classes for ω between 0 and 1, using a beta distribution (M7) and compares this to a model that also includes a site class with ω > 1 (M8). LRTs that accept models allowing for a class of selected sites indicate the action of positive selection; Bayes empricial Bayes (BEB) (Yang et al., 2005) posterior probabilities are then used to determine the probabilities of those sites. These tests were conducted on the 4 different functionally relevant partitions of exons, as described above. In this regard, the tests across these different partitions is considered a test of the robustness of the findings are do not require a correction for multiple tests.
Branch site tests determine whether particular sites are evolving adaptively on particular lineages (Yang, 2006). Here, partitions of branches are the same as in the branch tests (Fig. 2). For the branch site test, the model estimates parameters for these two sets of branches. For the non-Plasmodium affected branches, two site classes are estimated, those under neutral evolution (ω = 1) and purifying selection (ω < 1). The lineages harboring Plasmodium parasites have these two site classes, and importantly also have an additional site class of positively selected sites. The LRT is between a model where this final site class is neutral (ω = 1) and one under positive selection (ω≥1). These tests were conducted on the 4 different functionally relevant partitions of exons, as described above. In this regard, the tests across these different partitions is considered a test of the robustness of the findings are do not require a correction for multiple tests. However, the tests between the two sets of branches do require a correction for multiple tests, implemented as described above.
3. Results
3.1. Alignment
We collected SLC4A1 DNA sequences from 12 primates (Table 1). These sequences were added to publicly available DNA sequences from a range of primate and non-primate mammals. For two primates, we collected additional DNA sequences to cover unknown regions in the available genome sequences. In total, the alignment of SLC4A1 exons comprised 23 species and was 2898 basepairs in length. One noteworthy aspect of the alignment was a potentially exonic 27 basepair insertion in the African apes, but not in humans (Fig. 3). This insertion is in frame and it is likely to be coding due to its pattern of intron–exon splice sites. The African ape insertion obliterates one splice site but has added another. It is also unusual to find an insertion with this pattern because it suggests that this insertion was gained in the African ape ancestor and then subsequently lost in humans because of the phylogenetic relationships of humans, chimpanzees, and gorillas. Furthermore, the insertion and subsequent deletion was at the same position and length; also very unlikely. A second interpretation is that insertion occurred once in the ancestor of humans, chimpanzees, and gorillas. Subsequently, due to ancestral polymorphism and incomplete lineage sorting the derived condition is found in chimpanzees and gorillas, while the ancestral condition is found in humans. Because incomplete lineage sorting is a known problem within humans and African apes (Ruvolo, 1997), we find the second interpretation to be more likely for this short region. When the entire alignment is analyzed phylogenetically, the accepted phylogeny for apes is obtained, suggesting that the incomplete lineage sorting only affects this small region. Therefore, we used the accepted primate phylogeny in subsequent analyses requiring a user-generated tree. The alignment is available upon request.
Fig. 3.
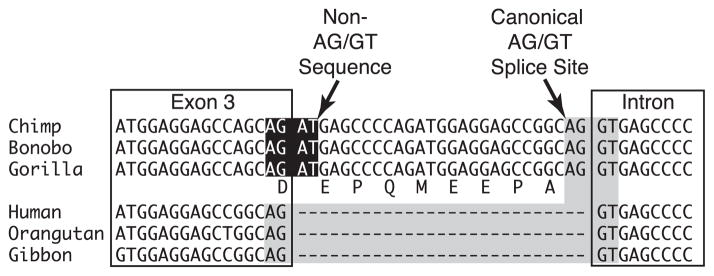
Detail of the insertion at the 3′ end of exon 3 that is reconstructed to have occurred in African Apes. There is clear homology among the sites in exon 3, the insertion, and following intron. The canonical splice sites are highlighted in gray; the obliterated splice site is highlighted in black. The in frame inferred amino acid sequence of the insertion is also given.
3.2. Analyses of adaptive evolution across mammals
Before examining patterns of evolution specifically within primates harboring different Plasmodium species, the pattern of adaptive evolution over the entire mammalian phylogeny was examined. Multiple data partitions were analyzed: the entire gene (911 AA), the cytosolic domain (403 AA in humans), the transmembrane domain (508 AA), and the external sites, a subset of transmembrane amino acids that occur on the cell surface (122 AA). As expected for a functional gene, none of the partitions were evolving neutrally (dn/ds = ω = 1); all had signatures of evolution consistent with purifying selection (Table 2) (ω ranged from 0.12 to 0.28). This level of purifying selection is consistent with average genome-wide estimates for ω in mammals (Ellegren, 2008). Comparing a model with a uniform ω across sites to a ‘nearly neutral model’ with two ω values for different sites (1 and <1) recovered a significant difference for all data partitions, showing that the amino acid sites are not evolving under a uniform neutral selective regime (‘Site tests’; Table 3). This ‘nearly neutral model’ was tested against a model that incorporates an additional class of sites under positive selection, and thus is able to detect particular amino acids under selection. The model incorporating positive selection was a significantly better fit for both the entire gene and the transmembrane partition. In these analyses, nearly 1% of the amino acids were found to be evolving with a ω of approximately 3, suggesting that a limited number of sites are evolving positively in SLC4A1. Two amino acids were identified as evolving under statistically significant positive selection (367,658). A related comparison, where ω is modeled using a beta distribution, found evidence for positively selected sites in multiple comparisons, including codon 658 and additional sites (Table 4). Overall, SLC4A1 is evolving in a manner consistent with a functional gene: the gene is under purifying selection and a limited number of sites are under positive selection.
Table 2.
Branch tests of adaptive evolution.
| Model | N parameters | All sites
|
Cytosolic sites
|
Transmembrane sites
|
External sites
|
||||
|---|---|---|---|---|---|---|---|---|---|
| lnL | ω | lnL | ω | lnL | ω | lnL | ω | ||
| A | |||||||||
| (A) ω = 1 | 44 | −18873.419 | 1.00 | −9687.625 | 1.00 | −9010.701 | 1.00 | −2581.484 | 1.00 |
| (B) Estimate one ω | 45 | −18039.469 | 0.19 | −9412.785 | 0.28 | −8413.696 | 0.12 | −2462.885 | 0.20 |
| (C) P. falciparum branches vs. others | 46 | −18036.433 | 0.37, 0.19 | −9407.341 | 0.86, 0.27 | −8413.530 | 0.09, 0.12 | −2462.808 | 0.14, 0.209 |
| (D) Plasmodium branches vs. others | 46 | −18039.340 | 0.21, 0.19 | −9410.411 | 0.38, 0.26 | −8412.150 | 0.09, 0.13 | −2462.599 | 0.15, 0.20 |
| Likelihood ratio tests | All sites
|
Cytosolic sites
|
Transmembrane sites
|
External sites
|
||||
|---|---|---|---|---|---|---|---|---|
| 2ΔlnL | P value | 2ΔlnL | P value | 2ΔlnL | P value | 2ΔlnL | P value | |
| B | ||||||||
| Neutral evolution (A vs B (df = 1)) | 1667.9 | 0.000 | 549.7 | 0.000 | 1194.0 | 0.000 | 237.2 | 0.000 |
| P. falciparum branches different (C vs B (df = 1))a | 6.07 | 0.014b | 10.9 | 0.001b | 0.3 | 0.565 | 0.2 | 0.694 |
| Plasmodium branches different (D vs B (df = 1))a | 0.3 | 0.611 | 4.7 | 0.029c | 3.1 | 0.079 | 0.6 | 0.449 |
A: parameters and likelihoods estimated for the different models.
B: likelihood ratio tests described in Methods and Results. Bold indicates P < 0.05.
A correction for multiple tests is used for C vs. B and D vs. B tests.
Significant after Bonferroni correction for multiple tests.
Not significant after correction for multiple tests.
Table 3.
Site tests of adaptive evolution, fixed class model.
| Model | N parameters | All sites
|
Cytosolic sites
|
Transmembrane sites
|
External sites
|
||||
|---|---|---|---|---|---|---|---|---|---|
| ln L | ω Values | lnL | ω Values | lnL | ω Values | lnL | ω Values | ||
| A | |||||||||
| (A) ‘M0’, one ω class | 45 | −18039.469 | 0.19 | −9412.785 | 0.28 | −8413.696 | 0.12 | −2462.885 | 0.20 |
| (B) ‘M1a’, two ω classes (<1,1) | 46 | −17475.971 | 0.07, 1.00 | −9178.578 | 0.10, 1.00 | −8172.866 | 0.05, 1.00 | −2367.630 | 0.07, 1.00 |
| (C) ‘M2a’, three ω classes (<1, 1,>1) | 48 | −17469.195 | 0.07, 1.00, 2.86 | −9178.578 | 0.10, 1.00, 1.00 | −8166.515 | 0.05, 1.00, 3.12 | −2364.868 | 0.07, 1.00, 2.88 |
| Likelihood ratio tests | All sites
|
Cytosolic sites
|
Transmembrane sites
|
External sites
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2ΔlnL | P value | Selected sites | 2ΔlnL | P value | Selected sites | 2ΔlnL | P value | Selected sites | 2ΔlnL | P value | Selected sites | |
| B | ||||||||||||
| Two ω classes vs. One ω class (A vs. B (df = 1)) | 1127 | 0 | n/a | 468.4 | 0 | n/a | 481.7 | 0 | n/a | 190.5 | 0 | n/a |
| Two ω classes vs. Three ω classes (B vs. C (df = 2)) | 13.6 | 0.001 | 367-1a, 658 | 0 | 1 | n/a | 12.7 | 0.002 | 658 | 5.5 | 0.063 | n/a |
A: parameters and likelihoods estimated for the different models.
B: likelihood ratio tests described in Sections 2 and 3. Bold indicates P < 0.05.
The position under selection is a gap in the human alignment corresponding to the codon before the 367th codon in human.
Table 4.
Site tests of adaptive evolution, beta distribution model.
| Model | N parameters | All sites
|
Cytosolic sites
|
Transmembrane sites
|
External sites
|
||||
|---|---|---|---|---|---|---|---|---|---|
| lnL | ω of selected class | lnL | ω of selected class | lnL | ω of selected class | lnL | ω of selected class | ||
| A | |||||||||
| (A) ‘M7’, ω beta distribution | 46 | −17452.735 | −9173.066 | −8159.716 | −2358.899 | ||||
| (B) ‘M8’, ω beta distribution with ω class < 1 | 48 | −17424.343 | 1.59 | −9164.167 | 1.25 | −8134.548 | 2.04 | −2352.06 | 1.79 |
| Likelihood ratio tests | All sites
|
Cytosolic sites
|
Transmembrane sites
|
External sites
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2ΔlnL | P value | Selected sites | 2ΔlnL | P value | Selected sites | 2ΔlnL | P value | Selected sites | 2ΔlnL | P value | Selected sites | |
| B | ||||||||||||
| Beta dist. For ω vs. Beta with a site class with ω < 1 (A vs. B (df = 2)) | 56.8 | 0 | 658 | 17.8 | 0 | None | 50.3 | 0 | 558, 560, 656, 658 | 13.7 | 0 | 658 |
These site tests are based on a beta distribution for site classes for ω values for each site. A: parameters and likelihoods estimated for the different models. B: likelihood ratio tests described in Sections 2 and 3. Bold indicates P < 0.05.
3.3. Analyses of adaptive evolution in primates harboring different Plasmodium parasites
Subsequently, we examined whether the lineages reconstructed to have harbored P. falciparum related parasites were evolving differently from lineages that are not reconstructed to have harbored these parasites (‘branch tests’; Fig. 2). When the entire coding region is tested, a model incorporating different ω values for these two sets of lineages is a better fit to the data than a model fitting only one ω to all lineages (P = 0.014; significant after correction for multiple tests) (Table 2). The ω of the lineages reconstructed to harbor P. falciparum-related species was higher (0.37) than the lineages reconstructed not to harbor these parasites (0.19). Of the different data partitions, only the cytosolic domain recovered a significant difference. In the cytosolic domain, the pattern of difference in ω was more marked (ω = 0.86 in P. falciparum related lineages vs. 0.27 in other lineages) than in the entire gene (P = 0.001; significant after correction for multiple tests). A phylogeny showing the reconstructed numbers of nonsynonymous and synonymous changes in humans and African apes reveals that all branches have higher absolute numbers of amino acid changes, except the P. troglodytes branch, which had no changes (Fig. 4).
Fig. 4.
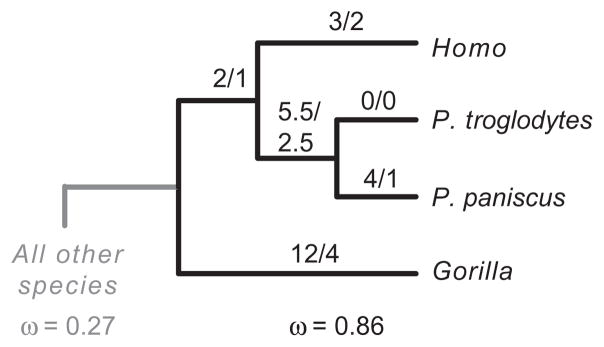
Summary of the significant branch test for the cytosolic domain. This simplified phylogeny shows the two branch classes (species harboring relatives of P. falciparum vs. all other species) and their respective estimated ω values for the cytosolic domain. The codeml program was used (with Rateancestor = 1) to infer the number of nonsynonymous (numerator) and synonymous (denominator) along each branch. These estimates are shown above each branch. Gapped regions were not included in these estimates.
A similar test was done to test whether primates harboring any type of Plasmodium species were evolving differently from lineages that were not reconstructed to have harbored these parasites (Fig. 2). When the entire coding region is tested, a model incorporating different ω values for these two sets of lineages is not a better fit to the data than a model fitting only one ω to all lineages (Table 2). In the cytosolic domain there is an elevated ω ratio in the species harboring any Plasmodium species (ω = 0.38 vs. 0.26), though this finding was not significant after correction for multiple tests. None of the other partitions yielded significant results.
Finally, we tested whether particular sites were evolving adaptively in particular lineages (i.e. the ‘branch site test’). First, positive evolution was tested at sites on the lineages reconstructed to have harbored P. falciparum and its relatives (Table 5). These analyses showed evidence for sites under positive selection in the lineages harboring P. falciparum relatives in the cytosolic region, but only using a less conservative critical value for the LRT (P < 0.05) (Yang, 2006). In this less conservative test, one site (111) was found to be evolving positively in lineages reconstructed to have harbored P. falciparum. However, correction for multiple tests would render this comparison insignificant. Second, we examined the sites on the lineages reconstructed to have harbored any Plasmodium species. None of these comparisons recovered sites under positive natural selection.
Table 5.
Branch-site tests of adaptive evolution.
| Null model
|
Selection model
|
Likelihood ratio tests
|
|||
|---|---|---|---|---|---|
| N parameters | lnL | N parameters | lnL | P value | |
| A | |||||
| All | 47 | −17473.9418 | 48 | −17472.7279 | 0.119 |
| Cytosolic sites | 47 | −9174.2083 | 48 | −9172.7565 | 0.088a |
| Transmembrane sites | 47 | −8172.8663 | 48 | −8172.8663 | 1.000 |
| External sites | 47 | −2367.4868 | 48 | −2367.4868 | 1.000 |
| B | |||||
| All | 47 | −17475.2612 | 48 | −17473.9605 | 0.107 |
| Cytosolic sites | 47 | −9176.0386 | 48 | −9175.5231 | 0.310 |
| Transmembrane sites | 47 | −8172.8663 | 48 | −8172.4822 | 0.381 |
| External sites | 47 | −2367.6301 | 48 | −2367.6301 | 1.000 |
A: tests on lineages harboring P. falciparum and related parasites.
B: tests on lineages harboring any plasmodium parasites.
See text for additional information regarding this comparison.
4. Discussion
The pattern of molecular evolution at the SLC4A1 gene offers insight into the differences in adaptations between primate species harboring different sets of Plasmodium parasites. Differential ω values were detected in humans and African apes—species harboring P. falciparum related parasites. The differential ω was most pronounced in the cytosolic domain of SLC4A1. Although the ω values for the cytosolic domain in humans and African apes are not greater than 1, the benchmark for positive evolution, they are three times higher than the values estimated for the rest of the tree (ω = 0.88 vs 0.27) and higher than average ω values estimated from mammalian genome-wide comparisons (Ellegren, 2008). This is a significant difference, despite the conservative nature of the test employed, and it shows that a shift in selective regimes has occurred in the African apes and humans at SLC4A1. A cautious interpretation of this finding is that the higher ω value in African apes is reflective of a relaxation of constraint or decreased purifying selection in the cytosolic domain. Less cautiously, these findings can be interpreted to support a hypothesis where P. falciparum and its relatives, found exclusively in humans and African apes, are a selective force acting on the cytosolic domain of SLC4A1. This latter interpretation is tenable based on the relationship the SAO mutation in humans and their relationship to malaria (Baer et al., 1976), the idea that Plasmodium is a selective force in the evolution of primates (Fooden, 1984), the presence of P. falciparum-like parasites throughout the African hominoid radiation (e.g. Duval et al., 2010), and the apparent restriction of this effect to the cytosolic domain. It may be the case that the small number of changes are under selection, a pattern of selection that is particularly difficult to detect (Yang, 2006). Furthermore, testing among the relaxed constraint and adaptive hypotheses will require additional work, from a combination of epidemiological, population genetic, and functional studies. Particular examination of the inferred nine amino acid insertion in African apes appears promising given its unique phylogenetic pattern and properties, including an inferred alternate start codon. Interestingly, a recent case report found evidence for an altered initiation site in SLC4A1 in a severely anemic individual, a mutation that leads to P. falciparum resistance in vitro (Perrotta et al., 2005). It is worth mentioning that the case for a relationship between this putatively adaptive evolution and Plasmodium will require additional functional work as a rigorous test, as it is possible that the evolution of this gene may be related to a wholly different selective pressure than Plasmodium.
Because it is restricted to humans and African apes, an adaptive interpretation supports the hypothesis that the SAO mutation of SLC4A1 is targeting P. falciparum in humans. Furthermore, it suggests that African apes and humans may share some common mechanisms of adaption to P. falciparum-related parasites. This is especially interesting because examination of other human malarial adaptations in non-human primates has found mixed support for common adaptations between humans and other primates. At the G6PD and β-globin loci of chimpanzees there is no evidence for selection (MacFie et al., 2009; Verrelli et al., 2006) and in orangutans the evidence for selection at the α-globin locus is limited (Steiper et al., 2005, 2006), though there is evidence for selection at these loci in humans (Allison, 1954; Flint et al., 1986). Tung et al. (2009) recently examined the relationship between variants of the baboon FY locus (known to be important in human malarial resistance) and infection with Hepatocystis (a relative of Plasmodium). Using multiple lines of evidence, Tung et al. clearly showed that particular FY variants conferred resistance to Hepatocystis, revealing that some mechanisms of parasitic adaptation are broadly similar between humans and non-human primates (2009). Further studies examining the population genetics of SLC4A1 in African apes and humans, including functional assessments of segregating variants and their relationships with particular Plasmodium parasites, will enable direct tests of the hypotheses forwarded here. Finally, the nine amino acid insertion inferred to have occurred in African apes, yet lost in humans, is worthy of special attention in future functional analyses of the primate SLC4A1 gene.
The results presented here also bear on our understanding of the evolutionary history of the human Wright blood group antigen. In humans, the Wr antigen is formed when the 658th amino acid at SLC4A1 links to the 61st amino acid of the GYPA protein (arginine) (Bruce et al., 1995). The human Wra allele is exceptionally rare (e.g. Arriaga et al., 2005). Interestingly, the 658th amino acid was identified in multiple tests as evolving positively in SLC4A1 across mammals. Furthermore, GYPA is one of the most rapidly evolving loci in the primate genome (Baum et al., 2005; Wang et al., 2003). The positive selection at this site linking SLC4A1 to GYPA suggests that these genes may be evolving adaptively and in a correlated manner. Previous evolutionary work exploring the relationship between GYPA and SLC4A1 in primates suggested that the specific amino acids bonding these proteins may differ across species (Huang et al., 1996). Because SLC4A1 amino acid 658 is evolving positively across all mammals studied, it is likely that the correlated evolution is not related to Plasmodium but is a more ancient or generalized selective force. Although a recent study of the population genetics of SLC4A1 in humans suggests the selective history of this gene may relate to anion transport (Wilder et al., 2009), the Wr antigens do not differ in this feature (Bruce et al., 1995). Future study will help to elucidate the relationship between the SLC4A1 and GYPA genes across primate evolution and also determine the selection pressure acting on these loci.
5. Conclusions
These results show that there has been a differential selective regime operating on SLC4A1 gene in humans and African apes relative to other primates and mammals. The effect is mainly found in the cytosolic domain of this protein. This finding has two interpretations. One interpretation is that the cytosolic domain of SLC4A1 is experiencing relaxed selective constraint in humans and African apes. A second interpretation is that there have been a small number of adaptive evolutionary changes within the human and African ape lineages, potentially in response to P. falciparum and its relatives. The adaptive hypothesis suggests that these African hominoid primates have been evolving to combat Plasmodium since their last common ancestor. Future studies investigating the relationship between SLC4A1 variants and Plasmodium in humans and wild African apes can further evaluate this hypothesis. Also of note is the finding that the amino acid in SLC4A1 that encodes the human Wright blood group antigen is under positive selection across mammals. The Wright blood group antigen is little studied, and additional research is required to determine the potential evolutionary, functional, and clinical significance of this finding.
6. Database ID
HM065568, HM065581, HM065569, HM065570, HM065571, HM065572, HM065580, HM065573, HM065573, HM065575, HM065576, HM065577, HM065578, HM065579 (all at Genbank, the Genetic sequence database at the National Center for Biotechnical Information (NCBI)).
Acknowledgments
The authors thank A. Lobell, W. Qiu, and the reviewers for comments on the manuscript and Z. Yang for statistical advice. The infrastructure of the Anthropological Genetics Lab at Hunter College was supported by Grant Number RR03037 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Contributor Information
Michael E. Steiper, Email: msteiper@hunter.cuny.edu.
Fiona Walsh, Email: fwalsh@hunter.cuny.edu.
Julia M. Zichello, Email: jzichello@gc.cuny.edu.
References
- Allen SJ, O’Donnell A, Alexander ND, Mgone CS, Peto TE, Clegg JB, Alpers MP, Weatherall DJ. Prevention of cerebral malaria in children in Papua New Guinea by southeast Asian ovalocytosis band 3. Am J Trop Med Hyg. 1999;60:1056–1060. doi: 10.4269/ajtmh.1999.60.1056. [DOI] [PubMed] [Google Scholar]
- Allison AC. Protection afforded by sickle-cell trait against subtertian malarial infection. Br Med J. 1954;1:290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimova M, Yang Z. Multiple hypothesis testing to detect lineages under positive selection that affects only a few sites. Mol Biol Evol. 2007;24:1219–1228. doi: 10.1093/molbev/msm042. [DOI] [PubMed] [Google Scholar]
- Arriaga F, Llopis F, de la Rubia J, Carpio N, Moscardo J, Marty ML. Incidence of Wra antigen and anti-Wra in a Spanish population. Transfusion. 2005;45:1324–1326. doi: 10.1111/j.1537-2995.2005.00196.x. [DOI] [PubMed] [Google Scholar]
- Baer A, Lie-Injo LE, Welch QB, Lewis AN. Genetic factors and malaria in the Temuan. Am J Hum Genet. 1976;28:179–188. [PMC free article] [PubMed] [Google Scholar]
- Baum J, Maier AG, Good RT, Simpson KM, Cowman AF. Invasion by P. falciparum merozoites suggests a hierarchy of molecular interactions. PLoS Pathog. 2005;1:e37. doi: 10.1371/journal.ppat.0010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2009;37:D26–31. doi: 10.1093/nar/gkn723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bininda-Emonds OR. TransAlign: using amino acids to facilitate the multiple alignment of protein-coding DNA sequences. BMC Bioinf. 2005;6:156. doi: 10.1186/1471-2105-6-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce L. Mutations in band 3 and cation leaky red cells. Blood Cells Mol Dis. 2006;36:331–336. doi: 10.1016/j.bcmd.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Bruce LJ, Ring SM, Anstee DJ, Reid ME, Wilkinson S, Tanner MJ. Changes in the blood group Wright antigens are associated with a mutation at amino acid 658 in human erythrocyte band 3: a site of interaction between band 3 and glycophorin A under certain conditions. Blood. 1995;85:541–547. [PubMed] [Google Scholar]
- Bruce LJ, Ring SM, Ridgwell K, Reardon DM, Seymour CA, Van Dort HM, Low PS, Tanner MJ. South-East Asian ovalocytic (SAO) erythrocytes have a cold sensitive cation leak: implications for in vitro studies on stored SAO red cells. Biochim Biophys Acta. 1999;1416:258–270. doi: 10.1016/s0005-2736(98)00231-4. [DOI] [PubMed] [Google Scholar]
- Cattani JA, Gibson FD, Alpers MP, Crane GG. Hereditary ovalocytosis and reduced susceptibility to malaria in Papua New Guinea. Trans R Soc Trop Med Hyg. 1987;81:705–709. doi: 10.1016/0035-9203(87)90001-0. [DOI] [PubMed] [Google Scholar]
- Coatney GR, Collines WE, Warren M, Contacos PG. The Primate Malarias. US Department of Health, Education, and Welfare; Bethesda: 1971. [Google Scholar]
- Cortés A, Benet A, Cooke BM, Barnwell JW, Reeder JC. Ability of Plasmodium falciparum to invade Southeast Asian ovalocytes varies between parasite lines. Blood. 2004;104:2961–2966. doi: 10.1182/blood-2004-06-2136. [DOI] [PubMed] [Google Scholar]
- Cortés A, Mellombo M, Mgone CS, Beck HP, Reeder JC, Cooke BM. Adhesion of Plasmodium falciparum-infected red blood cells to CD36 under flow is enhanced by the cerebral malaria-protective trait South-East Asian ovalocytosis. Mol Biochem Parasitol. 2005;142:252–257. doi: 10.1016/j.molbiopara.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Dluzewski AR, Nash GB, Wilson RJ, Reardon DM, Gratzer WB. Invasion of hereditary ovalocytes by Plasmodium falciparum in vitro and its relation to intracellular ATP concentration. Mol Biochem Parasitol. 1992;55:1–7. doi: 10.1016/0166-6851(92)90121-y. [DOI] [PubMed] [Google Scholar]
- Duval L, Fourment M, Nerrienet E, Rousset D, Sadeuh SA, Goodman SM, Andriaholinirina NV, Randrianarivelojosia M, Paul RE, Robert V, Ayala FJ, Ariey F. African apes as reservoirs of Plasmodium falciparum and the origin and diversification of the Laverania subgenus. Proc Natl Acad Sci USA. 2010;107:10561–10566. doi: 10.1073/pnas.1005435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval L, Nerrienet E, Rousset D, Sadeuh MbaSA, Houze S, Fourment M, Le Bras J, Robert V, Ariey F. Chimpanzee malaria parasites related to Plasmodium ovale in Africa. PLoS One. 2009;4:e5520. doi: 10.1371/journal.pone.0005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H. Comparative genomics and the study of evolution by natural selection. Mol Ecol. 2008;17:4586–4596. doi: 10.1111/j.1365-294X.2008.03954.x. [DOI] [PubMed] [Google Scholar]
- Escalante AA, Barrio E, Ayala FJ. Evolutionary origin of human and primate malarias: evidence from the circumsporozoite protein gene. Mol Biol Evol. 1995;12:616–626. doi: 10.1093/oxfordjournals.molbev.a040241. [DOI] [PubMed] [Google Scholar]
- Flint J, Hill AVS, Bowden DK, Oppenheimer SJ, Sill PR, Serjeantson SW, Bana-Koiri J, Bhatia K, Alpers MP, Boyce AJ, et al. High frequencies of α-thalassaemia are the result of natural selection by malaria. Nature. 1986;321:744– 750. doi: 10.1038/321744a0. [DOI] [PubMed] [Google Scholar]
- Foo LC, Rekhraj V, Chiang GL, Mak JW. Ovalocytosis protects against severe malaria parasitemia in the Malayan aborigines. Am J Trop Med Hyg. 1992;47:271–275. doi: 10.4269/ajtmh.1992.47.271. [DOI] [PubMed] [Google Scholar]
- Fooden J. Malaria in macaques. Intl J Primatol. 1984;15:573–596. [Google Scholar]
- Genton B, al-Yaman F, Mgone CS, Alexander N, Paniu MM, Alpers MP, Mokela D. Ovalocytosis and cerebral malaria. Nature. 1995;378:564–565. doi: 10.1038/378564a0. [DOI] [PubMed] [Google Scholar]
- Goel VK, Li X, Chen H, Liu SC, Chishti AH, Oh SS. Band 3 is a host receptor binding merozoite surface protein 1 during the Plasmodium falciparum invasion of erythrocytes. Proc Natl Acad Sci USA. 2003;100:5164–5169. doi: 10.1073/pnas.0834959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M, Porter CA, Czelusniak J, Page SL, Schneider H, Shoshani J, Gunnell G, Groves CP. Toward a phylogenetic classification of Primates based on DNA evidence complemented by fossil evidence. Mol Phylogenet Evol. 1998;9:585–598. doi: 10.1006/mpev.1998.0495. [DOI] [PubMed] [Google Scholar]
- Hadley T, Saul A, Lamont G, Hudson DE, Miller LH, Kidson C. Resistance of Melanesian elliptocytes (ovalocytes) to invasion by Plasmodium knowlesi and Plasmodium falciparum malaria parasites in vitro. J Clin Invest. 1983;71:780–782. doi: 10.1172/JCI110827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Arisue N, Udono T, Hirai H, Sattabongkot J, Toyama T, Tsuboi T, Horii T, Tanabe K. Identification of Plasmodium malariae, a human malaria parasite, in imported chimpanzees. PLoS One. 2009;4:e7412. doi: 10.1371/journal.pone.0007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Culleton R, Otani H, Horii T, Tanabe K. Big bang in the evolution of extant malaria parasites. Mol Biol Evol. 2008;25:2233–2239. doi: 10.1093/molbev/msn171. [DOI] [PubMed] [Google Scholar]
- Huang CH, Reid ME, Xie SS, Blumenfeld OO. Human red blood cell Wright antigens: a genetic and evolutionary perspective on glycophorin A-band 3 interaction. Blood. 1996;87:3942–3947. [PubMed] [Google Scholar]
- Hubbard TJ, Aken BL, Ayling S, Ballester B, Beal K, Bragin E, Brent S, Chen Y, Clapham P, Clarke L, Coates G, Fairley S, Fitzgerald S, Fernandez-Banet J, Gordon L, Graf S, Haider S, Hammond M, Holland R, Howe K, Jenkinson A, Johnson N, Kahari A, Keefe D, Keenan S, Kinsella R, Kokocinski F, Kulesha E, Lawson D, Longden I, Megy K, Meidl P, Overduin B, Parker A, Pritchard B, Rios D, Schuster M, Slater G, Smedley D, Spooner W, Spudich G, Trevanion S, Vilella A, Vogel J, White S, Wilder S, Zadissa A, Birney E, Cunningham F, Curwen V, Durbin R, Fernandez-Suarez XM, Herrero J, Kasprzyk A, Proctor G, Smith J, Searle S, Flicek P. Ensembl 2009. Nucleic Acids Res. 2009;37:D690–697. doi: 10.1093/nar/gkn828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarolim P, Palek J, Amato D, Hassan K, Sapak P, Nurse GT, Rubin HL, Zhai S, Sahr KE, Liu SC. Deletion in erythrocyte band 3 gene in malaria-resistant Southeast Asian ovalocytosis. Proc Natl Acad Sci USA. 1991;88:11022– 11026. doi: 10.1073/pnas.88.24.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay DG. Role of band 3 in homeostasis and cell shape. Cell. 1996;86:853–854. doi: 10.1016/s0092-8674(00)80160-9. [DOI] [PubMed] [Google Scholar]
- Kariuki MM, Li X, Yamodo I, Chishti AH, Oh SS. Two Plasmodium falciparum merozoite proteins binding to erythrocyte band 3 form a direct complex. Biochem Biophys Res Commun. 2005;338:1690–1695. doi: 10.1016/j.bbrc.2005.10.154. [DOI] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidson C, Lamont G, Saul A, Nurse GT. Ovalocytic erythrocytes from Melanesians are resistant to invasion by malaria parasites in culture. Proc Natl Acad Sci USA. 1981;78:5829–5832. doi: 10.1073/pnas.78.9.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochar DK, Saxena V, Singh N, Kochar SK, Kumar SV, Das A. Plasmodium vivax malaria. Emerg Infect Dis. 2005;11:132–134. doi: 10.3201/eid1101.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krief S, Escalante AA, Pacheco MA, Mugisha L, Andre C, Halbwax M, Fischer A, Krief JM, Kasenene JM, Crandfield M, Cornejo OE, Chavatte JM, Lin C, Letourneur F, Gruner AC, McCutchan TF, Renia L, Snounou G. On the diversity of malaria parasites in African apes and the origin of Plasmodium falciparum from Bonobos. PLoS Pathog. 2010;6:e1000765. doi: 10.1371/journal.ppat.1000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen H, Bahamontes-Rosa N, Kun JF, Traore B, Crompton PD, Chishti AH. Plasmodium falciparum signal peptide peptidase is a promising drug target against blood stage malaria. Biochem Biophys Res Commun. 2009;380:454– 459. doi: 10.1016/j.bbrc.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen H, Oh SS, Chishti AH. A Presenilin-like protease associated with Plasmodium falciparum micronemes is involved in erythrocyte invasion. Mol Biochem Parasitol. 2008;158:22–31. doi: 10.1016/j.molbiopara.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen H, Oo TH, Daly TM, Bergman LW, Liu SC, Chishti AH, Oh SS. A co-ligand complex anchors Plasmodium falciparum merozoites to the erythrocyte invasion receptor band 3. J Biol Chem. 2004;279:5765–5771. doi: 10.1074/jbc.M308716200. [DOI] [PubMed] [Google Scholar]
- Liu SC, Jarolim P, Rubin HL, Palek J, Amato D, Hassan K, Zaik M, Sapak P. The homozygous state for the band 3 protein mutation in Southeast Asian Ovalocytosis may be lethal. Blood. 1994;84:3590–3591. [PubMed] [Google Scholar]
- Liu SC, Zhai S, Palek J, Golan DE, Amato D, Hassan K, Nurse GT, Babona D, Coetzer T, Jarolim P, et al. Molecular defect of the band 3 protein in southeast Asian ovalocytosis. N Engl J Med. 1990;323:1530–1538. doi: 10.1056/NEJM199011293232205. [DOI] [PubMed] [Google Scholar]
- Liu W, Li Y, Learn GH, Rudicell RS, Robertson JD, Keele BF, Ndjango JB, Sanz CM, Morgan DB, Locatelli S, Gonder MK, Kranzusch PJ, Walsh PD, Delaporte E, Mpoudi-Ngole E, Georgiev AV, Muller MN, Shaw GM, Peeters M, Sharp PM, Rayner JC, Hahn BH. Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature. 2010;467:420–425. doi: 10.1038/nature09442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFie TS, Nerrienet E, Bontrop RE, Mundy NI. The action of falciparum malaria on the human and chimpanzee genomes compared: absence of evidence for a genomic signature of malaria at HBB and G6PD in three subspecies of chimpanzee. Infect Genet Evol. 2009;9:1248–1252. doi: 10.1016/j.meegid.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- Mgone CS, Koki G, Paniu MM, Kono J, Bhatia KK, Genton B, Alexander ND, Alpers MP. Occurrence of the erythrocyte band 3 (AE1) gene deletion in relation to malaria endemicity in Papua New Guinea. Trans R Soc Trop Med Hyg. 1996;90:228–231. doi: 10.1016/s0035-9203(96)90223-0. [DOI] [PubMed] [Google Scholar]
- Mohandas N, Winardi R, Knowles D, Leung A, Parra M, George E, Conboy J, Chasis J. Molecular basis for membrane rigidity of hereditary ovalocytosis. A novel mechanism involving the cytoplasmic domain of band 3. J Clin Invest. 1992;89:686–692. doi: 10.1172/JCI115636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy WJ, Pevzner PA, O’Brien SJ. Mammalian phylogenomics comes of age. Trends Genet. 2004;20:631–639. doi: 10.1016/j.tig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Okoye VC, Bennett V. Plasmodium falciparum malaria: band 3 as a possible receptor during invasion of human erythrocytes. Science. 1985;227:169–171. doi: 10.1126/science.3880920. [DOI] [PubMed] [Google Scholar]
- Okoyeh JN, Pillai CR, Chitnis CE. Plasmodium falciparum field isolates commonly use erythrocyte invasion pathways that are independent of sialic acid residues of glycophorin A. Infect. Immun. 1999;67:5784–5791. doi: 10.1128/iai.67.11.5784-5791.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollomo B, Durand P, Prugnolle F, Douzery E, Arnathau C, Nkoghe D, Leroy E, Renaud F. A new malaria agent in African hominids. PLoS Pathog. 2009;5:e1000446. doi: 10.1371/journal.ppat.1000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasvol G, Wainscoat JS, Weatherall DJ. Erythrocytes deficiency in glycophorin resist invasion by the malarial parasite Plasmodium falciparum. Nature. 1982;297:64–66. doi: 10.1038/297064a0. [DOI] [PubMed] [Google Scholar]
- Perkins SL, Schall JJ. A molecular phylogeny of malarial parasites recovered from cytochrome b gene sequences. J Parasitol. 2002;88:972–978. doi: 10.1645/0022-3395(2002)088[0972:AMPOMP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Perrotta S, Borriello A, Scaloni A, De Franceschi L, Brunati AM, Turrini F, Nigro V, del Giudice EM, Nobili B, Conte ML, Rossi F, Iolascon A, Donella-Deana A, Zappia V, Poggi V, Anong W, Low P, Mohandas N, Della Ragione F. The N-terminal 11 amino acids of human erythrocyte band 3 are critical for aldolase binding and protein phosphorylation: implications for band 3 function. Blood. 2005;106:4359–4366. doi: 10.1182/blood-2005-07-2806. [DOI] [PubMed] [Google Scholar]
- Prugnolle F, Durand P, Neel C, Ollomo B, Ayala FJ, Arnathau C, Etienne L, Mpoudi-Ngole E, Nkoghe D, Leroy E, Delaporte E, Peeters M, Renaud F. African great apes are natural hosts of multiple related malaria species, including Plasmodium falciparum. Proc Natl Acad Sci USA. 2010;107:1458–1463. doi: 10.1073/pnas.0914440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich SM, Leendertz FH, Xu G, Lebreton M, Djoko CF, Aminake MN, Takang EE, Diffo JL, Pike BL, Rosenthal BM, Formenty P, Boesch C, Ayala FJ, Wolfe ND. The origin of malignant malaria. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0907740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson SJ, Carter R. Severe vivax malaria: newly recognised or rediscovered. PLoS Med. 2008;5:e136. doi: 10.1371/journal.pmed.0050136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvolo M. Molecular phylogeny of the hominoids: inferences from multiple independent DNA sequence data sets. Mol Biol Evol. 1997;14:248–265. doi: 10.1093/oxfordjournals.molbev.a025761. [DOI] [PubMed] [Google Scholar]
- Serjeantson S, Bryson K, Amato D, Babona D. Malaria and hereditary ovalocytosis. Hum Genet. 1977;37:161–167. doi: 10.1007/BF00393579. [DOI] [PubMed] [Google Scholar]
- Sim BK, Chitnis CE, Wasniowska K, Hadley TJ, Miller LH. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- Steiper ME, Wolfe ND, Karesh WB, Kilbourn AM, Bosi EJ, Ruvolo M. The population genetics of the alpha-2 globin locus of orangutans (Pongo pygmaeus) J Mol Evol. 2005;60:400–408. doi: 10.1007/s00239-004-0201-x. [DOI] [PubMed] [Google Scholar]
- Steiper ME, Wolfe ND, Karesh WB, Kilbourn AM, Bosi EJ, Ruvolo M. The phylogenetic and evolutionary history of a novel alpha-globin-type gene in orangutans (Pongo pygmaeus) Infect Genet Evol. 2006;6:277–286. doi: 10.1016/j.meegid.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Tanner MJ. Molecular and cellular biology of the erythrocyte anion exchanger (AE1) Semin Hematol. 1993;30:34–57. [PubMed] [Google Scholar]
- Tung J, Primus A, Bouley AJ, Severson TF, Alberts SC, Wray GA. Evolution of a malaria resistance gene in wild primates. Nature. 2009;460:388–391. doi: 10.1038/nature08149. [DOI] [PubMed] [Google Scholar]
- Verrelli BC, Tishkoff SA, Stone AC, Touchman JW. Contrasting histories of G6PD molecular evolution and malarial resistance in humans and chimpanzees. Mol Biol Evol. 2006;23:1592–1601. doi: 10.1093/molbev/msl024. [DOI] [PubMed] [Google Scholar]
- Wang HY, Tang H, Shen CK, Wu CI. Rapidly evolving genes in human. I The glycophorins and their possible role in evading malaria parasites. Mol Biol Evol. 2003;20:1795–1804. doi: 10.1093/molbev/msg185. [DOI] [PubMed] [Google Scholar]
- WHO. World Malaria Report. World Health Organization; Geneva: 2008. [Google Scholar]
- Wilder JA, Stone JA, Preston EG, Finn LE, Ratcliffe HL, Sudoyo H. Molecular population genetics of SLC4A1 and Southeast Asian Ovalocytosis. J Hum Genet. 2009;54:182–187. doi: 10.1038/jhg.2009.12. [DOI] [PubMed] [Google Scholar]
- Wildman DE, Jameson NM, Opazo JC, Yi SV. A fully resolved genus level phylogeny of neotropical primates (Platyrrhini) Mol Phylogenet Evol. 2009;53:694– 702. doi: 10.1016/j.ympev.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Williamson RC, Toye AM. Glycophorin A: band 3 aid. Blood Cells Mol Dis. 2008;41:35–43. doi: 10.1016/j.bcmd.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Wolfe ND. PhD thesis. Harvard School of Public Health; Cambridge, MA: 1999. Pathogen evolution and exchange in Bornean orangutans. [Google Scholar]
- Yang Z. Computational Molecular Evolution. Oxford University Press; Oxford: 2006. [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wong WS, Nielsen R. Bayes empirical bayes inference of amino acid sites under positive selection. Mol Biol Evol. 2005;22:1107–1118. doi: 10.1093/molbev/msi097. [DOI] [PubMed] [Google Scholar]