Abstract
Scope
Ulcerative colitis (UC) is a chronic inflammatory disease of the colon. α-Mangostin (α-MG), the most abundant xanthone in mangosteen fruit, exerts anti-inflammatory and antibacterial activities in vitro. We evaluated the impact of dietary α-MG on murine experimental colitis and on the gut microbiota of healthy mice.
Methods and results
Colitis was induced in C57BL/6J mice by administration of dextran sulfate sodium (DSS). Mice were fed control diet or diet with α-MG (0.1%). α-MG exacerbated the pathology of DSS-induced colitis. Mice fed diet with α-MG had greater colonic inflammation and injury, as well as greater infiltration of CD3+ and F4/80+ cells, and colonic myeloperoxidase, than controls. Serum levels of granulocyte colony-stimulating factor, IL-6, and serum amyloid A were also greater in α-MG-fed animals than in controls. The colonic and cecal microbiota of healthy mice fed α-MG but no DSS shifted to an increased abundance of Proteobacteria and decreased abundance of Firmicutes and Bacteroidetes, a profile similar to that found in human UC.
Conclusion
α-MG exacerbated colonic pathology during DSS-induced colitis. These effects may be associated with an induction of intestinal dysbiosis by α-MG. Our results suggest that the use of α-MG-containing supplements by patients with UC may have unintentional risk.
Keywords: Diet, Gut microbiota, Inflammation, Mouse model
1 Introduction
Ulcerative colitis (UC) is a chronic disease characterized by mucosal and submucosal inflammation limited to the colon with cryptitis and crypt abscesses. Persistent progressive or relapsing inflammation results in bloody diarrhea and abdominal distress that are hallmarks of UC [1]. In addition, the relative risk of UC patients developing colorectal cancer is substantially increased with duration and severity of the disease [2]. The etiology of UC remains only partially understood, but it is thought to result from a dysregulated immune response to the gut microbiota in a genetically susceptible host [3]. Dysbiosis, an imbalance between putative species of “protective” versus “harmful” intestinal bacteria, has been implicated in UC [4].
The chemically induced dextran sulfate sodium (DSS) colitis model has been shown to mimic human UC pathology, and recent preclinical studies have supported its use as a system to evaluate the role of anti-inflammatory agents [2]. Oral ingestion of DSS induces diarrhea, rectal bleeding, ulceration of the colonic epithelium, loss of goblet cells, and immune cell infiltration, similar to the phenotypic changes observed in human UC [5]. Attenuation of the synthesis and secretion of proinflammatory mediators is expected to be beneficial during chronic inflammatory conditions such as inflammatory bowel disease (IBD). Medical therapy for the treatment of IBD has only modest success and is associated with adverse side effects [6]. This likely contributes to the use of complementary and alternative medicine (CAM), such as herbal preparations, by as many as 50% of IBD patients [7]. However, clinical evidence supporting the use of such products in UC management is limited or absent.
In vitro and in vivo studies have shown that dietary components can protect against inflammation [8]. Plant-derived phytochemicals, such as specific polyphenols, have been shown to inhibit cell signaling processes that are involved in the inflammatory response, thus attenuating synthesis of proinflammatory cytokines and cell adhesion molecules [9]. Garcinia mangostana is a tree native to Southeast Asia that produces a fruit known as mangosteen, which has been used in traditional medicine to treat inflammation, infections, wounds, and diarrhea. The bioactivities of mangosteen have been associated with a family of polyphenolic compounds referred to as xanthones [10]. α-Mangostin (α-MG; Fig. 1), the most abundant xanthone in the pericarp of mangosteen fruit [11], attenuates secretion of proinflammatory cytokines in colonic and immune human cell lines [12] and reduces the inflammatory response by human and rodent macrophages, as well as primary human adipocytes [13]. In vivo, α-MG attenuates paw edema and airway inflammation in rodents [14, 15]. However, α-MG stimulates tumor necrosis factor-α(TNF-α) secretion in primary human blood monocyte-derived macrophages [12], and ingestion of a mangosteen juice supplement by healthy individuals is associated with elevated serum levels of IL-1 and complement components [16]. α-MG also exerts antibacterial, antifungal, and antiviral activities [10]. For instance, α-MG inhibits pathogenic bacteria such as Staphylococcus aureus and Bacillus subtilis, but has no effect on Escherichia coli and Candida albicans [10], which suggests low selectivity against these pathogens. As a result of the aggressive marketing of purported health-promoting activities, numerous supplements, beverages, and food products containing mangosteen are now available. Mangosteen juice, for instance, has been promoted as beneficial for gastrointestinal and immune health. Although objective scientific data supporting these and other claims are lacking, sales of mangosteen-containing beverages alone exceeded $200 million in the United States in 2008 [17]. Indeed, many individuals suffering illness consume these products without the knowledge of their medical team. The potential for both adverse interactions with conventional medications and unintended effects on health outcomes is often overlooked.
Figure 1.
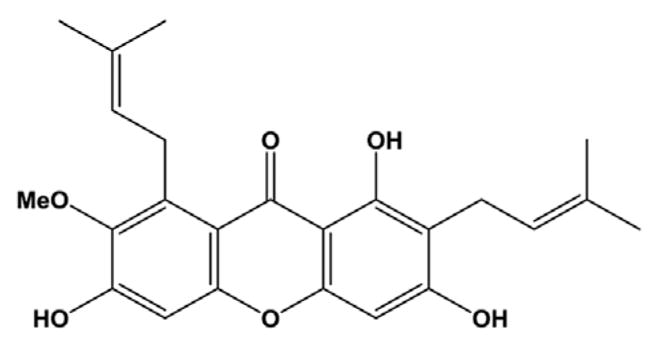
Chemical structure of α-mangostin.
The modulatory effects of α-MG in the context of intestinal inflammation remain unknown. Because greater concentrations of dietary bioactive components, such as α-MG, are found in the gastrointestinal tract than in peripheral tissues [18], this xanthone may exert protective effects in conditions such as UC. Thus, we hypothesized that α-MG would ameliorate colonic inflammation and injury during experimental colitis. The chemically induced DSS colitis model was used in the present study. C57BL/6 mice were fed standard diet or diet containing α-MG and disease severity was assessed based on body weight (BW) loss, diarrhea, and rectal or occult bleeding. Colonic infiltration of immune cells and epithelial cell proliferation, as well as systemic and colonic inflammation, were evaluated. Finally, because α-MG has been reported to exert antibacterial activities, its impact on the gut microbiota of healthy, noncolitic mice was also studied.
2 Materials and methods
2.1 Mice
For colitis studies, 10-week-old female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were housed in the animal facilities at The Ohio State University (OSU) under conventional conditions with controlled temperature at 23°C and a 12-h light/dark cycle. Mice were acclimatized for 1 week before entering the study and had free access to water and standard AIN93G diet. All procedures were approved under Protocol no. 2011A00000006 and followed the guidelines by the Institutional Animal Care and Use Committee (IACUC) at The Ohio State University.
2.2 Diet
α-MG was >98% pure [11, 19]. Gamma-irradiated AIN93G diet (control) and AIN93G diet containing 900 mg/kg α-MG and FDA approved dyes E102 and E133 for green color were prepared by Research Diets (New Brunswick, NJ). This dose has been reported to be safe and effective in reducing tumor mass in xenograft models of colon and prostate cancer. For a mouse weighing 20 g and ingesting 2.5 g diet per day, this dose equates to 112 mg/kg BW. The human equivalent dose (mg/kg) is calculated as animal dose (mg/kg) × (mouse Km/human Km), where Km is a correction factor reflecting the relationship between BW and body surface area [20]. Thus, the human equivalent dose [(112 mg/kg)/(3/37) = 9.12 mg/kg] for an adult weighing 60 kg equates to 547 mg/day. Intake of this amount could be achieved by ingesting one to two capsules of mangosteen extract [21] or one cup of 100% mangosteen juice [22].
2.3 Induction of colitis and experimental groups
DSS-induced colitis is a widely used model to evaluate the role of anti-inflammatory agents [2]. Murine strain, gender, microbiota, the molecular weight, concentration and batch of DSS, and the duration of administration of the chemical insult are known to affect susceptibility to DSS-induced colitis. We selected the C57BL/6J mouse strain as it has been shown that DSS-induced colitis in this strain mimics the chronicity observed in human UC [5]. Furthermore, female mice were selected as their response to DSS-induced inflammation has been reported to be less severe than males [23]. In addition, two pilot studies were performed to optimize the dose of DSS and duration of administration necessary to induce colitis. Accordingly, the dose of DSS in water was decreased from 3 to 2%, and DSS was administered for 4 rather than 5 days. These conditions were found to induce colitis without significant loss of BW. The experimental design is presented in Fig. 2. Colitis was chemically induced with DSS (MP Biomedicals; molecular weight 36 000–50 000) dissolved in the drinking water (2% wt/vol) and provided ad libitum for 4 days. One week prior to the induction of colitis, mice were randomized to be fed either the standard AIN93G diet or the AIN93G diet with α-MG. Mice continued to be fed their respective diets until the end of the study. We followed this approach to mimic chronic use of mangosteen-containing products during both inflammatory flare ups and remission periods. To induce colitis, mice in each diet group were provided ad libitum access to either tap water (control groups: control [n = 20] and α-MG [n = 20]), or water containing 2% DSS (DSS groups: DSS [n = 20] and DSS + α-MG [n = 20]) for 4 days. After DSS administration, all mice were given tap water without DSS and allowed to recover for either 1 or 2 weeks before euthanasia. Ten mice per group were used at each end point.
Figure 2.
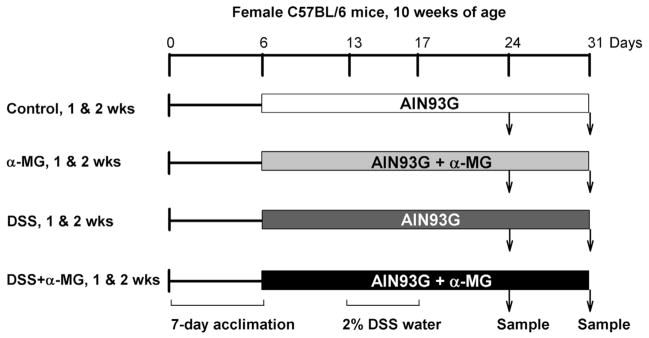
Experimental design. Clinical assessment and body weight were taken throughout the study. Dextran sulfate sodium (DSS) was provided in the drinking water for 4 days and mice were allowed to recover for 1 or 2 weeks. Ten mice per group were used at each time point.
2.4 Disease activity index (DAI) and histopathological evaluation of colitis
The DAI was calculated as previously described [24]. At necropsy, colons were excised, rinsed in PBS and divided into three segments of equal length (proximal, middle, and distal). Sections from each segment were fixed in 10% neutral buffered formalin, paraffin embedded, and stained with hematoxylin and eosin for examination by light microscopy (n = 9–10/group). Histological evaluation was performed in a blinded manner by a board-certified veterinary anatomic pathologist (L.D.B.B.). Inflammation, crypt lesions, ulceration, and hyperplasia, as well as lesion distribution, in each colon section were graded. A combination of the grading and scoring schemes established previously was used [25,26] (Supporting Information—methods).
2.5 Biochemical analyses
Distal colonic tissue was lysed (n = 8–10 mice per group) as previously described [5] and colonic myeloperoxidase (MPO) protein was measured by ELISA according to the manufacturer’s instructions (Hyccult biotech, Plymouth Meeting, PA). Serum amyloid A (SAA) levels were quantified by ELISA (n = 6–8 mice per group; Tridelta Development Ltd., Ireland). To assess possible liver toxicity, serum (n = 3–6 mice per group) was analyzed for aspartate and alanine aminotransferase enzyme activities using commercial assays (Pointe Scientific Inc., Canton MI). A panel of 15 cytokines was analyzed in plasma using high-throughput Luminex Mulitplex Cytokine Kits (Affymetrix, Santa Clara, CA; Supporting Information—methods).
2.6 Immunohistochemistry
For immunohistochemistry analysis, 5-μm-thick, paraffin-embedded sections of the distal colon from three mice per group were randomly selected. T cells and macrophages were identified using CD3 and F4/80, respectively, as markers. Avidin-biotin complex was used for detection (Vector), followed by 3,3′-diaminobenzidine chromogen, and hematoxylin as counterstain. To quantify CD3+ and F4/80+ cells, three randomly selected 200X fields for each sample were scanned to obtain pixel count using Aperio Smage Scope (v11.2). Data (expressed as percentage) represent the average number of weak positive, positive, and strong positive pixels per total pixels. To evaluate cell proliferation, tissue sections were immunostained for Ki67 using the streptavidin/horseradish peroxidase method, 3,3′-diaminobenzidine as chromogen followed by hematoxylin counterstain. For each colonic section, the percentage of Ki67-immunopositive cells in the crypts in three randomly selected 200X fields was calculated using Image-Pro Plus software (Supporting Information—methods).
2.7 Bacterial analyses
To study the effect of dietary α-MG on the gut micriobiota of healthy mice (i.e. non-DSS treated), 10-week-old female C57BL/6 mice (Jackson Laboratories) were housed under sterile conditions with controlled temperature at 23°C and a 12-h light/dark cycle. Mice were acclimatized for 1 week before entering the study with free access to water and AIN93G diet. Mice were randomly assigned to AIN93G diet (control group, n = 5 mice) or the AIN93G diet with α-MG (α-MG group, n = 5 mice) for 4 weeks ad libitum. At necropsy, cecum and distal colon were excised under aseptic conditions, gently rinsed in sterile cold PBS, and collected in sterile tubes. Tissue was frozen in liquid nitrogen and stored at −80°C until analysis. Bacterial analyses were performed using bacterial tag-encoded FLX amplicon pyrosequencing at the Research and Testing Laboratory (Lubbock, TX). Fasta and qual files obtained from pyrosequencing were uploaded into Quantitative Insights Into Microbial Ecology (QIIME) software [27]. Clustering of sequencing reads into operational taxonomic units was achieved at 97% identity. Operational taxonomic unit picking was performed in the QIIME pipeline using the Uclust algorithm [28]. Taxonomic assignment was achieved using the Ribosomal Database Project classifier [29], employing the GreenGenes database [30]. Sequences were aligned using python nearest alignment space termination [31]. QIIME was used to calculate α-diversity (Shannon index). Unifrac analysis [32] followed by principal coordinate analysis (based on unweighted Unifrac distance) was used to characterize the diversity in the bacterial populations (Supporting Information—methods).
2.8 Statistical analysis
All data are expressed as mean ± SD. For parametric data, statistical differences were determined by one-way analysis of variances followed by Tukey’s test. Nonparametric data were analyzed by Kruskal–Wallis test followed by selected mean comparisons with Bonferroni correction. Differences were considered statistically significant at p < 0.05. Analyses were performed using SPSS v. 20 (IBM, Armonk, NY). For pyrosequencing data, statistical differences in unweighted Unifrac distance were investigated by analysis of similarities, provided by R’s vegan package implemented into QIIME.
3 Results
3.1 Dietary α-MG exacerbates disease activity in DSS-induced colitis
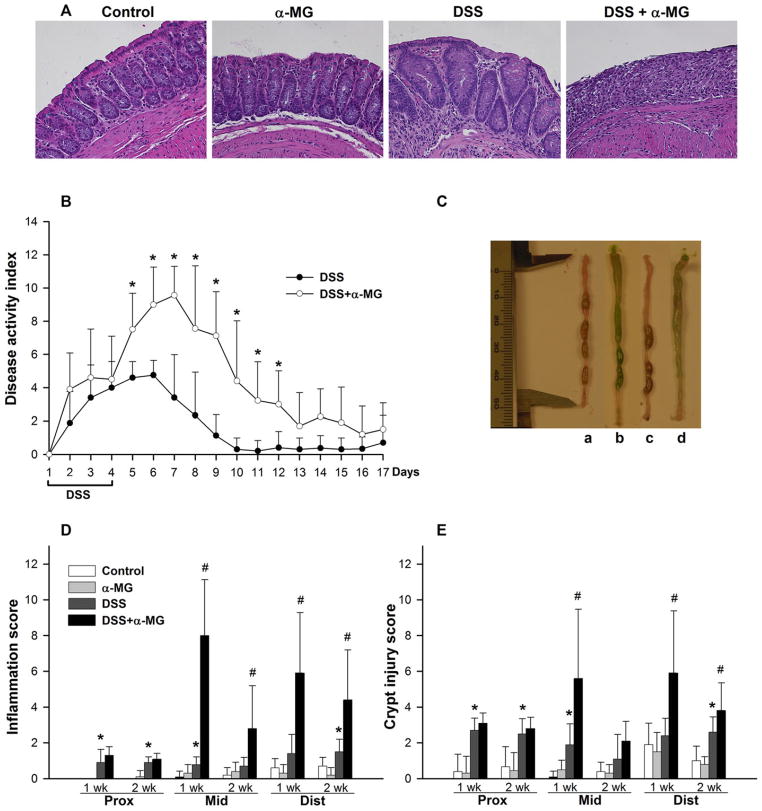
The DAI was calculated by adding the scores from the percentage of BW lost from baseline, stool consistency, and rectal bleeding [24]. As expected, DSS treatment increased DAI. However, DAI was significantly greater in mice that received DSS and were fed the AIN93G diet with α-MG (DSS + α-MG group) than in mice that received DSS while being fed a standard AIN93G diet (DSS group; p < 0.01). While DAI in the DSS group steadily declined to baseline 5 days after cessation of DSS treatment, increased severity of symptoms persisted in the DSS + α-MG group and remained significantly greater 8 days post-DSS administration (p < 0.01; Fig. 3B). Significant loss of BW (17% from baseline) only occurred in the DSS + α-MG group following DSS treatment but was recovered 1 week post-DSS (data not shown). Diarrhea and rectal bleeding were also more severe in the DSS + α-MG group.
Figure 3.
(A) Hematoxylin and eosin staining in distal colon from mice following 1-week recovery after administering dextran sulfate sodium (DSS). Magnification: 20×. (B) Dietary α-mangostin (α-MG) exacerbates disease activity index (*p < 0.05). (C) Increased fluid content in colonic lumen of mice fed AIN93G diet with α-MG; experimental groups: a, control; b, α-MG; c, DSS; d, DSS + α-MG. The green pigmentation in the colonic lumen of mice fed diet with α-MG is the dye added to the diet (see Methods). (D) Inflammation and (E) crypt injury scores in the mid and distal colon are greater in the DSS + α-MG group compared to DSS group after 1 and 2 weeks of recovery (*p < 0.05 against control; #p < 0.05 against DSS group). The data points represent the mean (±SD) of values from nine to ten mice per group.
Food intake was temporarily decreased in the DSS + α-MG group after cessation of DSS treatment but reverted to that of all other groups 5 days later. No alterations in liver aspartate aminotransferase and alanine aminotransferase enzyme activities or histological evidence of hepatic toxicity were detected in any experimental group (data not shown).
3.2 DSS-induced colonic inflammation and injury are aggravated by α-MG
Microscopic assessment of hematoxylin and eosin stained colon (Fig. 3A) confirmed induction of colitis by DSS, as mice in the DSS group had significantly greater inflammation (Fig. 3D) and crypt injury (Fig. 3E) scores in the mid colon 1 week after DSS administration compared to mice receiving tap water (p < 0.05). Although there was no significant difference in the scores for inflammation and crypt injury in the distal colon of mice in the DSS group after recovering for 1 week, these scores were significantly greater compared to the control group after 2 weeks of recovery (Fig. 3D and E, p < 0.05). Mice in the DSS + α-MG group had more severe inflammation in the mid and distal colon compared to DSS group 1 and 2 weeks after cessation of DSS treatment (Fig. 3D, p < 0.05). Crypt injury scores were also significantly higher in the mid and distal colon of mice in the DSS + α-MG group compared to DSS group after 1 week of recovery from DSS (Fig. 3E, p < 0.05). Ulcers were only present in the mid and distal colon of animals receiving DSS + α-MG (Table 1).
Table 1.
Effect of dietary α-mangostin (α-MG) on colonic ulceration and epithelial hyperplasia
| 1-week recovery
|
2-week recovery
|
|||
|---|---|---|---|---|
| Mid | Distal | Mid | Distal | |
| Ulceration score | ||||
| DSS | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| DSS + α-MG | 1.4 ± 1.0 (100%)a)b) | 1.4 ± 1.0 (90%)b) | 0.1 ± 0.3 (10%) | 0.5 ± 0.7 (40%) |
| Hyperplasia score | ||||
| DSS | 0.1 ± 0.3 (10%) | 0.1 ± 0.3 (10%) | 0 ± 0 | 0 ± 0 |
| DSS + α-MG | 2.3 ± 0.9 (100%)b) | 1.4 ± 1.1 (90%)b) | 0.9 ± 0.7 (70%)b) | 0.8 ± 0.6 (70%)b) |
Number in parenthesis indicates percentage of mice displaying ulceration or hyperplasia.
p < 0.05 against same colon section and recovery time in DSS group.
The data points represent the mean (± SD) of values from nine to ten mice per group.
Although administration of 3% DSS to C57BL/6 mice for 5 days has been reported to shorten the colon [5], colonic length of mice treated with 2% DSS was not altered in this study (data not shown). However, the ratio of colon weight (after removal of luminal contents) to colon length was significantly greater in non-DSS-treated animals receiving α-MG in the diet compared to mice fed standard diet after 1 and 2 weeks of recovery (p < 0.05). This ratio was also greater in the DSS + α-MG group than in the DSS group at 1 and 2 weeks post-DSS treatment (p < 0.05; Table 2). Increased fluid volume in the colonic lumen of mice fed diet with α-MG was evident especially in the proximal and mid colon, independently of DSS treatment (Fig. 3C).
Table 2.
Liver and spleen weight and ratio of colon weight to colon length
| 1-week recovery
|
2-week recovery
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | α-MG | DSS | DSS + α-MG | Control | α-MG | DSS | DSS + α-MG | |
| Liver (% BW)a) | 4.0 ± 0.3 | 3.9 ± 0.5 | 4.0 ± 0.4 | 6.0 ± 0.5** | 4.5 ± 0.4 | 4.3 ± 0.5 | 4.1 ± 0.3 | 4.8 ± 0.3** |
| Spleen (% BW) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.7 ± 0.1** | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.4 ± 0.2** |
| Colon weight/lengthb) | 19.5 ± 2.8 | 28.5 ± 2.9* | 23.8 ± 3.1 | 40.8 ± 7.1** | 18.3 ± 2.0 | 30.6 ± 1.6* | 19.7 ± 1.7 | 35.0 ± 1.7** |
BW, body weight.
Colon weigh to length ratio in milligram per centimeter.
p < 0.05 against control group;
p < 0.05 against DSS group.
The data points represent the mean (± SD) of values from ten mice per group.
3.3 Dietary α-MG stimulates colonic epithelial cell proliferation
Crypt distortion, increased epithelial cell proliferation, and dysplasia have been described in the DSS colitis model [25]. Mild hyperplasia was observed in both the mid and distal colon of mice in the DSS group 1 week after cessation of DSS treatment (Fig. 3A). Hyperplasia was reversible, as it was not observed in this group at 2 weeks after DSS treatment. In contrast, moderate hyperplasia in the mid and distal colon of mice in the DSS + α-MG group was present at 1 week after DSS treatment and mild hyperplasia remained evident following 2 weeks of recovery (Table 1).
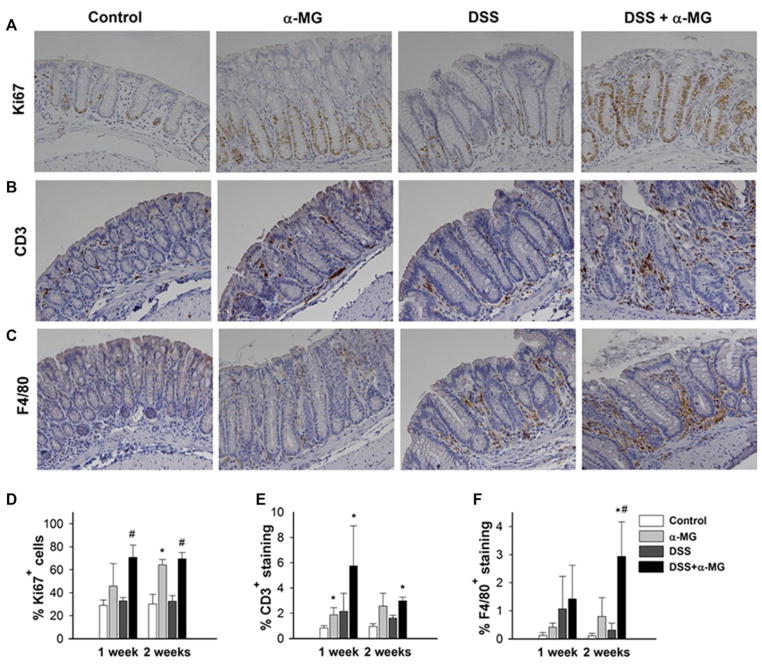
Colonic epithelial cell proliferation, as determined by the percentage of Ki67+ cells in the crypts (Fig. 4A), was significantly greater in the DSS + α-MG group compared to the DSS group after 1 and 2 weeks of recovery (p < 0.05). Interestingly, the percentage of Ki67 expressing cells was greater in the colonic epithelium of non-DSS-treated mice fed diet with α-MG as compared to control mice fed standard diet at 1 (p > 0.05) and 2 weeks (p < 0.05; Fig. 4D).
Figure 4.
Representative images of (A) Ki67, (B) CD3, and (C) F4/80 immunostaining in distal colon of mice 1 week after cessation of dextran sulfate sodium (DSS) treatment. Magnification: 20×. (D) Ki67+ immunostaining of colonic epithelial cells was increased in animals fed diet with α-mangostin (α-MG). (E) CD3+-stained tissue for T cells in the colonic lamina propria of mice fed diet with α-MG was greater than in the control group. (F) Significant macrophage infiltration, as determined by immunostaining for F4/80, in the DSS + α-MG 2 weeks after recovery. *p < 0.05 against control group; #p < 0.05 against DSS group). The data points represent the mean (±SD) of values from three mice per group.
3.4 Macrophage and T-cell infiltration in the colon are exacerbated by dietary α-MG
Increased colonic infiltration of T cells and macrophages in response to DSS ingestion has been reported [33, 34]. Thus, we examined the expression of CD3+ and F4/80+ in the distal colonic lamina propria as markers of T-cell and macrophage infiltration, respectively (Fig. 4B and C). The area of positively stained tissue for CD3+ cells was greater in mice fed the diet with α-MG compared to control mice at 1 week, independently of DSS treatment. At 2 weeks, only the DSS + α-MG group had significantly greater infiltration of CD3+ cells compared to control group (p < 0.05; Fig. 4E). Infiltration of F4/80+ macrophages, as determined by the area of F4/80 staining in the tissue section, also was significantly greater in the DSS + α-MG group compared to DSS group after 2 weeks of recovery (p < 0.05; Fig. 4F).
3.5 Dietary α-MG exacerbates DSS-induced colonic and systemic inflammation
We examined MPO protein expression in the distal colon as a surrogate indicator of neutrophil infiltration and activity [35]. Colonic MPO expression was significantly greater in the DSS + α-MG group compared to the DSS group after both 1 and 2 weeks post-DSS treatment (p < 0.05). MPO was also significantly greater in non-DSS-treated mice fed diet with α-MG compared to control mice fed standard diet at 1 and 2 weeks (p < 0.05; Table 3).
Table 3.
Colonic myeloperoxidase (MPO) protein and serum amyloid A (SAA) levels at 1 and 2 weeks post-DSS administration
| 1-week recovery
|
2-week recovery
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | α-MG | DSS | DSS + α-MG | Control | α-MG | DSS | DSS + α-MG | |
| Colonic MPOa) | 6.1 ± 2.7 | 25.2 ± 12.5* | 13.5 ± 4.1* | 81.7 ± 32.5** | 3.8 ± 1.3 | 22.0 ± 15.6* | 9.3 ± 5.2* | 25.3 ± 15.8** |
| SAAb) | 35.5 ± 3.8 | 30.5 ± 4.4 | 119 ± 26.4* | 1547 ± 968.3** | 37.0 ± 3.8 | 30.6 ± 3.4* | 120 ± 26.0* | 371 ± 257.7** |
Myeloperoxidase in nanogram per milligram protein. The data points represent the mean (±SD) of values from n = 8–10 mice per group.
SAA in microgram per milliliter. The data points represent the mean (±SD) of values from six to eight mice per group.
p < 0.05 against control group;
p < 0.05 against DSS group.
Splenomegaly and increased liver weight were observed in mice in the DSS + α-MG group (Table 2), thus we determined the effects of α-MG on systemic markers of inflammation by analyzing a panel of soluble inflammatory factors in serum. SAA has been shown to be well correlated with disease activity in the DSS-induced colitis model [5]. Consistent with these data, SAA was significantly higher in the DSS group compared to the control group (p < 0.05). SAA in the DSS + α-MG group was significantly greater than in the DSS group after 1 and 2 weeks of recovery (p < 0.05; Table 3). The acute inflammatory response in the DSS-induced colitis model also has been characterized by increased serum levels of TNF-α, IL-6, and IL-17, and elevated levels of IL-6, IFN-γ, IL-4, and IL-10 have been reported in chronic colitis [36]. Multiplex analysis of 15 cytokines involved in inflammatory and immune responses revealed that IL-6 and granulocyte colony-stimulating factor were significantly increased in mice receiving DSS + α-MG treatment compared to other groups after 1 week of recovery. Following 2 weeks of recovery, granulocyte colony-stimulating factor and IL-12p40 levels were significantly elevated in the DSS + α-MG group (p < 0.05; Supporting Information Table 1).
3.6 Dietary α-MG induces changes in tissue-associated colonic and cecal microbiota
Because alterations in the gut microbiota have been implicated in the pathogenesis of UC [4] and α-MG has been reported to exert antimicrobial activities [10], we examined if this xanthone was capable of inducing changes in the abundance of tissue-associated bacterial communities in the colon and cecum of C57BL/6 mice in the absence of DSS-induced colonic inflammation. Consistent with our initial findings (Fig. 3C), there was an increase in fluid content in the colonic lumen and stool of non-DSS-treated mice fed diet with α-MG (α-MG group) compared to mice fed standard diet (control group), while BW and food intake did not differ between the groups (data not shown).
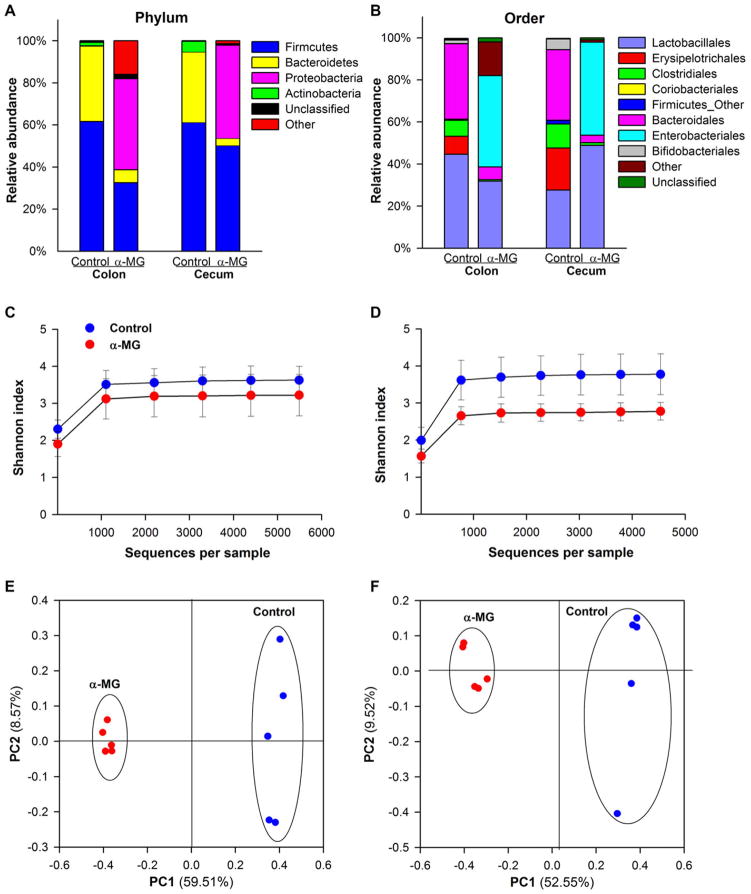
There was a change in the microbiota profile in the colon and cecum of mice fed diet with α-MG but no DSS. The Firmicutes and Bacteroidetes phyla comprised approximately 95–97% of the identified sequences in the colonic and cecal tissue of mice in the control group. Changes at the phylum taxonomic level were identified in mice in the α-MG group compared to animals in the control group (Fig. 5A). The relative abundances of Firmicutes and Bacteroidetes in the colon were significantly reduced in mice fed diet with α-MG (p < 0.01), as was the relative abundance of Bacteroidetes in cecum (p < 0.01). Conversely, there was a significant increase in the relative abundance of Proteobacteria in the colon and cecum of mice fed diet with α-MG (p < 0.01).
Figure 5.
Dietary α-mangostin (α-MG) changes the relative abundance of colonic and cecal bacterial communities at the (A) phylum and (B) order levels. Shannon index (α-diversity) in (C) colon and (D) cecum. Principal coordinate analysis (PCoA) in (E) colon and (F) cecum. Samples in the α-MG group (red) cluster together and away from those in control group (blue). Data are from five mice per group.
To investigate which bacterial populations accounted for the changes induced by dietary α-MG at the phylum level, the relative abundances of the top ten orders were analyzed (Fig. 5B). α-MG elicited a significant reduction in the abundance of Lactobacillales and Bacteroidales in the colon (p < 0.05). Only Bacteroidales abundance was significantly reduced in the cecum (p < 0.01). Within Lactobacillales, there was a significant increase in the genus Enterococcus and a decrease in the genus Lactobacillus in both colon and cecum of mice fed α-MG (p < 0.01, data not shown). Furthermore, significantly lower abundances of Erysipelotrichales, Clostridiales, and Bifidobacteriales were also found in colon and cecum of these mice (p < 0.05). In contrast, there was a significant increase in the relative abundance of an unclassified genus of bacteria in the family Enterobacteriaceae (order Enterobacteriales) in the colon and cecum of mice in the group fed diet with α-MG (p < 0.01).
The Shannon index was calculated to describe within-sample diversity. Although no significant changes were identified in the colon (Fig. 5C), a significant reduction in bacterial diversity was observed in the cecum of mice in the α-MG group compared to mice fed standard diet (p < 0.05; Fig. 5D). Principal coordinate analysis provided additional evidence of changes in colonic and cecal bacterial populations induced by dietary α-MG. Data corresponding to mice in the α-MG group (red) clustered together and away from those corresponding to control group (blue; Fig. 5E and F). There were significant differences in the α-diversity of the colonic and cecal microbiota between α-MG and control groups (p < 0.01).
4 Discussion
CAM has become increasingly popular in the last few years with nearly 40% of American adults reporting its use [37]. Given the marginal success and adverse side effects of conventional therapies, CAM is used by up to 50% of UC patients [6, 7]. Mangosteen-containing supplements are promoted as beneficial for gut health and the immune system. α-MG, the most abundant xanthone in mangosteen, has been shown to exert anti-inflammatory activities in vitro and in vivo [38] but its effect on experimental colitis has not been previously examined. In addition, antimicrobial activities of α-MG against pathogenic bacteria, fungi, and virus have been reported [10]. The aim of this study was to characterize the effects of dietary α-MG in the DSS-induced mouse model of colitis. The dose of α-MG was selected based on recent studies that showed decreased tumor growth in xenograft models of colon and prostate cancer [18,21]. Surprisingly, dietary α-MG exacerbated, rather than attenuated, DSS-induced inflammation and injury in the colon of C57BL/6 mice. Increased disease severity in animals receiving DSS and fed diet with α-MG included loss of BW, greater inflammatory and crypt injury scores, immune cell infiltration, ulceration, an increased degree of hyperplasia and epithelial cell proliferation in the colon, and greater systemic and colonic inflammation. Interestingly, increased colonic MPO protein levels, epithelial cell proliferation, and immune cell infiltration were also observed in non-DSS-treated mice fed diet with α-MG. Dietary α-MG also elicited a shift in the mucosa-associated microbiota profile in the colon and cecum of non-DSS-treated mice. This shift was similar to that seen in human UC [39]. We speculate that this alteration may be associated with the exacerbation of DSS-induced pathology.
We first observed that dietary α-MG exacerbated the pathology of DSS-induced colitis in two pilot studies. Because previous studies with transformed cells and rodents suggested that the compound has anti-inflammatory activity [38], the possibilities that the xanthone may have been degraded to toxic products or that there may have been other adverse changes in dietary quality were considered. Analyses showed that α-MG was stable in the diet during storage for at least 6 months. Also, fresh diet was prepared for use in the more comprehensive present study. The dose and time of exposure to DSS were adjusted to induce colitis without significant loss of BW in control mice. Despite such changes, the adverse effect of dietary α-MG on colonic pathology in mice-administered DSS was replicated. Contamination of diets with microbial pathogens also was unlikely because they were irradiated and handled aseptically until placed in cages. These findings led us to consider several reports that α-MG also has proinflammatory activity. This xanthone stimulated TNF-α output by normal human monocyte-derived macrophages [12] and increased serum levels of IL-1α, IL-1β, and complement components C3 and C4 in human subjects ingesting a mangosteen supplement [16]. Also, several other phytochemicals and plant extracts generally assumed to be health promoting have been reported to exacerbate DSS-induced colitis in mice. These included dietary luteolin, tomato lycopene, and green tea polyphenol extracts [40–42].
Mangosteen has been used as an antidiarrheal agent in traditional medicine for centuries [10]. However, mice fed diet with α-MG developed loose stools shortly after initiating feeding and this response was independent of the administration of DSS. Preparation of the antidiarrheal liquid involves boiling roasted mangosteen pericarp in water [43]. The differential effect of the hot water extract and dietary α-MG is likely due to the relative absence of lipophilic xanthones in the former. The effect of α-MG on consistency of stool in mice does align with the gentle laxative activity advertised online as a purported health benefit of ingested mangosteen products. It is interesting that mangosteen xanthones are structurally similar to anthraquinones, which are widely used as laxatives and have been shown to induce apoptosis of colonic epithelial cells [44]. α-MG also has antimicrobial activity. For example, this xanthone inhibited the growth of pathogenic bacteria, such as B. subtilis (IC50 3.9 μM), S. aureus (IC50 7.8 μM), with no effects against other pathogens, such as E. coli and C. albicans (IC50 >200 μM) [10]. α-MG also has been shown to inhibit bacterial species such as methicillin-resistant S. aureus, vancomycin-resistant Enterococci, Mycobacterium tuberculosis, and Helicobacter pylori at concentrations in the range of 1.6–12.5 μg/mL [45]. Although these studies have been done using a single species, the results suggest that α-MG at the levels found in the gut lumen after oral ingestion [18] may affect the balance between commensal and pathogenic bacterial communities. Perhaps more importantly, the relatively nonselective antimicrobial activities of α-MG could potentially impact commensal bacteria [46]. Altered consistency of stool and exacerbation of DSS-induced colitis by α-MG, as well as reported antimicrobial activities of α-MG [10], led us to investigate the possible effect of α-MG on the gut microbiota of healthy mice (i.e. non-DSS treated). Consistent with previous reports in both humans and mice [47, 48], 95–97% of tissue-associated colonic and cecal bacteria of mice fed the control diet belonged to the Firmicutes and Bacteroidetes phyla. In contrast, mice fed the diet with α-MG had reductions in the relative abundance of Firmicutes and Bacteroidetes and increased Proteobacteria, a shift similar to that seen in UC and Crohn’s disease [39, 49, 50].
The change in the gut microbiota may have enhanced colonic inflammation in response to the chemical insult. Bacteria in the Bacteroidetes and Firmicutes phyla ferment dietary fiber, generating short chain fatty acids (SCFA) that exert anti-inflammatory activity [51,52]. It is possible that the lower abundance of Bacteroidetes and Firmicutes associated with ingestion of α-MG resulted in reduced levels of colonic SCFA, thus exacerbating inflammation. In addition, some members of the microbiota, such as bacteria of the genus Lactobacillus, have immunoregulatory effects [53]. Ingestion of α-MG led to a decrease in the relative abundance of Lactobacillus in the colon and cecum, and a concomitant increase in the Enterobacteriaceae, which have known proinflammatory effects. This profile is often evident in various mouse models of colitis and in IBD patients [48,54,55]. Moreover, the increase in Enterococcus in the colon and cecum in the present study is similar to that seen during DSS-induced colitis [56]. The reduced microbial diversity in α-MG-fed animals is similar to reports of patients with active UC [39, 50]. The possibility that the overgrowth of pathogenic bacteria associated with administration of α-MG, coupled with the DSS-induced injury in the gut epithelial barrier, may contribute to the exacerbating effects of the xanthone merits investigation. However, because host-mediated inflammation is known to alter colonic microbial populations [49], secondary changes in the gut microbiota resulting from the host’s immune response to the dietary xanthone also need to be considered.
Despite similarities at the phylum level in the gut microbiota of mice and humans, it is known that there are differences at other taxonomic levels. Nevertheless, our finding that dietary α-MG induced changes in the microbiota at the phylum level is provocative. Because host genetics and environmental factors are known to affect the composition of the gut microbiota [57] and susceptibility to DSS-induced inflammation [58], we have examined the effect of dietary α-MG on the gut microbiome of healthy Balb/c, C3H, and athymic FoxN1nu mice. Interestingly, we have found that induction of dysbiosis by dietary α-MG is not strain specific, but rather a more generalized effect in mice (manuscript in preparation).
Because we only evaluated colonic pathology at two times after DSS administration, and the cecal and colonic microbiota after feeding diet with α-MG to healthy mice for 4 weeks, the effect of α-MG on earlier changes in the colonic epithelium and gut microbiota, as well as its mechanisms of action, will be a focus of future investigation. Whether a similar shift in the gut microbiota in response to dietary α-MG also occurs when DSS is administered to mice remains elusive, as does the effect of the host inflammatory response to the xanthone on the gut microbiota. Similar to the previously described hormetic response induced by flavonoids [59], dietary xanthones may act as hormetic agents by exerting beneficial effects at low concentrations, but having detrimental activities at higher levels. The effect of lower doses of α-MG and feeding of ground pericarp rather than the purified compound on experimental colitis also merit consideration.
In summary, our results show that dietary α-MG exacerbates DSS-induced colitis and modifies the gut microbiota in non-DSS-treated mice by shifting it to a profile resembling that found in UC. We suggest that chronic consumption of mangosteen supplements rich in pericarp, and therefore xanthones, should be considered with caution by those with inflammatory bowel disorders and perhaps even by healthy individuals.
Supplementary Material
Acknowledgments
The authors thank Alissa Wampler and Hannah Bills for their assistance with daily care and necropsy of mice and with laboratory analyses. This study was supported by grants from the Molecular Carcinogenesis and Chemoprevention Program and the Food Innovation Center at The Ohio State University (G.B.L., S.K.C., M.L.F.), and by the Conacyt doctoral scholarship (F.G.-O.).
Abbreviations
- α-MG
α-mangostin
- BW
body weight
- CAM
complementary and alternative medicine
- DAI
disease activity index
- DSS
dextran sulfate sodium
- IBD
inflammatory bowel disease
- MPO
myeloperoxidase
- QIIME
Quantitative Insights Into Microbial Ecology
- SAA
serum amyloid A
- SCFA
short chain fatty acids
- TNF-α
tumor necrosis factor-α
- UC
ulcerative colitis
Footnotes
The authors have declared no conflict of interest.
References
- 1.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007;28:1450–1459. doi: 10.1111/j.1745-7254.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 3.Ordás I, Eckmann L, Talamini M, Baumgart DC, et al. Ulcerative colitis. Lancet. 2012;380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 4.Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53:1–4. doi: 10.1136/gut.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melgar S, Karlsson A, Michaëlsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Gastrointest Liver Physiol. 2005;288:G1328–G1338. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- 6.Lanzoni G, Roda G, Belluzzi A, Roda E, et al. Inflammatory bowel disease: moving toward a stem cell-based therapy. World J Gastroenterol. 2008;14:4616–4626. doi: 10.3748/wjg.14.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahimi R, Mozaffari S, Abdollahi M. On the use of herbal medicines in management of inflammatory bowel diseases: a systematic review of animal and human studies. Dig Dis Sci. 2009;54:471–480. doi: 10.1007/s10620-008-0368-x. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Schauss AG. Mitigation of inflammation with foods. J Agric Food Chem. 2012;60:6703–6717. doi: 10.1021/jf3007008. [DOI] [PubMed] [Google Scholar]
- 9.Pan MH, Lai CS, Ho CT. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010;1:15–31. doi: 10.1039/c0fo00103a. [DOI] [PubMed] [Google Scholar]
- 10.Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem Toxicol. 2008;46:3227–3239. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Walker EB. HPLC analysis of selected xanthones in mangosteen fruit. J Sep Sci. 2007;30:1229–1234. doi: 10.1002/jssc.200700024. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez-Orozco F, Chitchumroonchokchai C, Lesinski GB, Suksamrarn S, et al. α-Mangostin: anti-Inflammatory activity and metabolism by human cells. J Agric Food Chem. 2013;61:3891–3900. doi: 10.1021/jf4004434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bumrungpert A, Kalpravidh RW, Chuang CC, Overman A, et al. Xanthones from mangosteen inhibit inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. J Nutr. 2010;140:842–847. doi: 10.3945/jn.109.120022. [DOI] [PubMed] [Google Scholar]
- 14.Chen LG, Yang LL, Wang CC. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol. 2008;46:688–693. doi: 10.1016/j.fct.2007.09.096. [DOI] [PubMed] [Google Scholar]
- 15.Jang HY, Kwon OK, Oh SR, Lee HK, et al. Mangosteen xanthones mitigate ovalbumin-induced airway inflammation in a mouse model of asthma. Food Chem Toxicol. 2012;50:4042–4050. doi: 10.1016/j.fct.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 16.Tang YP, Li PG, Kondo M, Ji HP, et al. Effect of a mangosteen dietary supplement on human immune function: a randomized, double-blind, placebo-controlled trial. J Med Food. 2009;12:755–763. doi: 10.1089/jmf.2008.0204. [DOI] [PubMed] [Google Scholar]
- 17.Sloan EW. Getting ahead of the curve: phytochemicals. Nutraceutical World. 2010;13:16–17. [Google Scholar]
- 18.Chitchumroonchokchai C, Thomas-Ahner JM, Li J, Riedl KM, et al. Anti-tumorigenicity of dietary α-mangostin in an HT-29 colon cell xenograft model and the tissue distribution of xanthones and their phase II metabolites. Mol Nutr Food Res. 2013;57:203–211. doi: 10.1002/mnfr.201200539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaivisuthangkura A, Malaikaew Y, Chaovanalikit A, Jaratrungtawee A. Prenylated xanthone composition of Garcinia mangostana (mangosteen) fruit hull. Chromatographia. 2009;69:315–318. [Google Scholar]
- 20.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JJ, Petiwala S, Syed D, Rasmussen J, et al. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis. 2012;33:413–419. doi: 10.1093/carcin/bgr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chitchumroonchokchai C, Riedl KM, Suksumrarn S, Clinton SK, et al. Xanthones in mangosteen juice are absorbed and partially conjugated by healthy adults. J Nutr. 2012;142:675–680. doi: 10.3945/jn.111.156992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding S, Walton K, Blue R, McNaughton K, et al. Mucosal healing and fibrosis after acute or chronic inflammation in wild type FVB-N mice and C57BL6 procollagen α1(I)-promoter-GFP reporter mice. PLoS One. 2012;7:e42568. doi: 10.1371/journal.pone.0042568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murthy SN, Cooper HS, Shim H, Shah RS, et al. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 25.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 26.Mähler M, Bristol I, Leiter E, Workman A, et al. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am J Physiol. 1998;274:G544–G551. doi: 10.1152/ajpgi.1998.274.3.G544. [DOI] [PubMed] [Google Scholar]
- 27.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald D, Price M, Goodrich J, Nawrocki E, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, et al. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12:1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 34.Sund M, Xu LL, Rahman A, Qian BF, et al. Reduced susceptibility to dextran sulphate sodium-induced colitis in the interleukin-2 heterozygous (IL-2) mouse. Immunology. 2005;114:554–564. doi: 10.1111/j.1365-2567.2005.02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren T, Grants I, Alhaj M, McKiernan M, et al. Impact of disrupting adenosine A3 receptors (A3−/−AR) on colonic motility or progression of colitis in the mouse. Inflamm Bowel Dis. 2011;17:1698–1713. doi: 10.1002/ibd.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alex P, Zachos N, Nguyen T, Gonzales L, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes PM, Bloom B, Nahin R. National health statistics reports, no 12. National Center for Health Statistics; Hyattsville, MD, USA: 2008. Complementary and alternative medicine use among adults and children: United States, 2007. [PubMed] [Google Scholar]
- 38.Gutierrez-Orozco F, Failla ML. Biological activities and bioavailability of mangosteen xanthones: a critical review of the current evidence. Nutrients. 2013;5:3163–3183. doi: 10.3390/nu5083163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frank DN, St Amand AL, Feldman RA, Boedeker EC, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joo YE, Karrasch T, Mühlbauer M, Allard B, et al. Tomato lycopene extract prevents lipopolysaccharide-induced NF-κB signaling but worsens dextran sulfate sodium-induced colitis in NF-κBEGFP mice. PLoS One. 2009;4:e4562. doi: 10.1371/journal.pone.0004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karrasch T, Kim JS, Jang BI, Jobin C. The flavonoid luteolin worsens chemical-induced colitis in NF-κBEGFP transgenic mice through blockade of NF-κB-dependent protective molecules. PLoS One. 2007;2:e596. doi: 10.1371/journal.pone.0000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim M, Murakami A, Miyamoto S, Tanaka T, et al. The modifying effects of green tea polyphenols on acute colitis and inflammation-associated colon carcinogenesis in male ICR mice. Biofactors. 2010;36:43–51. doi: 10.1002/biof.69. [DOI] [PubMed] [Google Scholar]
- 43.Yapwattanaphun C, Subhadrabandhu S, Sugiura A, Yonemori K, et al. Utilization of some Garcinia species in Thailand. Acta Hort. 2002;575:563–570. [Google Scholar]
- 44.Walker N, Bennett T, Axelsen RA. Melanosis coli A consequence of antraquinone-induced apoptosis of colonic epithelial cells. Am J Path. 1988;131:465–476. [PMC free article] [PubMed] [Google Scholar]
- 45.Chin Y, Kinghorn AD. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini-Rev Org Chem. 2008;5:355–364. doi: 10.2174/157019308786242223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sindermusk J, Deekijsermphong S. The antibacterial activities of crude extract from the fruit hull of Garcinia mangostana on enteric pathogens and intestinal commensal flora. Bull Depart Med Services. 1989;14:421–426. [Google Scholar]
- 47.Eckburg PB, Bik E, Bernstein C, Purdom E, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lupp C, Robertson M, Wickham M, Sekirov I, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Ott SJ, Musfeldt M, Wenderoth D, Hampe J, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maslowski KM, Vieira A, Ng A, Kranich J, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72. doi: 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- 53.Christensen HR, Frøkiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 54.Fite A, Macfarlane S, Furrie E, Bahrami B, et al. Longitudinal analyses of gut mucosal microbiotas in ulcerative colitis in relation to patient age and disease severity and duration. J Clin Microbiol. 2013;51:849–856. doi: 10.1128/JCM.02574-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pathmakanthan S, Thornley JP, Hawkey CJ. Mucosally associated bacterial flora of the human colon: quantitative and species specific differences between normal and inflamed colonic biopsies. Microb Ecol Health Dis. 1999;11:169–174. [Google Scholar]
- 56.Heimesaat MM, Fischer A, Siegmund B, Kupz A, et al. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS One. 2007;2:e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell J, Foster C, Vishnivetskaya T, Campbell A, et al. Host genetic and environmental effects on mouse intestinal microbiota. ISME J. 2012;6:2033–2044. doi: 10.1038/ismej.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitajima S, Morimoto M, Sagara E, Shimizu C, et al. Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp Anim. 2001;50:387–395. doi: 10.1538/expanim.50.387. [DOI] [PubMed] [Google Scholar]
- 59.Siow R, Mann GE. Dietary isoflavones and vascular protection: activation of cellular antioxidant defenses by SERMs or hormesis? Mol Aspects Med. 2010;31:468–477. doi: 10.1016/j.mam.2010.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.