Abstract
Purpose
Treatment decisions for patients with T1a,bN0M0 breast cancer are challenging. We studied the time trends in use of adjuvant chemotherapy and survival outcomes among these patients.
Patients and Methods
This was a prospective cohort study within the National Comprehensive Cancer Network Database that included 4,113 women with T1a,bN0M0 breast cancer treated between 2000 and 2009. Tumors were grouped by size (T1a, T1b), biologic subtype defined by hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status, and receipt of chemotherapy with or without trastuzumab.
Results
Median follow-up time was 5.5 years. Eight percent of patients with HR-positive/HER2-negative tumors were treated with chemotherapy. Fifty-two percent of those with HER2-positive or HR-negative/HER2-negative breast cancers received chemotherapy, with an increase over the last decade. Survival outcomes diverged by subtype and size, but the 5-year distant relapse-free survival (DRFS) did not exceed 10% in any subgroup. The 5-year DRFS for patients with T1a tumors untreated with chemotherapy ranged from 93% to 98% (n = 49 to 972), and for patients with T1b tumors, it ranged from 90% to 96% (n = 17 to 2,005). Patients with HR-positive/HER2-negative disease had the best DRFS estimates, and patients with HR-negative/HER2-negative tumors had the lowest. In this observational, nonrandomized cohort study, the 5-year DRFS for treated patients with T1a tumors was 100% for all subgroups (n = 12 to 33), and for patients with T1b tumors, it ranged from 94% to 96% (n = 88 to 241).
Conclusion
Women with T1a,b tumors have an excellent prognosis without chemotherapy. Size and tumor subtype may identify patients in whom the rate of recurrence justifies consideration of chemotherapy. These patients represent an optimal group for evaluating less toxic adjuvant regimens to maintain efficacy while minimizing short- and long-term risks.
INTRODUCTION
Over the past two decades, the incidence of stage I breast cancers has increased dramatically, and these tumors now comprise nearly half of stage I to III diagnoses.1 Between 1990 and 1998, there was an almost 15% increase in the rate of T1 (0 to 2 cm) tumors in the United States, as assessed by the Surveillance, Epidemiology, and End Results Program (from 143.5 to 163.5 per 100,000),2 largely attributed to detection of nonpalpable breast cancer associated with screening mammography.1–3
Patients with breast cancer who have T1a,b (≤ 1 cm) node-negative tumors generally have an excellent prognosis, with breast cancer–specific survival (BCSS) at 10 years exceeding 95%.4–6 However, outcomes for these patients may vary by biologic subtype.7–15 Compounding this problem, these patients have been excluded from adjuvant chemotherapy trials, which have resulted in uncertainty regarding the true risks and benefits of chemotherapy.
Since 1997, the National Comprehensive Cancer Network (NCCN) Breast Cancer Outcomes Database has collected detailed tumor and treatment data on a large cohort of women with newly diagnosed breast cancer presenting to many of its member institutions across the United States.16–18 In this study, we examined time trends of chemotherapy use in the last decade and outcomes of patients with breast cancer with T1a,bN0M0 tumors by biologic subtype, size, and treatment among women included in the NCCN database.
PATIENTS AND METHODS
Study Design and Data Source
This was a prospective cohort study performed in the NCCN Breast Cancer Outcomes Database. Patients were included if they received all or some of their treatment at a reporting center; those with one-time consultations were not included. Eight centers contributed data to this analysis: City of Hope National Medical Center, University of Texas MD Anderson Cancer Center (MDACC), Fox Chase Cancer Center, Dana-Farber Cancer Institute, Roswell Park Cancer Institute, H. Lee Moffitt Cancer Center, University of Michigan Cancer Center, and Ohio State University. All centers adhered to the data collection procedures and definitions developed by the NCCN Database, which have been subjected to rigorous quality assurance.19
Institutional review boards (IRBs) from participating centers approved data collection, transmission, and storage protocols. At centers where the IRB required signed informed consent for data collection, only patients who provided consent were included; elsewhere, the IRB granted a waiver of signed informed consent. An analytic cohort of 4,113 patients with T1a,bN0 breast cancer was identified (Figure 1).
Fig 1.
Flow diagram of patient population. HER2, human epidermal growth factor receptor 2; HR, hormone receptor; NCCN: National Comprehensive Cancer Network.
Key Variables
The database contains tumor information on tumor size, nodal status, grade, lymphovascular invasion (LVI), hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status, as abstracted from pathology reports. HR is considered positive if the estrogen receptor and/or progesterone receptor are positive. For HER2 classification, the fluorescent in situ hybridization result was used, if available. If only immunohistochemistry was available, 3+, high positive, or positive not otherwise specified were considered HER2-positive, and 2+, 1+, 0, or negative were considered HER2-negative; 1% (n = 32) of the patients were positive not otherwise specified. Tumors were grouped by biologic subtypes (HR-positive/HER2-positive, HR-positive/HER2-negative, HR-negative/HER2-positive, or HR-negative/HER2-negative) and tumor size (T1a [≤ 0.5 cm] or T1b [> 0.5 cm to ≤ 1 cm)]). Patients were classified as having received chemotherapy with or without trastuzumab or not. Six patients received trastuzumab alone. Tumor grade was categorized as high (according to histologic grade or, if not available, by nuclear grade) or low-intermediate. Data on race/ethnicity and comorbidity score20,21 came from patient surveys collected at initial presentation to the NCCN center. The following variables were abstracted by chart review: age at diagnosis, drug treatment, type and date of recurrence, vital status, and cause of death. Vital status and cause of death were also confirmed by using the Social Security Death Index and the National Death Index, current as of December 31, 2009. Invasive disease-free survival (IDFS), distant relapse-free survival (DRFS), BCSS, and overall survival (OS) were defined as time in years from diagnosis to date of death or last known vital status or recurrence (Appendix Table A1, online only).22
Statistical Analysis
Descriptive statistics were used to characterize the clinicopathologic and treatment characteristics. The percentage of patients who received chemotherapy with or without trastuzumab was calculated by subgroups. The Cochran-Armitage trend test was used to test the receipt of chemotherapy with or without trastuzumab over time. Estimates of 5-year survival were calculated among subgroups by using Kaplan and Meier estimates for OS, BCSS, IDFS, and DRFS. Besides stratification, no further adjustment for other clinicopathologic features was performed because of the small sample size of some subgroups.
Sensitivity analyses were performed. First, we included patients who did not have follow-up at the NCCN institution in the first 365 days after diagnosis (n = 589; Appendix Table A2 [online only] presents deaths and/or censuring among these patients). Second, we excluded the patients treated with trastuzumab only (n = 6). Finally, for patients with HR-positive/HER2-negative disease, we also examined the impact of grade on outcomes.
All P values presented are two-sided tests of statistical significance at .05. All statistical analyses were performed by using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Characteristics of the Study Population
Median follow-up time was 5.5 years (range, 1 to 13 years). Baseline clinicopathologic characteristics differed by subgroups (Table 1). As expected, compared with patients who received chemotherapy with or without trastuzumab, patients not treated with chemotherapy or trastuzumab were older and had more comorbidities. Moreover, fewer patients treated without chemotherapy with or without trastuzumab presented with adverse prognostic features, such as HER2 expression, high grade, and LVI.
Table 1.
Patient Demographics and Clinicopathologic Characteristics for T1a,bN0 Patients With Breast Cancer, NCCN 2000-2009
| Characteristic | T1aN0 Patients With Breast Cancer |
T1bN0 Patients With Breast Cancer |
||||||
|---|---|---|---|---|---|---|---|---|
| No Chemotherapy or Trastuzumab (n = 1,197) |
Chemotherapy With or Without Trastuzumab (n = 102) |
No Chemotherapy or Trastuzumab (n = 2,205) |
Chemotherapy With or Without Trastuzumab (n = 609) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||
| < 50 | 325 | 27 | 60 | 59 | 437 | 20 | 278 | 46 |
| 50-60 | 394 | 33 | 28 | 27 | 651 | 30 | 211 | 35 |
| 60-70 | 296 | 25 | 12 | 12 | 614 | 28 | 105 | 17 |
| ≥ 70 | 182 | 15 | 2 | 2 | 503 | 23 | 15 | 2 |
| Method of detection | ||||||||
| Abnormal screening mammogram | 957 | 80 | 57 | 56 | 1,705 | 77 | 349 | 57 |
| Symptoms | 177 | 15 | 36 | 35 | 423 | 19 | 230 | 38 |
| Other/unknown | 63 | 5 | 9 | 9 | 77 | 3 | 30 | 5 |
| Race/ethnicity | ||||||||
| Non-Hispanic white | 1,012 | 85 | 72 | 71 | 1,908 | 87 | 501 | 82 |
| Non-Hispanic black | 71 | 6 | 12 | 12 | 97 | 4 | 31 | 5 |
| Hispanic | 48 | 4 | 9 | 9 | 106 | 5 | 39 | 6 |
| Asian-Pacific Islander | 42 | 4 | 7 | 7 | 63 | 3 | 23 | 4 |
| American Indian | 2 | < 1 | 1 | 1 | 4 | < 1 | 2 | < 1 |
| Unknown | 14 | 1 | 1 | 1 | 19 | 1 | 8 | 1 |
| Other | 8 | 1 | 0 | 0 | 8 | 0 | 5 | 1 |
| Comorbidity score* | ||||||||
| 0 | 910 | 76 | 94 | 92 | 1,550 | 70 | 503 | 83 |
| 1 | 159 | 13 | 5 | 5 | 388 | 18 | 66 | 11 |
| ≥ 2 | 128 | 11 | 3 | 3 | 267 | 12 | 40 | 6 |
| Year of diagnosis | ||||||||
| 2000 | 82 | 7 | 2 | 2 | 134 | 6 | 37 | 6 |
| 2001 | 76 | 6 | 5 | 5 | 175 | 8 | 46 | 8 |
| 2002 | 103 | 9 | 6 | 6 | 175 | 8 | 35 | 6 |
| 2003 | 92 | 8 | 7 | 7 | 213 | 10 | 59 | 10 |
| 2004 | 115 | 10 | 3 | 3 | 233 | 11 | 59 | 10 |
| 2005 | 146 | 12 | 16 | 16 | 269 | 12 | 67 | 11 |
| 2006 | 145 | 12 | 15 | 15 | 249 | 11 | 80 | 13 |
| 2007 | 140 | 12 | 14 | 14 | 228 | 10 | 74 | 12 |
| 2008 | 172 | 14 | 14 | 14 | 287 | 13 | 81 | 13 |
| 2009 | 126 | 11 | 20 | 20 | 242 | 11 | 71 | 12 |
| Histology | ||||||||
| Ductal | 954 | 80 | 95 | 93 | 1,726 | 78 | 549 | 90 |
| Lobular | 100 | 8 | 2 | 2 | 153 | 7 | 24 | 4 |
| Mixed | 52 | 4 | 1 | 1 | 130 | 6 | 22 | 4 |
| Other | 91 | 8 | 4 | 4 | 196 | 9 | 14 | 2 |
| Subtype | ||||||||
| HR-positive, HER2-negative | 972 | 81 | 12 | 12 | 2,005 | 91 | 241 | 40 |
| HR-positive, HER2-positive | 102 | 9 | 33 | 32 | 89 | 4 | 110 | 18 |
| HR-negative, HER2-positive | 49 | 4 | 32 | 31 | 17 | 1 | 88 | 14 |
| HR-negative, HER2-negative | 74 | 6 | 25 | 25 | 94 | 4 | 170 | 28 |
| Grade | ||||||||
| Low-intermediate | 876 | 73 | 28 | 27 | 1,789 | 81 | 245 | 40 |
| High | 270 | 23 | 72 | 71 | 391 | 18 | 355 | 58 |
| Unknown | 51 | 4 | 2 | 2 | 25 | 1 | 9 | 1 |
| Lymphovascular invasion | ||||||||
| Yes | 41 | 3 | 11 | 11 | 115 | 5 | 97 | 16 |
| No | 1,132 | 95 | 89 | 87 | 2,047 | 93 | 506 | 83 |
| Unknown | 24 | 2 | 2 | 2 | 43 | 2 | 6 | 1 |
Nineteen percent of patients in the analytic cohort were deemed lost to follow-up (defined as > 2 years without a contact at an NCCN center). Age and year of diagnosis differed by group (P < .001). However, no other significant differences in patient or tumor characteristics were found between those lost to follow-up versus others (data not shown).
Treatment Characteristics and Time Trend in the Use of Chemotherapy
There were striking differences in chemotherapy use between tumor subtypes (Appendix Table A3, online only). Chemotherapy was rarely administered in patients with HR-positive/HER2-negative tumors, and their pattern of care was unchanged over the last decade. In contrast, a high proportion of patients with HER2-positive or HR-negative/HER2-negative tumors received adjuvant chemotherapy with or without trastuzumab, and there was an increase in the use of chemotherapy with or without trastuzumab among patients with HER2-positive and in patients with T1a HR-negative/HER2-negative tumors over the past decade (Table 2).
Table 2.
Use of Chemotherapy With or Without Τrastuzumab Over Time by Subtype
| Year | HR-Positive/HER2-Negative |
HR-Positive/HER2-Positive |
HR-Negative/HER2-Positive |
HR-Negative/HER2-Negative |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1a |
T1b |
T1a |
T1b |
T1a |
T1b |
T1a |
T1b |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| 2000 | 64 | 2 | 127 | 12 | 10 | 0 | 18 | 39 | 2 | 50 | 6 | 50 | 8 | 0 | 20 | 60 |
| 2001 | 61 | 0 | 173 | 9 | 8 | 38 | 22 | 36 | 8 | 25 | 5 | 80 | 4 | 0 | 21 | 86 |
| 2002 | 84 | 1 | 169 | 8 | 7 | 0 | 9 | 33 | 7 | 29 | 5 | 100 | 11 | 27 | 27 | 48 |
| 2003 | 76 | 3 | 210 | 10 | 8 | 12 | 22 | 36 | 4 | 50 | 17 | 76 | 11 | 18 | 23 | 70 |
| 2004 | 93 | 1 | 233 | 10 | 9 | 0 | 20 | 40 | 9 | 0 | 16 | 81 | 7 | 29 | 23 | 65 |
| 2005 | 113 | 1 | 267 | 11 | 20 | 25 | 24 | 50 | 16 | 38 | 13 | 77 | 13 | 31 | 32 | 50 |
| 2006 | 117 | 1 | 265 | 15 | 15 | 33 | 18 | 56 | 12 | 33 | 8 | 100 | 16 | 31 | 38 | 61 |
| 2007 | 114 | 1 | 236 | 10 | 22 | 18 | 29 | 76 | 8 | 88 | 13 | 100 | 10 | 20 | 24 | 67 |
| 2008 | 150 | 1 | 299 | 9 | 19 | 37 | 27 | 81 | 6 | 33 | 15 | 80 | 11 | 27 | 27 | 78 |
| 2009 | 112 | 2 | 267 | 13 | 17 | 47 | 10 | 100 | 9 | 67 | 7 | 100 | 8 | 50 | 29 | 69 |
| P* | .851 | .485 | .005 | < .001 | .028 | .038 | .042 | .551 | ||||||||
NOTE. Six patients received trastuzumab alone.
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor.
P values were calculated by Cochran-Armitage trend test per year.
Survival Outcomes
Table 3 lists the survival outcomes for patients treated and not treated with chemotherapy with or without trastuzumab by tumor subtype and size. In the overall cohort, the 5-year OS for patients not treated with chemotherapy with or without trastuzumab with T1a or T1b tumors exceeded 95%. The 5-year DRFS was 97% for T1a and 95% for T1b tumors.
Table 3.
Survival Outcomes of Patients With T1a,bN0 Breast Cancer, NCCN 2000-2009
| Outcome | Patients With T1aN0 Breast Cancer |
Patients With T1bN0 Breast Cancer |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Chemotherapy or Trastuzumab |
Chemotherapy With or Without Trastuzumab |
No Chemotherapy or Trastuzumab |
Chemotherapy With or Without Trastuzumab |
|||||||||
| 5-Year Estimate (%) | 95% CI | Total No. of Events | 5-Year Estimate (%) | 95% CI | Total No. of Events | 5-Year Estimate (%) | 95% CI | Total No. of Events | 5-Year Estimate (%) | 95% CI | Total No. of Events | |
| HR-positive/HER2-negative | (n = 972) | (n = 12) | (n = 2,005) | (n = 241) | ||||||||
| OS | 98 | 97 to 99 | 38 | 100 | 1 | 97 | 96 to 97 | 111 | 98 | 94 to 99 | 6 | |
| BCSS | 100 | 99 to 100 | 3 | 100 | 0 | 99 | 99 to 100 | 16 | 99 | 95 to 100 | 4 | |
| IDFS | 93 | 90 to 94 | 96 | 100 | 1 | 91 | 90 to 93 | 211 | 95 | 91 to 97 | 14 | |
| DRFS | 98 | 96 to 99 | 41 | 100 | 1 | 96 | 95 to 97 | 124 | 96 | 92 to 98 | 9 | |
| HR-positive/HER2-positive | (n = 102) | (n = 33) | (n = 89) | (n = 110) | ||||||||
| OS | 95 | 88 to 98 | 5 | 100 | 0 | 95 | 88 to 98 | 8 | 99 | 90 to 100 | 3 | |
| BCSS | 99 | 90 to 100 | 1 | 100 | 0 | 98 | 91 to 99 | 3 | 100 | 1 | ||
| IDFS | 86 | 76 to 92 | 13 | 100 | 0 | 86 | 76 to 92 | 17 | 90 | 81 to 95 | 8 | |
| DRFS | 96 | 89 to 98 | 5 | 100 | 0 | 94 | 86 to 98 | 10 | 96 | 88 to 99 | 5 | |
| HR-negative/HER2-positive | (n = 49) | (n = 32) | (n = 17) | (n = 88) | ||||||||
| OS | 93 | 79 to 98 | 3 | 100 | 0 | 100 | 2 | 95 | 86 to 98 | 6 | ||
| BCSS | 95 | 81 to 99 | 2 | 100 | 0 | 100 | 1 | 96 | 89 to 99 | 3 | ||
| IDFS | 84 | 69 to 92 | 7 | 89 | 70 to 96 | 4 | 68 | 40 to 86 | 6 | 94 | 86 to 97 | 9 |
| DRFS | 93 | 80 to 98 | 4 | 100 | 0 | 94 | 63 to 99 | 3 | 94 | 85 to 97 | 7 | |
| HR-negative/HER2-negative | (n = 74) | (n = 25) | (n = 94) | (n = 170) | ||||||||
| OS | 94 | 85 to 98 | 9 | 100 | 0 | 91 | 82 to 95 | 14 | 96 | 91 to 98 | 7 | |
| BCSS | 95 | 86 to 99 | 5 | 100 | 0 | 95 | 88 to 98 | 5 | 98 | 94 to 99 | 4 | |
| IDFS | 86 | 75 to 92 | 13 | 91 | 68 to 98 | 3 | 81 | 71 to 88 | 25 | 88 | 81 to 92 | 20 |
| DRFS | 93 | 84 to 97 | 10 | 100 | 0 | 90 | 81 to 95 | 15 | 96 | 90 to 98 | 8 | |
Abbreviations: BCSS, breast cancer–specific survival; DRFS, distant relapse-free survival; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IDFS, invasive disease-free survival; NCCN, National Comprehensive Cancer Network; OS, overall survival.
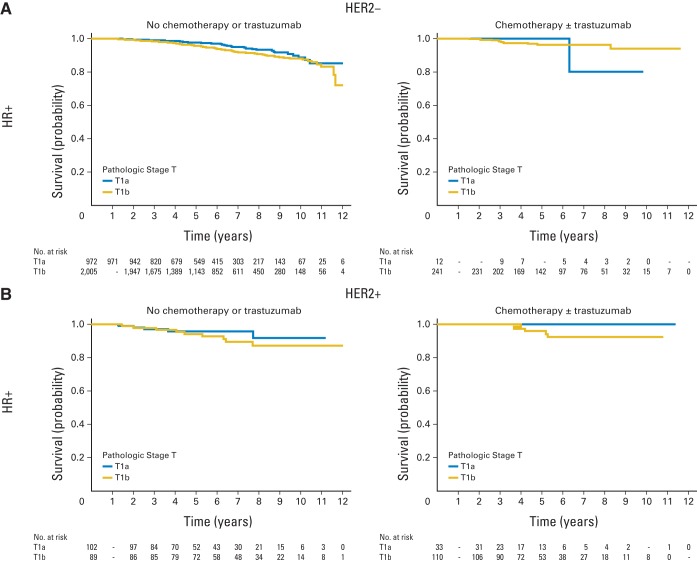
Among patients with HR-positive/HER2-negative breast cancer, 5-year OS and BCSS exceeded 95% in both T1a and T1b tumors and exceeded 90% for IDFS and DRFS, irrespective of whether the patient received chemotherapy or not. Among patients with HR-positive/HER2-negative tumors, those with high-grade tumors not treated with chemotherapy experienced numerically lower DRFS (94%) compared with patients treated with chemotherapy (98%), although the absolute difference, even in this high-grade subset, was small (Appendix Table A4, online only, and Appendix Fig A1, online only). For patients with HER2-positive tumors, the 5-year OS and BCSS were similarly high, and the 5-year distant recurrence rate did not exceed 7%, albeit with wide CIs for the HR-negative/HER2-positive subset, given the small sample size. The 5-year DRFS for patients with T1bN0 tumors who did not receive chemotherapy or trastuzumab was 94% for both HR-positive/HER2-positive tumors (95% CI, 86% to 98%; n = 89) and HR-negative/HER2-positive tumors (95% CI, 63% to 99%; n = 17). The 5-year DRFS for patients with T1b tumors who received chemotherapy with or without trastuzumab was 96% (95% CI, 88% to 99%; n = 110) for HR-positive/HER2-positive tumors and 94% (95% CI, 85% to 97%; n = 88) for HR-negative/HER2-positive tumors. For patients with HR-negative/HER2-negative tumors untreated with chemotherapy, the 5-year DRFS for T1a tumors was 93% (95% CI, 84% to 97%; n = 74) and for T1b tumors was 90% (95% CI, 81% to 95%; n = 94); however, in one of the sensitivity analyses, this point estimate for T1b tumors was 86% (Appendix Table A5, online only). The 5-year DRFS for treated HR-negative/HER2-negative T1a tumors was 100% (n = 25), and for HR-negative/HER2-negative T1b tumors, it was 96% (95% CI, 90% to 98%; n = 170; Fig 2). Additional results of sensitivity analyses were similar (data not shown).
Fig 2.
Distant relapse-free survival of T1a,bN0 patients with breast cancer, National Comprehensive Cancer Network, 2000 to 2009. (A) HR-positive/HER2-negative group; (B) HR-positive/HER2-positive group; (C) HR-negative/HER2-negative group; (D) HR-negative/HER2-positive group. HR, hormone receptor; HER2, human epidermal growth factor receptor 2.
DISCUSSION
Over the last decade, among patients with T1a,bN0M0 breast cancer treated at academic centers geographically distributed across the United States, there was an increase in chemotherapy usage for patients with HER2-positive or T1a HR-negative/HER2-negative breast cancers. Across all subgroups, these patients experienced an excellent prognosis, with the 5-year distant relapse rate for patients with T1a tumors untreated with chemotherapy ranging from 2% to 7% and for patients with T1b tumors from 4% to 10%; patients with HR-positive/HER2-negative tumors had the best prognosis, and patients with HR-negative/HER2-negative tumors had the lowest outcomes estimates. For treated patients with T1a tumors, there were no distant recurrence events at 5 years, and for T1b tumors the 5-year distant recurrence rate was 4% for patients with HR-positive and HR-negative/HER2-negative tumors and 6% for patients with HR-negative/HER2-positive tumors. This observational, nonrandomized cohort study calls into question what type of treatment is justified and appropriate for these patients.
In making decisions regarding adjuvant chemotherapy, the potential absolute benefits of treatment must be weighed against the treatment-related risks. Although the risk of death during breast cancer adjuvant chemotherapy is less than 1%, it is not absent, and there is a non-negligible risk of hospitalization or need for urgent evaluation for serious adverse effects related to chemotherapy.23,24 Other serious and lasting adverse effects include cardiomyopathy, secondary leukemias, and neuropathy.25–33 For example, for patients treated with chemotherapy and trastuzumab, the cardiotoxicity rate in clinical trials ranged from 1% to 4%, but population-based studies have raised concerns that this may be higher outside of trials.25,26,32,34–38 The absolute benefit of treatment is a function of the baseline prognosis and impact of therapy on risk of recurrence, with size and biologic subtype being factors in both.
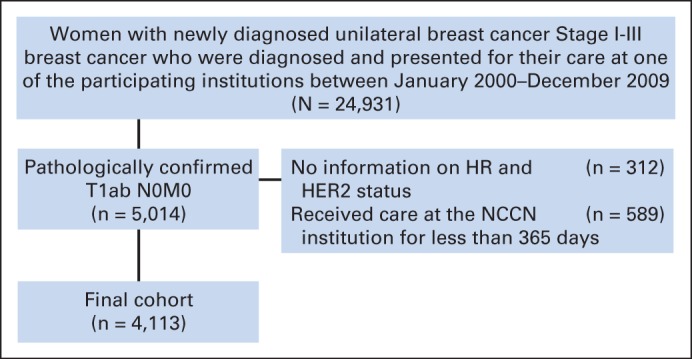
For patients with HR-positive/HER2-negative T1a,bN0 tumors, the 2013 NCCN guidelines indicate that a 21-gene recurrence score can be requested to predict the benefit of chemotherapy, with chemotherapy to be considered in patients with an intermediate or high score; in the absence of recurrence score data, consideration of chemotherapy is recommended. Our data, which demonstrate a less than 5% 5-year risk of distant recurrence in T1bN0 patients with HR-positive/HER2-negative tumors treated without chemotherapy (89% of patients with HR-positive/HER2-negative T1bN0 tumors in the cohort), strongly suggest that further prognostic tests are likely unnecessary in the vast majority of such patients. In the pivotal studies that examined the prognostic and predictive role of the 21-gene recurrence score among patients with HR-positive/HER2-negative node-negative breast cancer, the proportion of patients with T1a,b tumors with high recurrence score was only 16%. In addition, among patients with low-grade tumors only 5%-12% had high recurrence scores.39,40 These data complement our results and suggest that the default treatment in patients with T1bN0 HR-positive/HER2-negative breast cancer should be endocrine therapy alone and that molecular predictive testing beyond standard pathologic examination is not generally required in such patients, unless there are other high-risk features of concern (eg, a combination of features such as young age, high grade, and LVI). Notably, in a secondary analysis, the 5-year DRFS in T1a,bN0 HR-positive/HER2-negative high-grade tumors untreated with chemotherapy is still quite favorable (94%), even if lower than that of patients treated with chemotherapy (98%), and among patients with HR-positive/HER2-negative tumors with low-intermediate grade, patients untreated with chemotherapy had a 3% risk of distant recurrence.
The situation is somewhat different in patients who have HER2-positive tumors for whom the potential benefits for therapy among these subsets and the variability in prognostic estimates have resulted in uncertainty on the appropriate threshold for adjuvant chemotherapy with or without trastuzumab recommendations, with a steady evolution of the guidelines. Before 2003, NCCN guidelines recommended adjuvant chemotherapy for tumors more than 1 cm in size and/or with nodal involvement. In 2003, guidelines recommended adjuvant chemotherapy “to be considered” for patients with moderate to poorly differentiated T1bN0 breast cancers, regardless of subtype; in 2007, this was adjusted to encompass HER2-positive tumors, and since 2008, this recommendation has also covered HR-negative cancers. Finally, since 2010, the guidelines have recommended consideration of chemotherapy and trastuzumab for patients with HER2-positive T1bN0 tumors.41
The MDACC, the European Institute of Oncology (EIO), and the Kaiser Permanente Northern California (KPNC) group reported the largest experiences to date among patients with T1a,bN0M0 HER2-positive disease. The MDACC analyzed the risk of recurrence among 98 patients with T1a,bN0M0 HER2-positive disease who did not receive chemotherapy or trastuzumab and reported a 5-year recurrence-free survival of 77% (95% CI, 67% to 85%).10 In contrast, in the EIO series of 150 patients, the 5-year disease-free survival was 92% (95% CI, 86% to 99%) in patients with HR-positive/HER2-positive tumors and 91% (95% CI, 84% to 99%) in patients with HR-negative/HER2-positive tumors.9 Approximately one third of patients in this series received adjuvant chemotherapy, most of them with HR-negative disease.9 Finally, in an unpublished series of 237 patients from KPNC (116 T1a and 121 T1b), the 5-year recurrence-free survival was 96% (95% CI, 90% to 99%) for patients with T1a tumors and 91% (95% CI, 83% to 95%) for patients with T1b tumors. Twenty-five percent of those patients received adjuvant chemotherapy, and 9% received adjuvant trastuzumab.8
Our series, which included 520 HER2-positive patients, revealed a low 5-year risk of distant recurrence among untreated patients; however, given that most patients with HR-negative/HER2-positive tumors received chemotherapy with or without trastuzumab, the sample size for the untreated subgroups is small, leading to point estimates for recurrence in untreated patients with wide CIs. For untreated patients with T1aN0 HR-negative/HER2-positive tumors (n = 49), 5-year DRFS was 93%; for untreated patients with T1bN0 HR-negative/HER2-positive tumors (n = 17), 5-year DRFS was 94%. Among patients with HR-positive/HER2-positive tumors untreated with chemotherapy or trastuzumab, the 5-year DRFS was 96% in T1a (n = 102) tumors and 94% in T1b (n = 89) tumors, which may be impacted by the use of endocrine treatment (85% received endocrine therapy).42,43
Finally, despite the historical association between HR-negative/HER2-negative breast cancer and poor outcome,10 in our series, these patients fared well overall, with 5-year OS of 91% to 94% in untreated patients and 96% to 100% in treated patients. Among untreated T1aN0 (n = 74) and T1bN0 (n = 94) HR-negative/HER2-negative tumors, the 5-year DRFS was 93% for patients with T1a tumors and 90% for patients with T1b tumors, albeit with wide CIs and with a 5-year DRFS estimate for T1b HR-negative/HER2-positive tumors of 86% in one of the sensitivity analyses.
Overall, among patients with HER2-positive or HR-negative/HER2-negative tumors, we observed numerically higher 5-year DRFS in treated patients; however, formal comparison of outcomes between treated and nontreated subgroups cannot be made, given that this was not a randomized experience. We should note that there were differences between IDFS and DFRS estimates; however, we decided to focus on DRFS, because we believe that this is the primary driver of chemotherapy use.
Our study adds evidence to the understanding of clinical outcomes by biologic subtypes, tumor size, and treatment.44 It provides a starting point for estimating the baseline risk of recurrence among patients with small, node-negative tumors to guide discussions between patients and providers. Our data support the overall excellent prognosis across all subgroups; however, they also suggest that particularly those with larger and HR-negative tumors may derive benefit from treatment. Above all, it highlights the importance of clinical trials to identify regimens with a more favorable toxicity profile to improve the risk-benefit ratio for those who opt to proceed with adjuvant therapy and in whom the absolute benefits of treatment are likely to be small. Examples of such trials include the APT (Adjuvant Paclitaxel and Trastuzumab for Node-Negative HER2-Positive Breast Cancer; NCT00542451), RESPECT (Evaluation of Trastuzumab Without Chemotherapy as a Postoperative Adjuvant Therapy in HER2 Positive Elderly Breast Cancer Patients; NCT01104935), and ATEMPT (T-DM1 vs Paclitaxel/Trastuzumab for Breast; NCT01853748) clinical trials. Finally, it informs future clinical trials; for example, combinations of anti-HER2 therapy and endocrine therapy or of other noncytotoxic targeted only approaches may be appropriately studied in the years ahead.
We had access to a large prospectively collected data set with stringent quality assurance that drew from large academic centers distributed geographically across the United States. 16,19 Limitations include the lack of central pathologic review; however, it is likely that academic pathologists at the participating institutions reviewed specimens as part of clinical care. For the receptor definition, the cutoffs used were based on each center's standards applicable at time of diagnosis. For estrogen receptor status during the majority of the time frame of the study, many but not all, institutions used a threshold of greater than 10% for a positive result. For tumor size definition, we used cancer registry standards; however, for small tumors, tissue fixation, processing, and the rounding up or down of tumor size can affect the reliability of tumor metrics classification, and we cannot attest to the specific pathology practices used.45,46 Second, the classification of treated versus nontreated was done on an ever/never basis, not accounting for the impact of variability of regimens. Third, because the study was limited to patients who presented to NCCN academic centers, we cannot rule out a potential referral bias, which may have had an impact on the decision to use chemotherapy, the choice of chemotherapy regimens, and outcomes. Fourth, we have a limited follow-up time. Particularly in patients with HR-positive/HER2-negative tumors, we would expect additional recurrences with longer follow-up. However, it is unlikely that chemotherapy would have a major impact on late recurrences, and therefore, our data are still informative in terms of chemotherapy decision making.47 Most of the clinical trials and meta-analyses that examined recurrence rates reported 5-year recurrence rates.48 Fifth, we had more than 4,000 patients in the analytic cohort, but small numbers of patients in some subgroups. We were unable to perform further adjustment aside from stratification and this led to wide CIs in some of the outcomes estimates; however, for the HR-positive/HER2-negative tumors, we examined the role of histologic grade on outcomes. We acknowledge that treatment groups were not balanced. Perhaps, in light of the changes in clinical practice over time, we had somewhat shorter follow-up time for treated versus untreated patients in some subtypes, which may have had an impact on our estimates of outcome. Sixth, limiting the analyses to patients who received care for at least 365 days at an NCCN center has the potential for selection bias; however, the sensitivity analyses suggest that this exclusion did not have a major influence on outcomes. Finally, this study did not have a randomized design; therefore, comparisons between patients treated with chemotherapy with or without trastuzumab and patients not treated with chemotherapy or trastuzumab and treatment time trends should be interpreted with caution.
In conclusion, overall, women with T1a,bN0 tumors have an excellent prognosis without chemotherapy across all subgroups. Although chemotherapy with or without trastuzumab may be beneficial in some patients, the absolute benefit for most patients with T1a,bN0M0 is relatively small, and potential toxicities should be a factor in treatment decisions, which ultimately should be driven by a well-informed patient. In the long run, we need to develop better ways to identify patients at the highest risk of recurrence while simultaneously trying to minimize treatment-related toxicity.
Glossary Terms
- HER2/neu (human epidermal growth factor receptor 2):
also called ErbB2. HER2/neu belongs to the epidermal growth factor receptor (EGFR) family and is overexpressed in several solid tumors. Like EGFR, it is a tyrosine kinase receptor whose activation leads to proliferative signals within the cells. On activation, the human epidermal growth factor family of receptors are known to form homodimers and heterodimers, each with a distinct signaling activity. Because HER2 is the preferred dimerization partner when heterodimers are formed, it is important for signaling through ligands specific for any members of the family. It is typically overexpressed in several epithelial tumors.
- trastuzumab:
a humanized anti-ErbB2 monoclonal antibody approved for treating patients whose breast cancers overexpress the ErbB2 protein or demonstrate ErbB2 gene amplification. It is currently being tested in combination with other therapies.
Appendix
Table A1.
Breakdown of Events by Outcome
| Event/Site | Patients |
OS | BCSS | IDFS | DRFS | |
|---|---|---|---|---|---|---|
| No. | % | |||||
| Second primary* | 158 | 3.8 | X | |||
| Invasive ipsilateral tumor recurrence | 34 | 0.83 | X | |||
| Locoregional invasive recurrence | 17 | 0.41 | X | |||
| Chest wall | 5 | 0.12 | ||||
| Ipsilateral axillary lymph nodes | 8 | 0.19 | ||||
| Other locoregional lymph nodes | 4 | 0.10 | ||||
| Distant recurrence | 25† | 0.60 | X | X | ||
| Liver | 7 | 0.17 | ||||
| Lung | 2 | 0.05 | ||||
| Other distant visceral | 1 | 0.02 | ||||
| Pleural effusion | 2 | 0.05 | ||||
| Distant lymph nodes | 1 | 0.02 | ||||
| Bone | 11 | 0.27 | ||||
| Skin | 1 | 0.02 | ||||
| Other causes of death | 165 | 4.0 | X | X | X | |
| Breast cancer-specific death | 48 | 1.2 | X | X | X | X |
NOTE. This breakdown of events accounted for first events. A ranking system of events was performed as follow: Breast cancer–specific death, other causes of death, distant recurrence (brain, liver, lung, other distant visceral, pericardial effusion, intra-abdominal, pleural effusion, other distant lymph nodes, bone, skin, other), locoregional invasive recurrence, invasive ipsilateral tumor recurrence, second primary (breast, nonbreast).
Abbreviations: BCSS, breast cancer–specific survival; DRFS, distant relapse-free survival; IDFS, invasive disease-free survival; OS, overall survival.
Breast and nonbreast tumors included.
Four patients in addition to these 25 patients had a distant recurrence, after a second primary/ipsilateral or locoregional event (two, liver; one, lung; and one, distant lymph node).
Table A2.
Total Number of Deaths by Subtype Among Patients Without Follow-Up at the NCCN Institution in the First 365 Days After Diagnosis
| Tumor Subtype | No. of Deaths After Diagnosis |
No. of Patients Who Were Censored | |
|---|---|---|---|
| In First 365 Days | After the First 365 Days | ||
| HR-positive/HER2-negative | 5 | 35 | 429 |
| HR-positive/HER2-positive | 0 | 0 | 35 |
| HR-negative/HER2-positive | 0 | 0 | 17 |
| HR-negative/HER2-negative | 4 | 9 | 55 |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor; NCCN, National Comprehensive Cancer Network.
Table A3.
Treatment Characteristics for Patients With T1a,bN0 Breast Cancer, NCCN 2000-2009
| Treatment Characteristic | T1aN0 Breast Cancer |
T1bN0 Breast Cancer |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR-Positive/HER2-Negative (n = 984) |
HR-Positive/HER2-Positive (n = 135) |
HR-Negative/HER2-Positive (n = 81) |
HR-Negative/HER2-Negative (n = 99) |
HR-Positive/HER2-Negative (n = 2,246) |
HR-Positive/HER2-Positive (n = 199) |
HR-Negative/HER2-Positive (n = 105) |
HR-Negative/HER2-Negative (n = 264) |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Definitive surgery | ||||||||||||||||
| Mastectomy | 284 | 29 | 59 | 44 | 52 | 64 | 39 | 39 | 501 | 22 | 57 | 29 | 45 | 43 | 63 | 24 |
| Breast-conserving surgery | 699 | 71 | 76 | 56 | 29 | 36 | 60 | 61 | 1,745 | 78 | 142 | 71 | 60 | 57 | 201 | 76 |
| No definitive surgery | 1 | < 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Radiation therapy | ||||||||||||||||
| No | 330 | 34 | 62 | 46 | 52 | 64 | 40 | 40 | 644 | 29 | 69 | 35 | 45 | 43 | 67 | 25 |
| Yes | 654 | 66 | 73 | 54 | 29 | 36 | 59 | 60 | 1,602 | 71 | 130 | 65 | 60 | 57 | 197 | 75 |
| Hormone therapy | ||||||||||||||||
| No | 192 | 20 | 31 | 23 | 75 | 93 | 92 | 93 | 226 | 10 | 20 | 10 | 101 | 96 | 253 | 96 |
| Yes | 792 | 80 | 104 | 77 | 6 | 7 | 7 | 7 | 2,020 | 90 | 179 | 90- | 4 | 4 | 11 | 4 |
| Trastuzumab | ||||||||||||||||
| No | 984 | 100 | 110 | 81 | 60 | 74 | 98 | 99 | 2,239 | 100 | 127 | 64 | 57 | 54 | 259 | 98 |
| Yes | 0 | 0 | 25 | 19 | 21 | 26 | 1 | 1 | 7 | < 1 | 72 | 36 | 48 | 46 | 5 | 2 |
| Chemotherapy | ||||||||||||||||
| No | 972 | 99 | 103 | 76 | 50 | 62 | 75 | 76 | 2,005 | 89 | 92 | 46 | 17 | 16 | 94 | 36 |
| Yes | 12 | 1 | 32 | 24 | 31 | 38 | 24 | 24 | 241 | 11 | 107 | 54 | 88 | 84 | 170 | 64 |
| Type of chemotherapy | ||||||||||||||||
| Anthracycline-containing regimen | 7 | 58 | 9 | 28 | 13 | 42 | 14 | 58 | 144 | 60 | 40 | 37 | 36 | 41 | 98 | 58 |
| CMF-containing regimen | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 5 | 2 | 2 | 2 | 2 | 8 | 5 |
| Taxane-containing regimen | 4 | 33 | 13 | 41 | 10 | 32 | 1 | 4 | 40 | 17 | 25 | 23 | 18 | 20 | 26 | 15 |
| Anthracycline-containing regimen-taxane | 0 | 0 | 4 | 13 | 4 | 13 | 7 | 29 | 39 | 16 | 23 | 21 | 23 | 26 | 34 | 20 |
| Single chemotherapy agent | 1 | 8 | 6 | 19 | 4 | 13 | 2 | 8 | 6 | 2 | 17 | 16 | 9 | 10 | 4 | 2 |
| Other | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | < 1 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: CMF, cyclophosphamide, methotrexate, fluorouracil; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; NCCN, National Comprehensive Cancer Network.
Table A4.
DRFS of Patients With HR-Positive/HER2-Negative T1a,bN0 Breast Cancer by Histologic Grade, NCCN 2000-2009
| DRFS Grade | No Chemotherapy |
Chemotherapy |
||||
|---|---|---|---|---|---|---|
| No. of Patients | 5-Year Estimate (%) | 95% CI | No. of Patients | 5-Year Estimate (%) | 95% CI | |
| Low-intermediate | 2,485 | 97 | 96 to 97 | 156 | 95 | 90 to 98 |
| High | 434 | 94 | 91 to 96 | 92 | 98 | 91 to 99 |
Abbreviations: DRFS, distant relapse-free survival; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; NCCN, National Comprehensive Cancer Network.
Table A5.
Survival Outcomes of Patients With T1a,bN0 Breast Cancer by Tumor Subtype, NCCN 2000-2009
| Survival Outcome | Patients With T1aN0 Breast Cancer |
Patients With T1bN0 Breast Cancer |
||||||
|---|---|---|---|---|---|---|---|---|
| No Chemotherapy or Trastuzumab |
Chemotherapy With or Without Trastuzumab |
No Chemotherapy or Trastuzumab |
Chemotherapy With or Without Trastuzumab |
|||||
| 5-Year Estimate (%) | 95% CI | 5-Year Estimate (%) | 95% CI | 5-Year Estimate (%) | 95% CI | 5-Year Estimate (%) | 95% CI | |
| HR-positive/HER2-negative | (n = 1,027) | (n = 13) | (n = 2,134) | (n = 254) | ||||
| OS | 98 | 96 to 99 | 100 | 96 | 95 to 97 | 98 | 94 to 99 | |
| BCSS | 100 | 99 to 100 | 100 | 99 | 99 to 100 | 99 | 96 to 100 | |
| IDFS | 92 | 90 to 94 | 100 | 91 | 89 to 92 | 95 | 91 to 98 | |
| DRFS | 97 | 96 to 98 | 100 | 95 | 94 to 96 | 96 | 92 to 98 | |
| HR-positive/HER2-positive | (n = 105) | (n = 35) | (n = 89) | (n = 116) | ||||
| OS | 95 | 88 to 98 | 100 | 95 | 88 to 98 | 99 | 91 to 100 | |
| BCSS | 99 | 90 to 100 | 100 | 98 | 91 to 99 | 100 | ||
| IDFS | 86 | 77 to 92 | 100 | 86 | 76 to 92 | 90 | 81 to 95 | |
| DRFS | 96 | 89 to 98 | 100 | 94 | 86 to 98 | 96 | 89 to 99 | |
| HR-negative/HER2-positive | (n = 51) | (n = 33) | (n = 17) | (n = 92) | ||||
| OS | 93 | 80 to 98 | 100 | 100 | 95 | 86 to 98 | ||
| BCSS | 95 | 82 to 99 | 100 | 100 | 96 | 89 to 99 | ||
| IDFS | 85 | 70 to 92 | 90 | 71 to 97 | 68 | 40 to 86 | 93 | 85 to 97 |
| DRFS | 93 | 81 to 98 | 100 | 94 | 63 to 99 | 94 | 85 to 97 | |
| HR-negative/HER2-negative | (n = 79) | (n = 27) | (n = 103) | (n = 183) | ||||
| OS | 93 | 84 to 97 | 100 | 87 | 77 to 92 | 94 | 89 to 97 | |
| BCSS | 96 | 87 to 99 | 100 | 96 | 88 to 98 | 97 | 93 to 99 | |
| IDFS | 85 | 74 to 92 | 91 | 69 to 98 | 78 | 68 to 85 | 86 | 79 to 91 |
| DRFS | 92 | 83 to 96 | 100 | 86 | 76 to 91 | 94 | 88 to 97 | |
NOTE. Survival outcomes for all patients (including patients without follow-up at the NCCN institution in the first 365 days after diagnosis).
Abbreviations: BCSS, breast cancer–specific survival; DRFS, distant relapse-free survival; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; IDFS, invasive disease-free survival; NCCN, National Comprehensive Cancer Network; OS, overall survival.
Fig A1.
Distant relapse-free survival of patients with hormone receptor–positive/human epidermal growth factor receptor 2–negative T1a,bN0 breast cancer by histologic grade, National Comprehensive Cancer Network, 2000 to 2009. (A) Patients who did not receive chemotherapy; (B) patients who received chemotherapy.
Footnotes
Supported by Grant No. HMSP-ICS/0004/2011 from Fundacao para a Ciencia e Tecnologia, Grant No. NIH P50 CA089393 from the National Cancer Institute Specialized Program of Research Excellence in Breast Cancer, and by the National Comprehensive Cancer Network, Breast Cancer Research Foundation, Conquer Cancer Foundation, and Susan G. Komen for the Cure.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Presented in part at the 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Ana M. Gonzalez-Angulo, Genentech (C), Novartis (C); Eric P. Winer, Genentech (U) Stock Ownership: None Honoraria: Ana M. Gonzalez-Angulo, Genentech, Novartis Research Funding: Ana M. Gonzalez-Angulo, Genentech, Novartis, Genomic Health, Celgene, GlaxoSmithKline, Bristol-Myers Squibb, Bayer AG; Eric P. Winer, Genentech; Nancy U. Lin, Genentech, GlaxoSmithKline, Synta Pharmaceuticals, Array BioPharma, Incyte Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: Richard L. Theriault, UpToDate
AUTHOR CONTRIBUTIONS
Conception and design: Ines Vaz-Luis, Rebecca A. Ottesen, Melissa E. Hughes, Harold J. Burstein, Ana M. Gonzalez-Angulo, Jane C. Weeks, Eric P. Winer, Nancy U. Lin
Administrative support: Nancy U. Lin, Jane C. Weeks
Provision of study materials or patients: Beverly Moy, Richard L. Theriault, Hope S. Rugo, Jane C. Weeks, Eric P. Winer
Collection and assembly of data: Ines Vaz-Luis, Rebecca A. Ottesen, Melissa E. Hughes, Rizvan Mamet, Stephen B. Edge, Beverly Moy, Hope S. Rugo, Richard L. Theriault, Jane C. Weeks, Nancy U. Lin
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Kennedy T, Stewart AK, Bilimoria KY, et al. Treatment trends and factors associated with survival in T1aN0 and T1bN0 breast cancer patients. Ann Surg Oncol. 2007;14:2918–2927. doi: 10.1245/s10434-007-9441-5. [DOI] [PubMed] [Google Scholar]
- 2.Schootman M, Jeffe D, Reschke A, et al. The full potential of breast cancer screening use to reduce mortality has not yet been realized in the United States. Breast Cancer Res Treat. 2004;85:219–222. doi: 10.1023/B:BREA.0000025410.41220.67. [DOI] [PubMed] [Google Scholar]
- 3.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 4.Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, et al. Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. J Clin Oncol. 2007;25:4952–4960. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 5.Smart CR, Hartmann WH, Beahrs OH, et al. Insights into breast cancer screening of younger women: Evidence from the 14-year follow-up of the Breast Cancer Detection Demonstration Project. Cancer. 1993;72:1449–1456. doi: 10.1002/1097-0142(19930815)72:4+<1449::aid-cncr2820721406>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.Tabár L, Fagerberg G, Day NE, et al. Breast cancer treatment and natural history: New insights from results of screening. Lancet. 1992;339:412–414. doi: 10.1016/0140-6736(92)90090-p. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Dignam J, Tan-Chiu E, et al. Prognosis and treatment of patients with breast tumors of one centimeter or less and negative axillary lymph nodes. J Natl Cancer Inst. 2001;93:112–120. doi: 10.1093/jnci/93.2.112. [DOI] [PubMed] [Google Scholar]
- 8.Fehrenbacher L, Capra AM, Quesenberry CP, Jr, et al. Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2–positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: A cohort from an integrated health care delivery system. J Clin Oncol. 2014;32:2151–2158. doi: 10.1200/JCO.2013.52.0858. [DOI] [PubMed] [Google Scholar]
- 9.Curigliano G, Viale G, Bagnardi V, et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009;27:5693–5699. doi: 10.1200/JCO.2009.22.0962. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Angulo AM, Litton JK, Broglio KR, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27:5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McArthur HL, Mahoney KM, Morris PG, et al. Adjuvant trastuzumab with chemotherapy is effective in women with small, node-negative, HER2-positive breast cancer. Cancer. 2011;117:5461–5468. doi: 10.1002/cncr.26171. [DOI] [PubMed] [Google Scholar]
- 12.Chia S, Norris B, Speers C, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26:5697–5704. doi: 10.1200/JCO.2007.15.8659. [DOI] [PubMed] [Google Scholar]
- 13.Park YH, Kim ST, Cho EY, et al. A risk stratification by hormonal receptors (ER, PgR) and HER-2 status in small (< or = 1 cm) invasive breast cancer: Who might be possible candidates for adjuvant treatment? Breast Cancer Res Treat. 2010;119:653–661. doi: 10.1007/s10549-009-0665-x. [DOI] [PubMed] [Google Scholar]
- 14.Amar S, McCullough AE, Tan W, et al. Prognosis and outcome of small (<= 1 cm), node-negative breast cancer on the basis of hormonal and HER-2 status. Oncologist. 2010;15:1043–1049. doi: 10.1634/theoncologist.2010-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong FY, Yip CS, Chua ET. Implications of HER2 amplification in small, node-negative breast cancers: Do Asians differ? World J Surg. 2012;36:287–294. doi: 10.1007/s00268-011-1353-7. [DOI] [PubMed] [Google Scholar]
- 16.Weeks JC. Outcomes assessment in the NCCN. Oncology (Williston Park) 1997;11:137–140. [PubMed] [Google Scholar]
- 17.Weeks J. Outcomes assessment in the NCCN: 1998 update: National Comprehensive Cancer Network. Oncology (Williston Park) 1999;13:69–71. [PubMed] [Google Scholar]
- 18.Niland JC. NCCN outcomes research database: Data collection via the Internet. Oncology (Williston Park) 2000;14:100–103. [PubMed] [Google Scholar]
- 19.Punglia RS, Hughes ME, Edge SB, et al. Factors associated with guideline-concordant use of radiotherapy after mastectomy in the National Comprehensive Cancer Network. Int J Radiat Oncol Biol Phys. 2008;72:1434–1440. doi: 10.1016/j.ijrobp.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 23.Swain SM, Tang G, Geyer CE, Jr, et al. Definitive results of a phase III adjuvant trial comparing three chemotherapy regimens in women with operable, node-positive breast cancer: The NSABP B-38 trial. J Clin Oncol. 2013;31:3197–3204. doi: 10.1200/JCO.2012.48.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassett MJ, O'Malley AJ, Pakes JR, et al. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98:1108–1117. doi: 10.1093/jnci/djj305. [DOI] [PubMed] [Google Scholar]
- 25.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 26.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 27.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Postma TJ, Vermorken JB, Liefting AJ, et al. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6:489–494. doi: 10.1093/oxfordjournals.annonc.a059220. [DOI] [PubMed] [Google Scholar]
- 30.Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol. 2006;24:1633–1642. doi: 10.1200/JCO.2005.04.0543. [DOI] [PubMed] [Google Scholar]
- 31.Morton LM, Dores GM, Tucker MA, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121:2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karp J, Blackford A, Visvanathan K, et al. Myelodysplastic syndrome and/or acute myelogenous leukemia (MDS and/or AML) after a breast cancer diagnosis: The National Comprehensive Cancer Network (NCCN) experience. Cancer Res. 2012;72(suppl):S3–S5. abstr SABCS12-S3-5. [Google Scholar]
- 34.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 36.Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Long JB, Hurria A, et al. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60:2504–2512. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 38.Vaz-Luis I, Keating NL, Lin NU, et al. Duration and toxicity of adjuvant trastuzumab in older patients with early-stage breast cancer: A population-based study. J Clin Oncol. 2013;32:927–934. doi: 10.1200/JCO.2013.51.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 40.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 41.National Comprehensive Cancer Network. Breast Cancer, version 3.2014. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 42.Vaz-Luis I, Ottesen RA, Hughes ME, et al. Impact of hormone receptor status on patterns of recurrence and clinical outcomes among patients with human epidermal growth factor-2-positive breast cancer in the National Comprehensive Cancer Network: A prospective cohort study. Breast Cancer Res. 2012;14:R129. doi: 10.1186/bcr3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Untch M, Gelber RD, Jackisch C, et al. Estimating the magnitude of trastuzumab effects within patient subgroups in the HERA trial. Ann Oncol. 2008;19:1090–1096. doi: 10.1093/annonc/mdn005. [DOI] [PubMed] [Google Scholar]
- 44.Adjuvant! Online. Decision making tools for health care professionals. http://www.adjuvantonline.com/index.jsp.
- 45.Pritt B, Tessitore JJ, Weaver DL, et al. The effect of tissue fixation and processing on breast cancer size. Hum Pathol. 2005;36:756–760. doi: 10.1016/j.humpath.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Provencher L, Diorio C, Hogue JC, et al. Does breast cancer tumor size really matter that much? Breast. 2012;21:682–685. doi: 10.1016/j.breast.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Rosen PR, Groshen S, Saigo PE, et al. A long-term follow-up study of survival in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma. J Clin Oncol. 1989;7:355–366. doi: 10.1200/JCO.1989.7.3.355. [DOI] [PubMed] [Google Scholar]
- 48.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]