Abstract
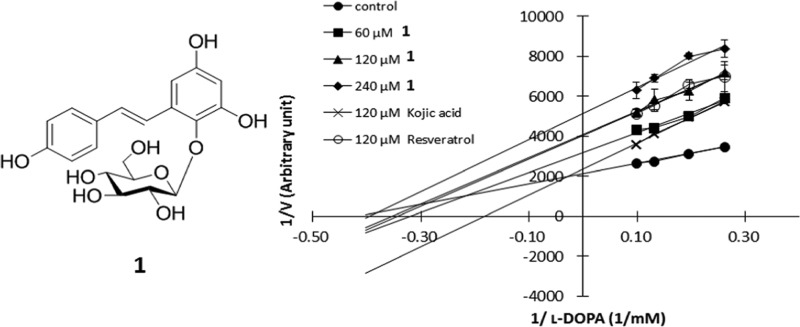
2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucopyranoside (1), isolated from Polygonum multiflorum, is a noncompetitive inhibitor of tyrosinase in cell-free kinetics; it reduced the Vmax values in a dose-dependent manner. Compound 1 inhibited PKA-induced melanogenesis, reduced the protein expression of tyrosinase and its transcription factor, the microphthalmia-associated transcription factor, and lowered the complex formation between tyrosinase and tyrosinase-related protein 1 (TRP-1). Immunofluorescence microscopy revealed no association of tyrosinase with the endoplasmic reticulum or lysosomes, implying the absence of a direct effect of 1 on the maturation process of the enzyme. The antimelanogenic activity of 1 is likely mediated through a noncompetitive inhibition on tyrosinase, down-regulation of the expression of melanogenic proteins, and reduction of tyrosinase/TRP-1 complex formation.
Melanocytes localize at the epidermal–dermal junction and produce pigmentary granules (e.g., eumalanin and pheomalanin), which are responsible for the coloration of skin and hair.1 Melanogenesis involves a cascade of enzymatic reactions in which the initial steps include the conversion of tyrosine and 3,4-dihydroxyphenylalanine (DOPA) to DOPA-quinone.2 During the process, tyrosinase serves as the key catalytic enzyme, which is synthesized and folded in the endoplasmic reticulum, matures in the Golgi apparatus, and becomes fully functional in the melanosome for melanin synthesis and storage.3−5 However, the enzyme will remain inactive until it is phosphorylated at two serine residues by the action of protein kinase C-β (PKC-β).2 In addition to tyrosinase, two other proteins, tyrosinase-related proteins 1 and 2 (TRP-1 and TRP-2), are involved in the process of melanogenesis. TRP-1 exhibits tyrosinase activity and forms a complex with the enzyme to induce melanin formation in murine melanocytes.2 TRP-2 catalyzes the conversion of dopachrome toward the formation of 5,6-dihydroxyindole and regulates the eumalanin antioxidant properties.6 The expression of these melanogenic proteins is regulated by a key transcription factor, the microphthalmia-associated transcription factor (MITF).7 In addition to its role as a transcriptional regulator, MITF is involved in melanosomal protein transport; it also activates a number of genes in a wide range of biological activities, e.g., cell growth and survival.7 Thus, the regulation of MITF has become a strategic target for controlling pigmentation, and herbal extracts and their ingredients have been reported to modulate its expression and activity.8
The dried tubers of Polygonum multiflorum Thunb. (Polygonaceae) (“He-Shao-Wu” in Chinese medicine) is used commonly as a tonic for the improvement of general body strength, and it is allegedly able to provide a rejuvenation effect during the aging process including the darkening of gray hair. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucopyranoside (1) is a water-soluble component extracted from this plant drug, and in the literature, the compound has been reported to induce pigmentation in melanoma cells through an induction of the cAMP–MITF–tyrosinase pathway.9,10 However, contrary to such observations, a considerable amount of literature data has been documented on the melanogenesis inhibitory activity of stilbenes and their glycosidic derivatives.1,11 It was, therefore, the objective of this study to verify the biological activities of 1 using mouse melan-a cells.
Results and Discussion
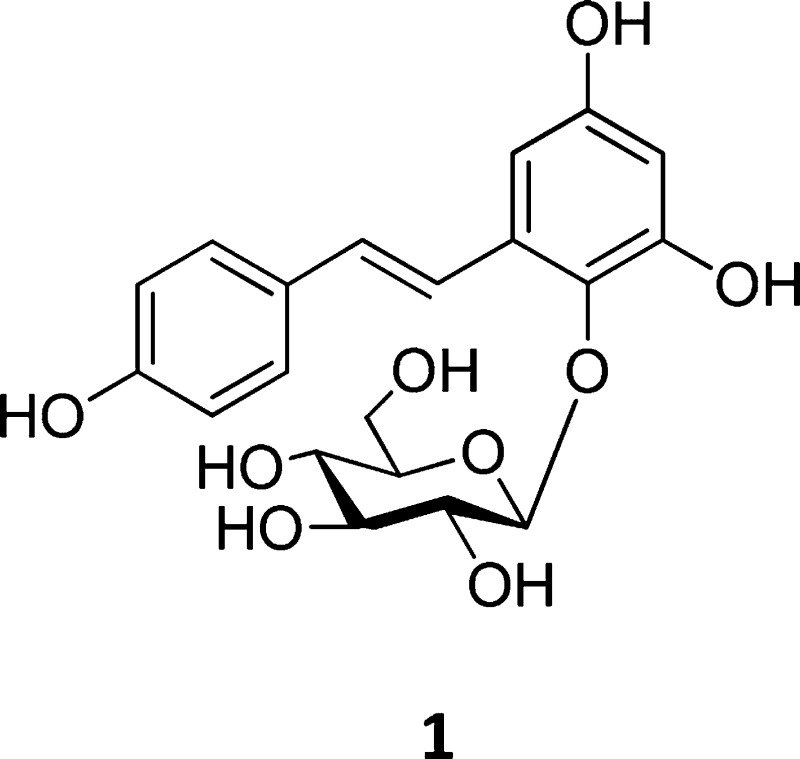
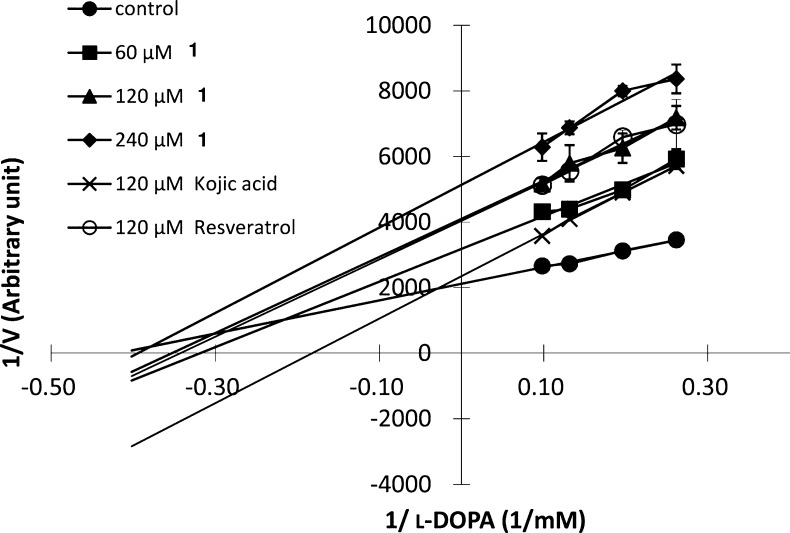
The inhibition of 1 on cell-free melan-a tyrosinase was demonstrated by a Lineweaver–Burk plot. The results shown in Figure 1 revealed both 1 and resveratrol to be noncompetitive inhibitors of tyrosinase, with kojic acid being a competitive inhibitor. Thus, the addition of 1 to the reaction mixture led to a reduction of the maximal velocity (Vmax = 4.7 × 10–4) and Km value (2.4 mM l-DOPA) of tyrosinase activity. The Vmax value decreased in a dose-dependent manner from 3.1 × 10–4 to 2.4 × 10–4 and 2.0 × 10–4 in the presence of 60, 120, and 240 μM of 1, respectively. The Ki value also decreased from 3.1 to 2.8 and 2.5 mM, respectively. Resveratrol, a stilbenoid of the same oxidative level as 1, exhibited comparable Ki and Vmax values at 120 μM (Table 1). These results are in agreement with a previous observation that the tyrosinase inhibitory activity of stilbenes varies with the extent of hydroxylation, having more potent inhibitory activity with a higher degree of hydroxylation.12 A pretreatment experiment was also performed in which tyrosinase was mixed with 1 for 24 h prior to kinetic analysis. The Ki and Vmax values of pretreated samples (data not shown) were similar to those without the pretreatment. This finding suggests that the binding between 1 and tyrosinase is likely reversible. Taken together, the available evidence indicated 1 to be a reversible noncompetitive inhibitor of melan-a tyrosinase showing a higher potency than kojic acid (Table 1).
Figure 1.
Inhibitory effects of 60, 120, and 240 μM 1, 120 μM kojic acid, or 120 μM resveratrol on tyrosinase activity in murine melan-a cells. The Vmax and Km values in the absence (control) or presence of 1 with l-DOPA as the substrate are analyzed using Lineweaver–Burk plots.
Table 1. Kinetic Parameters of Tyrosinase in the Presence of 1, Resveratrol, and Kojic Acida.
| compound | Km (M) | Vmax (ΔA490/s) | Ki (M) | |
|---|---|---|---|---|
| none | 2.4 × 10–3 | 4.7 × 10–4 | ||
| 1 | 60 μM | 3.1 × 10–4 | 3.1 × 10–3 | |
| 1 | 120 μM | 2.4 × 10–4 | 2.8 × 10–3 | |
| 1 | 240 μM | 2.0 × 10–4 | 2.5 × 10–3 | |
| resveratrol | 120 μM | 2.5 × 10–4 | 2.9 × 10–3 | |
| kojic acid | 120 μM | 4.3 × 10–4 | 5.5 × 10–3 | |
The kinetic parameters are obtained with l-DOPA as a substrate using the Lineweaver–Burk plot. Km (Michaelis constant) and Ki (inhibition constant) values are represented as molar concentration, and Vmax (maximum velocity) values are expressed as the change of absorbance at 490 nm per second.
Forskolin and 12-O-tetradecanoylphorbol-13-acetate (TPA) are inducers of melanogenesis via the PKA and PKC pathways, respectively.13 The present results showed that 1 reduces tyrosinase activity in melan-a cells in a dose-dependent manner in the presence or absence of these melanogenesis inducers (Figure 2a). In the presence of forskolin, the tyrosinase activity was reduced from 162% to 103% with 60 μM 1. Similarly, the enzyme activity was reduced from 100% to 65% in the presence of TPA. These results suggest that 1 is a more potent blocker of the PKA melanogenic pathway than the PKC pathway. Possible inhibitory mechanisms of 1 include the inhibition of melanocortin-1 receptor from forskolin binding, inhibition of cAMP synthesis, or inactivation of cAMP responding element (CRE) binding protein. Further study is warranted to elucidate the inhibitory mechanism of 1 on the PKA pathway.13
Figure 2.
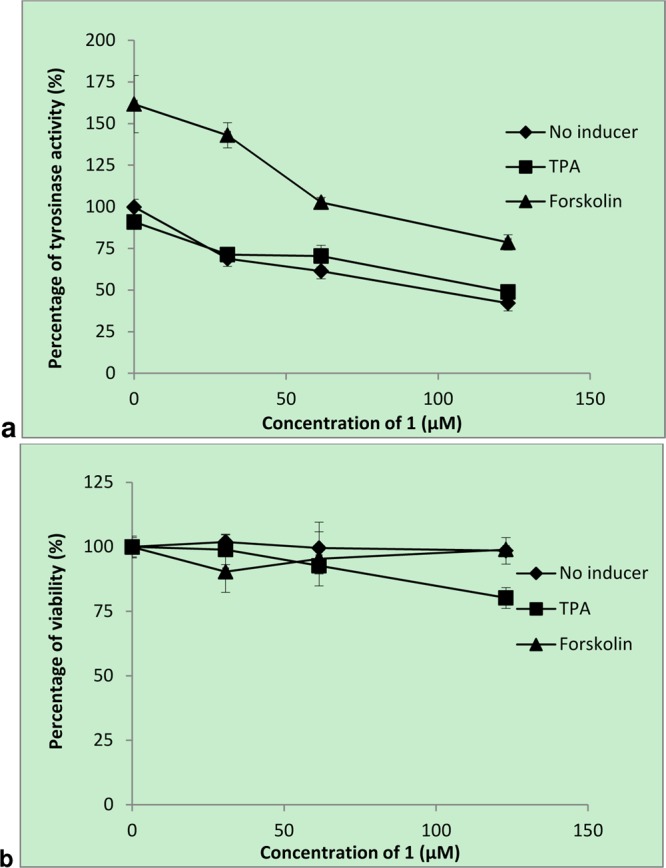
Tyrosinase inhibitory and cytotoxic activities of 1 on melan-a melanocytes. Cells were incubated with or without 1 in the medium containing 20 nM 12-O-tetradecanoylphorbol-13-acetate (TPA), 1 μM forskolin, or vehicle alone for 3 days. Tyrosinase activity was measured by l-DOPA oxidation (a), and cell viability was determined by the sulforhodamine B assay (b). The values are denoted as mean ± SD of triplicate experiments.
Among stilbene compounds, resveratrol and oxyresveratrol have demonstrated in vitro and in vivo activities against mammalian tyrosinase.1,14 The number and location of the hydroxy group(s), as well as the trans-olefin structure of the stilbene skeleton, have been shown to be associated with the inhibitory properties on murine tyrosinase.15 On the other hand, glycosylation of the hydroxy group did not show a significant effect.15,16 In our hands, 1 was the least cytotoxic compound among the hydroxystilbenes tested including resveratrol, oxyresveratrol, and picetanneol (Supporting Information, Figure S1). Moreover, it did not exert any cytotoxic effect in samples with or without forskolin (Figure 2).
Tyrosinase is biosynthesized in the endoplasmic reticulum (ER). The nascent tyrosinase core is a polypeptide of 60 kDa that is N-glycosylated to become a 70 kDa folding intermediate and further post-translationally modified to a mature form of 80 kDa in the Golgi apparatus.3 In cases where the polypeptide is misfolded or not properly glycosylated, it will remain bound to calnexin in the ER until a quality glycoprotein intermediate is formed. If there is misfolding or aberration trafficking from the late endosome to melanosome, the enzyme will likely be trapped in the lysosome.17 Consistent with the observations made by others,17,18 functional tyrosinase colocalizes with TRP-1 (Figure 3a and b), but not with Lamp-1 of the lysosome (Figure 3c and d) or calnexin of the ER (Figure 3f and g), regardless of the presence or absence of 1. These findings suggest that no aberrant tyrosinase is trapped in the ER and lysosome in 1-treated cells and tyrosinase is properly transported outside to the melanosomes where it forms a complex with TRP-1. As a positive control, calnexin and tyrosinase clearly colocalize to the ER in resveratrol-treated cells (Figure 3h), because resveratrol is capable of retarding the post-translational-modified tyronsinase in the ER.19 Colocalization of both tyrosinase and Lamp-1 were also observed (Figure 3e) in progesterone-treated cells in which misfolded tyrosinase was induced and retarded in the lysosomes.17
Figure 3.
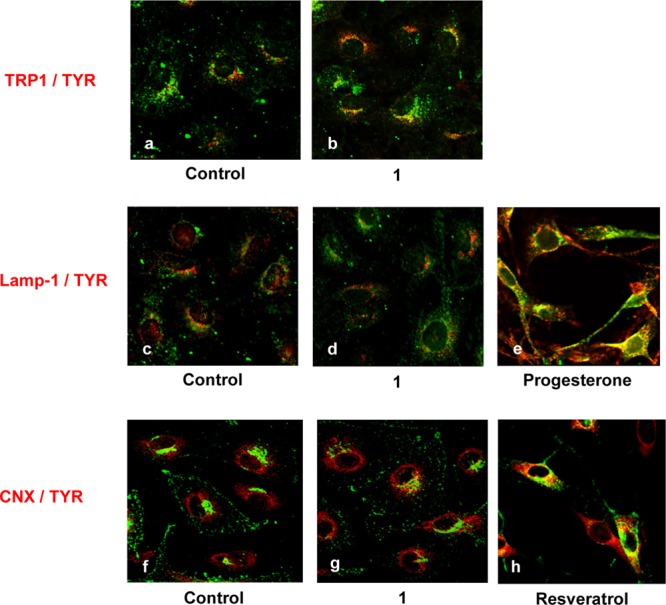
Confocal immunofluorescence microscopy of melan-a melanocytes incubated in the presence of 1, progesterone, or resveratrol. The locations of TRP-1 (red, a, b), Lamp-1 (red, c–e), or calnexin (CNX, red, f–h) are colabeled with tyrosinase (green). Overlay of the red and green images resulted in a yellow fluorescence, indicating strong colocalization of the targeted proteins. Cells were treated with vehicle alone (a, c, f), or 1 (60 μM, b, d, g) for 3 days, progesterone (15 μM) for 4 days (e), or resveratrol (85 μM) for 1 day (h).
Glycosylation analysis further confirmed the normal trafficking of tyrosinase in both untreated and 1-treated cells (Figure 4). The immature oligosaccharide added to tyrosinase in the ER can be digested only with endoglycosidase H (Endo H), whereas both mature and immature oligosaccharide added to tyrosinase in the Golgi apparatus can be removed by peptide-N-glycosidase F (PNGase F).5 Results of Western blotting revealed two bands: an upper band at 80 kDa for the mature and Endo H-resistant form of tyrosinase and a lower band at 60 kDa for the immature and Endo H-sensitive form (Figure 4). No immature Endo H-sensitive form of tyrosinase was present in both the untreated control and 1-treated murine cells after being digested with Endo H. These findings agree with the immunofluorescence results to indicate that 1 does not interfere with tyrosinase maturation in the ER, Golgi apparatus, and protein trafficking to the melanosome. The deglycosylated tyrosinase digested by PNGase F (60 kDa) represented the total amount of tyrosinase.
Figure 4.

Endoglycosidase H sensitivity of tyrosinase from 1-treated samples. Protein lysates were obtained from 1-treated melan-a cells in forskolin-supplemented medium. The lysates were incubated with either peptide-N-glycosidase F (PNGase F) or endoglycosidase H (Endo H) following the manufacturer’s instruction. The Western blot was analyzed for the amounts of glycosylated or deglycosylated tyrosinase. The upper band at 80 kDa and the lower band at 60 kDa represent the mature Endo H-resistant form and immature Endo H-sensitive form of tyrosinase, respectively. Identical results were obtained in a second independent experiment.
Stilbene derivatives such as resveratrol and 2,2′-dihydroxy-5,5′-dipropylbiphenyl have been associated with the unfolded protein response that triggers ER stress and subsequent apoptosis,19 but 1 seems to induce neither ER stress nor lysosomal storage stress as suggested by the above results. Despite the structural similarity between 1 and resveratrol, they do not share the same canonical maturation retardation and apoptotic properties.19 Being a weak acid as conferred from its phenolic groups, and unlike basic compounds such as quinolines that would alter the lysosomal pH and retard lysosomal function,201 appears not to alter the acidic lysosomal pH, or otherwise it would have resulted in lysosomal storage stress.
Melanogenesis is controlled by MITF, a key regulator of a number of melanogenic proteins such as tyrosinase, TRP-1, and TRP-2. Since melanogenesis is significantly increased through the forskolin–PKA pathway (Figure 2), the inhibitory action of 1 on these proteins was analyzed further in forskolin-supplemented melan-a cells. It was found that 1 suppressed the expression of MITF and tyrosinase in a dose-dependent manner, but the reduction of TRP-1 expression was not prominent (Figure 5). Such a down-regulation of MITF and tyrosinase protein could explain, at least partially, the reduction of l-DOPA oxidation with increasing doses observed in Figure 2a.
Figure 5.
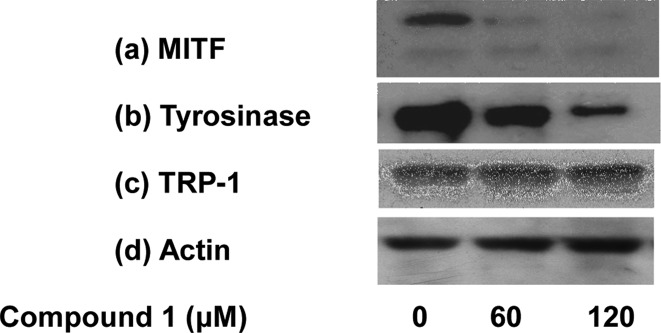
Expression of melanogenesis-related proteins in melan-a melanocytes after 1 treatment for 1 day in FSK-supplemented medium. Cell lysates were prepared from treated melanocytes following 1 treatment. The expressions of MITF (a), tyrosinase (b), and TRP-1 (c) were analyzed by Western blotting, and the expression of melanogenic proteins relative to actin is shown in panel (d).
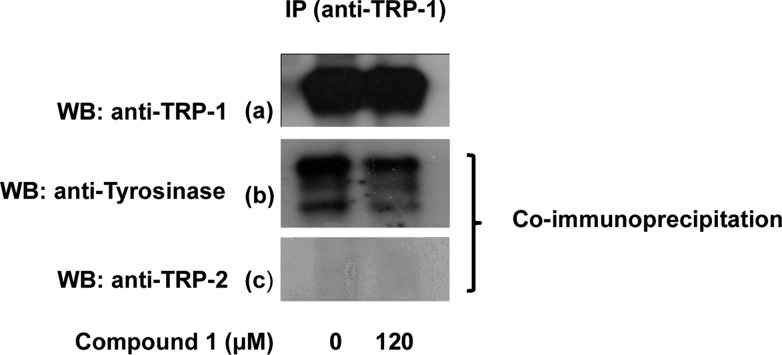
TRP-1 influences tyrosinase activity by stabilizing and forming complexes with tyrosinase. Without stabilization from TRP-1 during the processing and intracellular trafficking, tyrosinase will be prompted to degrade in the proteasome, leading to a reduction of pigment formation.21 This phenomenon has been demonstrated in mutant brown melan-b melanocytes in which the TRP-1 locus is mutated.3,22 Co-immunoprecipitation using antibodies against TRP-1 resulted in a reduction of immunoprecipitated tyrosinase after treatment with 1 (Figure 6b), whereas TRP-2 was not pulled down together with tyrosinase (Figure 6c). These results are in agreement with the findings of Hearing and associates that TRP-1 forms a complex with tyrosinase but not with TRP-2.22 Therefore, reduction of tyrosinase activity after treatment with 1 was not only caused by the down-regulation of tyrosinase expression but also affected by the reduction of tyrosinase/TRP-1 complex formation.
Figure 6.
Association of tyrosinase and TRP-1 in melan-a melanocytes after treatment with 1 for 3 days in forskolin-supplemented medium. Co-immunoprecipitation with antibody against TRP-1 proteins showed that TRP-1 (a) and tyrosinase (b), but not TRP-2 (c), proteins were co-immunoprecipitated.
In conclusion, compound 1 displayed antimelanogenic activity in murine melanocytes. It noncompetitively inhibited the enzyme activity of tyrosinase, down-regulated the expression of melanogenic proteins, and reduced the tyrosinase/TRP-1 complex formation. Studies of the antimelanogenic properties of 1 and its analogues in the future using human melanocytes are needed to confirm these activities.
Experimental Section
General Experimental Procedures
2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucopyranoside (1) was isolated from Polygonum multiflorum, and a larger quantity was obtained from the Hong Kong Jockey Club Institute of Chinese Medicine; the purity was determined to be >98% by HPLC. Kojic acid, forskolin, 12-O-tetradecanoylphorbol-13-acetate, progesterone, and resveratrol were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). All antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX, USA), unless otherwise specified.
Cell Culture
Mouse melan-a melanocytes derived from C57BL/6 mice were a gift from Professor D. C. Bennett (St George’s Hospital Medical School, London, UK). Cells were cultured in RPMI-1640 medium supplemented with 5% FBS, 100 IU/mL penicillin (Sigma), 100 μg/mL streptomycin sulfate (Sigma), and 200 nM TPA (Sigma) for cell attachment and proliferation. During treatment, cells were cultured in the absence of TPA unless specified. Cells used in the experiments were restricted to passages of <30 as recommended by the donor.
Cell Viability and Tyrosinase Activity
The sulforhodamine B (SRB, Sigma) colorimetric cytotoxicity assay was adopted in this study. Briefly, melan-a melanocytes (20 000 cells/0.1 mL/well) were treated with the test compounds in 96-well Corning Costar plates for 72 h. The reaction was stopped and subjected to SRB cytotoxicity assays as described previously.5 The 3,4-dihydroxy-l-phenylalanine (l-DOPA) (Sigma) conversion assay is a colorimetric assay in which the oxidation rate of l-DOPA specifically reflects the tyrosinase activity. Tyrosinase inhibitory property of 1 in the presence of various melanogenic inducers was investigated under the same conditions. After treatment, one volume cell lysate in 20 mM Tris/0.1% Triton X-100 (pH 6) reacted with one volume 1 μg/mL l-DOPA. The absorbance of enzymatic product was measured at 490 nm. Data represent the mean values and standard deviations of triplicate assays in at least three separate experiments.
Kinetic Analysis of Tyrosinase Activity
The melan-a cells were rinsed with phosphate buffer saline twice, then lysed with 20 mM Tris/0.1% Triton X-100 (pH 7). The protein lysate (50 μg) was preincubated with the test compounds, i.e., 1, resveratrol, or kojic acid, for 10 min at room temperature before reacting with l-DOPA (pH 6). After preincubation, l-DOPA was added and crimson dopachrome was produced. The rate of dopachrome formation was measured at 490 nm per 20 s for a period of 10 min at 37 °C using a FLUOstar microplate reader (BMG Labtech, Ortenberg, Germany). The tyrosinase activity was determined based on the rate of l-DOPA oxidation (absorbance at 490 nm per second); Michaelis–Menten constants (Km) and maximal velocity (Vmax) were calculated from the Lineweaver–Burk plot.23
Immunofluorescence Microscopy
For colocalization of tyrosinase and TRP-1, cells cultured on coverslips were fixed in 100% methanol, permeablized with 0.2% Triton X-100, and blocked in 2% bovine serum albumin before subjected to confocal immunofluorescence microscopy. The procedures for costaining tyrosinase with organelle marker proteins, i.e., Lamp-1 and calnexin (Sigma) for lysosome and endoplasmic reticulum, respectively, have been described in a previous paper.5
Immunoblotting Analysis
Cells cotreated with 1 μM forskolin with or without 1 before total proteins were extracted with PhosphoSafe extraction buffer (Novagen, Darmstadt, Germany). The proteins were resolved on a 10% SDS-PAGE gel and transferred onto a PVDF membrane using a Semidry Transfer Cell (Bio-Rad, Hercules, CA, USA). The blot was blocked with 3% bovine serum albumin before incubation with primary antibodies against tyrosinase, TRP-1 (generous gift from Dr. V.J. Hearing, NIH, Bethesda, MD, USA), TRP-2, MITF (Thermo Fisher Scientific, Pittsburgh, PA, USA), or β-actin. Signals were detected by incubating the blot for 1 h at room temperature with secondary antibodies, horseradish peroxidase-conjugated goat anti-mouse, or donkey anti-goat, or goat anti-rabbit (Dako Cytomation, Glostrup, Denmark), and visualized using an enhanced chemiluminescence system (ECL, GE Healthcare, Lower Chalfont, UK). For reprobing with other primary antibodies, previous antibodies were stripped away using the Reblot Plus Western blot recycling kit (Millipore, Temecula, CA, USA).
Glycosylation Analysis
Protein lysates (10 μg) obtained in the immunoblotting analyses were digested with either 1000 units of endoglycosidase H (New England Biolabs, Beverly, MA, USA) or 1000 units of peptide-N-glycosidase F (New England Biolabs) according to the manufacturer’s instruction. In brief, the protein lysates were subjected to denaturation by treating with glycoprotein denaturing buffer at 100 °C for 10 min. The denatured lysates were incubated with Endo H or PNGase F at 37 °C for 1 h and then subjected to Western blotting analysis using anti-TYR antibody.5
Co-immunoprecipitation
Tyrosinase and TRP-1 proteins were co-immunoprecipitated using a Dynabeads co-immunoprecipitation kit with minor modifications (Invitrogen, Grand Island, NY, USA). Briefly, harvested cell pellets were lysed with immunoprecipitation lysis buffer supplemented with 0.1 M NaCl, 0.5 mM PMSF, and 10 μg/mL apotinin on ice for 30 min. The extracted proteins of the same amount from 1-treated or untreated samples (1 mg each) were reacted with antibody-coupled Dynabeads overnight and separated by SDS-PAGE analysis. Following protein transfer on PVDF blot, melanogenic proteins were detected by corresponding antibodies using ECL Plus (GE Healthcare) or Supersignal West Dura chemiluminescent substrate (Thermo Fisher Scientific).
Acknowledgments
The authors thank Dr. M. Zhou and Dr. K. S. H. Tsoi for technical assistance. Professor D. C. Bennett (St George’s Hospital Medical School, London, UK) and Dr. V. J. Hearing (National Institutes of Health, Bethesda, MD) are acknowledged for the generous gifts of melan-a cells and TRP-1 antibody, respectively.
Supporting Information Available
Figure S1 showing the cytotoxic activity of compound 1 on murine melan-a melanocytes is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Chang T. S. Int. J. Mol. Sci. 2009, 10, 2440–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.; Park H. Y. Biochem. Biophys. Res. Commun. 2003, 311, 948–953. [DOI] [PubMed] [Google Scholar]
- Ando H.; Kondoh H.; Ichihashi M.; Hearing V. J. J. Invest. Dermatol. 2007, 127, 751–761. [DOI] [PubMed] [Google Scholar]
- Schallreuter K. U.; Hasse S.; Rokos H.; Chavan B.; Shalbaf M.; Spencer J. D.; Wood J. M. Exp. Dermatol. 2009, 18, 680–688. [DOI] [PubMed] [Google Scholar]
- Cheung F. W.; Guo J.; Ling Y. H.; Che C. T.; Liu W. K. Mar. Drugs 2012, 10, 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzella L.; Napolitano A.; d’Ischia M. Pigm. Cell Melanoma Res. 2011, 24, 248–249. [DOI] [PubMed] [Google Scholar]
- Vachtenheim J.; Borovanský J. Exp. Dermatol. 2010, 19, 617–627. [DOI] [PubMed] [Google Scholar]
- Kim D. S.; Park S. H.; Kwon S. B.; Li K.; Youn S. W.; Park K. C. Arch. Pharm. Res. 2004, 27, 334–339. [DOI] [PubMed] [Google Scholar]
- Guan S.; Su W.; Wang N.; Li P.; Wang Y. Phytother. Res. 2008, 22, 660–663. [DOI] [PubMed] [Google Scholar]
- Jiang Z.; Xu J.; Long M.; Tu Z.; Yang G.; He G. Life Sci. 2009, 85, 345–350. [DOI] [PubMed] [Google Scholar]
- Chang S. T. Materials 2012, 5, 1661–1685. [Google Scholar]
- Yokozawa T.; Kim Y. J. Biol. Pharm. Bull. 2007, 30, 2007–2011. [DOI] [PubMed] [Google Scholar]
- Buscà R.; Ballotti R. Pigm. Cell Res. 2000, 13, 60–69. [DOI] [PubMed] [Google Scholar]
- Smit N.; Vicanova J.; Pavel S. Int. J. Mol. Sci. 2009, 10, 5326–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohguchi K.; Tanaka T.; Kido T.; Baba K.; Iinuma M.; Matsumoto K.; Akao Y.; Nozawa Y. Biochem. Biophys. Res. Commun. 2003, 307, 861–863. [DOI] [PubMed] [Google Scholar]
- Kim Y. M.; Yun J.; Lee C. K.; Lee H.; Min K. R.; Kim Y. J. Biol. Chem. 2002, 277, 16340–16344. [DOI] [PubMed] [Google Scholar]
- Hall A. M.; Krishnamoorthy L.; Orlow S. J. Pigm. Cell Res. 2003, 16, 149–158. [DOI] [PubMed] [Google Scholar]
- Halaban R.; Svedine S.; Cheng E.; Smicun Y.; Aron R.; Hebert D. N. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 5889–5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton R. A.; Cook A. L.; Roberts D. W.; Leonard J. H.; Sturm R. A. J. Invest. Dermatol. 2007, 127, 2216–2227. [DOI] [PubMed] [Google Scholar]
- Ni-Komatsu L.; Tong C.; Chen G.; Brindzei N.; Orlow S. J. Mol. Pharmacol. 2008, 74, 1576–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedine S.; Wang T.; Halaban R.; Hebert D. N. J. Cell Sci. 2004, 117, 2937–2949. [DOI] [PubMed] [Google Scholar]
- Kobayashi T.; Hearing V. J. J. Cell Sci. 2007, 120, 4261–4268. [DOI] [PubMed] [Google Scholar]
- Kim J. H.; Baek S. H.; Kim D. H.; Choi T. Y.; Yoon T. J.; Hwang J. S.; Kim M. R.; Kwon H. J.; Lee C. H. J. Invest. Dermatol. 2008, 128, 1227–1235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.