Abstract
Importance
Modifying levels of factors associated with age-related macular degeneration (AMD) may decrease risk of visual impairment in older persons.
Objective
To examine the relationships of markers of inflammation, oxidative stress, and endothelial dysfunction to the 20-year cumulative incidence of early AMD.
Design
Longitudinal population-based cohort study.
Setting
Beaver Dam, Wisconsin.
Participants
A random sample of 975 persons in the Beaver Dam Eye Study without signs of AMD who participated in the baseline examination in 1988-1990 and up to four follow-up examinations in 1993-1995, 1998-2000, 2003-2005, and 2008-2010.
Exposures
Serum markers of inflammation (high sensitivity C-reactive protein [hsCRP], tumor necrosis factor-α receptor 2 [TNF-αR2], interleukin-6 [IL-6], and white blood cell count), oxidative stress (8-isoprostane and total carbonyl content), and endothelial dysfunction (soluble vascular cell adhesion molecule-1 [sVCAM-1] and soluble intercellular adhesion molecule-1) were measured. Interactions with Complement Factor H (rs1061170) and Age-Related Maculopathy Susceptibility 2 (rs10490924), C3 (rs2230199) and C2/CFB (rs4151667) were examined using multiplicative models. AMD was assessed from fundus photographs.
Main Outcome Measure
Early AMD defined by the presence of any size drusen and the presence of pigmentary abnormalities, or by the presence of large-sized drusen (≥125 μm diameter), in the absence of late AMD.
Results
The 20-year cumulative incidence of early AMD was 23.0%. Adjusting for age, sex, and other risk factors, hsCRP (odds ratio [OR] comparing 4th to 1st quartile 2.18, P=0.005), TNF-αR2 (1.78, P=0.04), and IL-6 (1.78, P=0.03) were associated with the incidence of early AMD. Increased incidence of early AMD was associated with sVCAM-1 (OR per standard deviation on the log ng/mL scale 1.21, P=0.04).
Conclusions and Relevance
We found modest evidence of relationships of serum hsCRP, TNF-αR2, and IL-6 and sVCAM-1 to the 20-year cumulative incidence of early AMD independent of age, smoking status, and other factors. It is not known whether these associations represent a cause and effect relationship or if other unknown confounders accounted for the findings. Even if inflammatory processes are a cause of early AMD, it is not known whether interventions that reduce systemic inflammatory processes will reduce the incidence of early AMD.
Introduction
Age-related macular degeneration (AMD), the most common cause of severe loss of vision in older persons of European ancestry, is a multifactorial disease with strong evidence of genetic determinants.1-6 Age, smoking, physical activity, and obesity have been found in most studies to be related to the incidence of AMD.6 Inflammation, oxidative stress, and endothelial dysfunction are also among the many host and environmental influences that have been hypothesized to affect the incidence and progression of AMD.7-25 There is a strong biological rationale supporting a role of inflammation, oxidative stress, and, to a lesser extent, endothelial dysfunction in the development and progression of AMD. There is accumulating evidence of a relationship of high sensitivity C-reactive protein (hsCRP) to late AMD but less consistent evidence of a similar relationship to the incidence of early AMD.26-36 Fewer epidemiological studies have examined the relationships of other systemic markers of inflammation (e.g., tumor necrosis factor-alpha receptor 2 [TNF-αR2]),32-34 oxidative stress,37 and endothelial dysfunction32,34,38 to the incidence of AMD.
In an earlier prospective substudy in the Beaver Dam Eye Study (BDES) cohort, we found no relationships of markers of systemic inflammation and endothelial dysfunction to the 10-year cumulative incidence of early AMD.33 Since that report, we have genotyped AMD candidate gene single nucleotide polymorphisms (SNPs) including Complement Factor H (CFH rs1061170), Age-Related Maculopathy Susceptibility 2 (ARMS2 rs10490924), Complement Component 2/Complement Factor B (C2/CFB rs4151667) and Complement Component 3 (C3 rs2230199) and remeasured the same markers as well as systemic markers of oxidative stress present in a random sample of the BDES cohort. We hypothesized that elevated levels of markers of systemic inflammation in the presence of 1 or 2 variant alleles for CFH rs1061170, ARMS2 rs10490924, C2/CFB rs4151667 and C3 rs2230199 and higher levels of markers of oxidative stress and endothelial dysfunction would be associated with greater risk of developing early AMD.
Methods
Population
Methods used to identify and descriptions of the population have appeared in previous reports.39-43 Of the 5,924 eligible persons identified by a private census, 4,926 (83%) persons aged 43-86 years participated in the baseline examination in 1988-1990. Ninety-nine percent of the population was white. The cohort was re-examined at 5- (n=3,722), 10- (n=2,962), 15- (n=2,375) and 20-year (n=1,913) follow-up examinations.40-43 There was greater than 80% participation among survivors at each examination.
All data were collected with Institutional Review Board approval from the University of Wisconsin-Madison in conformity with all federal and state laws, the work was compliant with the Health Insurance Portability and Accountability Act, and the study adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from each participant before every examination. Comparisons between participants and nonparticipants at each examination have appeared elsewhere.39-43 In general, those who participated in the follow-up were more likely to be younger than nonparticipants who were alive or those who died, and, while adjusting for age, were less likely to have AMD.
Procedures
A standardized interview and examination were administered at each visit. Information on demographic characteristics, medication use including history of use of lipid-lowering drugs by type and use of steroidal and nonsteroidal anti-inflammatory drugs (NSAIDs), and history of smoking and physical activity was obtained by questionnaire. Body weight and height were measured. Similar procedures were followed at baseline and follow-up examinations.44
Casual blood specimens were obtained at the baseline examination. An aliquot of blood was used immediately to determine the white blood cell (WBC) count. Remaining serum was stored for up to 17 years until being shipped on dry ice to the University of Minnesota laboratory for measurement of markers of inflammation (hsCRP, interleukin-6 [IL-6], and TNF-αR2), oxidative stress (8-isoprostane [8-ISO], an indicator of lipid oxidation, and total carbonyl content [TCC], an indicator of the amount of protein that has been oxidized by highly reactive free radicals), and endothelial dysfunction (soluble vascular cell adhesion molecule-1 [sVCAM-1] and soluble intercellular adhesion molecule-1 [sICAM-1]). The eMethods describe procedures to measure these markers and their inter-assay coefficients of variation as well as measurements of candidate gene SNPs.45
Fundus Photography and Grading
Stereoscopic 30° color film fundus photographs centered on the macula (Diabetic Retinopathy Study standard field 2) were taken of each eye.44,46,47 Gradings were performed for the pair of photographs of each macula at each examination using the Wisconsin Age-Related Maculopathy Grading System.46-51 Graders were masked to any information regarding the participant and the fellow eye.
Definitions
The severity of AMD was determined using the 5-step Three Continent AMD Consortium Severity Scale.52 Individuals were considered not to have AMD if both eyes had either hard drusen or small soft drusen (<125 μm in diameter) only, regardless of area of involvement and no pigmentary abnormalities (defined as increased retinal pigment or retinal pigment epithelial [RPE] depigmentation present); or no definite drusen with any pigmentary abnormality. Early AMD was defined by the presence of any sized drusen and the presence of any pigmentary abnormality; or by presence of large-sized drusen (≥125 μm in diameter), regardless of area of involvement, in the absence of late AMD defined by the presence of pure geographic atrophy (GA) or exudative macular degeneration. When one eye was ungradable, it was assumed to have the same AMD severity as the fellow eye.
Persons at risk for developing early AMD were those without early AMD in either eye at baseline. Incidence of early AMD was defined by developing signs of early AMD in at least 1 eye when both eyes had no AMD at the baseline examination. Incidence was determined for signs of early AMD, e.g., large drusen size ≥125 μm, drusen type (soft indistinct/reticular), and pigmentary abnormalities (increased retinal pigment and RPE depigmentation). Due to limited power, we did not examine the relationship of the markers and risk of developing late AMD.
All covariates were measured at baseline. Age was categorized into four groups: 43-54 years, 55-64 years, 65-74 years and 75 or more years. Body mass index (BMI) was calculated by dividing a participant's weight in kilograms by their height in meters squared. Obesity was defined as a BMI of 30 kg/m2 or greater. Current smokers were identified as persons having smoked 100 or more cigarettes in their lifetime and smoking at the time of the examination. Participants were considered physically active if they engaged in physical activity long enough to work up a sweat at least once per week. Use of statin drugs, steroidal anti-inflammatory drugs, and NSAIDs were determined from self-report.
Statistical Analysis
All analyses were performed with SAS version 9.2 (SAS Institute, Cary, North Carolina, USA). Cumulative incidence was estimated by the product-limit method,53 accounting for the competing risk of death.54 Discrete logistic hazard regression55 was used to estimate odds ratios (ORs) for associations between each marker of inflammation, oxidative stress, and endothelial dysfunction with incidence of early AMD, incidence of large drusen ≥125 μm in diameter, incidence of soft indistinct or reticular drusen, and incidence of pigmentary abnormalities. Each marker was examined using a natural logarithmic transformation and categorized into quartiles. P-values are reported per standard deviation (SD) increase on the logarithmic scale, for each higher quartile compared to the first quartile, and for a trend per increasing quartile. Models first adjust only for age and sex. Then models additionally adjust for smoking status, physical activity, BMI, statin use, and anti-inflammatory medication use. ORs were estimated for associations of having 1 and 2 versus 0 risk alleles of CFH and ARMS2 and having 1 or 2 versus 0 risk alleles for C3 and C2/CFB with the incidence of early AMD adjusting for the same factors. P-values were estimated for the relationship of age (older 2 versus younger 2 age groups), sex, obesity, current smoking, and physical activity to each marker using the Mann-Whitney U test. To test for interactions, we first modeled a multiplicative interaction between each inflammatory, oxidative stress, and endothelial dysfunction marker and having 0, 1, or 2 risk alleles for CFH and ARMS2 and having 0 or 1 or 2 risk alleles for C3 and C2/CFB; we then examined each relationship by stratifying by genotype for each SNP.
Change in area under the receiver operating characteristic curve (AUC) was used to measure improvement in prediction when a marker (e.g., hsCRP, modeled as trend per SD on the logarithmic scale) was added to a model based on traditional AMD risk factors and to a model based on traditional risk factors plus candidate SNPs using the method described by DeLong and colleagues.56
Results
Of the 4,926 BDES participants at baseline, 1,793 were included as part of a random sample in a sub-study of chronic kidney disease. Age, sex, BMI, history of smoking status, history of sedentary lifestyle, history of use of NSAIDs, presence of early and late AMD, and the distributions of the AMD candidate genotypes did not differ between those included in the random sample and those excluded, except for CFH variant allele (59% in those included vs. 62% in those excluded, P=0.03, eTable 1). To be included in analyses, a participant from the random sample must have had measures of markers of inflammation (hsCRP, IL-6, TNF-αR2, and WBC count), oxidative stress (8-ISO and TCC), and endothelial dysfunction (sVCAM-1 and sICAM-1), relevant genetic data, and no AMD at baseline as assessed from 30° stereoscopic color fundus photographs. Each participant also must have had at least one follow-up visit with photographs where at least one eye was gradable for AMD. Characteristics of the 975 persons who met these criteria and were included in analyses and those excluded are described in Table 2.
Table 2.
Characteristics of Participants Included and Excluded from Analysis.
| Measure | Included | Excluded | P valuea | ||
|---|---|---|---|---|---|
|
| |||||
| N | Mean ± SD, Median or % | N | Mean ± SD, Median or % | ||
| Mean age, years | 975 | 58.1 ± 9.8 | 3951 | 63.0 ± 11.3 | <.0001 |
| Sex, % men | 421 | 43.2 | 1743 | 44.1 | 0.60 |
| Mean BMI, kg/m2 | 972 | 28.8 ± 5.6 | 3909 | 28.7 ± 5.4 | 0.92 |
| Smoking status, % | |||||
| Former | 334 | 34.3 | 1413 | 35.8 | 0.63 |
| Current | 205 | 21.0 | 765 | 19.4 | 0.37 |
| Sedentary lifestyle, % | 707 | 72.5 | 3068 | 77.7 | 0.001 |
| Using NSAIDs, % | 303 | 31.1 | 1340 | 33.9 | 0.09 |
| CFH genotype, % | |||||
| C/T | 408 | 44.4 | 1701 | 48.2 | 0.009 |
| C/C | 115 | 12.5 | 486 | 13.8 | 0.06 |
| ARMS2 genotype, % | |||||
| G/T | 346 | 36.4 | 1245 | 34.5 | 0.45 |
| T/T | 35 | 3.7 | 193 | 5.3 | 0.05 |
| C2FB rs4151667 genotype, % | |||||
| T/A | 42 | 6.4 | 223 | 9.9 | 0.006 |
| A/A | 0 | 0.0 | 8 | 0.4 | 0.97 |
| C3 rs2230199 genotype, % | |||||
| C/G | 217 | 33.0 | 699 | 30.9 | 0.33 |
| G/G | 25 | 3.8 | 94 | 4.2 | 0.79 |
| Median hsCRP, mg/L | 956 | 1.9 | |||
| Median TNF-αR2, pg/mL | 950 | 2295.2 | |||
| Median IL-6, pg/mL | 944 | 2.1 | |||
| Median WBC count, 1000/μL | 957 | 7.0 | |||
| Median 8-ISO, pg/mL | 961 | 120.7 | |||
| Median TCC, nmol/mg | 971 | 0.1 | |||
| Median sVCAM-1, ng/mL | 973 | 758.8 | |||
| Median sICAM-1, ng/mL | 970 | 277.9 | |||
8-ISO, 8-isoprostane; ARMS2, age-related maculopathy susceptibility 2; C2/CFB, complement component 2/complement factor B; C3, complement component 3; CFH, complement factor H; hsCRP, high sensitivity C-reactive protein; IL-6, interleukin-6; NSAID, nonsteroidal anti-inflammatory drug; sICAM-1, serum intercellular adhesion molecule-1; sVCAM-1, serum vascular cell adhesion molecule-1; TCC, total carbonyl content; TNF-αR2, tumor necrosis factor alpha receptor 2; WBC, white blood cell.
Adjusted for age and sex.
Associations of markers with the 20-year incidence of early AMD
One hundred and ninety-eight of the 975 individuals developed early AMD. The 20-year cumulative incidence adjusting for the competing risk of death for early AMD was 23.0% (95% confidence interval [CI] 20.2%-25.8%). Log transformed serum hsCRP (P=0.004), IL-6 (P=0.02), and sVCAM-1 (P=0.04) were associated with the 20-year cumulative incidence of AMD while adjusting for age, sex, and other factors (Table 3). There were trends for increasing quartile of hsCRP (P for test of trend=0.01) and IL-6 (P for test of trend=0.04) and higher 20-year cumulative incidence of early AMD. Compared to those in the lowest quartile, those in the highest quartile for hsCRP (P=0.005), TNF-αR2 (P=0.04) and IL-6 (P=0.03) had greater odds of developing early AMD (Table 3). When all three of these markers plus sVCAM-1 were included in the same model, only hsCRP (P=0.04) and sVCAM-1 (P=0.04) remained associated with the incidence of early AMD. There were no other relationships of other markers to the 20-year cumulative incidence of early AMD (Table 3). Relationships for hsCRP and WBC count were similar when analyses were expanded to include all individuals in the population (data not shown). The relationships of the markers of inflammation, oxidative stress, and endothelial dysfunction to the incidence of drusen type and size and pigmentary abnormalities were similar to that of early AMD (eTable 4).
Table 3.
Relationship of Inflammatory, Oxidative Stress and Endothelial Dysfunction Markers to the 20-year Cumulative Incidence of Early Age-related Macular Degeneration.
| Marker | N at Risk | N Events | Model 1a | Model 2b | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |||
| hsCRP (mg/L) | ||||||||
| Per SD on log scale | 875 | 178 | 1.27 | 1.08-1.50 | 0.003 | 1.29 | 1.08-1.55 | 0.004 |
| ≤1.0 | 237 | 33 | 1.00 | 1.00 | ||||
| >1.0 - 2.0 | 226 | 54 | 1.77 | 1.12-2.80 | 0.02 | 1.94 | 1.19-3.14 | 0.007 |
| >2.0 - 4.5 | 247 | 50 | 1.55 | 0.98-2.46 | 0.06 | 1.76 | 1.07-2.87 | 0.03 |
| >4.5 | 165 | 41 | 2.14 | 1.31-3.48 | 0.002 | 2.18 | 1.27-3.74 | 0.005 |
| Trend over quartiles | 875 | 178 | 1.22 | 1.06-1.42 | 0.007 | 1.23 | 1.05-1.45 | 0.01 |
| TNF-αR2 (pg/mL) | ||||||||
| Per SD on log scale | 886 | 179 | 1.18 | 1.00-1.40 | 0.06 | 1.19 | 1.00-1.42 | 0.06 |
| ≤2000 | 252 | 36 | 1.00 | 1.00 | ||||
| >2000 - 2500 | 320 | 71 | 1.55 | 1.02-2.36 | 0.04 | 1.56 | 1.01-2.42 | 0.05 |
| >2500 - 3000 | 176 | 37 | 1.48 | 0.91-2.41 | 0.12 | 1.44 | 0.87-2.39 | 0.16 |
| >3000 | 138 | 35 | 1.76 | 1.04-2.99 | 0.04 | 1.78 | 1.03-3.08 | 0.04 |
| Trend over quartiles | 886 | 179 | 1.17 | 1.00-1.38 | 0.05 | 1.17 | 0.99-1.38 | 0.07 |
| IL-6 (pg/mL) | ||||||||
| Per SD on log scale | 880 | 176 | 1.22 | 1.05-1.42 | 0.009 | 1.22 | 1.03-1.44 | 0.02 |
| ≤1.6 | 274 | 43 | 1.00 | 1.00 | ||||
| >1.6 - 2.4 | 244 | 52 | 1.50 | 0.98-2.29 | 0.06 | 1.58 | 1.00-2.50 | 0.05 |
| >2.4 - 3.7 | 192 | 42 | 1.45 | 0.92-2.29 | 0.11 | 1.57 | 0.96-2.56 | 0.07 |
| >3.7 | 170 | 39 | 1.69 | 1.06-2.69 | 0.03 | 1.78 | 1.05-3.02 | 0.03 |
| Trend over quartiles | 880 | 176 | 1.17 | 1.01-1.35 | 0.03 | 1.19 | 1.01-1.39 | 0.04 |
| WBC count (1000/μL) | ||||||||
| Per SD on log scale | 892 | 181 | 1.23 | 1.05-1.45 | 0.01 | 1.13 | 0.95-1.35 | 0.16 |
| ≤6.0 | 241 | 40 | 1.00 | 1.00 | ||||
| >6.0 - 7.2 | 245 | 58 | 1.49 | 0.97-2.28 | 0.07 | 1.45 | 0.93-2.25 | 0.10 |
| >7.2 - 8.5 | 200 | 39 | 1.26 | 0.79-2.01 | 0.34 | 1.13 | 0.69-1.84 | 0.63 |
| >8.5 | 206 | 44 | 1.75 | 1.11-2.75 | 0.02 | 1.41 | 0.86-2.31 | 0.17 |
| Trend over quartiles | 892 | 181 | 1.16 | 1.01-1.33 | 0.04 | 1.08 | 0.92-1.26 | 0.34 |
| 8-ISO (pg/mL) | ||||||||
| Per SD on log scale | 879 | 175 | 0.95 | 0.81-1.12 | 0.55 | 0.92 | 0.78-1.08 | 0.30 |
| ≤97 | 232 | 43 | 1.00 | 1.00 | ||||
| >97 - 124 | 229 | 54 | 1.37 | 0.89-2.10 | 0.15 | 1.43 | 0.92-2.21 | 0.11 |
| >124 - 164 | 201 | 46 | 1.41 | 0.90-2.19 | 0.13 | 1.53 | 0.97-2.42 | 0.07 |
| >164 | 217 | 32 | 0.79 | 0.49-1.27 | 0.32 | 0.71 | 0.43-1.17 | 0.18 |
| Trend over quartiles | 879 | 175 | 0.94 | 0.82-1.08 | 0.41 | 0.92 | 0.80-1.07 | 0.28 |
| TCC (nmol/mg) | ||||||||
| Per SD on log scale | 888 | 180 | 0.98 | 0.83-1.15 | 0.80 | 0.97 | 0.82-1.15 | 0.75 |
| ≤0.1 | 255 | 51 | 1.00 | 1.00 | ||||
| >0.1 - 0.14 | 182 | 35 | 0.94 | 0.60-1.47 | 0.78 | 0.87 | 0.54-1.39 | 0.56 |
| >0.14 - 0.2 | 213 | 54 | 1.26 | 0.84-1.90 | 0.27 | 1.19 | 0.78-1.82 | 0.42 |
| >0.2 | 238 | 40 | 0.88 | 0.57-1.36 | 0.56 | 0.88 | 0.56-1.38 | 0.57 |
| Trend over quartiles | 888 | 180 | 0.99 | 0.87-1.14 | 0.92 | 0.99 | 0.86-1.14 | 0.92 |
| sVCAM-1 (ng/mL) | ||||||||
| Per SD on log scale | 890 | 180 | 1.17 | 0.99-1.39 | 0.06 | 1.21 | 1.01-1.44 | 0.04 |
| ≤660 | 256 | 44 | 1.00 | 1.00 | ||||
| >660 - 790 | 258 | 50 | 1.14 | 0.74-1.74 | 0.55 | 1.19 | 0.76-1.85 | 0.45 |
| >790 - 950 | 211 | 42 | 1.13 | 0.72-1.78 | 0.58 | 1.14 | 0.72-1.81 | 0.58 |
| >950 | 165 | 44 | 1.43 | 0.90-2.27 | 0.13 | 1.58 | 0.98-2.56 | 0.06 |
| Trend over quartiles | 890 | 180 | 1.11 | 0.96-1.29 | 0.16 | 1.14 | 0.98-1.33 | 0.09 |
| sICAM-1 (ng/mL) | ||||||||
| Per SD on log scale | 888 | 180 | 1.04 | 0.89-1.23 | 0.61 | 0.98 | 0.82-1.17 | 0.81 |
| ≤240 | 248 | 45 | 1.00 | 1.00 | ||||
| >240 - 280 | 207 | 48 | 1.36 | 0.88-2.09 | 0.17 | 1.49 | 0.95-2.34 | 0.08 |
| >280 - 330 | 212 | 43 | 1.07 | 0.69-1.66 | 0.77 | 1.08 | 0.68-1.72 | 0.74 |
| >330 | 221 | 44 | 1.26 | 0.81-1.96 | 0.30 | 1.10 | 0.68-1.78 | 0.70 |
| Trend over quartiles | 888 | 180 | 1.05 | 0.91-1.20 | 0.50 | 1.00 | 0.86-1.16 | 0.99 |
8-ISO, 8-isoprostane; AMD, age-related macular degeneration; hsCRP, high sensitivity C-reactive protein; IL-6, interleukin-6; OR, odds ratio; sICAM-1, serum intercellular adhesion molecule-1; sVCAM-1, serum vascular cell adhesion molecule-1; TCC, total carbonyl content; TNF-αR2, tumor necrosis factor alpha receptor 2; WBC, white blood cell.
Model 1 adjusted for age and sex.
Model 2 adjusted for age, sex, smoking habits, physical activity, statin use, anti-inflammatory medication use, and body mass index.
We examined the relationship of serum TNF-αR2 to the incidence of early AMD by sex and, in women, by menopausal status. The TNF-αR2 relationship was similar in women (OR per 1 SD increase on log scale 1.22, P=0.08) and men (OR 1.16, P=0.34), and was stronger in women who had gone through menopause (OR 1.45, P=0.01) compared to women who had not (OR 1.07, P=0.91) while adjusting for age, BMI, smoking status, physical activity levels, and use of statins and anti-inflammatory medications.
Associations of candidate gene SNPs with the 20-year incidence of early AMD and interactions with the markers
Both CFH (P=0.003) and ARMS2 (P=0.006) with 2 risk alleles were associated with the 20-year incidence of early AMD. However, neither C3 nor C2/CFB were related to the 20-year incidence of early AMD (eTable 5). The relationships of each marker per SD on the log scale, stratified by having 0, 1, and 2 risk alleles for CFH and ARMS2, to the incidence of early AMD after adjustment for age, sex, smoking status, and other factors at baseline are presented in the Figure. There were no interactions between C3 or C2/CFB and any of the markers (data not shown).
Figure.
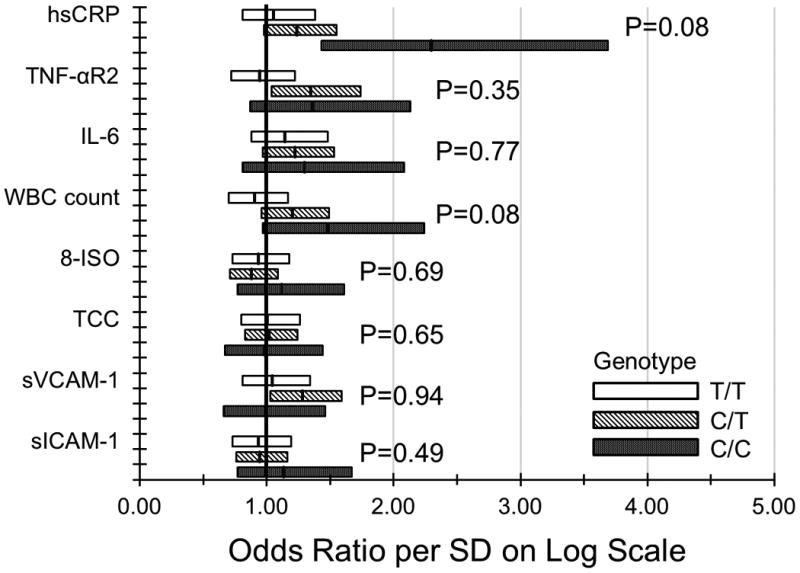
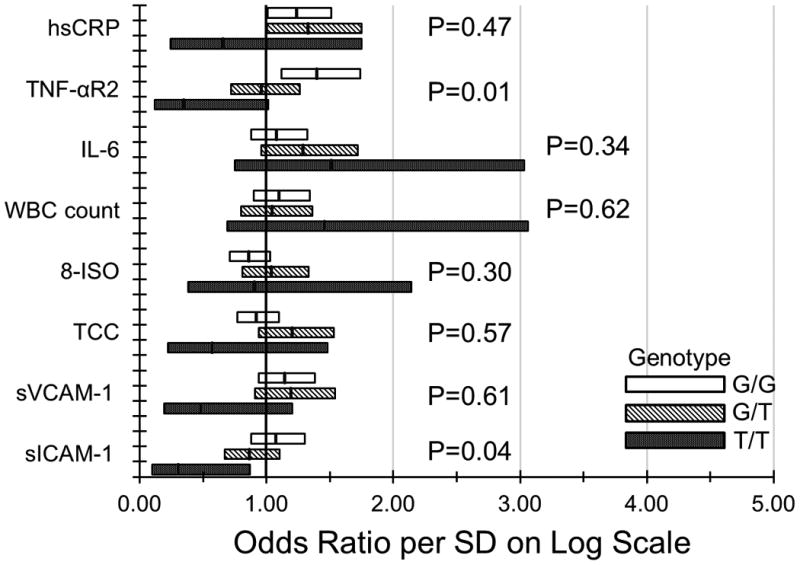
Relationship of markers of inflammation, endothelial dysfunction, and oxidative stress to the 20-year incidence of early age-related macular degeneration in the Beaver Dam Eye Study (1988-1990 to 2008-2010) stratified by A. CFH rs1061170 genotype; B. ARMS2 rs10490924 genotype.
Risk assessment
The models including smoking, physical activity, BMI, age, and sex discriminated poorly in predicting the incidence of early AMD (Table 6). The largest increase and incremental gain in the AUC occurred after including hsCRP in the model that included traditional risk factors for incidence of early AMD; however, it was not statistically significant (P=0.42, Table 6).
Table 6.
Effects of Markers of Inflammation, Endothelial Dysfunction, and Oxidative Stress on the Risk of Age-Related Macular Degeneration in Risk Assessment Models.
| Markera | Traditional Risk Factor Model | Traditional Risk Factors + Genetic Factors Model | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| AUC (95% CI) | Change in AUC, % | P value | AUC (95% CI) | Change in AUC, % | P value | |||
|
|
|
|||||||
| Without Markers in Model | With Marker in Model | Without Markers in Model | With Marker in Model | |||||
| hsCRP | 0.6714 (0.6212, 0.7216) | 0.6786 (0.6295, 0.7277) | 1.07 | 0.42 | 0.6923 (0.6411, 0.7435) | 0.6997 (0.6492, 0.7502) | 1.06 | 0.32 |
| TNF-αR2 | 0.6715 (0.6217, 0.7214) | 0.02 | 0.97 | 0.6926 (0.6417, 0.7435) | 0.04 | 0.93 | ||
| IL-6 | 0.6778 (0.6287, 0.7269) | 0.95 | 0.47 | 0.6992 (0.6483, 0.7500) | 0.98 | 0.38 | ||
| WBC count | 0.6747 (0.6239, 0.7255) | 0.49 | 0.59 | 0.6962 (0.6445, 0.7479) | 0.56 | 0.48 | ||
| 8-ISO | 0.6726 (0.6221, 0.7232) | 0.18 | 0.69 | 0.6927 (0.6412, 0.7443) | 0.06 | 0.86 | ||
| TCC | 0.6750 (0.6251, 0.7249) | 0.54 | 0.67 | 0.6953 (0.6443, 0.7462) | 0.42 | 0.65 | ||
| sVCAM-1 | 0.6720 (0.6215, 0.7226) | 0.09 | 0.92 | 0.6956 (0.6437, 0.7474) | 0.46 | 0.61 | ||
| sICAM-1 | 0.6719 (0.6214, 0.7225) | 0.08 | 0.82 | 0.6909 (0.6393, 0.7425) | -0.20 | 0.45 | ||
8-ISO, 8-isoprostane; AUC, area under the receiver operating characteristic curve; CI, confidence interval; hsCRP, high sensitivity C-reactive protein; IL-6, interleukin-6; TCC, total carbonyl content; TNF-αR2, tumor necrosis factor alpha receptor 2; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; WBC, white blood cell.
Traditional risk factors = age, sex, smoking status, physical activity, and body mass index.
Genetic factors = CFH rs1061770 and ARMS2 rs10490924.
Markers modeled per standard deviation on the logarithmic scale.
Discussion
In the BDES, higher levels of serum hsCRP, TNF-αR2, IL-6, and sVCAM-1 were modestly associated with the 20-year cumulative incidence of early AMD independent of age, sex, smoking, physical activity, obesity status, and history of use of statins and anti-inflammatory drugs.
Most studies have shown a consistent relationship between serum hsCRP and late AMD.26-28,31,33-36 There is less consistency in studies that have examined the relationship of hsCRP to the long-term incidence of early AMD; five studies did not find a relationship26,28,30,31,34 and two did.27,36 Our findings are consistent with those from a recent meta-analysis of five large studies that showed that participants with high hsCRP levels (>3 mg/L) had an increased risk of incident early AMD (OR 1.49; 95% CI 1.06-2.08) compared to participants with low hsCRP levels (<1 mg/L).36 When similar analyses were performed in the BDES cohort, while adjusting for age, sex, and other factors, those with high levels of hsCRP had greater odds of developing early AMD (OR >3 mg/dL vs. <1 mg/dL 1.69; P=0.04).57
The pathogenetic mechanisms underlying the role of hsCRP and other inflammatory biomarkers in the development of AMD are complex and not fully understood.26 Johnson and colleagues speculated that elevations of hsCRP during acute phase reactions over a lifetime in individuals homozygous for the CFH rs1061170 risk alleles resulted in increasing tissue damage to Bruch's membrane and the RPE, further increasing the risk of AMD compared to those homozygous for the wild type of CFH.58 There is emerging evidence that the association of elevated levels of hsCRP with early AMD is not due to hsCRP directly damaging the RPE and Bruch's membrane. Instead, when hsCRP levels increase (e.g., during an acute phase reaction), it has been shown that CRP is more likely to bind more strongly to the CFH gene site when 1 or 2 risk alleles are present compared to when no risk alleles are present.26,59,60 The stronger binding of hsCRP is thought to block the regulatory function of CFH in deactivating surface-bound C3b, a key factor in the response of the complement immune system to inflammatory stimuli.59,60 The finding in the BDES of a borderline multiplicative interaction of CFH with higher levels of hsCRP for the 20-year cumulative incidence of early AMD when 1 and 2 risk alleles are present is consistent with these observations.
Our study showed that TNF-αR2 was associated with the development of early AMD independent of BMI, smoking, and other factors. Their relationship was no longer statistically significant when hsCRP was included in the model. TNF-αR2 was not previously shown to be related to the prevalence of any AMD or the progression to late AMD.32,34 TNF-α is a cytokine involved in cell activation, differentiation and apoptosis, and has been shown to be related to AMD in some studies.61,62 The receptor TNF-αR2 is expressed in the choroid vascular cells, RPE, and Mueller cells in the retina. Its role in the pathogenesis of AMD is poorly understood, as is the reason the relationship was stronger in post-menopausal women than in pre-menopausal women. The reason for the inverse interaction in the BDES group with ARMS2 is not understood. It may be a chance finding.
In the BDES, there was no relation of two markers of oxidative stress, serum 8-ISO and TCC, to the incidence of AMD. This is consistent with the lack of a protective effect of antioxidant vitamins for the incidence of early AMD in the AREDS 1.63 The RPE has been shown to be vulnerable to oxidative damage by radical-catalyzed lipid peroxidation.64-66 The lack of an association may be due to oxidative stress not being related to incident early AMD or that the two markers do not reflect oxidative stress occurring at the cellular level at the RPE. The variability of these two oxidative stress measures may have affected our ability to find a relationship if it were present. Few other epidemiological studies have examined the relationships of these measures of oxidative stress to AMD. In one, a prospective case-control study involving 77 AMD patients and 75 controls, plasma F2 isoprostane was not related to AMD after adjustment for age, sex, and smoking.37
In the BDES, while adjusting for age, sex, smoking and other factors, sVCAM-1 but not sICAM-1 was associated with the incidence of early AMD. Both of these cellular adhesion molecules are transmembrane cell surface proteins with immunoglobulin superfamily domains. They regulate inflammation by attracting WBCs and controlling their migration into the blood vessel wall.67 Increased expression of these molecules in the cellular wall is reflected by increases in soluble forms of these molecules in the plasma. Increases in the number of WBCs have been shown in the choroid of eyes with early and late AMD.68-70 Complement mediated activation of choroidal endothelial secretion of sICAM-1 has been hypothesized to play a role in the pathogenesis of AMD.71 However, few associations were found in the studies that have examined these relationships.31,32,72
Our findings suggest that while there are statistically significant, clinically meaningful relationships of the inflammatory markers in persons without AMD, they have limited prognostic value for predicting the incidence of early AMD, independent of age, sex, smoking history, and other traditional risk factors. The increase in AUC of 1.02% for inclusion of hsCRP in the risk prediction model was small and not statistically significant. It compares unfavorably with other potential predictive factors used for other endpoints (e.g., hsCRP and serum high-density lipoprotein cholesterol levels) when added to the Framingham risk score for coronary heart disease in the Atherosclerosis Risk in Communities study.73-75
There are many strengths to our study, including the use of standard protocols to measure AMD from fundus photographs over a 20-year period in a representative population-based study. There are also limitations. First, the analyses were performed in a randomized sample of the cohort in an effort to minimize bias. It is possible that this sample may not be representative of the cohort. However, randomization appeared to minimize this possibility; there were few differences between those randomized and those not randomized. Additionally, hsCRP and WBC count were measured in the whole cohort at baseline and the findings were similar to those reported in the smaller randomized cohort. Second, selective survival may have obscured relationships if people with high levels of serum 8-ISO or TCC who developed early AMD were more likely to die before being examined than those with low levels of these markers. Those with higher levels of serum 8-ISO were not more likely to die than to be observed with or without early AMD (OR per SD on the log scale 0.98; 95% CI 0.80-1.15; P=0.80). However, those with higher levels of TCC were more likely to die than to be observed with or without early AMD (OR 1.22; 95% CI 1.06-1.38; P=0.02) after adjusting for age, sex, smoking status, physical activity, BMI, and anti-inflammatory medication use. Third, a single measure of a marker, e.g., hsCRP, may not be representative of lifetime exposure. However, Nash and colleagues evaluated the 10-year percent agreement between groups for levels of IL-6 (50.8%) and hsCRP (53.4%), and their data suggest that the levels of these inflammatory markers track over time and are fairly stable.76 Data from another study suggest modest variability of inflammatory markers over time, dependent partially upon changes in cardiovascular risk factors, eg, obesity, physical activity, and smoking status as people age.77 Fourth, the long period between freezing and measurement of samples may have resulted in the greater variability found in serum 8-ISO and TCC levels, reducing our ability to find a relationship. Serum samples were stored at -80°C. These tests were found to be stable with essentially no evidence of auto-oxidation in a pilot study from the Nurses' Health Study.78 Further, Schwedhelm and colleagues reported long-term storage of blood samples in prospective studies at -80°C to be stable with respect to these markers of oxidative stress.79
In summary, inflammatory markers and one marker of endothelial dysfunction were modestly associated with the 20-year cumulative incidence of early AMD in the BDES. These data provide further support for the role of inflammation in the pathogenesis of early AMD. It may be one of many mechanisms involved in the development of this complex multifactorial disease. It is unknown whether these associations represent a cause-and-effect relationship or if other unknown confounders accounted for the findings. Even if inflammatory processes are a cause of early AMD, it is unknown whether interventions that reduce systemic inflammatory processes will reduce the incidence of early AMD.
Supplementary Material
Acknowledgments
Financial Support: This study was supported by National Institutes of Health grant EY06594 (BEK Klein and R Klein), National Institute on Aging grant AG11099 (KJ Cruickshanks), and National Institute of Diabetes and Digestive and Kidney Diseases grant DK073217, National Institutes of Health, Bethesda, MD, USA and, in part, by Research to Prevent Blindness (RPB), New York, NY. The National Eye Institute and National Institute of Diabetes and Digestive and Kidney Diseases provided funding for study including collection and analyses of data; RPB provided additional support for data analyses. None of the funding organizations had any role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Data Access, Responsibility, and Analysis Statement: Dr. R. Klein had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Chelsea E. Myers (Department of Ophthalmology and Visual Sciences, University of Wisconsin School of Medicine and Public Health, Madison, WI) and Ronald E. Gangnon (Departments of Biostatistics and Medical Informatics and Population Health Sciences, University of Wisconsin School of Medicine and Public Health, Madison, WI) conducted and are responsible for the data analysis.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Eye Institute or the National Institutes of Health.
Conflict of Interest: No conflicting relationship exists for any author.
References
- 1.Ferris FL, III, Tielsch JM. Blindness and visual impairment: a public health issue for the future as well as today. Arch Ophthalmol. 2004;122(4):451–452. doi: 10.1001/archopht.122.4.451. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 3.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 4.Haddad S, Chen CA, Santangelo SL, Seddon JM. The genetics of age-related macular degeneration: a review of progress to date. Surv Ophthalmol. 2006;51(4):316–363. doi: 10.1016/j.survophthal.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Scholl HP, Fleckenstein M, Charbel IP, Keilhauer C, Holz FG, Weber BH. An update on the genetics of age-related macular degeneration. Mol Vis. 2007;13:196–205. [PMC free article] [PubMed] [Google Scholar]
- 6.Klein R. Epidemiology of age-related macular degeneration. In: Penfold PL, Provis JM, editors. Macular Degeneration: Science and Medicine in Practice. Berlin: Springer-Verlag; 2005. pp. 79–101. [Google Scholar]
- 7.Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60(5):324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration. The involvement of giant cells in atrophy of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1986;27(3):364–371. [PubMed] [Google Scholar]
- 9.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20(6):705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 10.Ernst E, Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA. Leukocytes and the risk of ischemic diseases. JAMA. 1987;257(17):2318–2324. [PubMed] [Google Scholar]
- 11.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 13.Edwards AO, Ritter R, III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 14.Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med. 2012;33(4):399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai X, McGinnis JF. Oxidative stress: the achilles' heel of neurodegenerative diseases of the retina. Front Biosci. 2012;17:1976–1995. doi: 10.2741/4033. [DOI] [PubMed] [Google Scholar]
- 16.Hollyfield JG. Age-related macular degeneration: the molecular link between oxidative damage, tissue-specific inflammation and outer retinal disease: the Proctor lecture. Invest Ophthalmol Vis Sci. 2010;51(3):1275–1281. doi: 10.1167/iovs.09-4478. [DOI] [PubMed] [Google Scholar]
- 17.Decanini A, Nordgaard CL, Feng X, Ferrington DA, Olsen TW. Changes in select redox proteins of the retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol. 2007;143(4):607–615. doi: 10.1016/j.ajo.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsao YP, Ho TC, Chen SL, Cheng HC. Pigment epithelium-derived factor inhibits oxidative stress-induced cell death by activation of extracellular signal-regulated kinases in cultured retinal pigment epithelial cells. Life Sci. 2006;79(6):545–550. doi: 10.1016/j.lfs.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 19.Handelman GJ. Evaluation of oxidant stress in dialysis patients. Blood Purif. 2000;18(4):343–349. doi: 10.1159/000014460. [DOI] [PubMed] [Google Scholar]
- 20.Davies MJ, Fu S, Wang H, Dean RT. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic Biol Med. 1999;27(11-12):1151–1163. doi: 10.1016/s0891-5849(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 21.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82(2):291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 22.Morrow JD, Frei B, Longmire AW, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332(18):1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 23.Pow DV, Sullivan RK, Williams SM, WoldeMussie E. Transporters and oxidative stress in AMD. In: Penfold PL, Provis JM, editors. Macular Degeneration: Science and Medicine in Practice. Berlin: Springer-Verlag; 2005. pp. 123–148. [Google Scholar]
- 24.Shaw PX, Zhang L, Zhang M, et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc Natl Acad Sci U S A. 2012;109(34):13757–13762. doi: 10.1073/pnas.1121309109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lip PL, Blann AD, Hope-Ross M, Gibson JM, Lip GY. Age-related macular degeneration is associated with increased vascular endothelial growth factor, hemorheology and endothelial dysfunction. Ophthalmology. 2001;108(4):705–710. doi: 10.1016/s0161-6420(00)00663-1. [DOI] [PubMed] [Google Scholar]
- 26.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134(3):411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 27.Boekhoorn SS, Vingerling JR, Witteman JC, Hofman A, de Jong PT. C-reactive protein level and risk of aging macula disorder: The Rotterdam Study. Arch Ophthalmol. 2007;125(10):1396–1401. doi: 10.1001/archopht.125.10.1396. [DOI] [PubMed] [Google Scholar]
- 28.Boey PY, Tay WT, Lamoureux E, et al. C-reactive protein and age-related macular degeneration and cataract: the singapore malay eye study. Invest Ophthalmol Vis Sci. 2010;51(4):1880–1885. doi: 10.1167/iovs.09-4063. [DOI] [PubMed] [Google Scholar]
- 29.Schaumberg DA, Christen WG, Buring JE, Glynn RJ, Rifai N, Ridker PM. High-sensitivity C-reactive protein, other markers of inflammation, and the incidence of macular degeneration in women. Arch Ophthalmol. 2007;125(3):300–305. doi: 10.1001/archopht.125.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dasch B, Fuhs A, Behrens T, et al. Inflammatory markers in age-related maculopathy: cross-sectional analysis from the Muenster Aging and Retina Study. Arch Ophthalmol. 2005;123(11):1501–1506. doi: 10.1001/archopht.123.11.1501. [DOI] [PubMed] [Google Scholar]
- 31.Hogg RE, Woodside JV, Gilchrist SE, et al. Cardiovascular disease and hypertension are strong risk factors for choroidal neovascularization. Ophthalmology. 2008;115(6):1046–1052. doi: 10.1016/j.ophtha.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 32.Seddon JM, George S, Rosner B, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. 2005;123(6):774–782. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- 33.Klein R, Klein BE, Knudtson MD, Wong TY, Shankar A, Tsai MY. Systemic markers of inflammation, endothelial dysfunction, and age-related maculopathy. Am J Ophthalmol. 2005;140(1):35–44. doi: 10.1016/j.ajo.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 34.Klein R, Knudtson MD, Klein BE, et al. Inflammation, complement factor h, and age-related macular degeneration: the Multi-ethnic Study of Atherosclerosis. Ophthalmology. 2008;115(10):1742–1749. doi: 10.1016/j.ophtha.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGwin G, Hall TA, Xie A, Owsley C. The relation between C reactive protein and age related macular degeneration in the Cardiovascular Health Study. Br J Ophthalmol. 2005;89(9):1166–1170. doi: 10.1136/bjo.2005.067397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitta VP, Christen WG, Glynn RJ, et al. C-reactive protein and the incidence of macular degeneration: pooled analysis of 5 cohorts. JAMA Ophthalmol. 2013;131(4):507–513. doi: 10.1001/jamaophthalmol.2013.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brantley MA, Jr, Osborn MP, Sanders BJ, et al. Plasma biomarkers of oxidative stress and genetic variants in age-related macular degeneration. Am J Ophthalmol. 2012;153(3):460–467. doi: 10.1016/j.ajo.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machalinska A, Kawa MP, Marlicz W, Machalinski B. Complement system activation and endothelial dysfunction in patients with age-related macular degeneration (AMD): possible relationship between AMD and atherosclerosis. Acta Ophthalmol. 2012;90(8):695–703. doi: 10.1111/j.1755-3768.2011.02295.x. [DOI] [PubMed] [Google Scholar]
- 39.Klein R, Klein BE, Linton KL, DeMets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98(8):1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 40.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103(8):1169–1178. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 41.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Chappell RJ. Changes in visual acuity in a population over a 10-year period: the Beaver Dam Eye Study. Ophthalmology. 2001;108(10):1757–1766. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 42.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142(4):539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Klein R, Lee KE, Gangnon RE, Klein BE. Incidence of visual impairment over a 20-year period: the Beaver Dam Eye Study. Ophthalmology. 2013;120(6):1210–1219. doi: 10.1016/j.ophtha.2012.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein BE, Klein R. The Beaver Dam Eye Study V Manual of Operations. Springfield, VA: National Technical Information Service; 2010. PB2010-114194. [Google Scholar]
- 45.Klein R, Myers CE, Meuer SM, et al. Risk alleles in CFH and ARMS2 and the long-term natural history of age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol. 2013;131(3):383–392. doi: 10.1001/jamaophthalmol.2013.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin Age-Related Maculopathy Grading System. Springfield, VA: National Technical Information Service; 1991. Accession No. PB91-184267. [DOI] [PubMed] [Google Scholar]
- 47.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98(7):1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 48.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 49.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology. 1997;104(1):7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 50.Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology. 2002;109(10):1767–1779. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 51.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114(2):253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 52.Klein R, Meuer SM, Myers CE, Buitendijk GH, Rochtchina E, Choudhury F, de Jong PT, McKean-Cowdin R, Iyengar SK, Gao X, Lee KE, Vingerling JR, Mitchell P, Klaver CC, Wang JJ, Klein BE. Harmonizing the classification of age-related macular degeneration in the Three Continent AMD Consortium. Ophthalmic Epidemiol. 2014;21(1):14–23. doi: 10.3109/09286586.2013.867512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 54.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 55.Hosmer DW, Jr, Lemeshow S. Applied logistic regression. New York, NY: John Wiley & Sons; 1989. Special topics; pp. 238–245. [Google Scholar]
- 56.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 57.Klein R, Myers CE, Meuer SM, et al. Risk alleles in CFH and ARMS2 and the long-term natural history of age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol. 2013;131(3):383–392. doi: 10.1001/jamaophthalmol.2013.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson PT, Betts KE, Radeke MJ, Hageman GS, Anderson DH, Johnson LV. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc Natl Acad Sci U S A. 2006;103(46):17456–17461. doi: 10.1073/pnas.0606234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeWan A, Bracken MB, Hoh J. Two genetic pathways for age-related macular degeneration. Curr Opin Genet Dev. 2007;17(3):228–233. doi: 10.1016/j.gde.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Perkins SJ, Nan R, Li K, Khan S, Miller A. Complement factor H-ligand interactions: self-association, multivalency and dissociation constants. Immunobiology. 2012;217(2):281–297. doi: 10.1016/j.imbio.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Black RA, Doedens JR, Mahimkar R, et al. Substrate specificity and inducibility of TACE (tumour necrosis factor alpha-converting enzyme) revisited: the Ala-Val preference, and induced intrinsic activity. Biochem Soc Symp. 2003;(70):39–52. doi: 10.1042/bss0700039. [DOI] [PubMed] [Google Scholar]
- 62.de Oliveira Dias JR, Rodrigues EB, Maia M, Magalhaes O, Jr, Penha FM, Farah ME. Cytokines in neovascular age-related macular degeneration: fundamentals of targeted combination therapy. Br J Ophthalmol. 2011;95(12):1631–1637. doi: 10.1136/bjo.2010.186361. [DOI] [PubMed] [Google Scholar]
- 63.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 65.Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19(2):205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 66.Fessel JP, Jackson RL. Isofurans: novel products of lipid peroxidation that define the occurrence of oxidant injury in settings of elevated oxygen tension. Antioxid Redox Signal. 2005;7(1-2):202–209. doi: 10.1089/ars.2005.7.202. [DOI] [PubMed] [Google Scholar]
- 67.Burger D, Touyz RM. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells. J Am Soc Hypertens. 2012;6(2):85–99. doi: 10.1016/j.jash.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(3):1606–1612. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grossniklaus HE, Ling JX, Wallace TM, et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis. 2002;8:119–126. [PubMed] [Google Scholar]
- 70.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol. 1985;223(2):69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- 71.Skeie JM, Fingert JH, Russell SR, Stone EM, Mullins RF. Complement component C5a activates ICAM-1 expression on human choroidal endothelial cells. Invest Ophthalmol Vis Sci. 2010;51(10):5336–5342. doi: 10.1167/iovs.10-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jonas JB, Tao Y, Neumaier M, Findeisen P. Monocyte chemoattractant protein 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 in exudative age-related macular degeneration. Arch Ophthalmol. 2010;128(10):1281–1286. doi: 10.1001/archophthalmol.2010.227. [DOI] [PubMed] [Google Scholar]
- 73.Chambless LE, Folsom AR, Sharrett AR, et al. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol. 2003;56(9):880–890. doi: 10.1016/s0895-4356(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 74.Folsom AR, Chambless LE, Ballantyne CM, et al. An assessment of incremental coronary risk prediction using C-reactive protein and other novel risk markers: the atherosclerosis risk in communities study. Arch Intern Med. 2006;166(13):1368–1373. doi: 10.1001/archinte.166.13.1368. [DOI] [PubMed] [Google Scholar]
- 75.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 76.Nash SD, Cruickshanks KJ, Klein R, et al. Long-term variability of inflammatory markers and associated factors in a population-based cohort. J Am Geriatr Soc. 2013;61(8):1269–1276. doi: 10.1111/jgs.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fontes JD, Yamamoto JF, Larson MG, et al. Clinical correlates of change in inflammatory biomarkers: The Framingham Heart Study. Atherosclerosis. 2013;228(1):217–223. doi: 10.1016/j.atherosclerosis.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu T, Rifai N, Roberts LJ, Willett WC, Rimm EB. Stability of measurements of biomarkers of oxidative stress in blood over 36 hours. Cancer Epidemiol Biomarkers Prev. 2004;13(8):1399–1402. [PubMed] [Google Scholar]
- 79.Schwedhelm E, Boger RH. Application of gas chromatography-mass spectrometry for analysis of isoprostanes: their role in cardiovascular disease. Clin Chem Lab Med. 2003;41(12):1552–1561. doi: 10.1515/CCLM.2003.238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


