Abstract
Atherosclerosis is the disease mechanism responsible for coronary heart disease (CHD), the leading cause of death worldwide. One strategy to combat atherosclerosis is to increase the amount of circulating high density lipoproteins (HDL), which transport cholesterol from peripheral tissues to the liver for excretion. The process, known as reverse cholesterol transport, is thought to be one of the main reasons for the significant inverse correlation observed between HDL blood levels and the development of CHD. This article highlights the most common strategies for treating atherosclerosis using HDL. We further detail potential treatment opportunities that utilize nanotechnology to increase the amount of HDL in circulation. The synthesis of biomimetic HDL nanostructures that replicate the chemical and physical properties of natural HDL provides novel materials for investigating the structure-function relationships of HDL and for potential new therapeutics to combat CHD.
Coronary Heart Disease and HDL
Coronary heart disease (CHD) remains the leading cause of death in the United States despite therapeutic advances, and is now the leading cause of death worldwide [1,2]. The most important underlying cause of CHD is atherosclerosis of the coronary arteries, the vessels that supply blood to the heart and are therefore essential for supporting cardiac function [1,3]. Atherosclerosis is a complex disease that results from the accumulation of cholesterol in macrophage cells within the arterial wall [4,5].
Cholesterol, therefore, is a central molecule in CHD pathogenesis, and strategies to modulate cholesterol levels in the body occupy a central role in CHD therapy [6]. Cholesterol is insoluble in aqueous solution and requires lipoprotein carriers for transport to peripheral tissues from the liver, where it is synthesized, or the gut, where it is absorbed [3,7]. Lipoproteins are a class of naturally occurring nanoparticles that solubilize lipids, especially cholesterol, for systemic circulation [3,7]. Low density lipoproteins (LDL) carry cholesterol from the liver to peripheral tissues [3,7]. In excess, LDLs increasingly adhere to arterial walls, traverse the vascular endothelial cell barrier, deposit within the intimal layers of the arterial wall, are oxidized, and initiate atherosclerosis [4,5]. Oxidized LDL is taken up by invading macrophages forming pathognomonic foam cells at arterial sites prone to lesion development [4,5]. Progressive vessel remodeling at these sites within the coronary arteries promotes the development of CHD [5,8].
Many drugs have been developed to lower LDL-cholesterol (LDL-c) [9]. One particular class of LDL-lowering medications, the hydroxymethylglutaryl CoA reductase inhibitors (more commonly known as “statins”), have been shown to decrease CHD-related deaths [10,11]. In one study, the risk of coronary death was reduced by 42% over the course of the study [12]. However, the benefits of statin therapy must be weighed against potential side effects such as muscle and liver toxicity that, in some cases, remove statins as a treatment option [13–15]. Furthermore, despite the success of statins and other pharamacologic and revascularization therapies designed to treat this devastating disease, [16], CHD remains the most common cause of death in the world [2]. Thus, a clear need exists for additional strategies to combat atherosclerosis.
LDL is only one of the molecules involved in cholesterol transport [3]. Another major cholesterol-transporting molecule is high density lipoprotein (HDL) [3,7]. Multiple studies show that levels of HDL are inversely correlated with incidence of CHD [3,17,18]. HDLs are thought to be involved in a process known as reverse cholesterol transport (RCT), removing cholesterol from the circulation and from developing atherosclerotic lesions, and transporting it to the liver for biliary excretion [3,7]. In addition to removing cholesterol from the body, HDL exerts other atheroprotective effects such as reducing inflammation of the endothelium, preventing endothelial dysfunction, and acting as an antioxidant [17,19]. Accordingly, increasing levels of HDL in the blood represents a promising therapeutic strategy to combat atherosclerosis [11,20].
In addition to therapeutic implications, HDL biology may have diagnostic implications as a method for the early detection of atherosclerosis. Many deaths due to CHD occur without warning and with few prior symptoms [21,22]. Therefore, the imaging and early identification of atherosclerotic plaques are of particular interest [23,24]. Molecular imaging, or imaging done on the cellular level, is now possible due to advances in imaging technology [23]. The development of novel computed tomography (CT) and magnetic resonance imaging (MRI) contrast agents has made possible the detection of small changes in specific tissues using non-invasive imaging techniques [23]. Combining these imaging modalities with structures that mimic molecules involved in cholesterol transport, such as HDL, may facilitate detection of changes in the arteries due to atherosclerosis and enable early identification and treatment of atherosclerosis [23,24].
Relationship between natural HDL structure and function
HDL is a dynamic natural nanoparticle that is constantly being remodeled as it transports and transfers cholesterol to cells and other lipoproteins [25]. Constant remodeling leads to HDL heterogeneity, which has been shown to influence its function (Box 1 and Figure 1) [17,19,26]. Natural HDL ranges in size from 6 to 13 nm in diameter and has a density of 1.063 to 1.210 g/mL [3,27].
Box 1. Formation of Different HDL Species.
Apo AI, the main protein component of HDL, modulates the form and function of HDL species [3,25] as supported by computational studies of nanodisc structure [53]. Initially, Apo A1 is secreted from cells of the liver and small intestine free of phospholipids and cholesterol [3,75]. Apo A1 acquires phospholipids and free cholesterol from cell membranes through interaction with membrane-bound ATP binding cassette A1 receptor (ABCA1) [3,7,17]. The HDL species formed is called nascent HDL and resembles a phospholipid bilayer disk with two molecules of Apo AI wrapped around the disk in a belt-like configuration [3,17,63]. Molecules of free cholesterol interdigitate the ~160 phospholipids present in the disc core [3,76]. Nascent HDL is the smallest, most cholesterol-poor HDL subspecies [3].
Nascent HDL acquires free cholesterol through interaction with ABCA 1, another ATP binding cassette receptor (ABCG1), and the scavenger receptor B1 (SR-B1) [3,7,17]. Maturing HDLs activate the enzyme lecithin:cholesterol acyltransferase (LCAT), which esterifies free cholesterol bound by the growing HDL particle [3,17]. Esterified cholesterol migrates to the center of the disk resulting in expansion and a more spherical shape [3,17,77]. Another enzyme, cholesteryl ester transfer protein (CETP), mediates the exchange of cholesteryl esters from HDL for triglycerides from triglyceride-rich lipoproteins, such as LDL [17,78]. The hydrophobic triglycerides also move to the center of HDL, maintaining the spherical shape [17]. During the remodeling process, there can also be an exchange of Apo A1 proteins, and HDL species contain between two and four proteins per particle [3,25,73]. The differential acquisition and movement of cholesterol into versus out of nascent HDL contributes to its growth into spherical, mature HDL, and this growth process accounts for the different subspecies of HDL (Figure 1) [3,17,77].
Figure 1. Growth of HDL from lipid-free Apo A1 to mature, spherical HDL.
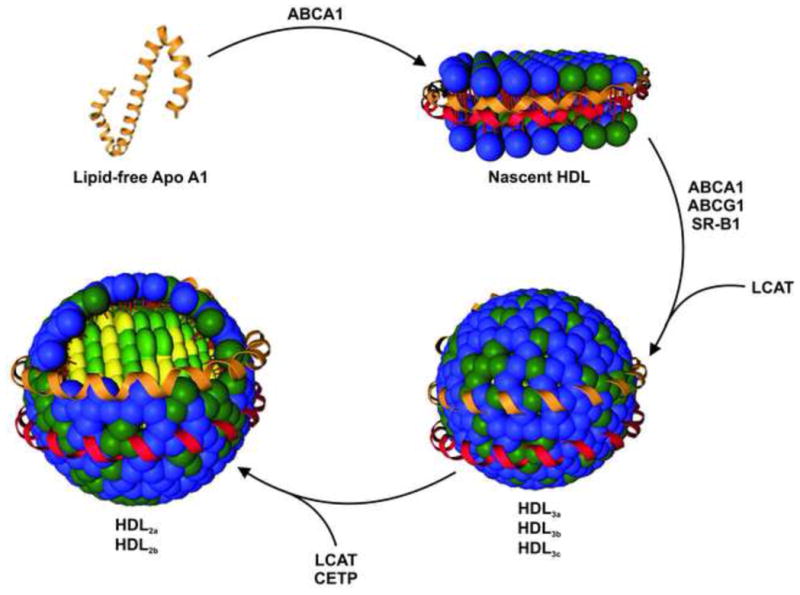
Lipid-free Apo A1 acquires phospholipids and cholesterol from cells through ABCA1 to form nascent HDL. Further interaction with ABCA1, as well as ABCG1 and SR-B1, provide nascent HDL with additional free cholesterol. The protein LCAT esterifies free cholesterol forming cholesteryl esters. Another protein, CETP, transfers cholesteryl ester from HDL in exchange for triglycerides. The conversion of free cholesterol into cholesteryl esters and the acquisition of triglycerides lead to the formation of spherical HDL species. Initially, spherical HDLs are small and lipid-poor. These are called HDL3a, HDL3b, and HDL3c. As HDL continues to grow, large, cholesterol-rich HDLs are generated, which are termed HDL2a and HDL2b. When classified using two-dimensional gel electrophoresis, nascent HDL occurs as pre-β HDL while HDL3 and HDL2 are found to be α or pre-α HDL [25,27]. Apo A1 – red and orange, phospholipids – blue, cholesterol and cholesteryl esters – green, and triglycerides – yellow.
It is being increasingly appreciated that all HDLs are not created equal. Studies demonstrate that the size and composition of HDL can be the determining factor in the role HDL plays in the body and influences its ability to exert atheroprotective effects [17,19]. Research shows a strong correlation between different subpopulations of HDL and CHD [28]. Specifically, Asztalos et al. reported that higher levels of α-3 and pre α-1 HDL particles are significantly associated with CHD and that individuals with CHD had a significantly lower concentration of α-1 HDL (Figure 1) [28]. Furthermore, the esterification of HDL-bound cholesterol, which ensures that a free cholesterol gradient is maintained between cells and HDL for RCT, occurs at different rates for different sizes of HDL [19,29]. There is also evidence that large, cholesterol rich HDL particles, such as HDL2 (Figure 1), have higher levels of antioxidant activity, another of the atheroprotective properties of HDL [30]. These findings demonstrate the importance of size and composition on the ability of HDL to protect against atherosclerosis and the notion that the most effective HDL therapeutic would ideally increase the amount of those HDL species that are most atheroprotective. To fully understand the structure-function relationship of HDL species and create novel HDL therapeutics, a synthetic strategy for producing spherical HDLs is needed.
Current technologies for increasing HDL
Generally, two strategies for increasing HDL levels have been pursued. First, small-molecule drugs have been developed that act on HDL metabolism and the RCT pathway to increase levels of circulating HDL [18,26]. Second, several forms of HDL replacement therapy have been studied [31,32]. Synthetic forms of HDL have been created by combining known biological components of HDL, Apo A1 and phospholipids, to create structures very similar to nascent HDL, termed reconstituted HDL (rHDL) [32–34]. In addition, administration of different forms of Apo A1, including Apo A1 mutants (e.g. Apo A1 Milano) and peptides that mimic the class A amphipathic helices of Apo A1, have been explored [31,35]. Administration of Apo A1 by itself has also been studied [36] but will only be discussed in terms of complexation with phospholipids in this article.
Small-molecule drugs
One of the most effective small-molecule drugs for increasing HDL levels is niacin [36]. Human studies show that niacin reduces the advancement of atherosclerosis and decreases cardiovascular events [36]. However, a significant side effect of niacin is dilation of the blood vessels in the skin of the face and upper body accompanied by a burning sensation [37]. This condition is known as flushing and limits tolerance and patient use [36,37].
Another class of small-molecule drugs being investigated is inhibitors of cholesteryl ester transfer protein (CETP). Inhibition of CETP prevents cholesterol transport from HDL to LDL, thus elevating HDL and HDL-cholesterol levels [36]. The most well-known CETP inhibitor is torcetrapib [36,38]. Human studies with torcetrapib have demonstrated that the drug increases levels of HDL compared to placebo [39–43]. However, torcetrapib was found to have little to no ability to reduce atherosclerosis, caused undesirable side effects, including increased blood pressure, and most importantly increased the risk of death in treated patients [43–45].
Two other CETP inhibitors are being studied, JTT-705 and anacetrapib. In human trials, both increase levels of HDL while decreasing levels of LDL compared to placebo and do not appear to have an effect on blood pressure [46–49]. The ability of these molecules to inhibit atherosclerosis in humans is still unknown, though studies with JTT-705 in rabbits exhibited mixed results for limiting the progression of atherosclerosis [36,50,51]. The long-term effects of JTT-705 and anacetrapib as well as the potential of CETP inhibitors to decrease atherosclerosis in humans remain to be seen.
HDL replacement therapies
Various methods exist for generating rHDL. These rely upon the amphipathic properties of Apo AI for self-assembly [17,35]. In all of these methods, Apo AI participates in a self-assembly process to form a disk-like structure very similar to nascent HDL [52,53]. There is some control of the diameter of the rHDL disks by varying the ratio of the phospholipids and Apo A1 [54,55]. rHDL particles range in size from approximately 7 to 13 nm, and monodisperse rHDL can be obtained by chromatography or gradient ultracentrifugation [17,33,54].
Numerous studies in animal models demonstrate the anti-inflammatory and anti-atherogenic properties of rHDL [17,32,56]. Two studies in humans with rHDL have shown the potential for using rHDL to treat atherosclerosis [57,58]. In the first study, no significant changes were observed in plaque volume compared to the placebo for the lower dose of rHDL (40 mg/kg), [57]. However, the plaque characterization index, a composite measurement of the characteristics of an atherosclerotic plaque as assessed by intravascular ultrasound, showed a modest but statistically significant improvement in the rHDL group as compared to placebo [57]. The highest dose of rHDL (80 mg/kg) was terminated due to elevations in transaminase levels, a sign of abnormal liver function and possible liver toxicity [57,59].
The second study examined plaque from the peripheral vasculature after a single injection of 80 mg/kg of rHDL [58]. In this study, administration of rHDL compared to the placebo resulted in a decrease in plaque lipid content, in the size of macrophage cells in the plaque, and in the expression of vascular cell adhesion molecule-1, indicating a decrease in inflammation [58]. Furthermore, enhanced in vitro cholesterol efflux to Apo B-depleted serum was observed for treated versus untreated patient serum [58]. These clinical studies demonstrate the therapeutic potential of increasing HDL levels through administration of synthetic HDL. Perceived drawbacks to rHDL therapies are the requirement of intravenous administration, the expense of producing rHDL, and limitations in the mass production of rHDL [32,38].
A mutant of Apo A1, called Apo A1Milano, has also shown many beneficial anti-atherogenic properties including the ability to efflux cholesterol from macrophage cells and a greater capacity to protect phospholipids from oxidation compared to wild-type Apo A1 [56,60]. Apo A1Milano has been combined with phospholipids to generate rHDL. In a human clinical trial, rHDL made with Apo A1Milano (ETC-216) that was administered weekly for 5 weeks resulted in a 4.2% decrease in plaque volume compared to baseline values [61]. As with rHDL made with wild-type Apo A1, studies of Apo A1Milano rHDL suggest that administration of rHDL initiates plaque regression, and may be used to treat CHD [32]. The use of Apo A1Milano rHDL as a therapeutic also faces similar disadvantages to rHDLs synthesized using wild-type Apo A1 [32]. Furthermore, rHDL species are restricted in the ability to manipulate chemical composition, size, and shape [17,54,55]—factors that may well impact therapeutic efficacy.
As an alternative to Apo A1 and rHDL, peptides that mimic the class A amphipathic helices of Apo A1 have been developed [35]. The peptide that has received the greatest amount of investigation is an 18 amino acid peptide that has been modified with different numbers of phenylalanine (F) residues on the hydrophobic face [35]. Studies have shown that the peptide 4F, which contains four phenylalanine residues, is the most biologically active, reducing atherosclerotic plaque and inflammation in mice [31,35]. One study has been done in humans. In this study, the peptide 4F made with D-amino acids (D-4F) caused a significant improvement in the inflammatory index of HDL compared to placebo and had a favorable side-effect profile [62]. This study shows that D-4F peptides may greatly enhance the anti-inflammatory properties of HDL but does not provide any information about their ability to decrease atherosclerosis and treat CHD. Further studies are needed to determine the effectiveness of Apo A1 mimetic peptides in treating atherosclerosis in humans.
Replacement HDL therapies have been largely restricted to Apo AI alone, nascent rHDL species, and Apo A1 mimetic peptides [31,38]. Less explored are more mature spherical forms of HDL. This is due, in part, to the complexity of the biological steps required for fabricating mature, spherical HDL species [52,63]. Biological maturation of discoidal rHDL species to spherical ones requires enzymes and intermediates [52,54]. Current synthetic routes provide no direct control over the final composition, presenting difficulties with reproducibly synthesizing and purifying spherical HDL products [52,63]. With more data emerging that indicate that size, shape, and composition of HDL largely dictates in vivo function [19,26], it is imperative to develop methods that provide exquisite control over these parameters. As such, new technologies are needed to generate synthetic, tailorable HDL structures that can accurately mimic specific species of HDL in order to identify the most optimal HDL mimics for treating atherosclerosis. In addition, the ability to modify these structures for imaging purposes may enable early detection of vulnerable plaques and decrease the occurrence of sudden coronary events.
Nanotechnology for synthetic HDL
Nanotechnology provides a new platform for the development of CHD therapeutics. Inorganic nanoparticles provide a scaffold for creating synthetic structures that mimic mature, spherical forms of HDL [64,65]. This approach provides a strategy for bypassing the biological and self-assembly methods currently used to produce spherical HDL. One useful inorganic nanoparticle platform for biological application is colloidal gold nanoparticles (Au NPs).
Monodisperse colloidal Au NPs can be synthesized with precise control over size and shape [66,67]. In addition, Au NPs can be surface functionalized with a myriad of biological molecules including nucleic acids, proteins, lipids, and other small molecules [67–70]. Au NPs generally appear to be non-toxic [71,72]; however, surface functionalities significantly impact the in vitro and in vivo behavior of nanomaterials [68]. Complete characterization of Au NP cytotoxicity, biodistribution, and efficacy will be necessary for each nanoparticle conjugate before an individual structure can be approved for use in humans.
Replacing the hydrophobic core of natural HDL with a Au NP has the potential to generate a robust and stable structure that accurately replicates the size, shape, and surface chemistry of mature spherical forms of HDL. Synthetic control over each of these parameters may allow for a more detailed investigation of the structure-function relationship of HDLs as well as provide access to particular properties of HDL. Furthermore, the Au NP core can be replaced by other inorganic nanoparticles such as iron oxide nanoparticles (FeO NPs) or quantum dots (QDs), lending additional capabilities (i.e., imaging) [64,69]. Overall, nanoparticles constitute a modular system with great potential to create an array of HDL mimetics with multimodal capabilities for detecting and treating CHD.
Synthetic HDL based on inorganic nanoparticles
Therapeutic
The first synthetic biomimetic HDL using a Au NP core (HDL Au NP) for therapeutic purposes was demonstrated by the Thaxton and Mirkin groups [65]. A 5 nm diameter Au NP was first surface-functionalized with Apo A1 and then two different phospholipids were added to create the lipid layer (Figure 2). This HDL Au NP has both surface-chemical and physical properties similar to that of natural HDL. The hydrodynamic diameter of the HDL Au NPs measured by dynamic light scattering (DLS) is 18 ± 3 nm. Furthermore, each HDL Au NP has 2 to 4 Apo A1 proteins and approximately 75 to 95 aminated phospholipids [65]. These values correlate well with those of natural mature spherical HDL [3,27,73].
Figure 2. Synthesis of biomimetic HDL using a Au NP core for use as a therapeutic.
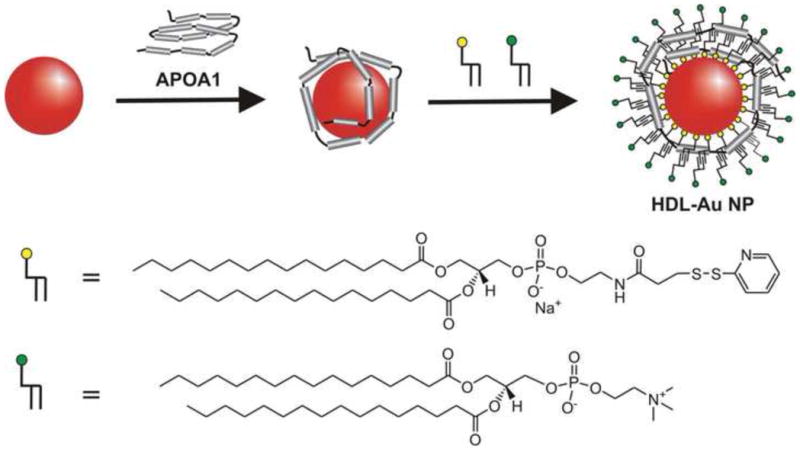
Citrate stabilized Au NPs 5 nm in diameter were surface-functionalized with Apo A1 and then with two phospholipids, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[3-(2-pyridyldithio)propionate] (yellow) and 1-2-dipalmitoyl-sn-glycero-3-phosphocholine (green). This HDL mimetic demonstrated tight cholesterol binding with a Kd of 3.8 ± 0.8 nM [65]. Reprinted from Journal of the American Chemical Society with permission from the American Chemical Society.
In order to quantify a functional baseline for cholesterol binding to a synthetic HDL Au NP, a fluorescently labeled cholesterol molecule, 25-[N-[(7-nitro-2-1,3-benzoxadiazol-4-yl)methyl]amino]-27-norcholesterol (NBD cholesterol) that is minimally fluorescent in aqueous environments and becomes highly fluorescent in lipid membranes (see [74]), was employed to evaluate the ability of the HDL Au NP to bind cholesterol in solution. HDL Au NPs were titrated with increasing concentrations of NBD cholesterol to develop a binding isotherm. The dissociation constant, Kd, was calculated from the binding isotherm as 3.8 ± 0.8 nM for the HDL Au NPs [65]. Dissociation constants, even for natural HDL, have not been reported in the literature. Accordingly, this value represents an initial baseline and serves as a point of comparison for future biomimetic HDL structures. This study demonstrates that biomimetic HDL Au NPs, with the size, shape, and surface chemistry of natural HDL and cholesterol binding capabilities, have the potential to be used to raise levels of circulating HDL acting as a novel therapeutic to treat atherosclerosis.
Imaging agent
Another example of HDL made with inorganic nanoparticles has been reported for imaging purposes. Cormode et al. demonstrated the use of three different inorganic nanoparticles to construct multi-modal imaging agents that mimic HDL (nanocrystal HDL) to image macrophages in atherosclerotic plaque [64]. Gold nanoparticles (Au HDL), iron oxide nanoparticles (FeO HDL), and quantum dots (QD HDL) were combined with Apo A1 and various phospholipids, including fluorescent and paramagnetic phospholipids, to create HDL mimetic structures that can be imaged by computed tomography (CT), magnetic resonance (MRI), and fluorescence (Figure 3) [64]. Control nanoparticles were also prepared using a pegylated phospholipid (Au PEG, FeO PEG, and QD PEG). The Au HDL and QD HDL both had ~3 Apo A1 proteins per nanoparticle, whereas the FeO HDL had ~4 Apo A1 proteins per nanoparticle. The sizes, as measured by TEM, were 9.7, 11.9, and 12.0 nm for the Au HDL, FeO HDL, and QD HDL, respectively [64]. DLS and gel electrophoresis data corroborated these values [64]. These analyses demonstrate that the nanocrystal HDLs closely resemble spherical HDL in terms of size and composition.
Figure 3. Synthetic HDLs with a nanocrystal core for imaging atherosclerotic plaque.

(a) Representations of the three nanocrystal HDLs. All three nanocrystal HDLs have Apo A1 on their surface to mimic natural HDL. The Au HDL has a Au NP core that is suitable for CT imaging and phospholipids modified with gadolinium (Gd) and rhodamine (Rhod) for MRI and fluorescence imaging, respectively. The FeO HDL contains a FeO nanoparticle core that is useful for MRI as well as Rhod-modified phospholipids for fluorescence imaging. The QD-HDL has a quantum dot core for fluorescence imaging and Gd-modified phospholipids for MRI. (b) Synthesis of the nanocrystal HDLs. 1. The phospholipids, myristoyl hydroxy phosphatidylcholine (MHPC), gadolinium dimyristoyl phosphoethanolamine diethylenetriamine pentaactic acid (Gd-DTPA-DMPE), and dimyristoyl phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) ammonium salt (Rhod-DMPE) were first added to the nanoparticles. 2. Apo A1 was added to the solution for incorporation into the phospholipid layer. 3. Ultracentrifugation was used to purify the nanocrystal HDL. These HDL mimetics demonstrated specificity for macrophage cells in vitro and in vivo and showed potential for imaging these cells through either MRI, CT, or fluorescence imaging [64]. Reprinted from Nano Letters with permission from the American Chemical Society
The nanocrystal HDLs were studied with macrophage cells and in the Apo E −/− atherosclerotic mouse model. Imaging of macrophage cells exposed to nanocrystal HDLs demonstrated much greater uptake of the nanocrystal HDL compared to the PEG control particles [64]. In the Apo E −/− mouse model, a significant change in MRI signal from the aortic wall was observed for the nanocrystal HDLs compared to the PEG controls [64]. Fluorescence and CT imaging of appropriate nanocrystal HDLs also verified the absorption of nanocrystal HDLs into the aorta wall compared to the PEG control particles [64]. This work establishes the ability to use different inorganic nanoparticles to create structures that accurately mimic natural HDL and can be employed to specifically image macrophage cells in the wall of a mouse aorta. By accurately mimicking HDL, the nanocrystal HDLs can potentially be used for the non-invasive diagnosis of atherosclerosis and CHD.
The studies reviewed above showcase the ability to use inorganic nanoparticles to create synthetic structures that mimic natural HDL in size, shape, composition, and function. They further highlight the importance and necessity of accurately mimicking natural HDL in order to obtain desired specificity. The first example binds cholesterol in solution and has the potential to be used as a therapeutic to combat atherosclerosis. The second example delivers imaging modalities to macrophage cells for the purpose of imaging atherosclerotic plaque. Without correctly mimicking natural HDL, it would be difficult to obtain the desired specificity and perform either of these functions. These investigations indicate that nanotechnology has the potential to provide synthetic control not available with existing strategies employed to make replacement HDL and to generate synthetic structures that accurately mimic natural spherical HDL.
Translational considerations
Questions remain regarding the in vivo safety and efficacy of inorganic nanoparticles for treating atherosclerosis, CHD, and other diseases. Safety and efficacy concerns are not unique to novel therapeutic or imaging agents under consideration for human use. Unique, however, is the relative lack of experience that pharmaceutical companies and regulatory agencies have with regard to evaluating and regulating nanoparticles. Furthermore, the ensemble of nanoparticle platform, surface functionality(s), and internal functionality(s) cooperates to dictate in vivo pharmacokinetics and efficacy such that each agent will require individual detailed attention by investigators, interested industrial partners, and regulatory agencies. Ultimately, successful application of nanotechnology to atherosclerosis and CHD will require multidisciplinary collaboration.
Concluding remarks
CHD is the leading cause of death in the world, and new strategies for the treatment of CHD are needed. Novel nanotechnology constructs present promising therapeutic possibilities and offer advantages that are lacking in current therapeutic strategies. Though the most widely explored methods for augmenting circulating levels of HDL have demonstrated some effectiveness clinically, administration requirements, synthetic challenges, and undesirable side effects hinder their therapeutic potential. The use of nanotechnology to accurately mimic natural HDL, thereby avoiding off-target effects and potentially imitating the atheroprotective abilities of natural HDL, holds great promise for creating a therapeutic to combat atherosclerosis. The synthetic processes used to create synthetic HDLs with an inorganic nanoparticle core provide control that may allow investigation of the structure-function relationship of HDL and identify the synthetic HDL structure most effective for reversing atherosclerosis and treating CHD. In addition, the inclusion of imaging modalities or other molecules lends the ability to generate multimodal structures with additional functionalities. The novel capabilities presented by nanotechnology hold great promise for improving the treatment of atherosclerosis and CHD.
Box 2. Outstanding Questions.
Will inorganic nanoparticle based HDL absorb cholesterol in vivo and transport it to the liver according to the RCT pathway?
If inorganic nanoparticle based HDLs do transport cholesterol in vivo, will they be effective at removing cholesterol from foam cells and decreasing or reversing the formation of atherosclerotic plaque?
Do inorganic nanoparticle based HDLs require intravenous injection, or can they be dosed orally?
What is the stability and lifetime of the inorganic nanoparticle based HDL in vivo? Does it accumulate in the body, in a particular tissue? How is it degraded and/or excreted?
Are there any side effects or toxicity related to inorganic nanoparticle based HDL?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, et al. Heart disease and stroke statistics-2010 update a report from the American Heart Association. Circulation. 2010;121:E46–E215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Mackay J, Mensah GA. Atlas of Heart Disease and Stroke. World Health Organization and Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 3.Peter Libby M, Robert O, Bonow MD, Douglas L, Mann MD, Douglas P, Zipes MD, editors. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. W. B. Saunders; 2007. [Google Scholar]
- 4.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falk E. Pathogenesis of atherosclerosis. Journal of the American College of Cardiology. 2006;47:C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 6.Khera AV, Rader DJ. Future therapeutic directions in reverse cholesterol transport. Current Atherosclerosis Reports. 2010;12:73–81. doi: 10.1007/s11883-009-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nature Reviews Molecular Cell Biology. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 8.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 9.Ucar M, et al. HMG-CoA reductase inhibitors and myotoxicity. Drug Safety. 2000;22:441–457. doi: 10.2165/00002018-200022060-00003. [DOI] [PubMed] [Google Scholar]
- 10.Gotto JAM, Grundy SM. Lowering LDL cholesterol: Questions from recent meta-analyses and subset analyses of clinical trial data - Issues from the Interdisciplinary Council on Reducing the Risk for Coronary Heart Disease, Ninth Council Meeting. Circulation. 1999;99:e1–e7. doi: 10.1161/01.cir.99.8.e1. [DOI] [PubMed] [Google Scholar]
- 11.Singh IM. High-density lipoprotein as a therapeutic target: a systematic review. Journal of the American Medical Association. 2007;298:786–798. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen TR, et al. Randomized trial of cholesterol-lowering in 4444 patients with Coronary-Heart-Disease - the Scandinavian Simvastatin Survival Study (4s) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 13.Bruckert E, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovascular Drugs and Therapy. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 14.Bays H. Statin safety: an overview and assessment of the data--2005. American Journal of Cardiology. 2006;97:6C–26C. doi: 10.1016/j.amjcard.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson TA. Toward “pain-free” statin prescribing: clinical algorithm for diagnosis and management of myalgia. Mayo Clinic Proceedings. 2008;83:687–700. doi: 10.4065/83.6.687. [DOI] [PubMed] [Google Scholar]
- 16.Unal B, et al. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004;109:1101–1107. doi: 10.1161/01.CIR.0000118498.35499.B2. [DOI] [PubMed] [Google Scholar]
- 17.Calabresi L, et al. Synthetic high density lipoproteins for the treatment of myocardial ischemia/reperfusion injury. Pharmacology & Therapeutics. 2006;111:836–854. doi: 10.1016/j.pharmthera.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Joy T, Hegele RA. Is raising HDL a futile strategy for atheroprotection? Nature Reviews Drug Discovery. 2008;7:143–155. doi: 10.1038/nrd2489. [DOI] [PubMed] [Google Scholar]
- 19.Movva R, Rader DJ. Laboratory assessment of HDL heterogeneity and function. Clinical Chemistry. 2008;54:788–800. doi: 10.1373/clinchem.2007.101923. [DOI] [PubMed] [Google Scholar]
- 20.Duffy D, Rader DJ. Emerging therapies targeting high-density lipoprotein metabolism and reverse cholesterol transport. Circulation. 2006;113:1140–1150. doi: 10.1161/CIRCULATIONAHA.105.593855. [DOI] [PubMed] [Google Scholar]
- 21.Naghavi M, et al. From vulnerable plaque to vulnerable patient - A call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 22.Zipes DP, Wellens HJJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 23.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 24.Fuster V, et al. Early identification of atherosclerotic disease by noninvasive imaging. Nature Reviews Cardiology. 2010;7:327–333. doi: 10.1038/nrcardio.2010.54. [DOI] [PubMed] [Google Scholar]
- 25.Jonas A, Phillips MC. Lipoprotein structure. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membranes. 5. Elsevier B. V; 2008. pp. 485–506. [Google Scholar]
- 26.Degoma EM, et al. Beyond high-density lipoprotein cholesterol levels - Evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. Journal of the American College of Cardiology. 2008;51:2199–2211. doi: 10.1016/j.jacc.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warnick GR, et al. Polyacrylamide gradient gel electrophoresis of lipoprotein subclasses. Clinics in Laboratory Medicine. 2006;26:803–846. doi: 10.1016/j.cll.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Asztalos BF, et al. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arteriosclerosclerosis, Thrombosis, and Vascular Biology. 2004;24:2181–2187. doi: 10.1161/01.ATV.0000146325.93749.a8. [DOI] [PubMed] [Google Scholar]
- 29.Dobiasova M, et al. Effect of labeling of plasma lipoproteins with H-3 cholesterol on values of esterification rate of cholesterol in apolipoprotein B-depleted plasma. Journal of Lipid Research. 2000;41:1356–1357. [PubMed] [Google Scholar]
- 30.Brites FD, et al. Paraoxonase 1 and platelet-activating factor acetylhydrolase activities in patients with low HDL-cholesterol levels with or without primary hypertriglyceridemia. Archives of Medical Research. 2004;35:235–240. doi: 10.1016/j.arcmed.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Navab M, et al. Structure and function of HDL mimetics. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30:164–168. doi: 10.1161/ATVBAHA.109.187518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy AJ, et al. Reconstituted HDL: a therapy for atherosclerosis and beyond. Clinical Lipidology. 2009;4:731–739. [Google Scholar]
- 33.Jonas A, et al. Defined apolipoprotein A-I conformations in reconstituted high-density lipoprotein disks. The Journal of Biological Chemistry. 1989;264:4818–4824. [PubMed] [Google Scholar]
- 34.Matz CE, Jonas A. Micellar complexes of human apolipoprotein a-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. The Journal of Biological Chemistry. 1982;257:4535–4540. [PubMed] [Google Scholar]
- 35.Sherman CB, et al. Apolipoprotein A-1 mimetic peptides: A potential new therapy for the prevention of atherosclerosis. Cardiology in Review. 2010;18:141–147. doi: 10.1097/CRD.0b013e3181c4b508. [DOI] [PubMed] [Google Scholar]
- 36.Duffy D, Rader DJ. Update on strategies to increase HDL quantity and function. Nature Reviews Cardiology. 2009;6:455–463. doi: 10.1038/nrcardio.2009.94. [DOI] [PubMed] [Google Scholar]
- 37.Benyo Z, et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. The Journal of Clinical Investigation. 2005;115:3634–3640. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conca P, Franceschini G. Synthetic HDL as a new treatment for atherosclerosis regression: has the time come? Nutrition, Metabolism and Cardiovascular Diseases. 2008;18:329–335. doi: 10.1016/j.numecd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Brousseau ME, et al. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. New England Journal of Medicine. 2004;350:1505–1515. doi: 10.1056/NEJMoa031766. [DOI] [PubMed] [Google Scholar]
- 40.Brousseau ME, et al. Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:1057–1064. doi: 10.1161/01.ATV.0000161928.16334.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKenney JM, et al. Efficacy and safety of torcetrapib, a novel cholesteryl ester transfer protein inhibitor, in individuals with below-average high-density lipoprotein cholesterol levels on a background of atorvastatin. Journal of the American College of Cardiology. 2006;48:1782–1790. doi: 10.1016/j.jacc.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 42.Davidson MH, et al. Efficacy and safety of torcetrapib, a novel cholesteryl ester transfer protein inhibitor, in individuals with below-average high-density lipoprotein cholesterol levels. Journal of the American College of Cardiology. 2006;48:1774–1781. doi: 10.1016/j.jacc.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 43.Barter PJ, et al. Effects of torcetrapib in patients at high risk for coronary events. New England Journal of Medicine. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 44.Bots ML, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 2007;370:153–160. doi: 10.1016/S0140-6736(07)61088-5. [DOI] [PubMed] [Google Scholar]
- 45.Kastelein JJP, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. New England Journal of Medicine. 2007;356:1620–1630. doi: 10.1056/NEJMoa071359. [DOI] [PubMed] [Google Scholar]
- 46.de Grooth GJ, et al. Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans - A randomized phase II dose-response study. Circulation. 2002;105:2159–2165. doi: 10.1161/01.cir.0000015857.31889.7b. [DOI] [PubMed] [Google Scholar]
- 47.Krishna R, et al. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomised placebo-controlled phase I studies. Lancet. 2007;370:1907–1914. doi: 10.1016/S0140-6736(07)61813-3. [DOI] [PubMed] [Google Scholar]
- 48.Bloomfield D, et al. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. American Heart Journal. 2009;157:352–360. doi: 10.1016/j.ahj.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Hermann F, et al. Cholesterylestertransfer protein inhibition and endothelial function in type II hyperlipidemia. Thrombosis Research. 2009;123:460–465. doi: 10.1016/j.thromres.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto H, et al. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature. 2000;406:203–207. doi: 10.1038/35018119. [DOI] [PubMed] [Google Scholar]
- 51.Huang ZP, et al. Cholesteryl ester transfer protein inhibitor (JTT-705) and the development of atherosclerosis in rabbits with severe hypercholesterolaemia. Clinical Science. 2002;103:587–594. doi: 10.1042/cs1030587. [DOI] [PubMed] [Google Scholar]
- 52.Jonas A. Reconstitution of high-density lipoproteins. Methods in Enzymology. 1986;128:553–582. doi: 10.1016/0076-6879(86)28092-1. [DOI] [PubMed] [Google Scholar]
- 53.Shih AY, et al. Disassembly of nanodiscs with cholate. Nano Letters. 2007;7:1692–1696. doi: 10.1021/nl0706906. [DOI] [PubMed] [Google Scholar]
- 54.Calabresi L, et al. Apolipoprotein-A-I conformation in discoidal particles - Evidence for alternate structures. Biochemistry. 1993;32:6477–6484. doi: 10.1021/bi00076a023. [DOI] [PubMed] [Google Scholar]
- 55.Silva RAGD, et al. A mass spectrometric determination of the conformation of dimeric apolipoprotein A-I in discoidal high density lipoproteins. Biochemistry. 2005;44:8600–8607. doi: 10.1021/bi050421z. [DOI] [PubMed] [Google Scholar]
- 56.Chiesa G, et al. Acute effects of high-density lipoproteins: biochemical basis and clinical findings. Current Opinion in Cardiology. 2008;23:379–385. doi: 10.1097/HCO.0b013e3283007ccd. [DOI] [PubMed] [Google Scholar]
- 57.Tardif JC, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis - A randomized controlled trial. Journal of the American Medical Association. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 58.Shaw JA, et al. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circulation Research. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 59.Bai J, Cederbaum AI. Adenovirus-mediated expression of CYP2E1 produces liver toxicity in mice. Toxicological Sciences. 2006;91:365–371. doi: 10.1093/toxsci/kfj165. [DOI] [PubMed] [Google Scholar]
- 60.Favari E, et al. A unique protease-sensitive high density lipoprotein particle containing the apolipoprotein A-I-Milano dimer effectively promotes ATP-binding cassette A1-mediated cell cholesterol efflux. The Journal of Biological Chemistry. 2007;282:5125–5132. doi: 10.1074/jbc.M609336200. [DOI] [PubMed] [Google Scholar]
- 61.Nissen SE, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes - A randomized controlled trial. Journal of the American Medical Association. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 62.Bloedon LT, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. Journal of Lipid Research. 2008;49:1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silva RAGD, et al. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12176–12181. doi: 10.1073/pnas.0803626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cormode DP, et al. Nanocrystal core high-density lipoproteins: A multimodality contrast agent platform. Nano Letters. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thaxton CS, et al. Templated spherical high density lipoprotein nanoparticles. Journal of the American Chemical Society. 2009;131:1384–1385. doi: 10.1021/ja808856z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Millstone JE, et al. Iodide ions control seed-mediated growth of anisotropic gold nanoparticles. Nano Letters. 2008;8:2526–2529. doi: 10.1021/nl8016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang ZX, Ma LN. Gold nanoparticle probes. Coordination Chemistry Reviews. 2009;253:1607–1618. [Google Scholar]
- 68.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chemical Society Reviews. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 69.Agasti SS, et al. Nanoparticles for detection and diagnosis. Advanced Drug Delivery Reviews. 2010;62:316–328. doi: 10.1016/j.addr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mirkin CA. The polyvalent gold nanoparticle conjugate-materials synthesis, biodiagnostics, and intracellular gene regulation. MRS Bulletin. 2010;35:532–539. doi: 10.1557/mrs2010.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esther RJ, et al. Gold nanoparticles do not affect the global transcriptional program of human umbilical vein endothelial cells: A DNA-microarray analysis. Journal of Biomedical Nanotechnology. 2005;1:328–335. [Google Scholar]
- 72.Fadeel B, Garcia-Bennett AE. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Advanced Drug Delivery Reviews. 2010;62:362–374. doi: 10.1016/j.addr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 73.Segrest JP, et al. Structure and function of apolipoprotein A-I and high-density lipoprotein. Current Opinion in Lipidology. 2000;11:105–115. doi: 10.1097/00041433-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 74.Chattopadhyay A. Chemistry and biology of N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled lipids - fluorescent-probes of biological and model membranes. Chemistry and Physics of Lipids. 1990;53:1–15. doi: 10.1016/0009-3084(90)90128-e. [DOI] [PubMed] [Google Scholar]
- 75.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. Journal of Clinical Investigation. 2006;116:3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Segrest JP, et al. A detailed molecular belt model for apolipoprotein A-I in discoidal high density lipoprotein. The Journal of Biological Chemistry. 1999;274:31755–31758. doi: 10.1074/jbc.274.45.31755. [DOI] [PubMed] [Google Scholar]
- 77.Shih AY, et al. Maturation of high-density lipoproteins. Journal of the Royal Society Interface. 2009;6:863–871. doi: 10.1098/rsif.2009.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barkowski RS, Frishman WH. HDL metabolism and CETP inhibition. Cardiology in Review. 2008;16:154–162. doi: 10.1097/CRD.0b013e31816a3b60. [DOI] [PubMed] [Google Scholar]


