Abstract
A method is described that allows noninvasive identification and quantitative assessment of lipid classes present in sebaceous excretions in rodents. The method relies on direct high-field proton NMR analysis of common group lipid protons in deuterated organic solvent extracts of fur. Extracts from as little as 15 mg of fur from rat, mouse, and hamster provided acceptable results on a 600 MHz NMR equipped with a cryogenically cooled proton-observe probe. In rats, sex- and age-related differences in lipid composition are larger than differences in fur collected from various body regions within an individual and much larger than interanimal differences in age- and sex-matched specimens. The utility of this method to noninvasively monitor drug-induced sebaceous gland atrophy in rodents is demonstrated in rats dosed with a stearoyl-CoA desaturase 1 (SCD1) inhibitor. In this model, a 35% reduction in sebum lipids, extracted from fur, was observed. Finally, structural elucidation of cholesta-7,24-dien-3β-ol ester as the most prominent, previously unidentified sebum sterol ester in male Syrian hamsters is described. The utility of this method for drug and cosmetic safety and efficacy assessment is discussed.
Keywords: metabolomics, skin lipid, fur sebum, fur lipid, sebum lipid, human sebum, rat sebum, mouse sebum, hamster sebum, NMR assay, lipid class measurement, stearoyl-CoA desaturase 1
Sebum is a complex lipid-rich fluid that is excreted by sebaceous glands, which are key components of the follicular organ and present in all fur- and hair-bearing mammals (1, 2). Fur or hair is coated with sebum as it emerges from the follicle and then is redistributed through periodic grooming. Sebaceous glands and the sebum they produce have numerous normal and pathophysiological functions (2, 3). For example, it is widely accepted that sebum serves as a lubricating and hydrophobic film (4) and is crucial in fetal development (2). The composition of sebum is highly species specific (2) and can be affected by both systemic and environmental perturbations, including bacterial invasion (3), oral agents that affect lipid biosynthesis (5), skin disease (2, 6, 7), and toxicity (8, 9). Inasmuch as sebaceous glands are a prominent component of mammalian skin, sebum composition and excretion rates are one measure of overall skin health. It is desirable to have a noninvasive method for measuring these parameters to support safety or efficacy assessments in pharmaceutical or cosmetic research where either skin sensitivity may be a concern or sebum reduction is the target.
Traditionally, many analytical methods for sebum assessment have been reported. These can be divided into two groups based on whether they 1) distinguish individual lipid molecules or 2) measure lipid classes independent of the exact FA substituents. Previously, we described an NMR spectroscopy-based method that falls into the second category for assessing the molecular constitution of sebum and compared this with other analytical approaches (5). The NMR method relies on accurate integration of specific protons on selected analytes in extracts of skin biopsies and absorbent films commonly used in clinical evaluation of sebum excretion. The fundamental advantage of this method is that by integrating a peak from a single headgroup proton (or protons) arising from a class of lipids (e.g., the H3 of esterified cholesterol), one can assess the molar concentration of the entire class, independent of the FA distribution. In contrast, chromatographic- or mass spectrometric-based methods must contend with multiple (up to dozens) of individual analytes within the class that have different masses and physical properties. In this work, we extend its utility to fur clippings that can be obtained noninvasively for use in preclinical studies. We have evaluated the fur lipid content in rodents as a function of species, gender, age, and body region from which the fur is collected and show that this method is in good agreement with reports based on established methods. We also apply the method to study fur lipid changes in rodents upon dosing with the previously reported stearoyl-CoA desaturase 1 (SCD1) inhibitor, compound 1 (10), as shown in Scheme 1. SCD1 catalyzes the biosynthesis of MUFAs from saturated FAs, and the modulation of its activity or expression level has been suggested to have varied therapeutic benefits (8, 11–13) and a role in proinflammatory activity in human sebocyte cultures (14). However, it is known that compromised SCD1 activity also has adverse effects in rodents. For example, mice that have a natural defect in the SCD1 gene (15), and mice that have the SCD1 gene knocked out globally (9) or specifically in the skin (13), show mechanism-based sebaceous gland, hair follicle, and ocular abnormalities. Furthermore, rodents treated with SCD1 inhibitors display similar adverse effects (8). In this work, we demonstrate a significant reduction of fur lipids upon treatment of rats and hamsters with compound 1, a compound known to cause alopecia and atrophy of sebaceous glands in mice (8). Finally, we demonstrate the power of the method to distinguish between subclasses of some lipids and thereby identify a sterol ester that was previously reported in the sebum of male Syrian hamsters using TLC (16) or NMR (5) but remained unidentified until now.
Scheme 1.
Structures of the SCD1 inhibitor (1) and key sterols referred to in this study. Atom numbering in referred to in the structure elucidation is shown.
METHODS
Animal husbandry and sample collection
All animal procedures for this experiment were approved by Bristol-Myers Squibb Animal Care and Use Committee. Proper temperature and humidity conditions were maintained, and animals were provided food and water ad libitum. All animals were supplied by Charles River Laboratories. For method development studies, Sprague Dawley [Crl:CD SD (IGS)] male and female rats were ∼9 weeks or 21–24 weeks of age (N = 6). Male mice [Crl:CD-1(ICR)] (N = 6) were ∼9 weeks of age, and male and female golden Syrian hamsters [Crl:LVG (SYR)] (N = 6) were ∼8 weeks of age at the time of sampling. For the SCD1 inhibitor study, male CD rats weighing ∼240 g at the beginning of the study were administered compound 1 in hydroxypropyl-betacyclodextrin (HPBC) vehicle at 3, 10, and 30 mg/kg po for 21 days, with N = 5 for all doses. Male golden Syrian hamsters [Crl:LVG (SYR)], 8–9 weeks old and weighing ∼110–120 g, were administered 60 mg/kg of compound 1 in N-methyl-2-pyrrolidone (NMP)/tocopheryl polyethylene glycol succinate (TPGS)/polyethylene glycol (PEG) 400/water 10:10:60:20 vehicle for 21 days. Dorsal fur samples were taken 1 day prior to necropsy.
Rats were singly housed in wire-bottom stainless steel cages, mice were singly housed in plastic shoeboxes with Alpha-dri™ bedding, and hamsters were group housed three per cage in plastic cage bins with Alpha-dri™ bedding. All fur sampling was performed by gently restraining the animal and clipping a sufficient amount of fur (typically 10–30 mg) as close to the skin surface as possible with blunt-tipped, curved surgical scissors (curve down) and wiping the scissors off between sampling with 70% isopropyl alcohol. Samples were immediately placed into 15 ml Falcon conical tubes (VWR) and placed on dry ice. Mouse and hamster fur samples were collected over the dorsal thoracic area. Rat samples, male and female, were collected from the dorsal thoracic, dorsal caudal (just anterior of the tail), ventral abdominal, and right/left flanks (combined).
Animal studies were conducted in accordance with the current guidelines for animal welfare (Guide for the Care and Use of Laboratory Animals, 1996). The procedures used in this study have been reviewed and approved by the Institutional Animal Care and Use Committee.
Fur sample preparation
For the SCD1 inhibitor studies, weighed fur samples (13–23 mg) were placed in a glass vial with 2.0 ml of CHCl3, capped with a Teflon-lined screw cap, and vortexed briefly to wet the entire fur sample. Then, the entire supernatant was removed and allowed to evaporate to dryness and reconstituted in 600 μl CDCl3.
For all the other quantitation studies, 20–30 mg of fur was transferred and weighed in glass screw-cap vials. A measured volume of CDCl3 containing 0.47 mM arachidonyl alcohol (AA) as an internal standard was added to provide 50.0 μl of solvent per mg of fur. The samples were vortexed for ∼30 s, and 700 μl was transferred to 5 mm NMR tubes for recording NMR spectra. Thus, each NMR sample contained the extracted lipids from 14 mg of fur.
Authentic reference standards
All reference standard compounds were either synthesized (as described subsequently) or purchased from Sigma, including 1,2-distearoyl-3-oleoyl-rac-glycerol (TG), cholesteryl stearate [cholesteryl ester (CE)], stearyl palmitate [wax ester (WE)], stearoyl chloride, desmosterol, and lathosterol.
Lathosteryl stearate and desmosteryl stearate synthesis
To the solution of stearoyl chloride (33.3 mg, 0.11 mmol) in CH2Cl2 (2 ml) was added the free sterol (lathosterol, 38.7 mg, 0.1 mmol) followed by triethylamine (10 mg, 0.1 mmol) and N,N-dimethylaminopyridine (1.23 mg, 0.01 mmol). The resulting mixture was stirred at room temperature for 16 h, then purified by silica gel chromatography and eluted with 10% hexane in ethyl acetate. After pooling fractions and evaporating solvent, the desired product was obtained as white solid (43%, 28 mg). Desmosteryl stearate was synthesized in an identical manner as lathosteryl stearate.
NMR data acquisition for the quantitative fur study
Proton NMR spectra were acquired with a Bruker Avance NMR spectrometer operating at 600.11 MHz for proton using a 5 mm liquid-helium-cooled 1H[13C,15N] probe (triple resonance cryoprobe) equipped with a z-axis gradient and an automatic sample changer with a capacity of 60 samples (BACS 60). The acquisition parameters common for all experiments included a 20 ppm spectral width and a 2.6 s acquisition time. Free induction decays represent the sum of 256 transients acquired into 65,536 data points. All data were acquired with the samples maintained at 27°C.
NMR data processing for the quantitative fur study
Proton free induction decay data were processed using Topspin 2.1 (Bruker Biospin). The data were Fourier transformed using exponential multiplication with 0.3 Hz line broadening. Spectra were phased, baseline corrected, and referenced to the TMS signal at 0 ppm. Individual peaks of interest were integrated using the multi-integration tool in AMIX 3.8 (Bruker Biospin) by carefully selecting chemical shift ranges that composed the peak of interest across all spectra. For the nondrug studies, the integrals thus obtained were normalized indirectly to the internal standard AA peak at 3.65 ppm. Because some samples had components that overlapped with this peak, internal TMS was used as a secondary concentration reference based on its comparison with the AA peak in samples that had no overlap via a normalization factor, M, which was calculated based on the ratio of intensities of the TMS peak and the AA peak across 20 samples where no overlap existed:
| (Eq. 1) |
where ATMS and AAA are the mean integrated intensities of TMS or AA over 20 samples, [AA] is the concentration of AA, and N is the number of equivalent protons (2) from AA at 3.65 ppm. Substituting the appropriate values, one gets M = 36.04 μM. The individual lipid concentrations [lipid] (micromolar) within each sample were then given by the integrals of selected lipid peaks and TMS within that sample:
| (Eq. 2) |
where Ilipid and ITMS are the integrals of the lipid peak of interest and the TMS in the sample being measured, and N is 1, 2, or 3 for peaks arising from methine, methylene, or methyl groups, respectively. Finally the fur lipid load, L, or nanomoles of lipid per milligram of fur, was calculated:
| (Eq. 3) |
where V is the volume of the solvent used (in milliliters) for extraction, and W is the weight of fur (in milligrams). Microsoft Excel was used for all the analyses and graphical presentations that enabled comparison of levels in the sebum lipid components, namely TGs, WEs, lathosterol esters (LEs) lathosterol-like sterol esters (LLEs), CEs, and total cholesterol (TC). For the SCD1 inhibitor study, peak integrals were normalized to the amount of fur by dividing raw spectral integrals by fur weight (milligram) to provide arbitrary relative concentration units.
TLC separation and purification of sterol ester from male hamster fur
To identify the sterol ester in male hamster fur, separation and purification of lipid fractions was carried out by TLC. Male and female hamster fur was freshly clipped and stored at −80°C until ready for chloroform extraction.
For the test run, ∼20 mg of male and female hamster fur was extracted as described previously with 1.0 ml of CHCl3, and the supernatant was completely dried. The residue was dissolved in 50 µl chloroform for TLC. Once the TLC separation conditions were optimized, as noted subsequently, ∼500 mg of male hamster fur was extracted in 25 ml chloroform for male hamster sterol ester purification.
The TLC separation method from Brind et al. (16) and the reference therein (17) was followed. First, the TLC plate was preactivated for 15 min at 130°C and then loaded with extracts and a mixture of standards (20 μg each). Second, the lipids were eluted with hexane to 20 cm, toluene to 20 cm, and hexane-diethyl ether-acetic acid (70:30:1) to ∼11 cm. For the test run, to optimize separation conditions and to obtain a picture with better contrast for the separated lipid bands, the plate was dyed with CuSO4 to visualize the exact location of bands of interest. Third, for the final purification run, the staining was done with iodine. After scraping off the spot that matched the elution position of CE and LE in the standards lane, the iodine was evaporated in air. When the silica gel lost all color, the lipid content was extracted from it using 0.6 ml of CDCl3, which was immediately placed into an NMR tube for spectroscopic analysis.
NMR characterization of TLC-isolated male hamster sterol ester
To identify the sterol ester in male hamster fur, an NMR sample in CDCl3 (containing chemical shift reference standard TMS) was prepared as described in the previous section from TLC-separated sterol ester band of ∼500 mg of male hamster fur. Additionally, standard samples were prepared in CDCl3 from desmosterol (Sigma) and lathosteryl stearate (synthesis described previously).
For the structure determinations, a standard suite of structure elucidation NMR experiments [proton 1D, two dimensional homonuclear correlated spectroscopy (COSY), edited two dimensional heteronuclear single quantum coherence spectroscopy (HSQC), and heternuclear multiple bond correlation (HMBC)] was carried out using the following acquisition parameters for the 2D NMR experiments: 1) gradient COSY: 16 scans, 256 free-induction decays (fids), 2,048 data points in the direct dimension with 12.5 ppm spectral width; 2) gradient multiplicity-edited HSQC: 64 scans, 256 fids, 2,048 data points with 17 ppm proton and 300 ppm carbon spectral width; and 3) gradient HMBC: 200 scans, 512 fids, 1,024 data points with 9 ppm proton and 220 ppm carbon spectral width. NMR data for authentic desmosterol, desmosteryl stearate, and lathosteryl stearate standard samples were acquired on a 500 MHz Bruker NMR system with a conventional 5 mm proton-observe probe. All the spectra were referenced to TMS at 0.0 ppm.
RESULTS
Fur lipid quantification from NMR spectra
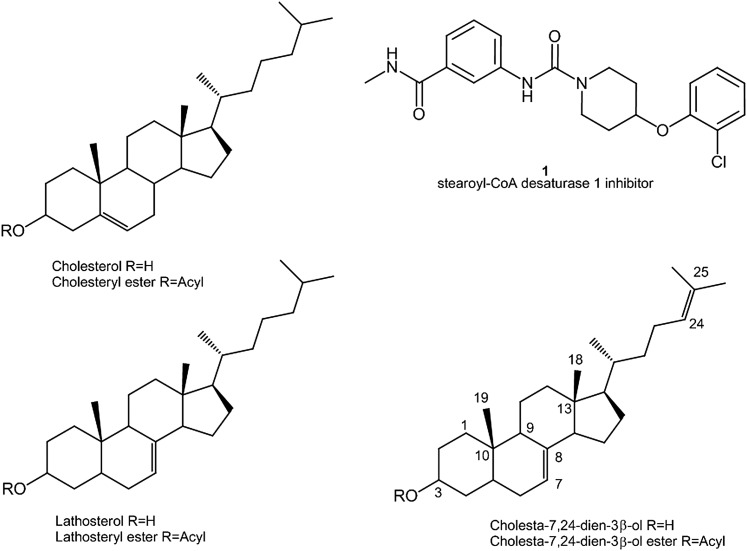
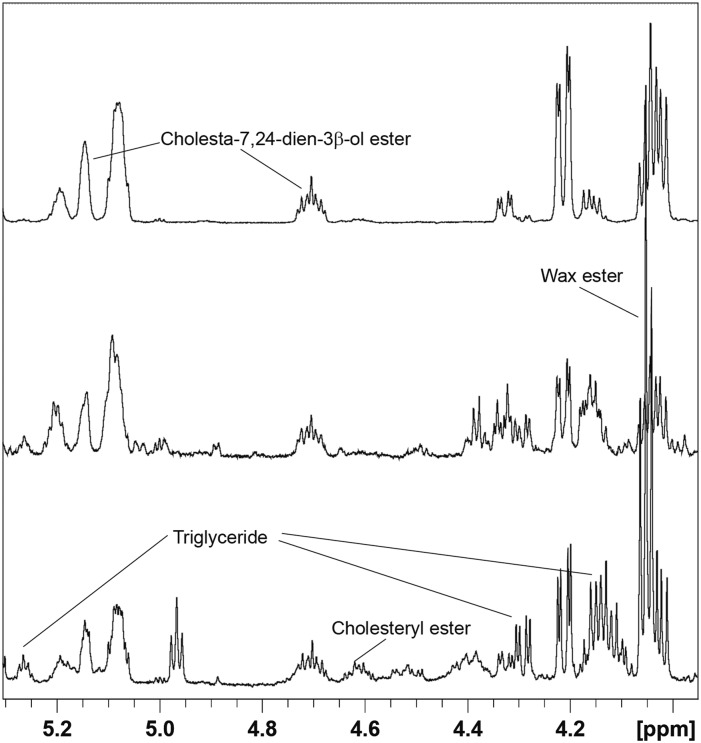
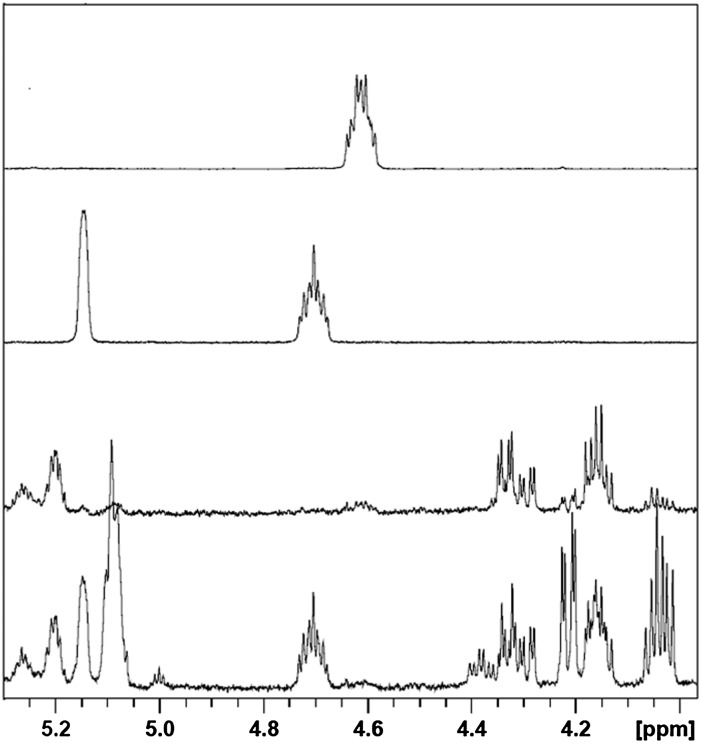
NMR spectra were recorded on chloroform extracts of rat, hamster, and mouse fur samples, and peaks from individual lipids were integrated as described previously. The mean absolute abundances derived from these integrals are presented in Table 1. Figure 1 shows the headgroup-containing region (3.9–5.5 ppm) of NMR spectra for representative dorsal thoracic fur extracts from the three species studied in this work, with proton resonances from various lipid classes labeled. Main peaks in this region are from WEs, sterol esters, glyceryl protons in TGs, and methylene-containing acyl ester chains. These were identified by comparison with literature values as well as by comparison with spectra from, or addition of, authentic reference standards for at least one member of each class of lipid measured in this study. In order to assign peaks in the 4.5–4.8 ppm region, which contains the methine proton from the 3 position of esterified sterols, the NMR spectra of synthetic stearoyl LE and CE were recorded (see Scheme 1 for cholesterol and lathosterol chemical structures, OH replaced by OR for CE and LE, where R = fatty acyl chain) and compared with fur extract NMR spectra (Fig. 2). Examples of the spectral differences between male and female hamsters are shown in Fig. 2, and sex, age, and body region differences in rats are shown in Fig. 3.
TABLE 1.
Abundance (nanomoles per milligram fur) of selected lipids in rodent fur extracts
| Species | Gender | Age (weeks) | Regiona | TG | LLEb | CE | WEc | 8-LEd | TC |
| Hamster | M | 8 | T | 1.64 ± 0.14 | 3.43 ± 1.06 | 0.77 ± 0.15 | 1.55 ± 0.51 | 1.46 ± 0.49 | 0.99 ± 0.21 |
| Hamster | F | 8 | T | 1.12 ± 0.25 | 0.34 ± 0.21 | 0.62 ± 0.26 | 0.37 ± 0.08 | 0.07 ± 0.07 | 0.89 ± 0.13 |
| Mouse | M | 9 | T | 1.58 ± 0.59 | 6.94 ± 1.82 | 0.83 ± 0.23 | 6.10 ± 1.68 | 0.13 ± 0.09 | 3.80 ± 0.62 |
| Rat | M | 9 | T | 2.72 ± 0.52 | 4.73 ± 0.62 | 3.35 ± 0.14 | 4.87 ± 0.55 | 2.03 ± 0.17 | 4.52 ± 0.40 |
| Rat | F | 9 | T | 2.15 ± 0.33 | 1.99 ± 0.82 | 2.25 ± 0.73 | 6.20 ± 2.06 | 0.68 ± 0.35 | 3.19 ± 0.86 |
| Rate | M | 9 | C | 3.63 ± 1.09 | 4.63 ± 1.24 | 3.52 ± 0.88 | 5.71 ± 1.10 | 2.13 ± 0.65 | 4.35 ± 0.87 |
| Rat | F | 9 | C | 2.28 ± 0.69 | 1.63 ± 1.18 | 1.63 ± 0.76 | 5.62 ± 0.87 | 0.50 ± 0.37 | 2.02 ± 0.82 |
| Rat | M | 9 | F | 2.72 ± 0.86 | 4.02 ± 0.76 | 3.10 ± 0.75 | 4.96 ± 1.38 | 1.76 ± 0.47 | 4.13 ± 1.12 |
| Rat | F | 9 | F | 2.07 ± 0.67 | 1.57 ± 0.72 | 1.70 ± 0.39 | 5.52 ± 0.93 | 0.54 ± 0.28 | 2.39 ± 0.63 |
| Rat | M | 9 | V | 2.06 ± 0.19 | 3.93 ± 0.58 | 2.62 ± 0.49 | 4.47 ± 0.73 | 2.26 ± 0.52 | 4.01 ± 0.69 |
| Rat | F | 9 | V | 2.28 ± 0.50 | 2.17 ± 0.47 | 2.03 ± 0.40 | 5.59 ± 0.83 | 0.26 ± 0.08 | 2.90 ± 0.60 |
| Rat | M | 21–24 | T | 4.24 ± 2.01 | 7.15 ± 2.99 | 4.94 ± 2.36 | 10.73 ± 4.83 | 2.63 ± 1.84 | 6.12 ± 3.71 |
| Rat | F | 21–24 | T | 0.71 ± 0.51 | 0.86 ± 0.31 | 1.32 ± 0.27 | 5.94 ± 0.90 | 0.26 ± 0.11 | 2.94 ± 0.34 |
| Rat | M | 21–24 | C | 7.72 ± 3.48 | 8.28 ± 3.52 | 4.62 ± 1.56 | 14.41 ± 7.03 | 4.11 ± 2.16 | 5.17 ± 2.50 |
| Rat | F | 21–24 | C | 0.98 ± 0.42 | 0.63 ± 0.20 | 0.83 ± 0.18 | 6.10 ± 0.59 | 0.23 ± 0.09 | 1.68 ± 0.23 |
| Rat | M | 21–24 | F | 2.62 ± 0.90 | 3.97 ± 1.56 | 2.67 ± 0.68 | 8.40 ± 3.99 | 1.55 ± 0.96 | 3.46 ± 1.39 |
| Rat | F | 21–24 | F | 1.01 ± 0.26 | 0.51 ± 0.31 | 0.80 ± 0.30 | 8.02 ± 1.78 | 0.10 ± 0.12 | 1.54 ± 0.42 |
| Rat | M | 21–24 | V | 2.24 ± 0.65 | 3.66 ± 0.96 | 2.17 ± 0.49 | 6.28 ± 2.22 | 1.54 ± 0.67 | 2.62 ± 0.63 |
| Rat | F | 21–24 | V | 1.31 ± 0.40 | 1.94 ± 1.04 | 2.10 ± 0.93 | 9.92 ± 3.18 | 0.12 ± 0.14 | 2.98 ± 0.91 |
LLE, lathosterol-like sterol ester. Absolute abundance calculated as described in Methods.
C, dorsal caudal; F, flank; T, dorsal thoracic; V, ventral abdominal.
Includes contributions from cholesta-7,24-dien-3β-ol and LEs.
Includes an overlapping multiplet integrating to ∼8% of the WE –OCH2 triplet.
Methyl singlet integrated at chemical shift 0.62–0.56 ppm; see text.
N = 5; all other N = 6.
Fig. 1.
Aliphatic, headgroup-containing region from proton NMR spectra of various rodent species. Traces from bottom to top correspond to chloroform extracts of dorsal thoracic fur from rat, hamster, and mouse. Peaks arising from different lipids are labeled.
Fig. 2.
Overlay of proton spectra of (bottom to top) male hamster fur extract, female hamster fur extract, authentic lathosteryl stearate, and authentic cholesteryl stearate.
Fig. 3.
Representative rat fur extract spectra illustrating sex, age, and body region differences. Bottom to top: 21- to 24-week-old female dorsal thoracic, 9-week-old female dorsal thoracic, 21- to 24-week-old male dorsal thoracic, 9-week-old male dorsal thoracic, 9-week-old male ventral, 9-week-old male caudal, and 9-week-old male flank. The top four spectra were obtained from fur clippings from the same male rat.
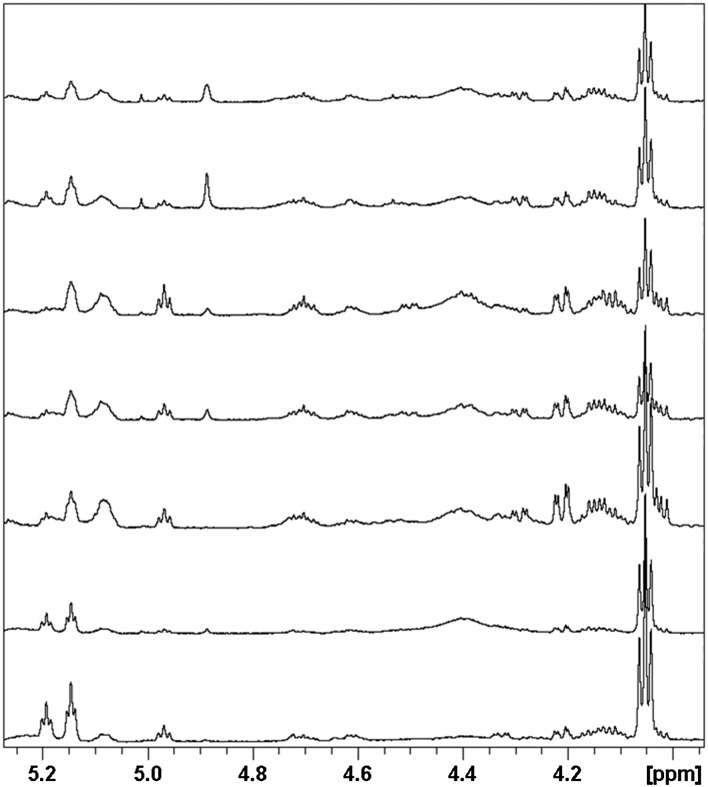
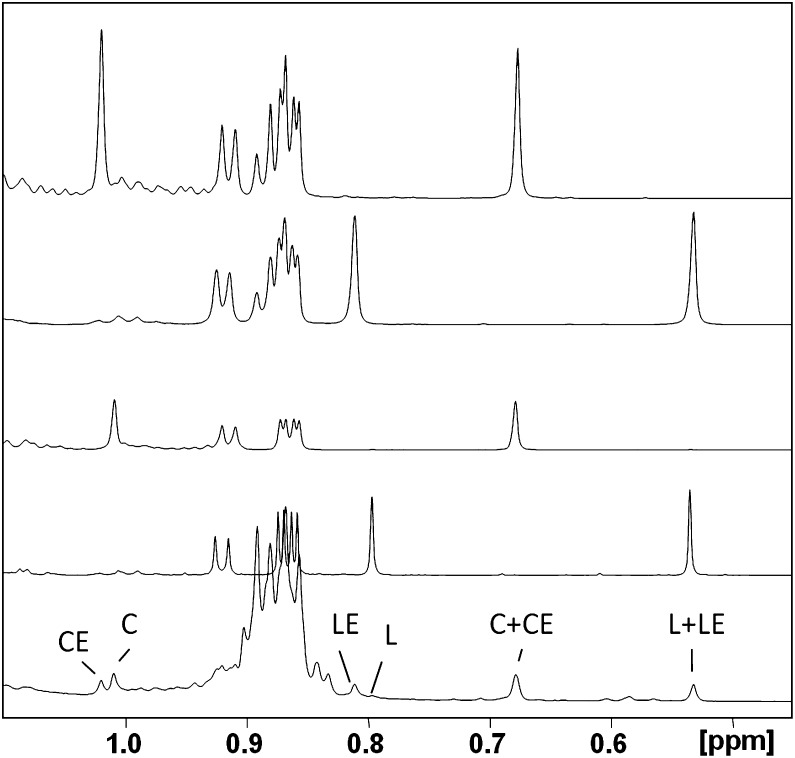
Because the methyl peaks for the major sterols (i.e., cholesterol and lathosterol and their esters) are well resolved, integrals of peaks from the methyl proton-containing region (0.5–0.8 ppm) representing these species were also analyzed for inclusion in the quantitative assessment (Fig. 4). The assignment of key sterol peaks in this and other regions was aided by previous work in this area (18, 19). As seen in Fig. 4, peaks correspond most closely with authentic standards for cholesterol, lathosterol, CEs, and LEs and are the predominant sterol species in fur extracts. The highest field methyl peaks at 0.53 and 0.66 ppm correspond to the lathosterol and cholesterol CH3 group at position 13, respectively (see Scheme 1). Because this is far removed from the site of esterification, these peak intensities have contributions from both the esterified and free sterols. In contrast, the methyl resonance at position 10 appears near 1.1 ppm for cholesterol and 0.8 ppm for lathosterol, but the chemical shift for this methyl peak is influenced enough by esterification that the free sterol and its corresponding ester can be differentiated. Hence, it is possible to obtain the relative concentrations of sterol species by simply integrating the corresponding high-field methyl peaks. Using this approach, the ratio of TC to total lathosterol is given in Table 2, along with the esterified/unesterified ratios for both sterols.
Fig. 4.
Methyl region of the 600 MHz NMR spectra of various standards and sample spectra. Bottom to top: male rat dorsal thoracic fur extract, lathosterol (L), cholesterol (C), lathosteryl stearate, and cholesteryl stearate.
TABLE 2.
Ratio of predominant sterol and sterol esters in rodent fur extracts
| Species | Gender | Age (weeks) | Regiona | TC/TLL | LL/LLE | Cholesterol/CE |
| Hamster | F | 8 | T | 9.58 ± 4.19 | 0.44 ± 0.04 | 1.10 ± 0.08 |
| Hamster | M | 8 | T | 0.37 ± 0.04 | 0.22 ± 0.02 | 1.36 ± 0.06 |
| Mouse | M | 8 | T | 0.58 ± 0.09 | 0.14 ± 0.02 | 2.50 ± 0.22 |
| Rat | F | 9 | C | 3.55 ± 0.85 | 0.43 ± 0.04 | 1.42 ± 0.11 |
| Rat | F | 9 | F | 2.64 ± 0.44 | 0.39 ± 0.05 | 1.49 ± 0.19 |
| Rat | F | 9 | T | 2.94 ± 0.53 | 0.40 ± 0.04 | 1.27 ± 0.07 |
| Rat | F | 9 | V | 1.85 ± 0.54 | 0.30 ± 0.04 | 1.30 ± 0.17 |
| Rat | F | 21–24 | C | 3.56 ± 1.11 | 0.40 ± 0.02 | 1.36 ± 0.14 |
| Rat | F | 21–24 | F | 3.38 ± 1.50 | 0.39 ± 0.04 | 1.27 ± 0.10 |
| Rat | F | 21–24 | T | 3.57 ± 0.91 | 0.40 ± 0.02 | 1.25 ± 0.09 |
| Rat | F | 21–24 | V | 1.67 ± 0.47 | 0.26 ± 0.05 | 0.98 ± 0.09 |
| Ratb | M | 9 | C | 1.73 ± 0.49 | 0.35 ± 0.04 | 1.39 ± 0.05 |
| Rat | M | 9 | F | 1.64 ± 0.30 | 0.32 ± 0.06 | 1.52 ± 0.14 |
| Rat | M | 9 | T | 1.54 ± 0.33 | 0.33 ± 0.05 | 1.38 ± 0.06 |
| Rat | M | 9 | V | 1.16 ± 0.15 | 0.22 ± 0.03 | 1.71 ± 0.17 |
| Rat | M | 21–24 | C | 1.08 ± 0.30 | 0.32 ± 0.07 | 1.26 ± 0.06 |
| Rat | M | 21–24 | F | 1.49 ± 0.41 | 0.32 ± 0.04 | 1.30 ± 0.06 |
| Rat | M | 21–24 | T | 1.65 ± 0.53 | 0.34 ± 0.03 | 1.21 ± 0.06 |
| Rat | M | 21–24 | V | 0.95 ± 0.18 | 0.27 ± 0.04 | 1.34 ± 0.08 |
LL, lathosterol-like sterol; TLL, total lathosterol-like sterol; TC, total cholesterol; LLE, lathosterol-like sterol esters. Ratio of methyl peak integrals from peaks at 1.00 (cholesterol), 0.79 (LL), 0.81 (LLE), and 1.02 (CE) ppm. Because of spectral overlap, these values only approximate the actual ratios but are useful for comparative purposes.
C, dorsal caudal; F, flank; T, dorsal thoracic; V, ventral abdominal.
N = 5; in all other cases N = 6.
Identification of cholesta-7,24-dien-3β-ol
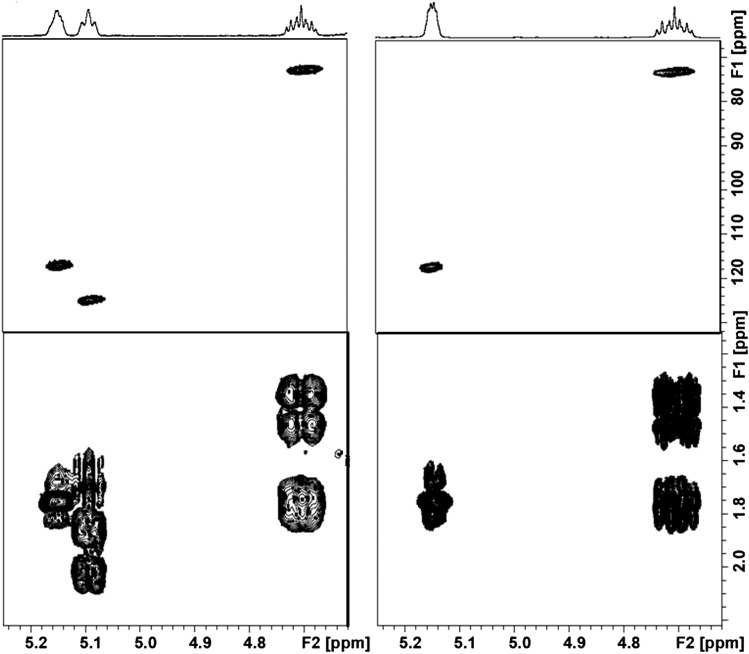
Initially, based on the previous analysis of resolved signals compared with available authentic standards, we tentatively assigned a predominant (noncholesterol) sterol ester observed in many of the samples (cf. Fig. 1) to LE. In order to confirm this, it was important to first isolate the sterol esters by TLC because in fur extract spectra, the resonances from many of the more aliphatic sterol protons are completely obscured by the resonances from all other FA chains of all the other lipid components. After isolation of this component, an exhaustive NMR structural elucidation was carried out (see below and the supplementary data), which revealed that this was, in fact, not lathosterol but the 24,25-dehydro analog, cholesta-7,24-dien-3β-ol ester (see Scheme 1). The following observations support this assignment. First, the doublet for cholesta-7,24-dien-3β-ol CH321 is downfield shifted by ∼0.03 ppm to 0.094 ppm as compared with LE CH321. Because the structure of the cyclic core is identical for both the esters, the other methyl singlets CH318 and CH319 at 0.53 ppm and 0.81 ppm have exactly the same chemical shift as LE. Second, upon examining the 1D and 2D NMR spectra of the TLC isolate, it was immediately apparent that triplet at 5.1 ppm was part of this molecule and could be assigned to H24 (Fig. 5). This, combined with the C24 chemical shift of 125.2 ppm (obtained from a multiplicity-edited HSQC 2D spectrum), indicated that the sterol was desaturated at C24/C25. Homonuclear COSY correlations from H24 to H23a/b and four-bond correlations to H26 and H27, along with HSQC peaks for C23, C26, and C27 at 25.0, 17.7, and 25.7, respectively, confirm the side chain double bond. Third, in order to rule out zymosterol ester (which differs from cholesta-7,24-dien-3β-ol in having a double bond at C8 and C24 instead of C7 and C24) as a possibility, chemical shifts of the isolate were compared with published values for zymosterol (20) that list the doubly bonded ring carbon C8 at 128.0 ppm, while we observed the doubly bonded ring carbon C7 at 117.4 ppm. Fourth, to rule out desmosteryl ester (which differs from cholesta-7,24-dien-3β-ol in having double bonds at C5 and C24), spectra of TLC-purified extract were compared with authentic desmosteryl ester. The proton chemical shift of H3 is 4.61 ppm for desmosteryl ester (the same as for CE), whereas we observed H3 in the TLC-purified fur extract at 4.70 ppm. Finally, comparison of the proton chemical shifts of the TLC isolate with those of cholesta-7,24-dien-3β-ol reported by Wilson et al. (18) gave good agreement for all measured protons. A more detailed description of the structure elucidation process, along with selected 1D and 2D NMR spectra and list of proton and carbon chemical shifts of differentiating and resolvable nuclei in the TLC isolate, can be found in the supplementary data.
Fig. 5.
Selected regions of 1H-13C HSQC (top) and COSY (bottom) NMR spectra for TLC-separated male hamster sterol ester (left) and authentic LE (right) showing the presence of a vinylic proton.
SCD1 inhibitor study
Table 3 displays specific fur lipid changes after treatment of rats and hamsters with different doses of the SCD1 inhibitor compound 1. Overall, a statistically significant (P < 0.05) reduction in most extractable lipids per milligram of fur of between 28% and 46% was seen for rats in the 10 mg/kg dose group relative to vehicle control. In the hamster, reductions in fur WE and cholesta-7,24-dien-3β-ol esters of 53% and 59%, respectively, were observed at 60 mg/kg.
TABLE 3.
Effect of SCD1 inhibitor on fur lipids in rodent models
| Spectra | Rat, 10 mg/kg | Hamster, 60 mg/kg | ||
| % Changea | Pb | % Changea | Pb | |
| TG | −46 | 0.02 | −33 | 0.59 |
| LLEc | −48 | 0.03 | −59 | 0.01 |
| CE | −33 | 0.07 | −8 | 0.57 |
| WE | −39 | 0.00 | −53 | 0.01 |
| TC | −28 | 0.01 | −25 | 0.14 |
| Cholesterol | −34 | 0.03 | −13 | 0.32 |
Percent change from vehicle control; N = 5 for rat, and N = 6 for hamster.
Student’s unpaired two-tailed t-test assuming unequal variance.
Includes contributions from cholesta-7,24-dien-3β-ol esters and LEs.
DISCUSSION
There are differences in the types of lipids, as well as their concentration, among the three species of commonly used lab animals studied here. These differences have been measured before using LC-based methods (21–24), but this is the first report where NMR spectroscopy has been used to compare fur lipid extracts. The three species of common laboratory animals used in this study all produced usable NMR data from which absolute concentration of fur lipids can be derived. Consistent with the literature, variables that influence the profile include species, gender, age, and the location from which the fur samples are clipped. The origins for the differences are unclear, but they presumably arise from either differences in the enzymes present in the sebaceous glands or postsecretion modifications to various sebum components. The former is almost certainly the case for the difference between species and genders within species. An example illustrating this difference is that the rat is clearly the only species that shows significant amounts of CE, whereas the mouse has a very low concentration of TGs compared with the other species (shown in Fig. 1).
Gender differences within a species are nicely exemplified in the Syrian hamster, a commonly used model for sebum-related studies. It has been previously reported that male and female Syrian hamsters have significantly different sterol ester composition in their sebum, with females having predominantly CE and males having an unidentified sterol ester that was referred to as “male hamster sterol ester” (16). Our data are consistent with this earlier finding, and the gender differences are evident from comparing the NMR spectra of female and male hamster fur extracts (Fig. 2) and from the abundance of quantified lipids in Table 1. Using high-resolution 1D and 2D NMR spectroscopy of the sterol ester band extracted from a TLC separation of male hamster sebum and comparing proton and 13C chemicals shifts with authentic standards and literature reports, we have definitively identified the “male hamster sterol ester” as esters of cholesta-7,24-dien-3β-ol. Because it is not easy to distinguish between LE and this 24,25-dehydro form based on 1D NMR of fur extracts due to the unfortunate overlap of critical distinguishing peaks, for the purposes of the following discussion, we will refer to these two sterols collectively as LL and similarly for the corresponding LLEs.
We also studied the effect of age on fur lipids in rats. The NMR method showed that in this study, male and female rats have different age-related changes. In all body regions, older male rats tend to have higher lipid levels, whereas the females tend to show an opposite trend, with decreased amounts of the various lipids from all body regions as they age (Table 1).
Our original intent in application of this method to sample from the dorsal thoracic region was based on the simple observation that it is one of the few areas in which rodents cannot lick themselves and that it might therefore be less susceptible to exposure to saliva. However, it was realized that this particular region might not provide enough fur in cases where many samples might need to be taken longitudinally. Therefore, we investigated how fur lipids varied across different body regions of rats. Lipid composition from different body regions in male and female rats is variable, but to a lesser extent than species- or gender-related differences. For males, order seems consistent for different lipids in terms of concentration: caudal > dorsal thoracic > flank > ventral. For females, ventral and dorsal thoracic regions have higher levels of lipids per milligram of fur than caudal and flank.
Quantification of sterols and sterol esters by integrating the high-field methyl region (Fig. 4), as described previously, allowed a comparison of the dependence of these molecules on the various parameters investigated in this study (Table 2). For example, in rats, the TC/TLL ratio ranges from about 1 to 3.5, with somewhat higher values in females, independent of age. Interestingly, the lowest values within each rat gender/age group are seen in fur taken from the ventral body region. In male mice and male hamsters, the TC/TLL is much lower, at 0.58 ± 0.09 and 0.37 ± 0.04, respectively, whereas in the female hamster it is much higher. The LL/LLE and cholesterol/CE ratios are relatively constant across all parameters, with the major excursion being the mouse.
Aside from the very intriguing differences in phenotypic (genetic) and morphological observations made here, a major motivation behind this work was to determine whether noninvasively obtained fur samples could be used to follow therapeutic interventions that may impact sebum production. To assess this, we selected SCD1 as an enzyme target, the inhibition of which is known to have deleterious side effects on sebaceous glands in rodents. Drug-induced changes in sebum-related lipids observed in the fur extracts of rodents after prolonged treatment with the SCD1 inhibitor compound 1 are summarized in Table 2. For rats, most of the measured lipids had significant reductions, whereas in hamsters the reductions were most significant for LLEs and WEs. Treatment of mice with this compound at similar doses was shown to induce dermal pathology characterized by alopecia and sebaceous gland atrophy (8), making it reasonable to associate this pathology with the reduced fur lipid levels observed in this work. It has previously been shown that topical flutamide and oral Accutane resulted in significant reduction in extractable skin lipids in hamsters using a similar method (5).
CONCLUSION
NMR is a useful tool for the characterization of skin lipids in both laboratory animals and humans (5). Clinically, this is most frequently done noninvasively with the use of absorbent films. In animal models, however, skin biopsies are the norm and almost always require euthanizing the animal. This work further extends the NMR approach to noninvasive evaluation of fur from animals, enabling serial sampling with significantly reduced numbers of animals for long-term studies. Furthermore, the unbiased nature and high-resolving power of NMR positions it as a powerful biomarker discovery tool, as evidenced in this work by the identification of cholesta-7,24-dien-3β-ol esters as the previously unidentified “male hamster sterol ester” noted almost three decades ago (16). As such, the use of this method will enable a more detailed understanding of species- and gender-based differences in dermatological physiology and pathology. As with almost any biochemical measure, it is clear that age and gender must be taken into account when comparing results from this assay. However, the biological variability problem is minimized by the noninvasive nature of this assay, which allows individuals to serve as their own controls in many cases. Additionally, while differences in lipids extracted from the fur from different body regions are small compared with some toxicity-, gender-, or certainly species-related differences, efforts should be taken to sample from similar body regions in longitudinal studies wherever possible. As part of any such longitudinal study, information on variation within an individual animal over different samplings should be collected to allow estimation of intra-animal variability across the body region of interest. It should also be emphasized that while multiple chemical shift alignment provides tempting evidence for annotation of components in complex mixtures, any such annotations should be considered tentative until rigorous confirmation is provided. Such was the case here where it was tempting to label the “male hamster sterol ester” as LE, whereas rigorous analysis demonstrated that it was actually the much less commonly reported cholesta-7,24-dien-3β-ol ester. In summary, the NMR assay described here provides results consistent with what is known from the literature, is highly quantitative and simple to implement, and provides the possibility of measuring unanticipated biomarkers.
Supplementary Material
Footnotes
Abbreviations:
- AA
- arachidonyl alcohol
- CE
- cholesteryl ester
- COSY
- two dimensional homonuclear correlated spectroscopy
- HSQC
- two dimensional heteronuclear single quantum coherence spectroscopy
- LE
- lathosterol ester
- LL
- lathosterol-like sterol
- LLE
- lathosterol-like sterol ester
- SCD1
- stearoyl-CoA desaturase 1
- TC
- total cholesterol
- WE
- wax ester
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one scheme, two tables, and four figures.
REFERENCES
- 1.Abramovits W., Gonzalez-Serva A. 2000. Sebum, cosmetics, and skin care. Dermatol. Clin. 18: 617–620 [DOI] [PubMed] [Google Scholar]
- 2.Zouboulis C. C. 2004. Acne and sebaceous gland function. Clin. Dermatol. 22: 360–366 [DOI] [PubMed] [Google Scholar]
- 3.Saint-Léger D. 2003. [Normal and pathologic sebaceous function. Research in a shallow milieu?]. Pathol. Biol. (Paris). 51: 275–278 French. [DOI] [PubMed] [Google Scholar]
- 4.Thiessen D. D., Kittrell E. M. W. 1980. The harderian gland and thermoregulation in the gerbil (Meriones unguiculatus). Physiol. Behav. 24: 417–424 [DOI] [PubMed] [Google Scholar]
- 5.Robosky L. C., Wade K., Woolson D., Baker J. D., Manning M. L., Gage D. A., Reily M. D. 2008. Quantitative evaluation of sebum lipid components with nuclear magnetic resonance. J. Lipid Res. 49: 686–692 [DOI] [PubMed] [Google Scholar]
- 6.Eisen D. B., Michael D. J. 2009. Sebaceous lesions and their associated syndromes: Part I. J. Am. Acad. Dermatol. 61: 549–560 [DOI] [PubMed] [Google Scholar]
- 7.Yosipovitch G., DeVore A., Dawn A. 2007. Obesity and the skin: skin physiology and skin manifestations of obesity. J. Am. Acad. Dermatol. 56: 901–916 [DOI] [PubMed] [Google Scholar]
- 8.Liu G. 2010. Recent advances in stearoyl-CoA desaturase 1 inhibitors for dyslipidemia and obesity. Curr. Top. Med. Chem. 10: 419–433 [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki M., Man W. C., Ntambi J. M. 2001. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J. Nutr. 131: 2260–2268 [DOI] [PubMed] [Google Scholar]
- 10.Xin Z., Zhao H., Serby M. D., Liu B., Liu M., Szczepankiewicz B. G., Nelson L. T. J., Smith H. T., Suhar T. S., Janis R. S., et al. 2008. Discovery of piperidine-aryl urea-based stearoyl-CoA desaturase 1 inhibitors. Bioorg. Med. Chem. Lett. 18: 4298–4302 [DOI] [PubMed] [Google Scholar]
- 11.Jiang G., Li Z., Liu F., Ellsworth K., Dallas-Yang Q., Wu M., Ronan J., Esau C., Murphy C., Szalkowski D., et al. 2005. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J. Clin. Invest. 115: 1030–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ntambi J. M., Miyazaki M. 2003. Recent insights into stearoyl-CoA desaturase-1. Curr. Opin. Lipidol. 14: 255–261 [DOI] [PubMed] [Google Scholar]
- 13.Sampath H., Flowers M. T., Liu X., Paton C. M., Sullivan R., Chu K., Zhao M., Ntambi J. M. 2009. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J. Biol. Chem. 284: 19961–19973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zouboulis C. C., Angres S., Seltmann H. 2011. Regulation of stearoyl-coenzyme A desaturase and fatty acid delta-6 desaturase-2 expression by linoleic acid and arachidonic acid in human sebocytes leads to enhancement of proinflammatory activity but does not affect lipogenesis. Br. J. Dermatol. 165: 269–276 [DOI] [PubMed] [Google Scholar]
- 15.Georgel P., Crozat K., Lauth X., Makrantonaki E., Seltmann H., Sovath S., Hoebe K., Du X., Rutschmann S., Jiang Z., et al. 2005. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect. Immun. 73: 4512–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brind J. L., Alani E., Wheatley V. R., Orentreich N. 1986. Analysis of ear sebum of the Syrian hamster (Mesocrecetus auratus) reveals pronounced sexual dimorphism. Comp. Biochem. Physiol. B. 84: 403–407 [DOI] [PubMed] [Google Scholar]
- 17.Lindholm J. S., McCormick J. M., Colton S. W., VI, Downing D. T. 1981. Variation of skin surface lipid composition among mammals. Comp. Biochem. Physiol. B. 69: 75–78 [DOI] [PubMed] [Google Scholar]
- 18.Wilson W. K., Sumpter R. M., Warren J. J., Rogers P. S., Ruan B., Schroepfer G. J., Jr 1996. Analysis of unsaturated C27 sterols by nuclear magnetic resonance spectroscopy. J. Lipid Res. 37: 1529–1555 [PubMed] [Google Scholar]
- 19.Oostendorp M., Engelke U. F. H., Willemsen M. A. A. P., Wevers R. A. 2006. Diagnosing inborn errors of lipid metabolism with proton nuclear magnetic resonance spectroscopy. Clin. Chem. 52: 1395–1405 [DOI] [PubMed] [Google Scholar]
- 20.Taylor U. F., Kisic A., Pascal R. A., Jr, Izumi A., Tsuda M., Schroepfer G. J., Jr 1981. Sterol synthesis: a simple method for the isolation of zymosterol (5α-cholesta-8,24-diene-3β-ol) from yeast and spectral properties of zymosterol. J. Lipid Res. 22: 171–177 [PubMed] [Google Scholar]
- 21.Wilkinson D. I., Karasek M. A. 1966. Skin lipids of a normal and mutant (asebic) mouse strain. J. Invest. Dermatol. 47: 449–455 [DOI] [PubMed] [Google Scholar]
- 22.Nikkari T., Valavaara M. 1970. The influence of age, sex, hypophysectomy and various hormones on the composition of the skin surface lipids of the rat. Hormones and sebum composition in the rat. Br. J. Dermatol. 83: 459–472 [DOI] [PubMed] [Google Scholar]
- 23.Lu G. W., Valiveti S., Spence J., Zhuang C., Robosky L., Wade K., Love A., Hu L. Y., Pole D., Mollan M. 2009. Comparison of artificial sebum with human and hamster sebum samples. Int. J. Pharm. 367: 37–43 [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R., Katare O. P., Vyas S. P. 2000. The pilosebaceous unit: a pivotal route for topical drug delivery. Methods Find. Exp. Clin. Pharmacol. 22: 129–133 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.