Abstract
Here, we investigated how LDL receptor deficiency (Ldlr−/−) modulates the effects of testosterone on obesity and related metabolic dysfunctions. Though sham-operated Ldlr−/− mice fed Western-type diet for 12 weeks became obese and showed disturbed plasma glucose metabolism and plasma cholesterol and TG profiles, castrated mice were resistant to diet-induced obesity and had improved glucose metabolism and reduced plasma TG levels, despite a further deterioration in their plasma cholesterol profile. The effect of hypogonadism on diet-induced weight gain of Ldlr−/− mice was independent of ApoE and Lrp1. Indirect calorimetry analysis indicated that hypogonadism in Ldlr−/− mice was associated with increased metabolic rate. Indeed, mitochondrial cytochrome c and uncoupling protein 1 expression were elevated, primarily in white adipose tissue, confirming increased mitochondrial metabolic activity due to thermogenesis. Testosterone replacement in castrated Ldlr−/− mice for a period of 8 weeks promoted diet-induced obesity, indicating a direct role of testosterone in the observed phenotype. Treatment of sham-operated Ldlr−/− mice with the aromatase inhibitor exemestane for 8 weeks showed that the obesity of castrated Ldlr−/− mice is independent of estrogens. Overall, our data reveal a novel role of Ldlr as functional modulator of metabolic alterations associated with hypogonadism.
Keywords: testosterone, metabolic syndrome, low density lipoprotein receptor-related protein 1, apolipoprotein E, diabetes, plasma glucose homeostasis, metabolic rate, uncoupling protein 1 • metabolic activation of white adipose tissue
Testosterone, a steroid hormone with an important role in male sexual differentiation, maintenance of libido, and erectile function (1, 2), is also a central modulator of key metabolic processes associated with metabolic syndrome. In humans, testosterone deficiency (TD) alters carbohydrate, lipid, and protein metabolism, thus contributing to oxidative stress, endothelial dysfunction, and increased production of proinflammatory factors (3). TD is considered as a primary risk factor for a number of disorders including obesity, metabolic syndrome, dyslipidemia, endothelial cell dysfunction, vascular disease, insulin resistance, and type 2 diabetes mellitus (4–8). Likewise, data from the Massachusetts Male Aging Study suggest that obesity leads to decreased levels of total and free testosterone, with follow-up levels lowest among men who remained or became obese (9).
Additional insights into the role of androgens in maintaining human health have been obtained following androgen deprivation therapy (ADT), which is often used in the treatment of prostate cancer (PCa). Metabolic complications of ADT, such as metabolic syndrome, insulin resistance, and type 2 diabetes mellitus have highlighted key avenues of human pathology associated with this treatment (10). ADT in men with PCa resulted in increased total cholesterol, LDL cholesterol (LDL-C), and TG levels (11, 12). Patients undergoing ADT exhibit significantly higher BMI (P < 0.01) and serum cholesterol levels (P = 0.03) than eugonadal subjects (13). On the contrary, testosterone replacement therapy in hypogonadal men resulted in reduced BMI; improved glucose tolerance and insulin sensitivity; decreased total cholesterol, LDL-C, and TG; and increased HDL cholesterol (HDL-C) levels (14). The realization that medical castration contributes to metabolic perturbations led to the reevaluation of ADT risks in PCa patients (15) but also emphasized the beneficial role that androgens may play in the general well-being of men. Overall, hypogonadism has been identified as an independent risk factor for cardiovascular morbidity and mortality (16, 17).
Though some cases of human obesity may be caused solely by genetic factors (18), Western-type diet combined with sedentary lifestyle, physical inactivity, and imbalance in caloric load are the most common contributors to the development of central obesity and metabolic syndrome (19, 20). Recent studies by us and others have suggested that the lipoprotein metabolic pathway is a pivotal contributor to the development of obesity and related metabolic dysfunctions in experimental mice. Specifically, mice deficient in ApoE, a key protein in the clearance of chylomicron remnants, LDL, and VLDL in plasma, are resistant to diet-induced obesity, insulin resistance, and glucose intolerance (21, 22), while knock-in ApoE3 transgenic mice are sensitized toward these conditions (21). Similarly, expression levels of LDL receptor-related protein 1 (Lrp1), a member of the LDL receptor (Ldlr) superfamily that binds lipoprotein-associated ApoE modulates the development of obesity and its related metabolic perturbations mainly by regulating the flux of dietary TGs to hepatic and adipose tissues and the energy expenditure (EE) of the tested mice (23). Along the same lines, we have also observed that lack of classical Ldlr-mediated clearance of chylomicron remnants slows down diet-induced obesity following feeding mice with standard Western-type diet (21), though it has no effect on TG deposition to the liver (24).
Despite the key role of plasma lipoproteins in the management of postprandial lipids and a number of clinical and epidemiological studies indicating that TD is associated with obesity and metabolic syndrome, the functional cross-talk between testosterone and lipoprotein metabolism in the development of diet-induced obesity remains vaguely defined. In the present study, we report that in contrast to studies in wild-type animals, TD in Ldlr−/− mice results in reduced body fat and body weight gain, improved glucose metabolism and plasma TG levels, and increased EE, despite an aggravation in plasma cholesterol profile. Our data suggest that Ldlr is a functional modulator of processes associated with hypogonadism-induced metabolic alterations.
MATERIALS AND METHODS
Animal studies
The Ldlr−/−, ApoE−/−, and C57BL/6 mice used in our studies were purchased from Jackson Labs (Bar Harbor, ME). Male mice 10–12 weeks old were used in these studies. Mice in each group were caged individually (one mouse per cage) and were allowed unrestricted access to food and water under a 12 h light/dark cycle. To ensure similar average cholesterol, TG, and glucose levels and starting body weights, groups of mice were formed after determining the fasting cholesterol, TG, and glucose levels and body weights of the individual mice. In all studies involving high-fat diet, we used the standard Western-type diet (Mucedola SRL, Milano, Italy) that is composed of 17.3% protein, 48.5% carbohydrate, 21.2% fat, and 0.2% cholesterol (0.15% added, 0.05% from fat source) and contains 4.5 kcal/g. The content of the main ingredients expressed as grams per kilogram of diet is the following: casein 195, DL-methionine 3, sucrose 341.46, corn starch 150, anhydrous milkfat 210, cholesterol 1.5, cellulose 50, mineral mix 35, calcium carbonate 4, vitamin mix 10, ethoxyquin, and antioxidant 0.04. For the dietary studies, two groups of mice of the same genotype were formed to have similar starting body weights and plasma cholesterol, TG, and glucose levels. One group was surgically castrated, and the other group was sham operated. Following a recovery period of 4 weeks on chow diet, to allow for testosterone clearance from the circulation of castrated mice, all mouse groups were switched to Western-type diet (week 0) for an additional period of 12 weeks. At the end of each experiment, mice were euthanized, and plasma and tissue samples were collected. Carcasses were stored at −80°C.
All animal studies were governed by the European Union guidelines of the Protocol for the Protection and Welfare of Animals. In our experiments, we took into consideration the 3Rs (reduce, refine, replace), and we minimized the number of animal experiments to the absolute minimum. To this date, there is no in vitro system to mimic satisfactorily the lipid and lipoprotein transport system and the in vivo effects of hypogonadism on metabolism, making the use of experimental animals mandatory. The work was authorized by the appropriate committee of the Laboratory Animal Center of the University of Patras Medical School and the Veterinary Authority of the Prefecture of Western Greece.
Surgical castration and treatment of mice with testosterone and exemestane
Surgical castration of mice was performed as described previously (25). Briefly, experimental mice were divided into two groups. One group was sham operated by performing only a skin incision. The other group of animals was bilaterally castrated through 1 cm scrotal incisions. Both groups were operated under isofluorane (CP Pharma, Burgdorf, Germany) anesthesia. The animals were allowed to recover from the operation for a period of 4 weeks, and plasma testosterone levels were determined to confirm the success of the operation. Then both groups were switched to Western-type diet for the indicated period of time.
For the testosterone replacement study, Ldlr−/− mice were surgically castrated, split into two groups, and switched to Western-type diet. Immediately after, one group was treated with intramuscular administration of 25 mg testosterone (Nebido, Bayer, Frankfurt, Germany) per kg of body weight, once a week, for a period of 8 weeks. The other group was treated with the carrier solution as placebo (a mix of castor oil and benzylbenzoate at a ratio of 4:1; Sigma-Aldrich, St. Louis, MO). Body weights of animals in both groups were monitored weekly. To confirm the success of the experiment, plasma testosterone levels were determined in both groups as described subsequently, immediately prior to castration, 4 weeks after castration (week 0), and then 8 weeks following testosterone supplementation. At the end of each experiment, mice were euthanized, and plasma and tissue samples were collected. Carcasses were stored at −80°C.
For exemestane treatment, sham-operated Ldlr−/− mice were split into two groups, and then all mice were switched to Western-type diet. Immediately after, one group was treated with 40 mg of exemestane (Sigma-Aldrich) per kg of body weight (dissolved in carrier solution containing 0.3% w/v hydroxypropyl cellulose; Sigma-Aldrich), once per week, as previously described (26), for a period of 8 weeks. The other group was treated with only carrier solution. During the course of the experiment, body weights of the animals of both groups were monitored weekly. To confirm the success of the experiment, plasma estradiol levels were determined in both groups as described subsequently, immediately prior to sham operation and 8 weeks following exemestane treatment. At the end of each experiment, mice were euthanized, and plasma and tissue samples were collected. Carcasses were stored at −80°C.
Plasma testosterone and estradiol determination
Blood was drawn from the tail vein at the indicated times, and serum samples were separated by centrifugation (4,000 rpm, 10 min) and stored at −20°C until used for testosterone and estradiol measurements. Plasma testosterone levels were determined using the Mouse/Rat Total Testosterone Elisa Kit (cat # TE187S-100, Calbiotech, Spring Valley, CA), and estradiol levels were determined using the Mouse/Rat Estradiol Kit (cat # ES180S-100, Calbiotech), according to the manufacturer’s instructions.
Indirect calorimetry studies
Indirect calorimetry studies were performed using the Calocage small animal colorimetry system (TSE Systems, Frankfurt, Germany). Groups of sham-operated and castrated mice were acclimated in the calocages for 48 h, and then EE was determined based on the carbon dioxide production expressed as a the difference of carbon dioxide exiting from carbon dioxide entering the metabolic cage [VCO2 (ml/h)] and oxygen consumption expressed as the difference of oxygen entering from oxygen exiting the metabolic cage [VO2 (ml/h)] over an additional 24 h period (12 h dark/12 h light). A total of 72 measurements of VCO2 and VO2 were collected. The EE per mouse (expressed in J/h) for each measurement was calculated by the formula EE = (15.818 × VO2) + (5.176 × VCO2) (27). Then, the average EE of the last 72 different measurements (1 every 20 min, starting at 7:00 PM on one day and ending at 7:00 PM on the following day) was determined and plotted as a function of body weight, and ANCOVA was performed between the two groups of mice (27). Respiratory exchange ratio (RER) was determined as the ratio of VCO2 over VO2.
Plasma lipid determination
Following a 16 h fasting period (starting at 6:30 PM on the first day and ending at 10:30 AM the next day), plasma samples were isolated from the experimental mice. Plasma cholesterol and TG levels were measured using the Cholesterol Kit (Thermo Fisher Scientific, Waltham, MA) and Triglyceride Determination Kit (Sigma-Aldrich), respectively, according to the manufacturer’s instructions and as described previously (28).
Fractionation of plasma lipoproteins by density gradient ultracentrifugation
In the present study, we used density gradient ultracentrifugation, the gold standard for the separation of plasma lipoproteins. Therefore, for the determination of plasma cholesterol and TG levels in various plasma lipoproteins, 0.5 ml of pools of plasma from five mice per group was fractionated by density gradient ultracentrifugation over a 10 ml KBr (Sigma-Aldrich) density gradient in a Beckmann-Coulter Ultracentrifuge (Indianapolis, IN) using an SW41 rotor, as described previously (28). The cholesterol and TG content of different density fractions was determined as described previously. The experiment was repeated three times with different sets of mice.
Body weight determination and body mass composition analysis
At the indicated time points during the course of the experiments, mice in each group were briefly anesthetized using isofluorane, and their body weight was determined by a Mettler-Toledo® precision microscale (Mettler-Toledo, Columbus, OH). At the end of each experiment, at least six mice from each group were euthanized. Mouse carcasses were weighed to determine wet weight, and then they were dehydrated at 65°C until a constant mass was achieved (dry weight). The dried carcasses were then dissolved completely in 200 ml of ethanolic potassium hydroxide solution (5 M KOH in 50% ethanol; Sigma-Aldrich), the pH of the solution was adjusted to 7, and the final volume was recorded. An aliquot of this solution was used for enzymatic determination of glycerol as a measure of TG content and for total protein determination. Total water content was calculated as wet weight minus dry weight, and lean body mass (LBM) was calculated as wet weight minus total lipid weight. Results are expressed as milligrams of TGs per gram of tissue ± SEM.
Determination of daily food consumption
Food intake was assessed by determining the difference in food weight during a 7 day period to ensure reliable measurements, as described previously (21, 29).
Fasting glucose determination and glucose tolerance test
For fasting plasma glucose determination, mice were fasted for 16 h (starting at 6:30 PM on the first day and ending at 10:30 AM the next day). Blood was drawn from the tail vein, and glucose was determined using the ACCU-CHECK AVIVA glucometer (Roche, Nutley, NJ) and comfort curve test strips. To determine the effects of TD on glucose tolerance, we performed a classical glucose tolerance test (GTT). Briefly, mice were fasted overnight (16 h), then dextrose (2 g/kg in PBS; Sigma-Aldrich) was injected intraperitoneally; serum samples were collected at 0, 15, 30, 60, and 120 min postinjection through the tail vein; and glucose levels were measured.
Isolation of mitochondria
Mitochondria from brown adipose tissue (BAT) and white adipose tissue (WAT) were isolated as described previously (30). Briefly, BAT and the contralateral fat pad from each depot site were dissected immediately after animals were euthanized. Tissues were minced in ice-cold sucrose buffer [0.25 M sucrose and 5.0 mM N-Tris, i.e., (hydroxymethyl)methyl-2-aminoethanesulfonic acid buffer, pH 7.2; Sigma-Aldrich]; diluted to 10% and 5% (w/v), respectively, in sucrose buffer; and homogenized with a glass-Teflon homogenizer. Homogenates were then centrifuged at 22,500 g for 20 min, and the pellet was resuspended in ice-cold sucrose buffer. After a low-speed spin at 850 g for 10 min, the supernatant, containing crude mitochondria, was decanted to a fresh tube and spun for 20 min at 48,000 g. The pelleted mitochondria were then resuspended in 2 ml solubilization buffer containing 20 mM Tris (pH 8), 1 mM EDTA, 100 Mm NaCl, and 0.9% sodium cholate (Sigma-Aldrich) and incubated on ice for 30 min. Mitochondria samples were then recentrifuged at 48,000 g for 30 min. The pellet was resuspended in solubilization buffer containing 1% Triton X-100 and incubated on ice for 30 min. The suspension was recentrifuged at 48,000 g, and the supernatant was retained for protein assay and Western blotting. The protein concentration of each mitochondrial sample was determined using the detergent-compatible protein assay (cat #500-0006, Bio-Rad Laboratories Inc.).
Western blot analysis
Western blotting for murine ApoE, cytochrome c, uncoupling protein 1 (Ucp1), and cytochrome c oxidase subunit 4 (Cox4) was performed using IgG primary rabbit anti-mouse antibodies (cat# K23100R, Meridian, Memphis, TN; cat# 4272, Cell Signaling, Danvers, MA; cat# GTX10983, Acris, Herford, Germany; cat# Ab165056, AbCam, Cambridge, UK, respectively). Briefly, mitochondrial samples from BAT (3 μg/lane) and WAT (10 μg/lane) were resolved by SDS-PAGE (12.5% acrylamide and 0.51% N,N’-diallyltartardiamide), transferred to polyvinylidene difluoride membranes, and probed with the corresponding primary antibodies. Semiquantitative determination of the relative protein amounts was performed by Image J free software.
Real-time PCR analysis of gene expression
Total RNA was extracted from fresh frozen liver tissue using the NucleoSpin RNA/Protein kit from Macherey-Nagel (cat# 740933.50, Dueren, Germany) according to the manufacturer’s instructions. Reverse transcription was performed using the PrimeScriptTM RT reagent kit from Takara Bio Inc. (cat# RR037A, Otsu, Shiga, Japan). Real-Time PCR for ApoE, Lrp1, and the housekeeping gene Rps18 was performed in a MicroAmp® 96-well reaction plate from Applied Biosystems (cat# 4346906, Foster City, CA) using the KAPA SYBR® FAST Universal qPCR kit from Kapa Biosystems (cat# KK4601, Woburn, MA), in an Applied Biosystems® StepOne™ cycler. Primers used are shown in Table 1. Primers were synthesized by Invitrogen-Life Technologies (Renfrewshire, Scotland). Data were normalized for Rps18 expression.
TABLE 1.
Primers used in the real-time PCR analysis of ApoE and Lrp1 gene expression
| Primer Name | Primer Sequence |
| Murine Lrp1 forward | 5′-GAC CAG GTG TTG GAC ACA GAT G-3′ |
| Murine Lrp1 reverse | 5′-AGT CGT TGT CTC CGT CAC ACT TC-3′ |
| Murine ApoE forward | 5′-CTG ACA GGA TGC CTA GCC G-3′ |
| Murine ApoE reverse | 5′-CGC AGG TAA TCC CAG AAG C-3′ |
| Murine Rps18 forward | 5′-GTA ACC CGT TGA ACC CCA TT-3′ |
| Murine Rps18 reverse | 5′-CCA TCC AAT CGG TAG TAG CG-3′ |
Statistical analysis
Data are reported as mean ± estimated SEM (* P < 0.05, ** P < 0.005; n indicates the number of animals tested in each experiment). Comparison of data from two groups of mice was performed using the Student’s t-test. Analysis of metabolic data was performed by ANCOVA, a statistical test that in our case evaluates whether population means of EE (our dependent variable) differ between mice of different genotype (our independent variable), while statistically controlling for the effects of body weight on EE (our covariate). The F value is the ratio of variability between groups (which in our case relates to genotype differences), divided by the variability within group (which in our case relates to body weight differences), and is a measure of the difference in EE between groups. The higher the F value, the higher the difference between groups when corrected for body weight, while P value indicates the statistical significance of the observed differences. All statistical tests were performed using SPSS software (released 2009, PASW statistics for Windows, Version 18.0; SPSS Inc., Chicago, IL).
RESULTS
Effects of hypogonadism on body weight gain and plasma lipid levels in Ldlr−/− mice
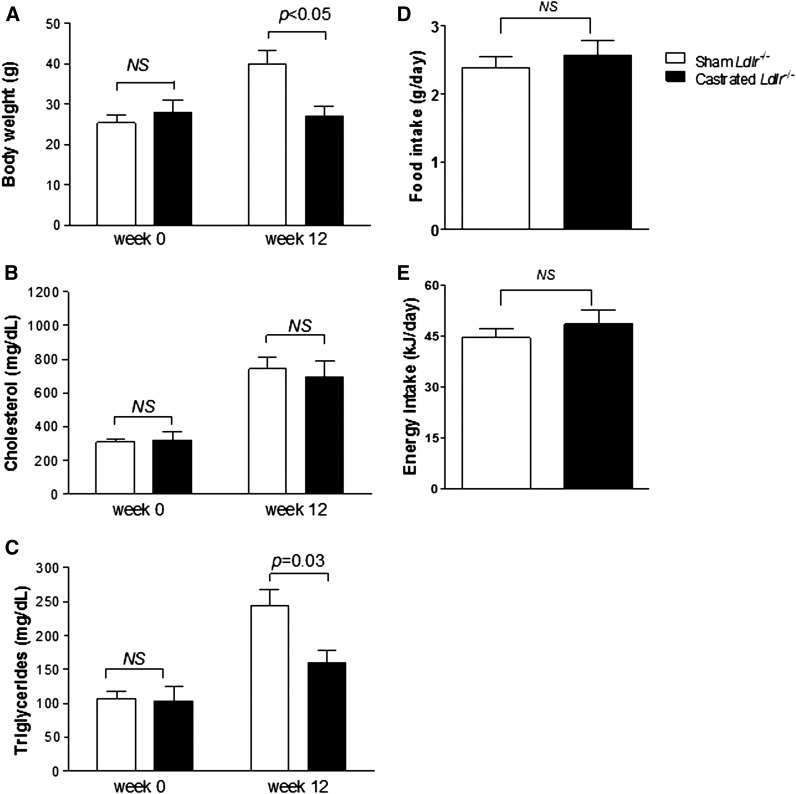
Twelve mice deficient for the classical Ldlr and 12 C57BL/6 mice were either surgically castrated or sham operated as described in Materials and Methods. Following a period of 4 weeks on chow diet, mice were placed for an additional 12 weeks on Western-type diet, and then biochemical and metabolic analyses were performed. In agreement with our previous data (21), sham-operated Ldlr−/− mice showed a considerable increase in their body weight after consuming Western-type diet for 12 weeks. However, to our great surprise castrated animals remained lean during the course of the experiment, and thus resistant to diet-induced body weight gain (Fig. 1A). Specifically, the sham-operated mice increased in body weight from 25.3 ± 1.6 g on week 0 to 39.9 ± 2.5 g (P < 0.05) on week 12, showing a 57.7 ± 1.1% increase that was statistically smaller than the 66.1 ± 1.7% seen in sham-operated C57BL/6 mice fed the same diet for the same period of time (from 25.1 ± 2.0 g at week 0 to 41.8 ± 1.2 g at week 12, P < 0.05), in agreement with our previously reported results (21). Contrasting with this observation, castrated Ldlr−/− mice exhibited no increase in their body weight, changing from 28.0 ± 4.0 g on week 0 to 27.0 ± 2.8 g on week 12 (P > 0.05), while castrated C57BL/6 mice increased from 22.3 ± 0.5 g on week 0 to 38.9 ± 3.4 g on week 12 (P < 0.05). Apparently, lack of Ldlr resulted in a modification of the effects of TD on body weight gain of mice.
Fig. 1.
Body weight gain (A), plasma cholesterol (B), plasma TGs (C), average daily food intake (D), and average daily energy consumption (E) in sham-operated (n = 12) and castrated (n = 12) Ldlr−/− mice fed Western-type diet for 12 weeks.
In a separate set of mice, body composition analysis showed that castrated Ldlr−/− animals had significantly lower body fat content than their sham-operated counterparts, while no significant differences were observed in LBM and protein and water content between the two groups (Table 2). Both Ldlr−/− mouse groups showed a similar average daily food and energy uptake. Castrated Ldlr−/− mice consumed 2.57 ± 0.22 g of food per day or 48.38 ± 4.06 kJ per day, while control Ldlr−/− mice consumed 2.39 ± 0.15 g of food per day or 44.39 ± 2.74 kJ per day (P > 0.05 for both measurements) (Fig. 1D, E).
TABLE 2.
Body composition of castrated and sham-operated Ldlr− /− mice that were fed Western-type diet for 12 weeks
| Mouse Group | Wet Body Weight | Dry Body Weight | Body Fat | Body Water | LBM | Protein |
| Sham Ldlr−/− (n = 12) | 31.6 ± 0.7 | 19.0 ± 1.4 | 8.8 ± 0.9 | 12.6 ± 0.8 | 22.8 ± 0.3 | 2.4 ± 0.3 |
| Castrated Ldlr−/− (n = 12) | 22.3 ± 1.3 | 9.1 ± 0.9 | 3.4 ± 0.4 | 13.2 ± 0.9 | 18.9 ± 1.6 | 2.2 ± 0.2 |
| P | <0.001 | <0.001 | <0.001 | >0.05 | >0.05 | >0.05 |
Values are expressed as mean ± estimated SEM.
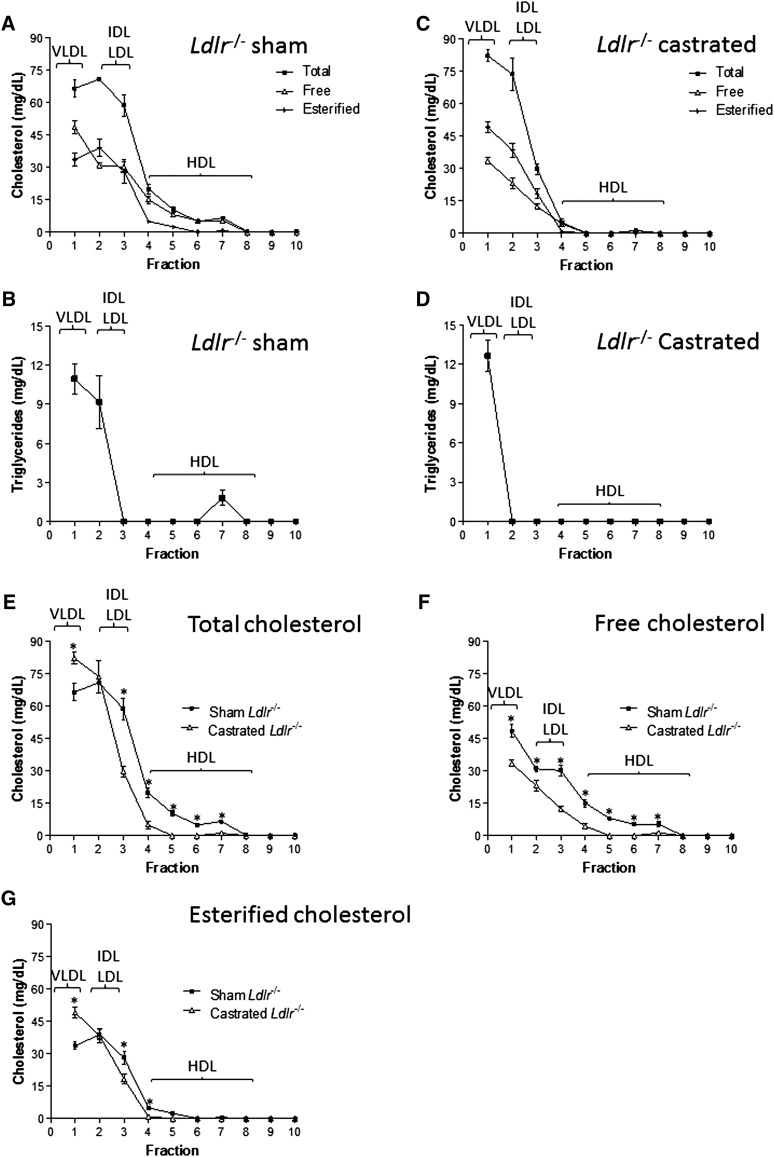
Measurements of fasting plasma lipid levels at the end of the experiment showed that both sham-operated and castrated Ldlr−/− mice showed a dramatic, yet comparable increase in their plasma cholesterol levels (P > 0.05) after 12 weeks of feeding Western-type diet (Fig. 1B). Likewise, plasma TG levels were increased in both groups. However, sham-operated mice showed a significantly larger increase in plasma TG levels than castrated animals (Fig. 1C). Fractionation of plasma lipoproteins by density gradient ultracentrifugation followed by determination of total cholesterol and TGs in the different density fractions revealed that deficiency in Ldlr had a profound effect on plasma lipoproteins of castrated mice fed Western-type diet for 12 weeks (Fig. 2A–D). Interestingly, castrated mice had higher levels of TG-rich VLDL and notably no measurable HDL-C present in their plasma (Fig. 2C, D) compared with the sham-operated group (Fig. 2A, B). Similarly, TD resulted in a quantitative decrease in the content of free versus esterified cholesterol in all lipoprotein fractions (Fig. 2F, G).
Fig. 2.
Total, esterified, and free cholesterol (A, C) and TG (B, D) levels of lipoprotein fractions isolated by KBr density gradient ultracentrifugation analysis of plasma from 12 sham-operated (A, B) and 12 castrated (C, D) Ldlr−/− mice fed Western-type diet for 12 weeks. E, F, and G indicate a comparative representation of the total (E), free (F), and esterified (G) cholesterol levels of various lipoprotein fractions, between sham-operated and castrated Ldlr−/− mice. The position of the various lipoprotein fractions is indicated. The data represent three separate experiments (n = 3) and are reported as mean ± estimated SEM. In E, F and G, * indicates P < 0.05 between sham and castrated Ldlr−/− groups.
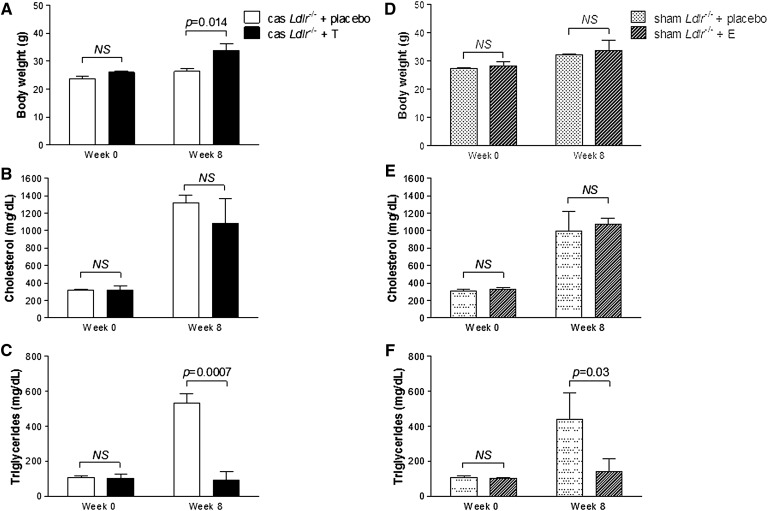
In order to distinguish between TD and other missing testicular functions that could possibly affect the phenotype of the castrated Ldlr−/− mice, we performed a testosterone replacement study, as described in Materials and Methods, for a period of 8 weeks. As shown in Fig. 3A, castrated Ldlr−/− mice treated with testosterone show a significantly higher body weight gain compared with castrated Ldlr−/− mice treated with placebo. Specifically, the body weight of the testosterone-treated animals increased from 26.07 ± 0.05 g at week 0 to 33.90 ± 2.34 g at week 8 of the experiment (30% increase, P < 0.005). In contrast, castrated Ldlr−/− mice treated with placebo were resistant to body weight gain during the 8 weeks of treatment, as expected. Indeed, the placebo group showed a much lower weight gain from 23.72 ± 0.86 g at week 0 to 26.37 ± 1.16 g at week 8 of the experiment (11.2% increase, P < 0.005), in agreement with the data of Fig. 1. Plasma cholesterol levels at week 0 were 319.86 ± 48.56 mg/dl and 314.45 ± 15.94 mg/dl for the testosterone-treated and placebo groups, respectively (P > 0.05), and on week 8, they increased to 1,079.64 ± 291.47 mg/dl and 1,316.43 ± 89.94 mg/dl, respectively (P > 0.05) (Fig. 3B). On week 0, plasma TG levels were 103.15 ± 21.23 mg/dl and 107.13 ± 9.85 mg/dl for the testosterone-treated and placebo groups, respectively (P > 0.05). Surprisingly, on week 8 of the experiment, plasma TG levels of the placebo group increased to 530.89 ± 57.10 mg/dl, while plasma TG levels of testosterone-treated animals were 92.11 ± 49.64 mg/dl (Fig. 3C). To confirm the effectiveness of castration and testosterone replacement protocol, plasma testosterone levels were determined at the initiation of the experiment, at week 0 prior to exogenous testosterone administration, and at week 8 following testosterone treatment. Castration resulted in nondetectable plasma testosterone levels, while testosterone replacement significantly increased plasma testosterone at the end of the experiment (581 ± 52 pg/ml). Plasma testosterone levels immediately prior to castration were 720 ± 41 pg/ml.
Fig. 3.
Body weight gain (A, D), plasma cholesterol (B, E), and plasma TGs (C, F) of castrated Ldlr−/− mice treated with testosterone or placebo (A–C) (n = 6), or sham-operated Ldlr−/− mice treated with exemestane or placebo (D–F) (n = 6) for a period of 8 weeks. All animal groups were fed Western-type diet as described in Materials and Methods.
In order to evaluate the contribution of estrogens in the observed phenotype, we treated sham-operated Ldlr−/− mice with exemestane, a selective nonreversible inhibitor of aromatase, as described in Materials and Methods, for a period of 8 weeks. As shown in Fig. 3D, exemestane-treated animals showed similar to placebo group diet-induced body weight gain during the course of the experiment. Specifically, the body weight of exemestane-treated animals increased from 28.40 ± 1.30 g at week 0 to 33.90 ± 3.65 g at week 8 of the experiment (P < 0.005) (Fig. 3D). The placebo-treated animals showed a similar weight gain from 27.27 ± 0.37 g at week 0 to 32.17 ± 0.47 g at week 8 of the experiment (P < 0.005). Similarly, no difference in cholesterol levels between exemestane-treated and placebo animals was observed at either week 0 or 8 of the experiment (Fig. 3E). Surprisingly, however, exemestane treatment resulted in a significant decrease in plasma TG levels at week 8 of the experiment. Specifically, the levels of exemestane group were 142.7 ± 73.20 mg/dl, while the levels of the placebo group were 438.85 ± 150.83 mg/dl (67.56% difference, P < 0.05) (Fig. 3F). To confirm the effectiveness of exemestane treatment, plasma estradiol and testosterone levels were determined at the initiation of the experiment and at week 8 following exemestane treatment. Treatment of mice with exemestane resulted in nondetectable levels of plasma estradiol with a concomitant slight increase in plasma testosterone levels (1,090 ± 86 pg/ml). Prior to exemestane treatment, testosterone and estradiol plasma levels were 750 ± 47 pg/ml and 17 ± 3 pg/ml, respectively.
Testosterone-deficient Ldlr−/− mice exhibit improved glucose metabolism
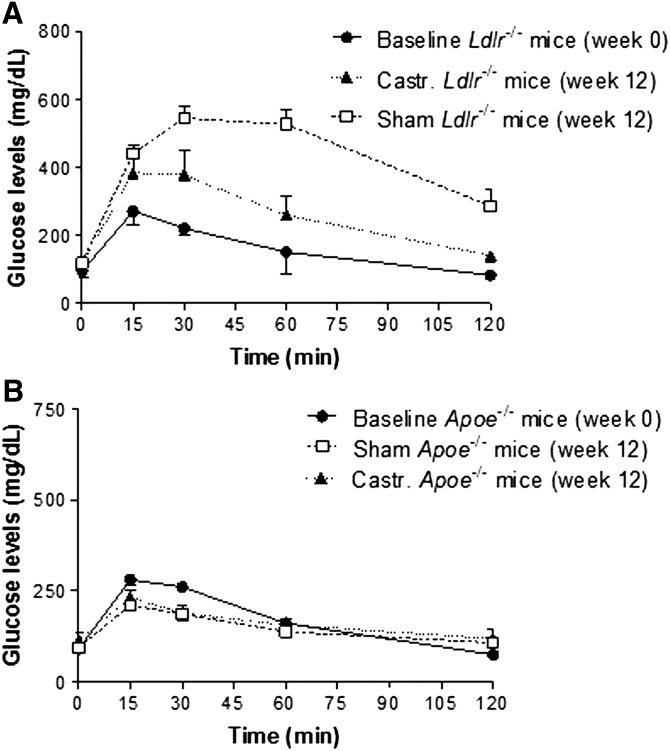
Given the reduced body fat and body weight of the castrated Ldlr−/− mice fed Western-type diet for 12 weeks (n = 12), we hypothesized that castrated animals would also have an improved glucose metabolism compared with sham-operated mice. To test our hypothesis, in the next set of experiments, control and castrated Ldlr−/− mice were challenged with intraperitoneal administration of glucose at a dose of 2 g per kg of body weight in PBS. As expected, both castrated and sham-operated mice had reduced glucose tolerance compared with nonoperated Ldlr−/− animals fed chow diet for the same period of time (baseline). Based on the area under the curve (AUC) values (30,896 ± 696 vs. 51,993 ± 458 for castrated and sham-operated mice, respectively, P < 0.001), castrated Ldlr−/− mice fed Western-type diet for 12 weeks exhibited a significantly improved capacity to respond to glucose challenge compared with sham-operated mice (Fig. 4A), a finding that coincides with the reduced body fat accumulation obtained in TD Ldlr−/− mice.
Fig. 4.
GTT of castrated and sham-operated Ldlr−/− mice (A) (n = 6 per group), and castrated and sham-operated ApoE−/− mice (B) (n = 6 per group) mice. Baseline refers to the GTT of the Ldlr−/− mice immediately before surgery (week 0). In A, AUCbaseline = 18,938 ± 239, AUCsham = 51,993 ± 458, and AUCcastrated = 30,896 ± 696 (all P < 0.05). In B, AUCbaseline = 20,173 ± 466, AUCsham = 18,987 ± 653, and AUCcastrated = 19,391 ± 573 (all P > 0.05).
Effects of TD on Lrp1 and ApoE gene expression in Ldlr−/− mice
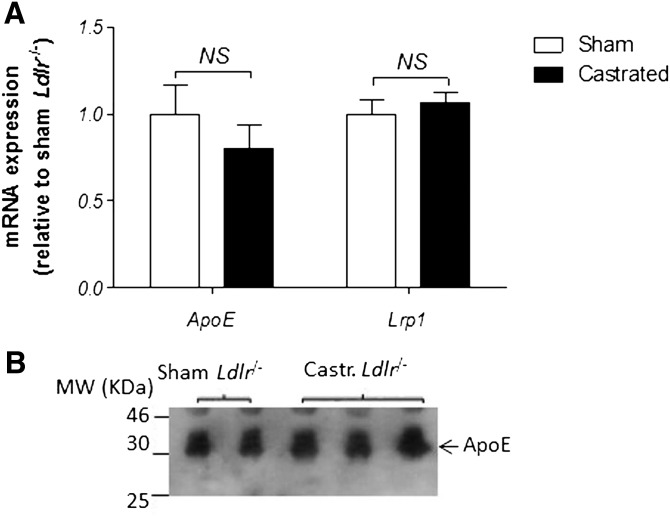
It is well established in the literature that in addition to the classical Ldlr, Lrp1 also binds lipidated ApoE (31), while deficiency in ApoE and diminished expression of Lrp1 have both been associated with reduced body fat accumulation and improved glucose metabolism in mice and humans (21, 22, 32, 33). In the next set of experiments, we sought a mechanistic interpretation of the observed phenotype of reduced body weight and fat in castrated Ldlr−/− mice. Given the role of ApoE and Lrp1 in the development of diet-induced obesity (21–23), we hypothesized that the effects of TD on body weight gain of the Ldlr−/− mice might be due to reduced ApoE and/or Lrp1 expression. Contrary to our hypothesis, quantitative real-time PCR analysis of hepatic mRNA (Fig. 5A) revealed no statistically significant changes in ApoE and Lrp1 gene expression following castration (P > 0.05). In addition, Western blot analysis of plasma from the two mouse groups indicated similar steady-state levels of ApoE for both sham and castrated Ldlr−/− mice (Fig. 5B).
Fig. 5.
Fold change in gene expression of Lrp1 and ApoE genes (A) in sham-operated and castrated Ldlr−/− mice (n = 12). The values of both sham and castrated mice were relative to those of the internal control gene Rps18. The values of the castrated mice represent the fold change relative to that of sham-operated mice (defined as 1). The fold-change in Lrp1 and ApoE gene expression was not statistically significant (both P > 0.05) using a two-tailed unpaired Student’s t-test. Shown in B is a representative Western blot analysis of plasma ApoE protein levels in sham-operated and castrated Ldlr−/− mice.
Effects of TD on body weight gain, plasma lipid levels, glucose tolerance, and hepatic lipid deposition of ApoE−/− mice
To confirm that ApoE plays no role in the resistance of castrated Ldlr−/− mice toward diet-induced obesity, we next tested how deficiency in ApoE might affect weight gain and glucose metabolism in TD mice.
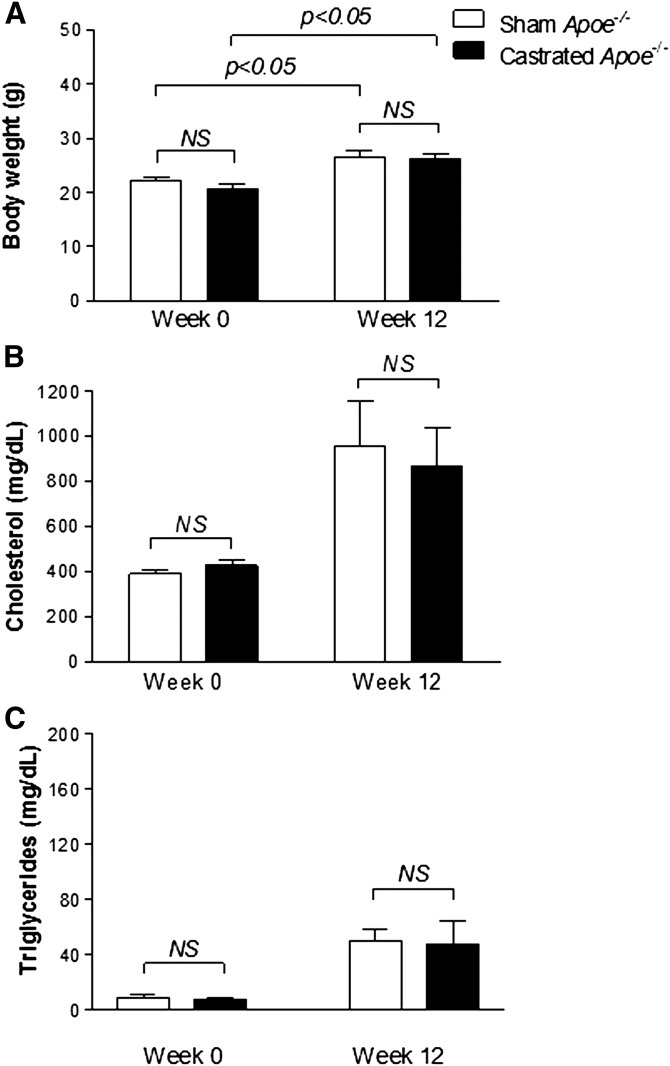
To address this question, ApoE-deficient (ApoE−/−) mice were either castrated (n = 12) or sham operated (n = 12), and following a recovery period of 4 weeks on chow diet, animals were fed Western-type diet for 12 weeks. As expected, ApoE−/− mice appeared to be resistant to diet-induced obesity showing only a modest increase in body weight, as reported previously (22). Specifically, the sham-operated ApoE−/− mice increased in body weight from 22.8 ± 1.2 g on week 0 to 27.4 ± 1.7 g on week 12 (P < 0.005), which compares to the increase observed in castrated Ldlr−/− mice (P > 0.05) (Fig. 1A). A similar increase in body weight was also obtained in the castrated ApoE−/− mice that changed from 20.6 ± 1.1 g to 26.1 ± 0.7 g (P < 0.05) (Fig. 6A). No statistically significant difference in the average body weight was observed between sham and castrated groups at weeks 0 and 12 of the experiment.
Fig. 6.
Body weight gain (A) and plasma cholesterol (B) and TG (C) levels in sham-operated (n = 6) and castrated (n = 6) ApoE−/− mice fed Western-type diet for 12 weeks.
Both sham-operated and castrated ApoE−/− mice showed a dramatic increase in their plasma cholesterol levels, though TG levels remained normal during the course of the experiment (Fig. 6B, C). At week 12 of the experiment, plasma cholesterol levels of the sham-operated and castrated ApoE−/− mice were 952.5 ± 403 mg/dl and 866.1 ± 384.1 mg/dl, respectively (P > 0.05) (Fig. 6B), while their plasma TG levels remained within normal range (50.0 ± 17.4 mg/dl and 48.0 ± 28.8 mg/dl, respectively, at week 12, P > 0.05) (Fig. 6C).
Similarly, in a GTT following 12 weeks on high-fat diet, sham-operated and castrated ApoE−/− mice showed no measurable changes in their ability to clear plasma glucose compared with week 0 (Fig. 4B), a finding consistent with the very modest increase in their body weight during the course of the experiment. The AUC values for each of the group were AUCbaseline = 20,173 ± 466, AUCsham = 18,987 ± 653, and AUCcastrated = 19,391 ± 573 (all P > 0.05).
Taken together, these data coupled with the data of Fig. 5 confirm that the effects of TD on Ldlr−/− mice are independent of ApoE-mediated signaling.
TD in Ldlr−/− mice is associated with increased metabolic rate, EE, and mitochondrial function, mainly due to increased thermogenesis
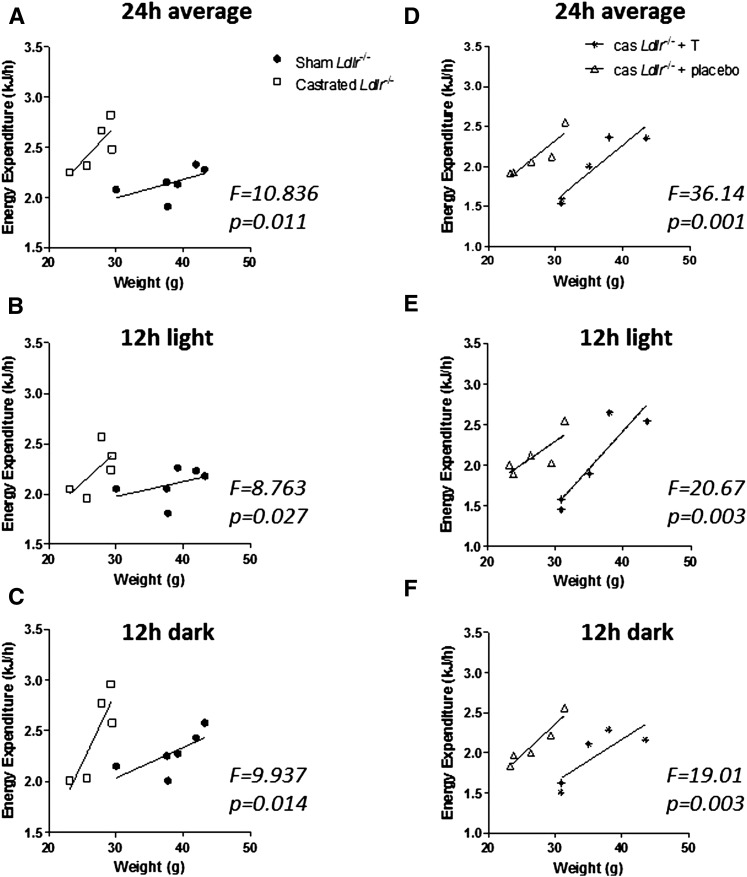
In an effort to determine whether the differences observed in the amount of adipose tissue deposition in sham and castrated Ldlr−/− mice correlate with differences in EE between these two groups, indirect calorimetry analysis was performed, as described in Materials and Methods, at week 12 of the experiment. We based our analysis on the recommendations of Tschöp and coworkers (27). During the 24 h period of the analysis, average EE was 2,508 ± 106 J/h for castrated (n = 5) and 2,155 ± 60 J/h for sham-operated (n = 6) mice (P < 0.05). ANCOVA analysis indicated that when controlled for body weight, the castrated group exhibits significantly higher EE (F = 10.836, P = 0.011) compared with the sham group (Fig. 7A). As expected, no significant difference was determined in the average RER between the two groups (0.78 ± 0.03 for sham and 0.84 ± 0.03 for castrated, P > 0.05) because animals in both groups consumed the same diet and similar types of fuel as energy. Separate ANCOVA analysis of the EE values collected during the 12 h light cycle and the 12 h dark cycle indicated that in both cycles castrated Ldlr−/− mice exhibit similarly increased metabolic rate, suggesting that variations in physical activity may not be a factor in the observed phenotype (Fig. 7B, C).
Fig. 7.
Indirect calorimetry analysis of sham-operated and castrated Ldlr−/− mice fed Western-type diet for 12 weeks (A–C), and castrated Ldlr−/− mice treated with testosterone or placebo and fed Western-type diet for 8 weeks (D–F). All panels represent linear regression plots of EE (J/h) versus mouse body weight (g) during a full day period of 24 h (12 h light/12 h dark) (A, D), or 12 h light (B, E), or 12 h dark (C, F). For each panel, ANCOVA analysis was performed, and the F and P values are indicated.
In agreement with the data from indirect calorimetry, Western blot analysis of mitochondrial lysates showed dramatic increase in mitochondrial cytochrome c levels, primarily in WAT and to a much lesser extent in BAT of castrated Ldlr−/− mice, suggesting elevated mitochondrial oxidative phosphorylation function in these mice (Fig. 8A, lanes 1–4). Moreover, substrate oxidative phosphorylation in these animals appears uncoupled from respiration due to the observed increased expression of Ucp1 primarily in WAT (Fig. 8B, lanes 1–4), resulting in the dissipation of energy as heat. Interestingly, Cox4, a marker of de novo mitochondrial biogenesis (34), does not appear to increase in either WAT or BAT following castration of Ldlr−/− (Fig. 8C, lanes 1–4).
Fig. 8.
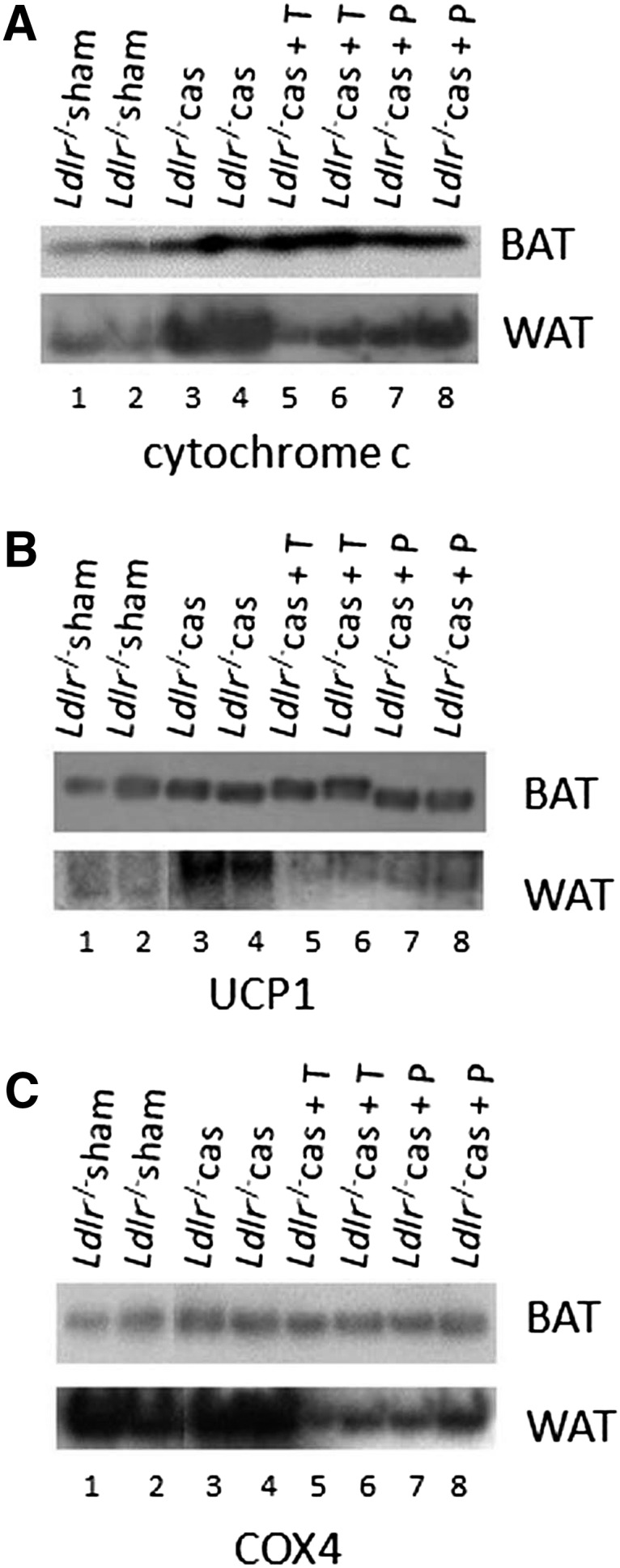
Representative Western blot analysis for cytochrome c (A), Ucp1 (B), and Cox4 (C) of mitochondrial extracts from WAT and BAT of sham-operated Ldlr−/− mice (lanes 1, 2), castrated Ldlr−/− mice (lanes 3, 4), castrated Ldlr−/− mice treated with testosterone (lanes 5, 6), and castrated Ldlr−/− mice treated with placebo (lanes 7, 8). Data were produced from the same blot probed with the indicated antibodies. The picture is the composite of two separate parts of the same film.
Testosterone replacement in castrated Ldlr−/− mice decreases metabolic rate, EE, and mitochondrial function
In an effort to determine whether the differences observed in the body weight of castrated Ldlr−/− mice treated with testosterone and placebo correlate with differences in EE between these two groups, indirect calorimetry analysis was performed, as described in Materials and Methods, at week 8 of the experiment. During the 24 h period of the analysis, average EE was 2,114 ± 117 J/h for castrated Ldlr−/− mice treated with placebo (n = 5) and 1,969 ± 117 J/h for castrated Ldlr−/− mice treated with testosterone (n = 5) (P < 0.05). ANCOVA analysis indicated that when controlled for body weight, the placebo-treated castrated group exhibits higher EE (F = 36.14, P = 0.001) compared with the testosterone-treated castrated group (Fig. 7D). As expected, no significant difference was determined in the average RER between the two groups (0.81 ± 0.02 for castrated Ldlr−/− mice treated with placebo, and 0.79 ± 0.04 for castrated Ldlr−/− mice treated with testosterone, P > 0.05) because animals in both groups consumed the same diet and similar types of fuel as energy. Separate ANCOVA analysis of the EE values collected during the light cycle and the dark cycle indicated that in both cycles castrated Ldlr−/− mice exhibit similarly increased metabolic rates, suggesting that variations in physical activity may not be a factor in the observed phenotype (Fig. 7E, F).
In agreement with the data from indirect calorimetry, Western blot analysis showed decreased cytochrome c levels in mitochondria of WAT, but not BAT, isolated from testosterone-treated castrated Ldlr−/− mice compared with placebo group (Fig. 8A, lanes 5–8), suggesting reduced mitochondrial oxidative phosphorylation function in WAT, but not in BAT, of these mice. This decrease in mitochondrial function was accompanied by a respective decrease in thermogenesis, documented by the marked decrease in mitochondrial Ucp1 expression levels (Fig. 8B, lanes 5–8). As control, Cox4 was not affected in either WAT or BAT of castrated Ldlr−/− mice treated with testosterone or placebo (Fig. 8C, lanes 5–8).
DISCUSSION
Epidemiological studies as well as studies in PCa patients undergoing ADT established that TD plays a causative role in the development of metabolic syndrome and cardiovascular disease (4–8). Given the key role of Ldlr in the management of plasma lipids, in the present study we sought to determine how TD affects diet-induced weight gain, EE, and glucose tolerance in mice lacking a functional Ldlr. TD in Ldlr−/− mice presented an intriguing phenotype. On one hand, sham-operated animals fed Western-type diet for 12 weeks became obese and showed decreased EE, disturbed plasma glucose metabolism, and increased plasma cholesterol and TG levels. On the other hand, castrated Ldlr−/− mice appeared resistant to diet-induced obesity and had elevated EE, improved glucose metabolism, and reduced plasma TG levels compared with sham-operated Ldlr−/− mice fed Western type diet for 12 weeks, though they exhibited a further deterioration in their already proatherogenic plasma cholesterol profile.
Based on classical biochemistry, it is well established that in males estradiol is produced mainly in testes through aromatization of testosterone and to a much lesser extent in extragonadal tissues such as brain, adrenal gland, bones, and adipose tissue. In males, however, estradiol produced in extragonadal tissues has an autocrine action and is not secreted to the circulation (35). In contrast, in the adrenal gland, progesterone is metabolized primarily to aldosterone through corticosterone and not to testosterone and estradiol. Consequently, in males there is no other metabolic pathway for plasma estradiol synthesis except the aromatization of testosterone by aromatase in testes (35). Thus, during the interpretation of our initial data from sham-operated and castrated mice, it became apparent that in addition to TD, estradiol deficiency could also be responsible for the phenotype of the castrated Ldlr−/− mice. To prove the direct role of testosterone in the observed phenotype, we performed a testosterone replacement study and an aromatase inhibitor treatment study. Castrated Ldlr−/− mice supplemented with testosterone showed a significant body weight gain, similar to sham-operated animals. This finding confirmed that lack of testosterone is the critical parameter in the resistance in diet-induced body weight gain exhibited by castrated Ldlr−/− mice (Fig. 3A). To exclude potential involvement of estradiol in the observed phenotype, we treated mice with exemestane, a third-generation irreversible aromatase inhibitor, one of the most commonly used currently in the clinic. Sham-operated Ldlr−/− mice treated with exemestane showed a similar body weight gain to placebo-treated animals, demonstrating that the resistance of castrated Ldlr−/− mice toward diet-induced obesity is directly due to TD and not to estrogen deficiency (Fig. 3D). Any additional low androgenic effect of exemestane would have an incremental impact on the phenotype of the treated animals given that sham-operated Ldlr−/− mice have already significant levels of endogenous testosterone.
One possibility explaining the weight gain resistance of castrated Ldlr−/− mice would be higher EE, due to higher physical activity. Given that mice with increased physical activity will manifest this phenotype primarily during the dark phase, we proceeded to separate ANCOVA analyses of the light cycle and dark cycle data. The findings indicated that in both cycles castrated Ldlr−/− mice exhibit similarly increased metabolic rates, suggesting that variations in physical activity may not be a factor in the observed phenotype.
Increased levels of mitochondrial cytochrome c have been associated with increased mitochondrial metabolic function (36). This function may relate to thermogenesis marked by increased mitochondrial Ucp1 expression (37). Our molecular analysis confirmed the data from indirect calorimetry, by showing that TD in the castrated Ldlr−/− mice resulted in increased mitochondrial function as judged by increased levels of cytochrome c protein expression primarily in WAT and to a much lesser extent in BAT. Further analysis showed elevated Ucp1 protein expression primarily in WAT and to a much lesser extent in BAT. This finding suggests that in the absence of Ldlr TD results in activation of thermogenesis mainly in WAT of castrated Ldlr−/− mice, and this increase may account for their elevated EE observed in metabolic cages. It is interesting that in WAT of placebo-treated castrated Ldlr−/− mice, we noted a higher Ucp1 expression compared with the testosterone-treated group (Fig. 8B, lanes 5–8), suggesting that in testosterone-treated mice the decreased EE is associated with reduced thermogenesis. Of note, the levels of Ucp1 expression in castrated Ldlr−/− mice after 12 weeks on Western-type diet (Fig. 8B, lanes 3, 4) were much higher than that of placebo-treated Ldlr−/− mice fed Western-type diet for 8 weeks (Fig. 8B, lanes 7, 8). It is possible that the longer exposure of the castrated Ldlr−/− mice to the diet may be responsible for this difference, though potential effects of placebo on Ucp1 expression cannot be excluded.
Contrary to studies from wild-type animals and humans in which TD is associated with increased fat mass and abnormal glucose metabolism (38), in the Ldlr−/− mouse model, where Lrp1 becomes the main receptor for ApoE-containing lipoproteins, TD results in resistance in body weight gain and improved glucose metabolism. In vitro and in vivo studies have shown that lipoprotein-bound ApoE is the natural ligand for Ldlr (39–41), which is the main receptor involved in the clearance of ApoE-containing lipoproteins in vivo (42). In addition to Ldlr, Lrp1 has also been shown to interact with ApoE and promote the clearance of ApoB48-containing lipoproteins, though it plays no significant role in the clearance of ApoB100-containing lipoproteins (43). Based on previous findings showing that both ApoE and Lrp1 expression levels modulate obesity and glucose metabolism in mice (21, 23, 32), here we tested whether the reduced body weight gain of castrated Ldlr−/− mice is associated with reduced ApoE and/or Lrp1 gene expression. Interestingly, we found that TD resulted in no significant change in hepatic ApoE and Lrp1 gene expression. Similarly, Western blot analysis of steady-state plasma ApoE levels showed no differences between sham and castrated Ldlr−/− mice. Given that steady-state plasma ApoE levels are a function of hepatic ApoE synthesis and receptor-mediated clearance of lipoprotein bound ApoE (40, 44–48), then from the data of Fig. 5A, B it is conceivable that TD does not affect the receptor-mediated clearance of plasma ApoE. Of course, our data do not exclude the possibility that TD serves as a modulator of secondary intracellular signals initiating from Lrp1 on the surface of the cell that tilts the energy balance toward increased EE and reduced body fat accumulation.
Plasma lipoproteins are classified solely based on their density (LDLs, HDLs, etc.). Though size exclusion chromatography of plasma fractions has been used extensively in the literature as an easy means for lipoprotein separation, the method is based solely on the assumption that size correlates with density. Of course, this is not always the case because often times lipoproteins of different densities have similar diameters (e.g., small dense LDL and large HDL), leading to mistaken conclusions. Thus, in the present study we used density gradient ultracentrifugation analysis as the method of choice for lipoprotein isolation. This analysis showed that in contrast to the prevailing view that plasma TG levels show an inverse correlation with plasma HDL-C levels, in our study castrated Ldlr−/− mice had reduced plasma TG and HDL-C levels compared with the control group. This may suggest that the regulation of plasma TG and HDL-C levels may not be mediated by a common mechanism, supporting the idea of Avogaro and coworkers (49) that regulation of plasma HDL-C and TG levels may occur as expression of two distinct primary metabolic effects. Apparently, in our experiments TD did not affect the total plasma cholesterol levels of Ldlr−/− mice, but it resulted in a deterioration of their lipoprotein profile by shifting cholesterol distribution toward proatherogenic lipoproteins. Castrated animals showed a further elevation in VLDL that combined with their reduced HDL levels (Fig. 2A, C) may provide a mechanistic interpretation of the results of a recent study showing that castrated Ldlr−/− mice fed a low-fat diet develop more severe atherosclerotic lesions than sham-operated animals (50). At first glance, it may appear that the reduction in plasma HDL levels is insignificant because sham-operated mice already have reduced HDL levels compared with control C57BL/6 mice. However, the observed changes are quite significant in terms of cardiovascular physiology and pathology given that it is well established through analysis of four American studies (two observational studies and the control groups of two clinical trials) that coronary heart disease risk decreases by 2% to 3% with each increment of 1 mg/dl in HDL-C levels (51). An interesting qualitative difference in plasma lipoproteins is that castrated Ldlr−/− mice had increased levels of esterified cholesterol in plasma, a finding that may be due to enhanced Lcat activity under conditions of hypogonadism in these mice.
Work by Casquero and coworkers (52) indicated that overexpression of human cholesteryl ester transfer protein (hCetp) in mice results in a significant attenuation of the increase in atherosclerosis, following surgical castration. However, these authors reported no significant difference in the body weight gain of the castrated hCetp-expressing mice compared with the non-hCetp-expressing castrated littermates. In agreement with our findings, in both mouse strains the authors reported a similar weight loss of ∼4% compared with the sham-operated littermates, with P > 0.05, suggesting that hCetp may not be a modulator of body weight gain following castration.
Despite a report stating that exemestane treatment of female ovariectomized rats decreases plasma cholesterol levels via estrogen-independent mechanism (26), in our experimental setup, treatment of sham-operated male Ldlr−/− mice with exemestane had no effect on plasma cholesterol levels, suggesting species-specific off-target effects of exemestane in rats. Of note, exemestane treatment of sham-operated Ldlr−/− mice resulted in a significant reduction in plasma TG levels of these mice, compared with placebo group (Fig. 3F). This finding is very interesting, considering the data from the testosterone replacement experiment where treatment of castrated Ldlr−/− mice with testosterone also resulted in reduced plasma TG levels (Fig. 3C). Based on these observations, it is conceivable to hypothesize that estradiol counteracts the functions of testosterone in the regulation of plasma TG levels in mice lacking a functional Ldlr.
The present study highlights a novel role of Ldlr as a functional modulator of processes associated with hypogonadism-induced metabolic alterations, such as diet-induced weight gain, EE, and glucose tolerance. In light of this information, it would be interesting to investigate how modulation of the number of functional Ldlr molecules on cell surface by pharmacological means (i.e., statins, proprotein convertase subtilisin/kexin type 9 inhibitors, etc.) may impact the metabolic effects of testosterone. Recent work by Sugiyama and coworkers (53) revealed increased caloric intake in statin-treated patients. However, it was not clear whether it was the increase in Ldlr expression or other factors (off-target effects of statins and/or psychological factors, such as increased patient confidence to the treatment) that were responsible for this observation.
Overall, our data indicate that under conditions of Ldlr deficiency, TD appears to reduce body fat accumulation and body weight gain due to increased thermogenesis and improved glucose tolerance despite a further aggravation of lipoprotein profile.
Footnotes
Abbreviations:
- ADT
- androgen deprivation therapy
- AUC
- area under the curve
- BAT
- brown adipose tissue
- Cox4
- cytochrome c oxidase subunit 4
- EE
- energy expenditure
- GTT
- glucose tolerance test
- hCetp
- human cholesteryl ester transfer protein
- HDL-C
- HDL cholesterol
- LBM
- lean body mass
- Ldlr
- LDL receptor
- Lrp1
- LDL receptor-related protein 1
- PCa
- prostate cancer
- RER
- respiratory exchange ratio
- TD
- testosterone deficiency
- Ucp1
- uncoupling protein 1
- VCO2
- carbon dioxide production expressed as the difference of carbon dioxide exiting from carbon dioxide entering the metabolic cage
- VO2
- oxygen consumption expressed as the difference between oxygen entering and oxygen exiting the metabolic cage
- WAT
- white adipose tissue
The research project has been implemented within the framework of the Action Supporting Postdoctoral Researchers of the Operational Program “Education and Lifelong Learning” (Action’s Beneficiary: General Secretariat for Research and Technology) and is cofinanced by the European Social Fund and the Greek state.
REFERENCES
- 1.Shahidi N. T. 2001. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin. Ther. 23: 1355–1390 [DOI] [PubMed] [Google Scholar]
- 2.Yassin A. A., Saad F. 2008. Testosterone and erectile dysfunction. J. Androl. 29: 593–604 [DOI] [PubMed] [Google Scholar]
- 3.Traish A. M., Abdou R., Kypreos K. E. 2009. Androgen deficiency and atherosclerosis: the lipid link. Vascul. Pharmacol. 51: 303–313 [DOI] [PubMed] [Google Scholar]
- 4.Traish A. M., Saad F., Guay A. 2009. The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance. J. Androl. 30: 23–32 [DOI] [PubMed] [Google Scholar]
- 5.Traish A. M., Saad F., Feeley R. J., Guay A. 2009. The dark side of testosterone deficiency: III. Cardiovascular disease. J. Androl. 30: 477–494 [DOI] [PubMed] [Google Scholar]
- 6.Traish A. M., Guay A., Feeley R., Saad F. 2009. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J. Androl. 30: 10–22 [DOI] [PubMed] [Google Scholar]
- 7.Kalyani R. R., Dobs A. S. 2007. Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr. Opin. Endocrinol. Diabetes Obes. 14: 226–234 [DOI] [PubMed] [Google Scholar]
- 8.Kapoor D., Jones T. H. 2008. Androgen deficiency as a predictor of metabolic syndrome in aging men: an opportunity for intervention? Drugs Aging. 25: 357–369 [DOI] [PubMed] [Google Scholar]
- 9.Derby C. A., Zilber S., Brambilla D., Morales K. H., McKinlay J. B. 2006. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin. Endocrinol. (Oxf.). 65: 125–131 [DOI] [PubMed] [Google Scholar]
- 10.Saylor P. J., Smith M. R. 2009. Prostate cancer: how can we improve the health of men who receive ADT? Nat. Rev. Urol. 6: 529–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith M. R., Lee H., Nathan D. M. 2006. Insulin sensitivity during combined androgen blockade for prostate cancer. J. Clin. Endocrinol. Metab. 91: 1305–1308 [DOI] [PubMed] [Google Scholar]
- 12.Smith M. R., Finkelstein J. S., McGovern F. J., Zietman A. L., Fallon M. A., Schoenfeld D. A., Kantoff P. W. 2002. Changes in body composition during androgen deprivation therapy for prostate cancer. J. Clin. Endocrinol. Metab. 87: 599–603 [DOI] [PubMed] [Google Scholar]
- 13.Braga-Basaria M., Dobs A. S., Muller D. C., Carducci M. A., John M., Egan J., Basaria S. 2006. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J. Clin. Oncol. 24: 3979–3983 [DOI] [PubMed] [Google Scholar]
- 14.Saad F., Gooren L. J., Haider A., Yassin A. 2008. A dose-response study of testosterone on sexual dysfunction and features of the metabolic syndrome using testosterone gel and parenteral testosterone undecanoate. J. Androl. 29: 102–105 [DOI] [PubMed] [Google Scholar]
- 15.Levine G. N., D’Amico A. V., Berger P., Clark P. E., Eckel R. H., Keating N. L., Milani R. V., Sagalowsky A. I., Smith M. R., Zakai N. 2010. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 121: 833–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svartberg J., von Mühlen D., Mathiesen E., Joakimsen O., Bønaa K. H., Stensland-Bugge E. 2006. Low testosterone levels are associated with carotid atherosclerosis in men. J. Intern. Med. 259: 576– 582. [DOI] [PubMed] [Google Scholar]
- 17.Aversa A., Bruzziches R., Francomano D., Rosano G., Isidori A. M., Lenzi A., Spera G. 2010. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J. Sex. Med. 7: 3495–3503 [DOI] [PubMed] [Google Scholar]
- 18.Barsh G. S., Farooqi I. S., O’Rahilly S. 2000. Genetics of body-weight regulation. Nature. 404: 644–651 [DOI] [PubMed] [Google Scholar]
- 19.Kopelman P. G. 2000. Obesity as a medical problem. Nature. 404: 635–643 [DOI] [PubMed] [Google Scholar]
- 20.Friedman J. M. 2000. Obesity in the new millennium. Nature. 404: 632–634 [DOI] [PubMed] [Google Scholar]
- 21.Karagiannides I., Abdou R., Tzortzopoulou A., Voshol P. J., Kypreos K. E. 2008. Apolipoprotein E predisposes to obesity and related metabolic dysfunctions in mice. FEBS J. 275: 4796–4809 [DOI] [PubMed] [Google Scholar]
- 22.Kypreos K. E., Karagiannides I., Fotiadou E. H., Karavia E. A., Brinkmeier M. S., Giakoumi S. M., Tsompanidi E. M. 2009. Mechanisms of obesity and related pathologies: role of apolipoprotein E in the development of obesity. FEBS J. 276: 5720–5728 [DOI] [PubMed] [Google Scholar]
- 23.Hofmann S. M., Zhou L., Perez-Tilve D., Greer T., Grant E., Wancata L., Thomas A., Pfluger P. T., Basford J. E., Gilham D., et al. 2007. Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. J. Clin. Invest. 117: 3271–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karavia E. A., Papachristou D. J., Kotsikogianni I., Giopanou I., Kypreos K. E. 2011. Deficiency in apolipoprotein E has a protective effect on diet-induced nonalcoholic fatty liver disease in mice. FEBS J. 278: 3119–3129 [DOI] [PubMed] [Google Scholar]
- 25.Traish A. M., Park K., Dhir V., Kim N. N., Moreland R. B., Goldstein I. 1999. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology. 140: 1861–1868 [DOI] [PubMed] [Google Scholar]
- 26.Goss P. E., Qi S., Cheung A. M., Hu H., Mendes M., Pritzker K. P. 2004. Effects of the steroidal aromatase inhibitor exemestane and the nonsteroidal aromatase inhibitor letrozole on bone and lipid metabolism in ovariectomized rats. Clin. Cancer Res. 10: 5717–5723 [DOI] [PubMed] [Google Scholar]
- 27.Tschöp M. H., Speakman J. R., Arch J. R., Auwerx J., Brüning J. C., Chan L., Eckel R. H., Farese R. V., Jr, Galgani J. E., Hambly C., et al. 2012. A guide to analysis of mouse energy metabolism. Nat. Methods. 9: 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kypreos K. E. 2008. ABCA1 promotes the de novo biogenesis of apolipoprotein CIII–containing HDL particles in vivo and modulates the severity of apolipoprotein CIII–induced hypertriglyceridemia. Biochemistry. 47: 10491–10502 [DOI] [PubMed] [Google Scholar]
- 29.Duivenvoorden I., Teusink B., Rensen P. C., Romijn J. A., Havekes L. M., Voshol P. J. 2005. Apolipoprotein C3 deficiency results in diet-induced obesity and aggravated insulin resistance in mice. Diabetes. 54: 664–671 [DOI] [PubMed] [Google Scholar]
- 30.Commins S. P., Watson P. M., Padgett M. A., Dudley A., Argyropoulos G., Gettys T. W. 1999. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology. 140: 292–300 [DOI] [PubMed] [Google Scholar]
- 31.Ruiz J., Kouiavskaia D., Migliorini M., Robinson S., Saenko E. L., Gorlatova N., Li D., Lawrence D., Hyman B. T., Weisgraber K. H., et al. 2005. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J. Lipid Res. 46: 1721–1731 [DOI] [PubMed] [Google Scholar]
- 32.Hofmann S. M., Perez-Tilve D., Greer T. M., Coburn B. A., Grant E., Basford J. E., Tschöp M. H., Hui D. Y. 2008. Defective lipid delivery modulates glucose tolerance and metabolic response to diet in apolipoprotein E-deficient mice. Diabetes. 57: 5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaefer E. J., Gregg R. E., Ghiselli G., Forte T. M., Ordovas J. M., Zech L. A., Brewer H. B., Jr 1986. Familial apolipoprotein E deficiency. J. Clin. Invest. 78: 1206–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puigserver P., Spiegelman B. M. 2003. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr. Rev. 24: 78–90 [DOI] [PubMed] [Google Scholar]
- 35.Simpson E., Rubin G., Clyne C., Robertson K., O’Donnell L., Davis S., Jones M. 1999. Local estrogen biosynthesis in males and females. Endocr. Relat. Cancer. 6: 131–137 [DOI] [PubMed] [Google Scholar]
- 36.Hüttemann M., Lee I., Grossman L. I., Doan J. W., Sanderson T. H. 2012. Phosphorylation of mammalian cytochrome c and cytochrome c oxidase in the regulation of cell destiny: respiration, apoptosis, and human disease. Adv. Exp. Med. Biol. 748: 237–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholls D. G., Locke R. M. 1984. Thermogenic mechanisms in brown fat. Physiol. Rev. 64: 1–64 [DOI] [PubMed] [Google Scholar]
- 38.Kelly D. M., Jones T. H. 2013. Testosterone: a metabolic hormone in health and disease. J. Endocrinol. 217: R25–R45 [DOI] [PubMed] [Google Scholar]
- 39.Brown M. S., Goldstein J. L. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science. 232: 34–47 [DOI] [PubMed] [Google Scholar]
- 40.Kypreos K. E., Teusink B., Van Dijk K. W., Havekes L. M., Zannis V. I. 2001. Analysis of the structure and function relationship of the human apolipoprotein E in vivo, using adenovirus-mediated gene transfer. FASEB J. 15: 1598–1600 [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto T., Choi H. W., Ryan R. O. 2008. Apolipoprotein E isoform-specific binding to the low-density lipoprotein receptor. Anal. Biochem. 372: 222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kypreos K. E., Zannis V. I. 2006. LDL receptor deficiency or apoE mutations prevent remnant clearance and induce hypertriglyceridemia in mice. J. Lipid Res. 47: 521–529 [DOI] [PubMed] [Google Scholar]
- 43.Véniant M. M., Zlot C. H., Walzem R. L., Pierotti V., Driscoll R., Dichek D., Herz J., Young S. G. 1998. Lipoprotein clearance mechanisms in LDL receptor-deficient “Apo-B48-only” and “Apo-B100-only” mice. J. Clin. Invest. 102: 1559–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zannis V. I., Chroni A., Kypreos K. E., Kan H. Y., Cesar T. B., Zanni E. E., Kardassis D. 2004. Probing the pathways of chylomicron and HDL metabolism using adenovirus-mediated gene transfer. Curr. Opin. Lipidol. 15: 151–166 [DOI] [PubMed] [Google Scholar]
- 45.Zannis V. I., Kypreos K. E., Chroni A., Kardassis D., Zanni E. E.2004. Lipoproteins and atherogenesis. In Molecular Mechanisms of Atherosclerosis. J. Loscalzo, editor. Taylor & Francis, New York. 111–174.
- 46.Li X., Kypreos K., Zanni E. E., Zannis V. 2003. Domains of apoE required for binding to apoE receptor 2 and to phospholipids: Implications for the functions of apoE in the brain. Biochemistry. 42: 10406–10417 [DOI] [PubMed] [Google Scholar]
- 47.Kypreos K. E., Li X., Van Dijk K. W., Havekes L. M., Zannis V. I. 2003. Molecular mechanisms of type III hyperlipoproteinemia: the contribution of the carboxy-terminal domain of ApoE can account for the dyslipidemia that is associated with the E2/E2 phenotype. Biochemistry. 42: 9841–9853 [DOI] [PubMed] [Google Scholar]
- 48.Kypreos K. E., Morani P., Van Dijk K. W., Havekes L. M., Zannis V. I. 2001. The amino-terminal 1–185 domain of apoE promotes the clearance of lipoprotein remnants in vivo. The carboxy-terminal domain is required for induction of hyperlipidemia in normal and apoE-deficient mice. Biochemistry. 40: 6027–6035 [DOI] [PubMed] [Google Scholar]
- 49.Avogaro P., Ghiselli G., Soldan S., Bittolo B. G. 1992. Relationship of triglycerides and HDL cholesterol in hypertriglyceridemia. Atherosclerosis. 92: 79–86 [DOI] [PubMed] [Google Scholar]
- 50.Hatch N. W., Srodulski S. J., Chan H. W., Zhang X., Tannock L. R., King V. L. 2012. Endogenous androgen deficiency enhances diet-induced hypercholesterolemia and atherosclerosis in low-density lipoprotein receptor-deficient mice. Gend. Med. 9: 319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordon D. J., Probstfield J. L., Garrison R. J., Neaton J. D., Castelli W. P., Knoke J. D., Jacobs D. R., Jr, Bangdiwala S., Tyroler H. A. 1989. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 79: 8–15 [DOI] [PubMed] [Google Scholar]
- 52.Casquero A. C., Berti J. A., Salerno A. G., Bighetti E. J., Cazita P. M., Ketelhuth D. F., Gidlund M., Oliveira H. C. 2006. Atherosclerosis is enhanced by testosterone deficiency and attenuated by CETP expression in transgenic mice. J. Lipid Res. 47: 1526–1534 [DOI] [PubMed] [Google Scholar]
- 53.Sugiyama T., Tsugawa Y., Tseng C. H., Kobayashi Y., Shapiro M. F. 2014. Different time trends of caloric and fat intake between statin users and nonusers among US adults: gluttony in the time of statins? JAMA Intern. Med. Epub ahead of print. April 24, 2014; 10.1001/jamainternmed.2014.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]