Abstract
2-Hydroxypropyl-β-cyclodextrin (HP-β-CD), a widely used excipient for drug formulation, has emerged as an investigational new drug for the treatment of Niemann-Pick type C1 (NPC1) disease, a neurodegenerative cholesterol storage disorder. Development of a sensitive quantitative LC-MS/MS assay to monitor the pharmacokinetics (PKs) of HP-β-CD required for clinical trials has been challenging owing to the dispersity of the HP-β-CD. To support a phase 1 clinical trial for ICV delivery of HP-β-CD in NPC1 patients, novel methods for quantification of HP-β-CD in human plasma and cerebrospinal fluid (CSF) using LC-MS/MS were developed and validated: a 2D-LC-in-source fragmentation-MS/MS (2D-LC-IF-MS/MS) assay and a reversed phase ultra performance LC-MS/MS (RP-UPLC-MS/MS) assay. In both assays, protein precipitation and “dilute and shoot” procedures were used to process plasma and CSF, respectively. The assays were fully validated and in close agreement, and allowed determination of PK parameters for HP-β-CD. The LC-MS/MS methods are ∼100-fold more sensitive than the current HPLC assay, and were successfully employed to analyze HP-β-CD in human plasma and CSF samples to support the phase 1 clinical trial of HP-β-CD in NPC1 patients.
Keywords: 2-hydroxypropyl-β-cyclodextrin, ultra performance liquid chromatography, two-dimensional liquid chromatography, in-source fragmentation, liquid chromatography-tandem mass spectrometry, Niemann-Pick C, cerebrospinal fluid
Cyclodextrins are cyclic oligosaccharides consisting of a varying number of α-1-4-linked glucose units. These glucose chains create a cone-like cavity into which compounds may enter and form a water-soluble complex, thus altering the drug’s physicochemical properties. The 2-hydroxypropyl-β-cyclodextrin (HP-β-CD), a hydroxyalkyl derivative of β-cyclodextrin, (Fig. 1A) has been widely utilized as an excipient to improve the solubility of poorly water-soluble drugs and to enhance physicochemical properties and chemical stability of drugs, because of its cavity size and greater hydrophilicity. HP-β-CD has also emerged as a promising experimental therapy for Niemann-Pick type C1 (NPC1) disease, a rare inherited neurodegenerative disorder with an estimated incidence in Western European and US populations in the order of one in 100,000 live births (1). NPC1 disease is characterized by an accumulation of cholesterol and other lipids in the endosomal/lysosomal system, resulting in hepatosplenomegaly, progressive neurologic dysfunction, and early death (2). Treatment with HP-β-CD ameliorates cholesterol storage, significantly reducing neurodegeneration and increasing lifespan in murine and feline models of NPC1 disease (3–7). Based on these studies, individual use investigational new drug applications for HP-β-CD have been allowed for eight pediatric patients in the US, and a phase 1 trial for delivery of HP-β-CD directly into the lateral ventricle of NPC1 patients was initiated in January 2013 at the National Institutes of Health (NIH) (8). To support the clinical trial, a reliable assay capable of quantifying HP-β-CD in human plasma and cerebrospinal fluid (CSF) was essential.
Fig. 1.
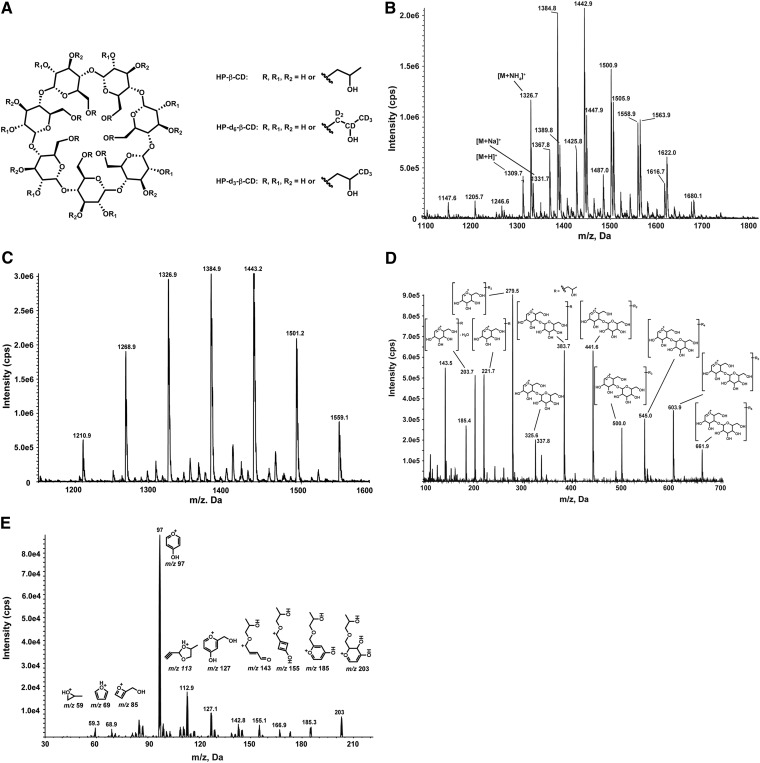
Structures of HP-β-CD, HP-d6-β-CD, and HP-d3-β-CD (A). Positive ion mode ESI mass spectrum of HP-β-CD in the mobile phase of the 2D-LC-IF-MS/MS method in which [M+NH4]+, [M+H]+, and [M+Na]+ species dominate, as highlighted in three substituted isomers (B). Positive ion mode ESI mass spectrum of HP-β-CD in the mobile phase of the RP-UPLC-MS/MS method in which [M+NH4]+ ions dominate (C). In-source fragmentation spectrum of HP-β-CD (D). Tandem mass spectrum of m/z 203 ion (E).
The only bioanalytical assay that has been reported for HP-β-CD quantification in human samples involves HPLC using inclusion complex formation and fluorescence detection. A limitation of this assay is the minimum requirement for 1 ml of plasma or urine to reach a lower limit of quantification (LLOQ) of 0.5 μg/ml (9). To address the need for a sensitive assay to determine the pharmacokinetics (PKs) of HP-β-CD in the context of a clinical trial, we initially developed and validated a novel method utilizing 2D-LC-in-source fragmentation-MS/MS (2D-LC-IF-MS/MS) for sensitivity enhancement with LLOQ for HP-β-CD of 10 ng/ml in human plasma and 100 ng/ml for HP-β-CD in human CSF, respectively. A more streamlined alternative approach was subsequently developed and validated according to generally accepted regulatory guidances (10–12) to support analysis of samples from the phase 1 trial. The latter assay uses a reversed phase ultra performance liquid chromatography (RP-UPLC)-MS/MS method for quantitative determination of HP-β-CD in human plasma and CSF with LLOQs at 50.0 ng/ml and 5.00 μg/ml, respectively. We found both assays were robust and reliable. While the 2D-LC-IF-MS/MS method was more sensitive and provided a more complete HP-β-CD PK profile, as many postdose plasma samples in clinical study were below the LLOQ of the RP-UPLC-MS/MS method, the RP-UPLC-MS/MS method proved easier to implement and afforded more rapid sample analysis. Herein, we report the development and validation of both assays.
MATERIALS AND METHODS
Chemicals and reagents
The HP-β-CD was obtained from Janssen Research and Development (Beerse, Belgium). β-Cyclodextrin, sodium hydroxide, ammonium formate, and ammonium carbonate were purchased from Sigma-Aldrich (St. Louis, MO). The d6-propylene oxide was purchased from Polymer Source (Dorval, Montreal, Quebec, Canada). Triethylamine, acetone, methanol, acetonitrile, and formic acid were purchased from EMD Chemicals (Gibbstown, NJ). Methanol was also purchased from Merck (Darmstadt, Germany). Acetonitrile was also purchased from Biosolv (Valkenswaard, The Netherlands). Milli-Q ultrapure water was prepared in-house with a Milli-Q integral water purification system (Billerica, MA). Pooled control human plasma (Na-heparin), human CSF, six lots of individual human plasma, and six lots of individual human CSF were purchased from BioChemed Services (Winchester, VA) or Bioreclamation (Westbury, NY).
Preparation of internal standards
HP-d6-β-CD for 2D-LC-IF-MS/MS method.
β-Cyclodextrin (0.57 g, 0.499 mmol, 1 equiv) was dissolved in 10% NaOH (1.35 ml, 3.37 mmol, 6.75 equiv) in water. The solution was cooled to 4°C, and d6-propylene oxide (0.411 g, 6.42 mmol, 12.9 equiv) was added over 3.5 h with a Harvard syringe pump (Harvard Apparatus, South Natick, MA). After addition of propylene oxide, the reaction mixture was stirred overnight at 4°C and neutralized with formic acid. The crude product was isolated with solid phase extraction on a Sep-Pak Vac12 cc C18-2g cartridge (Waters, Milford, MA) (20 –90% methanol in water) to yield 0.37 g of HP-d6-β-CD, which contained on average seven d6-hydroxypropyl units per cyclodextrin as determined by 1HNMR (Fig. 1A).
HP-d3-β-CD for RP-UPLC-MS/MS method.
A suspension of β-cyclodextrin hydrate (0.20 g, 0.17 mmol, 1 equiv) in water (1 ml) containing triethylamine (0.14 ml, 1.0 mmol, 6 equiv) and d3-propylene oxide (0.18 ml, 2.4 mmol, 14 equiv) was heated to 50°C for 1 h and then to 70°C for another hour. The reaction mixture was concentrated to residue. The crude product was suspended and washed repeatedly in acetone. White solid (0.26 g) was isolated, which contained on average 5.9 d3-hydroxypropyl units per cyclodextrin as determined by 1HNMR, as well as a salt in the form of 2-hydroxypropyl-d3-triethylammonium bicarbonate (Fig. 1A).
Solution preparation
The HP-β-CD and internal standard (IS) were accurately weighed and dissolved in water to obtain a 1.00 mg/ml stock solution. Two stock solutions for HP-β-CD were prepared independently, and the agreement between the solutions was verified. One solution was used for calibration standards and the other was used for quality control (QC) samples. These solutions were stored at −20°C. The IS spiking solutions were prepared by dilution with water.
Calibration standards and QC
The calibration standards (10.0–1,500 ng/ml and 0.100–15.0 μg/ml in the 2D-LC-IF-MS/MS method for the human plasma and CSF assays, respectively; 50.0–50,000 ng/ml and 5.00–5,000 μg/ml in the RP-UPLC-MS/MS method for the human plasma and CSF assays, respectively) and LLOQ, low QC (LQC), medium QC (MQC), high QC (HQC), and dilution QC samples (Tables 2, 3) were prepared by serial dilution after HP-β-CD stock solution was spiked into blank biological matrix. All the calibration standards and QCs in plasma and CSF were stored in cryogenic vials at −20 or −80°C after aliquoting.
TABLE 2.
Intra- and inter-run precision and accuracy for HP-β-CD in human plasma and human CSF samples
| Method | 2D-LC-IF-MS/MS | RP-UPLC-MS/MS | |||||||||||||||
| Analytical run number | Matrix | Human plasma | Human CSF | Human plasma | Human CSF | ||||||||||||
| QC level | LLOQ | LQC | MQC | HQC | LLOQ | LQC | MQC | HQC | LLOQ | LQC | MQC | HQC | LLOQ | LQC | MQC | HQC | |
| Nominal concentration | 10.0 ng/ml | 30.0 ng/ml | 600 ng/ml | 1,200 ng/ml | 0.100 μg/ml | 0.300 μg/ml | 6.00 μg/ml | 12.0 μg/ml | 50.0 ng/ml | 125 ng/ml | 1500 ng/ml | 40,000 ng/ml | 5.00 μg/ml | 12.8 μg/ml | 160 μg/ml | 4,000 μg/ml | |
| 1 | Intra-run mean (n = 6) | 9.07 | 28.2 | 596 | 1,190 | 0.0967 | 0.292 | 5.89 | 11.7 | 47.7 | 119 | 1,440 | 39100 | 4.87 | 12.4 | 155 | 4,230 |
| Intra-run SD | 0.466 | 1.39 | 25 | 48.9 | 0.00303 | 0.00666 | 0.1 | 0.225 | 1.59 | 3.73 | 47.2 | 575 | 0.377 | 0.988 | 11 | 223 | |
| Intra-run percent CV | 4.8 | 4.9 | 4.2 | 4.1 | 3.1 | 2.3 | 1.7 | 1.9 | 3.3 | 3.1 | 3.3 | 1.5 | 7.7 | 8.0 | 7.1 | 5.3 | |
| Intra-run percent bias | −2.8 | −5.9 | −0.7 | −0.6 | −3.3 | −2.7 | −1.8 | −2.8 | −4.6 | −4.8 | −4.0 | −2.3 | −2.6 | −3.1 | −3.1 | 5.8 | |
| 2 | Intra-run mean (n = 6) | 8.79 | 29.2 | 570 | 1150 | 0.0993 | 0.31 | 5.82 | 11.8 | 55.3 | 131 | 1,550 | 39,300 | 5.04 | 12.4 | 150 | 3,870 |
| Intra-run SD | 1.09 | 2.21 | 19.9 | 37.3 | 0.00481 | 0.00747 | 0.203 | 0.335 | 1.29 | 7.19 | 34.6 | 762 | 0.326 | 0.504 | 2.4 | 119 | |
| Intra-run percent CV | 12.4 | 7.6 | 3.5 | 3.3 | 4.8 | 2.4 | 3.5 | 2.8 | 2.3 | 5.5 | 2.2 | 1.9 | 6.5 | 4.1 | 1.6 | 3.1 | |
| Intra-run percent bias | −12.1 | −2.7 | −4.9 | −4.6 | −0.7 | 3.2 | −3.0 | −1.7 | 10.6 | 4.8 | 3.3 | −1.8 | 0.8 | −3.1 | −6.3 | −3.3 | |
| 3 | Intra-run mean (n = 6) | 10.0 | 29.2 | 581 | 1,150 | 0.0985 | 0.311 | 6.05 | 11.8 | 54.3 | 125 | 1,450 | 37,000 | 4.88 | 12.5 | 157 | 4,020 |
| Intra-run SD | 0.776 | 1.76 | 13.6 | 31.9 | 0.00346 | 0.0128 | 0.22 | 0.288 | 1.95 | 4.72 | 27.9 | 1240 | 0.289 | 0.354 | 5.1 | 80.4 | |
| Intra-run percent CV | 7.7 | 6.0 | 2.3 | 2.8 | 3.5 | 4.1 | 3.6 | 2.5 | 3.6 | 3.8 | 1.9 | 3.4 | 5.9 | 2.8 | 3.2 | 2.0 | |
| Intra-run percent bias | 0.3 | −2.8 | −3.2 | −4.3 | −1.5 | 3.7 | 0.8 | −2.1 | 8.6 | 0.0 | −3.3 | −7.5 | −2.4 | −2.3 | −1.9 | 0.5 | |
| Inter-run | Inter-run mean (n = 18) | 9.51 | 28.9 | 582 | 1,160 | 0.0982 | 0.304 | 5.92 | 11.7 | 52.4 | 125 | 1,480 | 38,500 | 4.93 | 12.4 | 154 | 4,030 |
| Inter-run SD | 0.938 | 1.77 | 21.7 | 43.9 | 0.00378 | 0.0126 | 0.197 | 0.275 | 3.78 | 7.36 | 62.5 | 1,360 | 0.323 | 0.633 | 7.31 | 204 | |
| Inter-run percent CV | 9.9 | 6.1 | 3.7 | 3.8 | 3.9 | 4.1 | 3.3 | 2.3 | 7.2 | 5.9 | 4.2 | 3.5 | 6.6 | 5.1 | 4.7 | 5.1 | |
| Inter-run percent bias | −4.9 | −3.8 | −2.9 | −3.1 | −1.8 | 1.4 | −1.4 | −2.2 | 4.8 | 0.0 | −1.3 | −3.8 | −1.4 | −3.1 | −3.8 | 0.8 | |
TABLE 3.
Precision and accuracy of dilution QC samples (n = 3) for HP-β-CD in human plasma and CSF samples
| Method | 2D-LC-IF-MS/MS | RP-UPLC-MS/MS | |||
| Matrix | Human plasma | Human CSF | Human plasma | Human CSF | |
| Nominal concentration | 20,000 ng/ml | 100 μg/ml | 100 μg/ml | 400,000 ng/ml | 40,000 μg/ml |
| Dilution factor | 20 | 20 | 200 | 10 | 10 |
| Mean (n = 3) | 21,100 | 94.8 | 94 | 396,000 | 34,900 |
| SD | 569 | 0.7 | 1.23 | 8,810 | 891 |
| Percent CV | 2.7 | 0.7 | 1.3 | 2.2 | 2.6 |
| Percent bias | 5.7 | −5.2 | −6.0 | −1.0 | −12.8 |
Sample preparation
For the plasma assay, to 200 μl of plasma sample, IS spiking solution (50 μl of 5 μg/ml HP-d6-β-CD in water in the 2D-LC-IF-MS/MS method or 20 μl of 2.00 μg/ml HP-d3-β-CD in water in the RP-UPLC-MS/MS method) was added, except for the blank without IS in which water (50 μl in the 2D-LC-IF-MS/MS method and 20 μl in the RP-UPLC-MS/MS method) was used, followed by adding methanol (750 and 800 μl in the 2D-LC-IF-MS/MS method and the RP-UPLC-MS/MS method, respectively). The mixtures were vortexed and then centrifuged. The supernatants were then transferred to clean tubes and evaporated to dryness under a stream of nitrogen. The residue was reconstituted with 200 μl of water.
For the CSF assay in the 2D-LC-IF-MS/MS method, 50 l of CSF sample was mixed well with 100 μl of IS spiking solution (2.5 μg/ml HP-d6-β-CD in water), and the blank without IS was mixed with 100 l of water. In the RP-UPLC-MS/MS method, 50 l of CSF sample was diluted with 1,900 μl of water, and 100 μl of the diluted sample was mixed well with 50 μl of IS spiking solution (20.0 μg/ml HP-d3-β-CD in water), except for the blank without IS in which 50 l of water was used, and to these samples another 100 μl water was added.
Each accuracy and precision run included eight calibration standards in duplicate for the 2D-LC-IF-MS/MS method, ten calibration standards in single for the RP-UPLC-MS/MS method, and six replicates of QC samples at the concentration levels of LLOQ, LQC, MQC, and HQC. At least one blank and one blank with IS were included in each run. The plasma samples with a concentration above the highest calibrator and dilution QC sample were diluted 10- or 20-fold with blank plasma prior to extraction. The CSF samples with concentrations above the highest calibrator and dilution QC sample were diluted 10-, 20-, or 200-fold with water or blank CSF prior to extraction.
MS conditions
A 4000QTRAP and an API 4000 mass spectrometer (AB/MDS-Sciex, Concord, Ontario, Canada) with TurboIonSprayTM interface operated in positive ionization mode were used in the 2D-LC-IF-MS/MS method and the RP-UPLC-MS/MS method, respectively. In the 2D-LC-IF-MS/MS method, the multiple reaction monitoring (MRM) mass transitions m/z 203.0→97.0 and m/z 209.0→97.0 were used for HP-β-CD and HP-d6-β-CD, respectively, with a dwell time of 100 ms for each mass transition. The following precursor product ion transitions were used in the RP-UPLC-MS/MS method for MRM: HP-β-CD, m/z 1326.5→383.0; and HP-d3-β-CD, m/z 1335.6→386.0; with a dwell time of 200 ms for each mass transition of the analyte and IS. The mass spectrometer was operated at unit mass resolution for both the first and third quadrupole. The optimized instrument parameters are listed in Table 1.
TABLE 1.
Optimized mass spectrometric parameters
| Method | Mass Spectrometer | CUR | TEM | GS1 | GS2 | ihe | CAD | IS | DP | EP | CE | CXP |
| 2D-LC-IF-MS/MS | 4000QTRAP | 20 | 500°C | 35 | 20 | ON | Medium | 5,000 | 254 | 9 | 16 | 15 |
| RP-UPLC-MS/MS | API 4000 | 30 | 450°C | 40 | 50 | ON | 6 | 5,000 | 81 | 10 | 45 | 12 |
CAD, collision gas; CE, collision energy; CUR, curtain gas; CXP, collision cell exit potential; DP, declustering potential; EP, entrance potential; GS1, ion source gas 1 (nebulizer gas); GS2, ion source gas 2 (auxiliary gas); ihe, interface heater; IS IonSpray voltage; TEM, temperature.
Chromatographic conditions
2D-LC-IF-MS/MS method.
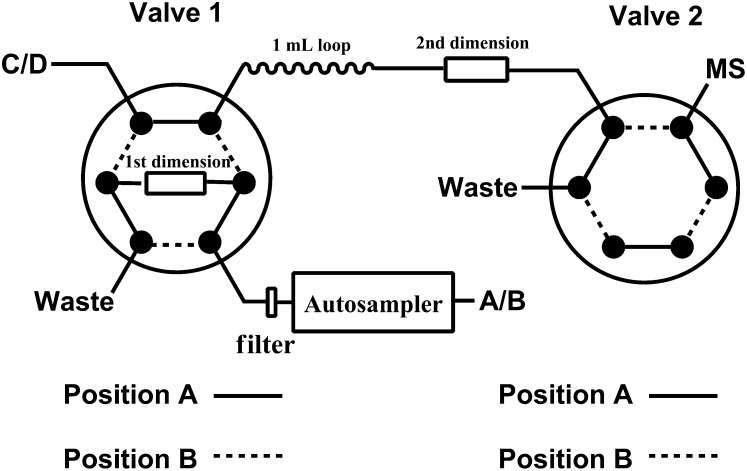
A Shimadzu Prominence HPLC system with a CBM-20A system controller, 4 LC-20AD pumps, a SIL-20ACHT autosampler, a rack changer (Shimadzu Scientific Instruments, Columbia, MD), and two six-port valves (Valco Instruments, Houston, TX) were used to separate HP-β-CD and HP-d6-β-CD from the biological matrix. The chromatography was performed at ambient temperature using a C18 guard column (4 × 3.0 mm, Phenomenex, Torrance, CA) and Atlantis hydrophilic interaction liquid chromatography (HILIC) silica column (3 × 50 mm, 3 μm; Waters) as first and second dimensions, respectively. The compartment of the autosampler was set at 4°C. Figure 2 is a schematic of the column and switching valve arrangement for 2D-LC. For the first dimension, mobile phase A (10 mM ammonium formate and 0.1% formic acid in water) and mobile phase B [0.1% formic acid in methanol-acetonitrile (4:1)] were operated with a gradient elution as follows: 0–0.3 min 0% B, 0.3–2.0 min 0–20% B, 2.0–2.1 min 20–100% B, 2.1–6.0 min 100% B, 6.0–6.1 min 100–0% B, and 6.1–7.5 min 0% B at a flow rate of 0.6 ml/min. The solvent gradient for second dimension LC using 10 mM ammonium formate and 0.1% formic acid in methanol-water (4:1) (phase C) and acetonitrile (phase D) at a flow rate of 0.60 ml/min was as follows: 0–2.1 min 88% D, 2.1–5.0 min 88–40% D, 5.0–5.5 min 40% D, 5.5–5.6 min 40–88% D, and 5.6–7.5 min 88% D. The valve 1 switched from position A to B at 1.6 min and back to position A at 1.8 min. The valve 2 switched from position A to B at 5.0 min and back to position A at 6.0 min.
Fig. 2.
Diagram of the 2D reversed phase and HILIC LC setup using binary pump A/B for reversed phase and binary pump C/D for HILIC.
RP-UPLC-MS/MS method.
The LC system was an Acquity UPLC® (Waters) consisting of binary solvent manager and sample manager. The separations were carried out using an Acquity UPLC BEH Shield RP18 column (2.1 × 100 mm, 1.7 μm; Waters) at 40°C. The components were eluted with a 4 min gradient program at a flow rate of 0.50 ml/min with an injection volume of 10 μl for plasma and 5 μl for CSF. The mobile phase was composed of mobile phase A (10.0 mM ammonium carbonate in water) and mobile phase B (acetonitrile). The linear gradient was as follows: 0–0.5 min 1% B; 0.5–2.0 min 1–55% B; 2.0–2.1 min 55–98% B; 2.1–2.8 min 98% B; 2.8–2.9 min 98–1% B, and 2.9–4.0 min 1% B.
Drug administration and sample collection in PK study
This clinical study was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Permission from guardians and assent, when possible, were obtained from all participants. The study was posted on ClinicalTrials.gov (NCT01747135) and use of HP-β-CD was covered under investigational new drug (IND) 113273. Three NPC1 subjects were admitted to the NIH Clinical Center Intensive Care Unit and an Ommaya reservoir was surgically placed on the nondominant side. The 50 mg HP-β-CD dose was prepared in 5 ml of an isotonic salt solution. Vehicle (saline) and HP-β-CD doses were administered ICV via the Ommaya reservoir. CSF and blood, 1.5 and 2 ml respectively, were collected at 0.25, 0.5, 1, 3, 8, 24, 36, and 48 h predose, and 72 h postdose. PK parameters of HP-β-CD for CSF and plasma were calculated with the noncompartmental approach using Phoenix WinNonlin software (version 6.2) (Certara, St. Louis, MO). Briefly, the area under the concentration-time curve (AUC) was calculated using the linear trapezoidal method. Where warranted, the slope of the apparent terminal phase was estimated by log-linear regression using at least three data points and the terminal rate constant (λ) was derived from the slope. AUC0-∞ was estimated as the sum of the AUC0-t (where t is the time of the last measurable concentration) and concentration after time t (Ct)/λ. The apparent terminal half-life (t1/2) was calculated as 0.693/λ. For the CSF data, the clearance (CL) of HP-β-CD was calculated as dose/AUC0-∞, and the volume of distribution at steady-state (Vdss) was calculated as (AUMC0-∞ /AUC0-∞) × CL, where AUMC0-∞ is the area under the first moment curve from zero to infinity. For the plasma data, the systemic exposure of HP-β-CD was assessed by the maximum drug concentration (Cmax), time to reach Cmax (Tmax), AUC, and t1/2.
RESULTS
MS
HP-β-CD is a heterogeneous complex mixture of homologs and isomers, in which there are a variable number of 2-hydroxypropyl groups substituted at different positions of the sugar moieties. Previously, unambiguous, sensitive, and quantitative detection of the polysaccharide mixture as a whole was not feasible due to the dispersity of the signals that are distributed over a large number of homologs and isomers. In theory, there are 2,097,151 possible homologs and isomers in the HP-β-CD mixture based on combination of variance in degree of substitution and the possible sites of the substitution (13).
In order to quantify total HP-β-CD concentrations with LC-MS/MS, innovative approaches needed to be developed and investigated. One approach was in-source fragmentation of all the HP-β-CD homologs and isomers present into common building blocks. Alternatively, it was investigated to determine whether one single homolog could be used as a probe compound for the quantification of the total of all the HP-β-CD homologs.
Mobile phase additives have a strong influence on HP-β-CD distribution profiles in their ESI mass spectra. Due to relatively weak proton affinity, even in the presence of 0.1% formic acid, HP-β-CD formed abundant sodium adducts and a low abundance of protonated molecular ions that were most often used for mass spectrometric detection in positive ion mode. In the mobile phase of second dimensional LC of 2D-LC-IF-MS/MS (10 mM of ammonium formate and 0.1% of formic acid as additives in mobile C), ammonium-, proton-, and sodium-adduct ions were observed; the protonated ions were probably generated from neutral loss of ammonia from ammonium adduct ions. In the mobile phase of the RP-UPLC-MS/MS method (10.0 mM ammonium carbonate as additive in mobile phase A), abundant ammonium adducts were formed. The number of 2-hydroxypropyl groups per β-cyclodextrin unit ranges from three to nine in the mobile phases of the second dimension LC of 2D-LC-IF-MS/MS (Fig. 1B), and one to seven in the mobile phase of the RP-UPLC-MS/MS method (Fig. 1C). Although the HP-β-CD with the same substitution degree appears as single peak in mass spectrum, it is a complex mixture composed of a large number of isobaric isomers. The efficiency of adduct formation for these isomers is different, leading to a different substitution pattern observed in the mass spectra of HP-β-CD in different solutions. Sodium adducts were easily formed in the ion source; however, they were not suitable for MS/MS detection due to their poor fragmentation. For our methods, ammonium- and proton-adduct ions were employed as precursor ions for MS/MS detection.
In-source fragmentation for the 2D-LC-IF-MS/MS approach.
When the voltage is significantly increased in the high-pressure region between the sample cone and skimmer in an atmospheric pressure ionization source, the ions passing through this region may dissociate when they collide with solvent or air molecules. This technique is called in-source fragmentation- or in-source collision-induced dissociation. In-source fragmentation has been used in structural elucidation (14), compound identification (15–17), compound profiling (18), and bioanalysis (19). In-source fragmentation of HP-β-CD, which was promoted by increasing declustering potentials on a 4000QTRAP mass spectrometer, led to glycosidic-bond cleavage and simultaneous fragmentation of all the HP-β-CD ammonium- and proton-adduct ions. The in-source fragmentation effectively generated abundant glucopyranose unit ions (Fig. 1D), and the complex mixture of homologs and isomers was simplified to several building blocks of HP-β-CD. The m/z 203 ion was a 2-hydroxypropyl substituted dihydropyrylium ion representing the simplest building block for HP-β-CD, and it was chosen as a surrogate for HP-β-CD and as the precursor for further collision-activated dissociation. The structure of the major component of the m/z 203 ion was proposed based on its product ion spectrum (Fig. 1E). The IS HP-d6-β-CD demonstrated similar in-source fragmentation and product ion spectra of the m/z 209 ion. The major product ion at m/z 97, a 4-hydroxypyrylium, was observed for the m/z 203 and m/z 209 ions. Hence, MRM transitions m/z 203→97 and m/z 209→97 were selected for detection of HP-β-CD and the IS, respectively.
Representative probe compound selection for the RP-UPLC-MS/MS approach.
As an alternative approach in the RP-UPLC-MS/MS method, individual HP-β-CD substituted forms, instead of the sum of all substituted forms, were monitored in MRM mode. Method development data revealed that monitoring any of the individual substituted forms was representative for the total of all HP-β-CD substituted forms and provided similar results as when using the in-source fragmentation approach or fluorescence detection on condition that the same lot of compound is used for the quantitation as for dosing in the study. Eventually, HP-β-CD containing three 2-hydroxypropyl groups was selected as a probe compound in MRM detection, as this isoform, although it is not the most abundant ion in the ESI mass spectrum, provided the best sensitivity in MRM mode. The MRM transitions m/z 1,326.5→383 and m/z 1,335.6→386 were chosen for detection of HP-β-CD and the IS, respectively.
Sample preparation
HP-β-CD is highly water soluble and dissolves poorly in most hydrophobic organic solvents. Liquid-liquid extraction was excluded for sample cleanup. A protein precipitation method was developed to replace the previous solid phase extraction method used for plasma (9) due to its simplicity, high extraction efficiency, high capacity, and low cost. For plasma samples in the 2D-LC-IF-MS/MS and RP-UPLC-MS/MS methods, protein precipitation with methanol was followed by evaporation of the supernatant and reconstitution of the extract in water. The reconstitution step reduces the elution strength of the injection solvent and provides additional clean-up, as part of the matrix constituents do not redissolve due the high polarity of the reconstitution solvent. The CSF samples were mixed with IS in water and submitted to 2D-LC-IF-MS/MS assay directly or to RP-UPLC-MS/MS assay after dilution with water and addition of IS.
Chromatography
2D-LC-IF-MS/MS method.
We found that HP-β-CD was eluted as a single sharp peak in HILIC on an underivatized bare silica column. An additional advantage of HILIC is increased sensitivity with ESI-MS due to the highly volatile organic mobile phase used. However, detection of HP-β-CD in plasma samples prepared with protein precipitation was hampered by ion suppression when HILIC was used alone. Glycerophosphocholine species, the major components in plasma that give rise to ion suppression (18), are well-separated from HP-β-CD on the HILIC column, suggesting that other polar endogenous matrix components such as inorganic salts, peptides, and amino acids may be responsible for the matrix effects.
Coupling of reversed phase and HILIC has been successfully used to reduce matrix effects and increase sensitivity for highly polar compounds (20, 21). A 2D-LC method was developed to separate HP-β-CD from the endogenous matrix using two orthogonal chromatographic separation mechanisms. Reconstitution of extracted samples in water allowed effective trapping of HP-β-CD on a reversed phase column when maximal injection volume was tried. A 2 min gradient from 0 to 20% mobile phase B was used at a flow rate of 0.6 ml/min to selectively remove the majority of polar matrix components. The first two-position switching valve was switched around the retention time of the HP-β-CD on the reversed phase column, and the HP-β-CD was back-flushed in a narrow peak window (about 0.2 min) from the reversed phase column to the second dimension, Alantis HILIC silica (3 × 50 mm, 3 μm), which further separated the HP-β-CD from other matrix components by gradient elution. Because HILIC has been found to be relatively sensitive to aqueous content, online dilution of the sample plug during transfer from the reversed phase column to the HILIC column was necessary to decrease the elution strength and thus provide sufficient retention and good peak shape of HP-β-CD on HILIC column. The online dilution was achieved by placing a 1 ml loop between the first and second dimensions prefilled with initial mobile phase for the second dimension. The acquisition window on the mass spectrometer was set for 1 min via a second two-position switching valve so that the mass spectrometer ion source was kept clean and well maintained. The overall run time was 7.5 min per sample.
RP-UPLC-MS/MS method.
HP-β-CD was eluted as a single peak on the size-exclusion chromatographic column (9); however, the highly aqueous mobile phase used in the size-exclusion chromatography was not conducive for achieving high sensitivity on LC-MS/MS systems. Although HP-β-CD, as a whole, yielded broad and unresolved peaks on reversed phase HPLC columns owing to unresolved homologs and isomers, HP-β-CD isomers with the same number of 2-hydroxypropyl groups were eluted as a single peak under a sharp gradient elution using UPLC. The UPLC technology uses sub 2 μm particles as column packing material and these particles allow for a higher optimum flow rate with higher theoretical plate number. Thus, improved resolution and narrower peaks are expected, which should translate to better sensitivity. As a consequence, a shorter run time (4 min per sample) was achieved even with this one-dimensional LC approach, and no significant matrix effects were observed.
Calibration curve and linearity
The assay linear calibration range of the 2D-LC-IF-MS/MS method was 10–1,500 ng/ml for human plasma and 0.1–15.0 μg/ml for human CSF. For three consecutive batches, the calibration curves showed an overall accuracy of 2.7–2.2% bias with precision of less than 9.3% coefficient of variance (CV) for human plasma, overall accuracy of −1.5 to 1.4% bias with precision of less than 4.8% CV for human CSF. The observed linear regression coefficient (R) was ≥0.9972 for human plasma and ≥0.9988 for human CSF.
For the RP-UPLC-MS/MS method, the assay linear calibration range was 50.0–50,000 ng/ml for human plasma and 5.00–5,000 μg/ml for human CSF. For three consecutive batches, the calibration curves showed an overall accuracy of −4.8 to 7.0% bias with precision of less than 5.7% CV for human plasma, and an overall accuracy of −2.8 to 2.2% bias with precision of less than 4.4% CV for human CSF. The observed linear regression coefficient (R) was ≥0.9990 for human plasma and ≥0.9998 for human CSF.
Assay selectivity and specificity
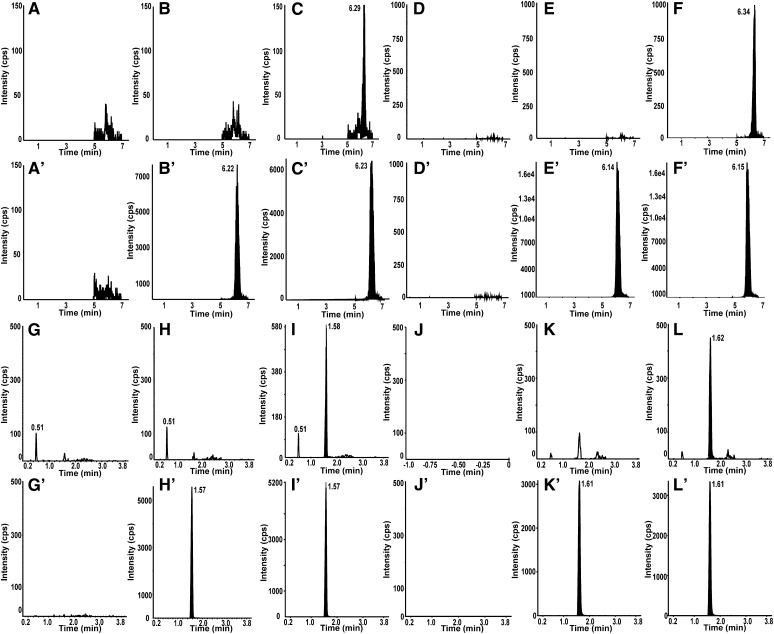
Six different lots of blank human plasma or human CSF were analyzed as blanks (no IS). In both the 2D-LC-IF-MS/MS and RP-UPLC-MS/MS assays, no peaks were detected in the blanks at the retention time of analyte and IS, indicating no interference from endogenous substances. The blank with IS showed no interference to the analyte detection (Fig. 3).
Fig. 3.
Representative 2D-LC-IF-MS/MS (A–F) and RP-UPLC-MS/MS (G–L) chromatograms for HP-β-CD (A–L) and IS (A′–L′). Blank (A, G), blank with IS (B, H), and LLOQ (C, I) are shown in human plasma. Blank (D, J), blank with IS (E, K), and LLOQ (F, L) are shown in human CSF.
Sensitivity
The LLOQ samples of the assays in human plasma (50.0 ng/ml for the RP-UPLC-MS/MS method and 10 ng/ml for the 2D-LC-IF-MS/MS method) and CSF (5 μg/ml for the RP-UPLC-MS/MS method and 0.1 μg/ml for the 2D-LC-IF-MS/MS method) were determined in 200 μl of human plasma and 50 μl of human CSF, respectively. Assay LLOQ was established as the lowest concentration that can be quantified with precision ≤20% CV and bias less than or equal to ±20% of nominal. Both intra- and inter-run variability at LLOQ were within the acceptable range for all the biological matrixes (Table 2). Representative chromatograms at the LLOQ concentration are shown in Fig. 3.
Accuracy and precision
The accuracy and precision of the method were evaluated by analyzing six replicates of QC samples at LLOQ, LQC, MQC, and HQC in three separate runs against calibration curves. A summary of the intra- and inter-run assay precision and accuracy of individual QC concentrations for human plasma and CSF is shown in Table 2. For all samples, the precision was <15% CV and the bias was in the range of ±15% in all three batches.
Table 3 shows the precision and accuracy of dilution QCs for human plasma and CSF. Accuracy of −5.7% bias with a CV of 2.7% for human plasma and accuracy of −6.0 to 5.2% bias with a CV of 0.7–1.3% for human CSF were obtained for the 2D-LC-IF-MS/MS method. For the RP-UPLC-MS/MS method, an accuracy of −1.0% bias with a CV of 2.2% for human plasma and an accuracy of −12.8% bias with a CV of 2.6% for human CSF were obtained.
Recovery and matrix effects
The recovery (referring to extraction efficiency) of HP-β-CD and its IS (HP-d6-β-CD or HP-d3-β-CD) from plasma was determined by comparing the mean peak area ratios of the pre-extract samples to the post-extract samples at LQC and HQC concentrations. The recovery of HP-β-CD and IS was >80% for plasma and was 100% for CSF. Matrix factors were in the range of 0.84–1.12 for the 2D-LC-IF-MS/MS method and 0.766–0.920 for the RP-UPLC-MS/MS method in the human plasma and human CSF assays. The IS-normalized matrix factors were close to 1 for both methods, indicating that the matrix effects were well-compensated by the IS. There was also no significant matrix effect in hemolyzed plasma.
Stability
The stability of HP-β-CD to freeze-thaw (n = 6 cycles) in human plasma and CSF, 72 h at room temperature, at −20°C (234 days for human plasma and 232 days for CSF), and at −80°C (254 days for human plasma and 259 days for CSF) were all established. Processed sample stability at 4°C and at room temperature was demonstrated for 145 h for plasma extracts and for 142 h for CSF extracts. Stock solution stability of HP-β-CD and IS in water was established for 3 days at room temperature, for 1 month at 4°C, and for 272 days at −20°C.
Carryover and run size evaluation
Carryover for both methods was found to be acceptable (<10% of the peak areas of the LLOQs) in the linear range of the assays. The assays were validated to successfully analyze batch sizes of approximately 140 samples.
Application of assays for PK studies in a phase 1 trial
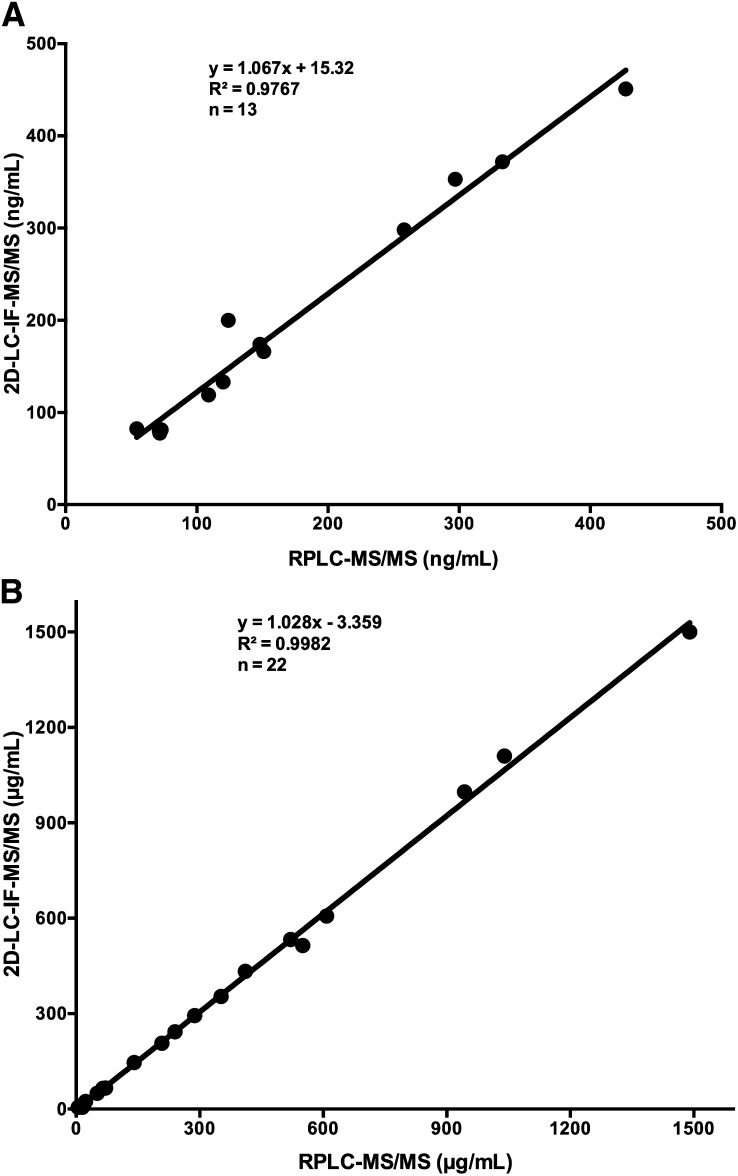
Both the 2D-LC-IF-MS/MS and RP-LC-MS/MS methods were used to analyze HP-β-CD in human plasma and CSF samples obtained from three subjects who were administered 50 mg of HP-β-CD ICV in a phase 1 study of HP-β-CD in NPC1 disease. For the 2D-LC-IF-MS/MS method, all of 27 CSF samples collected postdose were above the LLOQ of 0.1 μg/ml, and 24 of 27 plasma samples collected postdose were above the LLOQ of 10 ng/ml. This represents 100% and 89% coverage of the CSF and plasma samples, respectively. By contrast, using the RP-LC-MS/MS method, only 74% of CSF and 48% of plasma postdose samples were above the assay LLOQ. Thirteen human plasma and 22 CSF samples were quantifiable by both methods, and the quantifiable results obtained by both methods were in close agreement with slopes of 1.067 and 1.028 for plasma and CSF, respectively (Fig. 4).
Fig. 4.
Correlation between the 2D-LC-IF-MS/MS and RP-UPLC-MS/MS methods for the HP-β-CD measurement in human plasma (A) and CSF (B) samples.
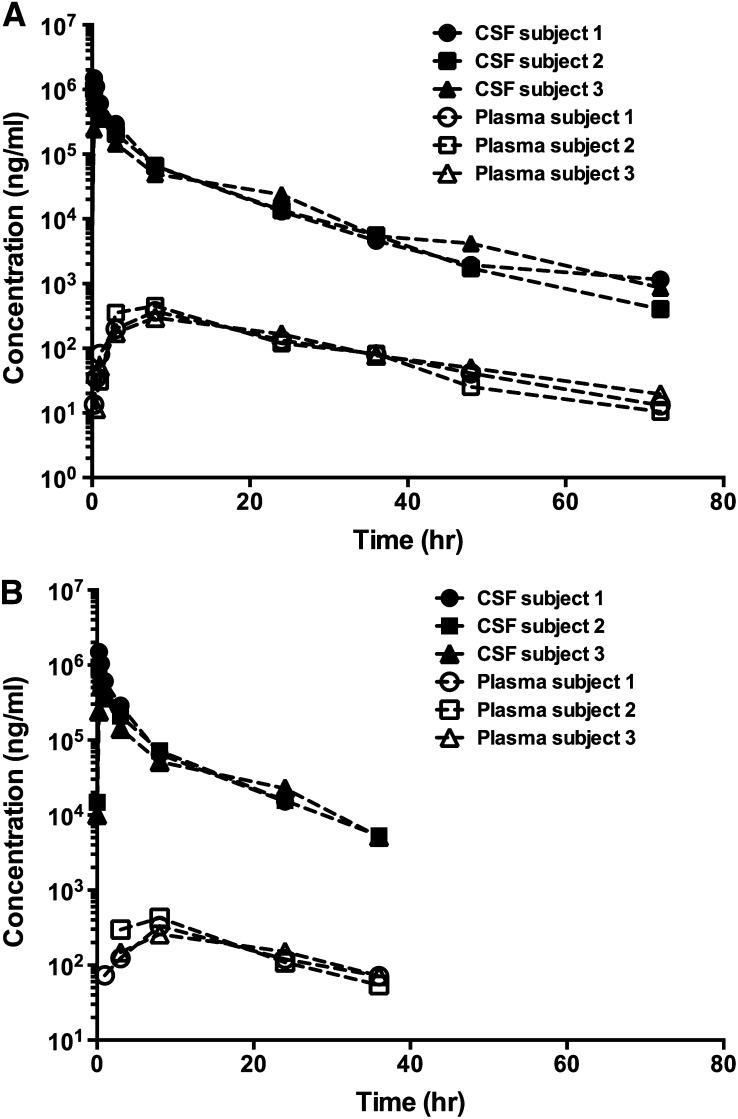
PK profiles were determined for HP-β-CD in CSF and plasma in the three patients treated with 50 mg HP-β-CD via ICV injection (Fig. 5). The CSF and plasma concentrations measured by both methods were used in the PK calculation. As shown in Table 4, the CSF PK parameters were comparable (within 10%) for all parameters except for the t1/2, in which a difference of 53% was observed between the two assay methods. A longer t1/2 was obtained based on the CSF concentrations measured by 2D-LC-IF-MS/MS, due to the fact that this method had a better sensitivity. Thus, the drug concentrations in CSF were measurable up to the last sampling time point of 72 h (Fig. 5A). Following the ICV injection of 50 mg HP-β-CD, high CSF concentrations of HP-β-CD were observed with the Cmax ranging from approximately 520 to 1,500 μg/ml in three patients (Table 4). The mean CL of 18–19 ml/h was slightly lower than the CSF turnover rate of approximately 21–25 ml/h in normal human subjects (22). The mean Vdss of ∼130 ml was approximately to the reported CSF volume in humans. The estimated mean t1/2 for HP-β-CD in CSF was 6.58 h based on the RP-UPLC-MS/MS method and 10.1 h based on the 2D-LC-IF-MS/MS method.
Fig. 5.
HP-β-CD PK profiles. HP-β-CD PK profiles are shown in human plasma and CSF from a phase 1 study of HP-β-CD in NPC1 subjects dosed with 50 mg HP-β-CD ICV. Data were obtained from 2D-LC-IF-MS/MS (A) and RP-UPLC-MS/MS (B) analyses.
TABLE 4.
CSF PK parameters of HP-β-CD in three patients treated ICV with 50 mg of HP-β-CD
| Patient ID | Cmax (μg/ml) | Tmax (h) | AUC0-∞ (μg.h/ml) | CL (ml/h) | Vdss (ml) | t1/2 (h) | ||||||
| 2D-LC-IF-MS/MS | RP-UPLC-MS/MS | 2D-LC-IF-MS/MS | RP-UPLC-MS/MS | 2D-LC-IF-MS/MS | RP-UPLC-MS/MS | 2D-LC-IF-MS/MS | RP-UPLC-MS/MS | 2D-LC-IF-MS/MS | RP-UPLC-MS/MS | 2D-LC-IF-MS/MS | RP-UPLC-MS/MS | |
| 1 | 1,500 | 1,490 | 0.25a | 0.25a | 3,568 | 3,434 | 14.0 | 14.6 | 78.9 | 65.2 | 10.9 | 4.64 |
| 2 | 997 | 943 | 0.25a | 0.25a | 2,601 | 2,671 | 19.2 | 18.7 | 129 | 124 | 8.69 | 7.45 |
| 3 | 533 | 521 | 0.50 | 0.50 | 2,330 | 2,205 | 21.5 | 22.7 | 204 | 182 | 10.8 | 7.65 |
| Mean | 1010 | 985 | 0.333 | 0.333 | 2,833 | 2,770 | 18.2 | 18.7 | 137 | 124 | 10.1 | 6.58 |
| SD | 484 | 486 | 0.144 | 0.144 | 651 | 620 | 3.82 | 4.06 | 62.7 | 58.2 | 1.25 | 1.68 |
| Percent CV | 47.9 | 49.3 | 43.2 | 43.2 | 23.0 | 22.4 | 21.0 | 21.7 | 45.8 | 46.9 | 12.4 | 25.5 |
PK calculation was performed on two sets of concentration data generated by 2D-LC-IF-MS/MS and RP-UPLC-MS/MS methods, respectively.
First sampling time point for CSF.
Similar to the CSF data, the plasma PK parameters were comparable using the concentrations generated by these two assay methods (Table 5). Following ICV injection, HP-β-CD transited into the blood circulation. However, the plasma exposure of HP-β-CD was much lower than in CSF. The mean Cmax and AUC of HP-β-CD in plasma were less than 0.3% of the corresponding CSF PK parameters. Again, a longer t1/2 was obtained from the 2D-LC-IF-MS/MS method, due to the fact that this method had a higher sensitivity and plasma drug concentrations were quantifiable up to the last sampling time point of 72 h. The estimated mean t1/2 values for HP-β-CD in plasma were 12.4 h based on the RP-UPLC-MS/MS method and 15.1 h based on the 2D-LC-IF-MS/MS method.
TABLE 5.
Plasma PK parameters of HP-β-CD in three patients treated with 50 mg of HP-β-CD via Ommaya reservoir. PK calculation was performed on two sets of concentration data generated by 2D-LC-IF-MS/MS and RP-UPLC-MS/MS methods respectively
| Patient ID | Cmax (μg/ml) | Tmax (h) | AUC0-∞ (μg.h/ml) | t1/2 (h) | ||||
| 2D-LC-IF-MS/MS | RP-UPLC-MS/MS | 2D-LC-IF-MS/MS | RP-UPLC-MS/MS | 2D-LC-IF-MS/MS | RP-UPLC-MS/MS | 2D-LC-IF-MS/MS | RP-UPLC-MS/MS | |
| 1 | 0.372 | 0.333 | 8.0 | 8.0 | 8.71 | 7.43 | 14.2 | 12.5 |
| 2 | 0.451 | 0.427 | 8.0 | 8.0 | 9.45 | 8.25 | 13.1 | 9.3 |
| 3 | 0.298 | 0.258 | 8.0 | 8.0 | 8.70 | 7.44 | 18.1 | 15.4 |
| Mean | 0.374 | 0.339 | 8.0 | 8.0 | 8.96 | 7.71 | 15.1 | 12.4 |
| SD | 0.077 | 0.085 | 0.0 | 0.0 | 0.431 | 0.471 | 2.64 | 3.04 |
| Percent CV | 20.6 | 25.1 | 0.0 | 0.0 | 4.8 | 6.1 | 17.5 | 24.5 |
PK calculation was performed on two sets of concentration data generated by 2D-LC-IF-MS/MS and RP-UPLC-MS/MS methods, respectively.
DISCUSSION
HP-β-CD has emerged as a promising potential therapy for NPC1 disease. A phase 1 trial is currently underway at the NIH Clinical Center to evaluate the safety of HP-β-CD to NPC1 patients. To support this clinical trial, two different novel assays for the quantification of HP-β-CD were developed and validated. Both methods circumvent the challenges posed by the heterogeneity of the HP-β-CD mixture and offer analytical approaches that are up to 100-fold more sensitive than the existing method (9). The availability of assays to accurately and sensitively measure HP-β-CD in CSF and plasma not only will assist in drug evaluation for treatment of NPC1 disease, but also will provide critical tools for exploring the utility of HP-β-CD in other cholesterol-related diseases, such as atherosclerosis and Alzheimer’s disease, in which HP-β-CD is being explored as a therapeutic (23).
The current standard for HP-β-CD quantification is based on an HPLC method with indirect fluorescence detection of inclusion complexes (9). Major limitations of this method are the requirement for large sample volumes and lack of sensitivity, which limited its utility in the current PK study where concentrations of HP-β-CD in all the plasma samples are below the LLOQ of the HPLC method. To circumvent these limitations, we have established rugged and sensitive 2D-LC-IF-MS/MS and RP-UPLC-MS/MS methods requiring only a small sample volume and involving rapid sample processing.
Compared with the 2D-LC-IF-MS/MS method, the RP-UPLC-MS/MS method is faster and easier to set up. Such features would facilitate implementation of HP-β-CD analytics outside the research laboratory, such as in clinical laboratories or good laboratory practice-compliant facilities. The 2D-LC-IF-MS/MS, on the other hand, proved more sensitive for quantification of HP-β-CD in the plasma and CSF samples. The critical design feature of the latter HP-β-CD assay is the combination of 2D-LC and in-source fragmentation MS/MS. Although the instrumentation and the method are more complex, the orthogonal 2D-LC system based on reversed phase liquid chromatography (RPLC) (C18) and HILIC (silica) phases, improves the separation of HP-β-CD from endogenous components, eliminates ion suppression, and increases analytical column life. The sensitivity and detection limit can be further improved by large volume injection of extracted samples reconstituted in water without compromising chromatographic peak shape and resolution. Compared with RPLC, utilization of HILIC as an analytical column offers enhanced ion spray in ESI-MS, and hence, increased sensitivity due to a large percentage of organic modifiers in the HILIC mobile phase. The HP-β-CD signals are distributed over a large number of homologs and isomers, reducing the achievable limits of quantification if only one homolog is chosen as probe for quantification. All the HP-β-CD homologs and isomers can be converted into several common building blocks during in-source fragmentation, and the signals split by parents with the same common building block are reconverged. The simplest building block for HP-β-CD at m/z 203 that generates a dominant and selective product ion at m/z 97 with high intensity was chosen to establish MRM transition. This MRM transition is not only sensitive but also selective in the presence of biological matrix without high MRM background noise or interference peaks. Furthermore, the methods established in the present work, which involve a simple protein precipitation sample cleanup, have a higher recovery than the previously reported method (9).
Validated assays were developed for human plasma and CSF to facilitate the PK study of HP-β-CD in a human phase 1 trial in the NPC1 patients. Data obtained from this study were used to generate information on the distribution and elimination of HP-β-CD after administration through an Ommaya reservoir to patients with NPC1. The enhanced sensitivity of the 2D-LC-IF-MS/MS and RP-UPLC-MS/MS assays provides optimal coverage for a broad range of HP-β-CD concentrations in human plasma and CSF samples from the phase 1 trial. An LLOQ of 10 ng/ml in human plasma achieved with 2D-LC-IF-MS/MS method is well-suited for measurement of drug concentration, given that the drug concentration is low due to the large volume of distribution following ICV administration of HP-β-CD to human subjects. Nevertheless, PK parameters calculated from the concentration data generated from both assays were comparable, except that a longer terminal t1/2 was obtained from the 2D-LC-IF-MS/MS method due to its higher sensitivity.
CONCLUSION
In this report, we present robust, sensitive, accurate, precise, and reproducible 2D-LC-IF-MS/MS and RP-UPLC-MS/MS methods that have been developed and validated for quantitative determination of HP-β-CD in human plasma and CSF. The extraction procedure is straight-forward and involves a one step protein precipitation extraction for plasma and dilution with water and/or IS solution for CSF. Both methods were used to quantify HP-β-CD concentrations in human plasma and CSF following ICV administration. While the 2D-LC-IF-MS/MS method was more sensitive, the RP-UPLC-MS/MS method was easier to implement and more rapid. Both methods provide a significant advance over existing methods for quantification of HP-β-CD, and are well-suited for clinical studies with ICV administration.
Acknowledgments
The authors thank Sarah Gale for assistance in preparation of figures.
Footnotes
Abbreviations:
- AUC
- area under the concentration-time curve
- AUMC
- area under the first moment curve
- Cmax
- maximum drug concentration
- CL
- clearance
- CSF
- cerebrospinal fluid
- CV
- coefficient of variance
- 2D-LC-IF-MS/MS
- 2D-LC-in-source fragmentation-MS/MS
- HILIC
- hydrophilic interaction liquid chromatography
- HP-β-CD
- 2-hydroxypropyl-β-cyclodextrin
- HQC
- high quality control
- ICV
- intracerebroventricular
- IS
- internal standard
- LLOQ
- lower limit of quantification
- LQC
- low quality control
- λ
- the terminal rate constant
- MQC
- medium quality control
- MRM
- multiple reaction monitoring
- NIH
- National Institutes of Health, NPC1, Niemann-Pick type C1
- PK
- pharmacokinetics
- QC
- quality control
- RPLC
- reversed phase liquid chromatography
- RP-UPLC-MS/MS
- reversed phase-ultra performance LC-MS/MS
- t1/2
- terminal half-life
- Tmax
- time to reach the maximum drug concentration
- Vdss
- volume of distribution at steady-state
The 2D-LC-IF-MS/MS assay was performed in the Metabolomics Facility at Washington University (P30 DK020579). This work was supported by the Bench to Bedside Program, the Office of Rare Diseases Research, Therapies for Rare and Neglected Diseases (TRND) Program in the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH), and NIH Grant 1ZIAHD008824-07 (F.D.P.). Additional support was provided by a grant from Dana’s Angels Research Trust (D.S.O).
REFERENCES
- 1.Vanier M. T. 2010. Niemann-Pick disease type C. Orphanet J. Rare Dis. 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanier M. T., Millat G. 2003. Niemann-Pick disease type C. Clin. Genet. 64: 269–281 [DOI] [PubMed] [Google Scholar]
- 3.Liu B., Turley S. D., Burns D. K., Miller A. M., Repa J. J., Dietschy J. M. 2009. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1-/- mouse. Proc. Natl. Acad. Sci. USA. 106: 2377–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson C. D., Ali N. F., Micsenyi M. C., Stephney G., Renault S., Dobrenis K., Ory D. S., Vanier M. T., Walkley S. U. 2009. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE. 4: e6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu B., Ramirez C. M., Miller A. M., Repa J. J., Turley S. D., Dietschy J. M. 2010. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J. Lipid Res. 51: 933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramirez C. M., Liu B., Taylor A. M., Repa J. J., Burns D. K., Weinberg A. G., Turley S. D., Dietschy J. M. 2010. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr. Res. 68: 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward S., O’Donnell P., Fernandez S., Vite C. H. 2010. 2-hydroxypropyl-beta-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann-Pick type C disease. Pediatr. Res. 68: 52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ottinger E. A., Kao M. L., Carrillo-Carrasco N., Yanjanin N., Shankar R. K., Janssen M., Brewster M., Scott I., Xu X., Cradock J., et al. 2014. Collaborative development of 2-hydroxypropyl-beta-cyclodextrin for the treatment of Niemann-Pick type C1 disease. Curr. Top. Med. Chem. 14: 330–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szathmary S. C. 1989. Determination of hydroxypropyl-beta-cyclodextrin in plasma and urine by size-exclusion chromatography with post-column complexation. J. Chromatogr. 487: 99–105 [DOI] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services Food and Drug Administration. 2001. Guidance for Industry: Bioanalytical Method Validations. Center for Drug Evaluation and Research, Center for Veterinary Medicine. Accessed June 5, 2014, at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM368107.pdf [Google Scholar]
- 11.Viswanathan C. T., Bansal S., Booth B., DeStefano A. J., Rose M. J., Sailstad J., Shah V. P., Skelly J. P., Swann P. G., Weiner R. 2007. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm. Res. 24: 1962–1973 [DOI] [PubMed] [Google Scholar]
- 12.European Medicines Agency (EMA). 2011. Guideline on Bioanalytical Method Validation. Accessed June 5, 2014, at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf [Google Scholar]
- 13.Szente L., Szejtli J. 1999. Highly soluble cyclodextrin derivatives: chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 36: 17–28 [DOI] [PubMed] [Google Scholar]
- 14.Hsu F. F., Turk J., Stewart M. E., Downing D. T. 2002. Structural studies on ceramides as lithiated adducts by low energy collisional-activated dissociation tandem mass spectrometry with electrospray ionization. J. Am. Soc. Mass Spectrom. 13: 680–695 [DOI] [PubMed] [Google Scholar]
- 15.Farwanah H., Wirtz J., Kolter T., Raith K., Neubert R. H., Sandhoff K. 2009. Normal phase liquid chromatography coupled to quadrupole time of flight atmospheric pressure chemical ionization mass spectrometry for separation, detection and mass spectrometric profiling of neutral sphingolipids and cholesterol. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2976–2982 [DOI] [PubMed] [Google Scholar]
- 16.Altman E., Li J. 2010. Characterization of polysaccharides using mass spectrometry for bacterial serotyping. Methods Mol. Biol. 600: 245–257 [DOI] [PubMed] [Google Scholar]
- 17.Prandi B., Farioli L., Tedeschi T., Pastorello E. A., Sforza S. 2012. Simulated gastrointestinal digestion of Pru ar 3 apricot allergen: assessment of allergen resistance and characterization of the peptides by ultra-performance liquid chromatography/electrospray ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 26: 2905–2912 [DOI] [PubMed] [Google Scholar]
- 18.Little J. L., Wempe M. F., Buchanan C. M. 2006. Liquid chromatography-mass spectrometry/mass spectrometry method development for drug metabolism studies: Examining lipid matrix ionization effects in plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 833: 219–230 [DOI] [PubMed] [Google Scholar]
- 19.Li H., Rose M. J., Holder J. R., Wright M., Miranda L. P., James C. A. 2011. Direct quantitative analysis of a 20 kDa PEGylated human calcitonin gene peptide antagonist in cynomolgus monkey serum using in-source CID and UPLC-MS/MS. J. Am. Soc. Mass Spectrom. 22: 1660–1667 [DOI] [PubMed] [Google Scholar]
- 20.Deng Y., Zhang H., Wu J. T., Olah T. V. 2005. Tandem mass spectrometry with online high-flow reversed-phase extraction and normal-phase chromatography on silica columns with aqueous-organic mobile phase for quantitation of polar compounds in biological fluids. Rapid Commun. Mass Spectrom. 19: 2929–2934 [DOI] [PubMed] [Google Scholar]
- 21.Liu A., Tweed J., Wujcik C. E. 2009. Investigation of an on-line two-dimensional chromatographic approach for peptide analysis in plasma by LC-MS-MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 1873–1881 [DOI] [PubMed] [Google Scholar]
- 22.Johanson C. E., Duncan J. A., 3rd, Klinge P. M., Brinker T., Stopa E. G., Silverberg G. D. 2008. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao J., Ho D., Calingasan N. Y., Pipalia N. H., Lin M. T., Beal M. F. 2012. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J. Exp. Med. 209: 2501–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]