Abstract
EPA and DHA are not biologically equivalent; however, their individual activity on B cells is unknown. We previously reported fish oil enhanced murine B-cell activity in obesity. To distinguish between the effects of EPA and DHA, we studied the ethyl esters of EPA and DHA on murine B-cell function as a function of time. We first demonstrate that EPA and DHA maintained the obese phenotype, with no improvements in fat mass, adipose inflammatory cytokines, fasting insulin, or glucose clearance. We then tested the hypothesis that EPA and DHA would increase the frequency of splenic B cells. EPA and DHA differentially enhanced the frequency and/or percentage of select B-cell subsets, correlating with increased natural serum IgM and cecal IgA. We next determined the activities of EPA and DHA on ex vivo production of cytokines upon lipopolysaccharide stimulation of B cells. EPA and DHA, in a time-dependent manner, enhanced B-cell cytokines with DHA notably increasing IL-10. At the molecular level, EPA and DHA differentially enhanced the formation of ordered microdomains but had no effect on Toll-like receptor 4 mobility. Overall, the results establish differential effects of EPA and DHA in a time-dependent manner on B-cell activity in obesity, which has implications for future clinical studies.
Keywords: n-3 polyunsaturated fatty acids, high fat diets, immunity, metabolism
Numerous studies in preclinical and clinical models suggest that dietary supplementation with marine long chain n-3 PUFAs, EPA and DHA, has potential for alleviating some of the complications associated with obesity (1–3). For example, prescription n-3 PUFA supplements are effective in treating moderate to severe hypertriglyceridemia that is prevalent in obese individuals (4, 5). Furthermore, extensive experiments in rodents demonstrate that n-3 PUFAs suppress cell-mediated immunity and inflammation (6). N-3 PUFAs diminish the activation of Th1/Th17 cells and promote an M2 over an M1 macrophage phenotype in adipose tissue, which contributes toward improved insulin sensitivity (7, 8). In contrast, much less is known about the role of n-3 PUFAs on B-cell driven humoral immunity, which is central in establishing host immune responses. This is essential to determine, given that obese individuals generally display suppressed humoral immunity (9).
It was previously reported that n-3 PUFAs from fish oil enhanced B-cell activity (10, 11). Initial studies in lean C57BL/6 mice showed that dietary administration of n-3 PUFAs as menhaden fish oil increased B-cell cytokine secretion (11, 12). Subsequent experiments demonstrated that menhaden fish oil elevated the frequency of splenic B cells and antibody production in lean mice and rescued the decrement in antibody production in mice consuming obesogenic diets in response to antigen stimulation (10). These results were consistent with more recent studies. For instance, Gurzell et al. (13) demonstrated DHA-enriched fish oil boosted B-cell antibody production of Smad3−/− mice and Tomasdottir et al. (14) showed fish oil enhanced B-cell responses in a model of murine peritonitis.
A major limitation of the aforementioned studies on n-3 PUFAs and B cells is that fish oil has various components and its composition can vary depending on the marine source (15). Therefore, in order to further develop n-3 PUFAs for clinical trials, here we focused on the individual activities of EPA and DHA. We first elucidated whether EPA and DHA ethyl esters, modeling prescription supplements, had any benefits on the obese phenotype. This was essential to determine because there is discrepancy in the literature on how n-3 PUFAs impact key aspects of obesity (16). We then determined whether EPA and DHA ethyl esters enhanced the frequency of select B-cell subsets, which led to studies on antibody production. Finally, we determined if EPA and DHA exerted equivalent immune enhancing effects on B-cell activation and potential membrane-mediated mechanisms. The studies also addressed the impact of time of intervention, which is a key variable to elucidate in order to develop future clinical trials on n-3 PUFAs.
MATERIALS AND METHODS
Mice and diets
Male C57BL/6 mice, about 5 weeks old, were fed experimental diets for 5 or 10 weeks. Mice were fed a low fat control diet, a high fat (HF) diet, or a HF diet enriched with oleic acid (OA) (HF-OA), EPA (HF-EPA), or DHA (HF-DHA) ethyl esters (Harlan Teklad). Ethyl esters were greater than 90% purity. The control diet was a 5% fat by weight diet and HF diets contained 45% of total kilocalories from fat. The ethyl esters of OA, EPA, or DHA accounted for 2% of total energy. This corresponded to levels of EPA and DHA that are achievable with dietary supplementation and used in clinical trials (5, 17). Supplementary Table I shows the composition of the diets. At the end of the feeding period, mice were euthanized with CO2 inhalation followed by cervical dislocation. Epididymal adipose tissue, cecum, and spleen were harvested following euthanasia. Mice were euthanized in accordance with the guidelines set forth by East Carolina University Brody School of Medicine for euthanasia and humane treatment.
Measurements of fat mass
Lean mass and fat mass were determined with EchoMRI (Active Field Resources, LLC, Houston, TX). In order to measure the size of adipocytes, epididymal adipose tissue was harvested and immediately fixed in 4% paraformaldehyde overnight, then dehydrated, and embedded in paraffin. Sections were cut at 5 μm and stained with hematoxylin and eosin. Images were acquired with a confocal microscope (Olympus, FV1000) using a 20× objective. Fluorescent microscopy images were digitized to 8-bits and the area was measured using National Institutes of Health ImageJ. For each mouse, 120 adipocytes were measured in four independent microscopic fields.
Glucose clearance and fasting insulin
Subsequent to a 6 h fast, baseline glucose levels were measured with a glucometer. Mice were injected ip with 2.5 g dextrose (Hospira, Inc., Lake Forest, IL) per kg lean mass and glucose measurements were made from the tail vein. Following a 6 h fasting period, serum insulin was measured according to manufacturer’s protocol via an ELISA (Crystal Chem, Inc., Downers Grove, IL). Briefly, 5 μl of sample combined with 95 μl of sample diluent were added to an antibody-coated microplate. Following a 2 h incubation, anti-insulin enzyme conjugate was added and incubated for 40 min. Stop solution was then added and absorbance measured at 450 nm.
Adipose inflammatory profile
RNA was extracted from 100 mg of adipose tissue with the RNeasy Mini kit (Qiagen, Hilden, Germany). cDNA was made following the manufacturer’s protocol using the RT2 First Strand kit (Qiagen). A custom RT2 PCR array plate (Qiagen), coated with the primers for the genes of interest and formulated for use on the Bio-Rad IQ5 real-time PCR machine, was combined with RT2 SYBR Green qPCR Mastermix (Qiagen) that contained buffer, HotStart DNA Taq polymerase, and SYBR Green dye. The genes measured were TNFα, IL-6, IL-10, IL-4, GAPDH, and β2m. GAPDH and β2m were chosen as reference genes because the copy number was constitutively expressed with all diets. ΔCt was calculated using the equation ΔCt = CtGOI − CtHSK, where GOI is the gene of interest and HSK is the average of both housekeeping genes. ΔΔCt was determined by subtracting the ΔCt of the control from the ΔCt of the experimental diets. The fold change from the control was calculated by the 2−ΔΔCt method.
Splenic B-cell isolation and phenotyping
Splenocytes were subjected to a negative selection microbead kit (Miltenyi Biotec, Auburn, CA) to purify B220+ B cells as previously described (11). Red blood cells were lysed with ACK lysis buffer (Life Technologies, Grand Island, NY). B cells (0.5 × 106) were transferred to a 96-well plate and blocked with FcR block (Miltenyi Biotec, San Diego, CA). Splenic B cells were then stained with an antibody cocktail containing B220-FITC (Miltenyi Biotec), IgM-PE (Southern Biotech, Birmingham, AL), IgD-APC (BioLegend, San Diego, CA), CD23-PE/Cy7 (BioLegend), and CD21-PerCp/Cy5.5 (BioLegend) in 1× PBS supplemented with 0.1% BSA. Dead cells were gated out with SYTOX Blue (Invitrogen, Carlsbad, CA). The major splenic B-cell populations analyzed were IgM+IgD−CD21lowCD23−, IgM+IgD+CD21midCD23+, IgM+IgD+CD21hiCD23+, IgM+IgD−CD21hiCD23−, and IgD+IgM−CD21midCD23+ (18, 19). The gating strategy is depicted in supplementary Fig. I.
Fatty acid analysis of B cells
Total lipids were extracted as previously described using the Folch method (12, 20). Isolated lipids from splenic B220+ B cells were methylated using 1 ml boron trifluoride-methanol solution (Sigma, St. Louis, MO) for 90 min at 100°C. The resulting methyl esters were extracted again with hexane and water and separated based on retention times by gas chromatography (Shimadzu GC-2010, Columbia, MD). Peaks were analyzed by area as shown previously (12).
IgM and IgA analyses
Serum was collected as previously described and analyzed for natural IgM with an ELISA (10). Anti-IgM (5 μg/ml) (Southern Biotech, Birmingham, AL) was used to coat 96-well plates at 4°C. The plate was blocked with 5% milk in 1× PBS for 24 h at 4°C. The serum was then diluted 1:400 in 1% milk in 1× PBS, 100 μl added to each well, and incubated at 37°C for 1 h. A 1:5,000 dilution of HRP-conjugated goat anti-mouse IgM (Southern Biotech) in 1% milk in 1× PBS was added to each well and incubated at 37°C for 1 h. TMB SureBlue (KPL, Gaithersburg, MD) was utilized to develop the plate followed by the addition of TMB stop solution (KPL). Absorbance was measured at 450 nm and a calibration curve was generated to convert absorbance values to absolute levels of IgM.
The cecum was harvested and cecal contents were collected in a preweighed microcentrifuge tube. Cecal contents were processed as previously described (13). Briefly, contents were frozen at −80°C until analysis. Once thawed, the cecal contents were placed in a 25% w/v solution of protease inhibitors in PBS. Samples were vortexed for 10 min and then centrifuged at 16,000 g for 10 min. Supernatants were collected, centrifuged once more, and collected for protein analysis. IgA was determined via an ELISA according to manufacturer’s instructions.
B-cell activation
B cells (1 × 106) were plated in 1 ml of RPMI-1640 1× media (Corning Cellgro, Manassas, VA) supplemented with 5% heat-inactivated defined FBS (Thermo Scientific, Waltham, MA), 2 mM l-glutamine (Corning Cellgro), 1% penicillin/streptomycin (Corning Cellgro), and 50 μM β-mercaptoethanol (Sigma) in a 24-well plate (Becton Dickinson, Franklin Lakes, NJ). B cells were stimulated with lipopolysaccharide (LPS) (Sigma) at a concentration of 1 μg/ml and incubated at 37°C in 5% CO2 for 24 h. Supernatants were collected after pelleting the cells by centrifugation at 300 g for 5 min, and TNFα, IL-6, and IL-10 were measured with an ELISA (BioLegend).
Two-photon polarization imaging
B cells (1 × 106) were washed twice with 1× PBS and stained with 1 μM Laurdan (Life Technologies) for 15 min at 4°C and then washed twice with 1× PBS. The staining was conducted at 4°C to induce the formation of an ordered plasma membrane. Paraformaldehyde (1 ml, 4%) (Electron Microscopy Sciences, Hatfield, PA) was used to fix the cells for 30 min on ice. The stained B cells were washed three times with 1× PBS and loaded into capillary tubes (Fiber Optic Center, New Bedford, MA). Multi-photon fluorescent imaging was conducted using an Olympus FV-1000 confocal microscope. Emission was measured at 400–460 nm and 495–540 nm. For each diet sample, a minimum of 10 cells were imaged in order to calculate the generalized polarization (GP). GP was calculated using
where I is the fluorescence intensity. The G factor was calculated using a control sample with a known GP (21).
Cell culture and fatty acid treatment
Ba/F3 cells, an IL-3-dependent murine pro-B-cell line expressing green fluorescent protein (GFP)-tagged Toll-like receptor 4 (TLR4), and Chinese hamster ovary cells were obtained from Dr. Daniel H. Hwang (University of California, Davis). Ba/F3 cells were maintained in RPMI 1640 (Corning Cellgro) supplemented with 70 U/ml recombinant murine IL-3, 10% heat-inactivated defined FBS (Hyclone), 2 mM l-glutamine (Corning Cellgro), 1% penicillin/streptomycin (Corning Cellgro), 100 μM β-mercaptoethanol, and 2 μg/ml puromycin at 37°C in a 5% CO2 incubator. The source of recombinant murine IL-3 was medium conditioned by Chinese hamster ovary cells that were engineered to produce up to 70,000 U/ml of murine IL-3. Ba/F3 cells (2.5 × 105) were treated for 24 h with fatty acid free BSA or 25 μM OA, EPA, or DHA in complete medium. Fatty acid free BSA and OA served as controls to ensure specificity of EPA and DHA.
Fluorescence recovery after photobleaching microscopy
Ba/F3 cells (2.5 × 105) were washed twice, added to poly-d-lysine-coated glass-bottom dishes, and washed twice to remove nonadhering cells. Adherent cells were immersed in phenol red-free RPMI supplemented with 10% FBS and stimulated with 1 μg/ml LPS (Sigma) for 20 min at 37°C. Fluorescence recovery after photobleaching (FRAP) measurements were made using an Olympus FV-1000 confocal microscope. An attenuated laser beam was focused through a 60× objective on the cell surface. For bleaching, a region of interest was defined as a circle of a 1 μm radius. Initial fluorescence was recorded followed by a 175 ms bleach with 50% laser power, then fluorescence recovery was measured for 50 s. For each experimental condition, 40 recovery curves were collected in four independent experiments. TLR4 mobile fraction and diffusion coefficients were then calculated using standard equations (22, 23).
Statistical analyses
The data sets were ensured to be parametric distributions using a Kolmogorov-Smirnov test. Statistical significance was then established using a one-way ANOVA followed by a Bonferroni multiple comparison t-test. The two-photon and FRAP microscopy data displayed nonparametric distributions and were therefore analyzed with a Kruskal-Wallis test followed by a Dunn’s multiple comparison test. For all data sets, P < 0.05 was considered to be significant.
RESULTS
EPA and DHA ethyl esters maintained the obesogenic phenotype
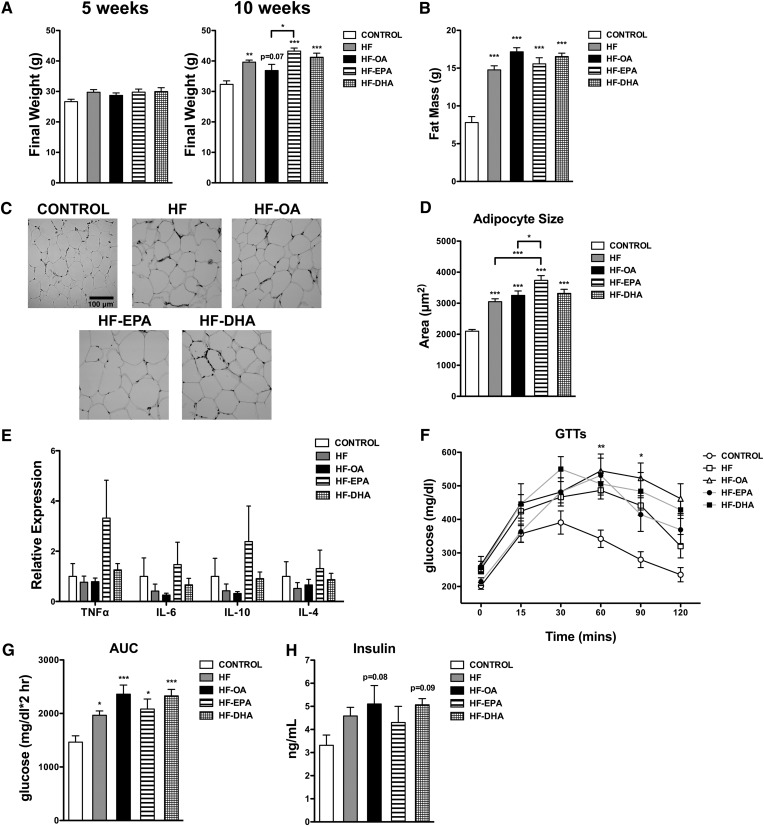
Given that we were studying the effects of EPA and DHA on B-cell activity in obesity, it was essential to establish the effects of the ethyl esters on fat mass, adipose inflammation, and glucose/insulin levels. After 5 weeks of feeding, the final body weights of the mice consuming control and HF diets remained the same (Fig. 1A). Obesity, defined here as an increase in body weight beyond that seen in the control group, was not observed until 10 weeks of feeding. The HF, HF-EPA, and HF-DHA diets increased body weight respectively by 22, 34, and 27% compared with the lean control (Fig. 1A). The HF-OA diet modestly increased the final weight by 14% (P = 0.07) (Fig. 1A).The HF-EPA diet elevated body weight by 17% compared with the HF-OA diet (Fig. 1A).
Fig. 1.
The obese phenotype is maintained with EPA and DHA ethyl esters. A: Final mouse body weights after 5 and 10 weeks of feeding control, HF, HF-OA, HF-EPA, and HF-DHA diets. Fat mass (B), paraffin-embedded sections (C) of epididymal adipose tissue, and average adipocyte size (D) following 10 weeks of feeding. E: Relative gene expression of epididymal adipose tissue after 10 weeks of feeding. F: Glucose tolerance tests (GTTs) used to calculate the area under the curve (AUC) (G) and fasting insulin (H) were measured after 6 h of fasting for the 10 week feeding period. Data are average ± SE from six to twelve animals per diet, except (D), which are three animals per diet. Asterisks indicate significance from control unless indicated by a bracket: *P < 0.05, **P < 0.01, ***P < 0.001.
The increase in body weight was driven by fat mass. Echo-MRI measurements showed the HF diet elevated fat mass by 89% compared with the control diet (Fig. 1B). The fat mass of mice consuming the HF-OA, HF-EPA, and HF-DHA diets were respectively elevated by 120, 100, and 112% compared with the lean control (Fig. 1B). There were no detectable differences in fat mass between mice fed the HF diets. Compared with the lean control, adipocyte size was increased respectively by 46, 55, 78, and 58% with the HF, HF-OA, HF-EPA, and HF-DHA diets (Fig. 1C, D). The HF-EPA diet increased adipocyte size by 23% compared with the HF diet and 15% compared with the HF-OA diet (Fig. 1D). Furthermore, we analyzed the inflammatory profile of the adipose tissue. The relative gene expression of pro-inflammatory (TNFα, IL-6) and anti-inflammatory (IL-10, IL-4) cytokines was not significantly elevated with any of the HF diets relative to the lean control (Fig. 1E).
Glucose tolerance tests revealed that all of the HF diets suppressed fasting glucose clearance compared with the lean control (Fig. 1F). The area under the curve for glucose clearance was increased respectively by 34–61% for all of the HF diets compared with the control diet (Fig. 1G). Fasting insulin was not significantly elevated for mice consuming HF diets compared with the control (Fig. 1H). However, the HF-OA and HF-DHA showed a trend in elevated fasting insulin (Fig. 1H). Altogether, these studies established that the mice consuming HF diets had elevated fat mass with poor glucose clearance.
EPA and DHA ethyl esters increased B-cell levels of EPA and DHA at the expense of linoleic and arachidonic acid
The uptake of EPA and DHA ethyl esters has never been tested on B cells; thus, we measured the total B-cell fatty acid content after dietary intervention. After 5 weeks of feeding, linoleic acid (18:2) was respectively decreased by 36, 39, and 43% with the HF, HF-OA, and HF-EPA diets compared with the control diet (Table 1). The HF-EPA diet decreased arachidonic acid (AA, 20:4) by 40% concomitant with a 46-fold increase in EPA (20:5) and a 6-fold increase in docosapentaenoic acid (22:5, n-3) compared with the control diet (Table 1). Compared with the control diet, the HF-DHA diet decreased AA by 51% and DHA constituted 17.3% of the total fatty acids. EPA, due to retroconversion from DHA, was also elevated (Table 1). Total n-6 PUFAs decreased with the HF-EPA and HF-DHA diets by 37–41% accompanied by an increase in total n-3 PUFAs with the HF-EPA and HF-DHA diets by 3-fold (Table 1).
TABLE 1.
Fatty acid composition of B cells after 5 weeks of treatment
| Fatty Acid | Control | HF | HF-OA | HF-EPA | HF-DHA |
| C14:0 | 0.6 ± 0.3 | 2.4 ± 0.7 | 2.8 ± 0.6a | 1.7 ± 0.4 | 1.6 ± 0.3 |
| C16:0 | 25.1 ± 1.8 | 23.2 ± 1.5 | 25.1 ± 1.2 | 24.8 ± 0.5 | 24.1 ± 1.2 |
| C16:1 | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.5 ± 0.3 | 1.5 ± 0.0 | 1.4 ± 0.1 |
| C18:0 | 22.4 ± 0.8 | 21.1 ± 0.5 | 21.7 ± 0.6 | 20.3 ± 0.2 | 20.2 ± 0.3 |
| C18:1 trans | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| C18:1 cis | 10.1 ± 0.3 | 10.9 ± 0.8 | 12.4 ± 1.6 | 9.4 ± 0.3 | 9.7 ± 0.2 |
| C18:2 (n-6) | 11.2 ± 0.2 | 7.2 ± 0.2b | 6.8 ± 0.1b | 6.4 ± 0.2b | 10.2 ± 0.5 |
| C20:4 (n-6) | 21.0 ± 1.0 | 25.3 ± 0.5 | 21.4 ± 2.4 | 12.7 ± 0.3b | 10.2 ± 0.2b |
| C20:5 (n-3) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 9.2 ± 0.4b | 3.6 ± 0.1b |
| C22:5 (n-3) | 1.7 ± 0.2 | 2.5 ± 0.2 | 2.4 ± 0.3 | 10.2 ± 0.4b | 1.8 ± 0.1 |
| C22:6 (n-3) | 6.2 ± 0.5 | 5.7 ± 0.3 | 5.4 ± 0.7 | 3.7 ± 0.2 | 17.3 ± 0.7b |
| ∑ SFA | 48.1 ± 1.5 | 46.7 ± 1.6 | 49.6 ± 1.9 | 46.8 ± 0.5 | 45.8 ± 1.3 |
| ∑ MUFA | 11.6 ± 0.3 | 12.3 ± 0.9 | 14.0 ± 1.6 | 11.0 ± 0.4 | 11.1 ± 0.1 |
| ∑ PUFA (n-3) | 8.1 ± 0.6 | 8.5 ± 0.5 | 8.1 ± 1.1 | 23.2 ± 0.6b | 22.6 ± 0.8b |
| ∑ PUFA (n-6) | 32.2 ± 1.0 | 32.5 ± 0.4 | 28.3 ± 2.4 | 19.1 ± 0.4b | 20.4 ± 0.7b |
Data are average ± SE from four to five animals per diet. SFA, saturated fatty acid.
P < 0.05.
P < 0.001.
After 10 weeks of feeding, the HF diet increased AA levels by 33% compared with the control diet (Table 2). The HF-EPA diet decreased linoleic acid and AA by 49 and 39%, respectively, compared with the control diet in addition to a 28-fold increase in EPA and a 5-fold increase in DPA (Table 2). The HF-DHA diet decreased AA by 43%, and 4.6% of total fatty acids were from EPA and 19.1% from DHA (Table 2). The HF-EPA diet and HF-DHA diet respectively increased the total n-3 PUFAs by 120 and 166% compared with the control diet (Table 2). Total n-6 PUFAs decreased by 43 and 27% with the HF-EPA and HF-DHA diets, respectively, compared with the control diet (Table 2). Overall, the data showed that EPA and DHA ethyl esters were effectively taken up by the B cells at the expense of n-6 PUFAs.
TABLE 2.
Fatty acid composition of B cells after 10 weeks of treatment
| Fatty Acid | Control | HF | HF-OA | HF-EPA | HF-DHA |
| C14:0 | 0.7 ± 0.3 | 1.1 ± 0.6 | 0.3 ± 0.2 | 1.3 ± 0.6 | 0.7 ± 0.3 |
| C16:0 | 26.9 ± 3.0 | 20.6 ± 2.7 | 18.9 ± 1.7 | 19.1 ± 2.0 | 17.4 ±3.2 |
| C16:1 | 1.7 ± 0.4 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.3 | 1.1 ± 0.3 |
| C18:0 | 18.4 ± 2.1 | 22.7 ± 0.7 | 22.4 ± 0.6 | 29.2 ± 7.7 | 20.6 ± 0.6 |
| C18:1 trans | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| C18:1 cis | 10.7 ± 0.5 | 10.8 ± 0.4 | 12.1 ± 0.6 | 9.2 ± 1.1 | 10.5 ± 0.5 |
| C18:2 (n-6) | 10.9 ± 1.0 | 7.1 ± 0.4 | 6.5 ± 0.3 | 5.6 ± 0.7a | 11.3 ± 2.5 |
| C20:4 (n-6) | 20.6 ± 0.7 | 27.3 ± 1.2a | 25.8 ± 0.3 | 12.5 ± 1.7b | 11.8 ± 2.0b |
| C20:5 (n-3) | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.7 ± 0.3 | 8.3 ± 1.2c | 4.6 ± 0.1c |
| C22:5 (n-3) | 2.1 ± 0.2 | 2.8 ± 0.3 | 3.2 ± 0.3 | 9.7 ± 1.2c | 2.9 ± 0.6 |
| C22:6 (n-3) | 7.6 ± 0.9 | 6.3 ± 0.8 | 9.1 ± 1.2 | 4.0 ± 0.8 | 19.1 ± 1.2c |
| ∑ SFA | 45.9 ± 1.2 | 44.4 ± 2.1 | 41.6 ± 1.6 | 49.7 ± 6.4 | 38.6 ± 3.0 |
| ∑ MUFA | 12.5 ± 0.5 | 11.9 ± 0.4 | 13.1 ± 0.7 | 10.3 ± 1.1 | 11.6 ± 0.5 |
| ∑ PUFA (n-3) | 10.0 ± 1.1 | 9.3 ± 0.9 | 12.9 ± 1.6 | 22.0 ± 3.0b | 26.6 ± 1.8c |
| ∑ PUFA (n-6) | 31.6 ± 1.2 | 34.4 ± 1.5 | 32.3 ± 0.3 | 18.0 ± 2.4c | 23.1 ± 2.2a |
Data are average ± SE from four to five animals per diet. SFA, saturated fatty acid.
P < 0.05.
P < 0.01.
P < 0.001.
EPA and DHA differentially increased the percentage and/or frequency of select B cell subsets
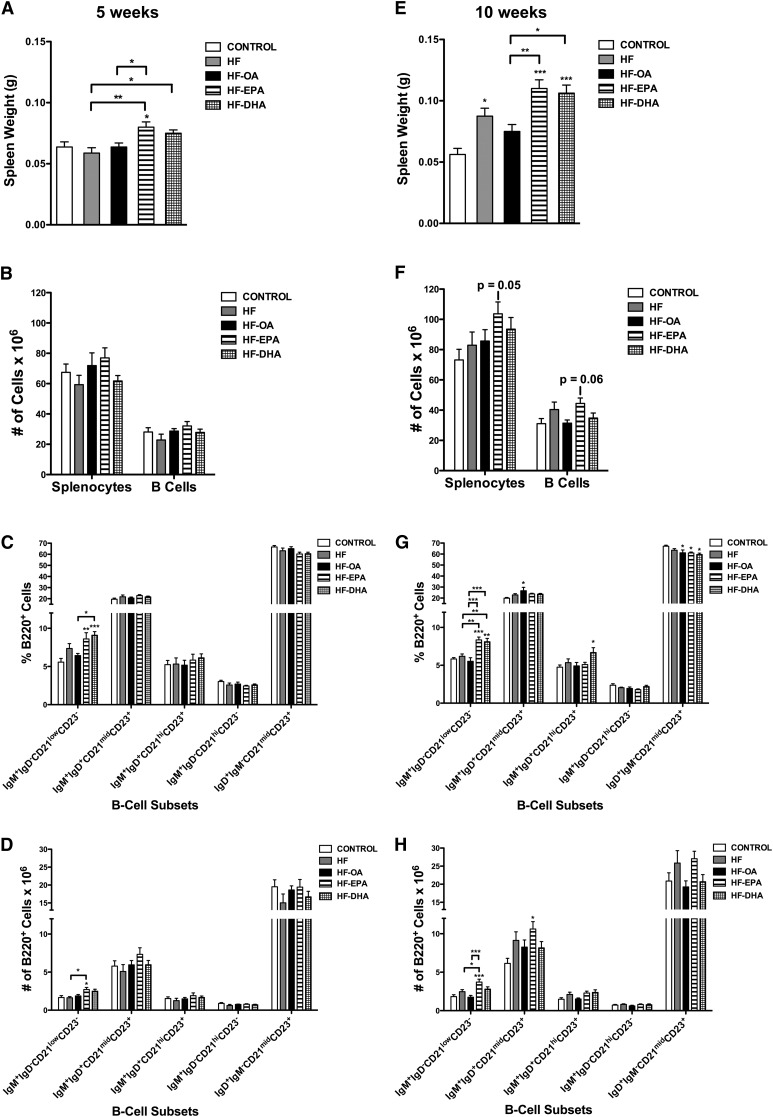
We previously showed that n-3 PUFAs from fish oil increased the frequency of select B-cell subsets (10). Here we determined whether EPA and DHA ethyl esters also enhanced the frequency of B-cell subsets. After 5 weeks of feeding, the HF-EPA diet increased spleen weight by ∼33% compared with the lean control, HF, and HF-OA diets (Fig. 2A). The HF-DHA diet increased spleen weight by 17% compared with the HF diet (Fig. 2A). No change was observed in the number of splenocytes or B cells at 5 weeks with the HF-EPA or HF-DHA diets compared with the control diet (Fig. 2B). We then quantified the percentage and frequency of splenic B-cell subsets. At 5 weeks, the HF-EPA and HF-DHA diets increased the percentage of IgM+IgD−CD21lowCD23− B cells by 54 and 63%, respectively, compared with the lean control (Fig. 2C). The HF-DHA diet increased the percentage of IgM+IgD−CD21lowCD23− B cells by 41% compared with the HF-OA diet (Fig. 2C). The HF-EPA diet increased the frequency of IgM+IgD−CD21lowCD23− B cells by 63% compared with the control diet and by 66% compared with the HF diet (Fig. 2D). The HF and HF-OA diets had no effect on the percentage or frequency of B cells relative to the control diet (Fig. 2C, D).
Fig. 2.
The percentage and frequency of select B-cell subsets is differentially enhanced with EPA and DHA. Spleen weight (A) and number of splenocytes (B) and B cells from mice fed control, HF, HF-OA, HF-EPA, and HF-DHA diets for 5 weeks. Corresponding percentage (C) and frequency (D) of IgM+IgD−CD21lowCD23−, IgM+IgD+CD21midCD23+, IgM+IgD+CD21hiCD23+, IgM+IgD−CD21hiCD23−, and IgD+IgM−CD21midCD23+ B-cell subsets at 5 weeks. Spleen weight (E) and number of splenocytes (F) and B cells and corresponding percentage (G) and frequency (H) of B-cell subsets from mice fed various diets for 10 weeks. Data are average ± SE from eight animals per diet. Asterisks indicate significance from control unless indicated by a bracket: *P < 0.05, **P < 0.01, ***P < 0.001.
Following 10 weeks of feeding, the HF diet increased spleen weight by 60% compared with the control diet (Fig. 2E). The HF-EPA and HF-DHA diets increased spleen weight by 96 and 89%, respectively, compared with the control diet and 47 and 41%, respectively, compared with the HF-OA diet (Fig. 2E). The HF-EPA diet had a tendency to increase the number of splenocytes (P = 0.05) and B cells (P = 0.06) compared with the lean control (Fig. 2F). The percentage of IgM+IgD−CD21lowCD23− B cells increased with the HF-EPA and HF-DHA diets by 43 and 39%, respectively, compared with the control diet (Fig. 2G). Compared with the HF diet, the HF-EPA and HF-DHA diets increased the percentage of IgM+IgD−CD21lowCD23− B cells by 35 and 31%, respectively (Fig. 2G). Compared with the HF-OA diet, the HF-EPA and HF-DHA diets increased the percentage of IgM+IgD−CD21lowCD23− B cells by 52 and 47%, respectively (Fig. 2G). The percentage of IgM+IgD+CD21midCD23+ B cells was elevated by 34% with the HF-OA diet relative to the lean control (Fig. 2G). The HF-DHA diet increased the percentage of IgM+IgD+CD21hiCD23+ B cells by 41% compared with the lean control (Fig. 2G). The percentage of IgD+IgM−CD21midCD23+ B cells was decreased by the HF-OA and HF-EPA diets by 9% relative to the control diet, and by 11% with the HF-DHA diet relative to the control diet (Fig. 2G).
IgM+IgD−CD21lowCD23− B cells increased in frequency by 103% with the HF-EPA diet compared with the control diet, and by 113% compared with the HF-OA diet (Fig. 2H). Compared with the HF diet, the HF-EPA diet increased the frequency of IgM+IgD−CD21lowCD23− B cells by 51% (Fig. 2H). The HF-EPA diet also increased the frequency of IgM+IgD+CD21midCD23+ B cells by 73% compared with the control diet (Fig. 2H). The frequency of B cells did not change in the remaining subsets with the HF diets compared with the control diet (Fig. 2H). Overall, the data showed that EPA and DHA had the most robust effect on the frequency and/or the percentage of IgM+IgD−CD21lowCD23− B cells.
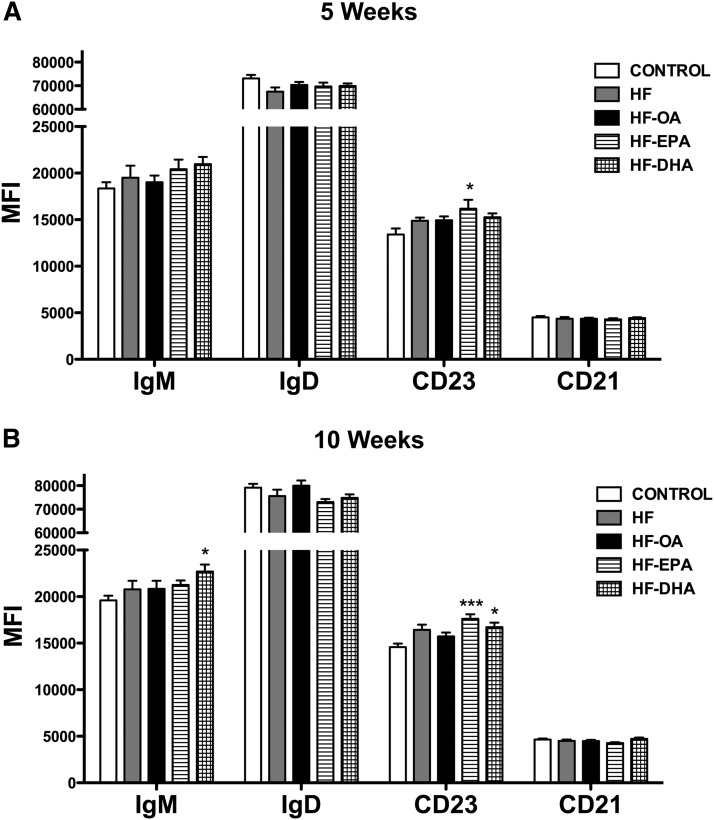
Surface IgM is elevated with DHA, but not EPA
We also analyzed the total surface levels of IgM, IgD, CD23, and CD21, the markers used to phenotype the B cells. After 5 weeks of feeding, the HF-EPA diet increased CD23 surface expression by 20% compared with the lean control, whereas the HF-EPA and HF-DHA diets had no effect on IgM, IgD, and CD21 (Fig. 3A). After 10 weeks, the HF-DHA diet increased IgM surface expression by 16% relative to the lean control (Fig. 3B). The HF-EPA and HF-DHA diets had no effect on IgD (Fig. 3B), but both increased CD23 surface expression by 20% and 15%, respectively (Fig. 3B). The HF-EPA and HF-DHA diets did not influence CD21 surface expression (Fig. 3B). The HF and HF-OA diets had no effect on IgM, IgD, CD23, and CD21 expression at 5 and 10 weeks. Overall, the increase in B-cell marker expression reflected an elevation in surface levels with EPA or DHA and not an increase in the number of CD23+ or IgM+ cells (data not shown).
Fig. 3.
Surface IgM and CD23 expression are differentially enhanced with EPA and DHA. Splenic B220+ B cells were isolated from mice consuming a control, HF, HF-OA, HF-EPA, or HF-DHA diet for 5 weeks or 10 weeks. Surface expression of IgM, IgD, CD23, and CD21 at 5 (A) and 10 weeks (B) were measured via flow cytometry. Data are average ± SE from seven to eight animals per diet. Asterisks indicate significance from control: *P < 0.05, ***P < 0.001.
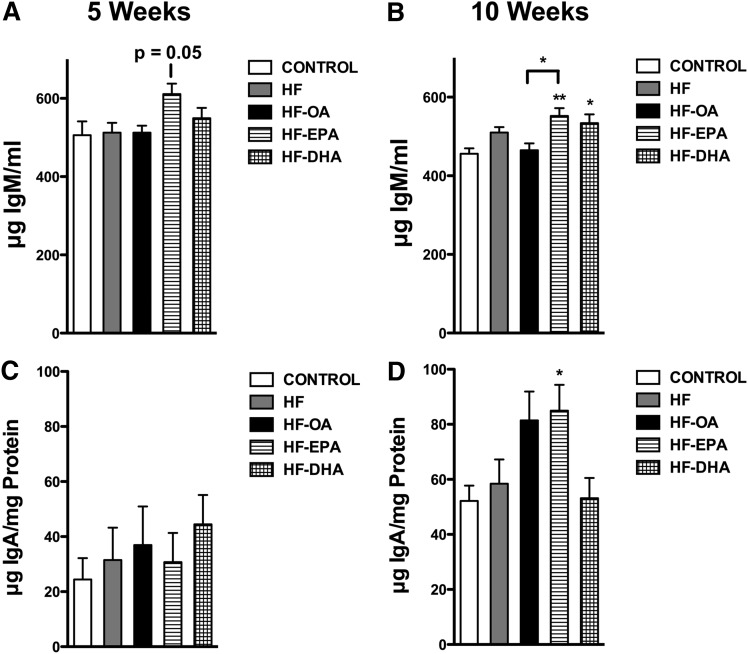
EPA and DHA differentially increased natural IgM and IgA
Natural IgM is produced in the absence of exogenous antigen stimulation (24). Therefore, we sought to determine whether EPA and DHA increased circulating levels of IgM in the absence of antigen. After 5 weeks of dietary intervention, the HF-EPA diet modestly increased total IgM in the serum by 19% (P = 0.05) compared with the control diet (Fig. 4A). After 10 weeks, the HF-EPA and HF-DHA diets modestly elevated total IgM by 20% and 15%, respectively, compared with the control diet (Fig. 4B). Compared with the HF-OA diet, the HF-EPA diet increased total IgM at 10 weeks by 20% (Fig. 4B). The HF and HF-OA diets had no effect on IgM levels relative to the lean control.
Fig. 4.
Antibody production is differentially enhanced with EPA and DHA. Natural IgM levels at 5 (A) and 10 weeks (B) and cecal IgA levels at 5 (C) and 10 weeks (D) of consuming a control, HF, HF-OA, HF-EPA, or HF-DHA diet. Data are average ± SE from eight animals per diet. Asterisks indicate significance from control unless indicated by a bracket: *P < 0.05, **P < 0.01.
We also measured cecal IgA. The levels of cecal IgA were not influenced by any of the HF diets after 5 weeks of dietary intervention (Fig. 4C). After 10 weeks, the HF-EPA diet increased IgA by 63% relative to the control diet (Fig. 4D). The other HF diets had no statistically significant effect on IgA levels compared with the control diet. In general, IgA levels were higher with all of the diets at 10 weeks compared with 5 weeks.
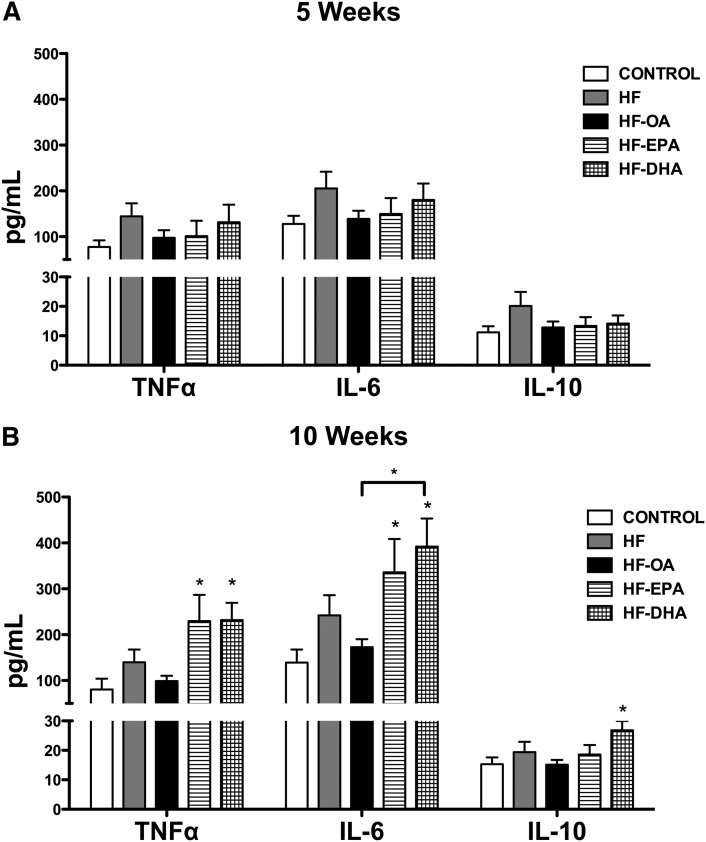
EPA and DHA differentially enhanced B-cell cytokine secretion ex vivo
We next conducted ex vivo experiments on B-cell cytokine secretion. The rationale was to directly compare with previous ex vivo studies in lean mice using fish oil (11, 12). The HF, HF-OA, HF-EPA, and HF-DHA diets did not effect cytokine production relative to the lean control after 5 weeks of feeding (Fig. 5A). After 10 weeks, TNFα secretion increased with the HF-EPA and HF-DHA diets by 186 and 188%, respectively, compared with the control diet (Fig. 5B). The HF-EPA and HF-DHA diets respectively increased IL-6 by 142 and 182% compared with the control at 10 weeks (Fig. 5B). Compared with the HF-OA diet, the HF-DHA diet increased IL-6 production by 127% (Fig. 5B). The HF-DHA diet increased the production of IL-10 by 75% compared with the lean control (Fig. 5B). These results established that EPA and DHA differentially boost B-cell cytokine secretion.
Fig. 5.
B-cell cytokine secretion is differentially enhanced with EPA and DHA. Splenic B220+ B cells were isolated from mice consuming control, HF, HF-OA, HF-EPA, or HF-DHA diets for 5 or 10 weeks and stimulated for 24 h with LPS. TNFα, IL-6, and IL-10 cytokine levels after 5 (A) and 10 weeks (B) of dietary intervention. Data are average ± SE from eight animals per diet. Asterisks indicate significance from control unless indicated by a bracket: *P < 0.05.
EPA and DHA ethyl esters differentially promote the molecular order of lipid microdomains without an effect on TLR4 lateral mobility
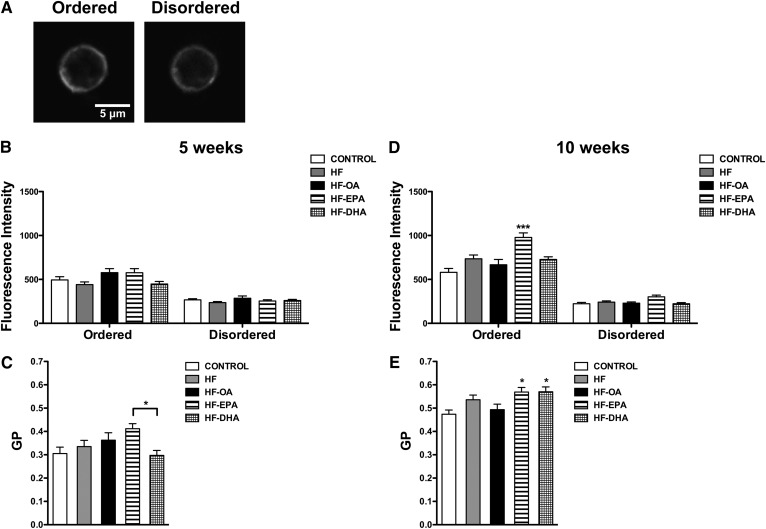
The mechanisms of EPA and DHA are pleiotropic and we focused on the potential effects of EPA and DHA on ex vivo B-cell membrane organization. The studies relied first on Laurdan, a fluorescent membrane probe that detects changes in membrane polarity, to quantify changes in B-cell plasma membrane order upon the formation of ordered membrane microdomains (Fig. 6A). After 5 weeks of feeding, there was no change in fluorescence intensity of Laurdan in the ordered and disordered fluorescence channels (Fig. 6B). GP values between all of the HF diets relative to the control diet were unchanged. However, the GP value for the HF-EPA diet was elevated relative to the HF-DHA diet by 39% (Fig. 6C).
Fig. 6.
B-cell plasma membrane molecular organization is differentially elevated with EPA and DHA. Splenic B220+ B cells were isolated from C57BL/6 mice consuming control, HF, HF-OA, HF-EPA, or HF-DHA diets for 5 or 10 weeks and stained with Laurdan. A: Sample images of Laurdan used to measure fluorescence intensities in the ordered (400–460 nm) and disordered (495–540 nm) channels and to calculate GP. Fluorescence intensities in the ordered and disordered channels (B) and GP values after 5 weeks of intervention (C). Fluorescence intensities in the ordered and disordered channels (D) and the associated GP values after 10 weeks of intervention (E). Data are average ± SE from four to five animals per diet. Asterisks indicate significance from control unless indicated by a bracket: *P < 0.05, ***P < 0.001.
After 10 weeks of feeding, the HF-EPA diet significantly increased the fluorescence intensity in the ordered channel by 68% relative to the control (Fig. 6D). There was no effect of the HF diets on fluorescence intensity in the disordered channel (Fig. 6D). The HF-EPA and HF-DHA diets, relative to the lean control, increased GP values by 20–21% (Fig. 6E). GP values for all of the diets were generally higher at 10 weeks of feeding compared with 5 weeks.
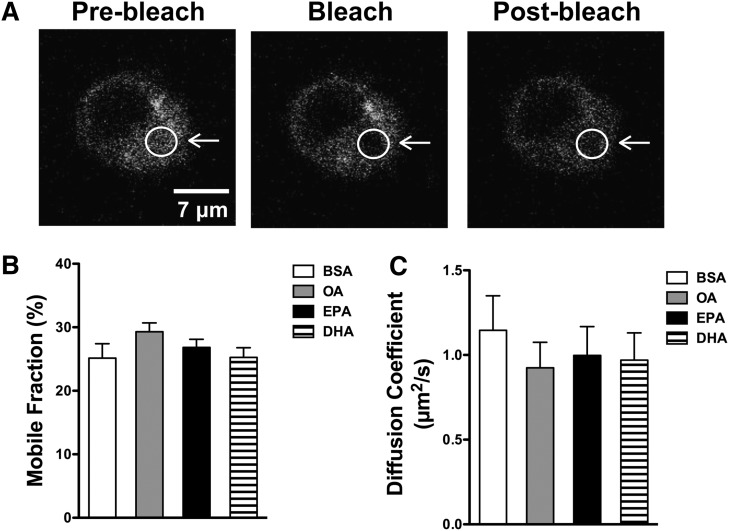
The increase in molecular order suggested that EPA and DHA may lower the long-range diffusion of TLR4 molecules as a mechanism by which LPS stimulation could enhance downstream B-cell activation. We therefore conducted FRAP microscopy of GFP-TLR4 upon LPS addition using an in vitro model system to calculate the fraction of mobile molecules and diffusion coefficients (Fig. 7A). We relied on treatment with fatty acids at 25 μM, which models the levels achieved in vivo (12, 25). TLR4 mobile fractions were not affected by treatment with OA, EPA, or DHA relative to the BSA control (Fig. 7B). Similarly, diffusion coefficients remained constant with OA, EPA, and DHA treatment compared with the BSA control (Fig. 7C).
Fig. 7.
TLR4 lateral mobility is not lowered by EPA and DHA. Ba/F3 stably transfected with GFP-TLR4 were treated for 24 h with BSA or 25 μM OA, EPA, or DHA complexed to BSA. A: Sample FRAP images used for measuring the mobile fraction and diffusion coefficients. Mobile fractions (B) and diffusion coefficients (C) of GFP-TLR4. Data are average ± SE from four independent experiments.
DISCUSSION
The field of immunity and diet has not addressed how EPA and DHA individually impact B-cell activity. This study advances the discipline by demonstrating that EPA and DHA ethyl esters, modeling human pharmacological intake, are not equivalent on enhancing B-cell activity in a murine model of diet-induced obesity. Furthermore, the data reveal that the effects of EPA and DHA are time-dependent and the underlying mechanism of action with B-cell cytokine secretion is independent of changes in TLR4 lateral diffusion.
EPA versus DHA ethyl esters on B-cell activity
The initial experiments on the obese phenotype established that we are studying mice with increased fat mass, poor glucose clearance, and no excessive adipose tissue inflammation. Fasting insulin levels tended to be elevated, but were not statistically significant. The lack of improvement in glucose clearance or fasting insulin with EPA and DHA was consistent with some clinical studies that reveal no benefits of n-3 PUFA consumption on glucose and insulin sensitivity (26).
The general paradigm is that EPA and DHA are immunosuppressive, anti-inflammatory, or inflammation-resolving molecules based on studies with macrophages, neutrophils, dendritic cells, and T cells in varying model systems (8, 27–32). Our lab initially identified immune-enhancing properties of n-3 PUFA-enriched fish oil on B-cell activity in lean mice, which was recently confirmed by others (11, 13). Here we tested the effects of EPA and DHA ethyl esters because they are used in clinical trials and for the treatment of elevated triglycerides (5). Elucidating the differences between EPA and DHA is of significance given that numerous studies at the preclinical and clinical levels, focused mostly on inflammation, have relied on fish oils (e.g., menhaden, tuna oil) and prescription ethyl ester supplements (Lovaza) that are mixtures of EPA and DHA, or more recently just one n-3 PUFA (Vascepa) (5, 33). This contributes toward conflicting results in the field because the individual activity of EPA compared with DHA is much less studied. Our data establish that EPA and DHA are not biologically equivalent on several aspects of B-cell activity.
We measured an increase in the percentage and/or frequency of IgM+IgD−CD21lowCD23− B cells with EPA and DHA after 10 weeks of feeding. IgM levels, which were modestly elevated with EPA and DHA, correlated with an increase in the percentage of IgM+IgD−CD21lowCD23− B cells at 10 weeks of feeding. Similarly, EPA significantly increased cecal IgA, which correlated with the increase in the frequency of IgM+IgD−CD21lowCD23− B cells. These studies suggest that changes in the frequency and/or percentage of select B-cell subsets may be contributing toward enhanced antibody production, and moreover, the effects of EPA and DHA are not equivalent. The IgM+IgD−CD21lowCD23− population of B cells, based on the flow cytometry gating strategy, represents a combination of B cells including transitional 1, B1a, and B1b cells (18, 19). Given that B1 B cells, in addition to marginal zone B cells, are the subtypes that readily produce IgM and class switch to IgA, more studies are needed in this area in response to EPA and DHA intervention (24, 34). Specifically, future studies will need to address whether the increase in the IgM+IgD−CD21lowCD23− compartment with EPA or DHA reflects an increase in B1 cells in addition to determining if B1a and B1b cells are elevated, particularly in peritoneal cavities.
The ex vivo B-cell activation studies also revealed differences between EPA and DHA at 10 weeks. EPA and DHA increased TNFα and IL-6 secretion; however, only DHA increased IL-10 secretion. We did not measure upregulation of cell surface molecules, given that our past studies with mice have consistently shown no effect of n-3 PUFAs on B-cell activation markers (11, 12). Interestingly, DHA, but not EPA, increased surface IgM, consistent with our previous studies with fish oil (10). The results with DHA increasing IL-10 secretion were consistent with a study by Olson et al. (35), which showed that DHA increased ex vivo secretion of IL-10 from LPS-stimulated splenocytes. The results open the door to the possibility that DHA may increase IL-10 secretion from B cells in vivo, which could be driven by natural IgM. Natural IgM increases phagocytosis of apoptotic cells in the marginal zone to increase IL-10-secreting B cells (24, 36). Furthermore, improving IL-10 secretion in the adipose is of particular significance because IL-10-secreting B cells improve insulin sensitivity in obesity (37).
The studies also reveal that timing of intervention had an effect on the measured outcomes. Administration of EPA or DHA for 5 weeks during the early stages of weight gain did not have a robust effect on B-cell frequency, antibody production, and ex vivo cytokine secretion. On the other hand, prolonged exposure for 10 weeks significantly increased the functional outcomes of EPA and DHA. These results may explain some of the inconsistencies in the literature on the efficacy of EPA and DHA. For instance, several clinical studies have relied on short-term intervention while others have used long-term intervention (38–40). The differences in time may also reflect the age and the metabolic profile of the animals. Some studies have focused on moderately overweight individuals while others on more obese people (26, 41). Thus, the data suggest that future clinical trials targeting humoral immunity and likely other aspects of immunity with n-3 PUFAs will have to account for timing of intervention and the cohort of individuals that are being targeted.
An unexpected finding was that the two HF diets, in the absence of EPA and DHA, had no influence on B-cell activity. Several studies report that HF diets dysregulate B-cell driven humoral immunity (42, 43). Winer et al. (43) demonstrated that HF diets promote the accumulation of B cells in the adipose tissue that secrete pathogenic autoantibodies. We measured CD19 mRNA expression in the adipose and did not find an increase in its expression (data not shown). DeFuria et al. (44) demonstrated that HF diets increase ex vivo B-cell cytokine secretion. The data from our lab are not in complete agreement with the studies by Winer et al. (43) and DeFuria et al. (44). The discrepancy between our findings and the two aforementioned studies may be due to differences between the composition and the levels of the fat source (43, 44). This study relied on a moderate dose of milkfat (45% of total kilocalories), modeling human consumption, whereas others have used HF diets (60% of total kilocalories from fat) enriched in lard (45). The unexpected results open a new area of investigation; that is, the possibility that dietary fat composition and content have differential effects on B-cell function.
Implications for the general public and clinical trials
The general public is increasingly consuming n-3 PUFA supplements of varying composition and intake of these fatty acids may impact their humoral immunity (5, 46). The preclinical data show that EPA and DHA are not biologically equivalent and may have potential benefits for boosting IgM and IgA levels in select clinical populations. Several diseases are associated with altered humoral immunity (47, 48). In particular, the data suggest that EPA and DHA may have benefits for modestly elevating antibody levels in obesity, which is associated with increased susceptibility to infections and poor responses to vaccinations (9, 49). Furthermore, we speculate that EPA and DHA could be developed for boosting IgM levels in atherosclerosis (50). Natural IgM suppresses the progression of atherosclerosis by clearance of oxidized low density lipoprotein and apoptotic cells (51). This is of value in select clinical populations, such as those with lupus, that have increased susceptibility to cardiovascular disease which is associated with low levels of IgM antibodies against phosphorylcholine (52, 53). Similarly, enhancing IgA levels with EPA likely has potential benefits for boosting immunity. IgA, some of which is derived from B1 cells, has been shown to contribute toward clearing microbes from the gut (54).
Potential mechanisms
We previously proposed a model, based on studies with lean mice, in which n-3 PUFAs promote the formation of ordered lipid microdomains, which we suggested would lower the lateral diffusion of B-cell antigen receptors (11). Therefore, we first tested the effects of EPA and DHA on the molecular packing of ordered lipid microdomains. EPA and DHA ethyl esters increased the molecular packing of lipid microdomains, consistent with data to show n-3 PUFAs increase molecular order of lipid microdomains on the T-cell side of the immunological synapse (55). Furthermore, EPA and DHA ethyl esters exerted differential effects on the intensity of Laurdan in the ordered channel. EPA, but not DHA, selectively increased the fluorescence intensity of Laurdan in the ordered channel at 10 weeks, suggesting that EPA may have a stronger influence on ordered domains. Our results validate recent NMR studies in model membranes that show EPA and DHA are not structurally equivalent (56, 57).
Although paradoxical to the general notion that EPA and DHA simply decrease membrane packing, the data suggest that when these fatty acids interact with ordered domains, they increase their molecular order and can adapt to the ordered environment (58, 59). This may be driven by several variables, which include the loss of AA, a highly disordered fatty acid, and a displacement of cholesterol between disordered and ordered domains (58, 60).
We next tested the effects of EPA and DHA on TLR4 diffusion. We relied on an in vitro model system with GFP-transfected TLR4 that would promote a high degree of TRL4 expression given that surface TLR4 expression is low on primary B cells. Contrary to our prediction, FRAP imaging showed that EPA and DHA did not lower TLR4 diffusion, as measured by the diffusion coefficient and mobile fraction. Thus, the data suggest EPA and DHA are not impacting long-range protein diffusion and may be influencing other aspects of protein lateral organization, likely on a nanometer scale. It is possible that EPA and DHA may also exert differential effects on B-cell activation downstream of the plasma membrane. One possibility is that EPA and DHA may differentially target downstream transcription factors, which would explain why DHA, but not EPA, enhances IL-10 secretion. For instance, in T cells, TNFα and IL-6 transcription proceeds through the NF-κB pathway, compared with IL-10 production regulated by the transcription factor CREB (61). Thus, it is possible that EPA and DHA target the membrane in different ways that eventually differentially impact downstream production of TNFα, IL-6, and IL-10.
CONCLUSION
In summary, this study showed that B-cell activity was differentially enhanced with EPA and DHA ethyl esters, modeling human pharmacological intake, in a time-dependent manner in diet-induced obesity. Furthermore, we demonstrated that the effects of EPA and DHA were accompanied by significant changes in membrane packing, independent of an effect on TLR4 diffusion. Our results show that EPA and DHA are not biologically equivalent on a key aspect of immunity, which has broad implications for the general public and select clinical populations that are increasingly consuming n-3 PUFAs.
Supplementary Material
Acknowledgments
The authors thank the late Dr. Bill Stanley (University of Sydney) for providing the EPA and DHA ethyl esters, Dr. Karen Haas (Wake Forest) for her assistance with evaluating the gating strategy of B-cell populations, and Dr. Sarah Comstock (Michigan State University) for assisting in the IgA measurements. The authors also appreciate Dr. Daniel Hwang (University of California, Davis) for providing the Ba/F3- and IL-3-secreting cells.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- FRAP
- fluorescence recovery after photobleaching
- GFP
- green fluorescent protein
- GP
- generalized polarization
- HF
- high fat
- LPS
- lipopolysaccharide
- OA
- oleic acid
- TLR4
- Toll-like receptor 4
This work was supported by National Institutes of Health Grant R15AT006122 (S.R.S), the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health Grants 1UL1TR001111 (NCTraCS to S.R.S.) and R03CA162427 (J.F). The authors declare no financial conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and one table.
REFERENCES
- 1.Kiecolt-Glaser J. K., Belury M. A., Andridge R., Malarkey W. B., Hwang B. S., Glaser R. 2012. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav. Immun. 26: 988–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itariu B. K., Zeyda M., Hochbrugger E. E., Neuhofer A., Prager G., Schindler K., Bohdjalian A., Mascher D., Vangala S., Schranz M., et al. 2012. Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: a randomized controlled trial. Am. J. Clin. Nutr. 96: 1137–1149 [DOI] [PubMed] [Google Scholar]
- 3.Spencer M., Finlin B. S., Unal R., Zhu B., Morris A. J., Shipp L. R., Lee J., Walton R. G., Adu A., Erfani R., et al. 2013. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes. 62: 1709–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dayspring T. D. 2011. Understanding hypertriglyceridemia in women: clinical impact and management with prescription omega-3-acid ethyl esters. Int. J. Womens Health. 3: 87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maki K. C., Lawless A. L., Kelley K. M., Dicklin M. R., Kaden V. N., Schild A. L., Rains T. M., Marshall J. W. 2011. Effects of prescription omega-3-acid ethyl esters on fasting lipid profile in subjects with primary hypercholesterolemia. J. Cardiovasc. Pharmacol. 57: 489–494 [DOI] [PubMed] [Google Scholar]
- 6.Shaikh S. R., Jolly C. A., Chapkin R. S. 2012. n-3 Polyunsaturated fatty acids exert immunomodulatory effects on lymphocytes by targeting plasma membrane molecular organization. Mol. Aspects Med. 33: 46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monk J. M., Hou T. Y., Turk H. F., Weeks B., Wu C., McMurray D. N., Chapkin R. S. 2012. Dietary n-3 polyunsaturated fatty acids (PUFA) decrease obesity-associated Th17 cell-mediated inflammation during colitis. PLoS ONE. 7: e49739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., Olefsky J. M. 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 142: 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheridan P. A., Paich H. A., Handy J., Karlsson E. A., Hudgens M. G., Sammon A. B., Holland L. A., Weir S., Noah T. L., Beck M. A. 2012. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. (Lond). 36: 1072–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teague H., Fhaner C. J., Harris M., Duriancik D. M., Reid G. E., Shaikh S. R. 2013. n-3 PUFAs enhance the frequency of murine B-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity. J. Lipid Res. 54: 3130–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rockett B. D., Teague H., Harris M., Melton M., Williams J., Wassall S. R., Shaikh S. R. 2012. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J. Lipid Res. 53: 674–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockett B. D., Salameh M., Carraway K., Morrison K., Shaikh S. R. 2010. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J. Lipid Res. 51: 1284–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurzell E. A., Teague H., Harris M., Clinthorne J., Shaikh S. R., Fenton J. I. 2013. DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. J. Leukoc. Biol. 93: 463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomasdottir V., Thorleifsdottir S., Vikingsson A., Hardardottir I., Freysdottir J. 2014. Dietary omega-3 fatty acids enhance the B1 but not the B2 cell immune response in mice with antigen-induced peritonitis. J. Nutr. Biochem. 25: 111–117 [DOI] [PubMed] [Google Scholar]
- 15.Strobel C., Jahreis G., Kuhnt K. 2012. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. 11: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalupahana N. S., Claycombe K. J., Moustaid-Moussa N. 2011. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv. Nutr. 2: 304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miles E. A., Calder P. C. 2012. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 107(Suppl 2): S171–S184 [DOI] [PubMed] [Google Scholar]
- 18.Pillai S., Cariappa A. 2009. The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 9: 767–777 [DOI] [PubMed] [Google Scholar]
- 19.Baumgarth N. 2011. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 11: 34–46 [DOI] [PubMed] [Google Scholar]
- 20.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509 [PubMed] [Google Scholar]
- 21.Gaus K., Zech T., Harder T. 2006. Visualizing membrane microdomains by Laurdan 2-photon microscopy. Mol. Membr. Biol. 23: 41–48 [DOI] [PubMed] [Google Scholar]
- 22.Shaikh S. R., Edidin M. 2007. Immunosuppressive effects of polyunsaturated fatty acids on antigen presentation by human leukocyte antigen class I molecules. J. Lipid Res. 48: 127–138 [DOI] [PubMed] [Google Scholar]
- 23.Kwik J., Boyle S., Fooksman D., Margolis L., Sheetz M. P., Edidin M. 2003. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc. Natl. Acad. Sci. USA. 100: 13964–13969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grönwall C., Vas J., Silverman G. J. 2012. Protective roles of natural IgM antibodies. Front. Immunol. 3: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim W., Khan N. A., McMurray D. N., Prior I. A., Wang N., Chapkin R. S. 2010. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog. Lipid Res. 49: 250–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kratz M., Kuzma J. N., Hagman D. K., van Yserloo B., Matthys C. C., Callahan H. S., Weigle D. S. 2013. n3 PUFAs do not affect adipose tissue inflammation in overweight to moderately obese men and women. J. Nutr. 143: 1340–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monk J. M., Jia Q., Callaway E., Weeks B., Alaniz R. C., McMurray D. N., Chapkin R. S. 2012. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J. Nutr. 142: 117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teague H., Rockett B. D., Harris M., Brown D. A., Shaikh S. R. 2013. Dendritic cell activation, phagocytosis and CD69 expression on cognate T cells are suppressed by n-3 long-chain polyunsaturated fatty acids. Immunology. 139: 386–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou T. Y., Monk J. M., Fan Y. Y., Barhoumi R., Chen Y. Q., Rivera G. M., McMurray D. N., Chapkin R. S. 2012. n-3 polyunsaturated fatty acids suppress phosphatidylinositol 4,5-bisphosphate-dependent actin remodelling during CD4+ T-cell activation. Biochem. J. 443: 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao W., Wong O. Y., Liu X., Lee P., Chen Y., Wong K. K. 2010. Omega-3 fatty acids suppress inflammatory cytokine production by macrophages and hepatocytes. J. Pediatr. Surg. 45: 2412–2418 [DOI] [PubMed] [Google Scholar]
- 31.Zeyda M., Saemann M. D., Stuhlmeier K. M., Mascher D. G., Nowotny P. N., Zlabinger G. J., Waldhausl W., Stulnig T. M. 2005. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-kappaB activation. J. Biol. Chem. 280: 14293–14301 [DOI] [PubMed] [Google Scholar]
- 32.Sperling R. I. 1998. The effects of dietary n-3 polyunsaturated fatty acids on neutrophils. Proc. Nutr. Soc. 57: 527–534 [DOI] [PubMed] [Google Scholar]
- 33.Nelson S. D., Munger M. A. 2013. Icosapent ethyl for treatment of elevated triglyceride levels. Ann. Pharmacother. 47: 1517–1523 [DOI] [PubMed] [Google Scholar]
- 34.Kaminski D. A., Stavnezer J. 2006. Enhanced IgA class switching in marginal zone and B1 B cells relative to follicular/B2 B cells. J. Immunol. 177: 6025–6029 [DOI] [PubMed] [Google Scholar]
- 35.Olson M. V., Liu Y. C., Dangi B., Paul Zimmer J., Salem N., Jr, Nauroth J. M. 2013. Docosahexaenoic acid reduces inflammation and joint destruction in mice with collagen-induced arthritis. Inflamm. Res. 62: 1003–1013 [DOI] [PubMed] [Google Scholar]
- 36.Notley C. A., Brown M. A., Wright G. P., Ehrenstein M. R. 2011. Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells. J. Immunol. 186: 4967–4972 [DOI] [PubMed] [Google Scholar]
- 37.Nishimura S., Manabe I., Takaki S., Nagasaki M., Otsu M., Yamashita H., Sugita J., Yoshimura K., Eto K., Komuro I., et al. 2013. Adipose natural regulatory B cells negatively control adipose tissue inflammation. Cell Metab. 18: 759–766 [DOI] [PubMed] [Google Scholar]
- 38.Ulven S. M., Kirkhus B., Lamglait A., Basu S., Elind E., Haider T., Berge K., Vik H., Pedersen J. I. 2011. Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA, in healthy volunteers. Lipids. 46: 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xin W., Wei W., Li X. 2012. Effects of fish oil supplementation on inflammatory markers in chronic heart failure: a meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 12: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johansen O., Seljeflot I., Hostmark A. T., Arnesen H. 1999. The effect of supplementation with omega-3 fatty acids on soluble markers of endothelial function in patients with coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 19: 1681–1686 [DOI] [PubMed] [Google Scholar]
- 41.Itariu B. K., Zeyda M., Leitner L., Marculescu R., Stulnig T. M. 2013. Treatment with n-3 polyunsaturated fatty acids overcomes the inverse association of vitamin D deficiency with inflammation in severely obese patients: a randomized controlled trial. PLoS ONE. 8: e54634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawasaki K., Abe M., Tada F., Tokumoto Y., Chen S., Miyake T., Furukawa S., Matsuura B., Hiasa Y., Onji M. 2013. Blockade of B-cell-activating factor signaling enhances hepatic steatosis induced by a high-fat diet and improves insulin sensitivity. Lab. Invest. 93: 311–321 [DOI] [PubMed] [Google Scholar]
- 43.Winer D. A., Winer S., Shen L., Wadia P. P., Yantha J., Paltser G., Tsui H., Wu P., Davidson M. G., Alonso M. N., et al. 2011. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17: 610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeFuria J., Belkina A. C., Jagannathan-Bogdan M., Snyder-Cappione J., Carr J. D., Nersesova Y. R., Markham D., Strissel K. J., Watkins A. A., Zhu M., et al. 2013. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. USA. 110: 5133–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacks F. M., Bray G. A., Carey V. J., Smith S. R., Ryan D. H., Anton S. D., McManus K., Champagne C. M., Bishop L. M., Laranjo N., et al. 2009. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 360: 859–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turchini G. M., Nichols P. D., Barrow C., Sinclair A. J. 2012. Jumping on the omega-3 bandwagon: distinguishing the role of long-chain and short-chain omega-3 fatty acids. Crit. Rev. Food Sci. Nutr. 52: 795–803 [DOI] [PubMed] [Google Scholar]
- 47.Yanaba K., Bouaziz J. D., Matsushita T., Magro C. M., St Clair E. W., Tedder T. F. 2008. B-lymphocyte contributions to human autoimmune disease. Immunol. Rev. 223: 284–299 [DOI] [PubMed] [Google Scholar]
- 48.Mariño E., Grey S. T. 2012. B cells as effectors and regulators of autoimmunity. Autoimmunity. 45: 377–387 [DOI] [PubMed] [Google Scholar]
- 49.Karlsson E. A., Beck M. A. 2010. The burden of obesity on infectious disease. Exp. Biol. Med. (Maywood). 235: 1412–1424 [DOI] [PubMed] [Google Scholar]
- 50.Baumgarth N., Herman O. C., Jager G. C., Brown L., Herzenberg L. A. 1999. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc. Natl. Acad. Sci. USA. 96: 2250–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cesena F. H., Dimayuga P. C., Yano J., Zhao X., Kirzner J., Zhou J., Chan L. F., Lio W. M., Cercek B., Shah P. K., et al. 2012. Immune-modulation by polyclonal IgM treatment reduces atherosclerosis in hypercholesterolemic apoE-/- mice. Atherosclerosis. 220: 59–65 [DOI] [PubMed] [Google Scholar]
- 52.Werwitzke S., Trick D., Kamino K., Matthias T., Kniesch K., Schlegelberger B., Schmidt R. E., Witte T. 2005. Inhibition of lupus disease by anti-double-stranded DNA antibodies of the IgM isotype in the (NZB x NZW)F1 mouse. Arthritis Rheum. 52: 3629–3638 [DOI] [PubMed] [Google Scholar]
- 53.de Faire U., Frostegard J. 2009. Natural antibodies against phosphorylcholine in cardiovascular disease. Ann. N. Y. Acad. Sci. 1173: 292–300 [DOI] [PubMed] [Google Scholar]
- 54.Kroese F. G., Butcher E. C., Stall A. M., Lalor P. A., Adams S., Herzenberg L. A. 1989. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int. Immunol. 1: 75–84 [DOI] [PubMed] [Google Scholar]
- 55.Kim W., Fan Y. Y., Barhoumi R., Smith R., McMurray D. N., Chapkin R. S. 2008. N-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J. Immunol. 181: 6236–6243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams J. A., Batten S. E., Harris M., Rockett B. D., Shaikh S. R., Stillwell W., Wassall S. R. 2012. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys. J. 103: 228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaikh S. R., Teague H. 2012. N-3 fatty acids and membrane microdomains: from model membranes to lymphocyte function. Prostaglandins Leukot. Essent. Fatty Acids. 87: 205–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaikh S. R. 2012. Biophysical and biochemical mechanisms by which dietary n-3 polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. J. Nutr. Biochem. 23: 101–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teague H., Ross R., Harris M., Mitchell D. C., Shaikh S. R. 2013. DHA-fluorescent probe is sensitive to membrane order and reveals molecular adaptation of DHA in ordered lipid microdomains. J. Nutr. Biochem. 24: 188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajamoorthi K., Petrache H. I., McIntosh T. J., Brown M. F. 2005. Packing and viscoelasticity of polyunsaturated omega-3 and omega-6 lipid bilayers as seen by (2)H NMR and X-ray diffraction. J. Am. Chem. Soc. 127: 1576–1588 [DOI] [PubMed] [Google Scholar]
- 61.Mills K. H. 2011. TLR-dependent T cell activation in autoimmunity. Nat. Rev. Immunol. 11: 807–822 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.