Abstract
Cell death-inducing DFF45-like effector b (Cideb), an endoplasmic reticulum (ER)- and lipid droplet (LD)-associated protein, has been shown to play a critical role in maintaining hepatic lipid homeostasis by promoting the lipidation and maturation of VLDL particles. Here, we observed that Cideb is expressed in the jejunum and ileum sections of the small intestine, and its expression was induced by high-fat diet. Intragastric gavage with lipids resulted in the retention of lipids in the intestine in Cideb-deficient mice. In addition, we observed that mice with Cideb deficiency exhibited reduced intestinal chylomicron-TG secretion and increased lipid accumulation in the enterocytes. The sizes of chylomicrons secreted from the small intestine of Cideb-deficient mice were also smaller than those from wild-type mice. Furthermore, the overexpression of Cideb increased TG secretion and reduced lipid accumulation in the enterocyte-like Caco-2 cells. In addition, we proved that Cideb was localized to the ER and LDs and could interact with ApoB48 in Caco-2 cells. Overall, these data revealed that Cideb plays an important role in controlling intestinal chylomicron lipidation.
Keywords: lipid droplet, lipid absorption, cell death-inducing DNA fragmentation factor-α-like effector b
The intestinal enterocytes absorb dietary lipids and export them as TG-rich chylomicrons, which are subsequently hydrolyzed in the circulation to provide fatty acids for other tissues (1). Chylomicrons contribute to the circulating lipids during the postprandial state (2). A key structural component of chylomicrons is the huge, hydrophobic, nonexchangeable apolipoprotein B, and other exchangeable apolipoproteins are also found in chylomicrons. Similar to VLDL assembly in hepatocytes, chylomicron assembly occurs via a two-step process in the secretory pathway (3, 4). The intestinal assembly of chylomicrons is initiated by the lipidation of ApoB by microsomal TG transfer protein (MTP) (5–7) in the endoplasmic reticulum (ER) of the enterocytes to form prechylomicron particles. Subsequently, prechylomicrons are transported from the ER to the Golgi apparatus to be lipidated by the incorporation of neural lipids before secretion (8, 9). Levels of serum chylomicrons have been connected to the development of metabolic disorders (10). However, the molecular mechanisms governing chylomicron lipidation and maturation remain elusive.
Cell death-inducing DFF45-like effector (CIDE) family members, Cidea, Cideb, and Fsp27 (Cidec in human), have been shown to play crucial roles in regulating various aspects of lipid metabolism (11, 12). Although Cidea is expressed at high levels in brown adipose tissue (BAT) (11) and Cidec is highly enriched in white adipose tissue (WAT) and BAT (13, 14), Cideb is more abundant in liver, kidney, small intestine, and colon (15). Mice with deficiency in either Cidea, Cideb, or Fsp27 exhibit a lean phenotype, increased energy expenditure, enhanced insulin sensitivity, and resistance to high-fat diet (HFD)-induced obesity, insulin resistance, and fatty liver formation (11, 13–15).
We have demonstrated that Cideb is localized to the surface of lipid droplets (LDs) and the ER, and promotes the lipidation of VLDL through interactions with ApoB in the liver (16, 17). In addition, Cideb is an important regulator of cholesterol homeostasis in the liver (18). Cideb-deficient mice exhibit decreased plasma TG and NEFA (15), as well as altered expression of genes involved in various metabolic and signaling networks (18). Interestingly, the livers of Cideb-deficient mice exhibit higher levels of TG accompanied by lower levels of VLDL secretion under fasting conditions. However, the role of Cideb in intestinal lipid metabolism remains unclear. Here, we demonstrate that Cideb is highly enriched in the jejunum and ileum of the small intestine and promotes lipid secretion in the small intestine by facilitating the lipidation and assembly of chylomicrons.
MATERIALS AND METHODS
Animals and tissue collection
Mice were handled according to the Care and Use of Laboratory Animals guideline, and the experimental protocols were approved by the animal ethical committee of the Fourth Military Medical University. Cideb-deficient mice were generated as previously described (15), and animals were maintained under a 12 h light/12 h dark cycle with ad libitum access to water and chow diet. Before tissue collection, animals were fasted for 4 h prior to anesthetization. Tissues were collected and fresh-frozen in liquid nitrogen. To analyze the distribution of Cideb, the intestine was cut into 5–6 cm segments from the pylorus to cecum. Intestinal segments of the duodenum, jejunum, and ileum were stored at −80°C before protein analyses or directly fixed in 4% formaldehyde solution to produce paraffin sections. Human jejunum samples were collected from patients without metabolic diseases from Xijing Hospital, the first affiliated hospital of the Fourth Military Medical University.
Immunohistochemistry and immunofluorescence
The anti-human Cideb monoclonal antibody (XA027) was generated by Dr. Boquan Jin (Department of Immunology, Fourth Military Medical University, China). Procedures for immunohistochemistry were essentially the same as those described previously (19). Briefly, the primary antibodies (1:200, anti-Cideb mAb) and negative control serum were applied to the 3 μm sections and incubated for 24 h at 4°C. The slides were then washed and incubated for 30 min with biotinylated anti-mouse IgG (GE Healthcare, Piscataway, NJ). After washing with PBS, the sections were incubated in streptavidin peroxidase conjugate for 45 min. After a final wash with PBS, the peroxidase activity was revealed by incubating the specimens in 3,3′-diaminobenzidine tetrahydrochloride solution containing 0.03% hydrogen peroxide. The slides were counter-stained with hematoxylin for 30 s before being examined and photographed under an Olympus BX51 microscope.
For immunofluorescence analysis, Caco-2 cells cultured on glass coverslips were fixed with 4% paraformaldehyde in PBS (pH 7.4). Fixed cells were washed twice with ice-cold PBS, incubated with PBS containing 0.1% Triton X-100 for 10 min, and washed three times with PBS. After blocking in BSA-blotting buffer (1% BSA, 0.1% Tween 20 in PBS) for 30 min, cells were incubated in BSA-blotting buffer containing anti-hemagglutinin (HA) antibody (Santa Cruz, USA) or anti-ApoB antibody (ab20737, 1:100; Abcam) in a humidified chamber overnight at 4°C. The cells were washed and incubated with secondary antibodies for 1 h. After washing, the slides were mounted using mounting medium with DAPI (4’,6-Diamidino-2-phenylindole) and photographed with an Olympus BX71 immunofluorescence microscope.
Western blot analysis
Cells or tissues were lysed in lysis buffer [25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 2 mM EDTA, 1% sodium deoxycholate, 0.5% SDS, 1% Triton X-100, and protease inhibitor], and soluble lysates were collected by centrifugation at 12,000 g for 30 min. The protein concentrations were measured using the Bio-Rad kit (Bio-Rad Laboratories, USA). Approximately 50–100 μg of protein was separated by SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membrane, and probed with the desired antibodies for visualization. β-Tubulin (antibody from Sigma, USA) was used as a loading control.
Oil Red O staining
Male wild-type and Cideb-deficient mice were fasted overnight, and 10 ml/kg olive oil was administered by gavage. Mice were euthanized 6 h after intragastric gavage, and the proximal jejunum was immediately removed and immersed in PBS containing 10% sucrose and embedded in OCT compound. Serial sections (10 μm in thickness) were made and stained with Oil Red O as well as counter-stained with hematoxylin.
Effect of Triton WR-1339 on the increase of postprandial serum TG
The postprandial increase in serum TG was evaluated after intravenous injection of Triton WR-1339, which blocks lipolysis of circulating lipoproteins. The male wild-type and Cideb-deficient mice (n = 6 per group) were fasted overnight and injected with Triton WR-1339 (Sigma; 12.5 mg/100 μl PBS per mouse) followed by gavage with an intragastric fat bolus containing olive oil (10 ml/kg). Blood samples were collected from the tail vein 1, 2, 3, and 4 h after the Triton WR-1339 injection. The serum TG levels were then determined as previously described (16), and ApoB levels were determined by Western blot.
Recombinant adenovirus generation
The pAdEasy-1 system (Stratagene) was used to create adenovirus as previously described (16). We generated pShuttle-CMV-Cideb by inserting HA-tagged full-length Cideb into pShuttle-CMV vector. After linearization with Pme1, pAdEasy-Cideb was generated by allowing pShuttle-CMV-Cideb to recombine with pAdEasy-1 in BJ5183 cells. Correct recombinants were selected, and the pAdEasy-Cideb was linearized by Pac1 and transfected into Ad293 cells to generate adenoviral particles. Recombinant viruses were propagated in HEK-293 cells, purified, and titered by standard methods as described elsewhere (16). The corresponding viruses were named Ad-Cideb. Recombinant adenovirus containing the green fluorescent protein (GFP) gene was used as a control adenovirus.
Caco-2 cell culture and TG secretion
Caco-2 human cell lines were purchased from the ATCC (USA) and were cultured in DMEM supplemented with 10% FBS (Invitrogen, USA) and penicillin/streptomycin. For experiments, Caco-2 cells were seeded onto transwell inserts (Corning, USA), and the medium was changed every 2–3 days for 3 weeks to induce the differentiation of Caco-2 cells into enterocyte-like cells that could secret chylomicrons. The differentiated Caco-2 cells were infected with the adenoviruses containing Cideb or GFP for 24 h. To measure TG secretion, DMEM containing 100 μM oleic acid (OA) (Sigma, USA) and 1 μCi [3H]labeled OA (Amersham Pharmacia Biotech) per well was added into the atypical side of differentiated Caco-2 cells. At the indicated time points, the basolateral side medium was collected, and lipids were extracted by the Folch method and separated by TLC (20). The TG fractions were scraped from TLC plates, collected in scintillation vials, and resuspended in 10 ml of liquid scintillation solution (Perkin-Elmer, USA). The radioactivity in each vial was measured with a scintillation counter. For ApoB secretion, 2 ml of serum-free medium was put on the basolateral side, and the ApoB concentration in the medium was measured using a commercial ELISA kit (Abnova, USA).
Analysis of intestinal TG content
The wild-type and Cideb-deficient mice were fasted overnight and received an intragastric gavage of 10 ml/kg olive oil. After 2 h, mice were euthanized, and the intestinal mucosa (jejunum), which consisted mostly of epithelial cells, was harvested by blunt light scraping. Lipids in the intestinal mucosa were extracted (21). Briefly, after homogenization of the mucosa with PBS, a mixture of hexane/isopropanol (3:2) was added, and then the mucosa samples were incubated for 30 min with occasional mixing. The upper part containing lipids was removed to a new glass tube. After evaporating the organic phase under nitrogen, lipids were dissolved in PBS supplemented with 2% Triton X-100. The levels of TGs were then determined using the Wako TG determination kit (Wako Chemical, Japan). The protein fractions were dried and dissolved in 1 M KOH, and the protein concentrations were measured using a Bio-Rad kit (Bio-Rad Laboratories). The TG contents were normalized to the protein concentrations.
Analysis of the size of chylomicrons
To measure the size of chylomicrons, we layered pooled aliquots of serum (200 μl) under 600 μl 0.15 M NaCl and subjected them to centrifugation at 117,320 g for 30 min in an ultracentrifuge. The chylomicron layer was removed and subjected to negative staining using aqueous uranyl acetate and visualized under a JEM-100SX electron microscope (22). The diameters of chylomicron particles were measured using the particle size analyzer, Zetasizer Nano ZS (Malvern, UK).
Coimmunoprecipitation assays
Twenty-four hours after infection with adenovirus carrying the indicated HA-tagged Cideb truncations, the cells were washed with ice-cold PBS and lysed in coimmunoprecipitation (CoIP) buffer [150 mM NaCl, 20 mM Tris-HCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, and proteinase inhibitor cocktails (pH 7.4)]. After centrifugation at 12,000 g for 30 min at 4°C, the supernatants were collected. A small proportion of the total lysate was used for the determination of the protein expression levels, and all of the remaining lysate was used for CoIP by incubation with the indicated antibodies and protein A/G agarose (Santa Cruz). The mixture was rotated overnight at 4°C. Following several washes with CoIP buffer, the beads were precipitated by centrifugation, and the proteins on the beads were analyzed by Western blot.
Statistics
Data are presented as means ± SEM. The Student’s t-test and Mann-Whitney U test were used for parametric and nonparametric data, respectively. All statistical analyses were performed using GraphPad Prism V4.0 (GraphPad Software, USA). The criterion for significance was set at P < 0.05.
RESULTS
Expression of Cideb in the small intestine
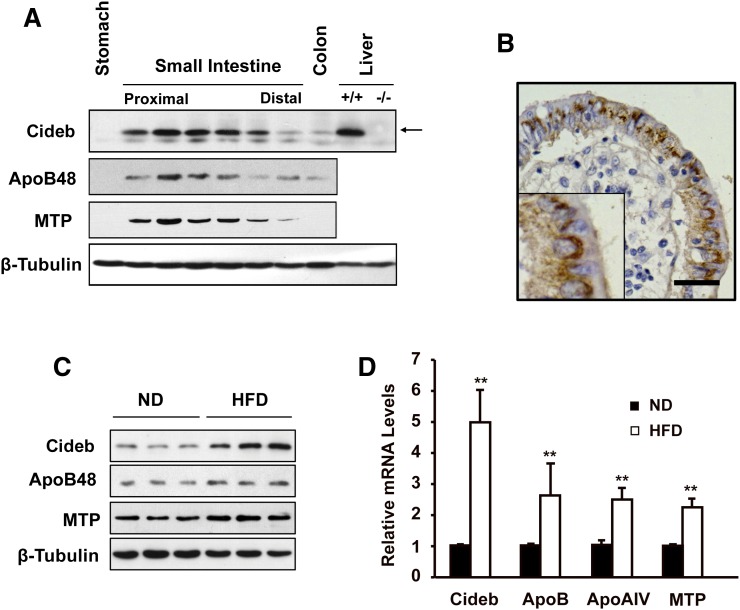
To determine the distribution of Cideb and its role in controlling lipid metabolism in the small intestine, we examined the expression levels of Cideb in different segments of the small intestine. Western blot analysis revealed that Cideb protein was detected in the small intestine, with the highest level in the jejunum, the major site for intestinal lipid absorption and secretion. A gradual decrease in Cideb expression was observed from the proximal to the distal side of the small intestine (Fig. 1A), which correlated with the distributions of MTP and ApoB48. Low levels of Cideb were detected in the colon, and no Cideb protein was detected in the stomach. Immunohistochemical analysis further confirmed the expression of Cideb in intestinal enterocytes (Fig. 1B). Interestingly, both the protein and mRNA levels of Cideb were significantly increased in the jejunums of mice fed with a HFD compared with those fed with a normal diet (ND) (Fig. 1C, D). As shown in Fig. 1C, ApoB48 and MTP, two chylomicron assembly-related proteins, were also increased in the jejunums of mice fed with a HFD. Consistently, levels of ApoB, ApoAIV, and MTP mRNA in the jejunums were also increased in mice fed with a HFD (Fig. 1D). These data suggest that Cideb is expressed at high levels in the small intestine and can be induced by HFD feeding.
Fig. 1.
Cideb is expressed in the small intestine and is induced by a HFD. A: Western blot analysis demonstrating the distribution of Cideb in the small intestine. The highest expression of Cideb was observed in the second and third sections, representing the jejunum, which is similar to the distribution of ApoB48 and MTP. The liver lysates of Cideb-deficient and wild-type mice were used as negative and positive controls, respectively. B: Cideb was detected in human enterocytes. Scale bar, 50 μm. C: Intestinal protein levels of Cideb, ApoB48, and MTP were induced in mice fed with a HFD. D: Intestinal mRNA levels of Cideb, ApoB, ApoAIV, and MTP increased in mice fed with a HFD (n = 3). Data are means ± SEM. **P < 0.01.
Increased lipid retention in the small intestine of Cideb-deficient mice
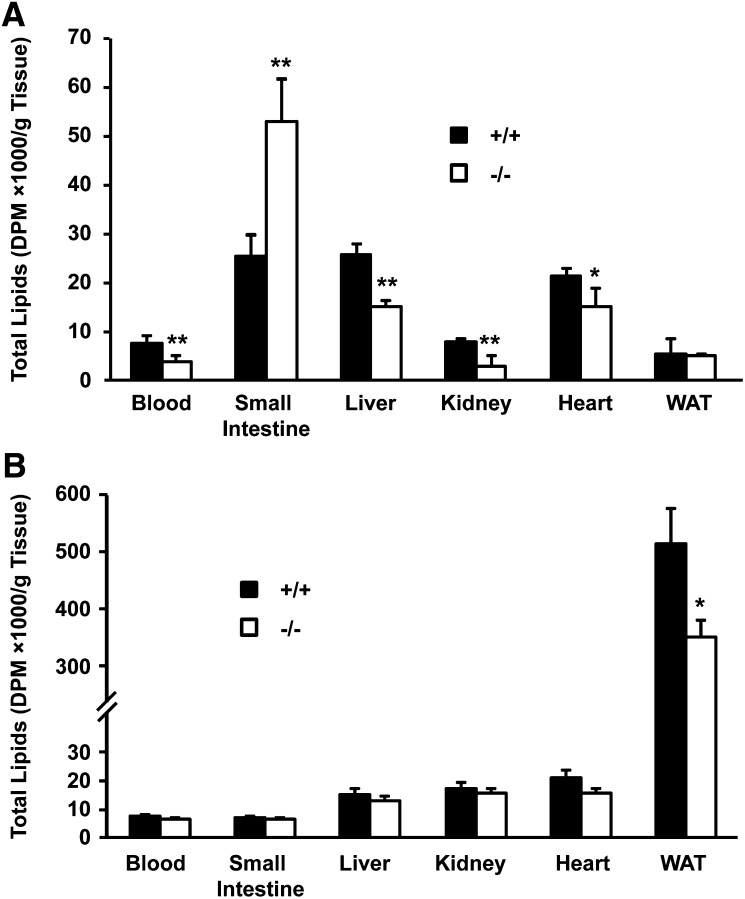
To determine the function of Cideb in intestinal lipid homeostasis, we fed animals with [3H]labeled OA by gavage and measured the levels of radio-labeled lipids in the intestine, blood, kidney, liver, heart and WAT. Four hours after gavage, the amount of radio-labeled lipids was significantly increased in the small intestine of Cideb-deficient mice compared with the levels in wild-type mice (Fig. 2A). Furthermore, the levels of radioactive lipids that accumulated in the blood, liver, kidney and heart were lower in Cideb-deficient mice (Fig. 2A). Forty-eight hours after gavage, the levels of radio-labeled lipids remained lower in the WAT of Cideb-deficient mice than in wild-type mice, but no difference in the levels of radioactive lipids was observed in the blood, small intestine, liver, kidney, or heart between the two phenotypes (Fig. 2B). The decreased levels of radioactive lipids in the blood, liver, kidney, and heart 4 h after gavage suggest slower rates of lipid absorption in Cideb-deficient mice, and the decreased levels of radioactive lipids in WAT 48 h after gavage suggest that the overall level of lipid absorption is lower in Cideb-deficient mice. These data indicate that the Cideb-deficient small intestine exhibits increased lipid retention after lipid gavage.
Fig. 2.
Increased lipid retention in the small intestine and decreased lipid accumulation in other tissues of Cideb-deficient mice after lipid gavage. The amounts of [3H]labeled lipids in various tissues were measured after intragastric gavage of 0.25 ml olive oil containing 5 μCi [3H]labeled OA. A: Increased accumulation of [3H]labeled lipids in the small intestine and reduced lipid accumulation in the blood, kidney, liver, heart, and WAT of Cideb-deficient mice 4 h after gavage. B: The amount of [3H]labeled lipids in the intestinal mucosa, blood, kidney, liver, heart, and WAT in Cideb-deficient mice 48 h after gavage. Data are means ± SEM. *P < 0.05, **P < 0.01.
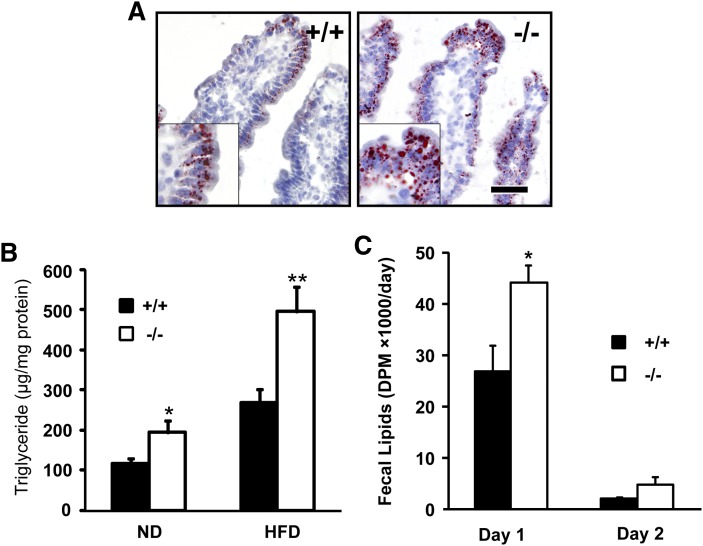
Next, we analyzed the TG content of the intestinal epithelium of wild-type and Cideb-deficient mice by Oil Red O staining and observed that intestinal epithelial cells of Cideb-deficient mice exhibited significantly stronger staining signals than those in wild-type mice (Fig. 3A). In addition, the amount of TG extracted from the intestinal mucosa was markedly elevated in Cideb-deficient mice compared with wild-type mice under both ND and HFD feeding conditions (1.6- and 1.8-fold, respectively; Fig. 3B). Next, we collected the feces of mice subjected to intragastric administration of [3H]labeled OA for 2 days and observed an increased amount of lipids in the feces of Cideb-deficient mice 1 day after treatment, suggesting increased lipid excretion in Cideb-deficient mice (Fig. 3C). These results suggest that deficiency of Cideb leads to the retention of lipids in the enterocytes and increased lipid excretion.
Fig. 3.
Cideb-deficient mice exhibited increased lipid accumulation in the small intestine and lipid excretion. A: Oil Red O staining demonstrating increased lipid accumulation in the intestinal mucosa of Cideb-deficient mice. Mice received an intragastric load of 10 ml/kg olive oil by gavage after overnight fasting. B: Increased levels of TG in the small intestine of Cideb-deficient mice under either ND or HFD conditions (n = 6). C: Increased lipid excretion in Cideb-deficient mice. Feces were collected over a period of 2 days after gavage with 10 ml/kg olive oil containing 5 μCi [3H]labeled OA. Radioactivity was measured in the extracted lipids (n = 4). Data are means ± SEM. *P <0.05, **P < 0.01.
Decreased intestinal lipid secretion in Cideb-deficient mice
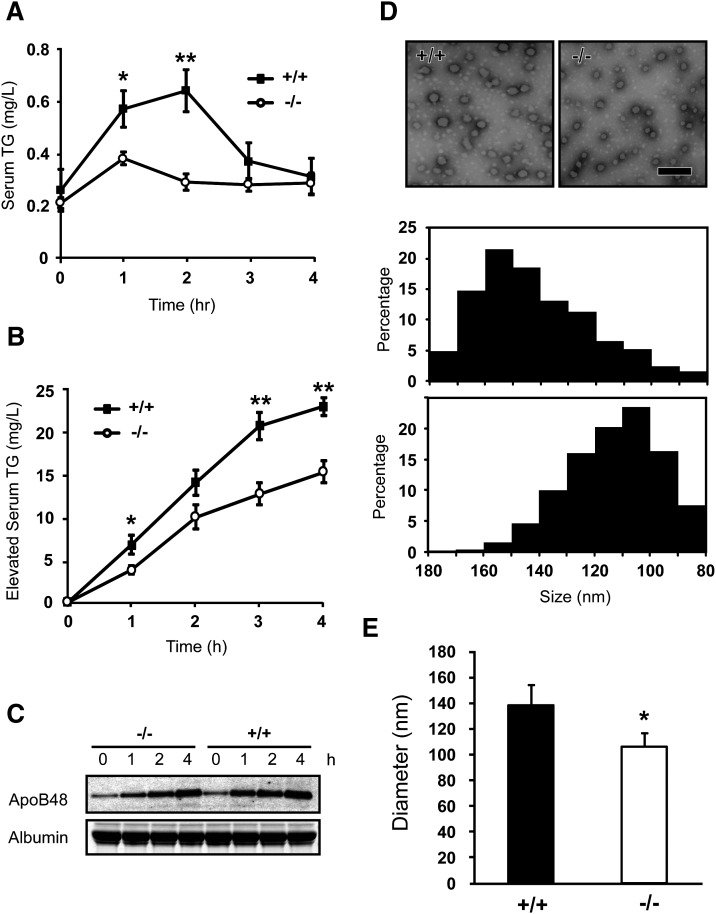
The increased intestinal lipid levels after lipid gavages suggest that Cideb may be involved in the regulation of intestinal lipid secretion. To test this hypothesis, we measured the plasma TG levels after lipid gavage. In wild-type mice, plasma TG levels were increased and became maximal after 2 h. However, plasma TG levels in Cideb-deficient mice were markedly lower than those in wild-type mice, with no obvious absorption peak observed (Fig. 4A). In the presence of Triton WR-1339, an inhibitor of serum LPL (23), the accumulation of serum TG was observed in both wild-type and Cideb-deficient mice, whereas the rate of accumulation was significantly lower in Cideb-deficient mice than in wild-type mice (Fig. 4B). However, the levels of plasma ApoB48 were similar in Cideb-deficient and wild-type mice (Fig. 4C). These data indicate that the Cideb-deficient small intestine exhibits reduced lipid secretion.
Fig. 4.
The levels of secreted lipids and the sizes of chylomicrons decreased in Cideb-deficient mice after lipid gavage. A: Reduced serum TG levels in Cideb-deficient mice (open cycles) compared with those in wild-type mice (filled cycles) after lipid gavage. B: Reduced lipid absorption in Cideb-deficient mice after lipid gavage. Mice were injected intravenously with Triton WR-1339 to block the lipolysis of circulating lipoproteins (n = 6). C: Similar protein levels of secreted ApoB48 in Cideb-deficient and wild-type mice. D, E: Reduction of chylomicron sizes in Cideb-deficient mice. Chylomicrons isolated from wild-type and Cideb-null mice after lipid gavage were observed under electron microscopy (D) and analyzed by particle size analyzer (E). Scale bars, 100 nm. Data are means ± SEM. *P < 0.05, **P < 0.01.
Next, we isolated the chylomicron-enriched fraction from the serum and analyzed the sizes of chylomicrons under electron microscopy. We observed that the sizes of chylomicrons from Cideb-deficient mice were significantly smaller than those from wild-type mice (Fig. 4D). After analysis, the peaks of the diameter distribution curves of chylomicrons isolated from Cideb-deficient mice were between 100 and 110 nm, whereas those from wild-type mice were between 150 and 160 nm (Fig. 4D, bottom panels). Using a particle size analyzer, we determined that the diameter of the chylomicron particles from Cideb-deficient mice was approximately 105 ± 11.2 nm, whereas the diameter of chylomicron particles from wild-type mice was 140 ± 8.6 nm (Fig. 4E). These data indicate that chylomicrons from Cideb-deficient mice are significantly smaller and contain fewer lipids, suggesting that Cideb deficiency leads to reduced chylomicron lipidation and lower lipid secretion. Consequently, the Cideb-deficient small intestine retains more lipids.
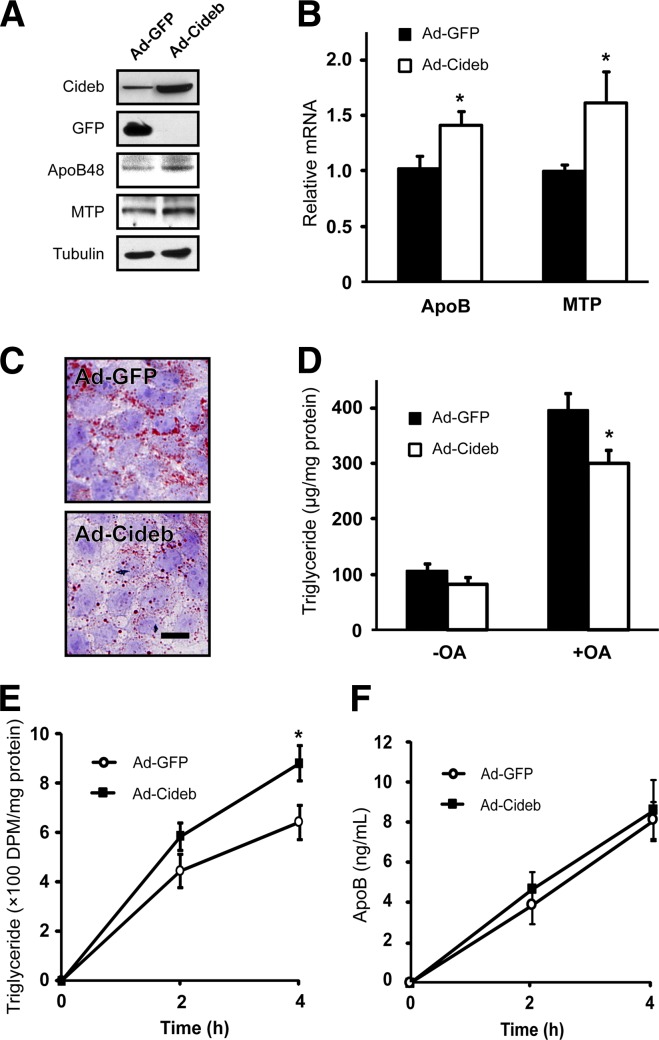
Cideb overexpression promotes lipid secretion in Caco-2 cells
To further demonstrate that Cideb promotes chylomicron lipidation and lipid secretion, we introduced Cideb into Caco-2 cells, which have been shown to acquire many features of absorptive intestinal enterocytes (24, 25), and evaluated the cellular lipid levels after overexpressing Cideb. After infection with adenovirus carrying Cideb, the protein levels of Cideb increased approximately 10-fold in Caco-2 cells (Fig. 5A). The mRNA and protein levels of ApoB48 and MTP increased slightly in Cideb-expressing Caco-2 cells compared with control cells expressing GFP (Fig. 5A, B). Interestingly, the amount of cellular lipid significantly decreased after overexpressing Cideb in Caco-2 cells (Fig. 5C). In addition, total TG levels were also decreased in cells expressing Cideb (Fig. 5D). Next, we measured the levels of TG secreted from Caco-2 cells incubated with [3H]labeled OA and observed that Caco-2 cells expressing Cideb secreted more [3H]labeled TG compared with cells expressing GFP (P < 0.05, Fig. 5E). However, the levels of secreted ApoB from Caco-2 cells expressing Cideb were similar to those from cells expressing GFP (Fig. 5F). These data suggest that Cideb overexpression indeed facilitates the lipidation of chylomicrons, resulting in increased lipid secretion and reduced TG retention in intestinal enterocytes.
Fig. 5.
Overexpression of Cideb enhanced lipid secretion and reduced lipid accumulation in Caco-2 cells. Caco-2 cells were infected with adenovirus to overexpress Cideb (Ad-Cideb) or GFP (Ad-GFP). The protein (A) and mRNA (B) levels of ApoB and MTP increased in Caco-2 cells overexpressing Cideb. C: Oil Red O staining demonstrating that Cideb overexpression could reduce intracellular lipid accumulation. D: Decreased intracellular TG levels in Caco-2 cells overexpressing Cideb when treated with OA (+OA). E: Increased TG secretion in Caco-2 cells overexpressing Cideb. Caco-2 cells were cultured in transwell plates and supplemented with serum-free DMEM containing 100 μM OA and 1 μCi [3H]labeled OA per well on the apical side. Two or four hours after incubation, lipids in the lateral side media were extracted and separated by TLC. The levels of radio-labeled TG were measured. F: Similar levels of ApoB were detected in the media from the lateral sides of Caco-2 cells overexpressing Cideb and GFP. Data are means ± SEM. *P < 0.05.
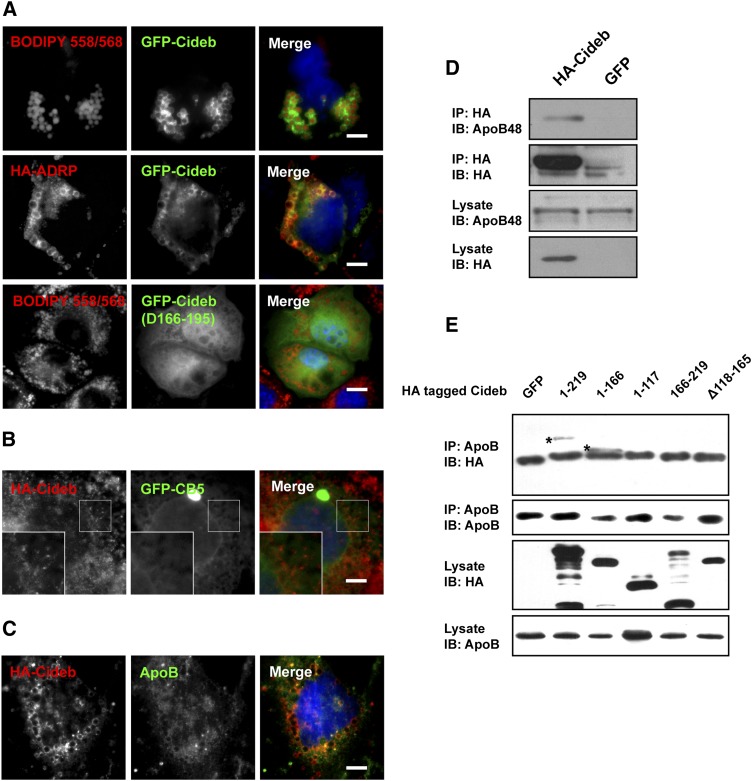
Cideb is localized to the ER and LDs and can interact with ApoB48 in Caco-2 cells
The recruitment of TGs during chylomicron maturation takes place in the ER/Golgi secretory compartments. Our previous data indicated that Cideb is localized to the ER and LDs and mediates the lipidation of VLDL by interacting with ApoB in the liver (16). To provide further insight into Cideb-regulated chylomicron lipidation, we determined the subcellular localization of Cideb in Caco-2 intestinal epithelial cells (Fig. 6A, B). The localization of Cideb in LDs was confirmed by introducing GFP-tagged Cideb into Caco-2 cells cultured in the presence of OA to promote TG synthesis and induce the formation of large LDs. GFP-tagged Cideb exhibited ring-like structures at the periphery of LDs (visualized by BODIPY 558/568 [D3835, Invitrogen, USA]) (Fig. 6A, top panel). Additional experiments revealed that Cideb colocalized with adipose differentiation-related protein (ADRP), a well-characterized LD-associated protein (Fig. 6A, middle panel). However, Cideb with deletion of amino acid (aa) 166-195 (Δ166-195) exhibited a diffused staining pattern (Fig. 6A, bottom panel), indicating that aa166-195 of Cideb is the ER- and LD-targeting domain. The subcellular localization of Cideb on the ER was further confirmed by overexpression of HA-tagged Cideb and the ER-specific marker GFP-cytochrome B5 (CB5) in Caco-2 cells (Fig. 6B). Cideb could colocalize with CB5 and exhibited intensive perinuclear staining and formed intensive granular structures along GFP-CB5 networks. These data suggest that Cideb is predominantly localized to the ER and LDs in Caco-2 cells.
Fig. 6.
Cideb was localized to the LD and ER and could interact with ApoB48. A: GFP-tagged Cideb (green) was localized on the surface of LDs (stained with BODIPY 558/568, red; upper panel) and colocalized with ADRP (red; middle panel). Cideb protein with deletion of aa166-195 (Δ166-195) exhibited a diffused staining pattern (bottom panel). Scale bars, 5 μm. B: HA-tagged Cideb (HA-Cideb) (red) formed granular structures along the ER network, overlapping with the ER-specific protein CB5 (green) in Caco-2 cells. Scale bars, 5 μm. C: Cideb (red) colocalized with ApoB (green) in ER networks. Scale bars, 5 μm. D: Cideb could interact with ApoB48. Caco-2 cells were infected with adenovirus expressing either HA-Cideb or GFP, and Cideb proteins were immunoprecipitated with anti-HA antibodies. ApoB48 proteins in the immunoprecipitates were detected with anti-ApoB antibodies. E: Amino acids 118-165 of Cideb mediated its interaction with ApoB48. Caco-2 cells were transfected with adenoviruses expressing HA-tagged Cideb truncations. ApoB48 proteins were immunoprecipitated with anti-ApoB antibodies, and the coimmunoprecipitated Cideb proteins were detected with anti-HA antibodies. IP, immunoprecipitation; IB, immunoblotting.
In the liver, Cideb can interact with ApoB to mediate the lipidation of VLDL (16). Here, we demonstrated that Cideb can interact with ApoB48 in intestinal epithelial cells. As shown in Fig. 6C, the distribution of Cideb was similar to that of ApoB48 in Caco-2 cells. To evaluate the interaction between Cideb and ApoB48, Caco-2 cells were infected with adenovirus carrying HA-tagged Cideb. Cideb was immunoprecipitated with an anti-HA antibody; and we observed that ApoB48 could be coimmunoprecipitated with Cideb (Fig. 6D). To determine the region(s) of Cideb that mediate its interaction with ApoB48, Caco-2 cells were infected with adenovirus carrying HA-tagged Cideb truncations. ApoB48 was immunoprecipitated by an anti-ApoB antibody. We observed that Cideb with deletion of aa118-165 could not be coimmunoprecipitated with ApoB48 (Fig. 6E), suggesting that aa118-165 of Cideb mediates its interaction with ApoB48. These data suggest that Cideb could interact with ApoB48 in the intestinal epithelium.
DISCUSSION
Intestinal enterocytes play important roles in lipid homeostasis by controlling lipid absorption from the intestinal lumen and lipid secretion into the lymphatics in the form of chylomicrons. Here, we observed that Cideb is expressed at high levels in mouse and human small intestine, and its deficiency results in reduced intestinal lipid secretion and increased lipid retention after lipid gavage. Furthermore, the overexpression of Cideb in enterocyte-like Caco-2 cells promotes lipid secretion. These data identify Cideb as a novel and important regulator in chylomicron secretion.
Cideb protein was detected in the entire small intestine with the highest expression in jejunum, the major site for intestinal lipid absorption and secretion. Specific and increased levels of Cideb were also detected in human intestinal enterocytes. Interestingly, intestinal levels of Cideb, as well as proteins that are important in lipid secretion (ApoB, ApoAIV, and MTP), increased in animals fed with a HFD. The coordinated increase in the expression of these molecules may help to effectively enhance chylomicron production and lipid secretion under HFD conditions.
Using the intragastric lipid gavage method, we have demonstrated that Cideb-deficient mice exhibit increased intestinal lipid retention and reduced plasma TG levels. Intravenous injection of Triton WR-1339, which blocks lipolysis of circulating lipoproteins, demonstrated that the rate of TG secretion of Cideb-deficient mice was lower than that of wild-type mice. Quantitative analysis of plasma chylomicron sizes indicated that Cideb-deficient enterocytes secreted smaller sized chylomicrons than wild-type enterocytes. However, the plasma levels of ApoB48 were similar between the two phenotypes after lipid gavage. Finally, we observed increased TG secretion in enterocyte-like Caco-2 cells overexpressing Cideb. Overall, these data strongly indicate that Cideb controls the lipidation and maturation of chylomicrons in small intestine.
We have previously reported that Cideb is localized to the ER and LDs and interacts with ApoB in the liver to control the lipidation of ApoB-containing VLDL particles (16). Here, we proved that Cideb is also localized to the ER and LDs in enterocyte-like Caco-2 cells and colocalized with ApoB48. Furthermore, we found that Cideb could interact with ApoB48 in Caco-2 intestinal epithelial cells, and aa118-165 of Cideb mediated the interaction between Cideb and ApoB48. It is likely that Cideb-controlled chylomicron lipidation in the small intestine is mediated by Cideb’s interaction with ApoB48, consistent with its role in the liver.
The liver and small intestine are two major organs that produce TG-rich lipoproteins (VLDLs and chylomicrons, respectively) to control lipid homeostasis. Our previous and current research reveals that Cideb is a crucial factor in controlling the lipidation and maturation of VLDLs and chylomicrons in the liver and small intestine, respectively, via its interaction with ApoB100 or ApoB48 (16).Thus, Cideb could serve as an important therapeutic target for treating celiac disease and malnutrition.
Acknowledgments
The authors thank Doctor Linkang Zhou in the laboratory of Peng Li at Tsinghua University for helpful assistance.
Footnotes
Abbreviations:
- aa
- amino acid
- ADRP
- adipose differentiation-related protein
- BAT
- brown adipose tissue
- CB5
- cytochrome B5
- CIDE
- cell death-inducing DNA fragmentation factor-α-like effector
- CoIP
- coimmunoprecipitation
- ER
- endoplasmic reticulum
- GFP
- green fluorescent protein
- HA
- hemagglutinin
- HFD
- high-fat diet
- MTP
- microsomal TG transfer protein
- ND
- normal diet
- OA
- oleic acid
- WAT
- white adipose tissue
This work was supported by grants from the National Natural Science Foundation of China [81100612 (L.Z.); 81070249, 31171132, and 81370958 (J.Y.); and 31030038 (P.L.)].
REFERENCES
- 1.Iqbal J., Hussain M. M. 2009. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 296: E1183–E1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gantz D., Bennett Clark S., Derksen A., Small D. M. 1990. Size and shape determination of fixed chylomicrons and emulsions with fluid or solid surfaces by three-dimensional analysis of shadows. J. Lipid Res. 31: 163–171 [PubMed] [Google Scholar]
- 3.Hamilton R. L., Wong J. S., Cham C. M., Nielsen L. B., Young S. G. 1998. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J. Lipid Res. 39: 1543–1557 [PubMed] [Google Scholar]
- 4.Cartwright I. J., Higgins J. A. 2001. Direct evidence for a two-step assembly of ApoB48-containing lipoproteins in the lumen of the smooth endoplasmic reticulum of rabbit enterocytes. J. Biol. Chem. 276: 48048–48057 [DOI] [PubMed] [Google Scholar]
- 5.Fisher E. A., Ginsberg H. N. 2002. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J. Biol. Chem. 277: 17377–17380 [DOI] [PubMed] [Google Scholar]
- 6.Rutledge A. C., Su Q., Adeli K. 2010. Apolipoprotein B100 biogenesis: a complex array of intracellular mechanisms regulating folding, stability, and lipoprotein assembly. Biochem. Cell Biol. 88: 251–267 [DOI] [PubMed] [Google Scholar]
- 7.White D. A., Bennett A. J., Billett M. A., Salter A. M. 1998. The assembly of triacylglycerol-rich lipoproteins: an essential role for the microsomal triacylglycerol transfer protein. Br. J. Nutr. 80: 219–229 [PubMed] [Google Scholar]
- 8.Siddiqi S. A., Siddiqi S., Mahan J., Peggs K., Gorelick F. S., Mansbach C. M., 2nd 2006. The identification of a novel endoplasmic reticulum to Golgi SNARE complex used by the prechylomicron transport vesicle. J. Biol. Chem. 281: 20974–20982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqi S. A., Mahan J., Siddiqi S., Gorelick F. S., Mansbach C. M., 2nd 2006. Vesicle-associated membrane protein 7 is expressed in intestinal ER. J. Cell Sci. 119: 943–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn J. S. 2008. Are we ready for a prospective study to investigate the role of chylomicrons in cardiovascular disease? Atheroscler. Suppl. 9: 15–18 [DOI] [PubMed] [Google Scholar]
- 11.Zhou Z., Yon Toh S., Chen Z., Guo K., Ng C. P., Ponniah S., Lin S. C., Hong W., Li P. 2003. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat. Genet. 35: 49–56 [DOI] [PubMed] [Google Scholar]
- 12.Xu L., Zhou L., Li P. 2012. CIDE proteins and lipid metabolism. Arterioscler. Thromb. Vasc. Biol. 32: 1094–1098 [DOI] [PubMed] [Google Scholar]
- 13.Nishino N., Tamori Y., Tateya S., Kawaguchi T., Shibakusa T., Mizunoya W., Inoue K., Kitazawa R., Kitazawa S., Matsuki Y., et al. 2008. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest. 118: 2808–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toh S. Y., Gong J., Du G., Li J. Z., Yang S., Ye J., Yao H., Zhang Y., Xue B., Li Q., et al. 2008. Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS ONE. 3: e2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J. Z., Ye J., Xue B., Qi J., Zhang J., Zhou Z., Li Q., Wen Z., Li P. 2007. Cideb regulates diet-induced obesity, liver steatosis, and insulin sensitivity by controlling lipogenesis and fatty acid oxidation. Diabetes. 56: 2523–2532 [DOI] [PubMed] [Google Scholar]
- 16.Ye J., Li J. Z., Liu Y., Li X., Yang T., Ma X., Li Q., Yao Z., Li P. 2009. Cideb, an ER- and lipid droplet-associated protein, mediates VLDL lipidation and maturation by interacting with apolipoprotein B. Cell Metab. 9: 177–190 [DOI] [PubMed] [Google Scholar]
- 17.Li X., Ye J., Zhou L., Gu W., Fisher E. A., Li P. 2012. Opposing roles of cell death-inducing DFF45-like effector B and perilipin 2 in controlling hepatic VLDL lipidation. J. Lipid Res. 53: 1877–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H., Song Y., Li F., Zhang L., Gu Y., Jiang L., Dong W., Ye J., Li Q. 2010. Identification of lipid droplet-associated proteins in the formation of macrophage-derived foam cells using microarrays. Int. J. Mol. Med. 26: 231–239 [DOI] [PubMed] [Google Scholar]
- 19.Yu M., Wang H., Zhao J., Yuan Y., Wang C., Li J., Zhang L., Li Q., Ye J. 2013. Expression of CIDE proteins in clear cell renal cell carcinoma and their prognostic significance. Mol. Cell. Biochem. 378: 145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509 [PubMed] [Google Scholar]
- 21.Hara A., Radin N. S. 1978. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 90: 420–426 [DOI] [PubMed] [Google Scholar]
- 22.Drover V. A., Ajmal M., Nassir F., Davidson N. O., Nauli A. M., Sahoo D., Tso P., Abumrad N. A. 2005. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J. Clin. Invest. 115: 1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borensztajn J., Rone M. S., Kotlar T. J. 1976. The inhibition in vivo of lipoprotein lipase (clearing-factor lipase) activity by triton WR-1339. Biochem. J. 156: 539–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy E., Mehran M., Seidman E. 1995. Caco-2 cells as a model for intestinal lipoprotein synthesis and secretion. FASEB J. 9: 626–635 [DOI] [PubMed] [Google Scholar]
- 25.Hussain M. M., Kancha R. K., Zhou Z., Luchoomun J., Zu H., Bakillah A. 1996. Chylomicron assembly and catabolism: role of apolipoproteins and receptors. Biochim. Biophys. Acta. 1300: 151–170 [DOI] [PubMed] [Google Scholar]