Abstract
The objective of this work was to evaluate the associations between levels of endogenous sex hormones in women at midlife and lipoprotein subclasses. One hundred and twenty women (68 late peri-/postmenopausal and 52 pre-/early perimenopausal) from the Study of Women’s Health Across the Nation (Pittsburgh site) were included. Lipoprotein subclasses were quantified using NMR spectroscopy. Participants (57.5% White and 42.5% Black) were 50.4 ± 1.9 years old. Adjusting for age, race, cycle day of blood draw, BMI, physical activity, and alcohol consumption, a negative correlation was found between estradiol (E2) and medium-small LDL particle (LDL-P) concentration (ρ = −0.19, P = 0.04). Further, E2 was positively correlated with HDL particle (HDL-P) size (ρ = 0.22, P = 0.02). For sex hormone binding globulin (SHBG), independent negative correlation was found with total small LDL-P concentration. SHBG was also positively correlated with LDL-P and HDL-P sizes (P < 0.05 for all). For free androgen index (FAI), positive correlations were found with concentrations of total VLDL particles, total LDL-Ps, and total small LDL-Ps. Additionally, FAI was negatively correlated with large HDL-P concentration, and HDL-P and LDL-P sizes (P < 0.05 for all). Lower levels of E2 and SHBG, and higher levels of FAI were associated with a more atherogenic profile of lipoprotein subclasses. Sex hormone levels at midlife may increase women’s risk of coronary heart disease.
Keywords: menopause, estradiol, free androgen index, sex hormone binding globulin
As women age, they are increasingly exposed to greater levels of CVD risk factors, including a poor lipid/lipoprotein profile (1–3). Previous studies reported adverse changes in lipoprotein profile around the time of menopause, including increases in total cholesterol, triglycerides, ApoB, and LDL cholesterol (LDL-C) (1, 4–7), which suggest a possible role of endogenous sex hormones. The reduction in plasma estradiol (E2) level that accompanies the menopausal transition has been suggested as a possible mechanism for this alteration of lipid profiles. This hypothesis was supported by the favorable influence of hormone therapy (HT) on lipids/lipoproteins (8, 9).
The conventional methods of measuring lipid/lipoprotein classes as VLDLs, LDL-C, and HDL cholesterol (HDL-C), provide concentrations of cholesterol carried by the lipoprotein particles rather than the concentration of the particles themselves (10). The amount of cholesterol per particle varies from person to person, simply because of differences in the relative amounts of cholesterol ester and triglycerides in the particle core, as well as the differences in particle diameter (11). The NMR spectroscopy method provides a direct measure of lipoprotein particle size, distribution, and concentrations, and therefore may provide a more accurate way to quantify CVD risk. High concentrations of small LDL particles (LDL-Ps) and small HDL particles (HDL-Ps) (12, 13), and total VLDL particles (VLDL-Ps) and large VLDL-Ps (13, 14) have been found to be associated with greater risk of CVD, while larger LDL- and HDL-P sizes are significantly associated with reduced incidence of CVD (14).
Most of the studies which evaluated the associations between endogenous sex hormones and lipid/lipoprotein levels were mainly limited to lipoprotein classes as measured by the conventional methods (15–21). Results from these studies were not consistent; some failed to report significant associations between lipids and sex hormones after adjusting for BMI (17, 18), while others showed significant associations of E2 (negative) and androgens (positive) with LDL-C and total cholesterol (15, 16, 18–20). Sex hormone binding globulin (SHBG) was positively associated with a high level of HDL-C independent of BMI (21).
The associations between levels of endogenous sex hormones and lipoprotein subclasses (size, distribution, and concentration), as measured by NMR in women transitioning through menopause, have never been evaluated before. To the best of our knowledge, only one study evaluated the associations between endogenous sex hormones and lipoprotein subclasses from NMR; however that study was based on postmenopausal women (22). The aim of this study was to assess the associations between levels of endogenous sex hormones [E2, SHBG, and free androgen index (FAI)] and lipoprotein subclasses from NMR in a sample of women undergoing the menopausal transition. We hypothesized that higher levels of E2 and SHBG would be associated with a less atherogenic profile (larger LDL- and HDL-P sizes and lower concentrations of total and large VLDL-Ps, total and small LDL-Ps, and small HDL-Ps), whereas FAI would be associated with a more atherogenic profile.
METHODS
Study participants
The Study of Women’s Health Across the Nation (SWAN) is an ongoing longitudinal multi-ethnic study of the biological, physical, psychological, and social changes during the menopausal transition. The study design has been previously reported (23). In brief, between 1996 and 1997, 3,302 participants aged 42–52 years were recruited from seven designated sites (Boston, MA; Detroit, MI; Oakland, CA; Los Angeles, CA; Pittsburgh, PA; Chicago, IL; and Newark, NJ). The eligibility criteria for the SWAN study were: 1) an intact uterus and at least one ovary; 2) not pregnant or breastfeeding; 3) at least one menstrual period within the past 3 months; and 4) no HT use within the past 3 months.
Participants of the current study were part of an ancillary study to SWAN at the Pittsburgh site where enrollment began between 1998 and 2003. Lipoprotein subclasses were available from blood samples (collected at SWAN core visits 1 through 5) of 120 participants who were selected from the larger Pittsburgh sample of participants who were not using HT (43.3% were premenopausal or early perimenopausal and 56.7% were late peri- or postmenopausal). Research protocols were approved by the University of Pittsburgh institutional review board and all the participants provided written informed consent prior to enrollment.
Study measures
Blood assays.
Phlebotomy was performed in the morning after 12 h of fasting. The participants were scheduled for venipuncture on days 2 to 5 of a spontaneous menstrual cycle. Two attempts were made to obtain a sample at days 2 to 5. If a timed sample could not be obtained (because menstrual cycles became less regular, samples tied to the early follicular phase were not feasible), a random fasting sample was taken within 90 days of the annual visit. Blood was maintained up to 1 h at 4°C until separated and then frozen (−80°C) and sent on dry ice to the Medical Research Laboratories. EDTA-treated plasma was used to analyze lipids and lipoprotein subclasses. Triglycerides were determined by enzymatic methods (Hitachi 747 analyzer; Boehringer Mannheim Diagnostics, Indianapolis, IN). HDL-C was isolated with heparin-2M manganese chloride. LDL-C was calculated by the Friedewald equation for all subjects with triglycerides <400 mg/dl (24). LDL-C was set to missing for those with triglycerides ≥400 mg/dl (only 1 participant). ApoA-1 and ApoB were measured by immunonephelometry (BN1A-100; Behring Diagnostics, Westwood, MA).
Lipoprotein subclasses were determined by an automated NMR spectroscopic assay (LipoScience Inc., Raleigh, NC), using the LipoProfile-3 algorithm (11, 25). Total particle concentrations of VLDLs and LDLs in nanomoles per liter units and HDLs in micromoles per liter units were determined by summing the concentrations of their respective subclasses [total VLDL-Ps as the sum of large, medium, and small VLDL-Ps; total LDL-Ps as the sum of large and small LDL-Ps and IDL particles; total HDL-Ps as the sum of large, medium, and small HDL-Ps], which were obtained from the measured amplitudes of the distinct lipid methyl group NMR signals they emit. Weighted-average VLDL-, LDL-, and HDL-P size was calculated by summing the diameter of each subclass multiplied by its relative mass percentage as estimated by the amplitude of its methyl NMR signal. Particle diameters and coefficients of variation (CVs) have been previously published for the NMR measures, with between-run CVs 7.1% or below for all particles except IDLs (13%) (25).
Endogenous sex hormones were measured at the University of Michigan Endocrine Laboratory using the Automated Chemilumisence System 180 automated analyzer (Bayer Diagnostics Corp., Norwood, MA). E2 was measured using a modified off-line Automated Chemilumisence System 180 (E2-6). The lower limit of detection (LLD) was between 1 and 7 pg/ml. The averaged inter- and intra-assay CVs were 10.6 and 6.4%, respectively. Serum testosterone concentration was evaluated with the Automated Chemilumisence System 180 total testosterone assay modified to increase precision in the low ranges. The LLD was between 2 and 2.2 ng/dl. The inter- and intra-assay CVs were 10.5 and 8.5%, respectively. SHBG was measured with a two-site chemiluminescent immunoassay. The LLD was between 1.9 and 3.2 nM. The inter- and intra-assay CVs were 9.9 and 6.1%, respectively. The FAI was used to estimate the amount of the free active (unbounded to SHBG) testosterone. FAI was calculated as (100 × testosterone)/(28.84 × SHBG) (26). Only E2 assays were conducted in duplicate. The arithmetic mean for the duplicate measures was calculated and reported (CVs of 3–12%). Hormone values below the LLD were replaced with a random value between zero and the LLD. For the current sample of SWAN women, only one participant had an E2 value that was below the LLD. None of the women had values for the other hormones that were below the LLD, and therefore the LLD was not an issue in the current analyses. Cycle day of blood draw was reported as days 2 to 5 (for regularly menstruating women) or outside of that period (for irregularly and nonmenstruating women).
Study covariates.
Weight and height were measured at each clinic visit. BMI was calculated as weight/height2. Race/ethnicity was self-reported and age, smoking status (current vs. past/never), physical activity, and alcohol use were derived from interviews administered during clinic visits. Physical activity was assessed via a modified Kaiser Permanente Health Plan Activity Survey (27). Alcohol use was reported as average weekly number of servings of beer, wine, liquor, or mixed drinks (none, <2 servings/week, ≥2 servings/week). Menopausal status was determined annually based on reports about frequency, regularity of menstrual bleeding, and use of HT as follow: 1) premenopause: monthly bleeding with no perceived change in cycle interval; 2) early perimenopause: monthly bleeding with a perceived change in cycle interval, but at least one menstrual period within the past 3 months; 3) late perimenopause: 3 consecutive months of amenorrhea; and 4) postmenopause: 12 consecutive months of amenorrhea. Both pre- and early perimenopausal women were combined in one group while late peri- and postmenopausal women were combined in a second group.
Statistical analysis
The distribution of each continuous variable was examined and variables were log transformed or categorized if the distribution was not normal. Two-sample t-test, two-sample Wilcoxon rank sum test, and chi-square test were utilized to compare participant characteristics between pre-/early perimenopausal and late peri-/postmenopausal.
Spearman correlations and partial Spearman correlations were used to assess the strength and direction of the associations between endogenous sex hormones and lipoprotein subclasses (separate model for each sex hormone with each lipoprotein subclass). Partial correlation coefficients were adjusted for covariates known to impact lipoprotein levels (physical activity, alcohol use, BMI) (18) in addition to age, cycle day of blood draw, and race. Quintiles of E2 were created and evaluated in relation to lipoprotein subclasses that were found to be significantly correlated with E2 level, using linear regression. Adjusted geometric means of lipoprotein subclasses were estimated for E2 quintiles and graphed. These graphs suggested a potential threshold effect of E2 at the third quintile (E2 ≥25.7 pg/ml), which was close to the median value of E2 among late peri-/postmenopausal participants in the current study. This threshold effect was evaluated by testing significant differences in lipoprotein subclasses among women with an E2 level below 25.7 pg/ml and those with an E2 ≥25.7 pg/ml. Effect modifications of menopausal status, BMI, and race on associations between sex hormones and lipoprotein subclasses were also evaluated. Analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Summary statistics of characteristics, lipoprotein subclasses, and endogenous sex hormones are presented for the total group and by menopausal status in Table 1. Participants were 50.4 (±1.9) years old with 42.5% being Black. About 57% of the study participants were late peri-/postmenopausal and 43% were pre-/early perimenopausal. In general, late peri-/postmenopausal participants had a worse lipoprotein profile compared with pre-/early perimenopausal participants. As expected, late peri-/postmenopausal participants had significantly lower levels of E2. They also had significantly higher levels of testosterone and marginally higher levels of FAI.
TABLE 1.
Characteristics and clinical measures of the study participants by menopausal status
| Total (N = 120) | Pre-/Early Peri (N = 52) | Late Peri/Post (N = 68) | P | |
| Age, years | 50.4 (1.9) | 50.0 (1.6) | 50.6 (2.1) | 0.11 |
| Black, n (%) | 51 (42.5) | 24 (46.2) | 27 (39.7) | 0.48 |
| Smoker, n (%) | 21 (17.7) | 8 (15.7) | 13 (19.1) | 0.63 |
| Alcohol consumption, n (%) | 0.20 | |||
| None | 71 (60.2) | 33 (66.0) | 38 (55.9) | |
| >2 servings/week | 21 (17.8) | 10 (20.0) | 11 (16.2) | |
| ≤2 servings/week | 26 (22.0) | 7 (14.0) | 19 (27.9) | |
| BMI, kg/m2 | 30.2 (7.5) | 29.7 (7.5) | 30.6 (7.6) | 0.53 |
| Physical activity | 7.8 (1.7) | 8.1 (1.5) | 7.6 (1.8) | 0.12 |
| HDL-C, mg/dla | 56.6 (14.9) | 56.7 (16.2) | 56.5 (14.1) | 0.94 |
| LDL-C, mg/dla | 124.9 (38.7) | 113.0 (33.3) | 133.7 (40.3) | 0.009 |
| ApoA, mg/dla | 165.0 (26.7) | 162.1 (27.7) | 167.2 (26.0) | 0.36 |
| ApoB, mg/dla | 114.0 (33.9) | 104.0 (27.2) | 121.5 (36.7) | 0.01 |
| Triglycerides, mg/dla | 107.0 (80.0,158.0) | 100.5 (77.0, 146.0) | 111.5 (80.0, 186.0) | 0.35 |
| VLDL-P concentration, nmol/l | ||||
| Total | 78.6 (38.2) | 69.4 (28.1) | 85.7 (43.2) | 0.01 |
| Large | 1.4 (0.3, 4.2) | 1.1 (0.3, 2.7) | 1.6 (0.3, 5.9) | 0.14 |
| Medium | 19.5 (8.9, 34.8) | 15.6 (7.8, 28.0) | 21.7 (10.0, 43.4) | 0.08 |
| Small | 49.9 (20.7) | 46.6 (15.6) | 52.4 (23.6) | 0.11 |
| LDL-P concentration, nmol/l | ||||
| Total | 1,180.6 (1,026.6, 1,420.9) | 1,154.2 (955.6, 1,297.9) | 1,231.9 (1,053.5, 1,458.5) | 0.006 |
| Large | 604.0 (478.0, 795.2) | 606.4 (480.8, 774.0) | 602.2 (476.0, 846.6) | 0.71 |
| Small (total) | 476.1 (291.3, 708.7) | 428.7 (291.3, 629.7) | 532.5 (290.6, 761.6) | 0.25 |
| Medium small | 90.8 (61.8, 134.2) | 86.8 (61.2, 124.4) | 108.0 (62.7, 147.0) | 0.23 |
| Very small | 373.3 (223.9, 570.5) | 354.9 (223.9, 517.7) | 408.8 (225.0, 599.8) | 0.32 |
| HDL-P concentration, umol/l | ||||
| Total | 32.7 (6.9) | 31.9 (6.6) | 33.3 (7.1) | 0.25 |
| Large | 6.8 (3.1) | 6.9 (2.7) | 6.7 (3.4) | 0.64 |
| Medium | 1.5 (0.3, 4.8) | 1.7 (0.6, 4.5) | 1.3 (0, 5.6) | 0.47 |
| Small | 22.9 (6.0) | 22.0 (0.6) | 23.7 (5.4) | 0.11 |
| VLDL-P size, nm | 48.1 (9.3) | 48.2 (9.8) | 48.0 (9.0) | 0.93 |
| LDL-P size, nm | 21.6 (0.7) | 21.7 (0.6) | 21.5 (0.7) | 0.28 |
| HDL-P size, nm | 9.2 (0.5) | 9.2 (0.4) | 9.1 (0.5) | 0.13 |
| E2, pg/ml | 31.8 (19.3, 83.1) | 48.4 (21.7, 109.0) | 27.4 (15.6, 47.9) | 0.007 |
| SHBG, nM | 37.0 (24.9, 51.2) | 36.9 (24.3, 51.2) | 37.4 (25.1, 51.3) | 0.77 |
| Testosterone, ng/dl | 32.2 (22.2, 49.15) | 29.7 (20.6, 36.1) | 38.8 (26.3, 53.9) | 0.007 |
| FAI | 3.2 (1.7, 6.1) | 2.5 (1.5, 5.1) | 3.6 (2.1, 6.6) | 0.11 |
Continuous variables were presented as mean (SD) or median (Q1, Q3) while categorical variables were presented as n (%).
Only available for 98 participants.
As hypothesized, E2 and SHBG were found to be significantly related to a less atherogenic profile, while FAI was found to be associated with a more atherogenic profile (Table 2). Significant negative correlations were found for E2 and medium-small LDL-P concentrations. Further, E2 was positively correlated with HDL-P size. For SHBG, independent negative correlations were found with concentrations of all small LDL-P subclasses. SHBG was also positively correlated with LDL-P and HDL-P sizes. Total testosterone was only negatively correlated with HDL-P size. For FAI, significant positive correlations were found with concentrations of total VLDL-P, total LDL-P, and all small LDL-P subclasses. Additionally, FAI was negatively correlated with large HDL-P concentration, and HDL-P and LDL-P sizes. The above results were independent of age, race, cycle day of the blood draw, BMI, physical activity, and alcohol consumption. Additional adjustment for menopausal status did not change the results except for medium-small LDL-P correlation with E2, which was slightly attenuated (ρ = −0.170, P = 0.07), (data not shown).
TABLE 2.
Partial correlations of lipoprotein subclasses and endogenous sex hormones
| E2 (pg/ml) | SHBG (nM) | Testosterone (ng/dl) | FAI | |||||
| Lipoprotein Subclasses | ρ | P | ρ | P | ρ | P | ρ | P |
| VLDL-P concentration, nmol/l | ||||||||
| Total | −0.049 | 0.61 | −0.128 | 0.18 | 0.141 | 0.14 | 0.218 | 0.02 |
| Large | −0.097 | 0.31 | −0.164 | 0.09 | 0.004 | 0.96 | 0.154 | 0.10 |
| Medium | −0.102 | 0.28 | −0.053 | 0.58 | 0.111 | 0.25 | 0.139 | 0.14 |
| Small | 0.045 | 0.64 | −0.041 | 0.67 | 0.175 | 0.07 | 0.150 | 0.11 |
| LDL-P concentration, nmol/l | ||||||||
| Total | −0.162 | 0.09 | −0.133 | 0.16 | 0.116 | 0.22 | 0.208 | 0.02 |
| Large | 0.032 | 0.74 | 0.119 | 0.31 | 0.003 | 0.97 | −0.112 | 0.24 |
| Small (total) | −0.143 | 0.14 | −0.193 | 0.04 | 0.104 | 0.28 | 0.249 | 0.008 |
| Medium small | −0.188 | 0.048 | −0.204 | 0.03 | 0.105 | 0.27 | 0.273 | 0.004 |
| Very small LDL | −0.133 | 0.17 | −0.188 | 0.048 | 0.120 | 0.21 | 0.244 | 0.01 |
| HDL-P concentration, umol/l | ||||||||
| Total | 0.043 | 0.66 | 0.026 | 0.78 | 0.000 | 0.99 | −0.081 | 0.39 |
| Large | 0.120 | 0.21 | 0.090 | 0.35 | −0.110 | 0.25 | −0.193 | 0.04 |
| Medium | −0.167 | 0.08 | 0.039 | 0.69 | −0.132 | 0.17 | −0.142 | 0.14 |
| Small | 0.052 | 0.59 | −0.032 | 0.74 | 0.083 | 0.39 | 0.030 | 0.75 |
| VLDL-P size, nm | −0.011 | 0.91 | −0.070 | 0.46 | −0.001 | 0.95 | 0.079 | 0.41 |
| LDL-P size, nm | 0.099 | 0.30 | 0.211 | 0.03 | −0.104 | 0.28 | −0.263 | 0.005 |
| HDL-P size, nm | 0.217 | 0.02 | 0.247 | 0.009 | −0.190 | 0.045 | −0.353 | 0.0001 |
All models adjusted for age, race, cycle day of the blood draw, BMI, physical activity, and alcohol consumption.
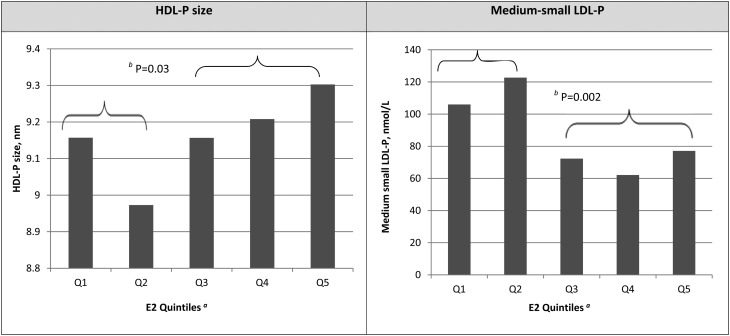
Geometric means of lipoprotein subclasses that were found to significantly correlate with E2 level are estimated and graphed in Fig. 1. Clear differences in HDL-P size and medium-small LDL-P concentration were observed at the third quintile of E2. Women with an E2 level <25.7 pg/ml had significantly smaller HDL-P size (β = −0.18, P = 0.03) and higher concentrations of medium-small LDL-Ps (β = 0.50, P = 0.002) compared with women with an E2 ≥25.7 pg/ml. Results did not change after further adjustment for menopausal status (data not shown). No significant interactions were found with menopausal status, BMI, or race and endogenous sex hormones in relation to lipoprotein subclasses after adjusting for multiple testing.
Fig. 1.
Estimated geometric means of selected lipoprotein subclasses by E2 quintiles. aQ1, E2 <15.6 pg/ml; Q2, 15.6 pg/ml ≤ E2 < 25.7 pg/ml; Q3, 25.7 pg/ml ≤ E2 < 42.05 pg/ml; Q4, 42.05 pg/ml ≤ E2 < 98.05 pg/ml; Q5, E2 ≥98.05 pg/ml. bP for comparisons between women with E2 ≥25.7 pg/ml and women with E2 <25.7 pg/ml.
DISCUSSION
Our results support the hypothesis that levels of endogenous sex hormones in women at midlife are associated with lipoprotein subclass profiles. We found that lower levels of E2 and SHBG and higher levels of FAI were independently associated with smaller/denser LDL-Ps and smaller LDL-P and/or HDL-P sizes in women at midlife. Further, higher levels of FAI were associated with higher total VLDL-P concentration. Previous reports showed that small LDL- and HDL-Ps (12, 13) and total VLDL- and large VLDL-Ps (13, 14) were associated with greater risk of CVD, while larger LDL- and HDL-P sizes were significantly associated with reduced incidence of CVD (14). Consistent with that, serum levels of triglycerides among the current study participants were found to be positively correlated with concentrations of lipoprotein subclasses related to CVD risk (total VLDL-Ps, small LDL-Ps, and small HDL-Ps) (data not shown). Taken together, our results suggest that the alterations in endogenous sex hormones that characterize the menopausal transition may render women’s lipoprotein profiles to be more atherogenic. This altered lipoprotein profile may in turn subject midlife women to a higher risk for coronary heart disease. Given the cross-sectional nature of the current report, future longitudinal studies are needed to evaluate this possibility.
Previous studies demonstrate adverse changes in lipoprotein profile around the time of menopause (1, 4–7). In these studies, decreases in large HDL-P (e.g., HDL2-C) (1, 6, 28) and possible shift in LDL-P size toward a smaller denser phenotype (5, 28) have been shown to accompany the menopausal transition. The current findings of significant correlations between endogenous sex hormones and lipoprotein subclasses are in agreement with the above studies and suggest sex hormones as a possible mechanistic pathway for the alteration in lipoprotein profile around the time of menopause.
Several studies have evaluated associations between endogenous sex hormones and lipid levels as measured by the conventional methods (15–21). However, to the best of our knowledge, no previous study has assessed associations between endogenous sex hormones and lipoprotein subclasses from NMR spectroscopy in women transitioning through menopause. Kuller et al. (29) evaluated the relationships between circulating estrogens and lipoprotein lipids and apolipoproteins in 120 midlife women of the Pittsburgh Healthy Women Study. Serum E2 was found to be positively related to larger HDL subfractions (HDL2-C) as measured by precipitation procedure. Additionally, a decline in E2 between the perimenopause and postmenopause was reported and accompanied by a decrease in HDL2-C (a decrease in larger HDL-Ps). Results from HT studies were consistent with the above. Oral 17β-E2 (2 mg/day) for 6 weeks shifted HDL-Ps from HDL3-C to HDL2-C (large HDLs) in healthy women (30). Additionally, 12 weeks of 17β-E2 and medroxyprogesterone acetate significantly increased average HDL-P size by 4.2% by altering the concentrations of two HDL subclasses in a sample of postmenopausal women (31). Consistent with these studies, we also reported positive correlations between E2 and HDL-P size. Additionally, we found a negative correlation between E2 and medium-small LDL-Ps. Although this latter finding was not evaluated in Kuller et al. (29), it was consistent with the independent negative association between E2 and LDL-C that was reported in a sample of women undergoing the menopausal transition (15). Thus, it appears that higher E2, whether endogenously measured or exogenously administered, is associated with larger HDL-Ps, a favorable profile. Conversely, a loss of E2 appears associated with a shift to smaller more atherogenic HDL-P sizes. However, the effect of HT on CVD risk is still controversial.
A reduction in plasma estrogens enhances the activity of lipolytic enzymes, such as lipoprotein lipase and hepatic triglyceride lipase, which play critical roles in lipoprotein catabolism. Lipoprotein lipase catalyzes the hydrolysis of VLDL-C to IDL-C and HDL-C. Hepatic triglyceride lipase hydrolyzes the triglycerides in IDL-C to produce LDL-C, accelerates the conversion of LDL-Ps from large to small and converts HDL2-C to HDL3-C (smaller HDLs) (16, 32, 33). Postmenopausal women have been found to have higher hepatic lipase activity (32). In a cross-sectional analysis in Japan that included 20 premenopausal, 10 postmenopausal, 10 bilateral oophorectomized women, a negative correlation between postheparin plasma lipase and E2 levels was reported (16). We postulate that changes in lipolytic enzymes may be a mechanistic pathway by which menopause and E2 influence lipoprotein characteristics.
Our finding that SHBG was associated with favorable lipoprotein subclass profiles (positively correlated with LDL-P and HDL-P sizes and negatively correlated with total small LDL-P concentration and its fractions) was consistent with other studies conducted either in women transitioning through menopause (34) or in postmenopausal women (22, 35). Significant positive relationships between SHBG and HDL-C and HDL2-C were reported in 352 pre- and perimenopausal women (34). In 172 postmenopausal women from the Atherosclerosis Risk in Communities Study, higher SHBG was significantly associated with lower total cholesterol and LDL-C and higher HDL-C (35). Higher SHBG was also associated with smaller and fewer VLDLs, larger and fewer LDLs, and larger and more numerous HDL-Ps among postmenopausal women from the Multi-Ethnic Study of Atherosclerosis baseline examination (22).
SHBG binds with higher affinity to androgens than estrogens, and therefore it could be used as a marker of androgenicity (36). Hepatic triglyceride lipase is known to be stimulated by androgens. Therefore, high SHBG would result in low free androgen which maintains low hepatic triglyceride lipase activity (37). Suppressing hepatic triglyceride lipase would decelerate the conversion of LDL-Ps from large to small and HDL2-C to HDL3-C (smaller HDLs) (16, 32, 33). However, the reported results for SHBG did not change even after further adjustment for androgenicity, as measured by total testosterone (data not shown). It is also well-known that SHBG is negatively associated with BMI (38), and therefore could impact lipoprotein subclasses via this path. Importantly, the reported results for SHBG in the current study were independent of BMI. Taken together, this supports different pathways by which SHBG may impact lipoprotein profile. Additional research is needed to better understand the mechanistic pathways by which SHBG may alter the lipoprotein subclass profile in women at midlife.
Consistent with the literature, we reported an unfavorable lipoprotein profile with higher levels of FAI. Higher FAI was associated with higher cholesterol, triglycerides, and LDL-C and lower HDL-C in premenopausal women at midlife (38). In postmenopausal women with minimal to severe carotid atherosclerosis from the Atherosclerosis Risk in Communities Study, FAI was associated with increased LDL-C, total cholesterol, and triglycerides (35). As we indicated above, androgens may impact lipoprotein catabolism via their positive effect on hepatic triglyceride lipase enzyme (37).
The main limitations of the current study include the cross-sectional design that prevents us from assessing temporality of the evaluated associations. The small sample size is another limitation, which reduced the statistical power to detect significant interactions with menopausal status, BMI, or race. Hormone assays were collected during the early follicular phase of the menstrual cycle, which might not be optimum for all hormones that were measured. All our analyses were adjusted for cycle day. Biologically active testosterone was not measured directly, although our estimation of FAI is a valid measure of free testosterone (26).
Future studies should evaluate the longitudinal changes in these lipoprotein subclasses over the menopausal transition and define patterns of these changes. It will be crucial to link these changes over time to CVD events and subclinical measures of atherosclerosis to better understand the potential role of alteration of lipoprotein subclass profiles on CVD risk after menopause.
In conclusion, in women transitioning through menopause, lower levels of E2 and SHBG, and higher levels of FAI were independently associated with smaller/denser LDL-Ps and smaller LDL-P and/or HDL-P sizes. Further, higher levels of FAI were associated with more total VLDL-Ps. These results suggest that sex hormone oscillation at midlife may increase women’s risk of coronary heart disease.
Acknowledgments
The authors thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Abbreviations:
- CV
- coefficient of variation
- E2
- estradiol
- FAI
- free androgen index
- HDL-C
- HDL cholesterol
- HDL-P
- HDL particle
- HT
- hormone therapy
- LDL-C
- LDL cholesterol
- LDL-P
- LDL particle
- LLD
- lower limit of detection
- SHBG
- sex hormone binding globulin
- SWAN
- Study of Women’s Health Across the Nation
- VLDL-P
- VLDL particle
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Health Office of Research on Women’s Health (grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, and U01AG012495). SWAN Heart is supported by the National Heart, Lung, and Blood Institute Grants HL065581 and HL065591.
REFERENCES
- 1.Stevenson J. C., Crook D., Godsland I. F. 1993. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 98: 83–90 [DOI] [PubMed] [Google Scholar]
- 2.Kannel W. B., Hjortland M. C., McNamara P. M., Gordon T. 1976. Menopause and risk of cardiovascular disease: the Framingham study. Ann. Intern. Med. 85: 447–452 [DOI] [PubMed] [Google Scholar]
- 3.Lerner D. J., Kannel W. B. 1986. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am. Heart J. 111: 383–390 [DOI] [PubMed] [Google Scholar]
- 4.Matthews K. A., Crawford S. L., Chae C. U., Everson-Rose S. A., Sowers M. F., Sternfeld B., Sutton-Tyrrell K. 2009. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J. Am. Coll. Cardiol. 54: 2366–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikenoue N., Wakatsuki A., Okatani Y. 1999. Small low-density lipoprotein particles in women with natural or surgically induced menopause. Obstet. Gynecol. 93: 566–570 [DOI] [PubMed] [Google Scholar]
- 6.Li Z., McNamara J. R., Fruchart J. C., Luc G., Bard J. M., Ordovas J. M., Wilson P. W., Schaefer E. J. 1996. Effects of gender and menopausal status on plasma lipoprotein subspecies and particle sizes. J. Lipid Res. 37: 1886–1896 [PubMed] [Google Scholar]
- 7.Anderson K. M., Wilson P. W., Garrison R. J., Castelli W. P. 1987. Longitudinal and secular trends in lipoprotein cholesterol measurements in a general population sample. The Framingham Offspring Study. Atherosclerosis. 68: 59–66 [DOI] [PubMed] [Google Scholar]
- 8.Godsland I. F. 2001. Effects of postmenopausal hormone replacement therapy on lipid, lipoprotein, and apolipoprotein (a) concentrations: analysis of studies published from 1974–2000. Fertil. Steril. 75: 898–915 [DOI] [PubMed] [Google Scholar]
- 9.Hulley S., Grady D., Bush T., Furberg C., Herrington D., Riggs B., Vittinghoff E. 1998. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 280: 605–613 [DOI] [PubMed] [Google Scholar]
- 10.Lamon-Fava S., Herrington D. M., Reboussin D. M., Sherman M., Horvath K. V., Cupples L. A., White C., Demissie S., Schaefer E. J., Asztalos B. F. 2008. Plasma levels of HDL subpopulations and remnant lipoproteins predict the extent of angiographically-defined coronary artery disease in postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 28: 575–579 [DOI] [PubMed] [Google Scholar]
- 11.Otvos J. D., Jeyarajah E. J., Cromwell W. C. 2002. Measurement issues related to lipoprotein heterogeneity. Am. J. Cardiol. 90(Suppl): 22i–29i [DOI] [PubMed] [Google Scholar]
- 12.St-Pierre A. C., Ruel I. L., Cantin B., Dagenais G. R., Bernard P. M., Després J. P., Lamarche B. 2001. Comparison of various electrophoretic characteristics of LDL particles and their relationship to the risk of ischemic heart disease. Circulation. 104: 2295–2299 [DOI] [PubMed] [Google Scholar]
- 13.Freedman D. S., Otvos J. D., Jeyarajah E. J., Barboriak J. J., Anderson A. J., Walker J. 1998. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 18: 1046–1053 [DOI] [PubMed] [Google Scholar]
- 14.Mora S., Otvos J. D., Rifai N., Rosenson R. S., Buring J. E., Ridker P. M. 2009. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 119: 931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg G., Mesch V., Boero L., Sayegh F., Prada M., Royer M., Muzzio M. L., Schreier L., Siseles N., Benencia H. 2004. Lipid and lipoprotein profile in menopausal transition. Effects of hormones, age and fat distribution. Horm. Metab. Res. 36: 215–220 [DOI] [PubMed] [Google Scholar]
- 16.Wakatsuki A., Sagara Y. 1995. Lipoprotein metabolism in postmenopausal and oophorectomized women. Obstet. Gynecol. 85: 523–528 [DOI] [PubMed] [Google Scholar]
- 17.Cauley J. A., Gutai J. P., Kuller L. H., Powell J. G. 1990. The relation of endogenous sex steroid hormone concentrations to serum lipid and lipoprotein levels in postmenopausal women. Am. J. Epidemiol. 132: 884–894 [DOI] [PubMed] [Google Scholar]
- 18.Shelley J. M., Green A., Smith A. M., Dudley E., Dennerstein L., Hopper J., Burger H. 1998. Relationship of endogenous sex hormones to lipids and blood pressure in mid-aged women. Ann. Epidemiol. 8: 39–45 [DOI] [PubMed] [Google Scholar]
- 19.Haffner S. M., Newcomb P. A., Marcus P. M., Klein B. E., Klein R. 1995. Relation of sex hormones and dehydroepiandrosterone sulfate (DHEA-SO4) to cardiovascular risk factors in postmenopausal women. Am. J. Epidemiol. 142: 925–934 [DOI] [PubMed] [Google Scholar]
- 20.Wild R. A., Applebaum-Bowden D., Demers L. M., Bartholomew M., Landis J. R., Hazzard W. R., Santen R. J. 1990. Lipoprotein lipids in women with androgen excess: independent associations with increased insulin and androgen. Clin. Chem. 36: 283–289 [PubMed] [Google Scholar]
- 21.Haffner S. M., Katz M. S., Stern M. P., Dunn J. F. 1989. Association of decreased sex hormone binding globulin and cardiovascular risk factors. Arteriosclerosis. 9: 136–143 [DOI] [PubMed] [Google Scholar]
- 22.Vaidya D., Dobs A., Gapstur S. M., Golden S. H., Hankinson A., Liu K., Ouyang P. 2008. The association of endogenous sex hormones with lipoprotein subfraction profile in the Multi-Ethnic Study of Atherosclerosis. Metabolism. 57: 782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sowers M., Crawford S., Sternfed B., Morganstein D., Gold E. B., Greendale G., Evans D., Neer R., Matthews K., Sherman S., et al. 2000. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In Menopause: Biology and Pathology. R. A. Lobo, J. Kelsey, and R. Marcus, editors. Academic Press, New York, NY. 175–188. [Google Scholar]
- 24.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502 [PubMed] [Google Scholar]
- 25.Jeyarajah E. J., Cromwell W. C., Otvos J. D. 2006. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 26: 847–870 [DOI] [PubMed] [Google Scholar]
- 26.van den Beld A. W., de Jong F. H., Grobbee D. E., Pols H. A., Lamberts S. W. 2000. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J. Clin. Endocrinol. Metab. 85: 3276–3282 [DOI] [PubMed] [Google Scholar]
- 27.Ainsworth B. E., Sternfeld B., Richardson M. T., Jackson K. 2000. Evaluation of the kaiser physical activity survey in women. Med. Sci. Sports Exerc. 32: 1327–1338 [DOI] [PubMed] [Google Scholar]
- 28.Matthews K. A., Wing R. R., Kuller L. H., Meilahn E. N., Plantinga P. 1994. Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Arch. Intern. Med. 154: 2349–2355 [PubMed] [Google Scholar]
- 29.Kuller L. H., Gutai J. P., Meilahn E., Matthews K. A., Plantinga P. 1990. Relationship of endogenous sex steroid hormones to lipids and apoproteins in postmenopausal women. Arteriosclerosis. 10: 1058–1066 [DOI] [PubMed] [Google Scholar]
- 30.Walsh B. W., Li H., Sacks F. M. 1994. Effects of postmenopausal hormone replacement with oral and transdermal estrogen on high density lipoprotein metabolism. J. Lipid Res. 35: 2083–2093 [PubMed] [Google Scholar]
- 31.Tangney C. C., Mosca L. J., Otvos J. D., Rosenson R. S. 2001. Oral 17beta-estradiol and medroxyprogesterone acetate therapy in postmenopausal women increases HDL particle size. Atherosclerosis. 155: 425–430 [DOI] [PubMed] [Google Scholar]
- 32.Berg G. A., Siseles N., González A. I., Ortiz O. C., Tempone A., Wikinski R. W. 2001. Higher values of hepatic lipase activity in postmenopause: relationship with atherogenic intermediate density and low density lipoproteins. Menopause. 8: 51–57 [DOI] [PubMed] [Google Scholar]
- 33.Homma Y., Nakaya N., Nakamura H., Goto Y. 1985. Increase in the density of lighter low density lipoprotein by hepatic triglyceride lipase. Artery. 13: 19–31 [PubMed] [Google Scholar]
- 34.Longcope C., Herbert P. N., McKinlay S. M., Goldfield S. R. 1990. The relationship of total and free estrogens and sex hormone-binding globulin with lipoproteins in women. J. Clin. Endocrinol. Metab. 71: 67–72 [DOI] [PubMed] [Google Scholar]
- 35.Mudali S., Dobs A. S., Ding J., Cauley J. A., Szklo M., Golden S. H. Atherosclerosis Risk in Communities Study. 2005. Endogenous postmenopausal hormones and serum lipids: the atherosclerosis risk in communities study. J. Clin. Endocrinol. Metab. 90: 1202–1209 [DOI] [PubMed] [Google Scholar]
- 36.Haffner S. M., Valdez R. A., Morales P. A., Hazuda H. P., Stern M. P. 1993. Decreased sex hormone-binding globulin predicts noninsulin-dependent diabetes mellitus in women but not in men. J. Clin. Endocrinol. Metab. 77: 56–60 [DOI] [PubMed] [Google Scholar]
- 37.Bataille V., Perret B., Evans A., Amouyel P., Arveiler D., Ducimetière P., Bard J. M., Ferrières J. 2005. Sex hormone-binding globulin is a major determinant of the lipid profile: the PRIME study. Atherosclerosis. 179: 369–373 [DOI] [PubMed] [Google Scholar]
- 38.Sutton-Tyrrell K., Wildman R. P., Matthews K. A., Chae C., Lasley B. L., Brockwell S., Pasternak R. C., Lloyd-Jones D., Sowers M. F., Torréns J. I. SWAN Investigators. 2005. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation. 111: 1242–1249 [DOI] [PubMed] [Google Scholar]