Abstract
Lysophosphatidic acid (LPA) is a small ubiquitous lipid found in vertebrate and nonvertebrate organisms that mediates diverse biological actions and demonstrates medicinal relevance. LPA’s functional roles are driven by extracellular signaling through at least six 7-transmembrane G protein-coupled receptors. These receptors are named LPA1–6 and signal through numerous effector pathways activated by heterotrimeric G proteins, including Gi/o, G12/13, Gq, and Gs. LPA receptor-mediated effects have been described in numerous cell types and model systems, both in vitro and in vivo, through gain- and loss-of-function studies. These studies have revealed physiological and pathophysiological influences on virtually every organ system and developmental stage of an organism. These include the nervous, cardiovascular, reproductive, and pulmonary systems. Disturbances in normal LPA signaling may contribute to a range of diseases, including neurodevelopmental and neuropsychiatric disorders, pain, cardiovascular disease, bone disorders, fibrosis, cancer, infertility, and obesity. These studies underscore the potential of LPA receptor subtypes and related signaling mechanisms to provide novel therapeutic targets.
Keywords: lysophosphatidic acid, lysophospholipid, autotaxin, brain lipids, cancer, fibrosis, reproduction, obesity, pain, atherosclerosis
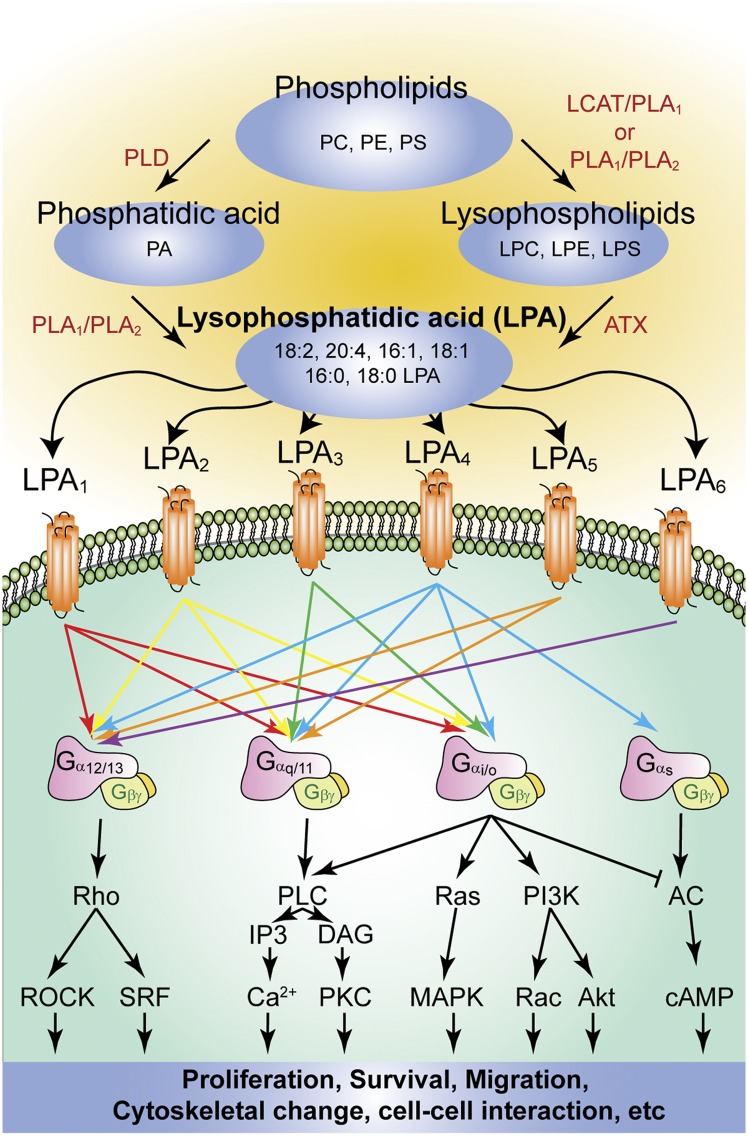
Lysophosphatidic acid (LPA) is a small glycerophospholipid (molecular mass: 430–480 Da) that acts as a potent extracellular signaling molecule through at least six cognate G protein-coupled receptors (GPCRs) in numerous developmental and adult processes involving virtually all vertebrate systems. All LPA molecules consist of a glycerol backbone connected to a phosphate head group and are commonly ester-linked to an acyl chain of varied length and saturation. These various chemical forms of LPA are derived from multiple sources, such as membrane lipids (1), and exist as bioactive ligands that signal through cognate receptors to produce a wide number of physiological responses (Fig. 1).
Fig. 1.
Summary of the major routes of LPA synthesis and the activated signaling pathways via the six cognate LPA receptors. Enzymes are indicated in red. PC, phosphatidylcholine; PE, phosphatidylethanolamine; PS, phosphatidylserine; PLD, phospholipase D.
In the 1960s, studies on smooth muscle and blood pressure hinted at the bioactivity of LPA (2, 3). In later years, various LPA species were isolated and identified from soy beans (4). This raised intriguing questions regarding this lipid’s mechanism of action, which was then thought to include membrane perturbation (5), calcium chelation (6), second messenger signaling (7), intracellular receptors (8), and cell-surface receptors (9). These contending theories were clarified upon the cloning and identification of the first lysophospholipid receptor, LPA1. This GPCR was previously known as “ventricular zone (VZ) gene-1” because of its enriched expression in the embryonic neuroproliferative layer of the cerebral cortex (10, 11). The cloning and functional identification of LPA1 led to the deorphanization of other putative receptor genes based upon sequence homology (12–14), particularly “endothelial differentiation gene” (EDG) members (15) that include LPA and sphingosine 1-phosphate (S1P) receptors. The significant homology between LPA1 and S1P1 was underscored by early reports of EDG-1 as a LPA receptor (16). At the time of LPA1 identification, the only functional homologous receptor was the cannabinoid receptor CB1 (encoded by CNR1) that interacts with the endogenous lipids anandamide and 2-arachidonoylglycerol (17, 18). Two other LPA receptors, LPA2 and LPA3, were subsequently discovered based on shared homology with LPA1. In the past ten years, three additional LPA receptors have been discovered (LPA4–6) (19–23) that are members of the P2Y purinergic family (Fig. 1). These receptors have significantly different encoding sequences than LPA1–3, yet still bind and mediate LPA signaling effects. All six current LPA receptors are class A rhodopsin-like GPCRs with 7-transmembrane (7-TM) domains. Every receptor couples to at least one or more of the four heterotrimeric Gα proteins (G12/13, Gq/11, Gi/o, and Gs) (Fig. 1), resulting in canonical downstream signaling that produces diverse physiological and pathophysiological effects. Other types of lysophospholipids, such as lysophosphatidylserine (LPS), lysophosphatidylinositol, and lysophosphatidylethanolamine (LPE), have some reported bioactivity and are being evaluated for the involvement of possible cognate receptors (24). An additional important aspect of LPA receptor biology is that various chemical forms of LPA may differentially activate LPA receptor subtypes (25). This finding has been supported by secondary readouts of receptor activity, because direct confirmation through classical receptor binding studies has been difficult.
LPA METABOLISM
There are two major synthetic pathways for LPA. In the first pathway, the precursor phospholipids (phosphatidylcholine, phosphatidylserine, or phosphatidylethanolamine) can be converted to their corresponding lysophospholipids such as lysophosphatidylcholine (LPC), LPS, or LPE. In platelets, this occurs via phosphatidylserine-specific phospholipase A1 (PS-PLA1) or secretory phospholipase A2 (sPLA2) activity. In plasma, LPC is produced by LCAT and PLA1 activity. In either location, lysophospholipids can then be converted to LPA via autotaxin (ATX) activity (Fig. 1). In the second major pathway, phosphatidic acid (PA) is first produced from phospholipids through phospholipase D or from diacylglycerol through diacylglycerol kinase. Then, PA is converted directly to LPA by the actions of either PLA1 or PLA2 (26) (Fig. 1).
Through a separate mechanism, LPA can be generated through the acylation of glycerol-3-phosphate by glycerophosphate acyltransferase and the phosphorylation of monoacylglycerol by monoacylglycerol kinase (27). Additional LPA-producing pathways also exist (26, 28). LPA generation from membrane phospholipids occurs in both intracellular and extracellular fashions (28). Intracellular LPA is an important intermediate for the de novo biosynthesis of complex glycerolipids, including mono-, di-, and triglycerides, as well as phospholipids (28). Extracellular LPA is thought to mediate bioactive effects through LPA receptors (29). Furthermore, a report supporting LPA as a functional ligand for the intracellular transcription factor PPARγ exists, although it remains to be examined (30).
LPA has been found in all eukaryotic tissues examined. The formation of an LPA species depends on its precursor phospholipid, which can vary by acyl chain length and degree of saturation. The term LPA most often refers to 18:1 oleoyl-LPA (1-acyl-2-hydroxy-sn-glycero-3-phosphate), as it is the most commonly used laboratory species. However, there is a growing range of recognized chemical forms of LPA in various biological systems (31, 32) that have been observed in concentrations spanning low nanomolar to micromolar levels. LPA concentrations in blood can range from 0.1 μM in plasma and up to 10 μM in serum, which is well over the apparent nanomolar Kd of LPA1–6 (23, 33–35). The 18:2, 20:4, 16:1, 16:0, and 18:1 LPA forms are particularly abundant in plasma (36–38). Aside from blood, LPA has been quantified in a variety of species, tissues, and fluids, including neural tissue, cerebrospinal fluid (CSF), seminal fluid, urine, saliva, and aqueous humor (7, 36, 39–45) (Table 1). Current methods to detect LPA include indirect enzymatic assays (34), TLC-GC, LC-MS, and LC-MS/MS (46–48). These techniques have a growing number of predictive, diagnostic, and therapeutic uses (29, 43, 49, 50).
TABLE 1.
Summary of LPA concentrations in various tissues and biological fluids
| Tissues/Fluids | LPA | LPC | Method of Measurement | References |
| Physiological conditions | ||||
| Embryonic brain | 0.32–0.35 pmol/mga | Not available | LC-MS | (43) |
| Adult brain | 3.7–35 pmol/mga | Not available | GC-MS, LC-MS/MS | (32, 47, 339, 340) |
| Nerve (spinal cord) | 0.79 pmol/mga | Not available | B103 bioassay | (42) |
| Plasma | 0.7 μMb/0.17–0.63 μMa | 100-140 μMb/440 μMa | LC-MS, RH7777 bioassay | (38, 239, 299, 341) |
| Serum | 4–15.5 μMb | 234 μMb | TLC-GC, enzymatic assay, LC-MS/MS | (166, 342, 343) |
| CSF | 0.025–0.2 pMa | Not detecteda | RH7777 bioassay | (344) |
| Seminal fluid | Negligible | 8–19 μMb | RH7777 bioassay, enzymatic cycling | (41) |
| Saliva | 0.785 nMb | Not available | FAB-MS | (345) |
| Lacrimal gland fluid | 1.3 μMa | Not available | MS | (45) |
| Aqueous humor | 0.2 μMb | Not available | MS | (45) |
| Follicular fluid | 25.3 μMb | 157 μMb | TLC-GC | (166) |
| Ascites | 0.62 μMb | Not available | RH7777 bioassay | (299) |
| Fertilized hen white | 8.0-9.6 μMa | Not available | LC-MS | (179) |
| Pathophysiological conditions | ||||
| Nerve (injury) | 74.8 pmol/mga | Not available | B103 bioassay | (42) |
| Serum (systemic sclerosis) | 5.5 μMb | 130 μMb | LC-MS/MS | (239) |
| Serum (sepsis) | Not available | 43.5 μMb | Enzymatic assay | (343) |
| Ascites (pancreatic cancer patients) | 2.7 μMa | Not available | RH7777 bioassay | (299) |
| Plasma (chronic liver injury) | 0.3-0.4 μMa | Not available | Enzymatic assay | (346) |
| Plasma (obesity) | Not available | 83.5–84.4 μMb/420–460 μMa | LC-MS | (341) |
Measurements were performed using a variety of techniques under numerous conditions, resulting in a range of values (associated errors were omitted for simplicity). References are indicated in parentheses. FAB-MS, fast atom bombardment MS.
Values from nonhuman organisms.
Values from humans.
LPA degradation is mediated by several classes of enzymes, including three types of lipid phosphate phosphatases (LPPs) (51), LPA acyltransferase, and various phospholipases (e.g., PLA1 or PLA2) (28, 52, 53). LPA may be converted back to PA by LPA acyltransferase, hydrolyzed by LPP (1–3), or converted by phospholipases into glycerol-3-phosphate (28, 54).
ATX
ATX is one of the best studied enzymes associated with LPA signaling. ATX was first identified and purified as “autocrine motility factor” from human melanoma cells and was perceived to be an ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Enpp2). Furthermore, because the addition of pertussis toxin reduced cellular motility, ATX’s effects were thought to involve Gi/o-mediated signaling (55). Extracellular LPA was found to be produced in sub-millimolar concentrations by lysophospholipase D activity in plasma (39). The responsible enzyme was subsequently identified as ATX (56, 57). It is clear now that ATX-mediated autocrine signaling induces cell motility through LPA production and Gi/o-mediated LPA receptor signaling (29, 58, 59).
ATX has broad tissue expression, with comparatively high levels in blood, brain (especially the choroid plexus and embryonic floor plate), kidney, and lymphoid organs (60–62). Secretion of ATX leads to high expression and concentration in CSF and in the high endothelial venules of lymphoid organs (63–66). Genetic deletion of ATX in mice (Enpp2−/− mutants) has contributed significant understanding to this enzyme’s physiological role. These mice die at embryonic day (E)9.5 with obvious vascular and neural tube defects (67, 68). In addition, they have a poorly formed yolk sac vascular network, as well as enlarged embryonic vessels, malformed allantois, reduced axial turning, kinked neural tube, and an asymmetric neural head fold (68, 69). Enpp2+/− heterozygotes appear normal, but have a 50% reduction in LPA plasma levels compared with wild-type mice. Thus, ATX activity is significantly responsible for the correct concentration of LPA in plasma, as well as for proper vascular and embryonic development.
ATX and LPA signaling are involved in the vasoregulation and vascular biology of multiple species (4, 70, 71). ATX expression can be induced by vascular endothelial growth factor (VEGF), and the resultant elevation of LPA induces both proliferation and migration of endothelial cells, as well as barrier formation in vitro (72–75). Such endothelial cell behaviors require a variety of additional activated-platelet mediators, such as growth factors and cytokines. LPA is also released from activated platelets (36), which can induce platelet activation through positive feedback (40, 76). In general, LPA production and signaling can induce mitogenic and migratory effects on numerous cell types that are involved in angiogenesis and tissue repair (73, 77–79). Despite these data, some controversy exists as to whether LPA and ATX are truly angiogenic, although both appear to help in vessel maintenance. Interestingly, increased LPA levels can rapidly and reversibly increase blood brain barrier permeability (80), suggesting a concentration-dependent mechanism. Finally, LPA can also regulate vascular tone, such as posthemorrhagic vasoconstriction in cerebral microvasculature (81) or vasodilation in the thoracic aorta (82). These opposing actions may be context dependent.
LPA RECEPTORS
The diverse and numerous physiological effects of LPA are mediated through six currently recognized LPA receptors, LPA1–6: protein names LPA1–LPA6 and gene names LPAR1–LPAR6 (human) and Lpar1–Lpar6 (nonhuman) (11, 83). These 7-TM GPCRs couple to at least one or more of the four Gα proteins (G12/13, Gq/11, Gi/o, and Gs) that initiate a variety of signaling cascades. The spatiotemporal distribution of LPA and LPA receptors, typically differential receptor subtypes in specific tissues, drives the abundance of the physiological and pathophysiological processes noted here.
LPA1
LPA1 was the first lysophospholipid receptor identified (10) and is the best studied of the six LPA receptors. The LPAR1 gene (human chromosome locus 9q31.3) encodes a 41 kDa protein containing 364 amino acids with 7-TM domains. In mice, the Lpar1 gene (mouse chromosome locus 4, 32.2 cM) encodes five exons with a conserved intron (shared among Lpar1–3) interrupting TM domain 6. There is a variant of Lpar1 (mrec1.3) that results in an 18 amino acid deletion of the N terminus (84), but its biological significance is unknown. LPA1 shares ∼50–60% amino acid sequence identity with LPA2 and LPA3. While no complete crystal structures have been reported for any LPA receptors to date, crystallization of other GPCRs have facilitated constraint modeling for LPA1, particularly in the second extracellular loop (85). Additionally, computer-modeled mutagenesis studies have identified three key residues in LPA1–3 signaling. R3.28A and K7.36A are both important for the efficacy and potency of LPA, while Q3.29A decreases ligand interaction and activation (86), based primarily on secondary readouts. Future structural analyses of LPA1 will help clarify key residues.
LPA1 couples with three types of Gα proteins: Gi/o, Gq/11, and G12/13 (Fig. 1), which initiate downstream signaling cascades though phospholipase C, MAPK, Akt, and Rho. LPA1 activation alters a range of cellular responses, including cell proliferation and survival, cell-cell contact through serum-response element activation, cell migration and cytoskeletal changes, Ca2+ mobilization, and adenylyl cyclase inhibition (15, 77, 87).
There is wide distribution of Lpar1/LPAR1 expression in both adult mice and humans, including in the brain, uterus, testis, lung, small intestine, heart, stomach, kidney, spleen, thymus, placenta, and skeletal muscle (13, 87, 88). Lpar1 expression is more restricted during embryonic development, including portions of the brain, dorsal olfactory bulb, limb buds, craniofacial region, somites, and genital tubercle (89, 90). Lpar1 expression is regulated both spatially and temporally. For example, in the developing nervous system (77, 87), Lpar1 is found in several areas, including the germinal neuroepithelium or VZ, portions of the midbrain, and superficially in the marginal zone and meninges (10, 43, 89, 91). Though the VZ disappears at birth, Lpar1 expression continues in the hindbrain and the white matter tracks during oligodendrocyte (OL) myelination of the postnatal brain (92). Visualization of LPA1 expression (as well as other LPA receptors) has been hampered by lack of validated antibodies.
LPA signaling has numerous effects on key neurodevelopmental processes, including cerebral cortex formation and function (93–95), neural cell proliferation, survival, growth cone retraction, migration, and adhesion (90, 95–102). Interestingly, studies have also found evidence of concentration-dependent LPA-mediated necrosis as well as apoptosis, possibly through oxidative stress (103–105). In vitro experiments have demonstrated the effects of LPA1-mediated stimulation that increase the proliferation and differentiation on primary neuroprogenitor cell (NPC) cultures, neuronal cell lines, and primary neurons (90, 106, 107). Additional LPA1-mediated neural effects have also been observed in nonmammalian organisms, in which analogs of LPA1 and LPA2 could regulate cortical actin assembly in Xenopus embryos (108).
LPA can generate diverse cellular responses, and the interacting proteins that mediate downstream signaling pathways are being uncovered. Toward elucidating these interactions, one study demonstrated that LPA1 (but not other tested LPA receptors) is trafficked to early endosomes through a PDZ-mediated motif that facilitates its binding to GAIP interacting protein, C-terminus (GIPC), which attenuates LPA-mediated Akt signaling from adaptor protein containing PH domain (APPL) signaling endosomes (109). Other studies identified LPA as a critical regulator of the Hippo-Yes-associated protein (YAP) pathway, which controls LPA-induced gene expression, cell migration, and cell proliferation. This pathway was predominantly activated through G13 stimulation, presumably via the RhoA-Rho-associated protein kinase (ROCK) pathway, while Gs activity inhibited YAP function (110, 111). While the critical LPA receptors in this pathway are still being investigated, there is evidence for varying LPA1, LPA2, and LPA3 involvement within different cell types.
Removal of Lpar1 by constitutive deletion has provided insight into the in vivo functions of this receptor. Lpar1−/− mouse litters have 50% perinatal lethality related to impaired suckling behavior (90), which was attributed to defective olfaction. Surviving Lpar1−/− mice have a significantly reduced body size, craniofacial dysmorphism with blunted snouts, and increased apoptosis in sciatic nerve Schwann cells (SCs) (90). When the original Lpar1−/− line was expanded, a spontaneous variant arose that was named “Málaga LPA1” (maLPA1) (93). The maLPA1 variant has negligible perinatal lethality, but displays more severe brain defects than the Lpar1−/− line. Neurodevelopment defects in maLPA1 include reduced NPC proliferation, increased cerebral cortical apoptosis, decreased cortical size, and premature expression of neuronal markers (93). Defects in the proliferation, differentiation, and survival of new neurons were also impacted during adult hippocampal neurogenesis (112). Additionally, maLPA1 mice showed several behavioral deficits, including inhibition of fear extinction (113) and aggravation of chronic stress-induced impairment to hippocampal neurogenesis (114). Recently, an Lpar1−/− rat hypomorph generated by N-ethyl-N-nitrosourea (ENU) mutation also showed some physical defects reminiscent of the mouse knockout (115). In addition to NPC and neurons, LPA1 signaling is also prominently involved in glial biology, which includes macroglia such as astrocytes, OLs, and SCs, as well as microglia. Astrocytes are the most abundant type of glia and play a significant role in developmental, functional, and pathological processes (116). While basal in vivo astrocytes have undetectable levels of LPA1 (91, 92, 117), cultured astrocytes express LPA1–5 (118) and are responsive to LPA. Such effects include calcium influx, phosphorylation of ERK, morphological changes, and stabilization of stress fibers (119–121). LPA1 is also implicated in astrocyte proliferation and astrogliosis, although the relevant receptors have not been conclusively identified (122). Lipopolysaccharide or interleukin (IL)-1β-primed astrocyte migration appears to involve a Gi/o/phosphatidylinositol 3-kinase (PI3K)/Rac1 response to LPA, in contrast to their normal proliferative response via G12/13 (123). However, some controversy regarding this proliferation pathway exists, possibly due to different culture systems (124). In addition, both LPA1 and LPA2 signaling can increase neuronal differentiation by LPA-primed astrocytes through unidentified astrocyte-derived soluble factors (125), possibly epidermal growth factor (EGF) (126) or nerve growth factor (127), hinting at a rich LPA-mediated biology between astrocytes and neurons.
Another glial cell type, the OL, also expresses LPA1 (91, 92, 128). These cells produce myelin, ensheathing neurons in the CNS. Basally, Lpar1 colocalizes with proteolipid protein and myelin basic protein (MBP), but not glial fibrillary acidic protein (91, 92). During development, Lpar1 expression in OLs emerges just prior to maturation/myelination, suggesting an important role in controlling these processes, although no effect of LPA on OL survival, maturation, myelination, or cytoskeleton organization was reported in vitro (129). However, both the rat OL precursor cell line CG-4 and freshly isolated precursors respond via Rho-ROCK cell rounding upon LPA exposure, in contrast to differentiated OLs (130). Similarly, LPA increases the network area of dendritic processes and MBP expression in differentiating OLs (131). Therefore, LPA may regulate OL function in a maturation stage-specific manner. Additional studies will better define LPA signaling activity in this cell type.
SCs, which are counterparts to OLs in the peripheral nervous system, have also been implicated in LPA signaling. Two main types of SCs exist: myelinating SCs express Lpar1 and possibly Lpar2 (100, 132), while terminal SCs express both Lpar1 and Lpar3 (100, 132). SC survival is increased by LPA through LPA1 and Gi/PI3K/Akt in vitro (133), which is supported in vivo by observations that Lpar1−/− mice have increased apoptosis of SCs in sciatic nerves (90). In addition to SC survival, LPA also induces morphological and cell adhesion changes. In vitro, LPA induces SC wreath formation and enhances focal adhesions, as well as promoting N-cadherin-dependent cell aggregation (100), responses that are dramatically reduced in Lpar1−/− SCs (100). LPA can also increase the expression of P0 protein in SCs through LPA2 signaling, possibly contributing to SC differentiation (134). Recently, LPA1 was shown to influence embryonic SC migration, adult myelination, and cell-to-axon segregation, processes that appear to be defective in Lpar1−/− mice (135).
LPA2
LPA2 was first identified through a gene sequence search for orphan GPCRs. It shows 55% amino acid similarity to LPA1. LPAR2 (human chromosome 19p12) encodes a 351 amino acid protein with a calculated molecular mass of 39.1 kDa (136), while mouse Lpar2 (mouse chromosome 8, 33.91 cM) encodes for a 348 amino acid protein with a molecular mass of 38.9 kDa. Mutagenesis studies of LPA2 have found two key residues that, when altered, can decrease LPA2 activation (Q3.29E and R5.38A) (86). During development, expression of Lpar2 is more diffuse than Lpar1, although it is clearly present in the embryonic brain, limb buds, craniofacial regions, and Rathke’s pouch (89, 137–139).
Compared with Lpar1/LPAR1, Lpar2/LPAR2 expression is more restricted in adult mice and humans, with high LPAR2 in leukocytes and the testis, and Lpar2 in the kidney, uterus, and testis (13, 87). More moderate levels of LPAR2 are found in the prostate, spleen, thymus, and pancreas, and lower levels of Lpar2 expression are found in the lung, stomach, spleen, thymus, postnatal brain, and heart (140).
LPA2 couples to the Gi/o, Gq/11, and G12/13 family of heterotrimeric G proteins, similar to LPA1 (Fig. 1). These G proteins initiate downstream signaling pathways through molecules such as Ras, Rac, PI3K, MAPK, phospholipase C (PLC), diacylglyerol, and Rho (90). LPA2 activation is associated with cell survival and cell migration. LPA2-initiated migration appears to be promoted through interactions with the focal adhesion molecule TRIP6 (141, 142) and several PDZ-domain and zinc finger proteins, through interactions with the carboxy-terminal tail of LPA2 (143). Notably, the PDZ-binding domain of LPA2 regulates Na+/H+ exchanger regulatory factor 2 (NHERF2) behavior, activating PLC-β3 and AKT/ERK signaling while inhibiting cystic fibrosis transmembrane conductance regulator (CTFR). These pathways stimulate cell migration, enhance survival, and alter gene expression, accounting for many of the functions attributed to LPA2. This intracellular coupling is a unique feature of LPA2. Also, LPA2 activation can inhibit EGF-induced migration and invasion of pancreatic cancer cells through the G12/13/Rho pathway (144). These data lend support to the pattern of transactivation and cross-regulation between GPCRs and receptor tyrosine kinases in a variety of settings (145) and expand the signaling effects of LPA beyond single subtype receptor mechanisms.
Phenotypically, Lpar2−/− mice appear mostly normal, with expected Mendelian birth ratios and normal prenatal and postnatal viability (146). Like LPA1, LPA2 is involved in several aspects of nervous system function and development. Activation of LPA2 increases P0 protein in cultured SCs, implicating this receptor in myelination. In fact, LPA2 is upregulated, along with LPA1, after neural injuries such as nerve transection and neuropathic pain (100, 147). The interaction of LPA2 with plasticity-related gene-1 (PRG-1) signaling has also been reported to modulate excitatory transmission in the hippocampus (148).
Mice that are Lpar1−/−/Lpar2−/− null have also been generated. Such mutants mostly phenocopy Lpar1−/− mice and have similar body size reduction, craniofacial dysmorphism with blunted snouts, and 50% perinatal lethality, but also exhibit an increased incidence of frontal hematomas. Studies in these mice have also shed light on LPA1 and LPA2 signaling redundancy in distinct neural and vascular phenotypes. Lpar1−/−/Lpar2−/− cells display altered responsiveness to LPA in vitro, ranging from significant reduction to complete abrogation (95, 149). Exogenous LPA exposure in ex vivo cerebral cortical cultures decreased cell death, increased NPC cell cycle exit, and produced early terminal mitosis. This resulted in precocious neuronal production, cortical thickening, and folding which resembles gyri in humans (95). These effects were abolished in Lpar1−/−/Lpar2−/− embryonic cerebral cortices.
Lpar1−/−/Lpar2−/− mice have also been helpful in understanding the effects of LPA on the vascular system. Specifically, LPA1 and LPA2 were found to exhibit opposite effects on primary vascular smooth muscle cells and injury-induced neointimal hyperplasia (149). Vascular smooth muscle cell (SMC) migration was increased in Lpar1−/− mice but was partially attenuated in Lpar1−/−/Lpar2−/− mice, demonstrating LPA1 and LPA2’s opposition as negative and positive chemotactic mediators, respectively. Additionally, exogenous LPA exposure can increase vascular permeability, which was blocked using the dual LPA1/3 receptor antagonist Ki16425 (150). Ki16425 also inhibited neointima formation and SMC recruitment to the injury site after wire-induced carotid injury (151). Neither LPA1 nor LPA2 was required for dedifferentiation of SMCs following vascular injury in vivo or LPA exposure ex vivo, nor were they required for LPA-induced blood pressure increases (149). These observations indicate the likely involvement of other LPA receptor subtypes in these processes.
A similar dynamic between LPA1 and LPA2 signaling also exists in immune system function. LPA receptors are found on virtually all immune cell types and organs, including lymphocytes (144), dendritic cells (DCs) (152, 153), thymus, and spleen (20, 77, 154). In T cells, LPA can stimulate or attenuate cellular activity, depending on the cell’s activation state and receptor expression. In unstimulated T cells, LPA augments chemotaxis and blocks IL-2 production through LPA2 (155, 156). In activated T cells, where LPA2 is downregulated and LPA1 is upregulated, the reverse is true; LPA inhibits chemotaxis, activates IL-2 and IL-13 production, and promotes cell proliferation (156, 157). In addition, LPA affects immature and mature DCs differently. LPA1–3 are expressed in both immature and mature DCs, and LPA appears to affect immature DCs by enhancing maturation and cytokine production (152, 153). Furthermore, LPA3-specific activation induces chemotaxis of immature, but not mature, DCs (158). DCs from Lpar2−/− mice are hyperactive compared with wild-type counterparts and less susceptible to inhibition by different LPA species, suggesting that LPA via LPA2 may inhibit DC activation (159). Thus, LPA may differentially affect DC maturity and stage-specific functions through multiple LPA receptors.
LPA3
Lpar3 was discovered and cloned in a similar fashion to Lpar2, using homology searches for orphan GPCRs and a degenerate PCR-based cloning method (14, 160). LPAR3/Lpar3 (human chromosomal locus 1p22.3; mouse chromosome locus 3, 71.03 cM) encodes a 353 amino acid sequence GPCR with a molecular mass of ∼40 kDa that, in humans, is ∼54% identical to LPA1 and ∼49% identical to LPA2 (14). Mutagenesis studies on LPA3 have identified two specific residues involved in LPA3 activation (W4.64A and R5.38N), as well as a residue that increased the EC50 of LPA3 by a factor of 10 (K7.35A) (86). During development, Lpar3 expression is observed in the heart, mesonephros, and in three spots in the otic vesicle (89). In the adult, LPAR3/Lpar3 expression is most prominent in the human heart, testis, prostate, and pancreas (14, 160), as well as mouse lung, kidney, uterus, and testis. LPAR3/Lpar3 is less highly expressed in the human lung, ovary, and brain, as well as mouse small intestine, brain, heart, stomach, placenta, spleen, and thymus (87, 140). Expression differences of LPA3 between human and mouse tissue warrant further study.
LPA3 couples with Gαi/o and Gαq/11 to mediate LPA-induced Ca2+ mobilization, adenylyl cyclase inhibition and activation, PLC activation, and MAPK activation (Fig. 1) (161). LPA3 has been reported to prefer 2-acyl-LPA containing unsaturated fatty acids (14, 162). In addition, Lpar3−/− mice are viable and appear normal, with no reported neural deficits, even though LPA3 is found in the frontal cortex, hippocampus, and amygdala (14, 160). However, female null mutants have a striking reproductive phenotype, with delayed embryo implantation, embryo crowding, and reduced litter size (163). In the uterus, Lpar3 is solely found in the endometrial epithelium of the lumen during the transient window of embryo implantation (163, 164) and is regulated by progesterone and estrogen (165). LPA is found in follicular fluid from healthy women, which underscores the LPA-LPA3 signaling axis in the uterus (166). During pregnancy, ATX concentration is upregulated in the serum and placenta of healthy women in the third trimester. Interestingly, patients with pregnancy-induced hypertension (preeclampsia) harbored decreased ATX levels in the third trimester compared with healthy women (56, 167, 168). LPA receptors are also expressed in the testis (19, 77, 88), suggesting a role for LPA signaling in male sperm behavior and fertility. These data support numerous LPA-mediated roles in reproduction.
In addition, LPA3 appears to be involved in determining vertebrate left-right patterning during embryogenesis, a crucial process for proper organ formation and function. Downregulation of Lpar3 or inhibition of LPA3 signaling with Ki16425 disrupted asymmetric gene expression and organ asymmetry in zebrafish, a phenotype which was rescued by Lpar3 overexpression (169). Downregulation of ATX by oligonucleotide knockdown or inhibition of ATX by HA130 similarly disrupted left-right patterning and developmental asymmetry.
LPA4
LPA4 was the first identified lysophospholipid receptor to show a dissimilar amino acid sequence from the other lysophospholipid receptors, LPA1–3, sharing only ∼20% amino acid identity with LPA1 (140) and slightly greater homology to other LPA receptors (19). It was previously known as the orphan GPCR P2Y9, based on its similarity to P2Y purinergic receptors. However, it was reclassified as LPA4 after demonstrating responsiveness to LPA, but not to any nucleotides or nucleosides during ligand screening using a calcium mobilization assay (11, 19). LPAR4/Lpar4 (human chromosome Xq21.1, mouse chromosome X region D) encodes a 370 amino acid protein with predicted molecular mass of 41.9 kDa.
During development, Lpar4 is found in the mouse embryonic brain, maxillary processes, branchial arches, limb buds, liver, and somites (89). In adults, LPAR4 is prominently found in the human ovary, while less prominent LPAR4 can be found in the thymus, pancreas, brain, heart, small intestine, testis, prostate, colon, and spleen. Lpar4 is present in mouse heart, ovary, skin, thymus, and bone marrow (89, 140).
LPA4 is a 7-TM GPCR that couples with Gα proteins, including G12/13, Gq/11, Gi/o, and Gs (170). This receptor induces neurite retraction and stress fiber formation through G12/13 and subsequent Rho/ROCK pathway activation, as seen with activation of other LPA receptors (170, 171). In addition, LPA4 facilitates both ROCK-dependent cell aggregation and N-cadherin-dependent cell adhesion, using a cell line that heterologously expresses this receptor (171). Moreover, LPA4 induces intracellular cAMP accumulation through Gs activation; currently this is the only known LPA receptor with this activity (170). LPA4 can also influence the differentiation of immortalized hippocampal progenitor cells (172). LPA-induced cell migration (e.g., via LPA1) is inhibited by activation of LPA4, and LPA4-deficient cells have increased sensitivity to LPA exposure, with increased lamellipodia formation and transwell movement (173). This noteworthy ability of LPA4 to negatively regulate cell motility opposes LPA’s traditional status as a chemoattractant and indicates that differential effects may be achieved through combinatorial LPA receptor expression.
Adult Lpar4−/− mice appear grossly normal (173), yet exhibit increased trabecular bone volume, number, and thickness (174, 175). This is in contrast to Lpar1−/− mice, which display decreased bone mass (176). This suggests that LPA4 negatively regulates osteogenesis and may counteract LPA1-initiated osteogenesis. Consistent with these observations, knockdown of LPAR4 in a human mesenchymal stem cell line resulted in the inhibition of its differentiation into osteoblasts (177). During embryo development, there is decreased prenatal survival for Lpar4−/− mutants. Observed phenotypes in these null mice include pericardial effusions, severe edema and hemorrhage, abnormally dilated blood and lymphatic vessels and lymph sacs, and impaired pericyte recruitment (178). This indicates important pleiotropic roles for LPA4 in circulatory and lymphatic system development. Vasculogenesis in extraembryonic membranes (such as the chorioallantoic membrane) has also been recently linked to an ATX-mediated product ion of LPA. The responsible receptor may be LPA4, although the concomitant presence of Lpar1, Lpar2, and Lpar6 make this subtype identification unclear (179).
LPA5
In 2006, LPA5 was identified through unbiased screening approaches that led to the deorphanization of GPR92 (20, 21). LPAR5 shares 35% homology with LPAR4, but only ∼22% homology to LPAR1–3 (21). LPAR5/Lpar5 (human chromosome 12p13.31; mouse chromosome 6, 59.21 cM) encodes a 372 amino acid protein. LPAR5 is highly expressed in spleen, and to a lesser degree in heart, small intestine, placenta, colon, and liver. Lpar5 in murine tissues is highly expressed in small intestine, and more moderately in lung, heart, stomach, colon, spleen, thymus, skin, liver, platelets, mast cells, gastrointestinal lymphocytes, and dorsal root ganglia (20, 21, 180, 181). Additionally, Lpar5 was found in the early embryonic mouse forebrain, rostral midbrain, and hindbrain. This expression pattern becomes more ubiquitous from E9.5–E12.5, showing signals throughout the embryo, as well as diffuse patterns in the developing brain and choroid plexus that suggest a possible role for LPA5 in brain development (89). Mutagenesis studies have found that several residues are likely involved in the binding of LPA to LPA5, including one mutant that abolished receptor activation (R2.60N) and three separate mutants that greatly reduced receptor activation (H4.64E, R6.62A, and R7.32A) (182).
LPA5 couples to G12/13 and Gq/11 (21). LPA5-expressing cell lines demonstrate LPA-induced neurite retraction, stress fiber formation, and receptor internalization in vitro through the G12/13 pathway (21). LPA5 can also activate Gq to increase intracellular calcium levels and induce cAMP accumulation (21). However, this cAMP accumulation is not changed by Gs minigene administration, suggesting the involvement of an alternative G protein (20, 21). In melanoma cells, LPA inhibits migration through LPA5 and the cAMP-protein kinase A pathway, acting as a chemorepellent that is similar to its LPA4-mediated effect. Moreover, LPA5 demonstrated a 10-fold preference for alkyl, rather than acyl, 18:1 LPA (183). LPA5 signaling may also affect the colon, where it may be involved in water absorption (184). In intestinal epithelial cells, LPA promoted Na+-dependent water absorption through Na+/H+ exchanger 3 (NHE3) via an interaction between LPA5 and Na+/H+ exchanger regulatory factor 2 (NHERF2), which then recruits NHE3 to the microvilli (184). In mast cells, LPA5 is the main LPA receptor responsible for LPA-induced release of MIP-1β (also known as chemokine ligand 4 or CCL-4) (181). The diverse expression patterns of LPA5 suggest that additional physiological effects will be uncovered in the future.
LPA6
The most recent addition to the LPA receptor family is LPA6. In 1993, receptor 6H1 was isolated from a chicken T cell library (185) and subsequently renamed to p2y5 because of sequence homology with P2Y receptors (186). Genetic studies indicated roles for LPA6 in hypotrichosis simplex, a complex of diseases involving familial hair loss (22). Most recently, studies using a chimeric G13 protein indicated that LPA induced multiple effects, including p2y5-mediated cAMP accumulation, Rho-dependent changes in cell morphology, [3H]LPA binding, and [35S]guanosine 5′-3-O-(thio)triphosphate binding (23). The p2y5 has now been reclassified as an LPA receptor and renamed LPA6 (22, 83). 2-Acyl-LPA appears to have a higher activity to LPA6 than 1-acyl-LPA. Interestingly, many of the performed assays require extraordinarily high concentrations of LPA (up to 10 μM) to show an effect, compared with the nanomolar concentrations needed for activating LPA1–5. When LPA6 was coexpressed with a promiscuous Gα protein, LPA6 was activated by LPA and resulted in increased intracellular Ca2+, reduced forskolin-stimulated [cAMP]I, and ERK1/2 activation (187).
While several studies have now identified LPA6 mutations in hypotrichosis patients (22, 188, 189), there have also been reports of lipase member H (LIPH) (also known as PA-PLA1α) mutations in hypotrichosis that are associated with both a decrease in LPA production as well as reduced or abrogated LPA6 activation in cells expressing the receptor (190, 191). An additional study has demonstrated that LIPH regulates hair follicle development by modulating epidermal growth factor receptor (EGFR) signaling through a tumor necrosis factor α converting enzyme and the transforming growth factor (TGF)-α pathway (192). These findings suggest LPA6, and possibly EGFR, as candidates for therapeutic intervention in forms of human hair loss.
LPA SIGNALING AGONISTS AND ANTAGONISTS
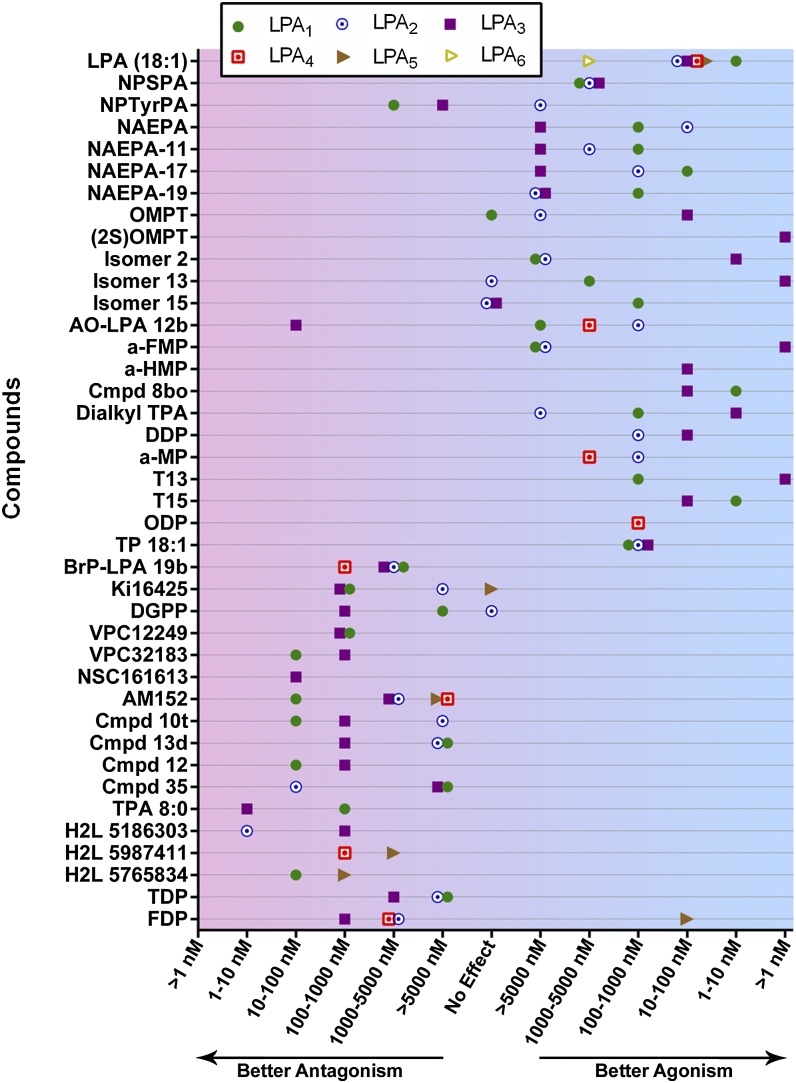
There is a rapidly growing number of reported LPA signaling agonists and antagonists, all with varying selectivity and potency (Fig. 2, Table 2). Most of these receptor modulators are directed at LPA receptors, particularly against LPA1–3, although a few compounds have demonstrated some LPA4 and LPA5 activity with limited selectivity (182, 193, 194). Additionally, several compounds target enzymes that regulate the production of LPA, notably ATX and LPPs (195, 196). The vast majority of these studies have relied heavily upon in vitro assays for validation thus far, but a growing number of compounds have some demonstrated in vivo efficacy (Table 2). An LPA1 antagonist named AM966 has shown efficacy in inhibiting lung fibrosis in a bleomycin injury model (197). The dual LPA1/3 antagonist Ki16425 has shown preventative efficacy in a mouse model of hydrocephalus (43), as well as therapeutic reduction of bone metastases from breast cancer in a xenograft tumor model (198). In murine renal ischemia-reperfusion injury, opposing effects can be seen in which the LPA3-selective agonist 1-oleoyl-2-O-methyl-rac-glycerophospho-thionate (OMPT) enhanced kidney damage, whereas the LPA1/3 dual antagonist VPC12249 reduced the injury via LPA3 inhibition (199). These compounds require further validation in vivo. LPA1 antagonists are also being tested clinically for disease amelioration: SAR-100842 for systemic sclerosis and AM-152 for idiopathic pulmonary fibrosis (200). Exogenous LPA exposure can also increase blood brain barrier permeability, which may be relevant to diseases and drug delivery into the brain (80). In the next section, LPA-mediated pathophysiologies across organ systems will be discussed, with specific mention of LPA signaling modulators in therapeutic contexts (Table 2).
Fig. 2.
Effects of LPA receptor agonists and antagonists. LPA receptor modulating compounds are classified according to their potency against each receptor, LPA1–LPA6. Ranges of compound EC50, IC50, or Ki values are given on the x axis, progressing from strong antagonism on the left to strong agonism on the right. A subset of these compounds is shown in more detail in Table 2. This graph was prepared using GraphPad Prism software. Cmpd, compound; NPSPA, N-palmitoyl serine phosphoric acid; NPTyrPA, N-palmitoyl tyrosine phosphoric acid; NAEPA, N-acyl ethanolamide phosphate; AO-LPA 12b, sn-2-aminooxy analog 12b; a-FMP, α-fluoromethylene phosphonate; a-HMP, α-hydroxymethylene phosphonate; TPA, thiophosphatidic acid; DDP, dodecyl phosphate; a-MP, α-methylene phosphonate; ODP, octadecenyl phosphate; TP 18:1, oleoyl-thiophosphate; BrP-LPA 19b, α-bromomethylene phosphonate analog 19b; TDP, tetradecyl phosphonate; FDP, farnesyl diphosphate.
TABLE 2.
Summary of LPA receptor modulators, receptor subtype target and activity, and disease relevance
| Compound | Target | Characteristics | Physiology or Disease Relevance | References |
| LPA (18:1) | LPA1–5 and weak LPA6 agonist | Lysophospholipid present in blood and tissues | Appropriate levels of signaling are essential for development, neurogenesis, cell migration, cell survival, vasculogenesis, and reproduction, among other processes | (25, 140, 319) |
| N-palmitoyl serine phosphoric acid | Weak LPA1–3 agonist | Lipid analog | Agonizes intracellular Ca2+ mobilization in mammalian cells (antagonizes in Xenopus) | (320–322) |
| N-acyl ethanolamide phosphate | LPA1/2 and weak LPA3 agonist | Lipid analog | Agonizes platelet aggregation | (25, 323) |
| Oleoyl-thiophosphate | LPA1–3 agonist | Thiophosphate lipid analog | Elevates intracellular Ca2+ mobilization | (322, 324) |
| Oleoyl-sn-glycero-3-phosphate | LPA1/2 agonist | LPA analog | Increases uterine smooth muscle contraction tension, amplitude, and frequency | (325) |
| Dialkyl thiophosphatidic acid 8:0 | LPA1/3 and weak LPA2 agonist | Thiophosphate lipid analog | Elevates intracellular Ca2+ mobilization | (322) |
| T13 | LPA1 and weak LPA3 agonist | Thiophosphate lipid analog | Elevates intracellular Ca2+ mobilization | (322, 326) |
| T15 | LPA1/3 agonist | Thiophosphate lipid analog | Activates cell migration | (322, 326) |
| Dodecyl phosphate | LPA2/3 agonist | Lipid analog | Elevates intracellular Ca2+ mobilization | (322, 327) |
| α-Methylene phosphonate | LPA2 and weak LPA4 agonist | Lipid analog | Elevates intracellular Ca2+ mobilization | (193, 322) |
| OMPT | LPA3 and weak LPA2 agonist | Lipid analog, S-enantiomer is more potent and LPA3-selective | Enhances kidney damage after renal ischemia-reperfusion injury, increases uterine smooth muscle contraction tension and amplitude but decreases frequency | (25, 199, 325) |
| Alkyl OMPT | LPA1/3/6 agonist | Lipid analog | Induces cancer cell migration and fibroblast proliferation, activates Ca2+ mobilization | (322, 328) |
| α-Fluoromethylene phosphonate | LPA3 and weak LPA1/2 agonist | Lipid analog | Activates LPARs in TGF-α shedding assay | (329) |
| α-Hydroxymethylene phosphonate | LPA3 agonist | Lipid analog | Activates LPA3 in TGF-α shedding assay | (329) |
| Octadecenyl phosphate | LPA4 agonist | Lipid analog | Induces platelet shape change | (182, 322) |
| N-palmitoyl tyrosine phosphoric acid | Weak LPA1/3 antagonist, weak LPA2/6 agonist | Lipid analog | Agonizes intracellular Ca2+ mobilization in mammalian cells (antagonizes in Xenopus) | (320–322) |
| sn-2-Aminooxy analog 12b | LPA2 and weak LPA1/4 agonist, LPA3 antagonist | Aminooxy lipid analog | Enhances intestinal endothelial cell migration, activates Rho/Rac1 signaling, potential use in repair of gastrointestinal epithelium | (194) |
| Farnesyl diphosphate | LPA5 agonist, LPA3 and weak LPA2/4 antagonist | Lipid analog | Farnesyl phosphates selectively induce and inhibit Ca2+ mobilization | (182, 322) |
| αbromomethylenephosphonate (BrP-LPA) | Antagonizes ATX and all LPA receptors | Lipid analog, diastereomer | Attenuates arthritis, reduces breast cancer migration and invasion, causes tumor regression, radiosensitizes tumor vasculature | (195, 196, 330) |
| HLZ-56 | Antagonizes all LPA receptors | Lipid analog | Prevents SMAD2/TGF-β activation in kidney fibrosis | (193, 253) |
| Tetradecyl-phosphonate | Weak LPA1–3 antagonist | Lipid analog | Inhibits intracellular Ca2+ mobilization | (322, 324) |
| Ki16425 | LPA1/3 and weak LPA2 antagonist | Small molecule, oral activity, high selectivity, developed by Kirin | Inhibits cell migration, attenuates autoimmune arthritis, inhibits cancer metastasis, inhibits progesterone and PGE2 secretion from bovine reproductive tract | (244, 262, 331–333) |
| Ki16198 | LPA1 and weak LPA2/3 antagonist | Orally active, methyl ester of Ki16425 | Attenuates pancreatic cancer invasion and migration | (316) |
| Debio 0719 | LPA1/3 antagonist | R-stereoisomer of Ki16425, higher potency | Inhibits dissemination of breast cancer cells, stops LPA1-stimulated Ca2+ flux | (317, 334) |
| VPC12249 | LPA1/3 antagonist | Lipid analog of NAEPA | Reduces lung fibrosis from irradiation therapy, reduces kidney ischemia-reperfusion injury | (25, 199, 252) |
| VPC32183 | LPA1/3 antagonist | Lipid analog of NAEPA | Intravaginal injection abrogates LPA-induced PGE2 production | (322) |
| Thiophosphatidic acid 8:0 | LPA1/3 antagonist | Lipid analog | May agonize LPA5-mediated platelet shape change | (182, 324) |
| Compound 12 | LPA1/3 antagonist | Small molecule | Inhibits intracellular Ca2+ mobilization | (322, 335) |
| H2L-5186303 | LPA2/3 antagonist | Small molecule | Inhibits intracellular Ca2+ mobilization | (322, 335) |
| H2L-5765834 | LPA1/5 antagonist | Small molecule | Inhibits LPA-induced platelet shape change and platelet activation | (182, 322) |
| H2L-5987411 | LPA4 and weak LPA5 antagonist | Small molecule | Inhibits LPA-induced platelet shape change and platelet activation | (182, 322) |
| AM095 | LPA1 antagonist | Small molecule, developed by Amira | Attenuates bleomycin-induced dermal/pulmonary/kidney fibrosis and reduces histamine release in mice, inhibits chemotaxis of human melanoma cells and LPA1-overexpressing CHO cells | (254, 336) |
| AM966 | LPA1 antagonist | Small molecule, developed by Amira | Inhibits chemotaxis of fibroblasts, attenuates bleomycin-induced pulmonary fibrosis in mice | (197) |
| AM152 | LPA1 and weak LPA2–5 antagonist | Small molecule, developed by Amira | Passed phase I clinical trials for idiopathic pulmonary fibrosis (Bristol Myers Squibb) | (200) |
| Compound 35 | LPA2 and weak LPA1/3 antagonist | Small molecule | Inhibits colon cancer cell growth and Erk activation | (25, 318) |
| diacylglycerol pyrophosphate (DGPP) 8:0 | LPA3 and weak LPA1 antagonist | Lipid analog | Does not affect progesterone or PGE2 production by endometrial cells. | (263, 322, 338) |
| NSC161613 | LPA3 antagonist | Small molecule | Inhibits intracellular Ca2+ mobilization | (322, 335) |
| SAR-100842 | LPA1 antagonist | Small molecule, developed by Sanofi | Passed phase I clinical trials for systemic sclerosis (Sanofi) | (200) |
Activity, compound characteristics, and effects in various disease models are emphasized. LPAR antagonists are listed first, followed by compounds with both agonist and antagonist activity, and antagonists listed last. If information in a disease context was not available, effects on cellular physiology were included instead. “Weak” effects are classified as having EC50, IC50, or Ki values greater than 1,000 nM. Note that not all compounds were tested against all LPA receptors (see Fig. 2 for known interactions). References are indicated in parentheses. NAEPA, N-acyl ethanolamide phosphate.
PATHOPHYSIOLOGY
Neurological disorders
Many neurodevelopmental disorders have unclear etiologies, yet are associated with prenatal and perinatal risk factors such as hemorrhage, hypoxia, infection, and immune activation. These include CNS malformations such as heterotopias, layer malformations, and hydrocephalus, as well as neurological disorders such as cerebral palsy, schizophrenia, and autism (201–203). LPA signaling can be significantly altered during all of these insults, and could induce or contribute to developmental disturbances.
Recently, a prominent effect of LPA signaling on the developing brain was shown in relation to posthemorrhagic fetal hydrocephalus (FH) (43). FH is a neurodevelopmental disorder characterized by the excessive accumulation of CSF, macrocephaly, enlarged ventricles, and neurological dysfunction. A main risk factor for FH is bleeding within the germinal matrix of the cerebral cortex. Prenatal injections of LPA directly into fetal brains induced most of the symptoms of FH in an LPA1-dependent manner. In a mouse model of intracranial hemorrhage (with lower levels of LPA), blood components induced FH with 25–50% penetrance, while Lpar1−/−/Lpar2−/− mice were protected from developing FH (43). Additionally, the effects of hypoxia involve LPA1 potentiation via the receptor kinase GRK2, linking LPA receptor signaling to another common FH risk factor (204). Postnatal brain exposure to blood appears to induce ventriculomegaly without hydrocephalus, possibly due to spatiotemporal LPA receptor and ligand distribution (205, 206).
Neuropsychiatric disorders are also thought to have a neurodevelopmental origin. Many share the same risk factors cited above, based on studies linking hypoxia, prenatal hemorrhage, and immune activation to diseases such as schizophrenia and autism (201, 208, 209). LPA, particularly through LPA1, is involved in both immune system function and hypoxia. Indeed, the removal of LPA1 signaling during development significantly alters the neuropsychiatric profile of mice. Lpar1−/− mice exhibit deficits in prepulse inhibition of the startle reflex, altered levels of serotonin, abnormalities in glutamatergic synapses (94, 210, 211), and a reduction in entorhinal cortex γ oscillations and parvalbumin-positive neurons (212). Furthermore, maLPA1−/− mice display defects in olfaction, pain sensing, exploration, anxiety, and memory retention in addition to cortical developmental defects. These syndromic dysfunctions are reminiscent of the pathological and behavioral symptoms exhibited by schizophrenic patients and animal models (93, 94, 213, 214). Expression of the LPA-synthesizing enzyme cytosolic (c)PLA2 is increased in schizophrenic patients, while cPLA2 inhibition in control groups reduces deficits in prepulse inhibition (215). These observations suggest that altered LPA1 signaling may be relevant to schizophrenia and related psychiatric symptoms. Lpar1−/− and maLPA1−/− mice also display craniofacial dysmorphism and defects in adult hippocampal neurogenesis, traits that are associated with autism (112). These data strongly implicate LPA and LPA1 receptor signaling in neurodevelopmental and neuropsychiatric disorders.
LPA and LPA1 signaling are also involved in nerve injury responses. Neuropathic pain, with symptomatic allodynia and hyperalgesia, is often associated with peripheral neuropathy and SC demyelination, commonly caused by trauma or inflammation of the nervous system. Intrathecal injections of LPA over-activate LPA1 and elicit both allodynia and hyperalgesia in wild-type mice, which were prevented in Lpar1−/− mice (147). LPA1 activation also induces a nociceptive response that appears to mediate the release of substance P, a neuropeptide implicated in inflammation and pain (216). Furthermore, partial sciatic nerve ligation (PSNL) nociception was completely blocked in Lpar1−/− mice. Allodynia and demyelination were also reduced using Rho pathway inhibitors, implicating LPA1-mediated Rho activation in these neuropathic pain-associated pathologies (147). Finally, PSNL-induced neuropathic pain was inhibited through a distinct pathway involving LPA5 and cAMP in the spinal cord dorsal horn neurons despite continued upregulation of several pain-related markers (217).
In earlier ex vivo studies, LPA induced demyelination in isolated dorsal root fiber and decreased myelin basic protein expression (218). ATX has also been shown to induce neuropathic pain through the conversion of LPC to LPA (219). This is supported by the fact that Enpp2+/− mice, which have a 50% decrease in LPA plasma concentration, also have a 50% recovery from PSNL-induced neuropathic pain (220). Interestingly, intrathecal LPC-mediated pain involved the feed forward production of LPA through ATX and LPA3 (221). Cyclic PA has been reported to attenuate PSNL-induced allodynia and hyperalgesia (222). LPA-mediated nociception may be dependent on specific LPA forms, as pain responses were strongly elicited by 18:1 LPA but not by 16:0 LPA or 18:0 LPA (223). Interestingly, intraperitoneal administration of Ki16425 (LPA1/3 antagonist) within a short window straddling the induction of pain appears to completely block LPA-induced nociception (223). These proof-of-concept studies indicate that LPA and LPA1 signaling can serve as important therapeutic targets for myelinating diseases, including neuropathic pain.
Atherosclerosis and cardiovascular disease
As LPA receptors are integral to vascular integrity and the immune response, it comes as no surprise that dysregulated LPA signaling may be implicated in atherosclerosis. In particular, LPA is involved in inflammatory cytokine release, monocyte attraction and adhesion, abnormal endothelial cell behavior, endothelial permeability, and LDL uptake for plaque formation (224–228). These factors can lead to the excessive macrophage invasion seen in atherosclerosis. Additionally, LPA accumulation has been observed within the thrombogenic core of atherosclerotic plaques (76, 229), potentially implicating the presence of LPA in disease progression.
LPA signaling may also be involved in cardiovascular disease and myocardial infarction, as excessive platelet activation is associated with cardiovascular disease (230). Activated platelets release LPA which can initiate a positive feedback loop where LPA induces platelet activation and aggregation (36, 40, 76). During myocardial infarction, cardiac myocytes can sustain major damage due to ischemia and hypoxia. LPA signaling has also been implicated in both of these conditions. For example, elevated LPA levels have been detected in ischemia and have been shown to increase cardiomyocyte and mesenchymal stem cell survival (231–234). During injury recovery, LPA stimulates the immune cell reaction, endothelial cell migration, cell survival, and proliferation processes involved in repair and angiogenesis (73, 77, 140). While prolonged LPA expression may exacerbate ischemia, the involvement of LPA in injury response may be important during myocardial infarction recovery. In addition to the potential cardioprotective effects of LPA, LPA signaling has been shown to be neuroprotective during ischemic injury. Treatment of a rat model of retinal ischemia/reperfusion injury with an LPA analog protected neural cells from apoptosis and improved recovery outcomes (235). However, these data are inconclusive, as LPA has also been shown to induce cell death in cerebral vascular cells, umbilical endothelial cells, brain explants, and retinas (236). This apoptotic response was reduced with an LPA1 antagonist
Inflammation and autoimmunity
LPA is a potent mediator of the immune response and can contribute to improper immune activation, upregulating the production of inflammatory cytokines such as IL-2 and IL-6 (140, 237). In addition, LPA1 and LPA2 signaling can activate microglial cells in the brain, triggering immune invasion. Importantly, LPA has been linked to several inflammatory and autoimmune disorders, including systemic sclerosis, asthma, and arthritis.
Abnormally high levels of LPA have been identified in fibroblasts from systemic sclerosis patients. These primary cells are hypersensitive to Cl−-current activation during LPA exposure (238, 239). The bronchoalveolar fluids of allergen-provoked asthmatic patients showed concomitant increases in ATX levels and LPA concentration with increased 22:5 and 22:6 polyunsaturated fatty acids (240). In a mouse model of allergic asthma, the blockade of ATX activity and LPA2 knockdown attenuated the Th2 cytokines and allergic lung inflammation (240).
LPA is also involved in several aspects and forms of arthritis. Rheumatoid arthritis patients display increased ATX concentrations in synovial fluid, and elevated cytokine production was found in patient fibroblast-like synoviocytes treated with LPA (241). Recent studies have also demonstrated that LPA/LPA1 contributes to the development of rheumatoid arthritis via cellular infiltration, Th17 differentiation, and osteoclastogenesis (242), underlining LPA’s effects on inflammation and immune regulation. Consistent with LPA receptor activation in this context, a functional single-nucleotide polymorphism discovered in the promoter region of LPA1 increased susceptibility to knee osteoarthritis, possibly through LPA1 upregulation (243). These results support the proposal that ATX and LPA are involved in facilitating immune system functions via modulation of lymphocyte trafficking and sensitization of affected cells during autoimmunity.
Understanding these pathways could lead to the use of LPA signaling modulators in the treatment of human autoimmune disorders. Use of Ki16425 or α-bromomethylene phosphonate (BrP-LPA) (a pan-LPA antagonist) attenuated arthritis in a mouse model of inflammatory joint disease (244). Specifically, treatment with Ki16425 reduced synovial inflammation, cell infiltration, and bone destruction, while decreasing the presence of inflammatory mediators in the joint tissue (242). These studies suggest that LPA receptor inhibition may be a future treatment option for arthritic patients.
Fibrosis
LPA signaling has been linked to injury response and fibrosis, which can involve the formation of excess fibrous connective tissues in a wide range of organ systems, resulting in complications such as pulmonary, renal, and tubulointerstitial fibrosis. Increased LPA has been found in bronchoalveolar fluid in pulmonary fibrosis after bleomycin-induced lung injury, which was associated with increased vascular leakage, fibroblast recruitment, and mortality. These pathologies were significantly reduced in Lpar1−/− mice, linking LPA1 signaling to this disorder (245). Renal fibrosis is also linked to LPA1 signaling, albeit through a slightly different mechanism that also involves LPA3 (246). In particular, LPA1 activation in mesothelial cells can lead to stimulated migration, cell proliferation, and upregulated expression of a profibrotic factor called connective tissue growth factor in epithelial cells and fibroblasts (247). The subsequent cell growth can result in the pathogenic fibroblast proliferation seen in fibrosis. Lpar2 may also be involved in pulmonary fibrosis, as knockout mice demonstrated protection against bleomycin-induced lung injury, fibrosis, and death (248). In addition, LPA and ATX levels are increased following hepatitis C-induced liver fibrosis, presumably through induction of stellate cell and hepatocyte proliferation (35, 249), which are the main contributors to extracellular matrix accumulation in the liver (250).
Furthermore, LPA2 signaling can activate αVβ6 integrin, causing TGF-β1 activation in epithelial cells and stimulating growth (200). Both LPA2 and αVβ6 integrin have been shown to be upregulated in animal models and patient samples of lung fibrosis. Not only does LPA mediate the progression of fibrosis via cell growth, but LPA1 has also been implicated in the initiation of disease through excessive fibroblast recruitment and persistent maintenance of leaky vasculature (245). While fibroblast presence and vascular permeability are important factors in the early stages of injury repair, high fibroblast levels and prolonged vascular leakage have both been implicated in disease.
Inhibition of LPA signaling has been demonstrated to attenuate many of the negative responses seen in fibrosis. For example, administration of Ki16425 in an animal model of lung fibrosis inhibited fibroblast chemotaxis (200). Usage of LPA1/LPA3 dual antagonist VPC12249 reduced the incidence of lung fibrosis from radiation therapy, counteracting the increased LPA1/LPA3 and connective tissue growth factor (CTGF) expression seen in these mice (252). Administration of this drug pre- and postirradiation resulted in a dose-dependent response including prolonged survival, inhibited fibroblast growth, reduced collagen deposits, and recovered lung structure. Another study utilized ischemia-reperfusion injury to the kidney as a model of fibrosis (253). This study implicated LPA2-stimulated Smad2 phosphorylation and TGF-β transactivation in proximal tubule cells as mediators of kidney fibrosis. In the presence of the pan-LPA receptor antagonist HLZ-56, but not the LPA1/LPA3-specific antagonist Ki16425, Smad2 activation was attenuated. Reduced Smad2 prevented the activation of pro-fibrotic factors TGF-β, CTGF, and PDGF-β. Another model simulated dermal fibrosis in the mouse by measuring changes in collagen content and dermal thickness (254). This study showed that the fibrosis was prevented in Lpar1−/− mice and its severity reduced by administration of the LPA1 antagonist AM095. In addition, myofibroblast differentiation into smooth muscle was diminished in Lpar1−/− mice and nuclear Smad2 reduced in null and drug-treated mice. The disease course was unaffected in Lpar2−/− mice. These data show that LPA1 or LPA2 inhibition may be therapeutically beneficial in patients with varying types of fibrosis. In particular, phase I clinical trials are already being carried out with LPA1 antagonists; Sanofi is testing SAR-100842 for systemic sclerosis and Bristol-Myers Squibb is assessing AM152 for idiopathic pulmonary fibrosis (200).
Bone development and disease
LPA signaling has also been implicated in aspects of bone development and pathology (255). The two major cell classes in bone are osteoblasts and osteoclasts, which build and remove bone, respectively. LPA can induce differentiation of human mesenchymal stem cells into osteoblasts through LPA1 and LPA4 (177), although these receptors appear to mediate opposing actions. Lpar4−/− mice have increased trabecular bone volume, number, and thickness (177). An interplay between osteoblasts and osteoclasts has also been hypothesized (256), based on observations that osteoblasts can produce LPA, while exposure to LPA causes osteoclast lamellipodia retraction, disrupted peripheral actin belts, increased survival, and resorptive activity (257). This process was driven mainly by LPA1 Gi/o coupling. However, G12/13 coupling with an as yet unidentified LPA receptor (LPA2, LPA4, LPA5, or LPA6) may also underlie osteoclast function (257).
Reproductive system and infertility
LPA3 signaling has an established role in regulating aspects of male and female reproduction and its dysregulation is implicated in mammalian infertility and reproductive problems (258). In fact, Lpar3−/− null mice have delayed embryo implantation, embryo crowding, and reduced litter size. These defects were traced to maternal influences, as wild-type embryos transferred into Lpar3−/− dams failed to implant normally (163). Mice lacking cyclooxygenase-2, an enzyme that produces prostaglandins downstream of LPA3, also have similar defective phenotypes. Mechanistically, exogenous prostaglandin administration to Lpar3−/− dams rescues delayed implantation and reduced litter size, demonstrating that LPA3-mediated signaling is upstream of prostaglandin synthesis (163). However, this treatment failed to rescue embryo crowding, indicating that LPA3 signaling mediates aspects of implantation in both prostaglandin-dependent and -independent fashions (259). The mechanism underlying spacing defects in Lpar3−/− mice remains to be fully understood, but may involve cytosolic phospholipase A2α (cPLA2α) or Wnt/β-catenin signaling, as cPLA2α removal or Wnt/β-catenin signaling inhibition result in similar embryo crowding phenotypes (260, 261). Injection of Ki16425 into bovine blood decreased progesterone and PGE2 secretion, suggesting yet another role for LPA signaling in the reproductive system and pregnancy (262). This change in progesterone and PGE2 secretion is LPA1-specific, because use of an LPA3-selective inhibitor, diacylglycerol pyrophosphate (DGPP), did not alter production (263).
In addition to being integral to female reproduction, LPA3 appears to play an important role in male fertility (88). One study demonstrated that LPP-1 overexpression resulted in impaired spermatogenesis (264), suggesting that the presence of LPA may be important in sperm development. There is also evidence for LPA involvement in sperm motility (265), although triple genetic deletion of LPA1–3 showed no detectable deficits in sperm motility. However, deletion of LPA1–3 did result in decreased germ cell survival and increased prevalence of azoospermia in aging mice (264). In addition to the above reproductive phenotypes, additional studies implicate LPA signaling in male sexual function, ovarian function, fertilization, decidualization, pregnancy maintenance, and parturition (88). This indicates that LPA1–3 signaling is important in both male and female reproduction.
Obesity
Obesity is quickly becoming one of the greatest problems in the developed world. Not only is obesity the most common childhood disorder, but it raises the risk of other potentially fatal comorbidities (266). The long-term effects of being overweight correlate with premature death, cardiovascular disease, metabolic morbidities, and asthma, among other problems (267). Many factors modulate the propensity to accumulate fat cells, including an increased ratio of adipocyte precursor cells to differentiated adipocytes (268). In this vein, LPA signaling may play a role in fat storage, as LPA influences adipocyte precursor differentiation. Additionally, ATX is secreted during adipocyte differentiation and LPA is produced by mature adipocytes (269–271). When obesity and type II diabetes are genetically induced in db/db mice, LPA production increases (241). Moreover, LPA and ATX production have been linked to preadipocyte proliferation (270, 272), a process which may be LPA1 dependent (195). LPA1-mediated activation of the mitogenic Erk1/2 pathway (273) and the differentiation-inhibiting PPARγ2 receptor (274) has been implicated in this response. These data are supported by the fact that Lpar1−/− mice have higher adiposity compared with wild-type littermates, despite lowered body weight (149, 275).
In addition to affecting adipocyte proliferation, LPA1 signaling may be more directly involved in mediating glucose and insulin in diabetic patients. LPA has been shown to lower glucose levels in normal and type I diabetic mice, though LPA production was unaltered (276). Furthermore, administration of LPA to obese prediabetic glucose-intolerant mice inhibited insulin secretion and lowered glucose tolerance (277, 278). This effect was prevented by the antagonist Ki16425. These studies also showed that circulating LPA levels were elevated in the high-fat diet mice compared with normal diet controls and that long-term administration of Ki16425 improved insulin tolerance, glycogen storage, and glucose use in these mice, possibly by increasing the population of pancreatic islets. Therefore, ATX, LPA production, and LPA1 signaling have been implicated in the propagation of adipose tissue and the instigation of abnormal glucose and insulin levels in obese patients and mice. These data suggest that reducing LPA levels or inhibiting LPA1 signaling in obese or diabetic patients may be a possible treatment option for these disorders.
Cancer
LPA and ATX signaling are implicated in the cancer field as important factors in the migration, invasion, metastasis, proliferation, survival, and angiogenesis processes of tumorigenic cells (140, 279–281). Increased LPA levels have been measured in ovarian cancer patients and several cell lines (282–286). In particular, LPA2 overexpression has been observed in several cancers in vivo and in vitro (287, 288). LPA2 signaling has been associated with invasion and metastasis of ovarian, endometrial, mesothelioma, and colon cancer cells (289, 290), likely through the activation and induction of VEGF, EGFR, metalloproteinases, urokinase-type plasminogen activator, cyclooxygenase-2, or Akt/Erk1/2 (289, 291–294). LPA3 may play a complementary role, initiating invasion and metastasis of the same cancer cell types (295–297).
LPA1 is also upregulated in many cancer cell lines and primary tumors and may play an important role in regulating cancer malignancy. Studies showed that induction of LPA1 expression induced metastasis in breast cancer cells (296, 298) and initiated motility in human pancreatic cancer cells (299). Additionally, genetic knockdown or LPA1 inhibition reduced cytokine production, tumor proliferation, and bone lesions or metastases with breast and ovarian cancer cells (198). LPAR1 mutations were also found in an osteosarcoma cell line (300) and in lung and liver tumors in rats (301). Moreover, LPA1-initiated colony scattering (a prerequisite for metastasis) occurred in some, but not all, gastrointestinal epithelial cancer lines (302), suggesting that this phenomenon may require cross-talk with other signaling molecules. It was even reported that LPA2 inhibited pancreatic cancer cell migration while LPA1 induced migration in response to LPA (144). LPA receptor signaling promotes invasion and metastasis of several cancer types, but the role of each receptor may be different in each case. These pro- and anti-tumorigenic effects of LPA1–3 may reflect the differential expression of various growth factors and receptors.
Finally, ATX itself has been implicated in stimulating cancer cell motility (58), and may promote bone metastasis (303). Additionally, ATX was shown to be overexpressed in several primary tumor types (175, 304–307). ATX expression may also confer resistance to cancer treatments such as Taxol (308). Overexpression of LPA1–4 and ATX has been reported to enhance tumor aggression for numerous cancer types, whereas knockdown studies mitigate tumorigenic behaviors (309, 310). These responses may be mediated, at least in part, by Gα-induced RhoA and Rac signaling leading to cytoskeletal remodeling and migration (311). In addition, LPA receptor signaling stimulates cell proliferation through Ras signaling and suppresses apoptosis through PI3K and Akt activation (279).
LPA is also a known vasculature-altering factor, participating in the formation and stabilization of new blood vessels (70). These properties may cause LPA signaling to assist invasion and metastasis more indirectly by stimulating the rapidly growing vasculature of the cancer microenvironment to form leaky blood vessels, feeding the cancer cells and allowing easier extravasation of malignant cells. To this end, LPA has been reported to stimulate VEGF production, a prominent angiogenic factor and target for cancer therapy research (312–314). In addition to enhancing blood supply to tumors, VEGF has been shown to increase cancer cell migration, proliferation, invasion, and survival. In a study involving epithelial ovarian cancer, LPA-induced VEGF expression was discovered to be mediated by NF-κB through Gi signaling (315).
Several LPA receptor antagonists have been utilized in the study of cancer development. Notably, these drugs seem to attenuate metastatic and migratory behaviors in cancer cells. In one study, the pan-LPA antagonist BrP-LPA was synthesized and applied to MDA-MB-231 breast cancer cells in vitro and in a mouse xenograft model (195). The treated cancer cells demonstrated reduced migration, inhibition of cancer cell invasion, tumor regression, and decreased blood vessel density surrounding the tumor. In addition, use of LPA1/LPA3-specific inhibitors reduced pancreatic and breast cancer invasion and metastasis, but not tumor size (316, 317), while administration of the LPA2-selective antagonist Compound 35 to HCT-116 colorectal cancer cells inhibited mitogenic Erk phosphorylation and reduced cell proliferation (318). Finally, BrP-LPA sensitized the radiation-resistant vascular endothelial cells surrounding mouse gliomas to radiation therapy (196). This evidence points to active LPA signaling as an important factor in the cancer field and a possible target for cancer therapies.
CONCLUSION
The past 20 years have seen the cloning and identification of a growing family of LPA receptors and signaling pathways, coupled with a rapidly burgeoning field aimed at understanding the physiological and pathophysiological relevance of these lipids. It is now an exception, rather than the rule, to find an organ system that is not impacted by LPA and LPA signaling. Moreover, there is ever-growing understanding and appreciation that numerous human disease mechanisms are implicitly or explicitly linked to altered LPA signaling. Most exciting is the development of novel pharmacological modulators that serve as both research tools and potential medicinal therapies aimed at reducing human suffering. The prospects are bright for expanding insights and contributions in LPA biology.
Acknowledgments
The authors thank Ms. Danielle Jones and Dr. Hope Mirendil for critical editing of this manuscript.
Footnotes
Abbreviations:
- ATX
- autotaxin
- BrP-LPA
- α-bromomethylene phosphonate
- cPLA
- cytosolic phospholipase A
- CSF
- cerebrospinal fluid
- DC
- dendritic cell
- DGPP
- diacylglycerol pyrophosphate
- EGF
- epidermal growth factor
- EGFR
- epidermal growth factor receptor
- Enpp2
- ectonucleotide pyrophosphatase/phosphodiesterase family member 2
- FH
- fetal hydrocephalus
- GPCR
- G protein-coupled receptor
- IL
- interleukin
- LPA
- lysophosphatidic acid
- LPC
- lysophosphatidylcholine
- LPE
- lysophosphatidylethanolamine
- LPP
- lipid phosphate phosphatase
- LPS
- lysophosphatidylserine
- maLPA1
- Málaga lysophosphatidic acid 1
- NPC
- neuroprogenitor cell
- OL
- oligodendrocyte
- OMPT
- 1-oleoyl-2-O-methyl-rac-glycerophospho-thionate
- PA
- phosphatidic acid
- PI3K
- phosphatidylinositol 3-kinase
- PLA1
- phospholipase A1
- PLA2
- phospholipase A2
- PLC
- phospholipase C
- PSNL
- partial sciatic nerve ligation
- ROCK
- Rho-associated kinase
- SC
- Schwann cell
- SMC
- smooth muscle cell
- TGF
- transforming growth factor
- 7-TM
- 7-transmembrane
- VEGF
- vascular endothelial growth factor
- VZ
- ventricular zone
This work was supported by National Institutes of Health Grant NS082092 (J.C.) and Training Grant T32 GM007752. (N.C.S.).
REFERENCES
- 1.van Meer G., Voelker D. R., Feigenson G. W. 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9: 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogt W. 1963. Pharamacologically active acidic phospholipids and glycolipids. Biochem. Pharmacol. 12: 415–420 [DOI] [PubMed] [Google Scholar]
- 3.Sen S., Smeby R. R., Bumpus F. M. 1968. Antihypertensive effect of an isolated phospholipid. Am. J. Physiol. 214: 337–341 [DOI] [PubMed] [Google Scholar]
- 4.Tokumura A., Fukuzawa K., Tsukatani H. 1978. Effects of synthetic and natural lysophosphatidic acids on the arterial blood pressure of different animal species. Lipids. 13: 572–574 [DOI] [PubMed] [Google Scholar]
- 5.Blankley C. J., Kaplan H. R. 1984. Biologically active phospholipids as potential cardiovascular drugs. Drug Develop. Res. 4: 351–372 [Google Scholar]
- 6.Pörn M. I., Akerman K. E., Slotte J. P. 1991. High-density lipoproteins induce a rapid and transient release of Ca2+ in cultured fibroblasts. Biochem. J. 279: 29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerrard J. M., Kindom S. E., Peterson D. A., Peller J., Krantz K. E., White J. G. 1979. Lysophosphatidic acids. Influence on platelet aggregation and intracellular calcium flux. Am. J. Pathol. 96: 423–438 [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder F. 1990. Platelet-activating factor and related acetylated lipids as potent biologically active cellular mediators. Am. J. Physiol. 259: C697–C708 [DOI] [PubMed] [Google Scholar]
- 9.van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. 1989. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 59: 45–54 [DOI] [PubMed] [Google Scholar]
- 10.Hecht J. H., Weiner J. A., Post S. R., Chun J. 1996. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell Biol. 135: 1071–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun J., Hla T., Lynch K. R., Spiegel S., Moolenaar W. H. 2010. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 62: 579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An S., Bleu T., Huang W., Hallmark O. G., Coughlin S. R., Goetzl E. J. 1997. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 417: 279–282 [DOI] [PubMed] [Google Scholar]
- 13.An S., Bleu T., Hallmark O. G., Goetzl E. J. 1998. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J. Biol. Chem. 273: 7906–7910 [DOI] [PubMed] [Google Scholar]
- 14.Bandoh K., Aoki J., Hosono H., Kobayashi S., Kobayashi T., Murakami-Murofushi K., Tsujimoto M., Arai H., Inoue K. 1999. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J. Biol. Chem. 274: 27776–27785 [DOI] [PubMed] [Google Scholar]
- 15.Fukushima N., Ishii I., Contos J. J., Weiner J. A., Chun J. 2001. Lysophospholipid receptors. Annu. Rev. Pharmacol. Toxicol. 41: 507–534 [DOI] [PubMed] [Google Scholar]
- 16.Lee M. J., Thangada S., Liu C. H., Thompson B. D., Hla T. 1998. Lysophosphatidic acid stimulates the G-protein-coupled receptor EDG-1 as a low affinity agonist. J. Biol. Chem. 273: 22105–22112 [DOI] [PubMed] [Google Scholar]
- 17.Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 258: 1946–1949 [DOI] [PubMed] [Google Scholar]
- 18.Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 1995. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 215: 89–97 [DOI] [PubMed] [Google Scholar]
- 19.Noguchi K., Ishii S., Shimizu T. 2003. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J. Biol. Chem. 278: 25600–25606 [DOI] [PubMed] [Google Scholar]
- 20.Kotarsky K., Boketoft A., Bristulf J., Nilsson N. E., Norberg A., Hansson S., Owman C., Sillard R., Leeb-Lundberg L. M., Olde B. 2006. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J. Pharmacol. Exp. Ther. 318: 619–628 [DOI] [PubMed] [Google Scholar]
- 21.Lee C. W., Rivera R., Gardell S., Dubin A. E., Chun J. 2006. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J. Biol. Chem. 281: 23589–23597 [DOI] [PubMed] [Google Scholar]
- 22.Pasternack S. M., von Kugelgen I., Aboud K. A., Lee Y-A., Ruschendorf F., Voss K., Hillmer A. M., Molderings G. J., Franz T., Ramirez A., et al. 2008. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat. Genet. 40: 329–334 [DOI] [PubMed] [Google Scholar]
- 23.Yanagida K., Masago K., Nakanishi H., Kihara Y., Hamano F., Tajima Y., Taguchi R., Shimizu T., Ishii S. 2009. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J. Biol. Chem. 284: 17731–17741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makide K., Kitamura H., Sato Y., Okutani M., Aoki J. 2009. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins Other Lipid Mediat. 89: 135–139 [DOI] [PubMed] [Google Scholar]
- 25.Kano K., Arima N., Ohgami M., Aoki J. 2008. LPA and its analogs-attractive tools for elucidation of LPA biology and drug development. Curr. Med. Chem. 15: 2122–2131 [DOI] [PubMed] [Google Scholar]
- 26.Aoki J., Inoue A., Okudaira S. 2008. Two pathways for lysophosphatidic acid production. Biochim. Biophys. Acta. 1781: 513–518 [DOI] [PubMed] [Google Scholar]
- 27.Bektas M., Payne S. G., Liu H., Goparaju S., Milstien S., Spiegel S. 2005. A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J. Cell Biol. 169: 801–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagès C., Simon M-F., Valet P., Saulnier-Blache J. S. 2001. Lysophosphatidic acid synthesis and release. Prostaglandins Other Lipid Mediat. 64: 1–10 [DOI] [PubMed] [Google Scholar]
- 29.Okudaira S., Yukiura H., Aoki J. 2010. Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie. 92: 698–706 [DOI] [PubMed] [Google Scholar]
- 30.McIntyre T. M., Pontsler A. V., Silva A. R., St Hilaire A., Xu Y., Hinshaw J. C., Zimmerman G. A., Hama K., Aoki J., Arai H., et al. 2003. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad. Sci. USA. 100: 131–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoki J. 2004. Mechanisms of lysophosphatidic acid production. Semin. Cell Dev. Biol. 15: 477–489 [DOI] [PubMed] [Google Scholar]
- 32.Sugiura T., Nakane S., Kishimoto S., Waku K., Yoshioka Y., Tokumura A., Hanahan D. J. 1999. Occurrence of lysophosphatidic acid and its alkyl ether-linked analog in rat brain and comparison of their biological activities toward cultured neural cells. Biochim. Biophys. Acta. 1440: 194–204 [DOI] [PubMed] [Google Scholar]
- 33.Aoki J., Taira A., Takanezawa Y., Kishi Y., Hama K., Kishimoto T., Mizuno K., Saku K., Taguchi R., Arai H. 2002. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J. Biol. Chem. 277: 48737–48744 [DOI] [PubMed] [Google Scholar]
- 34.Hosogaya S., Yatomi Y., Nakamura K., Ohkawa R., Okubo S., Yokota H., Ohta M., Yamazaki H., Koike T., Ozaki Y. 2008. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: strong correlation with lysophospholipase D activity. Ann. Clin. Biochem. 45: 364–368 [DOI] [PubMed] [Google Scholar]
- 35.Watanabe N., Ikeda H., Nakamura K., Ohkawa R., Kume Y., Aoki J., Hama K., Okudaira S., Tanaka M., Tomiya T., et al. 2007. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J. Clin. Gastroenterol. 41: 616–623 [DOI] [PubMed] [Google Scholar]
- 36.Sano T., Baker D., Virag T., Wada A., Yatomi Y., Kobayashi T., Igarashi Y., Tigyi G. 2002. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J. Biol. Chem. 277: 21197–21206 [DOI] [PubMed] [Google Scholar]
- 37.Yatomi Y., Igarashi K., Nakamura K., Ohkawa R., Masuda A., Suzuki A., Kishimoto T., Ikeda H., Aoki J. 2013. Clinical introduction of lysophosphatidic acid (LPA) and autotaxin assays. In Lysophospholipid Receptors Signaling and Biochemistry. J. Chun, T. Hla, W. H. Moolenaar, et al., editors. Wiley, Hoboken, NJ. 709–735. [Google Scholar]
- 38.Scherer M., Schmitz G., Liebisch G. 2009. High-throughput analysis of sphingosine 1-phosphate, sphinganine 1-phosphate, and lysophosphatidic acid in plasma samples by liquid chromatography-tandem mass spectrometry. Clin. Chem. 55: 1218–1222 [DOI] [PubMed] [Google Scholar]
- 39.Tokumura A., Harada K., Fukuzawa K., Tsukatani H. 1986. Involvement of lysophospholipase D in the production of lysophosphatidic acid in rat plasma. Biochim. Biophys. Acta. 875: 31–38 [PubMed] [Google Scholar]
- 40.Eichholtz T., Jalink K., Fahrenfort I., Moolenaar W. H. 1993. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem. J. 291: 677–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka M., Kishi Y., Takanezawa Y., Kakehi Y., Aoki J., Arai H. 2004. Prostatic acid phosphatase degrades lysophosphatidic acid in seminal plasma. FEBS Lett. 571: 197–204 [DOI] [PubMed] [Google Scholar]
- 42.Ma L., Uchida H., Nagai J., Inoue M., Aoki J., Ueda H. 2010. Evidence for de novo synthesis of lysophosphatidic acid in the spinal cord through phospholipase A2 and autotaxin in nerve injury-induced neuropathic pain. J. Pharmacol. Exp. Ther. 333: 540–546 [DOI] [PubMed] [Google Scholar]
- 43.Yung Y. C., Mutoh T., Lin M. E., Noguchi K., Rivera R. R., Choi J. W., Kingsbury M. A., Chun J. 2011. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci. Transl. Med. 3: 99ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tokumura A., Taira S., Kikuchi M., Tsutsumi T., Shimizu Y., Watsky M. A. 2012. Lysophospholipids and lysophospholipase D in rabbit aqueous humor following corneal injury. Prostaglandins Other Lipid Mediat. 97: 83–89 [DOI] [PubMed] [Google Scholar]
- 45.Liliom K., Guan Z., Tseng J. L., Desiderio D. M., Tigyi G., Watsky M. A. 1998. Growth factor-like phospholipids generated after corneal injury. Am. J. Physiol. 274: C1065–C1074 [DOI] [PubMed] [Google Scholar]
- 46.Smyth S. S., Cheng H-Y., Miriyala S., Panchatcharam M., Morris A. J. 2008. Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochim. Biophys. Acta. 1781: 563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aaltonen N., Laitinen J. T., Lehtonen M. 2010. Quantification of lysophosphatidic acids in rat brain tissue by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878: 1145–1152 [DOI] [PubMed] [Google Scholar]
- 48.Lee J. W., Nishiumi S., Yoshida M., Fukusaki E., Bamba T. 2013. Simultaneous profiling of polar lipids by supercritical fluid chromatography/tandem mass spectrometry with methylation. J. Chromatogr. A. 1279: 98–107 [DOI] [PubMed] [Google Scholar]
- 49.Dohi T., Miyauchi K., Ohkawa R., Nakamura K., Kishimoto T., Miyazaki T., Nishino A., Nakajima N., Yaginuma K., Tamura H., et al. 2012. Increased circulating plasma lysophosphatidic acid in patients with acute coronary syndrome. Clin. Chim. Acta. 413: 207–212 [DOI] [PubMed] [Google Scholar]
- 50.Ikeda H., Enooku K., Ohkawa R., Koike K., Yatomi Y. 2013. Plasma lysophosphatidic acid levels and hepatocellular carcinoma. Hepatology. 57: 417–418 [DOI] [PubMed] [Google Scholar]
- 51.Brindley D. N., Pilquil C. 2009. Lipid phosphate phosphatases and signaling. J. Lipid Res. 50(Suppl): S225–S230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saba J. D. 2004. Lysophospholipids in development: miles apart and edging in. J. Cell. Biochem. 92: 967–992 [DOI] [PubMed] [Google Scholar]
- 53.Kok B. P., Venkatraman G., Capatos D., Brindley D. N. 2012. Unlike two peas in a pod: lipid phosphate phosphatases and phosphatidate phosphatases. Chem. Rev. 112: 5121–5146 [DOI] [PubMed] [Google Scholar]
- 54.Bräuer A. U., Savaskan N. E., Kühn H., Prehn S., Ninnemann O., Nitsch R. 2003. A new phospholipid phosphatase, PRG-1, is involved in axon growth and regenerative sprouting. Nat. Neurosci. 6: 572–578 [DOI] [PubMed] [Google Scholar]
- 55.Stracke M. L., Krutzsch H. C., Unsworth E. J., Arestad A., Cioce V., Schiffmann E., Liotta L. A. 1992. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J. Biol. Chem. 267: 2524–2529 [PubMed] [Google Scholar]
- 56.Tokumura A., Majima E., Kariya Y., Tominaga K., Kogure K., Yasuda K., Fukuzawa K. 2002. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 277: 39436–39442 [DOI] [PubMed] [Google Scholar]
- 57.Umezu-Goto M., Kishi Y., Taira A., Hama K., Dohmae N., Takio K., Yamori T., Mills G. B., Inoue K., Aoki J., et al. 2002. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 158: 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee H. Y., Clair T., Mulvaney P. T., Woodhouse E. C., Aznavoorian S., Liotta L. A., Stracke M. L. 1996. Stimulation of tumor cell motility linked to phosphodiesterase catalytic site of autotaxin. J. Biol. Chem. 271: 24408–24412 [DOI] [PubMed] [Google Scholar]
- 59.Hama K., Aoki J., Fukaya M., Kishi Y., Sakai T., Suzuki R., Ohta H., Yamori T., Watanabe M., Chun J., et al. 2004. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J. Biol. Chem. 279: 17634–17639 [DOI] [PubMed] [Google Scholar]
- 60.Bächner D., Ahrens M., Betat N., Schröder D., Gross G. 1999. Developmental expression analysis of murine autotaxin (ATX). Mech. Dev. 84: 121–125 [DOI] [PubMed] [Google Scholar]
- 61.Lee H. Y., Murata J., Clair T., Polymeropoulos M. H., Torres R., Manrow R. E., Liotta L. A., Stracke M. L. 1996. Cloning, chromosomal localization, and tissue expression of autotaxin from human teratocarcinoma cells. Biochem. Biophys. Res. Commun. 218: 714–719 [DOI] [PubMed] [Google Scholar]
- 62.Savaskan N. E., Rocha L., Kotter M. R., Baer A., Lubec G., van Meeteren L. A., Kishi Y., Aoki J., Moolenaar W. H., Nitsch R., et al. 2007. Autotaxin (NPP-2) in the brain: cell type-specific expression and regulation during development and after neurotrauma. Cell. Mol. Life Sci. 64: 230–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakamura K., Ohkawa R., Okubo S., Yokota H., Ikeda H., Yatomi Y., Igarashi K., Ide K., Kishimoto T., Masuda A., et al. 2009. Autotaxin enzyme immunoassay in human cerebrospinal fluid samples. Clin. Chim. Acta. 405: 160–162 [DOI] [PubMed] [Google Scholar]
- 64.Nakasaki T., Tanaka T., Okudaira S., Hirosawa M., Umemoto E., Otani K., Jin S., Bai Z., Hayasaka H., Fukui Y., et al. 2008. Involvement of the lysophosphatidic acid-generating enzyme autotaxin in lymphocyte-endothelial cell interactions. Am. J. Pathol. 173: 1566–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanda H., Newton R., Klein R., Morita Y., Gunn M. D., Rosen S. D. 2008. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat. Immunol. 9: 415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bai Z., Cai L., Umemoto E., Takeda A., Tohya K., Komai Y., Veeraveedu P. T., Hata E., Sugiura Y., Kubo A., et al. 2013. Constitutive lymphocyte transmigration across the basal lamina of high endothelial venules is regulated by the autotaxin/lysophosphatidic acid axis. J. Immunol. 190: 2036–2048 [DOI] [PubMed] [Google Scholar]
- 67.Tanaka M., Okudaira S., Kishi Y., Ohkawa R., Iseki S., Ota M., Noji S., Yatomi Y., Aoki J., Arai H. 2006. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 281: 25822–25830 [DOI] [PubMed] [Google Scholar]
- 68.van Meeteren L. A., Ruurs P., Stortelers C., Bouwman P., van Rooijen M. A., Pradere J. P., Pettit T. R., Wakelam M. J., Saulnier-Blache J. S., Mummery C. L., et al. 2006. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 26: 5015–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koike S., Keino-Masu K., Masu M. 2010. Deficiency of autotaxin/lysophospholipase D results in head cavity formation in mouse embryos through the LPA receptor-Rho-ROCK pathway. Biochem. Biophys. Res. Commun. 400: 66–71 [DOI] [PubMed] [Google Scholar]
- 70.Teo S. T., Yung Y. C., Herr D. R., Chun J. 2009. Lysophosphatidic acid in vascular development and disease. IUBMB Life. 61: 791–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Y., Ramakrishnan D. P., Ren B. 2013. Regulation of angiogenesis by phospholipid lysophosphatidic acid. Front. Biosci. (Landmark Ed). 18: 852–861 [DOI] [PubMed] [Google Scholar]
- 72.Panetti T. S., Chen H., Misenheimer T. M., Getzler S. B., Mosher D. F. 1997. Endothelial cell mitogenesis induced by LPA: inhibition by thrombospondin-1 and thrombospondin-2. J. Lab. Clin. Med. 129: 208–216 [DOI] [PubMed] [Google Scholar]
- 73.Lee H., Goetzl E. J., An S. 2000. Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. Am. J. Physiol. Cell Physiol. 278: C612–C618 [DOI] [PubMed] [Google Scholar]
- 74.Lin C. H., Lu J., Lee H. 2011. Interleukin-1β expression is required for lysophosphatidic acid-induced lymphangiogenesis in human umbilical vein endothelial cells. Int. J. Inflam. 2011: 351010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.English D., Kovala A. T., Welch Z., Harvey K. A., Siddiqui R. A., Brindley D. N., Garcia J. G. 1999. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J. Hematother. Stem Cell Res. 8: 627–634 [DOI] [PubMed] [Google Scholar]
- 76.Siess W., Zangl K. J., Essler M., Bauer M., Brandl R., Corrinth C., Bittman R., Tigyi G., Aepfelbacher M. 1999. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. USA. 96: 6931–6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishii I., Fukushima N., Ye X., Chun J. 2004. Lysophospholipid receptors: signaling and biology. Annu. Rev. Biochem. 73: 321–354 [DOI] [PubMed] [Google Scholar]
- 78.Anliker B., Chun J. 2004. Cell surface receptors in lysophospholipid signaling. Semin. Cell Dev. Biol. 15: 457–465 [DOI] [PubMed] [Google Scholar]
- 79.Pilquil C., Dewald J., Cherney A., Gorshkova I., Tigyi G., English D., Natarajan V., Brindley D. N. 2006. Lipid phosphate phosphatase-1 regulates lysophosphatidate-induced fibroblast migration by controlling phospholipase D2-dependent phosphatidate generation. J. Biol. Chem. 281: 38418–38429 [DOI] [PubMed] [Google Scholar]
- 80.Lee S. J., Leoni G., Neumann P. A., Chun J., Nusrat A., Yun C. C. 2013. Distinct phospholipase C-beta isozymes mediate lysophosphatidic acid receptor 1 effects on intestinal epithelial homeostasis and wound closure. Mol. Cell. Biol. 33: 2016–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tigyi G., Hong L., Yakubu M., Parfenova H., Shibata M., Leffler C. W. 1995. Lysophosphatidic acid alters cerebrovascular reactivity in piglets. Am. J. Physiol. 268: H2048–H2055 [DOI] [PubMed] [Google Scholar]
- 82.Ruisanchez É., Dancs P., Kerék M., Németh T., Faragó B., Balogh A., Patil R., Jennings B. L., Liliom K., Malik K. U., et al. 2014. Lysophosphatidic acid induces vasodilation mediated by LPA1 receptors, phospholipase C, and endothelial nitric oxide synthase. FASEB J. 28: 880–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davenport A. P., Alexander S. P., Sharman J. L., Pawson A. J., Benson H. E., Monaghan A. E., Liew W. C., Mpamhanga C. P., Bonner T. I., Neubig R. R., et al. 2013. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol. Rev. 65: 967–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Contos J. J., Chun J. 1998. Complete cDNA sequence, genomic structure, and chromosomal localization of the LPA receptor gene, lpA1/vzg-1/Gpcr26. Genomics. 51: 364–378 [DOI] [PubMed] [Google Scholar]
- 85.Young J. K., Clayton B. T., Kikonyogo A., Pham T. C., Parrill A. L. 2013. Structural characterization of an LPA1 second extracellular loop mimetic with a self-assembling coiled-coil folding constraint. Int. J. Mol. Sci. 14: 2788–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valentine W. J., Fells J. I., Perygin D. H., Mujahid S., Yokoyama K., Fujiwara Y., Tsukahara R., Van Brocklyn J. R., Parrill A. L., Tigyi G. 2008. Subtype-specific residues involved in ligand activation of the endothelial differentiation gene family lysophosphatidic acid receptors. J. Biol. Chem. 283: 12175–12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Contos J. J., Ishii I., Chun J. 2000. Lysophosphatidic acid receptors. Mol. Pharmacol. 58: 1188–1196 [DOI] [PubMed] [Google Scholar]
- 88.Ye X. 2008. Lysophospholipid signaling in the function and pathology of the reproductive system. Hum. Reprod. Update. 14: 519–536 [DOI] [PubMed] [Google Scholar]
- 89.Ohuchi H., Hamada A., Matsuda H., Takagi A., Tanaka M., Aoki J., Arai H., Noji S. 2008. Expression patterns of the lysophospholipid receptor genes during mouse early development. Dev. Dyn. 237: 3280–3294 [DOI] [PubMed] [Google Scholar]
- 90.Contos J. J., Fukushima N., Weiner J. A., Kaushal D., Chun J. 2000. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc. Natl. Acad. Sci. USA. 97: 13384–13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Allard J., Barron S., Diaz J., Lubetzki C., Zalc B., Schwartz J. C., Sokoloff P. 1998. A rat G protein-coupled receptor selectively expressed in myelin-forming cells. Eur. J. Neurosci. 10: 1045–1053 [DOI] [PubMed] [Google Scholar]
- 92.Weiner J. A., Hecht J. H., Chun J. 1998. Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J. Comp. Neurol. 398: 587–598 [PubMed] [Google Scholar]
- 93.Estivill-Torrús G., Llebrez-Zayas P., Matas-Rico E., Santin L., Pedraza C., De Diego I., Del Arco I., Fernández-Llebrez P., Chun J., De Fonseca F. R. 2008. Absence of LPA1 signaling results in defective cortical development. Cereb. Cortex. 18: 938–950 [DOI] [PubMed] [Google Scholar]
- 94.Harrison S. M., Reavill C., Brown G., Brown J. T., Cluderay J. E., Crook B., Davies C. H., Dawson L. A., Grau E., Heidbreder C., et al. 2003. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol. Cell. Neurosci. 24: 1170–1179 [DOI] [PubMed] [Google Scholar]
- 95.Kingsbury M. A., Rehen S. K., Contos J. J., Higgins C. M., Chun J. 2003. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat. Neurosci. 6: 1292–1299 [DOI] [PubMed] [Google Scholar]
- 96.Campbell D. S., Holt C. E. 2001. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 32: 1013–1026 [DOI] [PubMed] [Google Scholar]
- 97.Fukushima N., Weiner J. A., Chun J. 2000. Lysophosphatidic acid (LPA) is a novel extracellular regulator of cortical neuroblast morphology. Dev. Biol. 228: 6–18 [DOI] [PubMed] [Google Scholar]
- 98.Yuan X. B., Jin M., Xu X., Song Y. Q., Wu C. P., Poo M. M., Duan S. 2003. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat. Cell Biol. 5: 38–45 [DOI] [PubMed] [Google Scholar]
- 99.Fukushima N., Weiner J. A., Kaushal D., Contos J. J., Rehen S. K., Kingsbury M. A., Kim K. Y., Chun J. 2002. Lysophosphatidic acid influences the morphology and motility of young, postmitotic cortical neurons. Mol. Cell. Neurosci. 20: 271–282 [DOI] [PubMed] [Google Scholar]
- 100.Weiner J. A., Fukushima N., Contos J. J., Scherer S. S., Chun J. 2001. Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J. Neurosci. 21: 7069–7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jalink K., Eichholtz T., Postma F. R., van Corven E. J., Moolenaar W. H. 1993. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 4: 247–255 [PubMed] [Google Scholar]
- 102.Tigyi G., Fischer D. J., Sebok A., Yang C., Dyer D. L., Miledi R. 1996. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and Rho. J. Neurochem. 66: 537–548 [DOI] [PubMed] [Google Scholar]
- 103.Holtsberg F. W., Steiner M. R., Bruce-Keller A. J., Keller J. N., Mattson M. P., Moyers J. C., Steiner S. M. 1998. Lysophosphatidic acid and apoptosis of nerve growth factor-differentiated PC12 cells. J. Neurosci. Res. 53: 685–696 [DOI] [PubMed] [Google Scholar]
- 104.Zheng Z. Q., Fang X. J., Qiao J. T. 2004. Dual action of lysophosphatidic acid in cultured cortical neurons: survival and apoptogenic. Sheng Li Xue Bao. 56: 163–171 [PubMed] [Google Scholar]
- 105.Steiner M. R., Holtsberg F. W., Keller J. N., Mattson M. P., Steiner S. M. 2000. Lysophosphatidic acid induction of neuronal apoptosis and necrosis. Ann. N. Y. Acad. Sci. 905: 132–141 [DOI] [PubMed] [Google Scholar]
- 106.Fukushima N., Kimura Y., Chun J. 1998. A single receptor encoded by vzg-1/lpA1/edg-2 couples to G proteins and mediates multiple cellular responses to lysophosphatidic acid. Proc. Natl. Acad. Sci. USA. 95: 6151–6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Svetlov S. I., Ignatova T. N., Wang K. K., Hayes R. L., English D., Kukekov V. G. 2004. Lysophosphatidic acid induces clonal generation of mouse neurospheres via proliferation of Sca-1- and AC133-positive neural progenitors. Stem Cells Dev. 13: 685–693 [DOI] [PubMed] [Google Scholar]
- 108.Lloyd B., Tao Q., Lang S., Wylie C. 2005. Lysophosphatidic acid signaling controls cortical actin assembly and cytoarchitecture in Xenopus embryos. Development. 132: 805–816 [DOI] [PubMed] [Google Scholar]
- 109.Varsano T., Taupin V., Guo L., Baterina O. Y., Jr, Farquhar M. G. 2012. The PDZ protein GIPC regulates trafficking of the LPA1 receptor from APPL signaling endosomes and attenuates the cell’s response to LPA. PLoS ONE. 7: e49227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu F. X., Zhao B., Panupinthu N., Jewell J. L., Lian I., Wang L. H., Zhao J., Yuan H., Tumaneng K., Li H., et al. 2012. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 150: 780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cai H., Xu Y. 2013. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun. Signal. 11: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matas-Rico E., García-Diaz B., Llebrez-Zayas P., López-Barroso D., Santín L., Pedraza C., Smith-Fernández A., Fernández-Llebrez P., Tellez T., Redondo M., et al. 2008. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol. Cell. Neurosci. 39: 342–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pedraza C., Sanchez-Lopez J., Castilla-Ortega E., Rosell-Valle C., Zambrana-Infantes E., Garcia-Fernandez M., Rodriguez de Fonseca F., Chun J., Santin L. J., Estivill-Torrus G. Fear extinction and acute stress reactivity reveal a role of LPA receptor in regulating emotional-like behaviors. Brain Struct. Funct. Epub ahead of print. June 18, 2013; 10.1007/s00429-013-0592-9. [DOI] [PubMed] [Google Scholar]
- 114.Castilla-Ortega E., Hoyo-Becerra C., Pedraza C., Chun J., Rodriguez De Fonseca F., Estivill-Torrus G., Santin L. J. 2011. Aggravation of chronic stress effects on hippocampal neurogenesis and spatial memory in LPA(1) receptor knockout mice. PLoS ONE. 6: e25522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Boxtel R., Vroling B., Toonen P., Nijman I. J., van Roekel H., Verheul M., Baakman C., Guryev V., Vriend G., Cuppen E. 2011. Systematic generation of in vivo G protein-coupled receptor mutants in the rat. Pharmacogenomics J. 11: 326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nedergaard M., Ransom B., Goldman S. A. 2003. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 26: 523–530 [DOI] [PubMed] [Google Scholar]
- 117.Cervera P., Tirard M., Barron S., Allard J., Trottier S., Lacombe J., Daumas-Duport C., Sokoloff P. 2002. Immunohistological localization of the myelinating cell-specific receptor LP(A1). Glia. 38: 126–136 [DOI] [PubMed] [Google Scholar]
- 118.Shano S., Moriyama R., Chun J., Fukushima N. 2008. Lysophosphatidic acid stimulates astrocyte proliferation through LPA1. Neurochem. Int. 52: 216–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Manning T. J., Jr, Sontheimer H. 1997. Bovine serum albumin and lysophosphatidic acid stimulate calcium mobilization and reversal of cAMP-induced stellation in rat spinal cord astrocytes. Glia. 20: 163–172 [DOI] [PubMed] [Google Scholar]
- 120.Suidan H. S., Nobes C. D., Hall A., Monard D. 1997. Astrocyte spreading in response to thrombin and lysophosphatidic acid is dependent on the Rho GTPase. Glia. 21: 244–252 [DOI] [PubMed] [Google Scholar]
- 121.Rao T. S., Lariosa-Willingham K. D., Lin F. F., Palfreyman E. L., Yu N., Chun J., Webb M. 2003. Pharmacological characterization of lysophospholipid receptor signal transduction pathways in rat cerebrocortical astrocytes. Brain Res. 990: 182–194 [DOI] [PubMed] [Google Scholar]
- 122.Sorensen S. D., Nicole O., Peavy R. D., Montoya L. M., Lee C. J., Murphy T. J., Traynelis S. F., Hepler J. R. 2003. Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol. Pharmacol. 64: 1199–1209 [DOI] [PubMed] [Google Scholar]
- 123.Sato K., Horiuchi Y., Jin Y., Malchinkhuu E., Komachi M., Kondo T., Okajima F. 2011. Unmasking of LPA1 receptor-mediated migration response to lysophosphatidic acid by interleukin-1β-induced attenuation of Rho signaling pathways in rat astrocytes. J. Neurochem. 117: 164–174 [DOI] [PubMed] [Google Scholar]
- 124.Noguchi K., Herr D., Mutoh T., Chun J. 2009. Lysophosphatidic acid (LPA) and its receptors. Curr. Opin. Pharmacol. 9: 15–23 [DOI] [PubMed] [Google Scholar]
- 125.Spohr T. C., Choi J. W., Gardell S. E., Herr D. R., Rehen S. K., Gomes F. C., Chun J. 2008. Lysophosphatidic acid receptor-dependent secondary effects via astrocytes promote neuronal differentiation. J. Biol. Chem. 283: 7470–7479 [Erratum. 2009. J. Biol. Chem. 284: 36720.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Sampaio e Spohr T. C. L., Dezonne R. S., Rehen S. K., Gomes F. C. 2011. Astrocytes treated by lysophosphatidic acid induce axonal outgrowth of cortical progenitors through extracellular matrix protein and epidermal growth factor signaling pathway. J. Neurochem. 119: 113–123 [DOI] [PubMed] [Google Scholar]
- 127.Furukawa A., Kita K., Toyomoto M., Fujii S., Inoue S., Hayashi K., Ikeda K. 2007. Production of nerve growth factor enhanced in cultured mouse astrocytes by glycerophospholipids, sphingolipids, and their related compounds. Mol. Cell. Biochem. 305: 27–34 [DOI] [PubMed] [Google Scholar]
- 128.Yu N., Lariosa-Willingham K. D., Lin F. F., Webb M., Rao T. S. 2004. Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia. 45: 17–27 [DOI] [PubMed] [Google Scholar]
- 129.Stankoff B., Barron S., Allard J., Barbin G., Noel F., Aigrot M. S., Premont J., Sokoloff P., Zalc B., Lubetzki C. 2002. Oligodendroglial expression of Edg-2 receptor: developmental analysis and pharmacological responses to lysophosphatidic acid. Mol. Cell. Neurosci. 20: 415–428 [DOI] [PubMed] [Google Scholar]
- 130.Dawson J., Hotchin N., Lax S., Rumsby M. 2003. Lysophosphatidic acid induces process retraction in CG-4 line oligodendrocytes and oligodendrocyte precursor cells but not in differentiated oligodendrocytes. J. Neurochem. 87: 947–957 [DOI] [PubMed] [Google Scholar]
- 131.Nogaroli L., Yuelling L. M., Dennis J., Gorse K., Payne S. G., Fuss B. 2009. Lysophosphatidic acid can support the formation of membranous structures and an increase in MBP mRNA levels in differentiating oligodendrocytes. Neurochem. Res. 34: 182–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kobashi H., Yaoi T., Oda R., Okajima S., Fujiwara H., Kubo T., Fushiki S. 2006. Lysophospholipid receptors are differentially expressed in rat terminal Schwann cells, as revealed by a single cell rt-PCR and in situ hybridization. Acta Histochem. Cytochem. 39: 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weiner J. A., Chun J. 1999. Schwann cell survival mediated by the signaling phospholipid lysophosphatidic acid. Proc. Natl. Acad. Sci. USA. 96: 5233–5238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Y., Gonzalez M. I., Meinkoth J. L., Field J., Kazanietz M. G., Tennekoon G. I. 2003. Lysophosphatidic acid promotes survival and differentiation of rat Schwann cells. J. Biol. Chem. 278: 9585–9591 [DOI] [PubMed] [Google Scholar]
- 135.Anliker B., Choi J. W., Lin M. E., Gardell S. E., Rivera R. R., Kennedy G., Chun J. 2013. Lysophosphatidic acid (LPA) and its receptor, LPA1, influence embryonic Schwann cell migration, myelination, and cell-to-axon segregation. Glia. 61: 2009–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Contos J. J., Chun J. 2000. Genomic characterization of the lysophosphatidic acid receptor gene, lp(A2)/Edg4, and identification of a frameshift mutation in a previously characterized cDNA. Genomics. 64: 155–169 [DOI] [PubMed] [Google Scholar]
- 137.Diez-Roux G., Banfi S., Sultan M., Geffers L., Anand S., Rozado D., Magen A., Canidio E., Pagani M., Peluso I., et al. 2011. A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol. 9: e1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dubin A. E., Herr D. R., Chun J. 2010. Diversity of lysophosphatidic acid receptor-mediated intracellular calcium signaling in early cortical neurogenesis. J. Neurosci. 30: 7300–7309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Magdaleno S., Jensen P., Brumwell C. L., Seal A., Lehman K., Asbury A., Cheung T., Cornelius T., Batten D. M., Eden C., et al. 2006. BGEM: an in situ hybridization database of gene expression in the embryonic and adult mouse nervous system. PLoS Biol. 4: e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Choi J. W., Herr D. R., Noguchi K., Yung Y. C., Lee C. W., Mutoh T., Lin M. E., Teo S. T., Park K. E., Mosley A. N., et al. 2010. LPA receptors: subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 50: 157–186 [DOI] [PubMed] [Google Scholar]
- 141.Lai Y. J., Chen C. S., Lin W. C., Lin F. T. 2005. c-Src-mediated phosphorylation of TRIP6 regulates its function in lysophosphatidic acid-induced cell migration. Mol. Cell. Biol. 25: 5859–5868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lai Y. J., Lin W. C., Lin F. T. 2007. PTPL1/FAP-1 negatively regulates TRIP6 function in lysophosphatidic acid-induced cell migration. J. Biol. Chem. 282: 24381–24387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin F. T., Lai Y. J. 2008. Regulation of the LPA2 receptor signaling through the carboxyl-terminal tail-mediated protein-protein interactions. Biochim. Biophys. Acta. 1781: 558–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Komachi M., Tomura H., Malchinkhuu E., Tobo M., Mogi C., Yamada T., Kimura T., Kuwabara A., Ohta H., Im D. S., et al. 2009. LPA1 receptors mediate stimulation, whereas LPA2 receptors mediate inhibition, of migration of pancreatic cancer cells in response to lysophosphatidic acid and malignant ascites. Carcinogenesis. 30: 457–465 [DOI] [PubMed] [Google Scholar]
- 145.Gschwind A., Zwick E., Prenzel N., Leserer M., Ullrich A. 2001. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene. 20: 1594–1600 [DOI] [PubMed] [Google Scholar]
- 146.Yang A. H., Ishii I., Chun J. 2002. In vivo roles of lysophospholipid receptors revealed by gene targeting studies in mice. Biochim. Biophys. Acta. 1582: 197–203 [DOI] [PubMed] [Google Scholar]
- 147.Inoue M., Rashid M. H., Fujita R., Contos J. J., Chun J., Ueda H. 2004. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med. 10: 712–718 [DOI] [PubMed] [Google Scholar]
- 148.Trimbuch T., Beed P., Vogt J., Schuchmann S., Maier N., Kintscher M., Breustedt J., Schuelke M., Streu N., Kieselmann O., et al. 2009. Synaptic PRG-1 modulates excitatory transmission via lipid phosphate-mediated signaling. Cell. 138: 1222–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Panchatcharam M., Miriyala S., Yang F., Rojas M., End C., Vallant C., Dong A., Lynch K., Chun J., Morris A. J., et al. 2008. Lysophosphatidic acid receptors 1 and 2 play roles in regulation of vascular injury responses but not blood pressure. Circ. Res. 103: 662–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sarker M. H., Hu D-E., Fraser P. A. 2010. Regulation of cerebromicrovascular permeability by lysophosphatidic acid. Microcirculation. 17: 39–46 [DOI] [PubMed] [Google Scholar]
- 151.Subramanian P., Karshovska E., Reinhard P., Megens R. T. A., Zhou Z., Akhtar S., Schumann U., Li X., van Zandvoort M., Ludin C., et al. 2010. Lysophosphatidic acid receptors LPA1 and LPA3 promote CXCL12-mediated smooth muscle progenitor cell recruitment in neointima formation. Circ. Res. 107: 96–105 [DOI] [PubMed] [Google Scholar]
- 152.Panther E., Idzko M., Corinti S., Ferrari D., Herouy Y., Mockenhaupt M., Dichmann S., Gebicke-Haerter P., Di Virgilio F., Girolomoni G., et al. 2002. The influence of lysophosphatidic acid on the functions of human dendritic cells. J. Immunol. 169: 4129–4135 [DOI] [PubMed] [Google Scholar]
- 153.Chen R., Roman J., Guo J., West E., McDyer J., Williams M. A., Georas S. N. 2006. Lysophosphatidic acid modulates the activation of human monocyte-derived dendritic cells. Stem Cells Dev. 15: 797–804 [DOI] [PubMed] [Google Scholar]
- 154.Oh D. Y., Yoon J. M., Moon M. J., Hwang J. I., Choe H., Lee J. Y., Kim J. I., Kim S., Rhim H., O’Dell D. K., et al. 2008. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J. Biol. Chem. 283: 21054–21064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng Y., Kong Y., Goetzl E. J. 2001. Lysophosphatidic acid receptor-selective effects on Jurkat T cell migration through a Matrigel model basement membrane. J. Immunol. 166: 2317–2322 [DOI] [PubMed] [Google Scholar]
- 156.Zheng Y., Voice J. K., Kong Y., Goetzl E. J. 2000. Altered expression and functional profile of lysophosphatidic acid receptors in mitogen-activated human blood T lymphocytes. FASEB J. 14: 2387–2389 [DOI] [PubMed] [Google Scholar]
- 157.Rubenfeld J., Guo J., Sookrung N., Chen R., Chaicumpa W., Casolaro V., Zhao Y., Natarajan V., Georas S. 2006. Lysophosphatidic acid enhances interleukin-13 gene expression and promoter activity in T cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 290: L66–L74 [DOI] [PubMed] [Google Scholar]
- 158.Chan L. C., Peters W., Xu Y., Chun J., Farese R. V., Jr, Cases S. 2007. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA). J. Leukoc. Biol. 82: 1193–1200 [DOI] [PubMed] [Google Scholar]
- 159.Emo J., Meednu N., Chapman T. J., Rezaee F., Balys M., Randall T., Rangasamy T., Georas S. N. 2012. Lpa2 is a negative regulator of both dendritic cell activation and murine models of allergic lung inflammation. J. Immunol. 188: 3784–3790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Im D. S., Heise C. E., Harding M. A., George S. R., O’Dowd B. F., Theodorescu D., Lynch K. R. 2000. Molecular cloning and characterization of a lysophosphatidic acid receptor, Edg-7, expressed in prostate. Mol. Pharmacol. 57: 753–759 [PubMed] [Google Scholar]
- 161.Ishii I., Contos J. J., Fukushima N., Chun J. 2000. Functional comparisons of the lysophosphatidic acid receptors, LP(A1)/VZG-1/EDG-2, LP(A2)/EDG-4, and LP(A3)/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol. Pharmacol. 58: 895–902 [DOI] [PubMed] [Google Scholar]
- 162.Sonoda H., Aoki J., Hiramatsu T., Ishida M., Bandoh K., Nagai Y., Taguchi R., Inoue K., Arai H. 2002. A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. J. Biol. Chem. 277: 34254–34263 [DOI] [PubMed] [Google Scholar]
- 163.Ye X., Hama K., Contos J. J., Anliker B., Inoue A., Skinner M. K., Suzuki H., Amano T., Kennedy G., Arai H., et al. 2005. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 435: 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liszewska E., Reinaud P., Dubois O., Charpigny G. 2012. Lysophosphatidic acid receptors in ovine uterus during estrous cycle and early pregnancy and their regulation by progesterone. Domest. Anim. Endocrinol. 42: 31–42 [DOI] [PubMed] [Google Scholar]
- 165.Hama K., Aoki J., Bandoh K., Inoue A., Endo T., Amano T., Suzuki H., Arai H. 2006. Lysophosphatidic receptor, LPA3, is positively and negatively regulated by progesterone and estrogen in the mouse uterus. Life Sci. 79: 1736–1740 [DOI] [PubMed] [Google Scholar]
- 166.Tokumura A., Miyake M., Nishioka Y., Yamano S., Aono T., Fukuzawa K. 1999. Production of lysophosphatidic acids by lysophospholipase D in human follicular fluids of in vitro fertilization patients. Biol. Reprod. 61: 195–199 [DOI] [PubMed] [Google Scholar]
- 167.Iwasawa Y., Fujii T., Nagamatsu T., Kawana K., Okudaira S., Miura S., Matsumoto J., Tomio A., Hyodo H., Yamashita T., et al. 2009. Expression of autotaxin, an ectoenzyme that produces lysophosphatidic acid, in human placenta. Am. J. Reprod. Immunol. 62: 90–95 [DOI] [PubMed] [Google Scholar]
- 168.Masuda A., Fujii T., Iwasawa Y., Nakamura K., Ohkawa R., Igarashi K., Okudaira S., Ikeda H., Kozuma S., Aoki J., et al. 2011. Serum autotaxin measurements in pregnant women: application for the differentiation of normal pregnancy and pregnancy-induced hypertension. Clin. Chim. Acta. 412: 1944–1950 [DOI] [PubMed] [Google Scholar]
- 169.Lai S. L., Yao W. L., Tsao K. C., Houben A. J., Albers H. M., Ovaa H., Moolenaar W. H., Lee S. J. 2012. Autotaxin/Lpar3 signaling regulates Kupffer’s vesicle formation and left-right asymmetry in zebrafish. Development. 139: 4439–4448 [DOI] [PubMed] [Google Scholar]
- 170.Lee C. W., Rivera R., Dubin A. E., Chun J. 2007. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J. Biol. Chem. 282: 4310–4317 [DOI] [PubMed] [Google Scholar]
- 171.Yanagida K., Ishii S., Hamano F., Noguchi K., Shimizu T. 2007. LPA4/p2y9/GPR23 mediates rho-dependent morphological changes in a rat neuronal cell line. J. Biol. Chem. 282: 5814–5824 [DOI] [PubMed] [Google Scholar]
- 172.Rhee H. J., Nam J. S., Sun Y., Kim M. J., Choi H. K., Han D. H., Kim N. H., Huh S. O. 2006. Lysophosphatidic acid stimulates cAMP accumulation and cAMP response element-binding protein phosphorylation in immortalized hippocampal progenitor cells. Neuroreport. 17: 523–526 [DOI] [PubMed] [Google Scholar]
- 173.Lee Z., Cheng C. T., Zhang H., Subler M. A., Wu J., Mukherjee A., Windle J. J., Chen C. K., Fang X. 2008. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol. Biol. Cell. 19: 5435–5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mansell J. P., Barbour M., Moore C., Nowghani M., Pabbruwe M., Sjostrom T., Blom A. W. 2010. The synergistic effects of lysophosphatidic acid receptor agonists and calcitriol on MG63 osteoblast maturation at titanium and hydroxyapatite surfaces. Biomaterials. 31: 199–206 [DOI] [PubMed] [Google Scholar]
- 175.Liu S., Umezu-Goto M., Murph M., Lu Y., Liu W., Zhang F., Yu S., Stephens L. C., Cui X., Murrow G., et al. 2009. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 15: 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gennero I., Laurencin-Dalicieux S., Conte-Auriol F., Briand-Mesange F., Laurencin D., Rue J., Beton N., Malet N., Mus M., Tokumura A., et al. 2011. Absence of the lysophosphatidic acid receptor LPA1 results in abnormal bone development and decreased bone mass. Bone. 49: 395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu Y. B., Kharode Y., Bodine P. V., Yaworsky P. J., Robinson J. A., Billiard J. 2010. LPA induces osteoblast differentiation through interplay of two receptors: LPA1 and LPA4. J. Cell. Biochem. 109: 794–800 [DOI] [PubMed] [Google Scholar]
- 178.Sumida H., Noguchi K., Kihara Y., Abe M., Yanagida K., Hamano F., Sato S., Tamaki K., Morishita Y., Kano M. R., et al. 2010. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood. 116: 5060–5070 [DOI] [PubMed] [Google Scholar]
- 179.Morishige J., Uto Y., Hori H., Satouchi K., Yoshiomoto T., Tokumura A. 2013. Lysophosphatidic acid produced by hen egg white lysophospholipase D induces vascular development on extraembryonic membranes. Lipids. 48: 251–262 [DOI] [PubMed] [Google Scholar]
- 180.Amisten S., Braun O. O., Bengtsson A., Erlinge D. 2008. Gene expression profiling for the identification of G-protein coupled receptors in human platelets. Thromb. Res. 122: 47–57 [DOI] [PubMed] [Google Scholar]
- 181.Lundequist A., Boyce J. A. 2011. LPA5 is abundantly expressed by human mast cells and important for lysophosphatidic acid induced MIP-1β release. PLoS ONE. 6: e18192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Williams J. R., Khandoga A. L., Goyal P., Fells J. I., Perygin D. H., Siess W., Parrill A. L., Tigyi G., Fujiwara Y. 2009. Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J. Biol. Chem. 284: 17304–17319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jongsma M., Matas-Rico E., Rzadkowski A., Jalink K., Moolenaar W. H. 2011. LPA is a chemorepellent for B16 melanoma cells: action through the cAMP-elevating LPA5 receptor. PLoS ONE. 6: e29260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lin S., Yeruva S., He P., Singh A. K., Zhang H., Chen M., Lamprecht G., de Jonge H. R., Tse M., Donowitz M., et al. 2010. Lysophosphatidic acid stimulates the intestinal brush border Na(+)/H(+) exchanger 3 and fluid absorption via LPA(5) and NHERF2. Gastroenterology. 138: 649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kaplan M. H., Smith D. I., Sundick R. S. 1993. Identification of a G protein coupled receptor induced in activated T cells. J. Immunol. 151: 628–636 [PubMed] [Google Scholar]
- 186.Webb T. E., Kaplan M. G., Barnard E. A. 1996. Identification of 6H1 as a P2Y purinoceptor: P2Y5. Biochem. Biophys. Res. Commun. 219: 105–110 [DOI] [PubMed] [Google Scholar]
- 187.Lee M., Choi S., Hallden G., Yo S. J., Schichnes D., Aponte G. W. 2009. P2Y5 is a G(alpha)i, G(alpha)12/13 G protein-coupled receptor activated by lysophosphatidic acid that reduces intestinal cell adhesion. Am. J. Physiol. Gastrointest. Liver Physiol. 297: G641–G654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shimomura Y., Garzon M. C., Kristal L., Shapiro L., Christiano A. M. 2009. Autosomal recessive woolly hair with hypotrichosis caused by a novel homozygous mutation in the P2RY5 gene. Exp. Dermatol. 18: 218–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nahum S., Morice-Picard F., Taieb A., Sprecher E. 2011. A novel mutation in LPAR6 causes autosomal recessive hypotrichosis of the scalp. Clin. Exp. Dermatol. 36: 188–194 [DOI] [PubMed] [Google Scholar]
- 190.Pasternack S. M., von Kugelgen I., Muller M., Oji V., Traupe H., Sprecher E., Nothen M. M., Janecke A. R., Betz R. C. 2009. In vitro analysis of LIPH mutations causing hypotrichosis simplex: evidence confirming the role of lipase H and lysophosphatidic acid in hair growth. J. Invest. Dermatol. 129: 2772–2776 [DOI] [PubMed] [Google Scholar]
- 191.Shinkuma S., Akiyama M., Inoue A., Aoki J., Natsuga K., Nomura T., Arita K., Abe R., Ito K., Nakamura H., et al. 2010. Prevalent LIPH founder mutations lead to loss of P2Y5 activation ability of PA-PLA1alpha in autosomal recessive hypotrichosis. Hum. Mutat. 31: 602–610 [DOI] [PubMed] [Google Scholar]
- 192.Inoue A., Arima N., Ishiguro J., Prestwich G. D., Arai H., Aoki J. 2011. LPA-producing enzyme PA-PLA(1)α regulates hair follicle development by modulating EGFR signalling. EMBO J. 30: 4248–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jiang G., Xu Y., Fujiwara Y., Tsukahara T., Tsukahara R., Gajewiak J., Tigyi G., Prestwich G. D. 2007. Alpha-substituted phosphonate analogues of lysophosphatidic acid (LPA) selectively inhibit production and action of LPA. ChemMedChem. 2: 679–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gajewiak J., Tsukahara R., Fujiwara Y., Tigyi G., Prestwich G. D. 2008. Synthesis, pharmacology, and cell biology of sn-2-aminooxy analogues of lysophosphatidic acid. Org. Lett. 10: 1111–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang H., Xu X., Gajewiak J., Tsukahara R., Fujiwara Y., Liu J., Fells J. I., Perygin D., Parrill A. L., Tigyi G., et al. 2009. Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res. 69: 5441–5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schleicher S. M., Thotala D. K., Linkous A. G., Hu R., Leahy K. M., Yazlovitskaya E. M., Hallahan D. E. 2011. Autotaxin and LPA receptors represent potential molecular targets for the radiosensitization of murine glioma through effects on tumor vasculature. PLoS ONE. 6: e22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Swaney J. S., Chapman C., Correa L. D., Stebbins K. J., Bundey R. A., Prodanovich P. C., Fagan P., Baccei C. S., Santini A. M., Hutchinson J. H., et al. 2010. A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br. J. Pharmacol. 160: 1699–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Boucharaba A., Serre C. M., Guglielmi J., Bordet J. C., Clezardin P., Peyruchaud O. 2006. The type 1 lysophosphatidic acid receptor is a target for therapy in bone metastases. Proc. Natl. Acad. Sci. USA. 103: 9643–9648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Okusa M. D., Ye H., Huang L., Sigismund L., Macdonald T., Lynch K. R. 2003. Selective blockade of lysophosphatidic acid LPA3 receptors reduces murine renal ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 285: F565–F574 [DOI] [PubMed] [Google Scholar]
- 200.Moll S., Chaykovska L., Meier M., Budd D. C., Formentini I., Pomposiello S., Prunotto M. 2013. Targeting the epithelial cells in fibrosis: a new concept for an old disease. Drug Discov. Today. 18: 582–591 [DOI] [PubMed] [Google Scholar]
- 201.Hultman C. M., Sparen P., Takei N., Murray R. M., Cnattingius S. 1999. Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: case-control study. BMJ. 318: 421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vukojević M., Soldo I., Granić D. 2009. Risk factors associated with cerebral palsy in newborns. Coll. Antropol. 33(Suppl 2): 199–201 [PubMed] [Google Scholar]
- 203.Whitelaw A. 2001. Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Semin. Neonatol. 6: 135–146 [DOI] [PubMed] [Google Scholar]
- 204.Herr K. J., Herr D. R., Lee C. W., Noguchi K., Chun J. 2011. Stereotyped fetal brain disorganization is induced by hypoxia and requires lysophosphatidic acid receptor 1 (LPA1) signaling. Proc. Natl. Acad. Sci. USA. 108: 15444–15449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Aquilina K., Hobbs C., Cherian S., Tucker A., Porter H., Whitelaw A., Thoresen M. 2007. A neonatal piglet model of intraventricular hemorrhage and posthemorrhagic ventricular dilation. J. Neurosurg. 107: 126–136 [DOI] [PubMed] [Google Scholar]
- 206.Lodhia K. R., Shakui P., Keep R. F. 2006. Hydrocephalus in a rat model of intraventricular hemorrhage. Acta Neurochir. Suppl. 96: 207–211 [DOI] [PubMed] [Google Scholar]
- 207.Deleted in proof.
- 208.Byrne M., Agerbo E., Bennedsen B., Eaton W. W., Mortensen P. B. 2007. Obstetric conditions and risk of first admission with schizophrenia: a Danish national register based study. Schizophr. Res. 97: 51–59 [DOI] [PubMed] [Google Scholar]
- 209.Brimacombe M., Ming X., Lamendola M. 2007. Prenatal and birth complications in autism. Matern. Child Health J. 11: 73–79 [DOI] [PubMed] [Google Scholar]
- 210.Roberts C., Winter P., Shilliam C. S., Hughes Z. A., Langmead C., Maycox P. R., Dawson L. A. 2005. Neurochemical changes in LPA1 receptor deficient mice–a putative model of schizophrenia. Neurochem. Res. 30: 371–377 [DOI] [PubMed] [Google Scholar]
- 211.Musazzi L., Di Daniel E., Maycox P., Racagni G., Popoli M. 2010. Abnormalities in α/β-CaMKII and related mechanisms suggest synaptic dysfunction in hippocampus of LPA1 receptor knockout mice. Int. J. Neuropsychopharmacol. 14: 941–953 [DOI] [PubMed] [Google Scholar]
- 212.Cunningham M. O., Hunt J., Middleton S., LeBeau F. E., Gillies M. J., Davies C. H., Maycox P. R., Whittington M. A., Racca C. 2006. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J. Neurosci. 26: 2767–2776 [Erratum. 2006. J. Neurosci. 26: table of contents.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Santin L. J., Bilbao A., Pedraza C., Matas-Rico E., Lopez-Barroso D., Castilla-Ortega E., Sanchez-Lopez J., Riquelme R., Varela-Nieto I., de la Villa P., et al. 2009. Behavioral phenotype of maLPA1-null mice: increased anxiety-like behavior and spatial memory deficits. Genes Brain Behav. 8: 772–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Castilla-Ortega E., Sánchez-López J., Hoyo-Becerra C., Matas-Rico E., Zambrana-Infantes E., Chun J., De Fonseca F. R., Pedraza C., Estivill-Torrús G., Santin L. J. 2010. Exploratory, anxiety and spatial memory impairments are dissociated in mice lacking the LPA1 receptor. Neurobiol. Learn. Mem. 94: 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ong W. Y., Farooqui T., Farooqui A. A. 2010. Involvement of cytosolic phospholipase A2, calcium independent phospholipase A2 and plasmalogen selective phospholipase A2 in neurodegenerative and neuropsychiatric conditions. Curr. Med. Chem. 17: 2746–2763 [DOI] [PubMed] [Google Scholar]
- 216.Renbäck K., Inoue M., Ueda H. 1999. Lysophosphatidic acid-induced, pertussis toxin-sensitive nociception through a substance P release from peripheral nerve endings in mice. Neurosci. Lett. 270: 59–61 [DOI] [PubMed] [Google Scholar]
- 217.Lin M. E., Rivera R. R., Chun J. 2012. Targeted deletion of LPA5 identifies novel roles for lysophosphatidic acid signaling in development of neuropathic pain. J. Biol. Chem. 287: 17608–17617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fujita R., Kiguchi N., Ueda H. 2007. LPA-mediated demyelination in ex vivo culture of dorsal root. Neurochem. Int. 50: 351–355 [DOI] [PubMed] [Google Scholar]
- 219.Inoue M., Xie W., Matsushita Y., Chun J., Aoki J., Ueda H. 2008. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience. 152: 296–298 [DOI] [PubMed] [Google Scholar]
- 220.Inoue M., Ma L., Aoki J., Chun J., Ueda H. 2008. Autotaxin, a synthetic enzyme of lysophosphatidic acid (LPA), mediates the induction of nerve-injured neuropathic pain. Mol. Pain. 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ma L., Uchida H., Nagai J., Inoue M., Chun J., Aoki J., Ueda H. 2009. Lysophosphatidic acid-3 receptor-mediated feed-forward production of lysophosphatidic acid: an initiator of nerve injury-induced neuropathic pain. Mol. Pain. 5: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kakiuchi Y., Nagai J., Gotoh M., Hotta H., Murofushi H., Ogawa T., Ueda H., Murakami-Murofushi K. 2011. Antinociceptive effect of cyclic phosphatidic acid and its derivative on animal models of acute and chronic pain. Mol. Pain. 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ma L., Nagai J., Chun J., Ueda H. 2013. An LPA species (18:1 LPA) plays key roles in the self-amplification of spinal LPA production in the peripheral neuropathic pain model. Mol. Pain. 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Siess W. 2002. Athero- and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim. Biophys. Acta. 1582: 204–215 [DOI] [PubMed] [Google Scholar]
- 225.Lin C. I., Chen C. N., Chen J. H., Lee H. 2006. Lysophospholipids increase IL-8 and MCP-1 expressions in human umbilical cord vein endothelial cells through an IL-1-dependent mechanism. J. Cell. Biochem. 99: 1216–1232 [DOI] [PubMed] [Google Scholar]
- 226.Palmetshofer A., Robson S. C., Nehls V. 1999. Lysophosphatidic acid activates nuclear factor kappa B and induces proinflammatory gene expression in endothelial cells. Thromb. Haemost. 82: 1532–1537 [PubMed] [Google Scholar]
- 227.Chang C. L., Hsu H. Y., Lin H. Y., Chiang W., Lee H. 2008. Lysophosphatidic acid-induced oxidized low-density lipoprotein uptake is class A scavenger receptor-dependent in macrophages. Prostaglandins Other Lipid Mediat. 87: 20–25 [DOI] [PubMed] [Google Scholar]
- 228.Neidlinger N. A., Larkin S. K., Bhagat A., Victorino G. P., Kuypers F. A. 2006. Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction. J. Biol. Chem. 281: 775–781 [DOI] [PubMed] [Google Scholar]
- 229.Rother E., Brandl R., Baker D. L., Goyal P., Gebhard H., Tigyi G., Siess W. 2003. Subtype-selective antagonists of lysophosphatidic acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 108: 741–747 [DOI] [PubMed] [Google Scholar]
- 230.Willoughby S., Holmes A., Loscalzo J. 2002. Platelets and cardiovascular disease. Eur. J. Cardiovasc. Nurs. 1: 273–288 [DOI] [PubMed] [Google Scholar]
- 231.Li Z-G., Yu Z-C., Wang D-Z., Ju W-P., Zhan X., Wu Q-Z., Wu X-J., Cong H-M., Man H-H. 2008. Influence of acetylsalicylate on plasma lysophosphatidic acid level in patients with ischemic cerebral vascular diseases. Neurol. Res. 30: 366–369 [DOI] [PubMed] [Google Scholar]
- 232.van Nieuw Amerongen G. P., Vermeer M. A., van Hinsbergh V. W. 2000. Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler. Thromb. Vasc. Biol. 20: E127–E133 [DOI] [PubMed] [Google Scholar]
- 233.Karliner J. S., Honbo N., Summers K., Gray M. O., Goetzl E. J. 2001. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J. Mol. Cell. Cardiol. 33: 1713–1717 [DOI] [PubMed] [Google Scholar]
- 234.Chen J., Baydoun A. R., Xu R., Deng L., Liu X., Zhu W., Shi L., Cong X., Hu S., Chen X. 2008. Lysophosphatidic acid protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis. Stem Cells. 26: 135–145 [DOI] [PubMed] [Google Scholar]
- 235.Savitz S. I., Dhallu M. S., Malhotra S., Mammis A., Ocava L. C., Rosenbaum P. S., Rosenbaum D. M. 2006. EDG receptors as a potential therapeutic target in retinal ischemia-reperfusion injury. Brain Res. 1118: 168–175 [DOI] [PubMed] [Google Scholar]
- 236.Brault S., Gobeil F., Jr, Fortier A., Honore J. C., Joyal J. S., Sapieha P. S., Kooli A., Martin E., Hardy P., Ribeiro-da-Silva A., et al. 2007. Lysophosphatidic acid induces endothelial cell death by modulating the redox environment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292: R1174–R1183 [DOI] [PubMed] [Google Scholar]
- 237.Tabuchi S., Kume K., Aihara M., Shimizu T. 2000. Expression of lysophosphatidic acid receptor in rat astrocytes: mitogenic effect and expression of neurotrophic genes. Neurochem. Res. 25: 573–582 [DOI] [PubMed] [Google Scholar]
- 238.Yin Z., Carbone L. D., Gotoh M., Postlethwaite A., Bolen A. L., Tigyi G. J., Murakami-Murofushi K., Watsky M. A. 2010. Lysophosphatidic acid-activated Cl- current activity in human systemic sclerosis skin fibroblasts. Rheumatology. 49: 2290–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tokumura A., Carbone L. D., Yoshioka Y., Morishige J., Kikuchi M., Postlethwaite A., Watsky M. A. 2009. Elevated serum levels of arachidonoyl-lysophosphatidic acid and sphingosine-1-phosphate in systemic sclerosis. Int. J. Med. Sci. 6: 168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Park G. Y., Lee Y. G., Berdyshev E., Nyenhuis S., Du J., Fu P., Gorshkova I. A., Li Y., Chung S., Karpurapu M., et al. 2013. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am. J. Respir. Crit. Care Med. 188: 928–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhao C., Fernandes M. J., Prestwich G. D., Turgeon M. l., Di Battista J., Clair T., Poubelle P. E., Bourgoin S. G. 2008. Regulation of lysophosphatidic acid receptor expression and function in human synoviocytes: implications for rheumatoid arthritis? Mol. Pharmacol. 73: 587–600 [DOI] [PubMed] [Google Scholar]
- 242.Miyabe Y., Miyabe C., Iwai Y., Takayasu A., Fukuda S., Yokoyama W., Nagai J., Jona M., Tokuhara Y., Ohkawa R., et al. 2013. Necessity of lysophosphatidic acid receptor 1 for development of arthritis. Arthritis Rheum. 65: 2037–2047 [DOI] [PubMed] [Google Scholar]
- 243.Mototani H., Iida A., Nakajima M., Furuichi T., Miyamoto Y., Tsunoda T., Sudo A., Kotani A., Uchida A., Ozaki K., et al. 2008. A functional SNP in EDG2 increases susceptibility to knee osteoarthritis in Japanese. Hum. Mol. Genet. 17: 1790–1797 [DOI] [PubMed] [Google Scholar]
- 244.Orosa B., Garcia S., Martinez P., Gonzalez A., Gomez-Reino J. J., Conde C. 2014. Lysophosphatidic acid receptor inhibition as a new multipronged treatment for rheumatoid arthritis. Ann. Rheum. Dis. 73 298–305 [DOI] [PubMed] [Google Scholar]
- 245.Tager A. M., LaCamera P., Shea B. S., Campanella G. S., Selman M., Zhao Z., Polosukhin V., Wain J., Karimi-Shah B. A., Kim N. D., et al. 2008. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 14: 45–54 [DOI] [PubMed] [Google Scholar]
- 246.Pradère J. P., Klein J., Grès S., Guigné C., Neau E., Valet P., Calise D., Chun J., Bascands J. L., Saulnier-Blache J. S., et al. 2007. LPA1 receptor activation promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 18: 3110–3118 [DOI] [PubMed] [Google Scholar]
- 247.Sakai N., Chun J., Duffield J. S., Wada T., Luster A. D., Tager A. M. 2013. LPA1-induced cytoskeleton reorganization drives fibrosis through CTGF-dependent fibroblast proliferation. FASEB J. 27: 1830–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Huang L. S., Fu P., Patel P., Harijith A., Sun T., Zhao Y., Garcia J. G., Chun J., Natarajan V. 2013. Lysophosphatidic acid receptor 2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice. Am. J. Respir. Cell Mol. Biol. 49: 912–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ikeda H., Yatomi Y., Yanase M., Satoh H., Nishihara A., Kawabata M., Fujiwara K. 1998. Effects of lysophosphatidic acid on proliferation of stellate cells and hepatocytes in culture. Biochem. Biophys. Res. Commun. 248: 436–440 [DOI] [PubMed] [Google Scholar]
- 250.Wu J., Zern M. A. 2000. Hepatic stellate cells: a target for the treatment of liver fibrosis. J. Gastroenterol. 35: 665–672 [DOI] [PubMed] [Google Scholar]
- 251. Deleted in proof. [Google Scholar]
- 252.Gan L., Xue J. X., Li X., Liu D. S., Ge Y., Ni P. Y., Deng L., Lu Y., Jiang W. 2011. Blockade of lysophosphatidic acid receptors LPAR1/3 ameliorates lung fibrosis induced by irradiation. Biochem. Biophys. Res. Commun. 409: 7–13 [DOI] [PubMed] [Google Scholar]
- 253.Geng H., Lan R., Singha P. K., Gilchrist A., Weinreb P. H., Violette S. M., Weinberg J. M., Saikumar P., Venkatachalam M. A. 2012. Lysophosphatidic acid increases proximal tubule cell secretion of profibrotic cytokines PDGF-B and CTGF through LPA2- and Gαq-mediated Rho and αvβ6 integrin-dependent activation of TGF-β. Am. J. Pathol. 181: 1236–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Castelino F. V., Seiders J., Bain G., Brooks S. F., King C. D., Swaney J. S., Lorrain D. S., Chun J., Luster A. D., Tager A. M. 2011. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 63: 1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Blackburn J., Mansell J. P. 2012. The emerging role of lysophosphatidic acid (LPA) in skeletal biology. Bone. 50: 756–762 [DOI] [PubMed] [Google Scholar]
- 256.Sims S. M., Panupinthu N., Lapierre D. M., Pereverzev A., Dixon S. J. 2013. Lysophosphatidic acid: a potential mediator of osteoblast-osteoclast signaling in bone. Biochim. Biophys. Acta. 1831: 109–116 [DOI] [PubMed] [Google Scholar]
- 257.Lapierre D. M., Tanabe N., Pereverzev A., Spencer M., Shugg R. P., Dixon S. J., Sims S. M. 2010. Lysophosphatidic acid signals through multiple receptors in osteoclasts to elevate cytosolic calcium concentration, evoke retraction, and promote cell survival. J. Biol. Chem. 285: 25792–25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ye X., Chun J. 2010. Lysophosphatidic acid (LPA) signaling in vertebrate reproduction. Trends Endocrinol. Metab. 21: 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hama K., Aoki J., Inoue A., Endo T., Amano T., Motoki R., Kanai M., Ye X., Chun J., Matsuki N., et al. 2007. Embryo spacing and implantation timing are differentially regulated by LPA3-mediated lysophosphatidic acid signaling in mice. Biol. Reprod. 77: 954–959 [DOI] [PubMed] [Google Scholar]
- 260.Song H., Lim H., Paria B. C., Matsumoto H., Swift L. L., Morrow J., Bonventre J. V., Dey S. K. 2002. Cytosolic phospholipase A2alpha is crucial [correction of A2alpha deficiency is crucial] for ‘on-time’ embryo implantation that directs subsequent development. Development. 129: 2879–2889 [DOI] [PubMed] [Google Scholar]
- 261.Mohamed O. A., Jonnaert M., Labelle-Dumais C., Kuroda K., Clarke H. J., Dufort D. 2005. Uterine Wnt/beta-catenin signaling is required for implantation. Proc. Natl. Acad. Sci. USA. 102: 8579–8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Woclawek-Potocka I., Komiyama J., Saulnier-Blache J. S., Brzezicka E., Bah M. M., Okuda K., Skarzynski D. J. 2009. Lysophosphatic acid modulates prostaglandin secretion in the bovine uterus. Reproduction. 137: 95–105 [DOI] [PubMed] [Google Scholar]
- 263.Woclawek-Potocka I., Kondraciuk K., Skarzynski D. J. 2009. Lysophosphatidic acid stimulates prostaglandin E2 production in cultured stromal endometrial cells through LPA1 receptor. Exp. Biol. Med. (Maywood). 234: 986–993 [DOI] [PubMed] [Google Scholar]
- 264.Ye X., Skinner M. K., Kennedy G., Chun J. 2008. Age-dependent loss of sperm production in mice via impaired lysophosphatidic acid signaling. Biol. Reprod. 79: 328–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Garbi M., Rubinstein S., Lax Y., Breitbart H. 2000. Activation of protein kinase Calpha in the lysophosphatidic acid-induced bovine sperm acrosome reaction and phospholipase D1 regulation. Biol. Reprod. 63: 1271–1277 [DOI] [PubMed] [Google Scholar]
- 266.Reilly J. J. 2005. Descriptive epidemiology and health consequences of childhood obesity. Best Pract. Res. Clin. Endocrinol. Metab. 19: 327–341 [DOI] [PubMed] [Google Scholar]
- 267.Reilly J. J., Kelly J. 2011. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int. J. Obes. (Lond). 35: 891–898 [DOI] [PubMed] [Google Scholar]
- 268.Isakson P., Hammarstedt A., Gustafson B., Smith U. 2009. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 58: 1550–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Valet P., Pages C., Jeanneton O., Daviaud D., Barbe P., Record M., Saulnier-Blache J. S., Lafontan M. 1998. Alpha2-adrenergic receptor-mediated release of lysophosphatidic acid by adipocytes. A paracrine signal for preadipocyte growth. J. Clin. Invest. 101: 1431–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ferry G., Tellier E., Try A., Gres S., Naime I., Simon M. F., Rodriguez M., Boucher J., Tack I., Gesta S., et al. 2003. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J. Biol. Chem. 278: 18162–18169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nobusue H., Kondo D., Yamamoto M., Kano K. 2010. Effects of lysophosphatidic acid on the in vitro proliferation and differentiation of a novel porcine preadipocyte cell line. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 157: 401–407 [DOI] [PubMed] [Google Scholar]
- 272.Boucher J., Quilliot D., Praderes J. P., Simon M. F., Gres S., Guigne C., Prevot D., Ferry G., Boutin J. A., Carpene C., et al. 2005. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia. 48: 569–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Holmström T. E., Mattsson C. L., Wang Y., Iakovleva I., Petrovic N., Nedergaard J. 2010. Non-transactivational, dual pathways for LPA-induced Erk1/2 activation in primary cultures of brown pre-adipocytes. Exp. Cell Res. 316: 2664–2675 [DOI] [PubMed] [Google Scholar]
- 274.Simon M. F., Daviaud D., Pradere J. P., Gres S., Guigne C., Wabitsch M., Chun J., Valet P., Saulnier-Blache J. S. 2005. Lysophosphatidic acid inhibits adipocyte differentiation via lysophosphatidic acid 1 receptor-dependent down-regulation of peroxisome proliferator-activated receptor gamma2. J. Biol. Chem. 280: 14656–14662 [DOI] [PubMed] [Google Scholar]
- 275.Dusaulcy R., Daviaud D., Pradere J. P., Gres S., Valet P., Saulnier-Blache J. S. 2009. Altered food consumption in mice lacking lysophosphatidic acid receptor-1. J. Physiol. Biochem. 65: 345–350 [DOI] [PubMed] [Google Scholar]
- 276.Yea K., Kim J., Lim S., Park H. S., Park K. S., Suh P. G., Ryu S. H. 2008. Lysophosphatidic acid regulates blood glucose by stimulating myotube and adipocyte glucose uptake. J. Mol. Med. 86: 211–220 [DOI] [PubMed] [Google Scholar]
- 277.Rancoule C., Attane C., Gres S., Fournel A., Dusaulcy R., Bertrand C., Vinel C., Treguer K., Prentki M., Valet P., et al. 2013. Lysophosphatidic acid impairs glucose homeostasis and inhibits insulin secretion in high-fat diet obese mice. Diabetologia. 56: 1394–1402 [DOI] [PubMed] [Google Scholar]
- 278.Rancoule C., Dusaulcy R., Treguer K., Gres S., Attane C., Saulnier-Blache J. S. 2014. Involvement of autotaxin/lysophosphatidic acid signaling in obesity and impaired glucose homeostasis. Biochimie. 96: 140–143 [DOI] [PubMed] [Google Scholar]
- 279.Lin M. E., Herr D. R., Chun J. 2010. Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 91: 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tigyi G. 2010. Aiming drug discovery at lysophosphatidic acid targets. Br. J. Pharmacol. 161: 241–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Mutoh T., Rivera R., Chun J. 2012. Insights into the pharmacological relevance of lysophospholipid receptors. Br. J. Pharmacol. 165: 829–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sedláková I., Vávrová J., Tošner J., Hanousek L. 2010. Lysophosphatidic acid (LPA)—a perspective marker in ovarian cancer. Tumour Biol. 32: 311–316 [DOI] [PubMed] [Google Scholar]
- 283.Xu Y., Fang X. J., Casey G., Mills G. B. 1995. Lysophospholipids activate ovarian and breast cancer cells. Biochem. J. 309: 933–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Nouh M. A. A. M., Wu X-X., Okazoe H., Tsunemori H., Haba R., Abou-Zeid A. M. M., Saleem M. D., Inui M., Sugimoto M., Aoki J., et al. 2009. Expression of autotaxin and acylglycerol kinase in prostate cancer: association with cancer development and progression. Cancer Sci. 100: 1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wu J. M., Xu Y., Skill N. J., Sheng H., Zhao Z., Yu M., Saxena R., Maluccio M. A. 2010. Autotaxin expression and its connection with the TNF-alpha-NF-kappaB axis in human hepatocellular carcinoma. Mol. Cancer. 9: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kishi Y., Okudaira S., Tanaka M., Hama K., Shida D., Kitayama J., Yamori T., Aoki J., Fujimaki T., Arai H. 2006. Autotaxin is overexpressed in glioblastoma multiforme and contributes to cell motility of glioblastoma by converting lysophosphatidylcholine to lysophosphatidic acid. J. Biol. Chem. 281: 17492–17500 [DOI] [PubMed] [Google Scholar]
- 287.Lee S. J., Yun C. C. 2010. Colorectal cancer cells - proliferation, survival and invasion by lysophosphatidic acid. Int. J. Biochem. Cell Biol. 42: 1907–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kitayama J., Shida D., Sako A., Ishikawa M., Hama K., Aoki J., Arai H., Nagawa H. 2004. Over-expression of lysophosphatidic acid receptor-2 in human invasive ductal carcinoma. Breast Cancer Res. 6: R640–R646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Jeong K. J., Park S. Y., Seo J. H., Lee K. B., Choi W. S., Han J. W., Kang J. K., Park C. G., Kim Y. K., Lee H. Y. 2008. Lysophosphatidic acid receptor 2 and Gi/Src pathway mediate cell motility through cyclooxygenase 2 expression in CAOV-3 ovarian cancer cells. Exp. Mol. Med. 40: 607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hope J. M., Wang F. Q., Whyte J. S., Ariztia E. V., Abdalla W., Long K., Fishman D. A. 2009. LPA receptor 2 mediates LPA-induced endometrial cancer invasion. Gynecol. Oncol. 112: 215–223 [DOI] [PubMed] [Google Scholar]
- 291.Wang G. L., Wen Z. Q., Xu W. P., Wang Z. Y., Du X. L., Wang F. 2008. Inhibition of lysophosphatidic acid receptor-2 expression by RNA interference decreases lysophosphatidic acid-induced urokinase plasminogen activator activation, cell invasion, and migration in ovarian cancer SKOV-3 cells. Croat. Med. J. 49: 175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yun C. C., Sun H., Wang D., Rusovici R., Castleberry A., Hall R. A., Shim H. 2005. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am. J. Physiol. Cell Physiol. 289: C2–C11 [DOI] [PubMed] [Google Scholar]
- 293.Huang M. C., Lee H. Y., Yeh C. C., Kong Y., Zaloudek C. J., Goetzl E. J. 2004. Induction of protein growth factor systems in the ovaries of transgenic mice overexpressing human type 2 lysophosphatidic acid G protein-coupled receptor (LPA2). Oncogene. 23: 122–129 [DOI] [PubMed] [Google Scholar]
- 294.Shida D., Fang X., Kordula T., Takabe K., Lepine S., Alvarez S. E., Milstien S., Spiegel S. 2008. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 68: 6569–6577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zeng Y., Kakehi Y., Nouh M. A., Tsunemori H., Sugimoto M., Wu X. X. 2009. Gene expression profiles of lysophosphatidic acid-related molecules in the prostate: relevance to prostate cancer and benign hyperplasia. Prostate. 69: 283–292 [DOI] [PubMed] [Google Scholar]
- 296.Yu S., Murph M. M., Lu Y., Liu S., Hall H. S., Liu J., Stephens C., Fang X., Mills G. B. 2008. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J. Natl. Cancer Inst. 100: 1630–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Masiello L. M., Fotos J. S., Galileo D. S., Karin N. J. 2006. Lysophosphatidic acid induces chemotaxis in MC3T3-E1 osteoblastic cells. Bone. 39: 72–82 [DOI] [PubMed] [Google Scholar]
- 298.Li T. T., Alemayehu M., Aziziyeh A. I., Pape C., Pampillo M., Postovit L. M., Mills G. B., Babwah A. V., Bhattacharya M. 2009. Beta-arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells. Mol. Cancer Res. 7: 1064–1077 [DOI] [PubMed] [Google Scholar]
- 299.Yamada T., Sato K., Komachi M., Malchinkhuu E., Tobo M., Kimura T., Kuwabara A., Yanagita Y., Ikeya T., Tanahashi Y., et al. 2004. Lysophosphatidic acid (LPA) in malignant ascites stimulates motility of human pancreatic cancer cells through LPA1. J. Biol. Chem. 279: 6595–6605 [DOI] [PubMed] [Google Scholar]
- 300.Okabe K., Hayashi M., Fujii M., Honoki K., Mori T., Fukushima N., Tsujiuchi T. 2010. Mutations of lysophosphatidic acid receptor genes in human osteosarcoma cells. Pathobiology. 77: 278–282 [DOI] [PubMed] [Google Scholar]
- 301.Obo Y., Yamada T., Furukawa M., Hotta M., Honoki K., Fukushima N., Tsujiuchi T. 2009. Frequent mutations of lysophosphatidic acid receptor-1 gene in rat liver tumors. Mutat. Res. 660: 47–50 [DOI] [PubMed] [Google Scholar]
- 302.Shin K. J., Kim Y. L., Lee S., Kim D. K., Ahn C., Chung J., Seong J. Y., Hwang J. I. 2009. Lysophosphatidic acid signaling through LPA receptor subtype 1 induces colony scattering of gastrointestinal cancer cells. J. Cancer Res. Clin. Oncol. 135: 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.David M., Wannecq E., Descotes F., Jansen S., Deux B., Ribeiro J., Serre C. M., Gres S., Bendriss-Vermare N., Bollen M., et al. 2010. Cancer cell expression of autotaxin controls bone metastasis formation in mouse through lysophosphatidic acid-dependent activation of osteoclasts. PLoS ONE. 5: e9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Yang S. Y., Lee J., Park C. G., Kim S., Hong S., Chung H. C., Min S. K., Han J. W., Lee H. W., Lee H. Y. 2002. Expression of autotaxin (NPP-2) is closely linked to invasiveness of breast cancer cells. Clin. Exp. Metastasis. 19: 603–608 [DOI] [PubMed] [Google Scholar]
- 305.Yang Y., Mou L-j., Liu N., Tsao M-S. 1999. Autotaxin expression in non-small-cell lung cancer. Am. J. Respir. Cell Mol. Biol. 21: 216–222 [DOI] [PubMed] [Google Scholar]
- 306.Kehlen A., Englert N., Seifert A., Klonisch T., Dralle H., Langner J., Hoang-Vu C. 2004. Expression, regulation and function of autotaxin in thyroid carcinomas. Int. J. Cancer. 109: 833–838 [DOI] [PubMed] [Google Scholar]
- 307.Kazama S., Kitayama J., Aoki J., Mori K., Nagawa H. 2011. Immunohistochemical detection of autotaxin (ATX)/lysophospholipase D (lysoPLD) in submucosal invasive colorectal cancer. J. Gastrointest. Cancer. 42: 204–211 [DOI] [PubMed] [Google Scholar]
- 308.Samadi N., Gaetano C., Goping I. S., Brindley D. N. 2009. Autotaxin protects MCF-7 breast cancer and MDA-MB-435 melanoma cells against Taxol-induced apoptosis. Oncogene. 28: 1028–1039 [DOI] [PubMed] [Google Scholar]
- 309.Houben A. J., Moolenaar W. H. 2011. Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 30: 557–565 [DOI] [PubMed] [Google Scholar]
- 310.Jonkers J., Moolenaar W. H. 2009. Mammary tumorigenesis through LPA receptor signaling. Cancer Cell. 15: 457–459 [DOI] [PubMed] [Google Scholar]
- 311.Willier S., Butt E., Grunewald T. G. 2013. Lysophosphatidic acid (LPA) signalling in cell migration and cancer invasion: a focussed review and analysis of LPA receptor gene expression on the basis of more than 1700 cancer microarrays. Biol. Cell. 105: 317–333 [DOI] [PubMed] [Google Scholar]
- 312.Lin C. I., Chen C. N., Huang M. T., Lee S. J., Lin C. H., Chang C. C., Lee H. 2008. Lysophosphatidic acid upregulates vascular endothelial growth factor-C and tube formation in human endothelial cells through LPA(1/3), COX-2, and NF-kappaB activation- and EGFR transactivation-dependent mechanisms. Cell. Signal. 20: 1804–1814 [DOI] [PubMed] [Google Scholar]
- 313.Sitohy B., Nagy J. A., Dvorak H. F. 2012. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 72: 1909–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Jeon E. S., Heo S. C., Lee I. H., Choi Y. J., Park J. H., Choi K. U., Park do Y, Suh D. S., Yoon M. S., Kim J. H. 2010. Ovarian cancer-derived lysophosphatidic acid stimulates secretion of VEGF and stromal cell-derived factor-1 alpha from human mesenchymal stem cells. Exp. Mol. Med. 42: 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Dutta S., Wang F. Q., Wu H. S., Mukherjee T. J., Fishman D. A. 2011. The NF-κB pathway mediates lysophosphatidic acid (LPA)-induced VEGF signaling and cell invasion in epithelial ovarian cancer (EOC). Gynecol. Oncol. 123: 129–137 [DOI] [PubMed] [Google Scholar]
- 316.Komachi M., Sato K., Tobo M., Mogi C., Yamada T., Ohta H., Tomura H., Kimura T., Im D. S., Yanagida K., et al. 2012. Orally active lysophosphatidic acid receptor antagonist attenuates pancreatic cancer invasion and metastasis in vivo. Cancer Sci. 103: 1099–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Marshall J. C., Collins J. W., Nakayama J., Horak C. E., Liewehr D. J., Steinberg S. M., Albaugh M., Vidal-Vanaclocha F., Palmieri D., Barbier M., et al. 2012. Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J. Natl. Cancer Inst. 104: 1306–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Beck H. P., Kohn T., Rubenstein S., Hedberg C., Schwandner R., Hasslinger K., Dai K., Li C., Liang L., Wesche H., et al. 2008. Discovery of potent LPA2 (EDG4) antagonists as potential anticancer agents. Bioorg. Med. Chem. Lett. 18: 1037–1041 [DOI] [PubMed] [Google Scholar]
- 319.Mirendil H., Lin M. E., Chun J. 2013. Lysophosphatidic acid (LPA) receptor signaling. In Lysophospholipid Receptors: Signaling and Biochemistry. J. Chun, T. Hla, S. Spiegel, et al., editors. Wiley, Hoboken, NJ. 1–39. [Google Scholar]
- 320.An S., Bleu T., Zheng Y., Goetzl E. J. 1998. Recombinant human G protein-coupled lysophosphatidic acid receptors mediate intracellular calcium mobilization. Mol. Pharmacol. 54: 881–888 [DOI] [PubMed] [Google Scholar]
- 321.Liliom K., Bittman R., Swords B., Tigyi G. 1996. N-palmitoyl-serine and N-palmitoyl-tyrosine phosphoric acids are selective competitive antagonists of the lysophosphatidic acid receptors. Mol. Pharmacol. 50: 616–623 [PubMed] [Google Scholar]
- 322.Im D. S. 2010. Pharmacological tools for lysophospholipid GPCRs: development of agonists and antagonists for LPA and S1P receptors. Acta Pharmacol. Sin. 31: 1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Sugiura T., Tokumura A., Gregory L., Nouchi T., Weintraub S. T., Hanahan D. J. 1994. Biochemical characterization of the interaction of lipid phosphoric acids with human platelets: comparison with platelet activating factor. Arch. Biochem. Biophys. 311: 358–368 [DOI] [PubMed] [Google Scholar]
- 324.Durgam G. G., Virag T., Walker M. D., Tsukahara R., Yasuda S., Liliom K., van Meeteren L. A., Moolenaar W. H., Wilke N., Siess W., et al. 2005. Synthesis, structure-activity relationships, and biological evaluation of fatty alcohol phosphates as lysophosphatidic acid receptor ligands, activators of PPARgamma, and inhibitors of autotaxin. J. Med. Chem. 48: 4919–4930 [DOI] [PubMed] [Google Scholar]
- 325.Markiewicz W., Kaminska K., Bogacki M., Maslanka T., Jaroszewski J. 2012. Participation of analogues of lysophosphatidic acid (LPA): oleoyl-sn-glycero-3-phosphate (L-alpha-LPA) and 1-oleoyl-2-O-methyl-rac-glycerophosphothionate (OMPT) in uterine smooth muscle contractility of the pregnant pigs. Pol. J. Vet. Sci. 15: 635–643 [DOI] [PubMed] [Google Scholar]
- 326.Tamaruya Y., Suzuki M., Kamura G., Kanai M., Hama K., Shimizu K., Aoki J., Arai H., Shibasaki M. 2004. Identifying specific conformations by using a carbohydrate scaffold: discovery of subtype-selective LPA-receptor agonists and an antagonist. Angew. Chem. Int. Ed. Engl. 43: 2834–2837 [DOI] [PubMed] [Google Scholar]
- 327.Virag T., Elrod D. B., Liliom K., Sardar V. M., Parrill A. L., Yokoyama K., Durgam G., Deng W., Miller D. D., Tigyi G. 2003. Fatty alcohol phosphates are subtype-selective agonists and antagonists of lysophosphatidic acid receptors. Mol. Pharmacol. 63: 1032–1042 [DOI] [PubMed] [Google Scholar]
- 328.Qian L., Xu Y., Simper T., Jiang G., Aoki J., Umezu-Goto M., Arai H., Yu S., Mills G. B., Tsukahara R., et al. 2006. Phosphorothioate analogues of alkyl lysophosphatidic acid as LPA3 receptor-selective agonists. ChemMedChem. 1: 376–383 [DOI] [PubMed] [Google Scholar]
- 329.Jiang G., Inoue A., Aoki J., Prestwich G. D. 2013. Phosphorothioate analogs of sn-2 radyl lysophosphatidic acid (LPA): metabolically stabilized LPA receptor agonists. Bioorg. Med. Chem. Lett. 23: 1865–1869 [DOI] [PubMed] [Google Scholar]
- 330.Nikitopoulou I., Kaffe E., Sevastou I., Sirioti I., Samiotaki M., Madan D., Prestwich G. D., Aidinis V. 2013. A metabolically-stabilized phosphonate analog of lysophosphatidic acid attenuates collagen-induced arthritis. PLoS ONE. 8: e70941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Ohta H., Sato K., Murata N., Damirin A., Malchinkhuu E., Kon J., Kimura T., Tobo M., Yamazaki Y., Watanabe T., et al. 2003. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol. Pharmacol. 64: 994–1005 [DOI] [PubMed] [Google Scholar]
- 332.Sato T., Sugimoto K., Inoue A., Okudaira S., Aoki J., Tokuyama H. 2012. Synthesis and biological evaluation of optically active Ki16425. Bioorg. Med. Chem. Lett. 22: 4323–4326 [DOI] [PubMed] [Google Scholar]
- 333.Bain G., Seiders J. 2013. LPA receptor subtypes LPA1 and LPA2 as potential drug targets. In Lysophospholipid Receptors: Signaling and Biochemistry. J. Chun, T. Hla, S. Spiegel, et al., editors. Wiley, Hoboken, NJ. 681–708. [Google Scholar]
- 334.David M., Ribeiro J., Descotes F., Serre C. M., Barbier M., Murone M., Clezardin P., Peyruchaud O. 2012. Targeting lysophosphatidic acid receptor type 1 with Debio 0719 inhibits spontaneous metastasis dissemination of breast cancer cells independently of cell proliferation and angiogenesis. Int. J. Oncol. 40: 1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Fells J. I., Tsukahara R., Fujiwara Y., Liu J., Perygin D. H., Osborne D. A., Tigyi G., Parrill A. L. 2008. Identification of non-lipid LPA3 antagonists by virtual screening. Bioorg. Med. Chem. 16: 6207–6217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Swaney J. S., Chapman C., Correa L. D., Stebbins K. J., Broadhead A. R., Bain G., Santini A. M., Darlington J., King C. D., Baccei C. S., et al. 2011. Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist. J. Pharmacol. Exp. Ther. 336: 693–700 [DOI] [PubMed] [Google Scholar]
- 337.Deleted in proof. [Google Scholar]
- 338.Fischer D. J., Nusser N., Virag T., Yokoyama K., Wang D., Baker D. L., Bautista D., Parrill A. L., Tigyi G. 2001. Short-chain phosphatidates are subtype-selective antagonists of lysophosphatidic acid receptors. Mol. Pharmacol. 60: 776–784 [PubMed] [Google Scholar]
- 339.Aaltonen N., Lehtonen M., Varonen K., Goterris G. A., Laitinen J. T. 2012. Lipid phosphate phosphatase inhibitors locally amplify lysophosphatidic acid LPA1 receptor signalling in rat brain cryosections without affecting global LPA degradation. BMC Pharmacol. 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Nakane S., Oka S., Arai S., Waku K., Ishima Y., Tokumura A., Sugiura T. 2002. 2-Arachidonoyl-sn-glycero-3-phosphate, an arachidonic acid-containing lysophosphatidic acid: occurrence and rapid enzymatic conversion to 2-arachidonoyl-sn-glycerol, a cannabinoid receptor ligand, in rat brain. Arch. Biochem. Biophys. 402: 51–58 [DOI] [PubMed] [Google Scholar]
- 341.Barber M. N., Risis S., Yang C., Meikle P. J., Staples M., Febbraio M. A., Bruce C. R. 2012. Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS ONE. 7: e41456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Kishimoto T., Soda Y., Matsuyama Y., Mizuno K. 2002. An enzymatic assay for lysophosphatidylcholine concentration in human serum and plasma. Clin. Biochem. 35: 411–416 [DOI] [PubMed] [Google Scholar]
- 343.Cho W. H., Park T., Park Y. Y., Huh J. W., Lim C. M., Koh Y., Song D. K., Hong S. B. 2012. Clinical significance of enzymatic lysophosphatidylcholine (LPC) assay data in patients with sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 31: 1805–1810 [DOI] [PubMed] [Google Scholar]
- 344.Sato K., Malchinkhuu E., Muraki T., Ishikawa K., Hayashi K., Tosaka M., Mochiduki A., Inoue K., Tomura H., Mogi C., et al. 2005. Identification of autotaxin as a neurite retraction-inducing factor of PC12 cells in cerebrospinal fluid and its possible sources. J. Neurochem. 92: 904–914 [DOI] [PubMed] [Google Scholar]
- 345.Sugiura T., Nakane S., Kishimoto S., Waku K., Yoshioka Y., Tokumura A. 2002. Lysophosphatidic acid, a growth factor-like lipid, in the saliva. J. Lipid Res. 43: 2049–2055 [DOI] [PubMed] [Google Scholar]
- 346.Watanabe N., Ikeda H., Nakamura K., Ohkawa R., Kume Y., Tomiya T., Tejima K., Nishikawa T., Arai M., Yanase M., et al. 2007. Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity. Life Sci. 81: 1009–1015 [DOI] [PubMed] [Google Scholar]