Abstract
In 2009, the National Academy for Engineering issued the Grand Challenges for Engineering in the 21st Century comprised of 14 technical challenges that must be addressed to build a healthy, profitable, sustainable, and secure global community (http://www.engineeringchallenges.org). Although crucial, none of the NEA Grand Challenges adequately addressed the challenges that face the biomaterials community. In response to the NAE Grand Challenges, Monty Reichert of Duke University organized a panel entitled Grand Challenges in Biomaterials at the at the 2010 Society for Biomaterials Annual Meeting in Seattle. Six members of the National Academies—Buddy Ratner, James Anderson, Allan Hoffman, Art Coury, Cato Laurencin, and David Tirrell—were asked to propose a grand challenge to the audience that, if met, would significantly impact the future of biomaterials and medical devices. Successfully meeting these challenges will speed the 60-plus year transition from commodity, off-the-shelf biomaterials to bioengineered chemistries, and biomaterial devices that will significantly advance our ability to address patient needs and also to create new market opportunities.
Keywords: biomaterial, grand challenges, biocompatibility testing, smart polymers, regenerative medicine
INTRODUCTION TO THE 2010 PANEL ON THE BIOMATERIALS GRAND CHALLENGES
Buddy D. Ratner, PhD
Member, National Academy of Engineering
The true measure of a man is not how he behaves in moments of comfort and convenience but how he stands at times of controversy and challenges.
Martin Luther King Jr., American Theologian
This inspirational quote from Dr. King is equally applicable to fields of endeavor as it is to individuals. Can the field of biomaterials embrace the challenges that confront the field, rather than continuing along well-trodden pathways? Can we rise to the occasion of challenges (and the controversies that they inevitably trigger) and advance the field technically, improve care for patients, and contribute to the economy?
Biomaterials is a young field—the roots of modern biomaterials go back to the late 1940’s and early 1950’s. The first medical devices were physician-driven. These early pioneers used off-the-shelf commodity materials that were also developed in that era—consider Teflon, high-density polyethylene, poly-(methyl methacrylate), stainless steel, polyurethane, titanium, and silicone elastomers. These efforts led to an innovative reparative medicine never before seen in the history of humans on this planet. But consider: biology had yet to learn about DNA, cell-surface receptors, or cytokines; materials science had barely coalesced as a discipline; bioengineering did not exist as a discipline; polymer chemistry was evolving its foundation principles. There was no scientific basis for medical devices—it was mostly seat-of-the-pants and opportunistic invention.
Now, in 2010, we have a sophisticated cell and molecular biology along with vibrant materials science, bioengineering, and polymer chemistry. In spite of having a much richer scientific portfolio at our disposal, the majority of our medical devices are still fabricated from Teflon, high-density polyethylene, poly(methyl methacrylate), stainless steel, polyurethane, titanium, and silicone elastomers.
Newer materials and ideas are slowly evolving. The path from laboratory bench to patient is rough and unpredictable with technical, regulatory, and market hurdles along the way. Some clinical devices have traversed this terrain and integrate sophisticated surface modifications or coatings. What will ultimately drive the development of a modern generation of biomaterials are patient need, clinical success, and market potential.
In response to the National Academy for Engineering Grand Challenges (http://www.engineeringchallenges.org), Professor Monty Reichert of Duke University organized a panel entitled Grand Challenges in Biomaterials at the at the 2010 Society for Biomaterials Annual Meeting in Seattle. Six members of the National Academies—Buddy Ratner, James Anderson, Allan Hoffman, Art Coury, Cato Laurencin, and David Tirrell—were asked to propose to the audience a grand challenge that, if met, would impact the future of biomaterials and medical devices. Successfully meeting these challenges will speed the 60-plus year transition from commodity, off-the-shelf biomaterials to bioengineered chemistries, and biomaterial devices that will significantly advance our ability to address patient needs and also to create new market opportunities (Fig. 1).
FIGURE 1.

Panel for the Biomaterials Grand Challenge, 2010 Society for Biomaterials annual meeting, Seattle, WA. Left to right: Art Coury, Allan Hoffman, Jim Anderson, Buddy Ratner (panel moderator), Cato Laurencin, David Tirrell, and panel organizer Monty Reichert. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Serving as moderator for the session, I, Professor Buddy Ratner led off with a challenge for redefining biocompatibility to address new materials that tap into the body’s own reconstructive mechanisms and show improved healing and integration. Professor James Anderson followed on this theme and challenged the community with the need for appropriate rationale and use of biological experimental design and methodology in biocompatibility studies. Dr. Art Coury offered a challenge in two parts (1) the necessity to continue to improve conventional “replacement” medicine by making better use of existing materials, developing better materials, producing better device designs, and improving surgical techniques; (2) the need to develop the materials and technologies that optimize the inevitable transition from replacement medicine to regenerative medicine and tissue engineering. Professor Allan Hoffman focused his challenge on in vitro applications and called for “smart new applications for smart polymers.” Professor Cato Laurencin’s vision for a grand challenge for moving medicine forward is full limb regeneration. Finally, Professor David Tirrell’s grand challenge exhorted us to harness the principles of developmental biology to control collective cell movement and differentiation in vivo and in vitro. These grand challenges address fundamental principles, materials development, medical devices, soft and hard tissue, in vivo and in vitro applications, and biomaterials testing—much of the spectrum of activity in our field.
The biomaterials community is clearly “at times of controversy and challenges.” The next step is to further define and embrace these challenges. The Biomaterials Grand Challenges are already the thematic focus of the 2012 Society for Biomaterials Meeting in New Orleans. The contest mechanism has proven to be a potent method for advancing nascent fields (consider the X-Prizes). These challenges could be the basis for an advanced biomaterials course in University settings. Perhaps a “Grand Challenges” text could spring from this movement. Federal agencies might review them as the basis for “calls for proposals.” There are many possibilities. Most importantly, though, they must not be ignored. The thinking behind formulating these challenges will take the biomaterials field up to the next plane. Lives depend on it!
PROPOSED GRAND CHALLENGES IN BIOMATERIALS
Rethink biomaterials biocompatibility Buddy D. Ratner, PhD
Member, National Academy of Engineering
Harmony makes small things grow, lack of it makes great things decay.
Gaius Sallustius Crispus, Roman Historian
The long-standing definition of biomaterials biocompatibility as stated succinctly by Professor David Williams is “the ability of a material to perform with an appropriate host response in a specific application.”1 This has been an excellent, functional definition that is both correct and accurate; however, this definition gives us little insight into critical questions like “How could we improve the biocompatibility of a material? How could we better test for biocompatibility?” So it guides us in just a limited way.
Tolerance, or mild “rejection” of an implanted biomaterial, characterized by an avascular fibrous encapsulation, is the current state of biocompatibility. This is the view held by the agencies that regulate biomedical devices, the physicians that implant them, the academics that study them, and the companies that make them. In cases where the implanted material degrades away, the stimulus that drives fibrous encapsulation is removed with it. But what if the material is nondegradable? What characteristics are necessary to make a nondegradable material, the substance of most of today’s implantable medical devices, become fully compatible with the surrounding tissue, and not just walled-off or isolated by it? This will lead to long-functioning implants, such as glucose sensors, stable implant electrodes, constant delivery implanted drug release devices, nonfibrotic hernia repair meshes, improved breast implants, and generally improvement for all medical implants.
Biocompatibility, as I see it, has four components. First is toxicology. Substances that leach out of our biomaterials, unless they are intended or designed to leach out, are probably going to be problematic. Second is bacteria and/or bacterial endotoxin. If a biomaterial is colonized with bacteria or has bacterial cell wall endotoxins on it, it will likely be inflamed, infected, or both. Third is mechanical irritation. If the biomaterial is rubbing or moving in the implant site, chances are it that mechanical irritation of the surrounding tissue will lead to a deleterious tissue reaction. Fourth is the interaction of proteins and cells with biomaterials, a subject that probably dominates half of the talks and papers on biomaterials, but is perhaps the least understood. Somehow we all sense that the early events of protein adsorption and cell interrogation at the material surface are connected to biocompatibility, but if I conducted a survey asking “How do protein and cell interactions with materials affect the tissue response to medical implants?” I do not think I will get a satisfying answer.
The term biocompatibility itself is also a conjunction of parts. First is bio, invoking a picture of proteins, DNA, cells, and tissues. Second is compatibility, implying a kind of feeling good together, in harmony. Being close, intimate, and part of the same thing. But the term compatibility appears to be in direct contradiction one of the central ideas in biomaterials: the foreign body response to implants. Here, a biomaterial is coated with a layer of proteins and cells, typically macrophages, which may be 2–3 days later, become activated. The cells spread out, attempt to engulf the material, and when unable to do so initiate the process that ultimately leads to the material being walled off in a relatively avascular and fibrous tissue capsule. This hardly seems like compatibility. The body is doing its best to isolate itself from this “biocompatible” biomaterial.
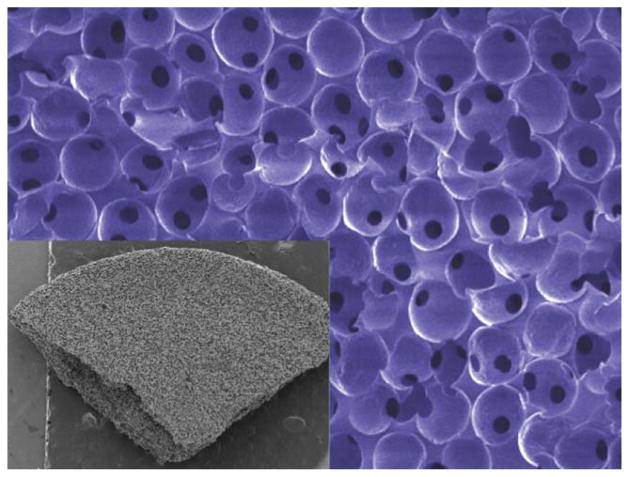
In recent years, we have been working with a new class of porous materials, called 6S, where every pore is the same size and are all part of an interconnected network. (Fig. 2) Originally, the work of Ph. D. student Andrew Marshall,2 it has been confirmed many times over that if the pores are 30–40 μm in diameter, about the size of a few cells, we obtain a well-integrated and vascularized healing response upon implantation (Fig. 3), whereas materials made with smaller or larger pores give us the classic foreign body reaction.
FIGURE 2.
Scanning electron micrographs of sphere-templated porous structures (6S) where all pores are the same size and ~35 μm. The inset shows a macroscopic section of this material to illustrate the pore uniformity throughout the structure.
FIGURE 3.
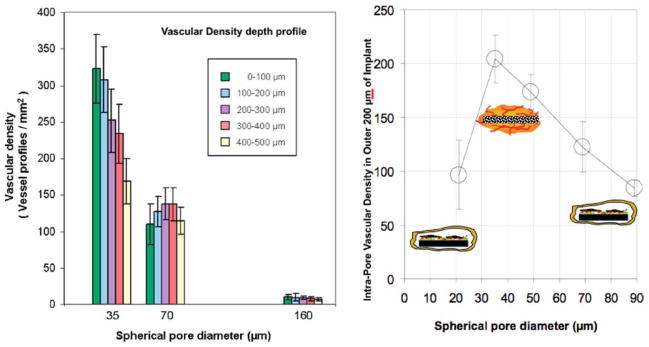
Vascularity as measured by endothelial staining for pHEMA sphere-templated porous structures (6S) implanted subcutaneously for one month in mice (data taken from Ref. 2).
Indicators of tissue compatibility for the 6S porous material are numerous.3,4 The tissue that surrounds the material exhibits blood vessels filled with red cells, so we know they are functional. These materials have shown good results in subcutaneous, intramuscular, sclera, vaginal wall, percutaneous, heart, and bone implant sites. It does not matter what “biocompatible” material they are made out of. We make them out of the polyHEMA hydrogel, silicone elastomer, polyurethane, or fibrin. All yield essentially the same results when implanted—that is, they exhibit tissue integration rather than fibrous encapsulation. There are no ligands or growth factors, no drug release, no hydroxyapatite, no stem cells, and no osteoblasts. Just 35-μm interconnected pores.
Recent results suggest that the pore structure of the 6S material is inducing cell phenotypic reprogramming and intracellular communication that leads to tissue integrating with the 6S material rather than attacking it. For example, microscopic images of the pore structure have shown the presence of macrophages, fibroblasts, polymorphonuclear leukocytes, T lymphocytes, and keratinocytes all in this same pore together. Recent data shows that the macrophages in these pores have considerable M2 character, in contrast to the profibrotic M1 phenotype. Possibly, the size of the pore allows macrophage entry, but does not permit the cell to go into the spread (attack) phenotype. Although we do not understand the details of the phenotypic reprogramming and cell–cell communication, it is clear that this communication is leading to a vascularized reconstructed tissue rather than an avascular fibrous encapsulation tissue. It is a kind of drug-free pharmacology, and the control of the macrophage does seem to be critical to make this happen.
Ultimately, what the 6S material is doing is promoting true integrated tissue reconstruction rather than the typical default case of foreign body capsule formation. In this case, the critical cue appears to be the presentation of a porous materials or correctly sized architecture that communicates “compatibility” to the cells that interpenetrate the material, rather than “foreignness” that is communicated by the same material if it is nonporous or if the pores are incorrectly sized.
This vascularized, nonfibrotic healing is striking compared with the normal foreign body reaction. Compare the healing of a slab of nonporous poly(HEMA) hydrogel to that of the same material with 35 μm pores in the context of the definition of biocompatibility: “ability of a material to perform with an appropriate host response in a specific application.” We have here two distinctly different host reactions in the same application with the same material. Can both these reactions be “biocompatible?” Others have seen such vascularized reconstruction using different approaches such as with decellularized tissues with the macrophage again being strongly implicated.5
“Thus, my grand challenge to biomaterials is to rethink the meaning of biocompatibility to our field, shifting away from a concept of tissue tolerance to one that that promotes the reconstruction of viable tissue around the implant.”
This will require us to re-examine this word, “biocompatible” and consider a new definition that embraces more appropriate host responses. We are on the verge of changing the way synthetic materials and medical devices heal in the body, and we need a more accurate term to describe this. This challenge is especially challenging because researchers, physicians, manufacturers, and regulatory agencies alike must embrace this new paradigm. Someone once quipped, “changing a curriculum is like moving a cemetery—the dead have many friends.” This thought may applicable for changing a definition too.
Design biologically accurate tests for biomaterials James Anderson, MD, PhD
Member, Institute of Medicine of the National Academies
Fast is fine, but accuracy is everything.
Wyatt Earp, American Peace Officer
As a pathologist and polymer scientist, I am concerned about the accuracy and validity of tests used by investigators in determining the biocompatibility of biomaterials. “Therefore, my grand challenge for biomaterials calls for the appropriate and accurate use of biological experimental design and methodology in biocompatibility testing.”
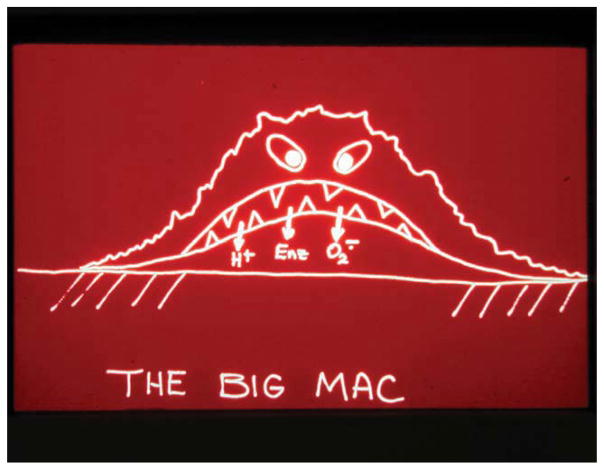
For the past 30-plus years, I have studied inflammation, wound healing, and the foreign-body reaction associated with implants. The central cellular mediators in these biological responses are macrophages and foreign body giant cells. Figure 4 is a cartoon of an activated macrophage attached to a material surface. Today, some 30 years later, our knowledge of the surface adherent macrophage is still limited. For example, many investigators do not appreciate the markedly different nature of the microenvironment beneath an activated macrophage. This region contains acids, enzymes, and oxygen radicals. The pH can drop to 3.5, which is almost 10,000 times more acid than what is commonly found in buffered solutions in the body. This zone is also immunologically privileged as it can exclude antibodies. Not shown in the Big Mac cartoon is the more recent observation that paracrine secretion from the macrophage of cytokines, chemokines, and growth factors can control cellular behavior occurring in the adjacent tissue.
FIGURE 4.
Big Mac the activated macrophage. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary. com.]
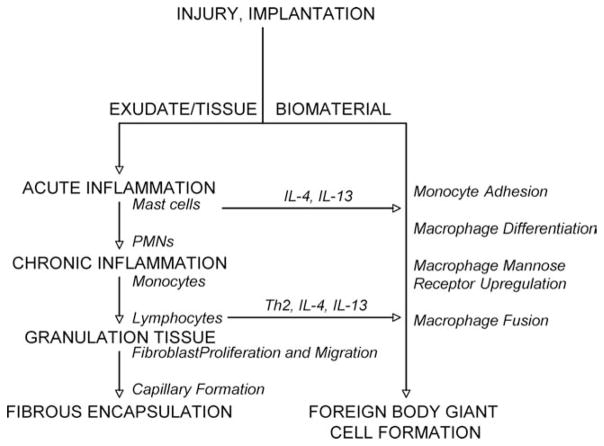
Figure 5 is a diagram showing the multiple cell types involved in the paracrine and juxtacrine signaling of wound healing. On the left, you see the normal sequence of events: acute inflammation, chronic inflammation, granulation tissue with neovascular formation, and then, finally, fibrous capsule formation. On the right, you have the presence of a biomaterial with subsequent monocyte adhesion, macrophage differentiation, fusion, and foreign body giant cell formation. The entire sequence can occur within 14–21 days of implantation, even though the implant may be designed for 5, 10, 15, and 20 years of in vivo use. Yet it is possible that the initial 2–3 week time period could control the ultimate success or failure of the device or biomaterial.
FIGURE 5.
Multiple cell types involved in the paracrine and juxtacrine signaling of wound healing and the foreign body response.
The questions we really need to ask are: What are the cellular and molecular mechanisms driving the foreign body response and can they be modified? Can we prevent leukocyte adhesion? Can we prevent macrophage fusion? Can we promote adherent cell death? Can we promote normal wound healing, or even reduce it, by manipulating cytokine production? And, lastly, can biomaterial surface chemistry influence adherent macrophage, apoptosis, and cytokine production? Over a number of years, we have been able to show that surface configuration, surface morphology, and surface chemistry can be used to partially control these interactions. But obviously, much more needs to be done in determining mechanisms that modulate the phenotypic expression of surface adherent macrophages and FBGCs.
The problem, from my perspective, is that much is lacking in the experimental design and methodology commonly used in biomaterials testing. Typically, the first thing one does is an in vitro cytotoxicity study. Many investigators use tumor derived cells because it is “fast and easy” with culture times of 6, 12, or 24 h, which are far too short to provide reliable information. If the tumor cells “look healthy” after a few hours then they claim biocompatibility. Well, if you are working on implants to go into tumors for a few hours, then may be that is a good protocol to use. It is also important to note that macrophages are a nonproliferating end-stage cell; whereas monocyte tumor cells are proliferating cells.
In our work, we draw human blood, isolate monocytes, and use primary derived cells. We carry out studies for several days and use immunohistochemistry and cell sorting to look for specific cell adhesion ligands and specific cell markers of the biological response at surfaces. Many investigators also are beginning to look at cytokine expression as part of the characterization of the cellular response. Typically, one assays for the mRNA that indicates upregulation of specific cytokine expression genes using gene microarrays or RT-PCR. We do not believe that gene upregulation is an accurate reflection of the proteins that the cells were actually secreting, plus it is expensive. We use proteomic arrays to screen broadly for cytokines secreted from cells on various surfaces and follow up the initial screening with much more sensitive ELISA techniques.
Finally, there are biocompatibility “hot buttons” that if not correctly defined can lead to problems and confusion; that is, chronic inflammation, immune response, host response, tissue response, innate immunity, and acquired immunity. For example, chronic inflammation commonly has been used to describe the foreign body reaction, and innate immunity is frequently substituted for inflammation. Misuse of this terminology is symptomatic of a poor understanding of the underlying biology.
In devising and using tests of the cellular response to biomaterials, it is important to understand the tissue response continuum and the cells that a pathologist uses to identify the presence of acute or chronic inflammation, the foreign body reaction, or granulation tissue (Table I). This is essential as you cannot accurately characterize a response to the presence of a biomaterial if you cannot, or do not, identify the type and phenotypic state of the cells associated with each stage. This can be accomplished only by applying biologically appropriate and rational experimental design and methodology with an understanding of the strengths and weaknesses of each type of biocompatibility test. Moreover, results and interpretations from in vitro assays should be confirmed by in vivo studies to establish claims of biocompatibility.
TABLE I.
Tissue Response Continuum
| Injury | Surgery or injection |
| Acute inflammation | Polymorphonuclear leukocytes |
| Chronic inflammation | Monocytes and lymphocytes |
| Foreign body reaction | Macrophages and foreign body giant cells |
| Granulation tissue | Fibroblasts and new blood vessels |
| Fibrous encapsulation | Fibroblasts and collagen |
In summary, it must be understood that “being biocompatible” is not a singular state characterized by the presence of the foreign body reaction because this is the normal host response to implanted biomaterials. Rather, biocompatibility exists across the tissue response continuum, the extent of which is determined by the activation and the phenotypic expression of the participating immune cells, particularly macrophages and FBGCs at the material surface. Therefore, learning to control the biology of these critical cellular mediators of the tissue response is the key to control biocompatibility.
Optimize the transition to regenerative medicine Art Coury, PhD
Member, National Academy of Engineering
When people ask me “What do you do?” I say we grow tissues and organs.
Anthony Atala, American Physician
The biomaterials community needs to become better at what we are currently doing in medicine and also lay the groundwork for the future of medicine. My grand challenge for medical implants therefore consists of two parts.
“First, we need to continue to improve conventional “replacement” medicine by making better use of existing materials, developing better materials, producing better device designs, and improving surgical techniques. Second, we need to develop the materials and technologies that optimize the inevitable transition from replacement medicine to regenerative medicine and tissue engineering.”
When I joined the biomaterials field in 1976, my company, Medtronic, had only three thermoplastic polymers: polyethylene, Dacron, and Noryl (a blend of polyphenylene oxide and polystyrene) and three types of thermoset polymers: silicone, polyurethane, and epoxy. One of the first things I did at Medtronic was to request our physiological research laboratories to qualify a set of materials to give us a larger arsenal of chronically implantable biomaterials that had the characteristics of being biostable and biocompatible as implant materials. This increased our available of thermoplastics by about a dozen and our thermosets by a few more polymers.
Early polymeric implant materials were essentially chosen from commercial sources, concentrated in the 1950s to 1970s. At Medtronic, these included polyolefins, epoxies, polyamides, polyurethanes, polyacetals, fluorocarbons, polysulfones and other rigid plastics, and softer elastomers. Other companies besides Medtronic (particularly orthopedic, ophthalmological, dental, and cardiovascular companies) were concurrently identifying and incorporating commercial polymers into their implantable devices. In essence, “if the material was stable, devoid of toxic extractables, and of a size, shape and surface texture that are tolerable to the organism, they could pass the test protocol for biocompatibility in long-term implants (Coury, 2005).”
In the more recent wave of biomaterial development (1970s to present), increasing numbers of “designer” polymers were conceived and produced for specific implantable devices (Table II). Perhaps the most important family of designer biomaterials is bioresorbable polyesters, such as polyglycolic acid, first introduced as sutures in 1970,6 then as orthopedic plates, pins, and screws.7
TABLE II.
Examples of “Designer” Polymer Compositions
| Bioresorbable Polyesters for Sutures, Plates, Pins, etc. |
| Hydrogels for implant and topical applications |
| Chemically or structurally modified natural polymers |
| Recombinant durable and resorbable polymers |
| Implantable elastomers (resorbable, biostable) |
| Orthopedic UHMW polyethylenes (modified) |
| Thrombosis and infection-resistant coatings, impregnants |
| Structural composites (orthopedic, dental, dermal, etc.) |
| Conjugates of polymers with bioactive agents |
| Polymer drugs with structural and pharmacologic action |
| Poly (phosphazenes, siloxanes, tyrosine carbonates, etc.) |
| Orthopedic bone graft substitutes |
| Tissue adhesives |
| Implantable epoxides |
Considering devices such as the artificial hip, artificial heart valve, knuckle prosthesis, intraocular lens, artificial knee, artificial heart and dental implants, commercial and “designer” biomaterials were developed for what I call “replacement medicine.” Even vascular stents meant to replace the lumen of a blood vessel or pacemakers meant to replace the electrical system of the heart are replacement medicine.
In recent years, I have begun noticing an evolution from replacement medicine to tissue engineering, which includes regenerative medicine. I define tissue engineering as “the generation, regeneration, augmentation or limitation of the structure and function of living tissues by the application of scientific and engineering principles (Coury, 2005). Put more simply, it is the systematic control of the body’s cells, matrices and fluids (Coury, 2006).”
The field of biomaterials has advanced dramatically in my last 35 years, even though we are still primarily in the era of replacing damaged or diseased tissues or functions with medical devices. Table III is a list of Medical “Holy Grails” that I have accumulated over the years that illustrates the evolution of clinical therapies. The table exemplifies the evolution in medical therapy toward tissue engineering and regenerative medicine. Although progress has been made on a number of these technologies, designated by “X” and “XX,” many remain full-blown Holy Grails.
TABLE III.
My List of Medical “Holy Grails” Entering the 1990sa
| Small diameter vascular prostheses | |
| Synthetic leaflet heart valves | |
| Bioartificial internal organs (liver, pancreas, kidney, intestine) | |
| Artificial trachea, esophagus, larynx | |
| Cellular heart pacemaker | |
| Artificial cells | |
| Devices to reconstruct sections of the digestive tract | |
| Venous valves | |
| Ligament replacement devices | |
| X | Devices to prevent surgical adhesions |
| X | Neuromuscular assist devices |
| X | Permanent cardiac assist devices |
| X | Fully functional artificial skin |
| X | Tissue adhesives, sealants |
| X | Cartilage (articular, meniscal, disc) regeneration techniques |
| X | Bone healing devices |
| X | Approaches to direct and control healing, growth, function of tissue |
| X | Artificial extracellular matrices |
| X | Artificial blood |
| X | Treatment to prevent infection, thrombosis |
| XX | Devices to treat cancer |
| XX | Percutaneous access, fixation devices |
| XX | Closed loop drug delivery devices |
| XX | Rectal, urinary function devices |
| XX | Artificial vision, hearing devices |
| XX | Nerve regeneration devices |
| XX | Electronic mind-body control |
| XX | Devices to treat chronic pain |
Removed from list due to progress from 1990 to 2000: (X) and from 2000 to present (XX).
The emergence of regenerative medicine represents both technical advances as well as an evolution of patient preferences. Personally, would I rather have a total artificial knee to replace my painfully degenerated meniscus? Or would I rather have an implanted scaffold that encourages repair of the meniscus in situ? This is what ReGen Biologics is already doing with a restorable collagen meniscal scaffold.8 My former company, Genzyme, has developed an injectable cartilage regeneration product with or without a scaffold.9 Even though it is only good for small areas now, I could imagine new methods being developed to specify and regenerate with a scaffold, the entire cartilage surface of a knee.
Would I rather have total shoulder replacement surgery? Or would I rather have my rotator cuff regenerated? I think I would rather have a decelluarized small intestine submucosa (SIS) scaffold from the Badylak group implanted? The SIS implant has been used in over two million surgeries by now.10
If my bladder went bad, would I rather have a urostomy bag for the rest of my life? Or would I go through a surgery to have my bladder regenerated? The Atala group from Wake Forest has developed the technology for in situ regeneration of an entirely functional bladder.11
Someday, the $15,000 defibrillator, the $5,000 pacemaker, and the $500 lead may be replaced by cardiac tissue constructs. The Gepstein group from Technion University in Israel has transformed embryonic human stem cells into pacemaker cells to stimulate the heart chambers to contract synchronously, which restored normal electrical activity to pigs with impaired cardiac electrical function.12
Clearly, the evolution conventional replacement medicine to regenerative medicine has begun in earnest; however with this evolution comes many technical, regulatory, ethical, and economic challenges. We need to know more about how to control biology, how to use our biomaterials to better implement our tissue engineering. We are in an era of tougher regulatory enforcement. All of the industrial people that I talk to will say that the pendulum has swung toward tougher enforcement. Ethical issues such as the use of stem cells, genetic engineering, gene therapy, nanotechnology, and so forth, need to be confronted and worked out to the greater benefit of society. We are in a period of tough economic times, from which we will emerge as we always have. Unfortunately, it looks like a return to good times of investment and easy adoption of advances are years away. Our noble commitment to solve these challenges on every front leaves me very optimistic about the future of our field of implant therapy.
Create new applications for stimuli-responsive “smart” polymers Allan S. Hoffman, PhD
Member, National Academy of Engineering
The point is that you want to have a system that is responsive.
William Nelson Joy, Cofounder Sun Microsystems
I really believe in implants. I have had an intraocular lens for almost 20 years and a hip implant for almost 10 years. But in addition to such therapeutic applications of traditional biomaterials, there is also a huge opportunity out there for in vitro use of what I am calling smart biomaterials in the home, in the research laboratory, and in the clinic.
“Smart” polymers undergo sharp and large transitions in their physical or chemical properties, such as solubility, in response to small environmental changes such as solution temperature, pH, or ionic strength.13 Figure 6 is an example of one such polymer that converts from a soluble clear polymer solution to a turbid insoluble precipitate when it passes through a critical phase transition temperature of 27–28°C.
FIGURE 6.
Light absorption versus T for a solution of a temperature-responsive “smart” polymer, such as polyNIPAAm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary. com.]
There are at least two examples of smart polymers that have been used in the clinic: the PEG-PLGA-PEG triblock copolymer of Sung Wan Kim14,15 and the surface-grafted polyNIPAAm cell sheet technology of Okano et al.16 both of which are thermally sensitive polymers. The Kim system transitions from a polymer drug solution at 45°C to form a gel when it encounters body conditions upon injection. The gel is a depot drug delivery system, and it releases drug gradually over the next hours to days. In the Okano system, cells are cultured to confluence at 37°C on polyNIPAAm-grafted surfaces. The confluent cell sheets are released fully intact when the temperature is lowered to room temperature, rehydrating the smart polymer surface. The polyNIPAAm remains on the surface and the cell sheets are suitable for transplantation.
“My grand challenge to the biomaterials community, particularly to the new members just joining our community, is to create new applications for smart polymers.”
Table IV shows what I call the toolbox of technologies that are available for creating new smart polymer compositions and structures. Such polymers can be synthesized using controlled free radical polymerizations that can produce polymers with very specific molecular weights and compositions. Random, block, graft, and hyperbranched smart polymers can be composed of two or more stimuli-responsive polymers. Smart polymers can be coated or grafted onto the surfaces of a wide variety of materials, including microparticles, nanoparticles, and patterned surfaces. For example, we have used RAFT controlled free radical polymerization to coat polyNIPAAm on onto magnetic nanoparticles,17,18 gold-shell magnetic nanoparticles,19 and porous hydroxylated membranes20 for uses in microfluidic point-of-care diagnostic assays.
TABLE IV.
Toolbox for Creating New Smart Polymers
| Controlled free radical polymerizations (RAFT, ATRP….) initiated in solution, on surfaces or at specific sites on biomolecules |
| Random, block, graft and hyperbranched smart copolymers that responded to 2 or more different stimuli in vivo or in vitro |
| Degradable linkages in smart copolymer compositions to permit release of captured targets from surfaces, release of drugs, or to enhance elimination from the body. |
| New chemistries and conditions for conjugating smart polymers to solid surfaces or biomolecules (Streptavidin[SA]-biotin, Click conjugations, Michael additions, reductive aminations…) |
| Cloning proteins to prepare mutants with specific sites where (a) smart polymerizations may be initiated or (b) end-reactive smart polymers may be directly conjugated to a specific site on proteins such as antibodies, antigens, enzymes, drugs, linkers (SA) |
| Coating smart polymers onto microparticles (lps), nanoparticles (nps), patterned surfaces, porous supports, or electrospun meshes made of polymers, natural materials, metals, metal oxides… |
| New uses for “under-used” stimuli such as light, ultrasound, electric or magnetic fields…and their combinations with each other or with T- or pH-responsive smart polymers |
Also, protein mutants can be cloned with specific sites where you can attach smart polymers for a variety of “on/off” applications. We used polyNIPAAm-conjugated antibodies to make a thermally driven immunoassay.20,21 In this assay, PolyNIPAAm was conjugated to a capture antibody, incubated with antigen and detection antibody in solution, forming a labeled “sandwich” immune complex. Raising the temperature triggered the polyNIPAAm-immune complex to phase separate out of solution and to adhere to a polyNIPAAm-coated porous membrane as the solution flowed through the pores, separating it from excess free detection antibody in solution. Subsequently lowering the temperature reversibly releases the membrane-bound immune complex that can then be assayed for the concentration of the antigen.
In another example, we produced an optical molecular switch by cloning a site directly adjacent to the active site of the enzyme endoglucanase.22 Then, we grafted a light-responsive polymer containing a UV–visible light sensitive azobenzene group pendant to the backbone chain. Under UV light, the azobenzene group is hydrophobic and the conjugated polymer collapses, blocking the active site and inactivating the protein. When illuminated with visible light, the azobenzene group is more polar, and the conjugated polymer is more extended, opening up the active site and activating the enzyme. This system can turn the enzyme on and off repeatedly by simply changing the illumination wavelength.
Smart systems can also be built around molecular recognition. We built a glucose sensing gel of glucosyl-ethyl methacrylate that is “crosslinked” by the glucose-binding lectin Concanavolin A (ConA).23 When free glucose is introduced, it competes with gel-bound ConA, releasing it from the polymer backbone and causing the polymer to swell in dose-response manner. This can be use as a glucose sensor. It can also be use as a swelling–deswelling hydrogel “gate” for delivery of insulin.
I want to close by mentioning three in vivo applications of smart polymers. A “smart” system example for in vivo use is an application of smart polymers as tissue engineering matrices.24 In this case, the smart polymers can be formed into degradable hydrogels that can be seeded with cells when they are swollen. Then they can be stimulated to collapse, entrapping the cells and allowing them to proliferate, as the matrix degrades. A second in vivo development is smart actuators that contain polymers capable of generating mechanical forces in response to in situ or externally applied stimuli. The third example is development of “smart” drug delivery systems that are responsive in vivo to metabolites, affinity-binding partners, or even to genetic characteristics of the individuals involved. This last field of “really smart systems” is coming. It is called pharmacogenetics.
Finally, I want to call your attention to an excellent article that came out this year by Brent Sumerlin entitled “Future Perspectives and Recent Advances in Stimuli Responsive Materials.”25 Have a look at it. It will also hopefully stimulate you to think more about using these very interesting smart polymers.
Engineer a human limb Cato T. Laurencin, MD, PhD
Member, Institute of Medicine of the National Academies
They struck her working tools, her legs, her arms, her hands.
Christine Arron, French Track and Field Athlete
Musculoskeletal defects arise from a variety of sources including trauma, disease, and birth defects. Collectively these give rise to over 34 million musculoskeletal or organ repair or replacement surgeries in the US each year. Orthopaedic surgeons generally choose from three strategies to repair such defects: physical therapy, bioactive agents or drugs, or surgical intervention. When considering the state of the art of surgical intervention, the choice is commonly between either a synthetic replacement/transplantation or a tissue replacement/transplantation. Transplantation of tissue can either be within an individual from a donor site to the site of repair, termed an autograft, or from a cadaver, termed an allograft.
The autograft state of the art includes not only transplantation of bone but also transplantation of cartilage from one region of an articulating surface to another. The allograft state of the art includes not only traditional allograft implantations but also allografts machined into specific shapes alone or in combination with synthetic substitutes. Therefore, the state of the art includes both the concept of transplantation and also the techniques underlying the transplantation. This is where we have come to over the last 15 years of clinical interventions, and it has poised us to move to the next level. As a chemical engineer with an acute understanding of what the state of care has been, currently is, and needs to be, I see the tremendous progress in the field of biomaterials playing a major role in regenerative medicine to establish a movement that unifies these two disciplines into one that is focused on directed in situ tissue repair. But we still have a long way to go.
One of the toughest surgical challenges is the repair of severely damaged or diseased limbs. When surgery fails to salvage a limb, or when surgery is not a viable option, the current solution is a prosthetic limb. The prosthetic limb will add some mobility and functionality but provides only low stability and limited capability. There has been a lot of excitement about robotic limbs and appendages. Robotics significantly increase functionality and capability but at a very high-economic cost. Of all the limitations of prosthetics, though, the most glaring is that prosthetics are not real tissue. Given the limitations to current strategies for limb loss, I believe a critical grand challenge for moving medicine forward is full limb regeneration. The need for limb regeneration is even more heightened now with our dual wars in Afghanistan and Iraq and our obligation to these soldiers. The central question is not “if” we should be doing this; rather, the question is “how” should we be doing this?
The traditional approach for biomaterial scientists would be tissue engineering that we defined as the application of biological, chemical, and engineering principles toward the repair restoration, regeneration of tissues using biomaterial cells, and factors alone or in combination.26 The human upper and lower extremities have a very complicated structure consisting of skin, bone, cartilage, muscle, nerves, and blood vessels. With such complex structures how can we begin to solve this challenge of regenerating multiple tissue types simultaneously into one intact and functioning organ? In my group we consider both a top-down or a bottom-up approach for reconstructing something this complex.
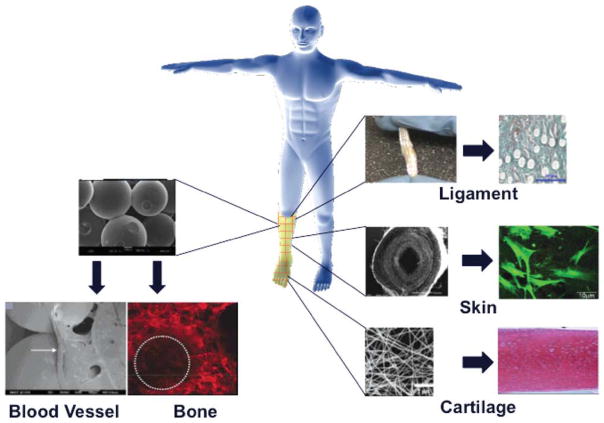
The top-down approach uses well-established elements of classical tissue engineering: biomaterials, cells, and growth factors. Over the last 20 years my laboratory and others have arrived at a point where we can create tissue specific scaffolds for pretty much every single portion of the upper or lower extremity.27 In my laboratory, we are currently using harvested tissues as autologous cell sources for regenerating different tissue types, including cartilage, bone, blood vessel, nerve, and ligament (Fig. 7). For instance, we have developed systems for in situ bone regeneration that use a sintered PLGA microsphere scaffold alone and impregnated with rhBMP-2 and seeded with bone marrow cells.28–37 We have also evaluated the efficacy of the sintered microsphere scaffold as a supporting construct for endothelial cell proliferation and vessel formation.38,39 Our work in ligament regeneration has led to the development of a three-dimensional braided structure made entirely from resorbable polymers. After numerous successful in vivo studies we are poised to begin clinical trials with our braided ligament structure.40–43 We have evaluated tubular nanofiber-based scaffolds for both blood vessel and nerve regeneration, again formed from entirely degradable materials. For cartilage and skin, the use of nanofiber scaffolds made of both conventional and next generation materials, such as polyphosphazenes, have shown great potential.44
FIGURE 7.
Variety of polymeric scaffolds used by the Laurencin laboratory to build different tissue types. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Although the complexity of tissues adds to the challenge of tissue regeneration, it also presents certain advantages. One significant advantage of complexity of extremity tissue is that it can provide a broad array of cell types that can be harvested for tissue engineering applications. These cell sources can also provide the necessary tools for the bottom up approach to tissue regeneration. The bottom-up approach calls on regenerative biology, where intact tissues are self-assembled from highly proliferative and differentiated cell layers. This is how blastema regenerates tissues and organs in amphibians. For example, if you chop off a newt’s limb it will regenerate a new one after a period of 7–10 weeks. The translational challenge here is, of course, we are not newts. Although we cannot simply use the same approach that newts use, studies of their regenerative capacity must be part of the arsenal we use to achieve our grand challenge.
The current dogma in developmental biology is that regenerative capacity is inversely related to organismal complexity. As such, humans have only limited regenerative capabilities; for example, a severed fingertip with periodic dressing changes will grow back. We can also surgically reattach severed appendages that heal nearly completely under certain conditions. The fact is that humans can exhibit some newt-like tendencies. The important question is: will we be able to expand this limited regenerative capability to repair tissue damage that currently results in amputation? Our strategy for overcoming barriers to regeneration in vertebrates is to “harness the newt in us” through the lessons learned and bottom up strategies used in regenerative biology in combination with classical top-down tissue engineering into what I am calling regenerative engineering.
Regenerative engineering is the integration of tissue engineering, advanced material science, stem cell science, and areas of developmental biology for the regeneration of complex tissues, organs, and organ systems. The integrated response of cells, especially stem cells to biomaterials is a key component of this approach. More specifically, a key element of regenerative engineering is to utilize biomaterials as guides for tissue development. We hope to engineer substrates that through topographical and physicochemical means will influence the differentiation of undifferentiated cell types. Through this approach, we feel that we can create location-specific topographies that will direct the same cell population towards different tissue types, depending on the physicochemical cues embedded in the substrate itself. To do this requires a comprehensive understanding of the influence of biomaterials on cells, and of the particular stimulus that evoke responses in particular cell types.
At the Institute for Regenerative Engineering at the University of Connecticut, we combine material design with developmental biology to regenerate complex tissues, organs, and organ systems. It is the intention of the Institute to find real solutions to real clinical problems. Although new discovery is essential and will always be a means to an end, it will not be the end itself, a direction that biomaterials research today seems to be trending toward.
In an eagerness to be innovative and to move the field to tissue engineering and tissue regeneration forward there has been a surge of “wheel of fortune” style research evident within the biomaterials community. The wheel consists of a cell type, a protein, factor, or peptide, and a substrate material and it is spun to choose the various elements of a new research endeavor. At times it seems a spin of the wheel ensures innovation because there will always be some combination of cell, factor, and material that has not previously been investigated. Clearly this is hyperbole, but it does bring to light an important manifestation of the overwhelming desire of researchers to find something new. The translation of these highly innovative and extremely creative research endeavors to the clinical realm, the goal of all biomaterials scientists and biomedical engineers, becomes so daunting that the potential clinical benefit of these innovative ideas is never realized. As we continue to make discoveries in the fields of tissue engineering and regenerative medicine, it is imperative that we keep one eye on the ultimate goal of our endeavors; patient care.
Harness the principles of developmental biology David Tirrell, PhD
Member, National Academy of Engineering and National Academy of Science
It is not birth, marriage, or death, but gastrulation which is truly the most important time in your life.
Lewis Wolpert, British Developmental Biologist
Briefly, gastrulation is the early stage of embryonic development when the multicelled blastula invaginates and “collectively” reorganizes to form the gastrula comprised of three germ layers: an outer cell layer, the ectoderm, and two inner cell layers, the endoderm and the mesoderm. The formation of the three germ layers is followed by differentiation and proliferation steps that give rise to all of the tissues and organs of the body. The gastrula follows a highly choreographed sequence of events that progresses through increasingly segregated, expanded, and differentially functional cellular constructs. Several videos available on the web (e.g., that presented by Eric Wieschaus: http://www.youtube.com/watch?v=ymRYxFYLsZ4) provide graphic demonstration of the remarkably cooperative processes of collective cell motion that comprise gastrulation.
Biology clearly knows how to do this and can do so routinely and flawlessly. However, to paraphrase Lewis Wolpert, if gastrulation goes poorly then your life is sure to go poorly as well. The classical tissue engineering paradigm is to take biomaterials, cells, growth factors, and so on, put them into an animal, and examine the evolution of that construct over time. The chief goal of introducing stem cells into the construct is to “engineer” complex tissues and organs from a common base of pluripotent cells. Although important progress has been made in identifying some of the cues that lead to directed stem cell differentiation, much remains to be learned about the control of collective cell growth and differentiation.
My grand challenge for biomaterials is to “harness the principles of developmental biology to control collective cell movement and differentiation in vivo and in vitro.” Meeting this challenge will teach us the tools that nature uses to build functional tissue and organs.
My training in chemistry prompts me to think about the elementary processes involved in going from a simpler system to a more complex system. Consider for example the molecule maitotoxin, a linear, polycyclic, multidomain neurotoxin.45 Just the ABCDEFG domain of maitotoxin (Fig. 8) contains 35 carbon atoms, 26 of which are chiral centers of one kind or another. Before chemists could attack the synthesis of such complex molecules, they had to address simpler, and perhaps more general questions, for example: “How do we make carbon–carbon bonds?” “How do we make carbon–oxygen bonds?” “How do we control stereochemistry at asymmetric centers?” It is only with these more elementary tools in hand that the chemist can productively approach the construction of a molecule as complex as maitotoxin.
FIGURE 8.

The ABCDEFG domain of maitotoxin, a linear, polycyclic, multidomain neurotoxin. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Organs and tissues, of course, present much more difficult construction challenges. So what I would like to advocate to the biomaterials community is to think about a complementary perspective as we continue to unite developmental biology with biomaterials science and engineering. Specifically, our community should think about the elementary steps underlie the more complex processes that coax tissue to regenerate or that allow tissue to heal.
In studying—and in learning how to control—tissue morphogenesis, we should consider deconstructing the overall process into simpler processes of collective cell motion. Figure 9 shows six of these processes: invagination, ingression, involution, epiboly, intercalation, and convergent extension.46 Breaking the more complicated system down to its constituent parts allows one to apply in vitro cell culture techniques to perform controlled studies on the factors that mitigate these phenomena.
FIGURE 9.
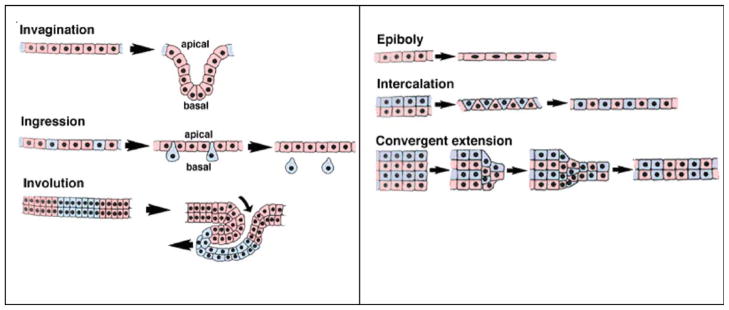
Six examples of the cooperative and collective cell motions that comprise gastrulation. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In my research group, we study wound closure on engineered biomaterials, which provides instructive examples of collective cell motion. Eileen Fong in my laboratory uses in vitro wound-healing models to study rates of epithelial cell boundary motion as a function of materials design parameters. The advantage of this approach is the ability to precisely titrate the cell culture and then use microscopy to follow the effects. Eileen’s studies show that increasing the density of RGD cell adhesion peptides on the surface dramatically increases the rate of wound closure, while—surprisingly—the cell migration speed remains relatively constant regardless of RGD surface density. These results suggest that the process of wound closure involves more than cell migration directed toward the wound boundary. Similar test protocols should be possible for studying the elementary cellular processes involved in tissue regeneration.
In sum, a critical grand challenge at the interface of biomaterials science and developmental biology would be to devise experimental test protocols that will allow us to understand the underlying elementary processes of collective cell motion, and then ultimately to control these elementary processes experimentally. Then, as we move to more complex systems, we will have a set of tools that will allow us to build the tissue and organ architectures that are needed for regenerative medicine.
References
- 1.Williams DF. The Williams Dictionary of Biomaterials. Liverpool: Liverpool University Press; 1999. [Google Scholar]
- 2.Marshall A. PhD Thesis. University of Washington; Dec, 2004. Porous Hydrogels with Well-Defined Pore Structure for Biomaterials Applications. [Google Scholar]
- 3.http://www.healionics.com/star-image-gallery.html.
- 4.Fukano Y, Usui ML, Underwood RA, Isenhath S, Marshall AJ, Hauch KD, Ratner BD, Olerud JE, Fleckman P. Epidermal and dermal integration into spheretemplated porous poly(2-hydrox-yethyl methacrylate) implants in mice. J Biomed Mater Res A. 2010;94:1172–1186. doi: 10.1002/jbm.a.32798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 6.Braun B. Copyright 2010; Available at: http://www.suturesbbraun.com/index.cfm?AD0744022A5AE62661619BE8AC9C4CEF.
- 7.Mohamed-Hashemand IK, Mitchell DA. Resorbable implants (Plates and Screws) in orthognathic surgery. Br J Orthod. 2000;27:198–199. doi: 10.1093/ortho/27.2.198. [DOI] [PubMed] [Google Scholar]
- 8.Regen Biologics Website. Copyright 2007; Available at: http://www.regenbio.com/usa/en/
- 9.Genzyme Corporation Website. Copyright 2010; Available at: http://www.carticel.com/patients/treatment.aspx.
- 10.Biotech Cook. Copyright 2010; Available at: http://www.cookbiotech.com/Tech_whatisbiodesign.php.
- 11.Tengion, Inc. Copyright May 21, 2009; Available at: http://www.tengion.com/
- 12.Rappaport Institute. Copyright 2006; Available at: http://www.rap-pinst.com/Rappaport/Templates/showpage.asp?DBID=1&TMID=178&FID=90&PID=860&IID=882.
- 13.Hoffman AS, Stayton PS. Conjugates of stimuli-responsive polymers and proteins. Prog Polymer Sci. 2007;32:922–932. [Google Scholar]
- 14.Jeong B-M, Bae Y-H, Lee D-S, Kim SW. Biodegradable block copolymers as injectable drug delivery systems. Nature. 1997;388:860–862. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- 15.Jeong B-M, Bae YH, Kim SW. In situ gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions and degradation thereof. J Biomed Mater Res A. 2000;50:171–177. doi: 10.1002/(sici)1097-4636(200005)50:2<171::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(NIPAAm) J Biomed Mater Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 17.Lai J, Hoffman AS, Stayton PS. Dual magnetic-temperature responsive nanoparticles for microfluidic separations and assays. Langmuir. 2007;23:7385–7391. doi: 10.1021/la062527g. [DOI] [PubMed] [Google Scholar]
- 18.Lai J, Nelson KE, Nash MA, Hoffman AS, Yager P, Stayton PS. Dynamic bioprocessing and microfluidic transport control with smart magnetic nanoparticles in laminar-flow devices. Lab Chip. 2009;4:1997–2002. doi: 10.1039/b817754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nash MA, Lai J, Hoffman AS, Yager P, Stayton PS. Smart diblock copolymers as templates for magnetic-core gold-shell nanoparticle synthesis. Nano Lett. 2010;10:85–91. doi: 10.1021/nl902865v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden AL, Battrell CF, Pennell S, Hoffman AS, Stayton PS. A simple fluidic system for purifying and concentrating diagnostic biomarkers using stimuli-responsive antibody conjugates and membranes 2010. Bioconjugate Chem. 2010;21:1820–1826. doi: 10.1021/bc100169y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monji N, Hoffman AS. A novel immunoassay system and bioseparation process based on thermal phase separating polymers. Appl Biochem Biotech. 1987;14:107–120. doi: 10.1007/BF02798429. [DOI] [PubMed] [Google Scholar]
- 22.Shimoboji T, Ding Z, Stayton PS, Hoffman AS. Mechanistic investigation of smart polymer-protein conjugates. Bioconjug Chem. 2001;12:314–319. doi: 10.1021/bc000107b. [DOI] [PubMed] [Google Scholar]
- 23.Miyata T, Jikihara A, Nakamae K, Hoffman AS. Preparation of reversibly glucose-responsive hydrogels by covalent immobilization of lectin in polymer networks having pendant glucose. J Biomater Sci Polym Ed. 2004;15:1081–1084. doi: 10.1163/1568562041753061. [DOI] [PubMed] [Google Scholar]
- 24.Galperin A, Long JT, Ratner BD. A degradable, thermo-sensitive poly(N-isopropyl acrylamide)-based scaffold with controlled porosity for tissue engineering applications. Biomacromolecules. 2010;11:2583–2592. doi: 10.1021/bm100521x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy D, Cambre JN, Summerlin BS. Future perspectives and recent advances in stimuli responsive materials. Prog Polymer Sci. 2010;35:278–301. [Google Scholar]
- 26.Laurencin CT, Ambrosio AA, Borden MD, Cooper JA. Tissue engineering: Orthopaedic applications. Ann Rev Biomed Eng. 1999;1:19–26. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 27.Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Progress in Polymer Science. 2007;32:762–798. [Google Scholar]
- 28.Laurencin CT, Khan Y, Kofron M, El-Amin S, Botchwey E, Yu X, Cooper JA., Jr The ABJS Nicolas Andry Award: Tissue engineering of bone and ligament: A 15-year perspective. Clin Orthop Relat Res. 2006;447:221–236. doi: 10.1097/01.blo.0000194677.02506.45. [DOI] [PubMed] [Google Scholar]
- 29.Jabbarzadeh E, Nair LS, Khan YM, Deng M, Laurencin CT. Apatite nano-crystalline surface modification of poly(lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering: Implications for protein adsorption. J Biomater Sci Polymer Ed. 2007;18:1141–1152. doi: 10.1163/156856207781554073. [DOI] [PubMed] [Google Scholar]
- 30.Cushnie E, Khan Y, Laurencin CT. Amorphous hydroxyapatite-sintered polymeric scaffolds for bone tissue regeneration: Physical characterization studies. J Biomed Mater Res. 2008;84:54–62. doi: 10.1002/jbm.a.31380. [DOI] [PubMed] [Google Scholar]
- 31.Khan Y, Cushnie E, Laurencin CT. In situ synthesized ceramic-polymer composites for bone tissue engineering: Bioactivity and degradation studies. J Mat Sci. 2007;42:4183–4190. [Google Scholar]
- 32.Khan Y, Yaszemski MJ, Mikos AG, Laurencin CT. Tissue engineering of bone: Material and matrix considerations. J Bone Joint Surg. 2008;90:36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 33.Jiang T, Khan Y, Nair LS, Abdel-Fattah WI, Laurencin CT. Functionalization of chitosan/poly(lactic acid-glycolic acid) sintered microsphere scaffolds via surface heparinization for bone tissue engineering. J Biomed Mater Res A. 2010;93:1193–1208. doi: 10.1002/jbm.a.32615. [DOI] [PubMed] [Google Scholar]
- 34.Cushnie E, Khan Y, Laurencin CT. Tissue engineered matrices as functional delivery systems: Adsorption and release of bioactive proteins from degradable composite scaffolds. J Biomed Mater Res A. 2010;94:568–575. doi: 10.1002/jbm.a.32722. [DOI] [PubMed] [Google Scholar]
- 35.Kofron MD, Cooper JA, Jr, Kumbar SG, Laurencin CT. Novel tubular composite matrix for bone repair. J Biomed Mater Res A. 2007;82:415–425. doi: 10.1002/jbm.a.31148. [DOI] [PubMed] [Google Scholar]
- 36.Kofron MD, Laurencin CT. Development of a calcium phosphate co-precipitate/poly(lactide-co-glycolide) DNA delivery system: release kinetics and cellular transfection studies. Biomaterials. 2004;25:2637–2643. doi: 10.1016/j.biomaterials.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 37.Lu HH, Kofron MD, El-Amin SF, Attawia MA, Laurencin CT. In vitro bone formation using muscle-derived cells: A new paradigm for bone tissue engineering using polymer-bone morphogenetic protein matrices. Biochem Biophys Res Commun. 2003;305:882–889. doi: 10.1016/s0006-291x(03)00858-1. [DOI] [PubMed] [Google Scholar]
- 38.Jabbarzadeh E, Jiang T, Deng M, Nair LS, Khan YM, Laurencin CT. Human endothelial cell growth and phenotypic expression on three dimensional poly(lactide-co-glycolide) sintered microsphere scaffolds for bone tissue engineering. Biotechnol Bioeng. 2007;98:1094–1102. doi: 10.1002/bit.21495. [DOI] [PubMed] [Google Scholar]
- 39.Jabbarzadeh E, Starnes T, Khan YM, Jiang T, Wirtel AJ, Deng M, Lv Q, Nair LS, Doty SB, Laurencin CT. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: A combined gene therapy-cell transplantation approach. Proc Natl Acad Sci USA. 2008;105:11099–11104. doi: 10.1073/pnas.0800069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper JA, Lu HH, Ko FK, Freeman JW, Laurencin CT. Fiber-based tissue-engineered scaffold for ligament replacement: Design considerations and in vitro evaluation. Biomaterials. 2005;26:1523–1532. doi: 10.1016/j.biomaterials.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Lu HH, Cooper JA, Jr, Manuel S, Freeman JW, Attawia MA, Ko FK, Laurencin CT. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: In vitro optimization studies. Biomaterials. 2005;26:4805–4816. doi: 10.1016/j.biomaterials.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 42.Cooper JA, Jr, Bailey LO, Carter JN, Castiglioni CE, Kofron MD, Ko FK, Laurencin CT. Evaluation of the anterior cruciate ligament, medial collateral ligament, achilles tendon and patellar tendon as cell sources for tissue-engineered ligament. Biomaterials. 2006;27:2747–2754. doi: 10.1016/j.biomaterials.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Cooper JA, Jr, Sahota JS, Gorum WJ, II, Carter J, Doty SB, Laurencin CT. Biomimetic tissue-engineered anterior cruciate ligament replacement. Proc Natl Acad Sci USA. 2007;104:3049–3054. doi: 10.1073/pnas.0608837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumbar SG, Nukavarapu SP, James R, Nair LS, Laurencin CT. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials. 2008;29:4100–4107. doi: 10.1016/j.biomaterials.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.http://pubs.acs.org/doi/abs/10.1021/ja102260q.
- 46.http://worms.zoology.wisc.edu/dd2/echino/gast/morph/morph.html.