Abstract
Background
Diabetic cardiomyopathy defined as either systolic or diastolic dysfunction in otherwise healthy diabetic persons is not clearly understood. The prevalence and outcomes of this disease in a community-based population have not been defined.
Methods
Cross-sectional survey of 2,042 randomly selected residents of Olmsted County, Minnesota, aged 45 years or older from June 1997 through September 2000. All patients underwent Doppler echocardiographic assessment of systolic and diastolic function. Diabetic cardiomyopathy was defined in a person with diabetes and any systolic or at least moderate diastolic dysfunction without a history of coronary disease, hypertension, significant valvular disease or congenital heart disease.
Results
The diagnosis of diabetic cardiomyopathy was made in 23 persons, corresponding to a community population prevalence rate of 1.1%. Among diabetic patients, 16.9% met criteria for diabetic cardiomyopathy, and 54.4% had diastolic dysfunction. Diabetes was associated with a 1.9 fold increase in risk of any left ventricular dysfunction, a 1.7 fold increase in risk of diastolic dysfunction, and a 2.2 fold increase in risk of systolic dysfunction. Among subjects with diabetic cardiomyopathy, the cumulative probability of death was 18%, development of heart failure was 22%, and development of death or heart failure was 31% at 9 years.
Conclusion
Diabetic cardiomyopathy is relatively common in the community with a prevalence of 1.1%. The morbidity and mortality of patients with diabetic cardiomyopathy is high.
Keywords: diabetes mellitus, diabetic cardiomyopathy, prevalence, left ventricular dysfunction, heart failure, Olmsted County
Introduction
Diabetes is associated with an increased risk of cardiovascular complications, including hypertension, coronary artery disease and the development of heart failure (HF) [1, 2]. However, there is growing recognition of a primary myocardial disease process or “diabetic cardiomyopathy” that predisposes diabetic patients to ventricular dysfunction in the absence of clinically significant coronary, valvular or hypertensive disease [3–11]. Diabetic cardiomyopathy (DCM), defined as either systolic or diastolic left ventricular dysfunction in otherwise healthy diabetic persons, is poorly understood from an epidemiologic and natural history standpoint.
First proposed by Rubler et al. in 1972 based on post-mortem findings, diabetic cardiomyopathy is thought to be secondary to underlying hyperglycemia resulting in a multitude of adverse downstream effects, including impaired myocyte calcium handling, increased oxidative stress, renin-angiotensin-aldosterone activation, microangiopathy and myocardial fibrosis [12–14]. Prior studies have attempted to characterize the prevalence of ventricular dysfunction among asymptomatic diabetic patients, but these were non-population-based studies or exhibited a referral bias of patients undergoing cardiovascular testing for clinical indications [15, 16]. In 2010, From and Chen demonstrated that pre-clinical diastolic dysfunction in diabetic patients was associated with an increased incidence of heart failure and higher mortality [17]. However, despite adjustment for co-morbidities, a large proportion of patients in the study had pre-existing hypertension and coronary artery disease. Thus, the true population prevalence and natural progression of diabetic cardiomyopathy is unknown.
In this study, we sought to determine a population-based prevalence of diabetic cardiomyopathy. Additionally, we planned to characterize the risk of systolic and/or diastolic left ventricular (LV) dysfunction in diabetic patients and assess the rates of long term survival and development of heart failure in patients with diabetic cardiomyopathy.
Methods
This study was approved by the Mayo Foundation institutional review board and informed consent was obtained by all subjects participating in the study. As previously described, the resources of the Rochester Epidemiology Project were utilized to identify a random sample of residents who were at least 45 years old as of January 1, 1997 [18, 19]. Participants were enrolled and studied during a 3-year period, ending September 30, 2000. Of the 4203 eligible residents invited, 2042 (47%) participated. An analysis of the medical records of 500 randomly selected residents who did not participate in the study revealed similar age and sex distribution to that observed in the participants and a similar prevalence of hypertension, coronary artery disease, previous myocardial infarction, diabetes, previous cardiovascular hospitalization, and congestive heart failure [18].
Each participant underwent a focused physical examination that included measurement of blood pressure, height, weight and BMI calculation (kg/m2). Community medical records for each participant were reviewed by trained nurse abstractors to record a history of hypertension or myocardial infarction using established criteria at the time of presentation [20, 21]. In addition, historical clinical diagnoses of coronary artery disease, valvular disease, congenital heart disease, and diabetes mellitus were recorded.
Each participant’s medical records were also reviewed to determine if any diagnosis of heart failure had been made. If so, each medical encounter was reviewed to determine whether the documented clinical information fulfilled Framingham criteria [25]. Participants with either systolic or diastolic dysfunction, but no formal heart failure diagnosis, were considered to have preclinical ventricular dysfunction. Such designation did not imply progression to symptomatic or clinical heart failure [18].
All subjects underwent echocardiography, performed using standard methods that have been previously described and validated [18, 22]. All echocardiograms were performed by 1 of 3 registered diagnostic cardiac sonographers and interpreted by a single echocardiologist (M.M.R.). Two dimensional and color Doppler imaging was performed to screen for valvular disease. Left ventricular ejection fraction measured by visual estimate was used for analysis. As previously described and validated, left ventricular diastolic dysfunction was assessed by pulsed-wave Doppler examination of mitral inflow (before and during Valsalva maneuver) and pulmonary venous inflow, as well as by Doppler tissue imaging of the mitral annulus. Diastolic dysfunction was categorized according to the progression of diastolic disease: normal (0.75<E/A<1.5 and E/e’<10); mild (defined as impaired relaxation without increased filling pressures, E/A≤0.75 and E/e’<10); moderate (defined as impaired relaxation associated with moderately elevated filling pressures or pseudonormal filling, 0.75<E/A<1.5 and E/e’≥10); and severe (defined as advanced reduction in compliance or reversible or fixed restrictive filling, E/A>1.5 and E/e’≥10) [18, 23, 24]. Participants were required to have two Doppler criteria consistent with moderate or severe diastolic dysfunction to be so classified. Subjects with one criterion for moderate or severe diastolic dysfunction or those whose parameters were borderline but not definitive for diastolic dysfunction were classified as indeterminate. In this study, left ventricular dysfunction is defined as an ejection fraction of <50% and/or moderate to severe diastolic dysfunction. Contrary to previous analyses utilizing this Olmsted County cohort, only diabetic patients with both systolic and diastolic ventricular assessments were included in this study [18].
In keeping with its previously described definitions, diabetic cardiomyopathy was diagnosed in patients with all of the following criteria: 1) the presence of diabetes mellitus 2) documented systolic or at least moderate diastolic dysfunction after the diagnosis of diabetes mellitus, 3) no history of clinical heart failure, 4) no history of coronary disease with or without a previous angiogram or stress test, 5) no history of hypertension, 6) no history of significant valvular disease and 7) no history of congenital heart disease [7–15].
Statistical Analysis
Categorical variables were summarized as percentages and continuous variables as mean ± standard deviation. Comparison between groups was based on a two sample t-test for continuous variables and Pearson’s chi-square test for categorical variables. The major endpoints were mortality and development of heart failure. Kaplan-Meier analysis was performed to estimate probabilities of events and the probabilities were compared between groups using the Log rank test. Healthy controls without diabetes, left ventricular dysfunction, hypertension or coronary disease were selected from the Olmsted County population for mortality comparison. Univariable and multivariable associations of clinical and echocardiographic variables with each endpoint were assessed with Cox’s proportional hazard modeling. Hypothesized trends in outcomes were tested using the following scoring within Cox’s models: 1= subjects with diabetes and no LV dysfunction (D0CM), 2= subjects with diabetic cardiomyopathy (DCM), 3= subjects with diabetes and hypertension or coronary artery disease and any LV dysfunction (D1CM). The presence of LV dysfunction was also assessed using univariable and multivariable logistic regression modeling. Analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
Diabetic cardiomyopathy (DCM) was diagnosed in 23 of the total 2,042 subjects, corresponding to an Olmsted County community population prevalence of 1.1% (95% CI 0.7% to 1.6%). However, among the 136 subjects with diabetes, 16.9% met the diagnostic criteria for diabetic cardiomyopathy. 83% of the subjects with DCM had LV diastolic dysfunction and preserved ejection fraction. The prevalence of LV diastolic dysfunction among diabetic patients in the community was 54.4%, while the prevalence of LV systolic dysfunction was 7.3%. Using multivariable logistic regression analysis, the presence of diabetes was associated with a 1.9 fold increase in risk of any left ventricular dysfunction (HR=1.87; 95% CI (1.32, 2.64), p=0.0004), a 1.7 fold increase in risk of diastolic dysfunction (HR=1.67; 95% CI (1.19, 2.34), p=0.0031), and a 2.2 fold increase in risk of systolic dysfunction (HR=2.23; 95% CI (1.27, 3.91), p=0.0051), after adjustment for age and sex.
Among subjects with diabetic cardiomyopathy, the cumulative probability of death was 18% (95% CI (0.3, 32.7)), the cumulative probability of the development of heart failure was 22% (95% CI (2.9, 37.3)), and of the development of death or heart failure was 31% (95% CI (8.9, 47.3)) at 9 years (Table 1).
Table 1.
Cumulative Probability of Death and Heart Failure in Diabetic Cardiomyopathy
| Variable | Diabetic Cardiomyopathy (n=23) |
95% Confidence Interval |
|---|---|---|
| Death | ||
| • 3 years | 0% | (0%, 0%) |
| • 6 years | 4% | (0%, 12.3%) |
| • 9 years | 18% | (0.3%, 32.7%) |
| Development of HF | ||
| • 3 years | 9% | (0%, 19.5%) |
| • 6 years | 17% | (0.4%, 31.5%) |
| • 9 years | 22% | (2.9%, 37.3%) |
| Death or HF | ||
| • 3 years | 9% | (0%, 19.5%) |
| • 6 years | 17% | (0.4%, 31.5%) |
| • 9 years | 31% | (8.9%, 47.3%) |
HF=heart failure
A secondary exploratory analysis of long term outcomes was performed comparing subjects with diabetes and no LV dysfunction (D0CM), subjects with diabetic cardiomyopathy (DCM) and subjects with diabetes and hypertension or coronary artery disease and any LV dysfunction (D1CM).
When comparing baseline characteristics among the three groups, subjects with DCM and D1CM were older than subjects with D0CM. Subjects with D1CM had a higher BMI compared to DCM and D0CM. There was no significant difference in left ventricular ejection fraction among the three groups, and only 17% of subjects with DCM had a left ventricular ejection fraction <50%. Left ventricular mass index was highest in D1CM. There was no significant difference in creatinine measurements among the three groups (Table 2).
Table 2.
General Characteristics
| Variable | Diabetic with No LV Dysfunction [D0CM] (N=52) |
Diabetic Cardiomyopathy [DCM] (N=23) |
Diabetic with CAD or HTN and Any LV Dysfunction [D1CM] (N=61) |
|---|---|---|---|
| Age (years) | 62.6 ± 9.1 | 68.5 ± 10.6b | 67.6 ± 9.2c |
| Gender (Male), No. (%) | 31 (60%) | 17 (74%) | 33 (54%) |
| BMI (kg/m2) | 29.9 ± 6.1 | 29.2 ± 4.3 | 32.3 ± 5.6ac |
| Hyperlipidemia, No. (%) | 18 (36%) | 4 (18%) | 24 (39%) |
| Smoking, No. (%) | 28 (54%) | 16 (70%) | 35 (57%) |
| LV Ejection Fraction (%) | 64.2 ± 5.2 | 61.7 ± 8.8 | 62.1 ± 8.9 |
| Reduced LVEF (<=50%), No. (%). | 0 (0%) | 4 (17%)b | 6 (10%)c |
| E/A Ratio | 1.0 ± 0.2 | 0.9 ± 0.4b | 0.9 ± 0.3c |
| E/e’ Ratio | 7.4 ± 1.3 | 10.5 ± 2.8b | 10.2 ± 2.4c |
| LV Mass Index (g/m2) | 94.6 ± 24.0 | 105.9 ± 19.9 | 108.0 ± 29.1c |
| Creatinine (mg/dL) | 1.1 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.2 |
Values are mean ± SD or n (%).
p<0.05 (DCM vs. D1CM);
p<0.05 (DCM vs. DOCM);
p<0.05 (D1CM vs. DOCM)
E=passive transmitral left ventricular inflow velocity; A=late transmitral left ventricular inflow during left atrial contraction; e’=tissue Doppler imaging velocity of the medial mitral annulus during passive filling.
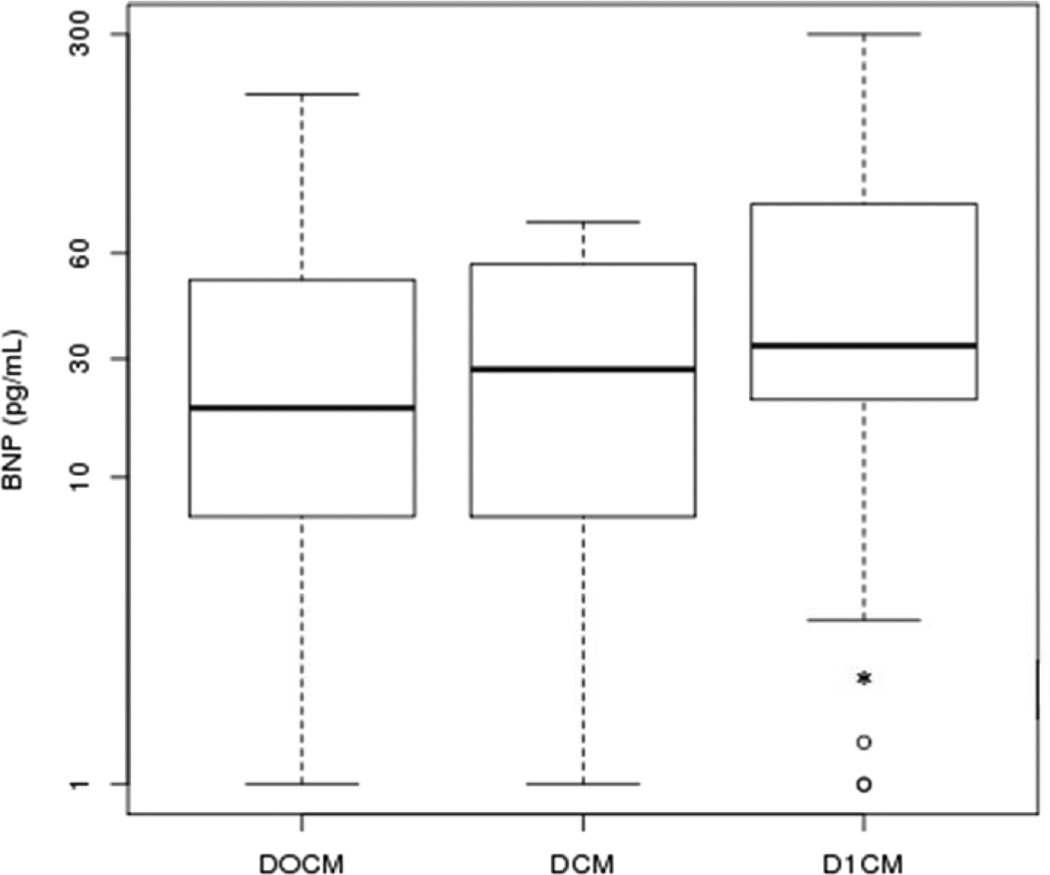
B-type natriuretic peptide (BNP) levels were highest in subjects with D1CM. There was no statistically significant difference in BNP levels in subjects with DCM compared to subjects with D0CM (Figure 1).
Figure 1. Comparison of B-type Natriuretic Peptide Levels.
The top of box is the 25% percentile, the middle bar in the box is the median and the bottom of box is the 75% percentile. The end lines outside the box are the statistical range. The open circles are the statistical outliers. The asterisk represents a statistically significant difference (p<0.05) when comparing D1CM to DOCM
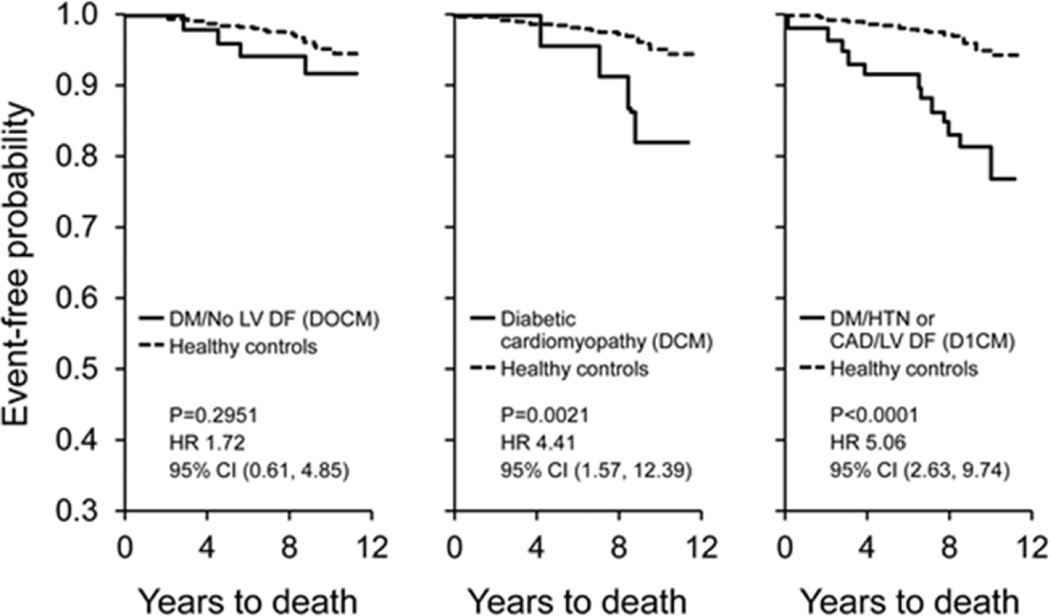
Using Kaplan-Meier analysis of survival among the three groups compared to healthy controls, there was a statistically significant increased risk of mortality in subjects with DCM compared to healthy controls and a trend toward increased risk after adjustment for age and sex (HR 1.25; 95% CI (0.42, 3.68), p=0.1377, adjusted for age/sex). There was a statistically significant increased risk of mortality in subjects with D1CM compared to healthy controls, before and after adjustment for age and sex (HR 2.09; 95% CI (1.05, 4.14), p=0.0012, adjusted for age/sex). There was no statistically significant difference in survival when comparing subjects with D0CM and healthy controls (HR 1.12; 95% CI (0.39, 3.18), p=0.9636, adjusted for age/sex) (Figure 2).
Figure 2. Kaplan-Meier Analysis of Survival.
The unadjusted P value is for comparison to the healthy controls.
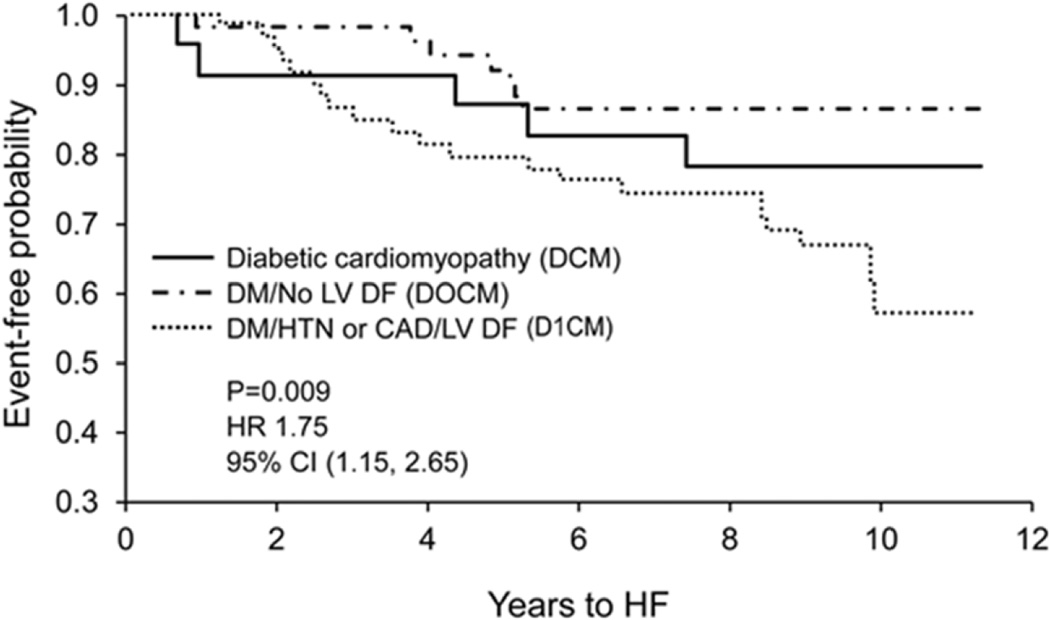
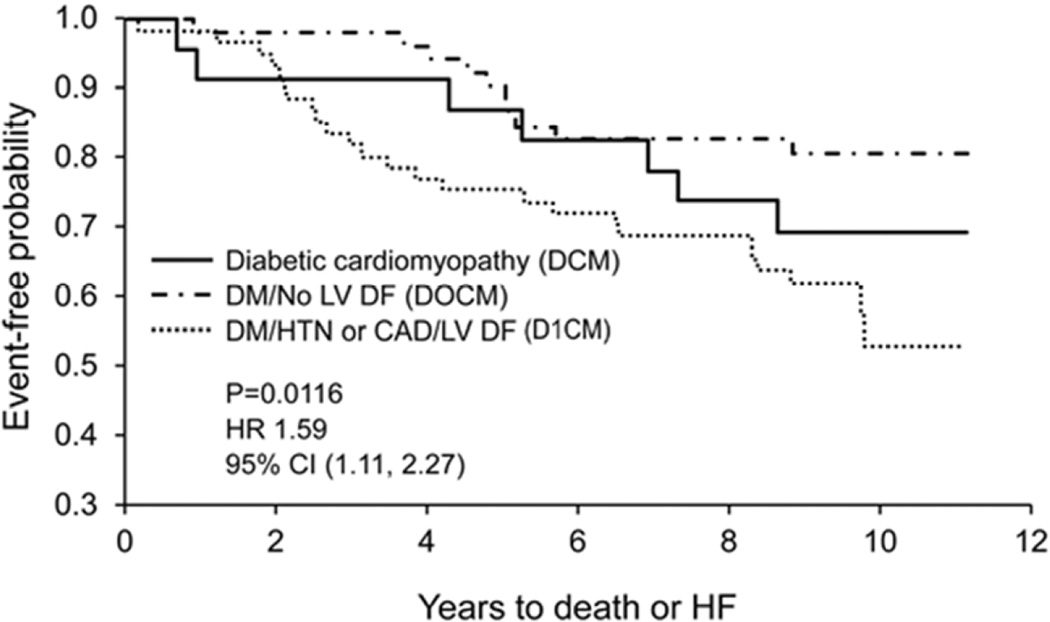
The probability of developing heart failure using Kaplan-Meier analysis was highest in subjects with D1CM, followed by subjects with DCM and lastly, D0CM (HR 1.60; 95% CI (1.03, 2.48), p=0.0364 for trend, adjusted for age/sex) (Figure 3). Similarly, the probability for the development of death or heart failure, based on Kaplan-Meier analysis, was highest in subjects with D1CM, followed by subjects with DCM, then D0CM; there remained a strong trend after adjustment for age and sex (HR 1.41; 95% CI (0.97, 2.07), p=0.0724 for trend, adjusted for age/sex) (Figure 4).
Figure 3. Kaplan-Meier Analysis of Development of Heart Failure.
The unadjusted P value is for trend.
Figure 4. Kaplan-Meier Analysis of Survival and Development of Heart Failure.
The unadjusted P value is for trend.
Discussion
This study is the first to determine a population-based prevalence of diabetic cardiomyopathy as defined by left ventricular dysfunction in diabetic patients in the absence of coronary, valvular or hypertensive disease. Using data from a large, prospectively enrolled cohort from Olmsted County, MN, we determined the community population prevalence of diabetic cardiomyopathy to be 1.1%. In addition, the prevalence of DCM in diabetic patients is 16.9% and the prevalence of diastolic dysfunction in diabetic patients is 54%. We estimated that the presence of diabetes was associated with an increased risk of systolic, diastolic and any left ventricular dysfunction, even after adjustment for age and gender. Lastly, we demonstrated that diabetic cardiomyopathy is associated with a relatively high cumulative probability of the development of heart failure and death.
The results of this study add to the growing evidence in support of a primary myocardial disease process predisposing diabetic patients to pre-clinical ventricular dysfunction, heart failure, and increased mortality. Several epidemiologic studies have confirmed that people with diabetes are more likely to develop heart failure compared with people without diabetes: a) The Framingham Heart Study investigators demonstrated that diabetes was an independent risk factor for heart failure [25]; b) The Cardiovascular Health Study reported a 2-fold increase in risk of development of heart failure associated with diabetes [26]; c) The Strong Heart Study also reported that diabetes is an independent risk factor for heart failure [7]. In a population-based cohort of 1204 subjects, the authors showed a 1.5-fold higher risk of heart failure in patients with diabetes after adjustment for multiple cofactors. Importantly, the survival of patients with diabetes and heart failure was also reduced relative to those without diabetes [27].
Despite several epidemiological studies demonstrating an increased risk of development of heart failure in diabetic patients, the prevalence and natural history of diabetic cardiomyopathy remains poorly defined. Recent studies have attempted to non-invasively detect and define the cardiovascular changes of diabetic cardiomyopathy with aggressive adjustment for multiple co-morbid diseases in biased selections of patients. The Strong Heart Study examined the left ventricular systolic and diastolic function of diabetic patients as compared to non-diabetic patients, but did not isolate groups of patients with or without confounding hypertension or coronary disease at enrollment as in our cohort. We previously reported that pre-clinical diastolic dysfunction in diabetic patients was associated with an increased incidence of heart failure and higher mortality [17]. However, despite adjustment for co-morbidities, a large proportion of patients in the study had pre-existing hypertension and coronary artery disease. In the current study, we report that the community population prevalence of diabetic cardiomyopathy is 1.1% and that the morbidity and mortality of patients with the DCM is high, approaching 31% over a decade.
Prior data suggests that LV diastolic dysfunction may precede LV systolic dysfunction in diabetic patients, which may explain why 83% of the patients with DCM in our cohort have diastolic dysfunction while only 17% have systolic dysfunction [28]. Previous studies of small or biased groups of patients have estimated the prevalence of diastolic dysfunction in diabetic patients to vary from 28% to 75% [29–32]. In the current study, we report that the prevalence of diastolic dysfunction among community population-based diabetic patients is 54%.
Recognizing that the number of patients with diabetic cardiomyopathy was modest, we still set out to perform an exploratory analysis of long term outcomes, comparing subjects with diabetic cardiomyopathy (DCM) to subjects with diabetes and LV dysfunction and co-morbidities (D1CM) and to subjects with diabetes and no LV dysfunction (D0CM). Through these analyses, we discovered that the cumulative probability of the development of heart failure and death is highest in diabetic patients with LV dysfunction and co-morbidities, followed by subjects with diabetic cardiomyopathy, then diabetic patients with no LV dysfunction. However, these secondary analyses of long term outcomes need to be confirmed by larger, prospective cohort studies.
While a great effort has been made in understanding some of the mechanisms involved in diabetic cardiomyopathy, future areas of research will need to focus on cost-effective screening modalities to identify this targeted population in addition to the development of novel therapeutic strategies to halt or slow the progression of disease once diagnosed.
Limitations
A study limitation is that the population of Olmsted County, MN may not be representative of the population of the United States, and therefore, these results may not be entirely generalizable. Secondly, the cohort is limited to persons age 45 years or older and therefore may underestimate the population prevalence. Thirdly, the presence of subjects with asymptomatic coronary artery disease cannot be fully excluded and is a potential confounding limitation of the study. Lastly, the number of subjects meeting diagnostic criteria for diabetic cardiomyopathy was relatively small, and thus the data does not allow for definitive conclusions regarding long term outcomes or disease progression without confirmatory prospective studies. Additionally, the cause of death information was not available to supplement our study data.
Conclusions
Diabetic cardiomyopathy is relatively common in the community. In the current study, we report that the community population prevalence of diabetic cardiomyopathy is 1.1% and that the morbidity and mortality of patients with the DCM is high, approaching 31% over a decade. Furthermore, diabetes is independently associated with left ventricular dysfunction.
Acknowledgments
Grant support: This work was supported by the National Institutes of Health [HL76611 and HL84155 to H.H.C] and [HL RO1-55502 to R.R].
Abbreviations
- HF
heart failure
- DCM
diabetic cardiomyopathy
- LV
left ventricular
- E
passive transmitral left ventricular inflow velocity
- A
late transmitral left ventricular inflow during left atrial contraction
- e’
tissue Doppler imaging velocity of the medial mitral annulus during passive filling
- D0CM
subjects with diabetes and no left ventricular dysfunction
- D1CM
subjects with diabetes and hypertension or coronary artery disease and any left ventricular dysfunction
- HR
hazard ratio
- CI
confidence interval
- BNP
B-type natriuretic peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that there is no conflict of interest associated with this manuscript.
References
- 1.Kannel WB, McGee DL. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59:8–13. doi: 10.1161/01.cir.59.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: as statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 3.Liu JE, Palmieri V, Roman MJ, Bella JN, Fabsitz R, Howard BV, et al. The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: the Strong Heart Study. J Am Coll Cardiol. 2001;37:1943–1949. doi: 10.1016/s0735-1097(01)01230-x. [DOI] [PubMed] [Google Scholar]
- 4.Lee M, Gardin JM, Lynch JC, Smith VE, Tracy RP, Savage PJ, et al. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: The Cardiovascular Health Study. Am Heart J. 1997;133:36–43. doi: 10.1016/s0002-8703(97)70245-x. [DOI] [PubMed] [Google Scholar]
- 5.Bella JN, Devereux RB, Roman MJ, Palmieri V, Liu JE, Paranicas M, et al. Separate and joint effects of systemic hypertension and diabetes mellitus on left ventricular structure and function in American Indians (the Strong Heart Study) Am J Cardiol. 2001;87:1260–1265. doi: 10.1016/s0002-9149(01)01516-8. [DOI] [PubMed] [Google Scholar]
- 6.Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, et al. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation. 2001;103:102–107. doi: 10.1161/01.cir.103.1.102. [DOI] [PubMed] [Google Scholar]
- 7.Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, et al. Impact of diabetes on cardiac structure and function: the Strong Heart Study. Circulation. 2000;101:2271–2276. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 8.Galderisi M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J Am Coll Cardiol. 2006;48:1548–1551. doi: 10.1016/j.jacc.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 9.Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study) Am J Cardiol. 1991;68:85–89. doi: 10.1016/0002-9149(91)90716-x. [DOI] [PubMed] [Google Scholar]
- 10.Ilercil A, Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Welty TK, et al. Relationship of impaired glucose tolerance to left ventricular structure and function: The Strong Heart Study. Am Heart J. 2001;141:992–998. doi: 10.1067/mhj.2001.115302. [DOI] [PubMed] [Google Scholar]
- 11.Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 12.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 13.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 14.Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121:748–757. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 15.Mildenberger RR, Bar-Shlomo B, Druck MN, Jablonksy G, Morch JE, Hilton JD, et al. Clinically unrecognized ventricular dysfunction in young diabetic patients. J Am Coll Cardiol. 1984;4:234–238. doi: 10.1016/s0735-1097(84)80207-7. [DOI] [PubMed] [Google Scholar]
- 16.Paillole C, Dahan M, Paycha F, Solal AC, Passa P, Gourgon R. Prevalence and significance of left ventricular filling abnormalities determined by Doppler echocardiography in young type I (insulin-dependent) diabetic patients. Am J Cardiol. 1989;64:1010–1016. doi: 10.1016/0002-9149(89)90799-6. [DOI] [PubMed] [Google Scholar]
- 17.From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol. 2010;55:300–305. doi: 10.1016/j.jacc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 19.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 20.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1998;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 21.Gillum RF, Fortmann SP, Prineas RJ, Kottke TE. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J. 1984;108:150–158. doi: 10.1016/0002-8703(84)90558-1. [DOI] [PubMed] [Google Scholar]
- 22.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41:1036–1043. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 23.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's Rosetta Stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 25.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 26.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 27.From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119:591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Raev DC. Which left ventricular function is impaired earlier in the evolution of diabetic cardiomyopathy? An echocardiographic study of young type I diabetic patients. Diabetes Care. 1994;17:633–639. doi: 10.2337/diacare.17.7.633. [DOI] [PubMed] [Google Scholar]
- 29.Brooks BA, Franjic B, Ban CR, Swaraj K, Yue DK, Celermajer DS, et al. Diastolic dysfunction and abnormalities of the microcirculation in type 2 diabetes. Diabetes Obes Metab. 2008;10:739–746. doi: 10.1111/j.1463-1326.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 30.Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870–875. doi: 10.1016/j.amjcard.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 31.Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA. Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol. 2001;87:320–323. doi: 10.1016/s0002-9149(00)01366-7. [DOI] [PubMed] [Google Scholar]
- 32.Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care. 2001;24:5–10. doi: 10.2337/diacare.24.1.5. [DOI] [PubMed] [Google Scholar]