Abstract
Importance
Increases in fructose consumption have paralleled the increasing prevalence of obesity, and high-fructose diets are thought to promote weight gain and insulin resistance. Fructose ingestion produces smaller increases in circulating satiety hormones compared with glucose ingestion, and central administration of fructose provokes feeding in rodents, whereas centrally administered glucose promotes satiety.
Objective
To study neurophysiological factors that might underlie associations between fructose consumption and weight gain.
Design, Setting, and Participants
Twenty healthy adult volunteers underwent 2 magnetic resonance imaging sessions at Yale University in conjunction with fructose or glucose drink ingestion in a blinded, random-order, crossover design.
Main Outcome Measures
Relative changes in hypothalamic regional cerebral blood flow (CBF) after glucose or fructose ingestion. Secondary outcomes included whole-brain analyses to explore regional CBF changes, functional connectivity analysis to investigate correlations between the hypothalamus and other brain region responses, and hormone responses to fructose and glucose ingestion.
Results
There was a significantly greater reduction in hypothalamic CBF after glucose vs fructose ingestion (–5.45 vs 2.84 mL/g per minute, respectively; mean difference, 8.3 mL/g per minute [95% CI of mean difference, 1.87-14.70]; P=.01). Glucose ingestion (compared with baseline) increased functional connectivity between the hypothalamus and the thalamus and striatum. Fructose increased connectivity between the hypothalamus and thalamus but not the striatum. Regional CBF within the hypothalamus, thalamus, insula, anterior cingulate, and striatum (appetite and reward regions) was reduced after glucose ingestion compared with baseline (P<.05 significance threshold, family-wise error [FWE] whole-brain corrected). In contrast, fructose reduced regional CBF in the thalamus, hippocampus, posterior cingulate cortex, fusiform, and visual cortex (P<.05 significance threshold, FWE whole-brain corrected). In whole-brain voxel-level analyses, there were no significant differences between direct comparisons of fructose vs glucose sessions following correction for multiple comparisons. Fructose vs glucose ingestion resulted in lower peak levels of serum glucose (mean difference, 41.0 mg/dL [95% CI, 27.7-54.5]; P<.001), insulin (mean difference, 49.6 μU/mL [95% CI, 38.2-61.1]; P<.001), and glucagon-like polypep-tide 1 (mean difference, 2.1 pmol/L [95% CI, 0.9-3.2]; P=.01).
Conclusion and Relevance
In a series of exploratory analyses, consumption of fructose compared with glucose resulted in a distinct pattern of regional CBF and a smaller increase in systemic glucose, insulin, and glucagon-like polypeptide 1 levels.
Obesity is increasingly prevalent. This is an environmental phenomenon and one related to the types of foods ingested in modern society. Substantial increases in the use of fructose as a sweetener may play a role in the current obesity epidemic.1-4 Fructose and glucose are both monosaccha-rides, but fructose is sweeter and metabolized differently.1,5 In contrast to glucose ingestion, fructose ingestion only weakly stimulates secretion of insulin,6 a hormone that acts centrally to increase satiety and blunt the reward value of food.7,8 Compared with glucose ingestion, fructose ingestion attenuates increases in circulating levels of the satiety hormone glucagon-like polypeptide 1 (GLP-1)9 and does not attenuate levels of ghrelin, an appetite-stimulating hormone.10,11 Thus, fructose possibly increases food-seeking behavior and increases food intake.3
Fuel sensing and appetite are controlled by the hypothalamus.4 Hunger is regulated by the hypothalamus in conjunction with an integrated network of other brain regions such as the striatum, orbitofrontal cortex, amygdala, and insula, which control motivation-reward systems associated with the hedonic drive to eat.4 Intraventricular administration of fructose provokes feeding in rodents, whereas centrally administered glucose decreases food intake via differential effects on hypothalamic malonyl coenzyme A–signaling pathways.12 How brain regions associated with fructose- and glucose-mediated changes in animal feeding behaviors translates to humans is not completely understood. New technologies are available to facilitate translation of animal to human studies. Functional magnetic resonance imaging (fMRI) provides a noninvasive way to assess the effects of glucose and fructose ingestion on regional cerebral blood flow (CBF), an indirect marker of neuronal activation. It is known that glucose ingestion decreases hypothalamic activity in humans.13-15 It remains unknown what the effects of fructose ingestion are on the homeo-static and brain reward circuitry or its influence on functional connectivity between the hypothalamus and other reward regions in the brain.
We hypothesized that fructose ingestion results in greater hypothalamic activity (measured as blood flow) than glucose ingestion. Fructose and glucose might result in differential activation of other brain regions. Similarly, fructose and glucose ingestion might differentially affect circulating levels of the satiety hormones GLP-1 and insulin. To examine these questions, we used pulsed arterial spin labeling and resting-state fMRI to investigate the brain response to acute ingestion of equal quantities of fructose and glucose in healthy volunteers. Studies in rats were performed to demonstrate the ability of fructose to cross the blood-brain barrier and to determine if the hypothalamus can transport and metabolize fructose.
METHODS
Human Neuroimaging Studies
Participants. Twenty (10 men,10 women) normal-weight healthy volunteers without diabetes and with a mean age of 31 (SD, 7) years participated in this study (TABLE 1). Participants were recruited by posting advertisement flyers in the New Haven area. Participants were excluded if taking medications known to alter metabolism, and they must have maintained a stable weight for at least 3 months prior to participation. Women participants were studied during the follicularphaseoftheirmenstrualcycle.The protocol was approved by the Yale University Human Investigation Committee. All participants provided informed, written consent before participation in the study.
Table 1.
Participant Characteristics
| Characteristic | Mean (SD) |
|---|---|
| Age, y | 31 (7) |
| Sex, No. | |
| Men | 10 |
| | |
| Women | 10 |
| Body mass indexa | 22 (2.5) |
| HbA1c, % | 5.1 (0.4) |
Abbreviation: HbA1c, glycated hemoglobin.
Calculated as weight in kilograms divided by height in meters squared.
Experimental Protocol
Volunteers underwent 2 MRI sessions together with ingestion of either a fructose or glucose drink in a blinded, random-order crossover design. The order of the drink types was randomized. A block-randomized, computer-generated sequence was developed and kept by the study statistician. Allocation of assignment was conducted on the morning before the first test day. The time between the 2 sessions was between 1 week and 2 months. Weight was measured and diet assessed at both sessions to ensure that these variables remained stable between sessions.
Participants arrived at the Yale Magnetic Resonance Research Center at 8 AM after an overnight fast. MRI was performed using a 3-Tesla Siemens Trio scanner (Siemens Medical Systems). A catheterwasplacedinanantecubitalvein for blood sampling prior to initiating the study. Participants underwent baseline MRI acquisitions, including pulsed arterial spin labeling to determine regional CBF and blood oxygen level– dependent fMRI sequences to determine functional connectivity. Subsequently, they drank 75 g (300 kcal) of either sugar in 300 mL of cherry-flavored water, followed by a 60-minute postdrink acquisition and blood-sampling period. To assess the effect of fructose and glucose ingestion on appetite, participants completed a visual analog scale (score range, 0 to 10) before and after the scan. Participants rated feelings of hunger, satiety, and fullness on a scale from 1 to 10, where 1 was “not at all” and 10 was “very much.” Prior studies have demonstrated good reproducibility and validity of visual analog scale scores for assessing subjective sensations of hunger and satiety.16
Blood samples were obtained for measurement of plasma glucose, lac-tate, insulin, leptin, ghrelin, peptide YY (PYY), and GLP-1 levels at baseline (before drink ingestion) and at 10-minute intervals during the MRI sessions. Samples were obtained for measurement of plasma fructose levels at baseline and at 15, 25, and 65 minutes following glucose and fructose ingestion.
The prespecified primary outcome was relative changes in hypothalamus cerebral blood flow in response to acute glucose vs fructose ingestion. Secondary outcomes included (1) functional connectivity analysis to investigate brain regions with MRI signal responses that were correlated with the hypothalamic response; (2) use of whole-brain analyses to explore brain areas with relative increases or decreases in regional CBF; and (3) changes in systemic hormone levels and ratings of hunger and satiety in response to the acute ingestion of fructose and glucose.
Multiparticipant Analysis. A standard whole-brain template (Montreal Neurological Institite [MNI] 1-mm) was used for participant spatial normalization of the individual data. Participant integration and registration were carried out using the BioimageSuite software package (http://www.bioimagesuite.org)17 for the images under fructose and glucose conditions. The 2 transformations calculated and used in multiple subject integration are included in the eMethods available at http://www.jama.org.
Voxel-wise contrasts between conditions were estimated in the common space on the pooled participant data using a t statistic to test for differences in the regional CBF response to glucose and fructose ingestion. Main-effect group contrast maps were performed separately for each condition (fructose and glucose) by subtracting after-drink from before-drink ingestion at a significance threshold set at P<.05, 2-sided, with family-wise error (FWE) whole-brain correction. Another group comparison map was performed comparing glucose with fructose conditions (P<.05, 2-sided, FWE whole-brain corrected). The association of changes in circulating hormones with brain CBF response to fructose and glucose was assessed using whole-brain, voxel-based correlation analyses.
Functional connectivity analysis was performed to assess brain regions that are temporally and thus functionally related.18 The hypothalamus was selected as the seed region to assess connections between the homeostatic control region with other regions involved in the regulation of feeding behavior. Significance threshold was set at P<.05, 2-sided, with FWE whole-brain correction. Details on imaging procedures, parameters, and analysis (preprocessing of images and calculation of CBF) are reported in the eMethods.
An a priori region-of-interest analysis of the hypothalamus regional CBF response to fructose and glucose was conducted using a mixed-model repeated-measures analysis (PROC MIXED; SAS version 9.2, SAS Institute Inc). Fixed factors in the model included drink type (glucose vs fructose), time (before vs after ingestion), and period (ie, first or second session) and their interactions. Interactions with period were used to evaluate whether the order of receiving glucose or fructose modified the differences between glucose and fructose. No significant ordering effects were observed. A random effect for participant was included to accommodate correlation between repeated measures. A linear contrast with a significance threshold of .05 was used to compare changes from before to after ingestion between glucose and fructose conditions. The time series were collapsed across the 1-hour period (ie, CBF difference maps to glucose or fructose ingestion averaged across the 5 runs postdrink vs baseline predrink run).
Our power calculations are based on our prior study,19 which showed a difference in hypothalamus CBF between 2 conditions, euglycemia and hypoglycemia, at an effect size (ratio between the mean difference and the pooled standard deviation) between 0.72 and 0.96. A sample size of 18 participants was required to detect a standardized effect of 0.72 at the 2-sided P<.05 significance level with a power of 80%. Twenty participants were enrolled to accommodate a 10% dropout. Given that this was an exploratory study, the clinical significance of the results is uncertain.
Laboratory Analysis. Plasma glucose levels were measured by an enzymatic reaction using glucose oxidase (YSI Inc). Plasma insulin, ghrelin, PYY (total), and leptin levels were measured with double-antibody radioimmunoassay (Millipore). GLP-1 (active) assays were performed by Millipore services using enzyme-linked immunosorbent assay. Plasma fructose levels were measured using gas chromatography–tandem mass spectrometry. Area under the curve was calculated for metabolites and hormones using the trapezoid method. Plasma metabolite and hormone levels were analyzed using the mixed-model repeated-measures analysis. The models included fixed effects for drink type, time (0, 15, 25, 35, 45, 55, and 65 minutes), and period, along with their interactions. A random effect was included for participant along with a first-order autoregressive covariance pattern that was allowed to vary with drink and period. Linear contrasts were used to compare glucose and fructose conditions at each individual time point. P values were adjusted for multiple comparisons using the Bonferroni correction.
Behavioral Ratings Analysis. The effect of treatment (ie, fructose and glucose ingestion) on appetite ratings was calculated by subtracting the score in the fasted state from the score after ingestion. The change in appetite score was analyzed using a mixed-model repeated-measures analysis. Fixed factors in the model included drink type, period, and their interaction. Conditions were compared with a significance threshold of P<.05.
Animal Studies
In a series of complementary studies in rodents, we infused fructose peripherally and used microdialysis to measure fructose concentrations in hypothalamic extracellular fluid as a means to assess whether fructose crosses from the blood into the brain and, more specifically, into the hypothalamus. In addition, we used polymerase chain reaction to identify whether genes for GLUT5, a fructose transporter, and ketohexokinase, which is responsible for the first step in the metabolism of fructose, are expressed in the hypothalamus. Principles of laboratory animal care were followed, and experimental protocols were approved by the Yale University Institutional Animal Care and Use Committee (methods used in the animal experiments are detailed in the eMethods).
RESULTS
Hypothalamus Region-of-Interest Analysis: Hypothalamic CBF Response to Glucose vs Fructose Ingestion
Although there was no difference in baseline hypothalamic CBF between the glucose and fructose conditions (mean, 39.7 [SD, 2] vs 38.6 [SD, 4] mL/g per minute, respectively), 15 minutes after drink ingestion the hypothalamic responsetoglucoseandfructosemarkedly differed.Within15minutes,glucosesignificantly reduced hypothalamic CBF, whereas fructose did not (mean, –6.84 mL/g per minute [95% CI, –11.85 to –1.84];P=.008vs5.10mL/gperminute [95%CI,–0.67to10.88];P=.08,respectively). There was a significant main effectofdrinkacrossalltimepointswhereby therewasagreaterreductioninhypothalamicCBFafterglucosevsfructose(–5.45 vs 2.84 mL/g per minute, respectively [mean difference, 8.3 mL/g [95% CI of mean difference, 1.87 to 14.70]; P=.01) (FIGURE 1A).
Figure 1. Mean Change in Hypothalamic Cerebral Blood Flow, Mean Plasma Glucose Response, and Mean Plasma Insulin Response.
X-axis represents time points when hypothalamic cerebral blood flow (CBF) was measured (A) or when blood was sampled (B,C). Error bars indicate 95% CIs. To convert glucose values to mmol/L, multiply by 0.0555; insulin values to pmol/L, multiply by 6.945.
Metabolic and Hormone Responses
Baseline levels of plasma glucose, fructose, insulin, GLP-1, PYY, leptin, ghrelin,andlactatewerenotdifferentbetween the glucose and fructose conditions (TABLE 2).Glucoseingestioncausedsignificantly greater elevations in plasma glucose (mean difference, 41.0 mg/dL [95% CI, 27.7-54.5]; P<.001), insulin (49.6 μU/mL [95% CI, 38.2-61.1] P<.001) (Figure 1B and C), and GLP-1 (2.1 pmol/L [95% CI, 0.90-3.2]; P=.01) concentrations compared with fructose ingestion,whereasplasmafructose,lac-tate, and PYY levels were greater after fructose ingestion compared with glucoseingestion(Table2).Levelsofleptin and ghrelin were not significantly dif ferent following ingestion of fructose compared with ingestion of glucose.
Table 2.
Metabolic and Hormonal Responses
| Hormone or Metabolite | Mean (95% CI)a |
P Valueb | |||||
|---|---|---|---|---|---|---|---|
| Glucose Drink |
Fructose Drink |
||||||
| Baseline | Peak | AUC | Baseline | Peak | AUC | ||
| Glucose, mg/dL | 96 (83 to 108) | 153 (140 to 166) | 8539 (7446 to 9632) | 94 (90 to 98) | 112 (104 to 119) | 7148 (8110 to 6185) | <.001 |
| Fructose, mg/dL | 0.6 (–0.4 to 1.6) | 1.2 (0.2 to 2.3) | 36 (13 to 59) | 0 (–2.2 to 2.1) | 5.6 (3.4 to 7.7) | 312 (191 to 433) | .002 |
| Insulin, μU/mL | 9.6 (–1.0 to 20.3) | 71.3 (60.4 to 82.2) | 3294 (2629 to 3958) | 9.1 (5.6 to 12.5) | 21.7 (18.1 to 25.1) | 1185 (967 to 1402) | <.001 |
| GLP-1, pmol/L | 2.2 (1.0 to 3.5) | 5.4 (4.2 to 6.7) | 265 (189 to 341) | 2.0 (0.8 to 3.2) | 3.4 (2.1 to 4.6) | 195 (155 to 235) | .01 |
| Lactate, mmol/L | 0.8 (0.6 to 1.0) | 1.4 (1.1 to 1.6) | 54 (40 to 69) | 0.8 (0.4 to 1.1) | 2.4 (2.0 to 2.7) | 101 (83 to 119) | <.001 |
| PYY, pg/mL | 92 (76 to 109) | 107 (83 to 131) | 5515 (4045 to 6984) | 92 (77 to 107) | 130 (95 to 165) | 7471 (5819 to 9124) | .001 |
| Ghrelin, pg/mL | 854 (735 to 974) | 655 (536 to 774) | 800 (681 to 918) | 635 (517 to 754) | .74 | ||
| Leptin, ng/mL | 7.4 (4.7 to 10.0) | 7.2 (4.5 to 9.8) | 7.9 (5.2 to 10.6) | 7.7 (5.0 to 10.4) | .17 | ||
Abbreviations: AUC, area under the curve; GLP-1, glucagon-like polypeptide 1; PYY, peptide YY.
SI conversion factors: To convert glucose values to mmol/L, multiply by 0.0555; fructose values to μmol/L, multiply by 55.506; insulin values to pmol/L, multiply by 6.945.
Plasma concentrations of metabolites and hormones after ingestion of 75-g glucose and fructose drink. Note that peak suppression of plasma ghrelin is shown in response to glucose and fructose drink and that AUC for ghrelin and leptin are not presented because ghrelin and leptin levels decreased in response to ingestion of glucose and fructose.
For comparisons of mean peak fructose vs glucose levels.
Whole-Brain Regional CBF and Connectivity Reponses and Neuroendocrine Correlations
As shown in FIGURE 2 and eTable 1, regional CBF within the hypothalamus, thalamus, insula, anterior cingulate, and striatum was significantly reduced after glucose ingestion compared with baseline (P<.05 significance threshold, 2-tailed FWE whole-brain corrected). In contrast, fructose produced a significant reduction in regional CBF in the thalamus, hippocampus, posterior cingulate cortex, fusiform gyrus, and visual cortex (P<.05 significance threshold, 2-tailed FWE whole-brain corrected) compared with baseline. Glucose ingestion (compared with baseline) increased functional connectivity between the hypothalamus (the seed region) and the thalamus, caudate, and putamen (FIGURE 3 and eTable 2), whereas fructose ingestion resulted only in increased connectivity between the hypothalamus and thalamus (P<.05 significance threshold, FWE whole-brain corrected). When whole-brain contrast maps were directly compared between fructose and glucose sessions, no differences remained significant following correction for multiple comparisons.
Figure 2. Regional Cerebral Blood Flow Response to Ingestion of Glucose or Fructose.
A, Regional cerebral blood flow (CBF) response to glucose ingestion. B, Regional CBF response to fructose ingestion. The images represent paired t tests for postdrink vs baseline for 20 participants. The blue regions identify the areas in the brain with significantly decreased regional CBF after glucose or fructose ingestion compared with baseline. There were no brain regions with increased regional CBF after either fructose or glucose ingestion. Significance threshold set at P<.05, 2-tailed, family-wise error whole-brain corrected. Z is defined from top to bottom on the Montreal Neurological Institute (MNI) atlas with the origin passing through the anterior commissure- posterior commissure (AC-PC) line. MNI coordinates were used to define brain regions.
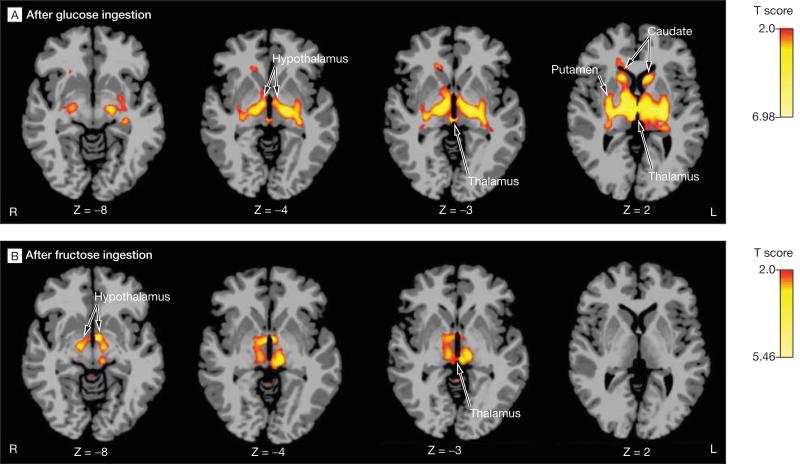
Figure 3. Functional Connectivity Analysis.
A, Functional connectivity analysis for glucose ingestion at baseline, with bilateral hypothalamus as the seed region. Hypothalamus response to glucose ingestion was functionally connected to the caudate, putamen, and thalamus response. B, Functional connectivity analysis for fructose ingestion at baseline, with bilateral hypothalamus as the seed region. Hypothalamus response to fructose ingestion was functionally connected to the thalamus response. The images represent paired t tests for postdrink vs baseline for 20 participants. Yellow and red regions identify areas in the brain with magnetic resonance imaging signal responses correlated with the hypothalamic response. Significance threshold set P<.05, 2-sided, family-wise error whole-brain corrected. Montreal Neurological Institute (MNI) coordinates were used to define brain regions.
Changes in levels of plasma insulin, but not of other hormones, correlated with changes in regional CBF in the cau-date and putamen motivation/reward regions in response to glucose ingestion (r=–0.62, P=.005) (FIGURE 4). There was no correlation between changes in glucose or hormone levels with changes in regional CBF in response to fructose ingestion.
Figure 4. Region-of-Interest Correlation Analysis.
A, Axial brain slice representing averaged data for 19 participants. Pink regions illustrate brain areas with a change in regional cerebral blood flow (CBF) that correlated with the change in plasma insulin levels after glucose ingestion. Montreal Neurological Institute (MNI) coordinates were used to define brain regions. B, Corresponding scatterplot showing the correlation between change in plasma insulin levels and the change in regional CBF to the left caudate and putamen following glucose ingestion for the 19 participants. The solid line in the scatterplot corresponds to the regression line (line of best fit). Difficulties with blood sampling in 1 participant limited analysis to 19 participants. To convert insulin values to pmol/L, multiply by 6.945.
Behavioral Ratings
There was no significant difference between glucose vs fructose ingestion on predrink-postdrink changes in hunger (mean difference, 0.7 [95% CI, –0.4 to 1.7]; P=.22), fullness (mean difference, –0.9 [95% CI, –2.1 to 0.2]; P=.09), or satiety (mean difference, –1.0 [95% CI, –2.3 to 0.4]; P=.15). Glucose ingestion resulted in a significant difference in predrink-postdrink changes in fullness (mean difference, 1.6 [95% CI, –0.6 to 2.7]; P=.005) and satiety (mean difference, 1.2 [95% CI, –0.1 to 2.3]; P=.03), whereas fructose ingestion did not have asignificanteffectonpredrink-postdrink changesinfullness(meandifference,0.7 [95% CI, –0.4 to 1.7]; P=.20) or satiety (mean difference, 0.3 [95% CI, –0.8 to 1.3]; P=.64).
Animal Studies. Intravenous infusion of 20% fructose increased mean plasma fructose levels from 0.16 (95% CI, 0.1 to 0.3) mg/dL to 22 (95% CI, 7.0 to 37.0) mg/dL (to convert to μmol/L, multiply by 55.506) (P<.01) (eFigure). This was accompanied by an increase in the mean concentration of fructose in dialysis fluid obtained from the ventromedial hypothalamus from 0.13 (95% CI, 0.1 to 0.2) mg/dL to 0.30 (95% CI, 0.2 to 0.4) mg/dL (P<.02). Neither plasma nor ventromedial hypothalamus microdialysate fructose concentrations changed during infusion of saline. Both GLUT5 and ketohexokinase mRNA were expressed in the hypothalamus as well as in the liver and kidney (positive controls) but not in the negative control lacking template RNA.
COMMENT
Increases in fructose consumption have paralleled the increasing prevalence of obesity, and high-fructose diets are thought to promote weight gain and insulinresistance.1-3,20,21Inthisstudy,ingestion of glucose but not fructose reduced cerebral blood flow and thus activity in specificbrainregionsthatregulateappetite and reward processing. In keeping with these data, ingestion of glucose but not fructose produced increased ratings of satiety and fullness.
Our results showing a reduction in hypothalamic CBF in response to glucose ingestion are consistent with prior fMRI studies in healthy volunteers, which observed a decrease in hypothalamic activity in response to ingestion of glucose13-15,22 or a liquid meal,23 a finding posited to reflect a central biomarker of satiety. Although 1 recent fMRI study did not detect significant changes in hypothalamic activity during intravenous administration of glucose or fructose,24 this could be related to the small sample size, the mode of delivery, or both, because orally administered glucose has been shown to inhibit hypothalamic activity more effectively than an intravenous glucoseinfusion.22 Inourwhole-brainanalyses, glucose ingestion produced a reduction in CBF within the thalamus, insula, anterior cingulate, and striatum as well as the hypothalamus—brain regions that act in concert to “read” the metabolic state of an individual and drive motivation and reward.
Brain responses markedly differed following ingestion of an equivalent amount of fructose. Not only did fructose fail to diminish hypothalamic activity, but it instead induced a small, transient increase in hypothalamic activity, a response similar to insulin-induced decrements in levels of circulating glucose.25 Furthermore, unlike glucose ingestion, fructose ingestion did not result in deactivation of the striatum. Hypothalamic and striatal deactivation occur when initially hungry individuals reach satiety.23,26 Of note, fructose ingestion reduced CBF in the hippocampus. The hippocampus not only plays an important role in memory function but also influences emotional responses to food intake,27 and in rodents this brain region was found to inhibit appetitive behavior.28
Homeostatic and hedonic brain regions are tightly interconnected and form an integrated network that dictates feeding behavior. Functional connectivity analyses were performed using the hypothalamus as a seed region to determine its functional connectivity to other brain regions implicated in food motivation and reward. Brain regions with an MRI signal response that was temporally and thus functionally correlated with the hypothalamic response to glucose ingestion were the thalamus, caudate, and putamen, whereas fructose ingestion resulted only in increased connectivity between the hypothalamus and thalamus but not the striatum. These findings suggest that ingestion of glucose, but not fructose, initiates a coordinated response between the homeostatic-striatal network that regulates feeding behavior.
It is notable that functional connectivity increased throughout the network, whereas regional CBF decreased within the network components. More commonly, increases in functional connectivity are accompanied by increases in blood oxygen level– dependent signal, which in turn is accompanied by increases in regional CBF. Note that changes in synchrony do not have to be directly coupled to local changes in flow or metabolism. There also could be an overall net reduction in connectivity to other circuits that is diffuse and thus subthreshold in concert with this increase in connectivity within these highly localized circuits. Recent work has focused on variable rates of aerobic glycolysis in different brain regions, and it is possible that modifications in the rate of aerobic glycolysis under these different conditions could account for these signal changes.29
Our neuroimaging findings are consistent with animal studies reporting that the central administration of fructose provokes feeding in rodents, whereas centrally administered glucose suppresses food intake.12 For fructose to exert such effects under physiological conditions it must first cross the blood-brain barrier and be metabolized. We used microdialysis to measure fructose concentrations in dialysate samples obtained from ventromedial hypothalamus extracellular fluid after peripheral infusion of fructose in rats. Fructose levels in ventromedial hypothalamus dialysate samples immediately increased after the start of the peripheral fructose infusion. Given the relatively low efficiency of fructose extraction by micro-dialysis, it is likely that higher concentrations of fructose reached the brain and thus could potentially directly act to influence brain homeostatic responses. Furthermore, the hypothalamus was found to express both Glut5 and ketohexokinase mRNA, the necessary cellular machinery for fructose metabolism. Our findings are consistent with studies showing that fructose is metabolized in hippocampal microglia30 and neurons in the cerebellum31 and demonstrate the capacity of fructose to cross the blood-brain barrier32,33 into the hypothalamus, where it can be metabolized and used as an energy source.
As anticipated,10,11 fructose ingestion caused a smaller increase in levels of plasma glucose, insulin, and GLP-1 than glucose ingestion. Levels of plasma fructose and lactate, on the other hand, increased to higher levels following fructose ingestion. Similar fructose increases have been reported in healthy volunteers who consumed fructose loads between 0.5 and 0.75 g/kg34 and in individuals who consumed fructose-sweetened beverages with mixed meals.35 Leptin and ghrelin levels were indistinguishable following acute ingestion of glucose or fructose, a finding possibly attributable to the short time interval of observation; leptin levels typically change 4 to 6 hours after glucose administration.36 Although fructose was previously reported to be less effective than glucose in suppressing ghrelin, such differences may be attributable to the different conditions and timing of ghrelin measurements.10 Little is known about the acute PYY response to fructose ingestion compared with glucose ingestion, although 1 study in rats found higher rather than lower PYY levels after 24 hours of glucose but not fructose feeding.11 Whether such disparities are related to study design or species differences remains uncertain.
Higher plasma insulin levels were correlated with decreased regional CBF in the striatum following glucose but not fructose ingestion. This finding supports animal studies showing that insulin acts centrally to reduce the reward properties of food37 and suggests that the human striatum may be responsive to hyperinsulinemia.
Limitations
The fMRI technology used provides an exploratory method to evaluate changes in neuronal activity associated with local changes in blood flow and blood oxygenation. It does not provide a direct measure of neuronal activity, and it cannot localize the activation of specific neurons. Instead, hemodynamic changes are localized to brain regions referred to as voxels or volume units of brain tissue. Small brain structures, such as the hypothalamus, require a large change in blood flow to detect significant differences between conditions. In region-of-interest analysis, we found that glucose and fructose produced significantly different hypothalamic responses, and we observed that different brain regions were responding to fructose and glucose ingestion in whole-brain contrast mapping performed separately for each sugar. When whole-brain contrast maps were directly compared between fructose and glucose sessions, fructose ingestion produced greater activation in the hypothalamus and striatum, but these regions did not survive whole-brain correction analysis. It should be emphasized that the clinical implications of the fMRI-based outcomes reported in this exploratory study remain to be determined.
CONCLUSIONS
Glucose but not fructose ingestion reduced the activation of the hypothalamus, insula, and striatum—brain regions that regulate appetite, motivation, and reward processing; glucose ingestion also increased functional connections between the hypothalamicstriatal network and increased satiety. The disparate responses to fructose were associated with reduced systemic levels of the satiety-signaling hor mone insulin and were not likely attributable to an inability of fructose to cross the blood-brain barrier into the hypothalamus or to a lack of hypothalamic expression of genes necessary for fructose metabolism.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported in part by grants from the National Institutes of Health (DK 20495, P30 DK 45735, T32 DK07058) and the Yale Center for Clinical Investigation supported by the Clinical Translational Science Award (grant UL1 RR024139).
Role of the Sponsors: The funding agencies had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Drs Page and Sherwin had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Page, Chan, Roehmholdt, Constable, Sherwin.
Acquisition of data: Page, Chan, Arora, Belfort-DeAguiar, Roehmholdt, Cline, Constable.
Analysis and interpretation of data: Page, Chan, Arora, Belfort-DeAguiar, Dzuira, Roehmholdt, Naik, Sinha, Constable, Sherwin.
Drafting of the manuscript: Page, Chan, Arora, Dzuira, Roehmholdt, Naik, Constable, Sherwin.
Critical revision of the manuscript for important intellectual content: Page, Belfort-DeAguiar, Dzuira, Cline, Sinha, Constable, Sherwin.
Statistical analysis: Page, Chan, Arora, Belfort-DeAguiar, Dzuira, Naik, Constable, Sherwin.
Obtained funding: Sherwin.
Administrative, technical, or material support: Page, Sinha, Constable, Sherwin.
Study supervision: Page, Sinha, Constable, Sherwin.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Online-Only Material: The eMethods, eTables 1 and 2, and the eFigure are available at http://www.jama.com
REFERENCES
- 1.Havel PJ. Dietary fructose: implications for dys-regulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63(5):133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 2.Jürgens H, Haass W, Castañeda TR, et al. Consuming fructose-sweetened beverages increases body adiposity in mice. Obes Res. 2005;13(7):1146–1156. doi: 10.1038/oby.2005.136. [DOI] [PubMed] [Google Scholar]
- 3.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79(4):537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 4.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 5.Hanover LM, White JS. Manufacturing, composition, and applications of fructose. Am J Clin Nutr. 1993;58(5)(suppl):724S–732S. doi: 10.1093/ajcn/58.5.724S. [DOI] [PubMed] [Google Scholar]
- 6.Curry DL. Effects of mannose and fructose on the synthesis and secretion of insulin. Pancreas. 1989;4(1):2–9. doi: 10.1097/00006676-198902000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Woods SC, Chavez M, Park CR, et al. The evaluation of insulin as a metabolic signal influencing behavior via the brain. Neurosci Biobehav Rev. 1996;20(1):139–144. doi: 10.1016/0149-7634(95)00044-f. [DOI] [PubMed] [Google Scholar]
- 8.Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: update 2008. Am J Physiol Regul Integr Comp Physiol. 2009;296(1):R9–R19. doi: 10.1152/ajpregu.90725.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong MF, Chapman I, Goble E, et al. Effects of oral fructose and glucose on plasma GLP-1 and appetite in normal subjects. Peptides. 1999;20(5):545–551. doi: 10.1016/s0196-9781(99)00006-6. [DOI] [PubMed] [Google Scholar]
- 10.Teff KL, Elliott SS, Tschöp M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89(6):2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 11.Lindqvist A, Baelemans A, Erlanson-Albertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Pept. 2008;150(1-3):26–32. doi: 10.1016/j.regpep.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Cha SH, Wolfgang M, Tokutake Y, Chohnan S, Lane MD. Differential effects of central fructose and glucose on hypothalamic malonyl-CoA and food intake. Proc Natl Acad Sci U S A. 2008;105(44):16871–16875. doi: 10.1073/pnas.0809255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda M, Liu Y, Mahankali S, et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes. 1999;48(9):1801–1806. doi: 10.2337/diabetes.48.9.1801. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Gao JH, Liu HL, Fox PT. The temporal response of the brain after eating revealed by functional MRI. Nature. 2000;405(6790):1058–1062. doi: 10.1038/35016590. [DOI] [PubMed] [Google Scholar]
- 15.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage. 2005;24(2):363–368. doi: 10.1016/j.neuroimage.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 16.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 17.Papademetris X, Jackowski AP, Schultz RT, Staib LH, Duncan JS. Integrated intensity and point-feature nonrigid registration. Med Image Comput Comput Assist Interv. 2001;3216(2004):763–770. doi: 10.1901/jaba.2001.3216-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page KA, Arora J, Qiu M, Relwani R, Constable RT, Sherwin RS. Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes. 2009;58(2):448–452. doi: 10.2337/db08-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76(5):911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 21.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smeets PA, Vidarsdottir S, de Graaf C, et al. Oral glucose intake inhibits hypothalamic neuronal activity more effectively than glucose infusion. Am J Physiol Endocrinol Metab. 2007;293(3):E754–E758. doi: 10.1152/ajpendo.00231.2007. [DOI] [PubMed] [Google Scholar]
- 23.Tataranni PA, Gautier JF, Chen K, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A. 1999;96(8):4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purnell JQ, Klopfenstein BA, Stevens AA, et al. Brain functional magnetic resonance imaging response to glucose and fructose infusions in humans. Diabetes Obes Metab. 2011;13(3):229–234. doi: 10.1111/j.1463-1326.2010.01340.x. [DOI] [PubMed] [Google Scholar]
- 25.Page KA, Seo D, Belfort-DeAguiar R, et al. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest. 2011;121(10):4161–4169. doi: 10.1172/JCI57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(pt 9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 27.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horne MR, Iordanova MD, Pearce JM. Spatial learning based on boundaries in rats is hippocampus-dependent and prone to overshadowing. Behav Neurosci. 2010;124(5):623–632. doi: 10.1037/a0020824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107(41):17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shu HJ, Isenberg K, Cormier RJ, Benz A, Zorumski CF. Expression of fructose-sensitive glucose transporter in the brains of fructose-fed rats. Neuroscience. 2006;140(3):889–895. doi: 10.1016/j.neuroscience.2006.02.071. [DOI] [PubMed] [Google Scholar]
- 31.Funari VA, Herrera VL, Freeman D, Tolan DR. Genes required for fructose metabolism are expressed in Purkinje cells in the cerebellum. Brain Res Mol Brain Res. 2005;142(2):115–122. doi: 10.1016/j.molbrainres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Cha SH, Lane MD. Central lactate metabolism suppresses food intake via the hypothalamic AMP kinase/malonyl-CoA signaling pathway. Biochem Biophys Res Commun. 2009;386(1):212–216. doi: 10.1016/j.bbrc.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Thurston JH, Levy CA, Warren SK, Jones EM. Permeability of the blood-brain barrier to fructose and the anaerobic use of fructose in the brains of young mice. J Neurochem. 1972;19(7):1685–1696. doi: 10.1111/j.1471-4159.1972.tb06213.x. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald I, Keyser A, Pacy D. Some effects, in man, of varying the load of glucose, sucrose, fructose, or sorbitol on various metabolites in blood. Am J Clin Nutr. 1978;31(8):1305–1311. doi: 10.1093/ajcn/31.8.1305. [DOI] [PubMed] [Google Scholar]
- 35.Teff KL, Grudziak J, Townsend RR, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009;94(5):1562–1569. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havel PJ, Townsend R, Chaump L, Teff K. High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes. 1999;48(2):334–341. doi: 10.2337/diabetes.48.2.334. [DOI] [PubMed] [Google Scholar]
- 37.Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R388–R394. doi: 10.1152/ajpregu.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.