Abstract
The current study developed prognostic tools to identify dementia caregivers at-risk for clinically relevant burden or depressive symptoms following nursing home admission (NHA) of their family members. A retrospective, longitudinal design was used that included 1,610 dementia caregivers who provided data prior to and up to 6 months following nursing home admission. Response operant characteristic (ROC) curves were constructed to test and validate two prognostic tools: the NHA-Burden and NHA-Depression tools. An ROC curve yielded a sensitivity of 77% and a specificity of 62.5% at a cut-off score of 5.41 for the NHA-Burden Prognostic tool. A second ROC curve indicated a sensitivity of 75.4% and a specificity of 62.5% at a cut-off score of 7.45 for the NHA-Depression tool. Clinicians may wish to utilize cutpoints on the NHA-Burden and NHA-Depression tools to ensure that more persons who are at-risk for clinically significant burden or depression during NHA are identified.
While dementia caregiving research has evolved to include evidence-based interventions that alleviate caregiver distress and delay nursing home admission (NHA) for care recipients (Gaugler, Mittelman, Hepburn, & Newcomer, 2009; Gaugler, Mittelman, Hepburn, & Newcomer, 2010; Gold, Reis, Markiewicz, & Andres, 1995; Grasel, 2002; Mausbach et al., 2007; Schulz et al., 2004; Zarit & Whitlatch, 1992), there remain key gaps in the literature that preclude the effective linking of at-risk caregivers to appropriate clinical interventions. For example, much attention in dementia caregiving research has focused on the development and testing of assessment tools to better measure stress or other relevant constructs (Family Caregiver Alliance, 2006). However, few studies have established cut-points on these measures that optimize the ability of clinicians to determine whether dementia caregivers are at-risk for negative outcomes or their appropriateness for subsequent interventions. To begin to fill this gap, the current study developed prognostic tools to identify dementia caregivers most at-risk for clinically relevant burden or depressive symptoms up to 6 months after institutionalization of their family members.
Burden, Depression, and Dementia Caregiving
Although defined in various ways, the concept of “burden” is considered the negative psychosocial, economic, or physical effects of providing care to a relative suffering from dementia. As one of the most frequently studied outcomes in dementia caregiving, burden is linked with an array of critical outcomes including expedited nursing home admission of the person with dementia, psychological or psychiatric concerns on the part of the caregiver, and increased caregiver mortality (Liu & Gallagher-Thompson, 2009; Sorensen, Duberstein, Gill, & Pinquart, 2006; Vitaliano, Zhang, & Scanlan, 2003). In conceptual models of stress and coping (Aneshensel, Pearlin, Mullan, Zarit, & Whitlatch, 1995; Pearlin, Mullan, Semple, & Skaff, 1990), caregiver depression is often positioned as a global outcome of caregiver burden, or an outcome that is generally applicable to caregiving and non-caregiving samples. Various meta-analyses report that dementia caregivers who experience greater burden are more likely to suffer from depressive symptoms and more severe depression than non-caregivers (Liu & Gallagher-Thompson, 2009; Sorensen et al., 2006; Vitaliano et al., 2003).
Based on the prominence of burden and depression in the dementia caregiving literature, developing prognostic tools to identify dementia caregivers at-risk for these outcomes would extend current research and be of use to care providers in residential settings who deliver services to families. There are a number of evidence-based interventions that can benefit family caregivers following a relative’s admission to a residential long-term care facility such as a nursing home (NH) (Gaugler, 2005b). Utilizing appropriate prognostic tools could assist facility staff target and deliver these interventions to families in need prior to or during the NH transition.
Nursing Home Admission and Dementia Caregiving
Although NHA is often considered the end of caregiving, several qualitative and quantitative studies have implied that dementia caregivers’ emotional distress and negative mental health may worsen after the institutionalization of a family member (Davies & Nolan, 2006; Dellasega & Mastrian, 1995; Grant, Adler, Patterson, Dimsdale, Ziegler, & Irwin, 2002; Marziali, Shulman, & Damianakis, 2006; Matsuda, Hasebe, Ikehara, Futatsuya, & Akahane, 1997; Ryan & Scullion, 2000; Schulz et al., 2004; Tornatore & Grant, 2002). Family members continue to provide care after a relative’s NHA (Gaugler, 2005a; Port et al., 2005) and therefore must effectively coordinate their own involvement with that of direct care workers. Negative interactions between family caregivers and institutional staff and poor perceptions of care can have powerful and negative impacts on family members (Almberg, Grafstrom, Krichbaum, & Winblad, 2000; Townsend, 1990; Zarit & Whitlatch, 1992). In contrast, NHA can also result in reduced emotional or psychological distress as well as improvements in physical health for caregivers as indicated in studies that rely on larger samples and longer-term follow-up (Gaugler et al., 2009, 2010; Gold et al., 1995; Grasel, 2002; Mausbach et al., 2007). These seemingly contradictory findings suggest that while the NH transition may offer relief to many families, there are a considerable number of caregivers who remain at continued risk for negative emotional or psychosocial outcomes in the months immediately following NHA.
Research Focus
Several efforts have established clinical cut-offs on measures of caregiver burden in community samples (Rankin, Haut, Keefover, & Franzen, 1994). In addition, risk screens have been developed for dementia caregivers. For example, the Risk Appraisal Measure (RAM) was developed by the investigators of the Resources for Enhancing Alzheimer’s Caregiver Health (REACH I and II) initiatives (Czaja et al., 2009) and is a brief 16-item assessment tool that includes dimensions associated with negative outcomes for the person with dementia or the caregiver. The RAM was created from well-established measures used throughout the dementia caregiving literature. The RAM includes a small number of items that have face validity, are modifiable with clinical intervention, and place caregivers or persons with dementia at risk for negative outcomes. Acceptable reliability and concurrent validity were apparent for the RAM (Czaja et al., 2009). Tools such as the RAM greatly advance the state-of-the-art of dementia caregiving, as they provide brief, validated options for researchers and clinicians to assess the well-being of caregivers and care recipients with dementia. However, the utility of tools such as RAM to: 1) predict or prognosticate dementia caregivers at-risk for stress, depression, or similar negative outcomes; and 2) identify at-risk caregivers across key transition points during the course of dementia, such as institutionalization, is not known.
Our objective in this study was to build on our previous work to determine whether prognostic tools could determine dementia caregivers’ risk for burden and depression in the months following NHA (Gaugler et al., 2009, 2010). To our knowledge, no assessment tools are available prior to or during the institutionalization event to aid clinical providers (e.g., NH geriatricians, psychiatrists, social work staff, or nurses) in such endeavors. The aim of this study was to fill this clinical gap by developing prognostic tools (administered by NH staff) that identify caregivers at-risk of burden or depression in the months following NH placement.
Methods
Procedure
The Medicare Alzheimer’s Disease Demonstration (MADDE) was conducted in eight catchment areas in the United States (Champaign, Illinois; Cincinnati, Ohio; Memphis, Tennessee; Miami, Florida; Parkersburg, West Virginia; Portland, Oregon, Rochester, New York; Minneapolis/St. Paul, Minnesota). In the original study, a randomized, experimental design was used to evaluate whether a combination of case management services and Medicare-covered home care benefits was effective in reducing caregiver burden and depression and delaying NH placement for care recipients when compared to usual care participants (who were not provided with MADDE services). The following criteria governed participants’ inclusion in MADDE: all older adults (a) had a physician-certified diagnosis of an irreversible dementia, (b) were enrolled or eligible for Parts A and B of Medicare, (c) had service needs, and (d) resided at home in 1 of the 8 MADDE catchment areas. Persons living in a nursing home or in a hospital at the time of application were ineligible. Trained nurses and social workers conducted in-person interviews with caregivers every 6-months over 3 years. The baseline interview (n = 5,831) was in-person, and semi-annual assessments and post-nursing home placement interviews were conducted by telephone. The date of enrollment in MADDE was considered the baseline date. Further details on MADDE and its initial evaluation are available elsewhere (Newcomer, Yordi, DuNah, Fox, & Wilkinson, 1999). The current study received University of Minnesota Institutional Review Board approval as an exempt protocol (0611E96989).
Sample
The expanded case management benefits of MADDE did not exert direct effects on caregiver burden and depressive symptoms nor did they delay institutionalization (Newcomer et al., 1999). For these reasons we pooled the data from the treatment and control groups to create a larger subsample for development of the NHA prognostic tools. A considerable number of care recipients entered NHs throughout the 3-year course of MADDE (n = 2,557; 43.9%). Time to NHA, on average, was 426.88 days from baseline (SD = 292.53). Data were available from 1,610 primary caregivers up to 6 months after NHA (the 6-month post-placement sample). The interview conducted immediately prior to NHA was considered the pre-placement interview for analytic purposes.
Measures
Table 1 displays descriptive data for the 6-month post-placement sample. Variables considered when constructing prognostic tools included those that are significant predictors of institutionalization as well as those that are associated with caregiver distress following NHA (Almberg et al., 2000; Gaugler, Yu, Krichbaum, & Wyman, 2009; Majerovitz, 2007; Schulz et al., 2004; Whitlatch, Schur, Noelker, Ejaz, & Looman, 2001). Cronbach alpha levels are presented for all pre-placement measures, below.
Table 1.
Descriptive Information, Six-Month Post-Placement Panel (N = 1,610)
| M/% | SD | Range | |
|---|---|---|---|
| Context of Care | |||
| CR is female | 59.3% | ||
| CR is Caucasian | 92.0% | ||
| CR age (years) | 71.47 | 7.62 | 30.63–102.10 |
| CR lives with CG | 73.7% | ||
| Kin Relationship | |||
| Wife | 33.4% | ||
| Husband | 17.8% | ||
| Daughter | 26.6% | ||
| Other | 22.2% | ||
| CG age (years) | 63.56 | 14.42 | 23.00–100.00 |
| CG income (1 = under $4,999; 11 = $50,000 and above) | 5.81 | 2.91 | 1.00–11.00 |
| CG is employed | 33.1% | ||
| CG education (1 = elementary school; 6 = post-graduate) | 3.54 | 1.33 | 0.00–6.00 |
| Duration of care at baseline (in months) | 44.21 | 38.53 | 0.00–360.00 |
| Dementia Severity | |||
| MMSE at baseline | 14.29 | 7.89 | 0.00–30.00 |
| Pre-placement behavior problems | 9.67 | 4.06 | 0.00–19.00 |
| Functional Impairment | |||
| Pre-placement ADLs | 4.34 | 2.55 | 0.00–10.00 |
| Pre-placement IADLs | 7.13 | 1.20 | 0.00–8.00 |
| Pre-placement caregiving hours (typical week) | 74.67 | 61.43 | 0.00–988.00 |
| Pre-placement unmet needs | 2.92 | 4.31 | 0.00–18.00 |
| Resources | |||
| Pre-placement chore service use (times) | 29.76 | 98.08 | 0.00–1300.00 |
| Pre-placement personal care use (times) | 67.56 | 193.55 | 0.00–1456.00 |
| Pre-placement adult day service use (days) | 19.74 | 37.06 | 0.00–120.00 |
| Pre-placement overnight hospital use (times) | 2.05 | 7.97 | 0.00–120.00 |
| Pre-placement secondary caregiving hours (typical week) | 6.42 | 20.97 | 0.00–168.00 |
| CG pre-placement self-reported health (1 = excellent; 4 = poor) | 2.05 | .83 | 1.00–4.00 |
| CG pre-placement ADLs | .24 | .69 | 0.00–5.00 |
| CG pre-placement IADLs | .78 | 1.51 | 0.00–8.00 |
| Pre-Placement Burden | 13.76 | 7.06 | 0.00–28.00 |
| Post-Placement Burden | 9.61 | 6.90 | 0.00–28.00 |
| Pre-Placement Depression | 4.69 | 3.55 | 0.00–15.00 |
| Post-Placement Depression | 4.17 | 3.74 | 0.00–15.00 |
NOTE: M = mean; SD = standard deviation; CR = care recipient; CG = caregiver; NHA = nursing home admission; ADL = activities of daily living; IADLs = instrumental activities of daily living; MMSE = Mini-Mental State Examination
Outcomes
Caregiver depressive symptoms were measured with the 15-item Geriatric Depression Scale (GDS; α = .75) (Sheikh & Yesavage, 1986) and caregiver burden was assessed with a 7-item short form of the Zarit Burden Inventory (ZBI; α = .89) (Zarit, Todd, & Zarit, 1986). This 7-item version of the ZBI was developed specifically for MADDE to include items appropriate for assessing burden before and after NHA. Prior analyses found that a score of 6 or greater on the 15-item version of the GDS maximized sensitivity and specificity to detect clinically relevant depression (Lyness, Noel, Cox, King, Conwell, & Caine, 1997). Our prior work using the full baseline sample of MADDE (N = 5,831) found that a cutoff score of 13.00 or higher (range = 0.00–28.00) was indicative of clinically relevant burden (Gaugler et al., 2009, 2010). For this study, clinical burden and clinical depression were operationalized as a score at or above the clinically significant cut-points of “high” burden (13) and depression (6) at 6-months post-placement for participating caregivers.
Context of care
Table 1 demonstrates the various demographic and contextual characteristics available in MADDE.
Dementia severity and functional impairment
Cognitive status of care recipients was assessed at baseline only with the Mini-Mental State Examination (MMSE; α = .94) (Folstein, McHugh, & McHugh, 1975) The 19-item Memory and Behavior Problems Checklist, which measured the frequency of behavior problems (α = .77) was assessed at the pre-placement interview (Zarit, Orr, & Zarit, 1985). Caregivers reported care recipients’ functional impairment in 10 activities of daily living (ADLs; α = .89) (Katz, Ford, Moskowitz, Jackson, & Jaffe, 1963) and 8 instrumental activities of daily living (IADLs; α = .84) (Lawton & Brody, 1969). Caregivers also indicated the number of hours they typically spent providing help to the care recipient (primary informal caregiving hours). After caregivers were asked whether care recipients needed “no help,” “some help,” or “maximum help” to perform each ADL or IADL task, caregivers were asked whether the care recipient was receiving enough help for that particular ADL or IADL (yes = 1; no = 0). Unmet need responses for each ADL or IADL item were summed to create the unmet needs construct (α = .87).
Resources
Caregivers reported on various types of service use at pre-placement. Three community-based services (chore, personal care, and adult day care) and overnight hospital use for the care recipient were examined in this analysis. Caregivers indicated how many hours they typically received help from other family members or friends when assisting the care recipient (secondary caregiving hours). Additional resource variables included caregivers’ self-reported health status and caregivers’ own functional dependency on 5 ADLs (α = .62) and 8 IADLs (α = .80).
Analysis
The initial step in developing a prognostic tool for clinical burden (i.e., the “NHA-Burden” tool) was to examine the bivariate associations (via logistic regression models) between pre-placement context of care, indicators of care recipients’ functional impairment, indicators of dementia severity, resources, pre-placement burden, pre-placement depressive symptoms, and clinical burden up to 6 months after NHA. These initial analyses were conducted in a development sample (i.e., a random split of the cases in the 6-month post-placement panel; n = 836). In addition to identifying those variables that were significantly associated with persistent burden at the bivariate level, we examined a series of cut-points to maximize the strength of relationship/Odds Ratios between the selected predictor and clinical burden. Following the identification of cut-points, we conducted a full logistic regression model in the development sample that included the predictors identified above. A stepwise approach was utilized to screen out collinear predictors and to yield the most parsimonious grouping of predictors. For those variables that emerged as significant (p < .05), Odds Ratios were extracted as scores to include in the NHA-Burden prognostic tool to identify those caregivers at greatest risk for clinical burden up to 6 months after NHA. Specifically, these Odds Ratios were summed to create a pre-placement prognostic score for caregivers who scored above or below the cut-point values that were significant in the full logistic regression model. Those caregivers below a variable’s cut-point were given a score of 0; those at or above the cut-point were given a score that reflected the variable’s Odds Ratio.
Using the summed NHA-Burden prognostic score derived from the development sample, we tested the sensitivity and specificity of the predictive classification using a response operant characteristic (ROC) curve (Simon, 2008) in a validation sample (n = 774; or those remaining cases in the 6-month post-placement panel who were not randomly selected for inclusion in the development panel). The ROC approach was chosen because it allows the researcher to determine: 1) the empirical association between a measure and a criterion (e.g., clinical judgment, a “gold standard” measure); and 2) the binary threshold that maximizes the accuracy of the measure in question to create a “cut point” to classify respondents appropriately. Unlike correlational or similar bivariate analyses, the ROC curve approach offers researchers or clinicians the opportunity to determine whether a score exists to effectively classify individuals as having or experiencing some phenomena of interest (e.g., a disease) while also identifying those who do not. In the context of this analysis, the sensitivity of cut points was calculated as the percent of clinical burden cases correctly classified by our prediction rule. Specificity was the percent of cases correctly predicted as not having clinical burden in the validation sample.
Identical to the creation of the NHA-Burden prognostic tool, we examined the bivariate associations between pre-placement context of care, indicators of functional impairment, indicators of dementia severity, resources, pre-placement burden, pre-placement depressive symptoms, and clinical depression up to 6 months after NHA in a new development sample randomly drawn from the 6-month post-placement panel (n = 797). These steps facilitated the construction of a prognostic tool to identify dementia caregivers at risk for clinical depression after NHA (i.e., the “NHA-Depression” tool). Using the summed NHA-Depression prognostic score, we conducted a ROC curve in a new validation sample (n = 813) to identify the clinical threshold that was most sensitive and specific in identifying caregivers at risk for clinical depression following institutionalization.
Results
Prognostic Tool Development: NHA-Burden
Table 2 shows the cut points and results of the logistic regression model used to predict 6-month post-placement clinical burden in the development sample. Using the stepwise approach, the following variables emerged as significant pre-placement predictors of clinical burden up to 6 months following NHA: duration of care, unmet needs, depression, and burden. The following cut-points on these variables were found to exert the strongest effects on clinical burden after testing various dichotomous and categorical (i.e., quartiles) scores on each: over 36 months on duration of care (OR = 1.41, p = .04; reference = less than 36 months); any unmet need indicated (OR = 1.48, p = .02; reference = no unmet need indicated); a score of 2–4, 4–7, and over 7 on the GDS (OR = 2.59, p = .001; OR = 2.83, p <= .001; OR = 2.93, p <= .001, respectively; reference = 0–2); and a score of 9–14, 14–19, and over 19 on the ZBI (OR = 2.10, p = .01; OR = 2.41, p <= .001; OR = 4.93, p <= .001, respectively; reference = 0–9).
Table 2.
Establishing the NHA-Burden Prognostic Tool: Parsimonious Logistic Regression Model Predicting Clinical Burden up to Six Months Following Nursing Home Admission (N = 836; Development Sample)
| OR | 95% CI Lower | 95% CI Upper | P-value | |
|---|---|---|---|---|
| Duration of Care | ||||
| Over 36 months | 1.41 | 1.01 | 1.97 | .04 |
| Pre-Placement Unmet Needs | ||||
| Any unmet need indicated | 1.48 | 1.06 | 2.07 | .02 |
| Pre-Placement Depression | ||||
| 0–2 on GDSa (reference) | ||||
| 2–4 on GDS | 2.59 | 1.51 | 4.45 | .001 |
| 4–7 on GDS | 2.83 | 1.65 | 4.85 | < .001 |
| Over 7 on GDS | 2.93 | 1.64 | 5.25 | < .001 |
| Pre-Placement Burden | ||||
| 0–9 on ZBIb (reference) | ||||
| 9–14 on ZBI | 2.10 | 1.19 | 3.71 | .01 |
| 14–19 on ZBI | 2.41 | 1.36 | 4.26 | < .001 |
| Over 19 on ZBI | 4.93 | 2.65 | 9.18 | < .001 |
NOTE: OR = Odds Ratio; CI = Confidence Interval; GDS = Geriatric Depression Scale; ZBI = Zarit Burden Inventory
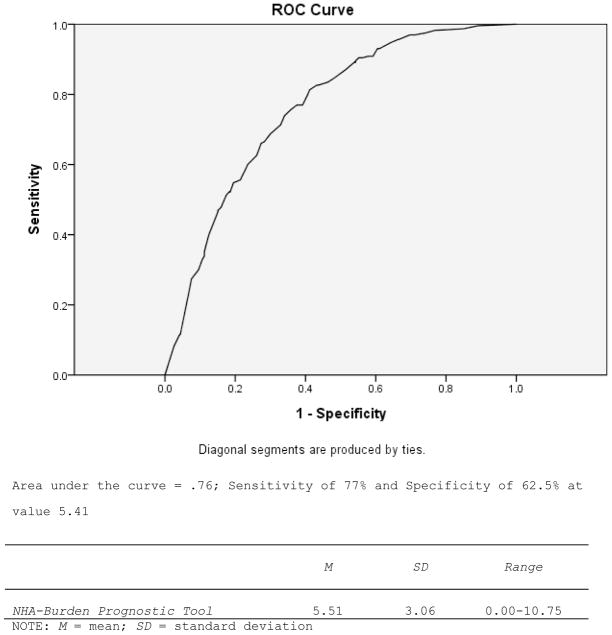
As shown in Figure 2, a sum of the Odds Ratios reported in Table 2 was constructed for each dementia caregiver in the validation sample for sensitivity and specificity testing. This represents the NHA-Burden prognostic tool score. The NHA-Burden average score for dementia caregivers in the validation sample was 5.51. The area under the ROC curve in Figure 2 was .76, which is considered fair (e.g., see http://gim.unmc.edu/dxtests/roc3.htm). The value on NHA-Burden at which sensitivity (77%) and specificity (62.5%) were optimized was 5.91.
Figure 2.
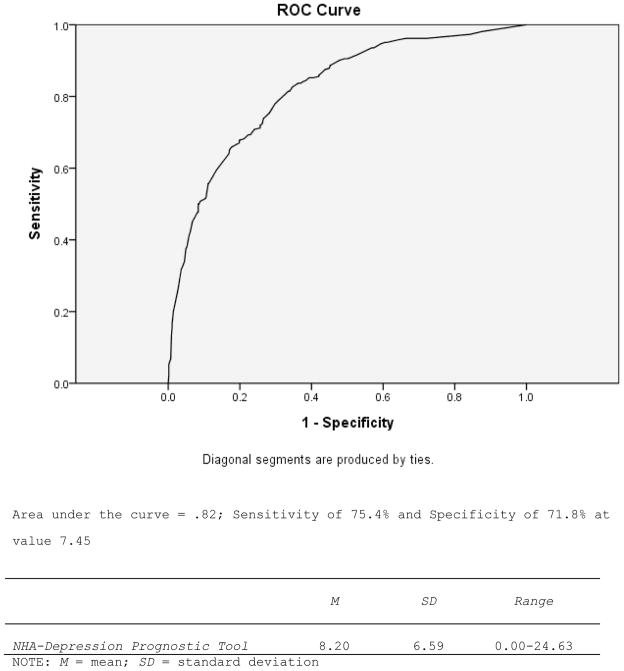
NHA-Depression Prognostic Tool: Sensitivity and Specificity (Validation Sample n = 813).
Prognostic Tool Development: NHA-Depression
Table 3 presents the cut points used in the logistic regression model that predicted clinical depression in a new development sample. The listwise approach resulted in the following significant pre-placement predictors of clinical depression up to 6 months post-placement: kin relationship, chore service use, subjective health, and depression. The following cut-points were found to result in the highest Odds Ratios when predicting clinical depression: caregivers who were spouses (OR = 2.50, p = .001; OR = 1.90, p =.046, respectively; reference = other relationship), any chore service use (OR = 1.81, p = .004; reference = no chore service use); a subjective health of good, fair, or poor (OR = 1.83, p = .02; OR = 1.88, p = .04; OR = 5.65, p <= .001, respectively; reference = excellent); and a score of 2–4, 4–7, and over 7 on the GDS (OR = 2.37, p = .008; OR = 5.56, p <= .001; OR = 14.67, p <= .001, respectively; reference = 0–2).
Table 3.
Establishing the NHA-Depression Prognostic Tool: Parsimonious Logistic Regression Model Predicting Clinical Depression up to Six Months Following Nursing Home Admission (N = 797; Development Sample)
| OR | 95% CI Lower | 95% CI Upper | P-value | |
|---|---|---|---|---|
| Kin Relationship | ||||
| Other (reference) | ||||
| Wife | 2.50 | 1.45 | 4.30 | .001 |
| Husband | 1.90 | 1.03 | 3.52 | .046 |
| Daughter | 1.28 | 0.71 | 2.31 | .41 |
| Pre-Placement Chore Service Use | ||||
| Any use | 1.81 | 1.21 | 2.69 | .004 |
| Pre-Placement Subjective Health | ||||
| Excellent (reference) | 1.83 | 1.09 | 3.09 | .02 |
| Good | 1.88 | 1.04 | 3.38 | .04 |
| Fair | 5.65 | 2.27 | 14.07 | < .001 |
| Poor | ||||
| Pre-Placement Depression | ||||
| 0–2 on GDSa (reference) | ||||
| 2–4 on GDS | 2.37 | 1.26 | 4.46 | .008 |
| 4–7 on GDS | 5.56 | 3.06 | 10.13 | < .001 |
| Over 7 on GDS | 14.67 | 7.95 | 27.09 | < .001 |
NOTE: OR = Odds Ratio; CI = Confidence Interval; GDS = Geriatric Depression Scale
Figure 3 illustrates descriptive information from the summed Odds Ratios reported in Table 3 for dementia caregivers in a new validation sample; this score represents the NHA-Depression prognostic tool score. The average NHA-Depression score for dementia caregivers in the validation sample was 8.20. The area under the ROC curve in Figure 2 was .82, which is considered good (e.g., see http://gim.unmc.edu/dxtests/roc3.htm). The value on NHA-Burden at which sensitivity (75.4%) and specificity (71.8%) were optimized was 7.45.
Figure 3.
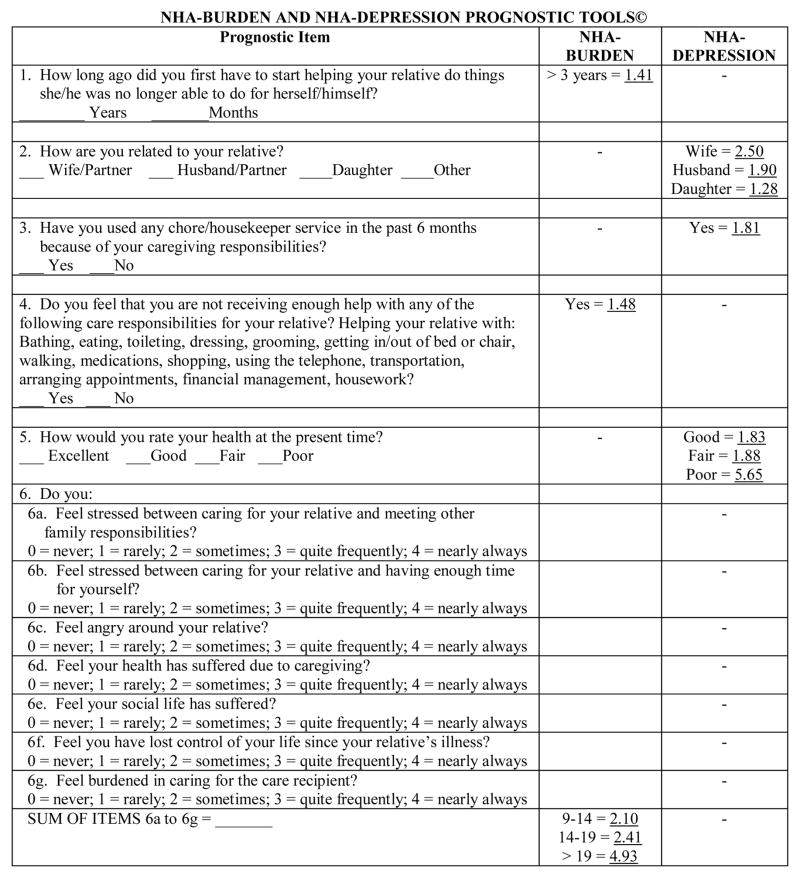
The NHA-Burden and NHA-Depression Prognostic Tools.
Discussion
The NHA-Burden tool demonstrated moderate sensitivity and specificity in classifying dementia caregivers at-risk for clinically high burden following institutionalization. The NHA-Burden tool could be used by residential care staff with caregivers of relatives who are in the process of entering a facility or in the months or weeks prior to this event to identify family members at-risk for negative psychosocial outcomes. Prognostic tools such as the ones developed here can help facility care managers (e.g., social work staff, nurses, or others) identify those families most at risk for increased stress during the months after NHA so that they can provide more targeted services such as individual/family consultation or other evidence-based family involvement interventions (Gaugler, 2005b) to enhance at-risk families’ adaptation to placement. It is important to note that the cut-off score we provide here (5.91) is one that optimizes both sensitivity and specificity. Clinicians may wish to maximize sensitivity at the expense of specificity to ensure that a larger proportion of persons who are at-risk for clinical burden are identified; if so, we would recommend using a cut-off score of 4.96 (sensitivity = 85%; specificity = 52%).
Similar findings occurred for the NHA-Depression prognostic tool. When optimizing sensitivity and specificity, the NHA-Depression tool showed appropriate predictive capability when identifying caregivers at-risk for clinical depression up to 6 months after NHA. However, as with the NHA-Burden tool, providers may wish to select a cut-off score that casts “a wider net” and captures as many dementia caregivers as possible among those at-risk for depressive symptoms in the months following institutionalization (we recommend a score of 4.26, with a specificity of 91% and a sensitivity of 51%). Dementia caregivers identified as at-risk for depression could then be provided an appropriate preventive intervention (e.g., individual consultation, referral to psychotherapeutic services, or similar types of support). As both the NHA-Burden and NHA-Depression prognostic tools have similar item content, the tools could be deployed together to identify dementia caregivers at-risk for post-placement burden or depressive symptoms prior to or during the institutionalization event.
Although this analysis was conducted on a large multi-regional data set, there are several limitations that are important to consider. Measures such as the ZBI and GDS, while effective general assessments of dementia caregiver stress and negative mental health, may not address issues more directly related to institutionalization. Similarly, we did not have access to “gold standards” of burden (e.g., clinical expert assessment) when developing the NHA-Burden tool; for this reason, we were relegated to validating burden with the clinical cut-off of the GDS (i.e., depressive symptoms). Although the MADDE data set is one of the largest of its kind and drew from multiple regions across the United States, it did not select a random sample of the dementia caregiver population. Similarly, while there is a relatively large number of African-American and Latino dementia caregivers in the MADDE sample (Gaugler, Kane, Kane, & Newcomer, 2006; Gaugler, Leach, Clay, & Newcomer, 2004), there is a need to test the validity of the NHA-Burden and NHA-Prognostic tools across racially and ethnically diverse dementia caregivers as well as in other residential long-term care settings (such as assisted living). There was variability in terms of when caregivers completed pre-placement and post-placement interviews. As we have reported in our prior analyses of the MADDE placement cohorts (Gaugler et al., 2009, 2010), time to NHA was significantly associated with rate of change in burden at 6-months post-placement (e.g., dementia caregivers who completed pre-placement interviews earlier were more likely to indicate an increase in burden); however, these results did not occur when we examined clinically significant burden.
Much of the literature on dementia caregiving has focused on establishing psychometrically-sound measures of caregiver stress or other domains, but it is not immediately apparent how such information can be used to inform the targeting of an appropriate support strategy. Such barriers are crucial to overcome; the current financial crisis has placed considerable pressure on publicly-financed social services in the U.S. and there is a demonstrable need to provide improved risk assessments to assist clinicians when linking families in need to the most appropriate services available. Evidence-based, comprehensive psychosocial interventions are often presented as “one-size fits all” solutions, and whether all dementia caregivers are likely to benefit from or even prefer such comprehensive support protocols is often not considered. The NHA-Burden or NHA-Depression Prognostic Tools could potentially overcome these challenges by allowing clinicians to more effectively classify those dementia caregivers most at-risk for negative outcomes following NHA. This could reduce the potential costs and inefficiency of administering complex, multi-component interventions to all dementia caregivers regardless of their potential risk for subsequent negative outcomes.
The NHA-Burden and NHA-Depression Tools, along with scoring guidance, are presented in Figure 3. As noted above, we recommend that these tools be either administered in the weeks prior to (i.e., while the care recipient is on a wait list) or during a relative’s admission to a long-term care facility in order to identify family caregivers at-risk for negative emotional or mental health outcomes in the months following this transition. The short, brief structure of the NHA-Burden and NHA-Depression tools (which can be combined on one back-to-back page; see Figure 3) as well as the overlap of some items could allow clinicians to determine dementia caregivers’ risk for subsequent burden or depression simultaneously. Clinicians simply have to sum the item-responses demonstrated in Figure 3 to create the NHA-Burden or NHA-Depression prognostic scores in order to classify family caregivers at-risk for either outcome, respectively. We would anticipate that the NHA-Burden or NHA-Depression tools would have considerable utility due to their brevity (they should not take longer than 5–10 minutes). These prognostic tools could be routinely administered by a facility social worker or director or nursing during an intake or care planning meeting with families. While an ongoing practical issue may involve reimbursement of such assessment activities, we believe that the efficiency and ease of use of the NHA-Burden and NHA-Depression tools would allay such concerns and allow for a quick integration of these tools into standard social work or nursing intake protocols (for reprints of the tools, please contact the first author).
While the empirical approaches detailed in this paper provide a strong foundation for the NHA-Burden and NHA-Depression tools, more work is needed to establish their utility and to refine their incorporation into routine, residential long-term care practice. We intend to disseminate these tools to local long-term care providers and staff, including directors of nursing, social workers, and intake coordinators (e.g., see http://www.leadingageiowa.org/i4a/pages/index.cfm?pageid=5727). The feedback we obtain from these dissemination activities will help us determine how the NHA-Burden and NHA-Depression tools operate in terms of scoring ease, perceptions of utility, administration (e.g., timing; whether the tools are best utilized face-to-face, over the telephone, or as an online survey, etc.), and whether use of the tools modify the types of services and interventions care staff recommend for families at-risk. This feasibility testing will likely require a mixed methods stance, as both qualitative and quantitative data will elucidate the strengths of the NHA-Burden and NHA-Depression tools as well as areas to refine. In addition, we plan to offer the NHA-Burden and NHA-Depression tools to providers across various residential long-term care environments (memory care units in assisted living facilities and family care homes in addition to nursing homes) to further determine how the NHA-Burden and NHA-Depression tools operate across a range of settings. With the proliferation of evidence-based interventions for dementia caregivers, continued refinement and testing of the NHA-Burden and NHA-Depression tools will allow for the improved identification of family caregivers at-risk during a key transition point in dementia with the aim of effectively linking families in need to appropriate support.
Figure 1.
NHA-Burden Prognostic Tool: Sensitivity and Specificity (Validation Sample n = 774).
Acknowledgments
This research was supported by grant R21 AG025625 from the National Institute on Aging/National Institutes of Health.
Footnotes
A version of this paper was presented at the 2008 Gerontological Society of America Meeting, National Harbor, MD.
Contributor Information
Joseph E. Gaugler, School of Nursing, Center on Aging, University of Minnesota.
Mary S. Mittelman, Department of Psychiatry, New York University Langone Medical Center.
Kenneth Hepburn, School of Nursing, Emory University.
Robert Newcomer, Department of Social and Behavioral Science, University of California-San Francisco.
References
- Almberg B, Grafstrom M, Krichbaum K, Winblad B. The interplay of institution and family caregiving: Relations between patient hassles, nursing home hassles and caregivers’ burnout. International Journal of Geriatric Psychiatry. 2000;15:931–939. doi: 10.1002/1099-1166(200010)15:10<931::aid-gps219>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Aneshensel CS, Pearlin LI, Mullan JT, Zarit SH, Whitlatch CJ. Profiles in caregiving: The unexpected career. San Diego: Academic Press; 1995. [Google Scholar]
- Brody E. Long-term care of older people: A practical guide. New York: Human Sciences Press; 1977. [Google Scholar]
- Czaja SJ, Gitlin LN, Schulz R, Zhang S, Burgio LD, Stevens AB, Gallagher-Thompson D. Development of the Risk Appraisal Measure: A brief screen to identify risk areas and guide interventions for dementia caregivers. Journal of the American Geriatrics Society. 2009;57(6):1064–1072. doi: 10.1111/j.1532-5415.2009.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S, Nolan M. ‘Making it better’: Self-perceived roles of family caregivers of older people living in care homes: A qualitative study. International Journal of Nursing Studies. 2006;43(3):281–291. doi: 10.1016/j.ijnurstu.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Dellasega C, Mastrian K. The process and consequences of institutionalizing an elder. Western Journal of Nursing Research. 1995;17(2):123–140. doi: 10.1177/019394599501700202. [DOI] [PubMed] [Google Scholar]
- Family Caregiver Alliance. Caregiver assessment: Principles, guidelines and strategies for change. San Francisco, CA: National Center on Caregiving at Family Caregiver Alliance; 2006. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Kane RL, Kane RA, Newcomer R. Predictors of institutionalization in latinos with dementia: A cross-cultural analysis. Journal of Cross-Cultural Gerontology. 2006;21:139–155. doi: 10.1007/s10823-006-9029-8. [DOI] [PubMed] [Google Scholar]
- Gaugler JE. Family involvement in residential long-term care: A synthesis and critical review. Aging & Mental Health. 2005a;9(2):105–118. doi: 10.1080/13607860412331310245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler JE. Promoting family involvement in long-term care settings: A guide to programs that work. Baltimore, MD: Health Professions Press; 2005b. [Google Scholar]
- Gaugler JE, Leach CR, Clay T, Newcomer RC. Predictors of nursing home placement in African Americans with dementia. Journal of the American Geriatrics Society. 2004;52(3):445–452. doi: 10.1111/j.1532-5415.2004.52120.x. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Mittelman MS, Hepburn K, Newcomer R. Predictors of change in caregiver burden and depressive symptoms following nursing home admission. Psychology and Aging. 2009;24(2):385–396. doi: 10.1037/a0016052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler JE, Mittelman MS, Hepburn K, Newcomer R. Clinically significant changes in burden and depression among dementia caregivers following nursing home admission. BMC Medicine. 2010;8:85. doi: 10.1186/1741-7015-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler JE, Yu F, Krichbaum K, Wyman JF. Predictors of nursing home admission for persons with dementia. Medical Care. 2009;47(2):191–198. doi: 10.1097/MLR.0b013e31818457ce. [DOI] [PubMed] [Google Scholar]
- Gold DP, Reis MF, Markiewicz D, Andres D. When home care giving ends: A longitudinal study of outcomes for caregivers of relatives with dementia. Journal of the American Geriatrics Society. 1995;43:10–16. doi: 10.1111/j.1532-5415.1995.tb06235.x. [DOI] [PubMed] [Google Scholar]
- Grant I, Adler KA, Patterson TL, Dimsdale JE, Ziegler MG, Irwin MR. Health consequences of Alzheimer’s caregiving transitions: Effects of placement and bereavement. Psychosomatic Medicine. 2002;64:477–486. doi: 10.1097/00006842-200205000-00012. [DOI] [PubMed] [Google Scholar]
- Grasel E. When home care ends--changes in the physical health of informal caregivers caring for dementia patients: A longitudinal study. Journal of the American Geriatrics Society. 2002;50(5):843–849. doi: 10.1046/j.1532-5415.2002.50209.x. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA : The Journal of the American Medical Association. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Liu W, Gallagher-Thompson D. Impact of dementia caregiving: Risks, strains, and growth. In: Qualls SH, Zarit SH, editors. Aging families and caregiving. Hoboken, NJ: John Wiley & Sons, Inc; 2009. pp. 85–112. [Google Scholar]
- Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression scale and the Geriatric Depression Scale. Archives of Internal Medicine. 1997;157(4):449–454. [PubMed] [Google Scholar]
- Majerovitz SD. Predictors of burden and depression among nursing home family caregivers. Aging & Mental Health. 2007;11(3):323–329. doi: 10.1080/13607860600963380. [DOI] [PubMed] [Google Scholar]
- Marziali E, Shulman K, Damianakis T. Persistent family concerns in long-term care settings: Meaning and management. Journal of the American Medical Directors Association. 2006;7(3):154–162. doi: 10.1016/j.jamda.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Matsuda O, Hasebe N, Ikehara K, Futatsuya M, Akahane N. Longitudinal study of the mental health of caregivers caring for elderly patients with dementia: Effect of institutional placement on mental health. Psychiatry and Clinical Neurosciences. 1997;51(5):289–293. doi: 10.1111/j.1440-1819.1997.tb03200.x. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Aschbacher K, Patterson TL, von Kanel R, Dimsdale JE, Mills PJ, Grant I. Effects of placement and bereavement on psychological well-being and cardiovascular risk in Alzheimer’s caregivers: A longitudinal analysis. Journal of Psychosomatic Research. 2007;62(4):439–445. doi: 10.1016/j.jpsychores.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Newcomer R, Yordi C, DuNah R, Fox P, Wilkinson A. Effects of the Medicare Alzheimer’s Disease Demonstration on caregiver burden and depression. Health Services Research. 1999;34(3):669–689. [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: An overview of concepts and their measures. The Gerontologist. 1990;30:583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- Port CL, Zimmerman S, Williams CS, Dobbs D, Preisser JS, Williams SW. Families filling the gap: Comparing family involvement for assisted living and nursing home residents with dementia. The Gerontologist. 2005;45(Spec No 1 1):87–95. doi: 10.1093/geront/45.suppl_1.87. [DOI] [PubMed] [Google Scholar]
- Rankin ED, Haut MW, Keefover RW, Franzen MD. The establishment of clinical cutoffs in measuring caregiver burden in dementia. Gerontologist. 1994;34(6):828–832. doi: 10.1093/geront/34.6.828. [DOI] [PubMed] [Google Scholar]
- Ryan AA, Scullion HF. Nursing home placement: An exploration of the experiences of family carers. Journal of Advanced Nursing. 2000;32(5):1187–1195. doi: 10.1046/j.1365-2648.2000.01589.x. [DOI] [PubMed] [Google Scholar]
- Schulz R, Belle SH, Czaja SJ, McGinnis KA, Stevens A, Zhang S. Long-term care placement of dementia patients and caregiver health and well-being. Journal of the American Medical Association. 2004;292:961–967. doi: 10.1001/jama.292.8.961. [DOI] [PubMed] [Google Scholar]
- Sheikh J, Yesavage J. Geriatric depression scale: Recent evidence and development of a shorter version. Clinical Gerontologist. 1986;5:165–173. [Google Scholar]
- Simon S. Stats: ROC curve. 2008 Retrieved April 9, 2009, from http://www.childrensmercy.org/stats/ask/roc.asp.
- Sorensen S, Duberstein P, Gill D, Pinquart M. Dementia care: Mental health effects, intervention strategies, and clinical implications. Lancet Neurology. 2006;5(11):961–973. doi: 10.1016/S1474-4422(06)70599-3. [DOI] [PubMed] [Google Scholar]
- Strang VR, Koop PM, Dupuis-Blanchard S, Nordstrom M, Thompson B. Family caregivers and transition to long-term care. Clinical Nursing Research. 2006;15(1):27–45. doi: 10.1177/1054773805282356. [DOI] [PubMed] [Google Scholar]
- Tornatore JB, Grant LA. Burden among family caregivers of persons with Alzheimer’s disease in nursing homes. The Gerontologist. 2002;42:497–506. doi: 10.1093/geront/42.4.497. [DOI] [PubMed] [Google Scholar]
- Townsend AL. Nursing home care and family caregivers’ stress. In: Stephens MAP, Cowther JH, Hobfoll SE, Tennebaum DL, editors. Stress and coping in later-life families. New York: Hemisphere; 1990. [Google Scholar]
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychological Bulletin. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- Whitlatch CJ, Feinberg LF, Stevens EJ. Predictors of institutionalization for persons with Alzheimer’s disease and the impact on family caregivers. Journal of Mental Health and Aging. 1999;5:275–288. [Google Scholar]
- Whitlatch CJ, Schur D, Noelker LS, Ejaz FK, Looman WJ. The stress process of family caregiving in institutional settings. The Gerontologist. 2001;41:462–473. doi: 10.1093/geront/41.4.462. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Orr N, Zarit J. Understanding the stress of caregivers: Planning an intervention. In: Zarit SH, Orr N, Zarit J, editors. Hidden victims of alzheimer’s disease: Families under stress. New York: NYU Press; 1985. pp. 69–86. [Google Scholar]
- Zarit SH, Todd PA, Zarit JM. Subjective burden of husbands and wives as caregivers: A longitudinal study. The Gerontologist. 1986;26(3):260–266. doi: 10.1093/geront/26.3.260. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Whitlatch CJ. Institutional placement: Phases of transition. The Gerontologist. 1992;32:665–672. doi: 10.1093/geront/32.5.665. [DOI] [PubMed] [Google Scholar]