Abstract
Despite advances in surgical techniques over the past three decades, tendon repairs remain prone to poor clinical outcomes. Previous attempts to improve tendon healing have focused on the later stages of healing (i.e., proliferation and matrix synthesis). The early inflammatory phase of tendon healing, however, is not fully understood and its modulation during healing has not yet been studied. Therefore, the purpose of this work was to characterize the early inflammatory phase of flexor tendon healing with the goal of identifying inflammation-related targets for future treatments. Canine flexor tendons were transected and repaired using techniques identical to those used clinically. The inflammatory response was monitored for 9 days. Temporal changes in immune cell populations and gene expression of inflammation-, matrix degradation-, and extracellular matrix-related factors were examined. Gene expression patterns paralleled changes in repair-site cell populations. Of the observed changes, the most dramatic effect was a greater than 4000-fold up-regulation in the expression of the pro-inflammatory factor IL-1β. While an inflammatory response is likely necessary for healing to occur, high levels of pro-inflammatory cytokines may result in collateral tissue damage and impaired tendon healing. These findings suggest that future tendon treatment approaches consider modulation of the inflammatory phase of healing.
Keywords: inflammation, intrasynovial flexor tendon, matrix metalloproteinase, extracellular matrix, collagen
INTRODUCTION
Over the past few decades, flexor tendon repair research has focused on improving rehabilitation protocols and surgical techniques which has led to decreased adhesion formation and repair-site elongation. Despite these advances, clinical outcomes continue to be variable and result in 1.5 million days lost from work per year1-6. As research advances resulting from altering operative or rehabilitation techniques have plateaued, our laboratory has shifted to focusing on biologic manipulation of tendon healing with the goals of further improving tendon strength, decreasing gap formation, and preventing adhesion formation.
After surgical repair, tendon healing progresses through three overlapping phases: inflammation (days 1-7), proliferation (days 3-14), and remodeling (day 10 onward). Prior attempts to improve tendon repair have focused on the later stages of healing by enhancing tendon fibroblast proliferation or promoting extracellular matrix synthesis7-9. Growth factors have successfully been used to stimulate biologic activity during flexor tendon healing7-9. Despite increased proliferation and matrix remodeling, however, healing tendons failed to accrue strength during the first 3 weeks following suture. Therefore, the repair-site remains at risk for gap formation or rupture following current treatment approaches.
In contrast to vascular and highly cellular tissues such as skin, where healing has been described in detail, tendons are relatively avascular10-12 and paucicellular15. Given that a typical wound healing response is initiated by an infiltration of inflammatory cells from the nearby vasculature, tendons may have a diminished healing response compared to skin. Articular cartilage, for example, which is avascular, has a very limited wound healing response13,14. Furthermore, due to the relative paucity of cells in the intrasynovial flexor tendon, early tendon healing relies on the migration of tendon surface cells and/or extrinsic cells from the synovial sheath to the repair site. This process may delay the overall healing process in comparison to highly cellular skin healing models.
Based on these results, the focus of the current study is to examine the early inflammatory stage of healing, including enhancement of cell survival, migration, and proliferation immediately following tendon suture, in order to provide a basis for future treatments. A clinically relevant large animal model of flexor tendon injury and repair was used to investigate temporal changes in immune cell populations, and gene expression of inflammation-, matrix remodeling-, extracellular matrix-(ECM), and differentiation-related factors during the early post-repair period.
MATERIALS AND METHODS
Flexor tendon animal model
All procedures were approved by the Washington University Animal Studies Committee. Flexor tendon injury and repair was performed in the clinically relevant canine animal model (n=11, female non-castrated)16. The sheaths of the second and fifth digits of the right forelimb in the region between the annular pulleys proximal and distal to the proximal interphalangeal joint were exposed through midlateral incisions. The sheaths were entered and the flexor digitorum profundus tendons were transected sharply. The tendons were repaired using surgical techniques identical to those used in humans (4-0 Supramid core suture, 6-0 Proline running epitenon suture). Both the second and fifth digits of the right forelimb were repaired. After surgery, the operative limb was immobilized using a fiberglass shoulder spica cast with the elbow flexed to 90° and the wrist flexed to 70° and a controlled passive motion rehabilitation protocol, similar to that used clinically, was applied. The protocol consists of two five-minute rehabilitation sessions performed five days a week starting on the first postoperative day17. The dogs were euthanized on days 1, 3 or 9 post-operatively, and the operated tendons were removed by dissection and prepped for either gene expression analysis (N=3 per timepoint) or histologic analysis (N=3-5 per timepoint). Tendons from the contralateral paw were also dissected to serve as normal/uninjured controls.
Histology
Upon dissection, tendons allocated for histology were fixed in 4% paraformaldehyde overnight, embedded in paraffin, and cut into 5 μm sections. To examine the immune response, sections were stained with hematoxylin and eosin (H&E) and assessed for various immune cells (i.e., PMNs, monocytes, macrophages) by an independent certified pathologist (NH), blinded to group. Fibroblasts were characterized as spindle-shaped cells with an elliptic nucleus and thin cytoplasm. PMNs were identified as cells containing nuclei with two to four lobules and a granulated cytoplasm, while cells with a single-lobed or kidney-shaped nucleus were classified as monocytes/macrophages. A standard scoring system was used to determine the levels of each outcome (see below). Overall cellularity, the prevalence of certain cell types, vascularity, and the percentage of fibroblasts compared to overall cellularity were assessed. All assessments were done using a 20x objective and 12-21 fields of view were averaged. Sections were also viewed under polarized light for the assessment of collagen alignment. Proliferating cell nuclear antigen (PCNA) staining (Invitrogen, NY) was performed according to the manufacturers’ protocols to access cell proliferation.
A standard scoring system was used to determine the levels of each outcome (-no prevalence, + mild prevalence, ++ moderate prevalence, +++ marked prevalence). For overall cellularity and the prevalence of certain cell types, the number of cells per high powered field (HPF, 20x) was counted and assigned a score as follows: + <50 per HPF, ++ 51-100 per HPF, +++ 101-150 per HPF, ++++ >150 per HPF. Vascularity was measured on the following scale: + <5 per HPF, ++ 6-10 per HPF, +++ > 10 per HPF. The percentage of fibroblasts compared to overall cellularity was also assessed and scored as follows: + = less than 5%, ++ = 5-50%, +++ > 50%.
Gene expression
Tendons allocated for gene expression were dissected and 10 mm sections (5 mm on each side of the repair) were isolated and immediately flash frozen in liquid nitrogen. RNA was extracted from the tendons using the RNeasy mini kit (Qiagen, CA) following the manufacturer’s protocol. RNA yield was quantified using a NanoDrop spectrophotometer (Thermo Scientific, DE) and 500 ng of RNA was reverse transcribed to cDNA using the Superscript VILO cDNA synthesis kit (Invitrogen Corporation, CA) following manufacturer’s instructions. Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) was performed using SYBR Green chemistry on a StepOnePlus Real-Time PCR System (Applied Biosystems, CA). All primers for real-time PCR were purchased (Qiagen, CA). Gene expression changes were measured for genes related to inflammation (IL-1β, TNFα, COX2), matrix degradation (MMP1a, 1b, 3, 13), extracellular matrix (collagen type 1 (COL1), collagen type 3 (COL3), lysyl oxidase (LOX), decorin (DCN)), differentiation (scleraxis (SCX), tenomodulin (TNMD), lubricin (LUB)), and vascular endothelial growth factor (VEGF). Data was analyzed using the delta delta Ct method. The results were first normalized to a housekeeping gene (glyceraldehyde 3-phosphate dehydrogenase (GAPDH)) and then again to normal/uninjured group. The delta Ct values were compared using a 2-way ANOVA (for treatment and time), followed by a Fisher’s post-hoc test. Significance was set to p < 0.05. Consistent Ct values were observed for GAPDH across groups (the standard deviation of GAPDH Ct values was less than 1).
RESULTS
Inflammation
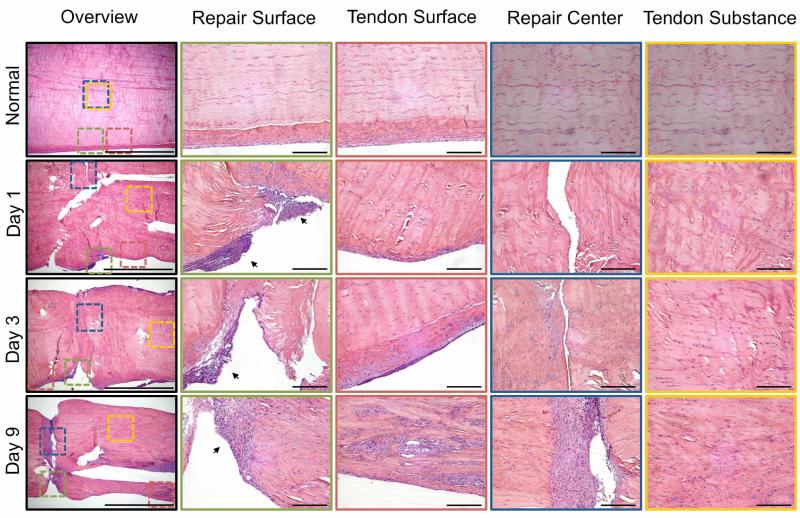
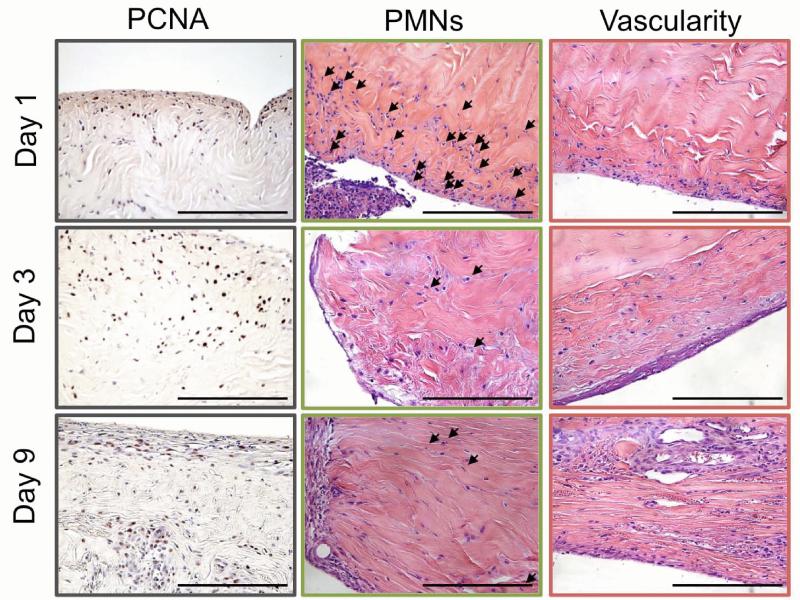
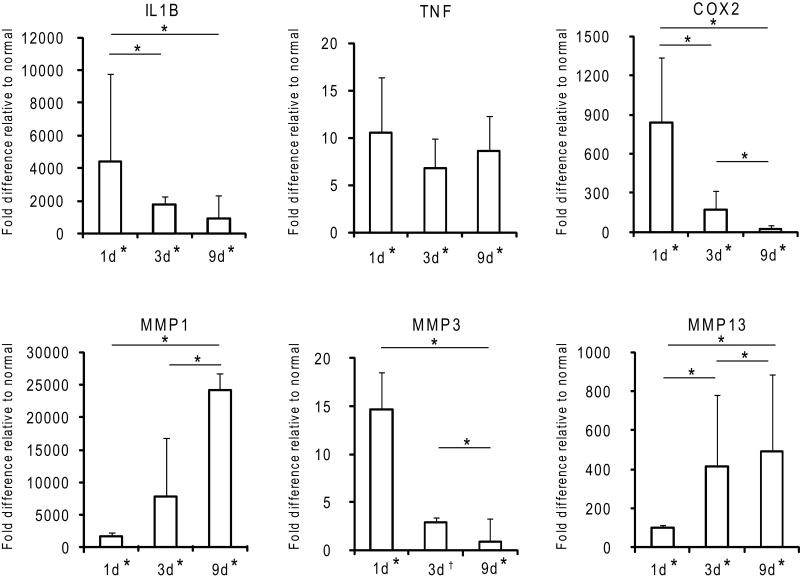
Cellularity at the repair site was significantly increased 1 day post-operatively (Table 1). The dominant cell type contributing to increased cellularity was the PMN (Figures 1-2, Table 1). The majority of the PMNs were within the fibrin clot in the superficial aspects of the repair. PMNs were also present in the tendon tissue near the surface of the repair (Figures 1-2). A corresponding spike in pro-inflammatory genes (TNFα, IL-1β, COX2) was evident. TNFα was up-regulated 10.6-fold compared to normal, while IL-1β and COX2 genes were up-regulated 4000 and 800-fold (Figure 3).
Table 1.
Histological analysis of the early inflammatory response 1, 3, and 9 days post-operatively. The prevalence and type of immune cells at the repair site was assessed over time. A standard scoring system was used to determine the levels of each outcome. Cellularity/PMN/Mono: + <50 per HPF, ++ 51-100 per HPF, +++ 101-150 per HPF, ++++ >150 per HPF. Vascularity: + <5 per HPF, ++ 6-10 per HPF, +++ > 10 per HPF. Fibroblast percentage: + = less than 5%, ++ = 5-50%, +++ > 50%. PMN = Polymorphonuclear cells, Mono = monocytes/macrophages, Fibro% = fibroblast percent compared to overall cellularity, HPF = High powered field (20x), (N=3-5).
| Cellularity | PMN | Mono | Fibro % | Vascularity | |
|---|---|---|---|---|---|
| Day 1 | ++ | ++ | − | + | −/+ |
| Day 3 | + | + | + | + | −/+ |
| Day 9 | + | + | + | ++/+++ | ++ |
Figure 1.
Representative histologic sections of normal and repaired tendons 1, 3, and 9 days post-operatively. The sections were stained with H&E and viewed under bright field for cell identification. An overview of a representative section from each timepoint is shown to the left (4x objective, 2 mm scale bar). Colored squares indicate the regions of interest (repair surface, tendon surface, repair center, tendon substance) on the overview images and corresponding high magnification images (20x objective, 200 μm scale bar) are shown to the right with corresponding border colors. Inflammatory cells are seen infiltrating the repair site via the tendon surface on day 1 (black arrows). The Inflammatory response decreases over time and fibroblasts invaginate and fill the repair by day 9. New blood vessel formation on the tendon surface is evident on day 9.
Figure 2.
Representative histologic sections of repaired tendons 1, 3, and 9 days post-operatively. The sections were stained with either H&E or PCNA and viewed under bright field for identification of immune cells (PMN), vascularity, and cell proliferation (PCNA). Changes in cell proliferation were most evident near the surface of the tendon. PMNs (black arrows) infiltrated the repair site on day 1, but decreased over time. New blood vessel formation was evident on the surface of the tendon away from the repair site by day 9. Images for PMNs and vascularity are the same regions as those presented in Figure 2.1 (see border color for region of image). 40x objective, 200 μm scale bar, (N=3-5).
Figure 3.
Fold changes in gene expression of pro-inflammation- and matrix degradation-related factors relative to normal 1, 3, and 9 days post-operatively. * p < 0.05, † p <0.10, 2-way ANOVA, significant effect of time (except for TNFα), bars signify Fisher’s post-hoc comparisons, * by x-axis signifies a significant difference compared to normal tendons, (N=3).
On day 3, overall cellularity remained higher than that noted in normal, uninjured tendons, but was decreased compared to day 1. A shift in the immune cell population was noted at this timepoint, with fewer PMNs evident at the repair site compared to day 1 (Figures 1-2, Table 1). The most prominent immune cell type at day 3 was from the monocyte/macrophage lineage (Figures 1-2, Table 1). Expression levels of pro-inflammatory genes remained significantly elevated at day 3 compared to levels noted in normal/uninjured control tendon (IL-1β, COX2, and TNFα were up-regulated 2000-, 200-, and 6.78-fold, respectively, compared to normal/uninjured tendons) (Figure 3).
The number of monocytes/macrophages at the repair site remained stable through day 9 (Figures 1-2, Table 1). IL-1β and COX2 expression continued to decrease, but remained significantly up-regulated compared to levels in normal/uninjured tendons (900- and 26.3-fold for IL-1β and COX2, respectively). TNFα levels were similar to those at the earliest timepoint (8.57-fold greater than normal) (Figure 3). While the expression of inflammatory factors decreased over time, expression levels remained significantly higher than normal at 9 days post-repair.
Proliferation
PCNA staining showed proliferation of epitenon cells (i.e., tendon surface cells) on day 1 (Figure 2). By day 3, the epitenon was thickened as the tendon surface cells continued to proliferate. Proliferation of cells deeper in the tendon substance was also observed on day 3 and continued through day 9 (Figure 2). By day 9, fibroblasts infiltrated the repair site, between the tendon stumps (Figure 1, Table 1). The source of the fibroblasts that invaginate and occupy the repair appear to be derived from the epitenon.
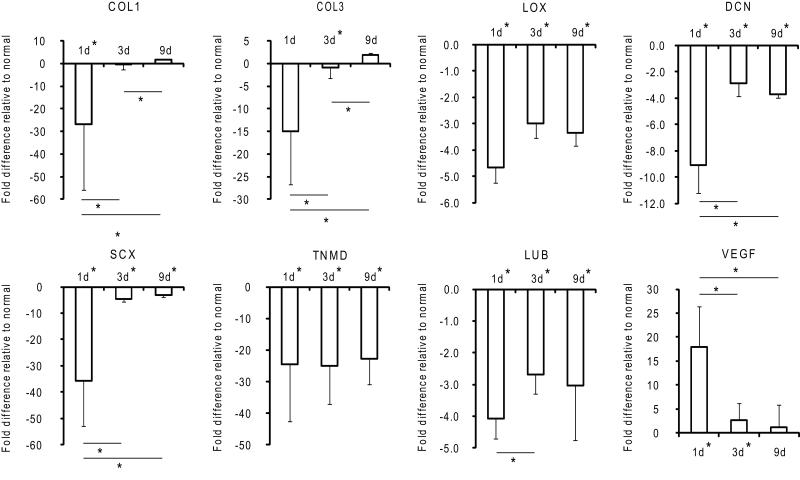
Expression of tendon-specific and extracellular matrix genes was significantly down-regulated 1 day post-operatively (27.0-, 14.9-, 35.6-, and 24.5-fold for COL1, COL3, SCX, and TNMD, respectively). As the cellular environment shifted from immune cell-dominant on day 1 to fibroblast-dominant on day 9 (Table 2.1), the expression of all tendon-specific and tendon extracellular matrix genes, aside from TNMD, returned to baseline (Figure 4).
Figure 4.
Fold changes in gene expression of tendon ECM and tendon-specific factors relative to normal 1, 3, and 9 days post-operatively. * p < 0.05, † p <0.10, 2-way ANOVA, significant effect of time (except for LOX and TNMD), bars signify Fisher’s post-hoc comparisons, * by x-axis signifies a significant difference compared to normal tendons, (N=3).
Remodeling
Matrix degradation, as evidenced by MMP expression and polarized light microscopy, was seen as early as day 1. MMPs 1, 3, and 13 were up-regulated 2000-, 14.7-, and 100-fold, respectively, compared to levels in normal/uninjured tendons (Figure 3). Polarized light microscopy revealed rapid loss of collagen fiber organization (Figure 5). On day 3, MMPs involved in the degradation of collagen types 1 and 3 (MMP1, MMP13) were further up-regulated (Figure 3) as collagen fiber alignment was disrupted further (Figure 5). By day 9, the expression of MMPs 1 and 13 continued to rise, reaching 24,000- and 500-fold increases compared to normal/uninjured tendon (Figure 3). Collagen fiber alignment remained similar to that noted on day 3 (Figure 5). Other genes related to later stages of tissue remodeling, LOX and LUB were significantly down-regulated (4.7- and 4.1-fold) 1 day following injury and repair and remained down-regulated throughout the 9 day study. VEGF gene expression was up-regulated (17.9-fold) on day 1. This is consistent with the appearance of newly formed blood vessels on the surface of the tendon (i.e., at the epitenon a few centimeters away from the repair) 9 days post-operatively (Figures 1-2, Table 1).
Figure 5.
Representative histologic sections of normal and repaired tendons 1, 3, and 9 days post-operatively. The sections were stained with H&E and viewed with polarized light to assess collagen fiber alignment. White regions indicate fiber alignment along the long axis of the tendon. Dark regions indicate a loss of fiber alignment. Some collagen fiber alignment was lost as early as 1 day post-operatively. Alignment was further disrupted by day 3 and remained steady through day 9. 4× objective, 2 mm scale bar, (N=3-5).
DISCUSSION
The temporal changes in cell population and gene expression of inflammation-, matrix remodeling-, extracellular matrix-, and differentiation-related factors were examined during the first 9 post-operative days following intrasynovial flexor tendon injury and repair. During this period, the expression of pro-inflammatory and matrix remodeling genes was significantly up-regulated. TNFα, IL-1β, and COX2 levels rose dramatically as early as day 1. Expression of these genes subsequently decreased over time, coincident with changes in cell populations from immune cells to tendon fibroblasts. In contrast to the marked elevation of pro-inflammatory genes, extracellular matrix- and differentiation-related genes were significantly down-regulated on day 1, an interval during which immune cells were prominent at the repair site. Matrix and differentiation gene expression values increased toward baseline levels as the number of PMNs decreased and tendon fibroblasts began to populate the repair site. Simultaneous with these early changes in gene expression, cellular proliferation, and cellular migration, MMP levels increased beginning at the time of injury. MMP levels increased further as macrophages cleared the wound of debris and dead tissue and as fibroblasts proliferated within the repair, initiating the remodeling phase of healing. The temporal increase in MMP expression coincided well with morphological changes in collagen fiber alignment. VEGF gene expression, which is related to the onset of neovascularization, was up-regulated on day 1. This is consistent with the appearance of newly formed blood vessels 9 days post-operatively. On the other hand, LOX and LUB remained down-regulated throughout the 9 day study. Given that LOX is important in cross-linking collagen and LUB is involved in tendon lubrication and gliding function, these results are not surprising; we anticipate that these genes to play a larger role in the remodeling stage of healing (i.e., 10 days after injury or later), which our study did not examine.
Prior studies of flexor tendon healing have focused for the most part on morphological changes at the repair site,18,19 and on the cellular source contributing to tendon healing.19-21 While these studies have provided key data supporting advances in surgical techniques and rehabilitation protocols, the early hemorrhagic and inflammatory processes have not been explored thoroughly. While Gelberman et al. described fibrinous bridges overlying a mesh of erythrocytes, macrophages, and other inflammatory-type cells on day 3, earlier timepoints were not investigated18. The expression of inflammatory and catabolic factors was not described until the past decade. Using a rabbit model of extrasynovial flexor tendon injury and repair, Berglund et al. revealed a significant elevation in the expression of pro-inflammatory factors (IL-1β and COX2) 3 days post-operatively22. IL-1β and COX2 levels were noted to remain elevated through day 6 and returning to baseline by day 12. Concurrently, the matrix degradation factor, MMP13, was significantly up-regulated on day 3 and remained elevated through day 2422. These results are generally in agreement with those of the current study. However, the animal model used in the previous study was limited with regard to clinical relevance: (1) the small size of rabbit flexor tendons precluded the use of standard repair methods used on humans, (2) a clinically appropriate postoperative rehabilitation protocol was not implemented, and (3) an extrasynovial tendon was used rather than the more commonly injured intrasynovial flexor tendon. In the current study, using a clinically relevant canine model, histological and gene expression experiments were performed to examine the cellular populations and gene expression patterns at in the early phase of flexor tendon healing.
Further experiments are needed to elucidate the cellular sources contributing to the changes in gene expression. As the gene expression changes were due to a combination of inflammatory cells and fibroblasts, the specific expression patterns for individual cell types remain unknown. However, histological assessment of the various cell types partly addresses this uncertainty. The percentage of fibroblasts (compared to overall cellularity) was estimated based on a morphological assessment and quantification. Given the striking rise in cellularity at the repair site and the relatively low number of fibroblasts noted on day 1, we postulate that the up-regulation of pro-inflammatory factors at this early timepoint was primarily due to the infiltration of immune cells (i.e., PMNs and monocytes/macrophages). Similarly, the initial down-regulation of tendon-specific and tendon ECM genes is likely due to a diluted fibroblast population. RNA extracted from normal/uninjured tendons was mainly from fibroblasts. These cells express SCX, TNMD, COL1, and COL3. In contrast, RNA extracted from the repaired tendons on day 1 was mainly derived from cells that do not express those genes. As the cell population shifted from immune cell-dominant to fibroblast-dominant, the expression of tendon-specific and tendon ECM-related factors returned to baseline levels. While the histological assessment of fibroblast percentage helped to interpret the gene expression data, immunohistochemical analyses would further elucidate the cellular source of the differential gene expression seen over time. Immunohistochemistry also would assist in verifying the relationship of the gene expression levels and subsequent changes in protein expression. However, the canine antibodies required to carry out these studies are not yet commercially available.
There were a number of limitations to the current study. First, additional timepoints are needed to more precisely determine temporal changes, as there appeared to be dramatic changes in cellular and gene expression patterns between days 3 and 9. Second, protein-level assessment is necessary to validate gene expression changes. However, protein expression assessment using techniques such as Western immunoblot analysis requires canine-specific antibodies, which have limited availability. Third, due to the use of digits from the contralateral (uncasted) paw for normal controls, we are unable to say with certainty that the effects seen in the injured and repaired digits were solely due to the injury and not due to post-operative immobilization. This is a minor concern, however, as the cast-immobilized limbs received twice-daily passive motion rehabilitation, as is done clinically. There is substantial evidence demonstrating that the optimal scenario for flexor tendon healing is low (but not zero) loading.23 Cast immobilization without passive motion rehabilitation or complete unloading of the repair site is detrimental to healing, but high loads can also lead to gapping or rupture and poor healing.23 Furthermore, the timepoints analyzed in the current study are relatively short, especially when considering the time frames within which negative immobilization effects are typically observed.24 The most remarkable effects in the current study were seen on day 1 and lessened with time, further supporting the conclusion that they were related to the injury and not to the immobilization.
The results of our study have implications for developing strategies to improve tendon repair outcomes. Previous in vitro studies have shown that pro-inflammatory factors, such as IL-1β and TNFα, induce tendon fibroblasts to up-regulate their own expression of inflammatory and catabolic enzymes25 and to down-regulate their expression of type 1 collagen26. At the tissue level, these changes may result in reductions in ultimate tensile strength and elastic modulus and increases in the maximum strain of repaired tendons. Gulotta et al. demonstrated that inhibition of TNFα during tendon healing improved the overall strength of tendon repair repair27. Similarly, De la Durantaye et al. found that macrophage depletion during tendon healing leads to enhanced material properties of the healed tendons.28 Dagher et al. showed a correlation between a shift in the macrophage population (from pro-inflammatory M1 macrophages to anti-inflammatory M2 macrophages) improved ligament healing29. Classical dermal wound healing literature suggests that neutrophils are not necessary for proper wound healing30 and more recent evidence suggests that neutrophils may impede skin healing31. Modulation of inflammation in a skin wound healing model resulted in an enhanced healing response characterized by accelerated wound healing and organized dermis and collagen bundles.32 Taken together, these studies suggest that high levels of pro-inflammatory cytokines may be detrimental to tendon healing and modulation of the early inflammatory phase of healing may be beneficial to tendon healing.
Despite evidence that suppressing inflammation can lead to improved wound healing, some pro-inflammatory cytokines are likely necessary for recruitment of immune cells to the site of injury and subsequent attraction of tendon fibroblasts.33,34 Synthesis of numerous potent growth factors, such as TGF-β and PDGF, by immune cells promotes cell proliferation and synthesis of extracellular matrix.33,34 Immune cells also play a pivotal role in angiogenesis through the secretion of VEGF.33,34 Several prior experiments have shown that complete depletion of certain immune cells and pro-inflammatory factors during wound healing leads to retarded wound repair35,36. Specifically, macrophages appear to play an important role in debridement of the wound37,38. Therefore, based on these studies, global IL-1β blockade or immune cell depletion is unlikely to be an effective strategy for improving tendon healing. Fine modulation of the inflammatory environment is likely necessary to enhance tendon healing. This could potentially be achieved using mesenchymal stem cells, which have recently been shown to modulate inflammation by controlling macrophages phenotype39,40, or targeted therapies that protect tendon fibroblasts from the detrimental effects of cytokines such as IL-1β.
ACKNOWLEDGMENTS
This study was funded by the National Institutes of Health (NIH R01 AR062947). Histological sections were prepared by the In Situ Molecular Analysis Core at the Washington University Musculoskeletal Research Center (NIH P30 AR057235).
REFERENCES
- 1.Boyer MI, Goldfarb C a, Gelberman RH. Recent Progress in Flexor Tendon Healing. Journal of Hand Therapy. 2005;18(2):80–85. doi: 10.1197/j.jht.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Boyer M, Gelberman R, Burns M, et al. Intrasynovial flexor tendon repair. An experimental study comparing low and high levels of in vivo force during rehabilitation in canines. The Journal of bone and joint surgery. 2001;83(A):891–899. [PubMed] [Google Scholar]
- 3.Kelsey J. Upper extremity disorders: frequency, impact and cost. Churchill Livingstone; New York, NY: 1997. [Google Scholar]
- 4.Khan U, Kakar S, Akali A, et al. Modulation of the formation of adhesions during the healing of injured tendons. Journal of Bone and Joint Surgery, British. 2000;82:1054–8. doi: 10.1302/0301-620x.82b7.9892. [DOI] [PubMed] [Google Scholar]
- 5.Zhao C, Amadio P, Paillard P, et al. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. The Journal of Bone and Joint Surgery. 2004;86(A):320–327. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Zhao C, Amadio PC, Paillard P, et al. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. The Journal of bone and joint surgery. American. 2004;86-A(2):320–7. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Thomopoulos S, Harwood FL, Silva MJ, et al. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. The Journal of hand surgery. 2005;30(3):441–7. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Thomopoulos S, Das R, Silva MJ, Harwood FL, Zampiakis E, Kim HM, Amiel D, GR Enhanced flexor tendon healing through controlled delivery of PDGF-BB. Journal of Orthopaedic Research. 2009;27(9):1209–1215. doi: 10.1002/jor.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomopoulos S, Zaegel M, Das R, et al. PDGF-BB Released in Tendon Repair Using a Novel Delivery System Promotes Cell Proliferation. Journal of Orthopaedic Research. 2007 Oct;25:1358–1368. doi: 10.1002/jor.20444. [DOI] [PubMed] [Google Scholar]
- 10.Lundborg G, Rank F. Experimental Intrinsic Healing of Flexor Tendons Based on Synovial Fluid Nutrition. Journal of Hand Surgery. 1978;3:21–31. doi: 10.1016/s0363-5023(78)80114-2. [DOI] [PubMed] [Google Scholar]
- 11.Lundborg G. Experimental Flexor Tendon Healing without Adhesion Formation - A New Concept of Tendon Nutrition and Intrinsic healing Mechanisms. A Preliminary Report.Hand. 1976;8:235–238. doi: 10.1016/0072-968x(76)90007-3. [DOI] [PubMed] [Google Scholar]
- 12.Fenwick S a, Hazleman BL, Riley GP. The vasculature and its role in the damaged and healing tendon. Arthritis research. 2002;4(4):252–60. doi: 10.1186/ar416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckwalter Ja. Articular cartilage: injuries and potential for healing. The Journal of orthopaedic and sports physical therapy. 1998;28(4):192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 14.Rai MF, Hashimoto S, Johnson EE, et al. Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis and rheumatism. 2012;64(7):2300–10. doi: 10.1002/art.34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gleberman RH, Vandeberg JS, Lundborg GN, Akeson WH. Flexor Tendon Healing and Restoration of the Gliding Surface. An Ultrastructural Study in Dogs. The Journal of Bone and Joint Surgery. 1983;65(1):70–80. [PubMed] [Google Scholar]
- 16.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, et al. The Early Effects of Sustained Platelet-Derived Growth Factor Administration on the Functional and Structural Properties of Repaired Intrasynovial Flexor Tendons: An In Vivo Biomechanic Study at 3 Weeks in Canines. Hand Surgery. 2007;32:373–379. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Gelberman R, Amifl D, Gonsalves M, et al. The influence of protected passive mobilizationon the healing of flexor tendons: a biochemical and microangiographic study. The Hand. 1981;13:120–128. doi: 10.1016/s0072-968x(81)80051-4. [DOI] [PubMed] [Google Scholar]
- 18.Gelberman R, JS V, Manske P, Akeson W. The Early Stages of Flexor Tendon Healing: A Morphologic Study of the First Fourteen Days. The Journal of Hand Surgery. 1985;10(A):776–784. doi: 10.1016/s0363-5023(85)80151-9. [DOI] [PubMed] [Google Scholar]
- 19.Potenza A. Tendon Healing Within the Flexor Digital Sheath in the Dog. The Journal of Bone and Joint Surgery. 1962;44-A(1):49–64. [PubMed] [Google Scholar]
- 20.Lindsay W, Birch J. The Fibroblast in Flexor Tendon Healing. Plastic and reconstructive surgery. 1964;34:223–232. doi: 10.1097/00006534-196409000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Peacock E. Biological Principles in the Healing of Long Tendons. Surgical Clinics of North America. 1965;45:461–476. doi: 10.1016/s0039-6109(16)37543-0. [DOI] [PubMed] [Google Scholar]
- 22.Berglund M, Hart D, Wiig M. The inflammatory response and hyaluronan synthases in the rabbit flexor tendon and tendon sheath following injury. Journal of hand surgery (European Volume) 2007;32:581–587. doi: 10.1016/J.JHSE.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Silva MJ, Brodt MD, Boyer MI, et al. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. Journal of Orthopaedic Research. 1999;17:777–783. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- 24.Hettrich C, Gasinu S, Beamer B, et al. The Effect of Immobilization on the Native and Repaired Tendon-to-Bone Interface. Journal of Bone and Joint Surgery. 2013;95:925–930. doi: 10.2106/JBJS.K.01329. [DOI] [PubMed] [Google Scholar]
- 25.Tsuzaki M, Guyton G, Garrett W, et al. IL-1beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1beta and IL-6 in human tendon cells. Journal of Orthopaedic Research. 2003;21:256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 26.Thampatty B, Li H, IM H, Wang J. EP4 receptor regulates collagen type-I, MMP-1, and MMP-3 gene expression in human tendon fibroblasts in response to IL-1beta treatment. Gene. 2007;386:154–161. doi: 10.1016/j.gene.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulotta LV, Kovacevic D, Cordasco F, Rodeo SA. Evaluation of tumor necrosis factor α blockade on early tendon-to-bone healing in a rat rotator cuff repair model. Arthroscopy: the journal of arthroscopic & related surgery. 2011;27(10):1351–7. doi: 10.1016/j.arthro.2011.03.076. [DOI] [PubMed] [Google Scholar]
- 28.De la Durantaye M, Piette AB, van Rooijen N, Frenette J. Macrophage depletion reduces cell proliferation and extracellular matrix accumulation but increases the ultimate tensile strength of injured Achilles tendons. Journal of Orthopaedic Research:EPUB ahead of print. 2013 doi: 10.1002/jor.22504. [DOI] [PubMed] [Google Scholar]
- 29.Dagher E, Hays PL, Kawamura S, et al. Immobilization modulates macrophage accumulation in tendon-bone healing. Clinical orthopaedics and related research. 2009;467(1):281–7. doi: 10.1007/s11999-008-0512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peacock E, Van Winkle W. Wound Healing. 2nd ed. W.B. Saunders Company; 1976. [Google Scholar]
- 31.Dovi JV, He L, Dipietro LA. Accelerated wound closure in neutrophil-depleted mice. 2002 doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 32.Morin C, Roumegous A, Carpentier G, et al. Modulation of inflammation by Cicaderma ointment accelerates skin wound healing. The Journal of pharmacology and experimental therapeutics. 2012;343(1):115–24. doi: 10.1124/jpet.111.188599. [DOI] [PubMed] [Google Scholar]
- 33.Williamson D, Harding K. Wound healing. Medicine. 2004;32(12):4–7. [Google Scholar]
- 34.Enoch S, Leaper DJ. The basic science of wound healing. Surgery. 2007;26(2):31–37. [Google Scholar]
- 35.Mirza R, Dipietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. The American journal of pathology. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin TW, Cardenas L, Glaser DL, Soslowsky LJ. Tendon healing in interleukin-4 and interleukin-6 knockout mice. Journal of biomechanics. 2006;39(1):61–9. doi: 10.1016/j.jbiomech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. The American journal of pathology. 1975;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- 38.Leibovich SJ, Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. The American journal of pathology. 1976;84(3):501–14. [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson WM, Nesti LJ, Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem cell research & therapy. 2012;3(3):20. doi: 10.1186/scrt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ylöstalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem cells. 2012;30(10):2283–96. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]