Abstract
The mechanisms by which MUC1 and p120 catenin contribute to progression of cancers from early transformation to metastasis are poorly understood. Here we show that p120 catenin ARM domains 1, 3–5 and 8 mediate interactions between p120 catenin and MUC1, and that these interactions modulate dynamic properties of cell adhesion, motility and metastasis of pancreatic cancer cells. We also show that different isoforms of p120 catenin when co-expressed with MUC1 create cells that exhibit distinct patterns of motility in culture (motility independent of cell adhesion, motility within a monolayer while exchanging contacts with other cells, and unified motility while maintaining static epithelial contacts) and patterns of metastasis. The results provide new insight into the dynamic interplay between cell adhesion and motility and the relationship of these to the metastatic process.
Keywords: p120 catenin, MUC1, E-cadherin, cell motility, pancreatic cancer metastasis
Introduction
Human MUC1, a type I transmembrane glycoprotein expressed at the apical surface of normal glandular epithelial cells (1, 2), is overexpressed and aberrantly glycosylated by most adeoncarcinomas (3–6). The MUC1 precursor undergoes an autoproteolytic cleavage (7), resulting in two associated subunits: a large extracellular domain; and a smaller membrane-associated domain that includes a short juxtamembrane region of the extracellular domain, a transmembrane domain, and a cytoplasmic tail (8, 9). The extracellular domain is extensively glycosylated by mucin-type O-linked oligosaccharides (10) and can protrude 200–500 nm above the cell membrane, providing lubrication of luminal spaces, protection against microbial infection, and general control of the biochemical environment at the cell surface (11). This structure also mediates cell-cell and cell-extracellular matrix (ECM) interactions by binding to or competing with other adhesion molecules (12–15). Overexpression of MUC1 promotes tumor cell invasion and metastasis (15, 16).
The carboxyl terminal cytoplasmic tail of MUC1 (MUC1 CT) is highly conserved among different species and contains several sites that are differentially phosphorylated by receptor tyrosine kinases and other kinases (17) leading to oncogenic effects (10, 18–20) including morphogenetic signaling through β-catenin and the Wnt signaling pathway (18, 21) (22). MUC1 has also been reported to interact with and affect nuclear localization of p120 catenin (23). However, the significance of interactions of MUC1 CT and p120 catenin remain poorly understood.
P120 catenin contributes to cadherin-mediated cell adhesion and actin cytoskeletal organization (24) by binding to the juxtamembrane domain of the cadherin cytoplasmic tail, separated from the conserved catenin-binding domain to which β-catenin binds (25). P120 catenin stabilizes E-cadherin at the cell surface by facilitating E-cadherin trafficking to the cell surface, protecting E-cadherin from endocytic degradation and regulating E-cadherin recycling (26–30).
P120 catenin has a central domain consisting of 10 ARM repeats, flanked by N-terminal coiled-coil and regulatory domains, and a C-terminal tail of unknown function (24). There are four isoforms that differ in amino termini due to alternative splicing and usage of alternate translation initiating codons, and each has a distinct start codon in the N-terminal region (24, 31–33). Isoform 1 has a coiled-coil domain at the N-terminus and is mostly expressed in highly motile cells (macrophages, fibroblasts); isoform 2 contains the entire “regulatory region”; isoform 3 has most of regulatory region and is preferentially expressed by epithelial cells; isoform 4 lacks the majority of the regulatory region and is rarely detected in normal tissues.
Here we report that p120 catenin is not detected in pancreatic cancer cell line S2-013, and that re-expression of p120 catenin in these cells stabilized and enhanced E-cadherin and β-catenin expression. Expression of different p120 catenin isoforms with and without MUC1 induced distinct morphologies, cell adhesion, and dynamic properties of motility along with different metastatic properties in vivo. The results demonstrate that MUC1 and p120 catenin play a coordinated role in regulating cell adhesion, motility and metastasis in pancreatic cancer.
Materials and Methods
Detailed materials and methods are reported in the supplementary information. All materials and methods employed previously reported reagents and techniques.
Results
Endogenous p120 expression in several pancreatic cell lines
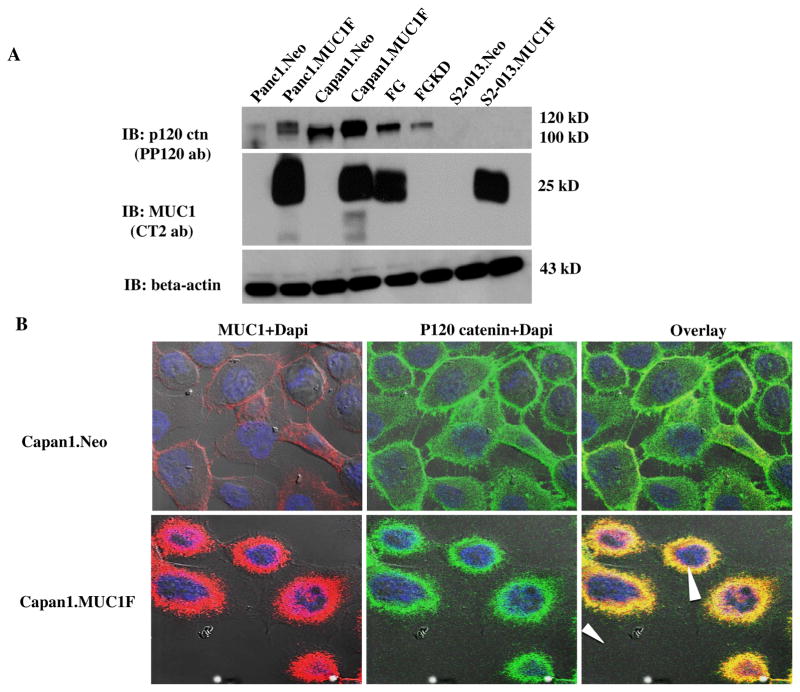
We evaluated the effect of MUC1 expression on the endogenous steady state expression of p120 catenin in several pancreatic cancer cell lines including Panc1, Capan1, FG, S2-013. Whole cell protein lysates were evaluated by western blotting (Fig. 1A). There was increased p120 catenin expression when MUC1 was highly expressed in the Panc1 and Capan1 cell lines (to levels that are consistent with that observed in primary cancers) and decreased p120 catenin levels upon knockdown of MUC1 in FG cells. No forms of p120 catenin were detected in S2-013 cells with an antibody to the carboxyl-terminus.
Fig. 1. Expression of P120 catenin is stabilized by high level expression of MUC1 in several pancreatic cancer cell lines.
(A) Total cell lysates from control (neo), MUC1 overexpressing (MUC1) or MUC1 knockdown (KD) pancreatic cancer cell lines Panc1, Capan1, FG and S2-013 were western blotted for MUC1, p120 catenin and beta actin. (B) Immunofluorescence analysis of expression of MUC1 and p120 catenin in Capan1 cells expressing MUC1 as compared to control Capan1.Neo cells. The red color indicates MUC1 and the green stains for p120 catenin. Yellow indicates the co-localization signal when the images are overlayed. Blue indicates DAPI staining and the arrowhead indicates co-localization in the nucleus.
Association of MUC1 and endogenous P120 catenin in pancreatic cancer cells
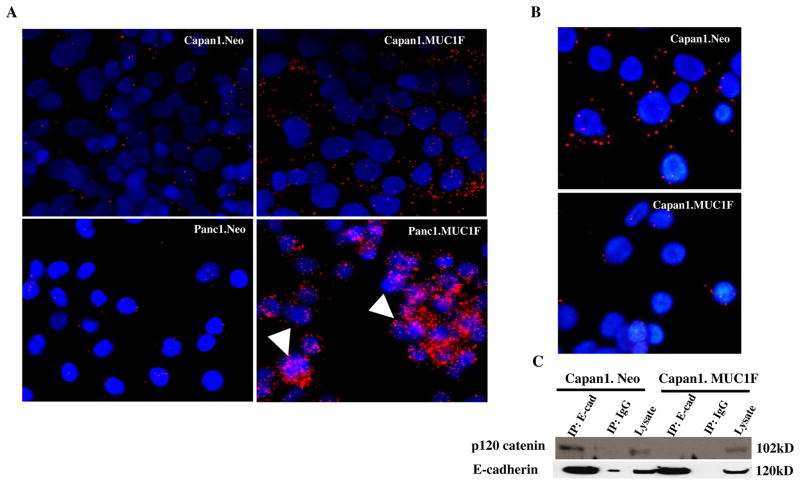
We evaluated the potential of interaction between MUC1 with p120 catenin by confocal immunofluorescence microscopy analysis of these two proteins in Capan1 cells (Fig. 1B). Antibodies against the C-terminal cytoplasmic tail of MUC1 were co-localized with antibodies to p120 catenin in both cytoplasm and nucleus. We confirmed and quantified interactions between p120 catenin and MUC1 by proximity ligation assays (Fig. 2A). The results showed that high levels of MUC1 increased the interactions between p120 catenin and MUC1. We also detected interactions between p120 catenin and MUC1 in both the cytoplasm and nucleus of Panc1 cells (Fig. 2A).
Fig. 2. MUC1 expression increases interactions between p120 catenin and MUC1 but decreases p120 catenin association with E-cadherin.
(A) Association of p120 catenin and MUC1 was evaluated by proximity ligation assay in pancreatic cell lines. Capan1.Neo vs Capan1.MUC1F; Panc1.Neo vs Panc1.MUC1F. Red dots indicate positions of interaction between p120 catenin and MUC1. Blue color (DAPI stain) indicates nuclei. The arrow indicates p120 catenin and MUC1 interactions in the nucleus. The images are single confocal planes, and additional interactions were observed in additional z-stacks. (B) Proximity Ligation assay was used to detect interactions between p120 catenin and E-cadherin in control (neo) and MUC1 overexpressing (MUC1) Capan1 cells. The red dots indicate interactions between p120 catenin and E-cadherin. Blue (DAPI) staining indicates nuclei. (C) Lysates of control and MUC1 overexpressing Capan1 cells were immunoprecipitated (IP) with an antibody to E-cadherin and blotted for p120 catenin and E cadherin. Loading controls for total lysates are also shown.
Interactions of MUC1 and p120 catenin decrease p120 catenin association with E-cadherin
Expression of MUC1 resulted in increased steady state levels of p120 catenin and increased interaction between P120 catenin and the C-terminal cytoplasmic tail of MUC1 (MUC1 CT). Given that p120 catenin has been shown to interact with E-cadherin through ARM domains 1–5 and 7 (34), we sought to investigate the possibility that MUC1 competes with E-cadherin for binding to p120 catenin. We performed proximity ligation assays (Fig. 2B) and co-immunoprecipitations (Fig. 2C) in Capan1 cells to determine whether MUC1 expression decreased the association of p120 catenin with E-cadherin. The results show that high levels of MUC1 decreased the relative number of detectable p120 catenin/E-cad complexes. Decreased association of p120 catenin and E-cadherin is predicted to impact adhesion junctions and increase potential EMT activity by epithelial cells.
Stable expression of mp120 constructs in S2-013 under conditions of high MUC1 expression
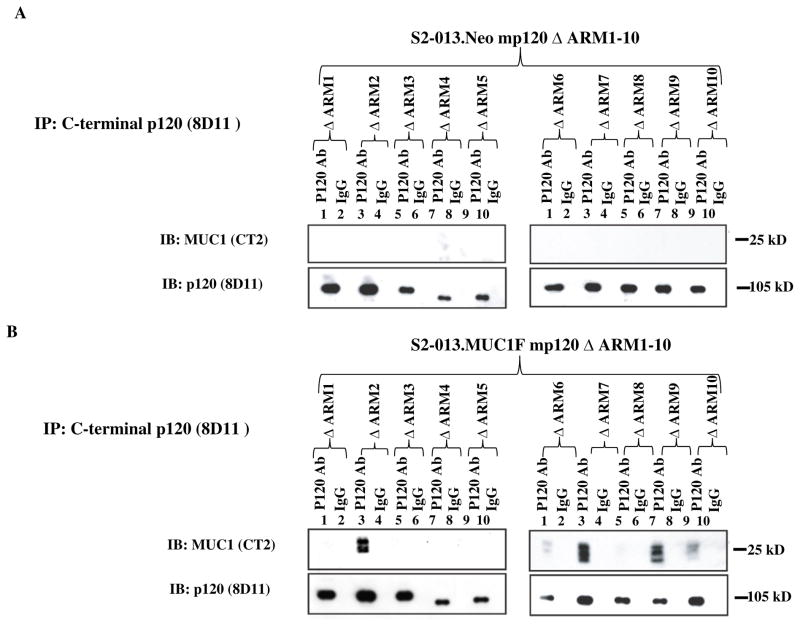
P120 catenin was not detected by a p120 antibody specific for the C-terminus in S2-013 cells. Given that S2-013 cells do not express high levels of endogenous MUC1, we concluded that this cell line would represent a unique model system to map the domains of interaction between MUC1 and p120 catenin and to determine the biological effects of re-expression of p120 catenin in the context of low expression and high expression of MUC1. We therefore expressed murine p120 constructs (mp120) (Figure S. 1) encoding full length isoform 1A (1A-WT), isoform 3A (3A-WT), N-terminal deleted isoform 4A (4A-WT), and a C-terminal deleted construct (1AΔC1) in MUC1 expressing S2-013.MUC1F (35) and control S2-013.Neo (35) cell lines by transduction with LZRS retroviral particles encoding the p120 ctn constructs. We sought to identify the critical p120 sequences that mediate interactions with MUC1. Expression of the constructs were verified by western blotting using mAb 8D11, which recognizes the C-terminus of mp120 (mp120 3A-WT and 4A-WT); and 6H11, which recognizes the 1A isoform (mp120 1AΔC1) (Fig. 3A). We also expressed mp120 constructs in which each of 10 different ARM repeat domains were deleted (ΔARM1-10) in S2-013.MUC1F and S2-013.Neo cell lines. These constructs were derived from the 3A-WT isoform. Expression was confirmed by western blotting using mAb 8D11.
Fig. 3. The amino and carboxyl termini of p120 catenin are dispensible for interaction with MUC1.
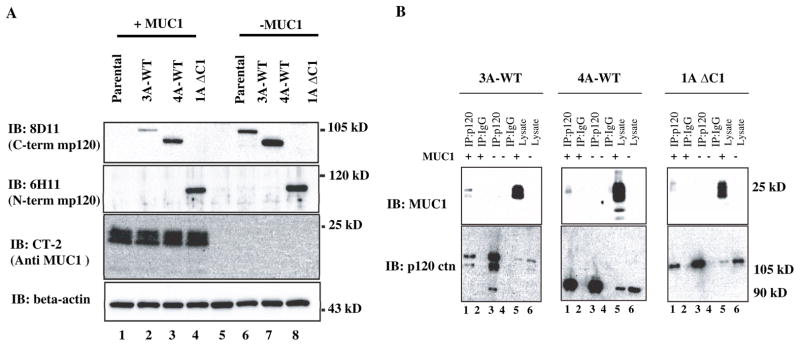
(A) Expression of 3A-WT, 4A-WT and C-terminal deleted (1A-ΔC1) mp120 in S2-013.MUC1F and S2-013.Neo. Equal amounts of protein lysates from control S2-013 cells and stable derivative clones expressing the indicated recombinant forms of murine p120 catenin were western blotted (IB) with antibodies to the carboxyl terminus of mp120 (8D11), the amino terminus of mp120 catenin (6H11), the carboxyl terminus of MUC1 (CT-2) and beta actin. (B) Lysates from control (neo) and MUC1 overexpressing (MUC1) S2-013 cells that expressed type 3 mp120 (3A-WT); N-terminal deleted type 4 mp120 (4A-WT) or C-terminal deleted type 1 mp120 (1AΔC1) were immunoprecipitated with an antibody to mp120 catenin (or IgG control) and western blotted for MUC1 or mp120 catenin.
N-terminal and C-terminal domains are dispensable for association between MUC1 and p120 catenin
To determine whether the N-terminal and C-terminal domains of p120 catenin are required for association with MUC1, we immunoprecipitated complexes from cell lysates containing the different constructs, separated the complexes on SDS-PAGE, and probed western blots for full-length, N-terminal deleted, C-terminal deleted mp120 and MUC1. We used mAb 8D11 that specifically recognizes the C-terminus of mp120 to immunoprecipitate full-length and N-terminal deleted mp120, and mAb 6H11 that recognizes the N-terminus of the 1A isoform to immunoprecipitate the C-terminal deleted mp120 1AΔC1. The results showed that all three mp120 constructs interacted with MUC1 (Fig. 3B). Therefore, we concluded that the interaction between p120 and MUC1 does not require the N-terminal regulatory domain or the C-terminal domain of p120.
Association between MUC1 and mp120 by its ARM domains
We next investigated the role of specific ARM domains in the association of MUC1 and p120 catenin. Cell lysates of S2-013.MUC1F and S2-013.Neo expressing 10 different deletion constructs (mp120 ΔARMs 1–10) were immunoprecipitated with mAb 8D11 that specifically recognizes the C-terminus of mp120 (Fig. 4), followed by western blotting with mAb CT-2 against MUC1 CT. The results revealed that mp120 ΔARM2, 6–7 and 9–10 associated with MUC1. The associations of ΔARM2, 7 and 9 with MUC1 were strong as significant amounts of protein were co-immunoprecipitated, and the associations of ΔARM6 and 10 were detected at lower levels, given that less MUC1 protein was co-immunoprecipitated with 8D11. ΔARM1, 3–5 and 8 did not co-IP MUC1. The results suggest that ARM1, 3–5 and 8 were required for association and ARM6 and 10 may contribute to MUC1 binding, but ARM2, 7 and 9 were dispensable for association between MUC1 and p120 catenin.
Fig. 4. Interaction between MUC1 and mp120 ARM domain deletion constructs.
Lysates of (A) control S2-013.Neo and (B) MUC1 overexpressing S2-013.MUC1F that expressed mp120 with the indicated ARM domain deleted (ΔARM1-10) were immunoprecipitated with mAb 8D11 or mouse IgG control. Immunoprecipitates were split into two equal fractions: one was resolved on an 8% Tris-Glycine gel to detect mp120 by western blotting, and one was resolved on a 14% Tris-Glycine gel to detect MUC1 CT by western blotting.
Reexpressing p120 catenin stabilizes E-cadherin and alters morphology in S2-013 cells, and overexpression of MUC1 stabilizes beta catenin in the context of p120 catenin expression
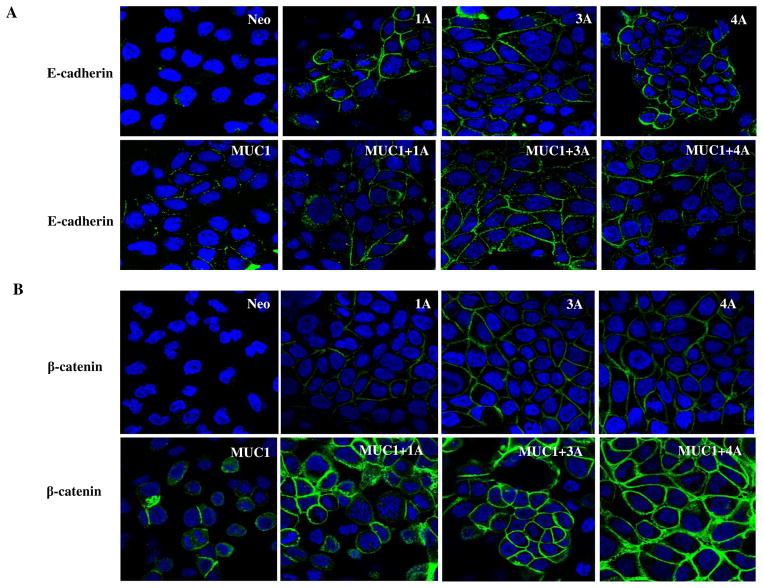
We elected to evaluate the effects of re-expressing the different amino terminal variant isoforms of p120 catenin in the context of low and high expression of MUC1 using the S2-013 cells, which are low in MUC1 expression and deficient in p120 catenin. We observed that E-cadherin and β-catenin expression were extremely low in the S2-013 cell line that lacked p120 catenin (Fig. 5A, Fig. 5B, and Figure S. 2). It had not been previously determined if the low levels of β-catenin and E-cadherin are directly linked to the deficiency of p120 catenin in this cell line. Re-expression of each p120 isoform in S2-013 cells enhanced surface E-cadherin expression in the context of low levels of MUC1, and overexpression of MUC1 did not affect surface E-cadherin levels in any of these cells (Fig. 5A). This suggests that overexpression of MUC1 alone does not induce turnover of E-cadherin, even though it may decrease the association of p120 catenin with E-cadherin (Fig. 2B, C). Interestingly, overexpression of MUC1 alone enhanced slightly the levels of β-catenin that were observed at cell junctions (Fig. 5B); however, there was a dramatic increase in levels of junctional beta catenin in cells in which MUC1 and any of the P120 catenin isoforms (Fig. 5B) were co-expressed. We previously reported that MUC1 stabilizes beta catenin (22), and our results here show that this stabilization is enhanced at the cell surface junctions by the presence of any p120 catenin isoform. This implies that there is a MUC1-p120 catenin axis at the cell surface that is capable of modulating Wnt signaling by stabilizing beta catenin under conditions of high level expression of MUC1.
Fig. 5. Immunofluorescence of E-cadherin and β-catenin expression in control (neo) and MUC1 expressing (MUC1) S2-013 cells with and without re-expression of the indicated isoforms of p120 catenin.
(A) E-cadherin is not stabilized in the absence of p120 catenin in S2-013.Neo cells. Re-expression of p120 isoforms 1A, 3A, 4A in S2-013 cells restored E-cadherin expression. Green indicates E-cadherin staining, Blue are nuclei. (B) β-catenin expression was extremely low in control S2-013 cells that lacked p120 catenin. Expression of MUC1 alone or re-expression of p120 catenin isoforms enhanced slightly the levels of β-catenin at cell junctions. There was a dramatic increase in levels of junctional β-catenin in cells expressing MUC1 and any of the p120 catenin isoforms. β-catenin staining is shown as green and nuclei are blue.
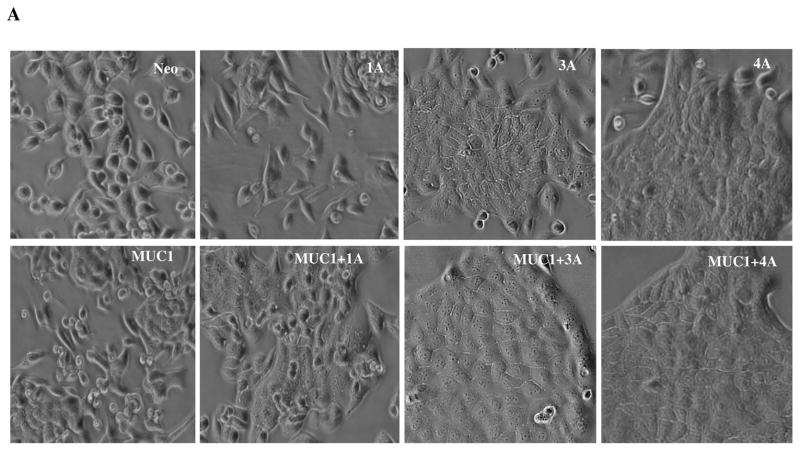
Different p120 catenin isoforms had a dramatic effect on the morphology of cells in culture. Parental and control vector transfected S2-013 cells exhibit a moderately differentiated morphology with cells showing a moderate mesenchymal morphology that lacks tight cellular junctions (Fig. 6A). Re-expression of p120 catenin 1A enhanced the mesenchymal phenotype with cells exhibiting elongated shapes. Re-expression of p120 catenin 3A and 4A induced epithelial changes in cell morphology. High expression of MUC1 induced epithelial changes in S2-013, and MUC1 also enhanced the epithelial appearance of cultures in the context of expression of all three isoforms of p120 catenin.
Fig. 6. Expression of different p120 catenin isoforms affects epithelial morphology and cell motility of S2-013 cells.
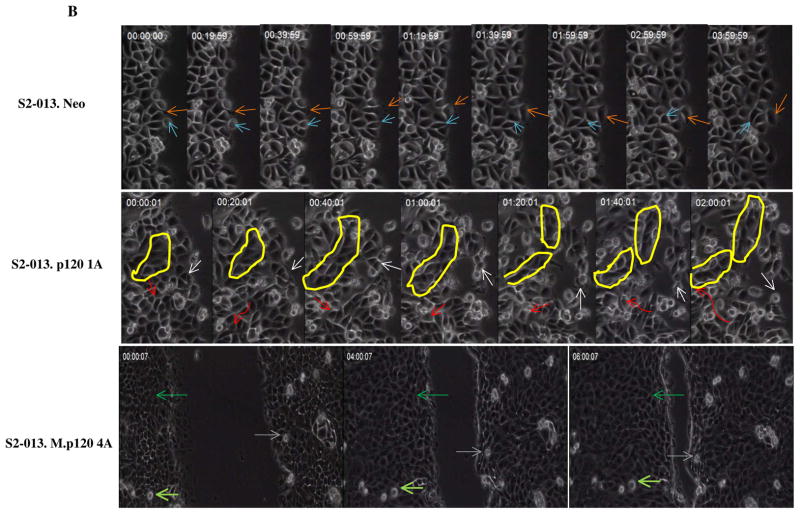
(A) Phase contrast photographs of control (neo) S2-013 cells and MUC1-expressing S2-013 cells with re-exepression of different p120 catenin isoforms. (B) Time lapse microscopy images showing three different types of cell motility that were produced upon expression of different p120 catenin isoforms (elapsed time indicated in each panel and arrows indicate position of individual cells in the monolayer at the different time points): control cells S2-013 neo showed weak and pliable cell-cell contacts that are maintained as a monolayer; dynamic changes of cell motility were seen in the S2-013 cells with p120 catenin 1A expression, which showed a high degree of motility in limited space but generally remained associated with other cells in the monolayer by highly pliable and exchangeable contacts; there was a high rate of organized and unified growth and motion in the direction of wound closure by S2-013 cells expressing MUC1 and p120 catenin 4A. Yellow circle shows dynamic features of movement of a group of cells. Red arrow indicates a cell migrating underneath the neighboring cells with greatly enhanced motility. Scale, 10 μm.
Different isoforms of p120 catenin induce distinct patterns of cell motility that are further modified by overexpression of MUC1
Given the importance of beta-catenin, p120 catenin and E-cadherin in cell adhesion, we predicted that the effects of re-expression of p120 catenin isoforms on cell morphology and adhesion would also affect cell motility. We examined these parameters by video microscopy of cells undergoing an in vitro wound healing assays, in which confluent monolayers were scratched and cell behavior in the monolayer was observed during closure of the wound.
Results from time-lapse videos showed that control S2-013 neo cells act independently in monolayer cultures and exhibit weak and pliable cell-cell contacts that are maintained as a monolayer (Movie S 1). It was notable that for control S2-013 cells, single cells seldom entered the wound area (only at later stages when the advancing fronts were proximal) and instead that the wound was filled by mass action of cells that were advancing in contact with each other and exhibiting low levels of localized random motion. Expression of MUC1 without p120 catenin in S2-013 cells created cells with enhanced cell motility in a localized manner within the monolayer and, of note, also produced a number of cells that migrated into the wounded area alone or in small groups without maintaining contacts to the monolayer. MUC1 expression enhanced the overall rate of wound closure compared to control cells (Movie S 5).
Strikingly, re-expression of p120 catenin isoform 1A in S2-013 cells induced a highly spindle shaped morphology (Fig. 6A) and dramatically increased cell motility within the monolayer (Movie S 2 and Fig. 6B): most cells exhibited a high degree of motility in limited space but generally remained associated with other cells in the monolayer by highly pliable and exchangeable contacts. There were occasional cells that explored free space in the wound area. Expression of MUC1 in the context of p120 catenin 1A yielded cells with high local motility in the monolayer (but slightly reduced as compared to p120 catenin 1A alone) and a high propensity to enter the wounded area as single cells or small groups of cells (Movie S 6). There was a subtle increase in the epithelial character of cells expressing MUC1 and a slight but statistically insignificant decrease in rate of wound closure.
Re-expression of p120 catenin 3A in the S2-013 cells induced moderate epithelial-like changes in cell morphology (Fig. 6A) with modest increases in localized cell motility compared to control cells (but lower than p120 catenin 1A cells) and pliable cell contacts that exchanged with other cells at a rate that was lower than that seen with p120 catenin 1A (Movie S 3). There were projections of groups of cells that advanced to cover the wounded area. Expression of MUC1 in the context of p120 catenin 3A (Movie S 7) produced a slightly more epithelial morphology in the cells and slightly decreased the rate of closure of the wound as compared to p120 catenin 3A cells.
Re-expression of p120 catenin 4A produced a pronounced epithelial morphology of the cells, which also maintained a relatively high degree of localized cell motility and pliable cell contacts with adjacent cells. These cells closed the wound quickly but did not produce a large number of cells that explored the wounded area in the absence of other cellular contacts (Movie S 4). Remarkably, expression of MUC1 and p120 catenin 4A produced cells that were highly epithelial and organized in appearance, with much lower levels of local motion within the monolayer but a high rate of organized and unified growth and motion in the direction of wound closure (Fig. 6B and Movie S 8).
Overall, our analysis of cell behavior in wound healing assays by video microscopy revealed that expression of different isoforms of p120 catenin alone and in the context of high level expression of MUC1 created dramatically different cellular behaviors that are not seen by analysis of static photomicrographs and are not revealed by biochemical analysis of the status of associated proteins. The results demonstrate that different isoforms of these two proteins dramatically affect cell morphology and motility independently when expressed alone, or in a coordinated manner when co-expressed. The results have important implications for our conceptualization of the relationship between cell adhesion, cell motility, and the process of epithelial to mesenchymal transition (EMT) or mesenchymal to epithelial transition (MET).
Different isoforms of p120 catenin in the context of expression of MUC1 induce distinct patterns of tumor growth and metastasis
The results described above present two-dimensional growth of monolayer cultures. Thus, we sought to further examine the influence of these two molecules on tumor growth and metastasis in the context of pancreatic microenvironment by examining growth of orthotopic implants into athymic mice. S2-013 cells (2.5 × 105) re-expressing different p120 catenin isoforms with and without expression of MUC1 were implanted into the pancreas of nude mice and allowed to grow for 30 days, at which time the animals were sacrificed and tumors were analyzed for morphology, growth and metastatic properties.
Unmanipulated S2-013 tumors produce a locally advanced tumor in the pancreas and, as previously reported (36), show metastasis to lymph nodes (Table S1). High level expression of MUC1 or re-expression of any single isoform of p120 catenin affected primary tumor volume (Fig. 7A) and metastatic processes. MUC1 enhanced lymph node metastasis (data not shown) (36). We observed that re-expression of p120 catenin 1A produced tumors that showed greater rates of localized invasion to the spleen (Table S 1) and that p120 catenin 4A produced greater rates of invasion and colonization to the walls of the peritoneal cavity, along with invasion into the muscle and connective tissue of that area. Expression of MUC1 and re-expression of p120 catenin 1A and 4A produced significantly larger tumors in the pancreas (Fig. 7A) that showed even greater rates of local invasion into the peritoneal cavity and colonization of organs surrounding the pancreas. Remarkably, expression of p120 catenin 3A alone did not affect the size of the primary tumor or the metastatic process; however, re-expression of p120 catenin 3A in the context of MUC1 expression produced a phenotype we have not previously observed in this experimental system: large metastatic lesions to the liver, which exceeded the volume of the primary tumor (Fig. 7B).
Fig. 7. Orthotopic implantation of tumor cells in athymic nude mice.
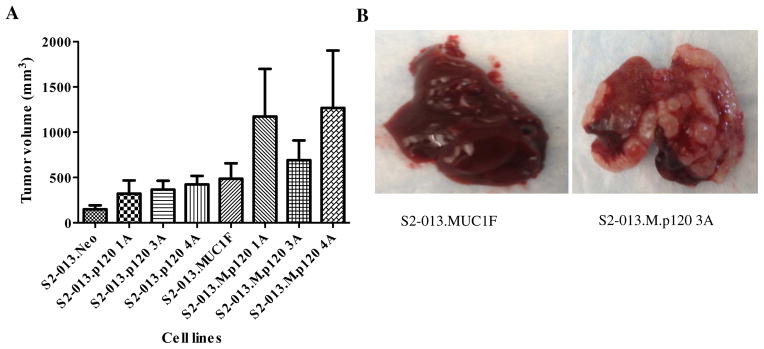
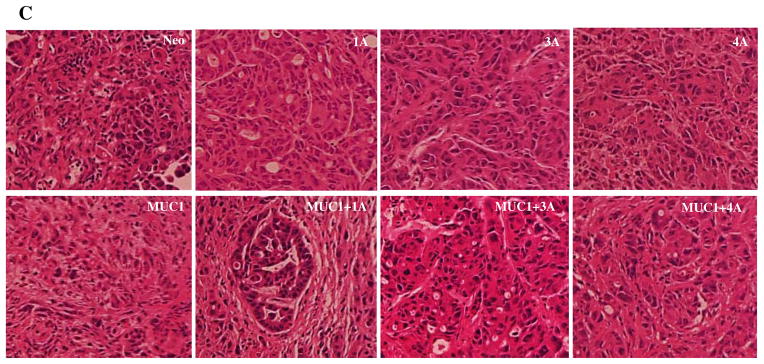
(A) Tumor volume following orthotopic implantation of S2-013 cell lines re-expressing different p120 catenin isoforms with and without overexpression of MUC1. S2-013 cells (2.5×105) re-expressing the indicated different p120 catenin isoforms with and without overexpression of MUC1 were injected into the pancreas of nude mice and allowed to grow for 30 days, at which time the animals were sacrificed and tumor volumes are quantified. (B) Orthotopic implantation of S2-013 cells with reexpression of p120 ctn 3A in the context of MUC1 overexpression exhibit liver metastasis. (C) H&E staining of formalin-fixed paraffin-embedded sections of tumors from orthotopically implanted S2-013 cells with and without expression of MUC1 and different p120 catenin isoforms.
Histological analysis of the primary tumors confirmed that orthotopic control S2-013 cells grow as a moderately differentiated carcinoma with substantial mesenchymal features and a disorganized stromal microenvironment. Expression of MUC1 does not significantly alter the morphology of S2-013 tumors (Fig. 7C); However re-expression of p120 catenin isoforms with and without expression of MUC1 affected the histological appearance of the tumors. Re-expression of p120 1A alone created tumors with a more epithelial appearance, and in the context of MUC1 expression created well differentiated tumors that showed evidence of tumor glandular formation and a highly organized stromal microenvironment. Re-expression of p120 catenin 3A or 4A produced tumors that were more epithelial in appearance than parental S2-013 cells, and expression of MUC1 did not dramatically affect the morphological appearance of these tumors (Fig. 7C).
Discussion
MUC1 and p120 catenin have been implicated as contributing to progression of cancers from early transformation to metastasis. P120 catenin stabilizes cadherins at the surface of epithelial cells, and paradoxically, isoforms that contain different amino termini are known to influence cell migration through interactions with RhoA, Rac and Cdc42 (37–39). As such, p120 catenin is hypothesized to influence the establishment of a polarized epithelial state and transition to a motile mesenchymal state and vice versa. Conditional deletion of p120 catenin in the oral cavity, esophagus and squamous forestomach of mice produced dysplasia, hyperplasia and invasive squamous cell cancers with accompanying desmoplasia and immune cell proliferation (40). Tumor suppressor activity for p120 catenin in this context was ascribed to the finding that tumor cells lacking p120 catenin activated NFkapppaB, Akt and Stat-3, which in turn produced a pro-inflammatory environment that drove tumor progression. The manner by which loss of p120 catenin upregulated these proinflammatory and protumorigenic pathways has not been defined. It was suggested that re-expression of native p120 isoforms in tumors deficient in these may be a way to ameliorate tumor progression (40). MUC1 is known to influence and configure the cell surface properties of cells and to engage in morphogenetic signaling in a manner that influences programs of transcription related to epithelial cell biology. MUC1 is overexpressed during tumor progression leading to oncogenic properties that are ascribed to its influence on cell surface properties of cells and to its capacity to directly conduct signals that influence transcriptional programs in the cell, thereby modulating survival, proliferation, differentiation status, and oncogenesis (4). It was previously documented that MUC1 and p120 catenin associate, but the nature of this association and their coordinated influence on the biological properties of normal and transformed cells is poorly understood (23).
Here we extend the initial report that MUC1 interacts with and stabilizes steady state cytoplasmic and nuclear levels of p120 catenin (23) by documenting the domains of interactions that contribute to this association and revealing significant biological consequences of these interactions. Our results show that MUC1 interacts with p120 catenin in several pancreatic cancer cell lines including S2-013, Panc-1, Capan-1 and that overexpressing MUC1 increased steady state p120 protein levels in Panc-1 cells and Capan-1 cells. We show that p120 catenin protein is not expressed by the S2-013 pancreatic tumor cell line, which was used to map domains of interaction between p120 catenin and MUC1, and to investigate the functional consequences of re-expressing p120 catenin in the context of low levels and high levels of expression of MUC1.
S2-013 is the second epithelial tumor cell line reported to date to not express functional p120 catenin. Daniel and Reynolds previously established that the SW48 cell line does not express p120 catenin and used this cell line to map interactions between p120 and E-cadherin. These investigators found that p120 bound to E-cadherin by ARM domains 1–5 and 7 (41, 42), and to Kaiso by ARM domains 1–7 (42). The N and C termini of p120 were not required for association with either E-cadherin or Kaiso, and we show here that the N and C termini of p120 catenin are dispensable for association with MUC1. Results with different ARM domain deletion constructs revealed that ΔARM 2, 7 and 9 interacted strongly with MUC1, whereas there were weaker interaction with ΔARM 6 and 10, suggesting that ARM 2, 7, 9 are not required for binding between p120 and MUC1, and that ARM 6 and 10 are not required but may contribute to the interaction. The interactions between p120 and MUC1 require ARM 1, 3–5 and 8.
A current concept is that E-cadherin is frequently lost in human tumors through different mechanisms at the transcriptional level and translational level (43, 44), and that this loss is associated with epithelial to mesenchymal transition, in which epithelial cells with established cellular polarity undergo a morphological transformation into cells with a mesynchymal shape in order to engage in a program of cell motility that impacts cell migration and metastasis. Previous results suggest that increased E-cadherin expression does not result from effects of p120 catenin on E-cadherin transcription (34) and that p120 catenin stabilizes E-cadherin through protein interactions at cell-cell junctions. MUC1 is not normally expressed at the adherens junction where p120 binds to E-cadherin. There is often loss of apical-basal cellular polarity in tumor cells, which enables the formation of novel complexes between MUC1 and cell surface proteins that are normally expressed at spatially distinct sites (basolateral) in the cell (45). One possibility is local competition for binding to p120 between MUC1 and E-cadherin, which may promote E-cadherin recycling or degradation, and cause a loss of cell-cell adhesion properties that accompanies epithelial – mesenchymal transition (46). Our results support the hypothesis that increased MUC1 expression leads to increased interactions between p120 catenin and MUC1 and decreased binding of p120 catenin to E-cadherin. This is consistent with published data showing that primary human bronchial epithelial (HBE) cells treated with smoke-concentrated medium (Smk) displayed increased interactions between P120 catenin and the cytoplasmic tail of MUC1 (MUC1 CT) and decreased P120 catenin/E-cad/complexes (47). Decreased p120 catenin stabilization of E-cadherin at the cell surface is predicted to disrupt adhesion junctions. Importantly, we did not observe decreases in E-cadherin levels at the cell surface under conditions of MUC1 expression, but instead observed stabilization of E-cadherin and beta catenin. Furthermore, we observed significant stabilization of beta catenin at the cell surface when MUC1 was expressed together with any of three different isoforms of p120 catenin, which also enhanced the epithelial morphology of these cell types (following fixation and viewing as static photomicrographs).
The static pictures showing epithelial cell morphology and our static analysis of molecular associations between MUC1, p120 catenin and E cadherin do not tell the whole story. Analysis of video microscopy of these cells together with observations of tumor growth properties in vivo provide additional insight into the overall influence of these molecules on cell morphology, motion, motility, tumor growth and metastasis.
Control S2-013 cells deficient in p120 catenin isoforms and MUC1 exhibit a mesenchymal phenotype that when grown as a xenograft presents as a moderate to poorly differentiated carcinoma. Video microscopy of these cells in wound healing assays reveal a monolayer that shows a modest degree of local cellular motility in which the cells slowly exchanged contacts with other cells but maintained contacts within the monolayer. Expression of different isoforms of p120 catenin with or without MUC1 modified this behavior and tumor growth in patterns that were isoform specific. P120 catenin 1A created cells with very high rates of local motility that remained within the monolayer. P120 catenin 3A created cells that were more epithelial in appearance than the parental cells and exhibited local cellular motility within the monolayer; however, these cells were highly metastatic to the liver when MUC1 was expressed. Re-expression of p120 catenin 4A enforced an epithelial morphology on the cells, which also showed some local motility and exchange of cell contacts and coexpression of MUC1 created cells that appeared highly epithelial in culture and showed little exchange of cell contacts, but instead showed a remarkable and distinctive type of rapid, unified motility. Tumors from these cells showed a high degree of local invasion and capacity to colonize and invade local organs and structures in the peritoneal cavity.
Taken together the results in this report reveal several new and important features about the oncogenic and biological properties of p120 catenin and MUC1. Firstly, although p120 catenin has been characterized as having tumor suppressor functions (40, 48) and MUC1 has been characterized as an oncogene (49), it is clear that neither of these characterizations is completely accurate. Re-expression of p120 catenin in a deficient cell line did not suppress tumor growth and instead modified the tumor growth and metastatic properties, particularly in the context of high level expression of MUC1, and in some cases rendered tumors much more invasive or metastatic to certain sites. Given that these analyses were conducted in isogenic cell lines, the results strongly suggest that MUC1 and p120 catenin modulate the oncogenic growth properties of tumors in a context dependent manner, by influencing the capacity to move and grow in certain patterns or environments.
The fact that expression of different p120 catenin isoforms with and without MUC1 created cells that showed different morphologies, cell adhesion and motility in cell culture demonstrates that these molecules play a coordinated role in regulating these activities. Previously, this role has been largely ascribed to structural functions of associations between p120 catenin and cadherins at the cell surface, and their posited roles in regulating aspects of epithelial cell adhesion and lack of adhesion during the process of EMT. Our video results highlight that the apparently static cell adhesions stabilized by E cadherin and p120 catenin and observed on fixed epithelial cells are in fact not static, but are instead pliable and associated with different types of motility in the context of maintaining contacts with other cells.
Thus, epithelial appearance, cell adhesion and motility are not strictly linked to cadherin status at the cell surface. Instead, cells can exhibit adhesions that appear epithelial (and include expression of E-cadherin) and simultaneously undertake distinct types of motility including motility within a monolayer while exchanging contacts with other cells, and motility within a monolayer while maintaining static epithelial contacts. We propose that these differences result from combinations of expression of structural-signaling molecules such as different isoforms of p120 catenin and MUC1. The net effects on cell behavior are related to both structural effects in stabilizing molecular complexes at the cell surface, and signaling that contributes to localized motility and also apprise the cell of its structural status and location within a tumor or tissue microenvironment. We do not report here on the consequences of expression of p120 catenin and MUC1 on signaling through Wnt, RhoA, Rac, Cdc42 and other morphogenetic signaling pathways that involve nuclear translocation of MUC1 and p120 catenin. It should not be surprising that these pathways are affected, given previous publications in this subject (37–39).
Finally, the consequences of expression of MUC1 and p120 catenin on tumor invasion and metastasis are only partly revealed by analysis of cells in culture. A remarkable example of this is provided by the fact that the combination of re-expression of p120 catenin 3A and overexpression of MUC1 created an aggressive metastatic phenotype that produced large liver metastases, even though there were not significant effects on cell behavior noted in culture. This suggests that a combination of the adhesion and signaling properties conferred by co-expression of MUC1 and p120 catenin 3A was highly favorable for producing cells capable of metastasis to and growth in the liver microenvironment, further suggesting that effects of adhesion to liver cells or growth in the cytokine environment of the liver are also processed through signaling and structural effects mediated by MUC1 and specific isoforms of p120 catenin.
In summary, we present the first report that maps the interaction between MUC1 and p120 catenin to ARM domains 1, 3–5, and 8. Interactions between p120 catenin and MUC1 are common in pancreatic cancer. We show that p120 catenin is not expressed in the S2-013 cell line, and that re-expressing different isoforms of p120 catenin restores E-cadherin stability in that cell line and affects the epithelial morphology and motile properties of these cells in the context of MUC1 expression. Expression of MUC1 induced motility independent of cell adhesion, whereas overexpression of p120 catenin induced motility while maintaining contacts with other cells, and expression of MUC1 and p120 catenin isoform 4A induced motility while maintaining static epithelial contacts. There were also distinct yet concomitant effects on tumor growth and metastatic properties. Taken together our results demonstrate that structural signaling proteins including MUC1 and p120 catenin influence cell adhesion and motility in important and dynamic ways that are not fully understood. For example, p120 ctn is known to associate with microtubules through the armadillo domain (50) and to regulate microtubule dynamics (51). Microtubules target focal adhesions to serve as a conduit for proteins that degrade the focal adhesions (52). This together with the results reported here raise the possibility that MUC1 modulates microtubule organization in concert with p120 catenin and that interactions between p120 ctn with MUC1 affect microtubule stability. The disruption of microtubule dynamics due to the interaction of p120 ctn and MUC1.CT may in turn influence the turnover of focal adhesions and thus affect cell motility. These and other hypotheses that address the biological significance and function of interactions between MUC1 and p120 catenin with respect to structural effects, signaling, transcriptional regulation and microtubule regulation during cell motility and metastasis should be further investigated in the future.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health. The National Cancer Institute: R01CA57362, SPORE (1P50CA127297), Early Detection Research Network (5U01CA111294), Tumor Microenvironment Network (U54 CA163120), Cancer Center Support Grant 5P30 CA036727. The National Institute of General Medical Sciences: 5R01GM051188, Institutional Development Award (IDeA) Grant 5P20GM103489 and CoBRE 1P30GM106397.
The authors gratefully acknowledge Dr. Albert Reynolds for providing constructs and antibody reagents, and Dr. Ashley Mohr and Thomas Caffrey for helpful advice and technical assistance.
Footnotes
Conflict of interest:
The authors declare that they have no conflict of interest.
References
- 1.Hilkens J, Buijs F, Hilgers J, Hageman P, Calafat J, Sonnenberg A, et al. Monoclonal antibodies against human milk-fat globule membranes detecting differentiation antigens of the mammary gland and its tumors. Int J Cancer. 1984;34:197–206. doi: 10.1002/ijc.2910340210. [DOI] [PubMed] [Google Scholar]
- 2.Taylor-Papadimitriou J, Burchell J, Miles DW, Dalziel M. MUC1 and cancer. Biochim Biophys Acta. 1999;1455:301–13. doi: 10.1016/s0925-4439(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 3.Price MR, Pugh JA, Hudecz F, Griffiths W, Jacobs E, Symonds IM, et al. C595--a monoclonal antibody against the protein core of human urinary epithelial mucin commonly expressed in breast carcinomas. Br J Cancer. 1990;61:681–6. doi: 10.1038/bjc.1990.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 5.Kim YS, Gum J, Jr, Brockhausen I. Mucin glycoproteins in neoplasia. Glycoconj J. 1996;13:693–707. doi: 10.1007/BF00702333. [DOI] [PubMed] [Google Scholar]
- 6.Dong Y, Walsh MD, Cummings MC, Wright RG, Khoo SK, Parsons PG, et al. Expression of MUC1 and MUC2 mucins in epithelial ovarian tumours. J Pathol. 1997;183:311–7. doi: 10.1002/(SICI)1096-9896(199711)183:3<311::AID-PATH917>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Macao B, Johansson DG, Hansson GC, Hard T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat Struct Mol Biol. 2006;13:71–6. doi: 10.1038/nsmb1035. [DOI] [PubMed] [Google Scholar]
- 8.Baeckstrom D, Hansson GC, Nilsson O, Johansson C, Gendler SJ, Lindholm L. Purification and characterization of a membrane-bound and a secreted mucin-type glycoprotein carrying the carcinoma-associated sialyl-Lea epitope on distinct core proteins. J Biol Chem. 1991;266:21537–47. [PubMed] [Google Scholar]
- 9.Ligtenberg MJ, Buijs F, Vos HL, Hilkens J. Suppression of cellular aggregation by high levels of episialin. Cancer Res. 1992;52:2318–24. [PubMed] [Google Scholar]
- 10.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia. 2001;6:339–53. doi: 10.1023/a:1011379725811. [DOI] [PubMed] [Google Scholar]
- 11.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–34. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 12.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–77. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–65. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinkman-Van der Linden EC, Varki A. New aspects of siglec binding specificities, including the significance of fucosylation and of the sialyl-Tn epitope. Sialic acid-binding immunoglobulin superfamily lectins. J Biol Chem. 2000;275:8625–32. doi: 10.1074/jbc.275.12.8625. [DOI] [PubMed] [Google Scholar]
- 15.McDermott KM, Crocker PR, Harris A, Burdick MD, Hinoda Y, Hayashi T, et al. Overexpression of MUC1 reconfigures the binding properties of tumor cells. Int J Cancer. 2001;94:783–91. doi: 10.1002/ijc.1554. [DOI] [PubMed] [Google Scholar]
- 16.Satoh S, Hinoda Y, Hayashi T, Burdick MD, Imai K, Hollingsworth MA. Enhancement of metastatic properties of pancreatic cancer cells by MUC1 gene encoding an anti-adhesion molecule. Int J Cancer. 2000;88:507–18. doi: 10.1002/1097-0215(20001115)88:4<507::aid-ijc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Spicer AP, Duhig T, Chilton BS, Gendler SJ. Analysis of mammalian MUC1 genes reveals potential functionally important domains. Mamm Genome. 1995;6:885–8. doi: 10.1007/BF00292441. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Bharti A, Chen D, Gong J, Kufe D. Interaction of glycogen synthase kinase 3beta with the DF3/MUC1 carcinoma-associated antigen and beta-catenin. Mol Cell Biol. 1998;18:7216–24. doi: 10.1128/mcb.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Ren J, Yu W, Li Q, Kuwahara H, Yin L, et al. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. J Biol Chem. 2001;276:35239–42. doi: 10.1074/jbc.C100359200. [DOI] [PubMed] [Google Scholar]
- 20.Ren J, Li Y, Kufe D. Protein kinase C delta regulates function of the DF3/MUC1 carcinoma antigen in beta-catenin signaling. J Biol Chem. 2002;277:17616–22. doi: 10.1074/jbc.M200436200. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J Biol Chem. 1997;272:12492–4. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- 22.Wen Y, Caffrey TC, Wheelock MJ, Johnson KR, Hollingsworth MA. Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J Biol Chem. 2003;278:38029–39. doi: 10.1074/jbc.M304333200. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Kufe D. The Human DF3/MUC1 carcinoma-associated antigen signals nuclear localization of the catenin p120(ctn) Biochem Biophys Res Commun. 2001;281:440–3. doi: 10.1006/bbrc.2001.4383. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds AB, Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23:7947–56. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- 25.Peifer M, Yap AS. Traffic control: p120-catenin acts as a gatekeeper to control the fate of classical cadherins in mammalian cells. J Cell Biol. 2003;163:437–40. doi: 10.1083/jcb.200310090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis MA, Reynolds AB. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev Cell. 2006;10:21–31. doi: 10.1016/j.devcel.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, et al. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–45. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, et al. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol Biol Cell. 2005;16:5141–51. doi: 10.1091/mbc.E05-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Kojima S, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol. 2003;163:547–57. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kowalczyk AP, Reynolds AB. Protecting your tail: regulation of cadherin degradation by p120-catenin. Curr Opin Cell Biol. 2004;16:522–7. doi: 10.1016/j.ceb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Mo YY, Reynolds AB. Identification of murine p120 isoforms and heterogeneous expression of p120cas isoforms in human tumor cell lines. Cancer Res. 1996;56:2633–40. [PubMed] [Google Scholar]
- 32.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol. 1994;14:8333–42. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci. 2000;113 (Pt 8):1319–34. doi: 10.1242/jcs.113.8.1319. [DOI] [PubMed] [Google Scholar]
- 34.Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, et al. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–76. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burdick MD, Harris A, Reid CJ, Iwamura T, Hollingsworth MA. Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J Biol Chem. 1997;272:24198–202. doi: 10.1074/jbc.272.39.24198. [DOI] [PubMed] [Google Scholar]
- 36.Kohlgraf KG, Gawron AJ, Higashi M, Meza JL, Burdick MD, Kitajima S, et al. Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer Res. 2003;63:5011–20. [PubMed] [Google Scholar]
- 37.Anastasiadis PZ, Reynolds AB. Regulation of Rho GTPases by p120-catenin. Curr Opin Cell Biol. 2001;13:604–10. doi: 10.1016/s0955-0674(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 38.Grosheva I, Shtutman M, Elbaum M, Bershadsky AD. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J Cell Sci. 2001;114:695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- 39.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–80. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, et al. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer cell. 2011;19:470–83. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniel JM, Reynolds AB. The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol Cell Biol. 1995;15:4819–24. doi: 10.1128/mcb.15.9.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daniel JM, Reynolds AB. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19:3614–23. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berx G, Becker KF, Hofler H, van Roy F. Mutations of the human E-cadherin (CDH1) gene. Human mutation. 1998;12:226–37. doi: 10.1002/(SICI)1098-1004(1998)12:4<226::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 44.Berx G, Van Roy F. The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast cancer research : BCR. 2001;3:289–93. doi: 10.1186/bcr309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollingsworth PKSaMA. Cell surface-associated mucins in signal transduction. TRENDS in Cell Biology. 2006 doi: 10.1016/j.tcb.2006.07.006. In press. [DOI] [PubMed] [Google Scholar]
- 46.Nollet F, Berx G, van Roy F. The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Mol Cell Biol Res Commun. 1999;2:77–85. doi: 10.1006/mcbr.1999.0155. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Gallup M, Zlock L, Basbaum C, Finkbeiner WE, McNamara NA. Cigarette smoke disrupts the integrity of airway adherens junctions through the aberrant interaction of p120-catenin with the cytoplasmic tail of MUC1. J Pathol. 2013;229:74–86. doi: 10.1002/path.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thoreson MA, Reynolds AB. Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation; research in biological diversity. 2002;70:583–9. doi: 10.1046/j.1432-0436.2002.700911.x. [DOI] [PubMed] [Google Scholar]
- 49.Kufe DW. Functional targeting of the MUC1 oncogene in human cancers. Cancer Biol Ther. 2009;8:1197–203. doi: 10.4161/cbt.8.13.8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franz CM, Ridley AJ. p120 catenin associates with microtubules: inverse relationship between microtubule binding and Rho GTPase regulation. J Biol Chem. 2004;279:6588–94. doi: 10.1074/jbc.M312812200. [DOI] [PubMed] [Google Scholar]
- 51.Ichii T, Takeichi M. p120-catenin regulates microtubule dynamics and cell migration in a cadherin-independent manner. Genes to cells : devoted to molecular & cellular mechanisms. 2007;12:827–39. doi: 10.1111/j.1365-2443.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 52.Stehbens S, Wittmann T. Targeting and transport: how microtubules control focal adhesion dynamics. J Cell Biol. 2012;198:481–9. doi: 10.1083/jcb.201206050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.