Abstract
Herpes simplex viruses (HSV) are highly pervasive pathogens in the human host with a seroconversion rate upwards of 60% worldwide. HSV type 1 (HSV-1) is associated with the disease, herpetic stromal keratitis, the leading cause of infectious corneal blindness in the industrialized world. Individuals suffering from genital herpes associated with HSV type 2 (HSV-2) are found to be two to three fold more susceptible in acquiring human immunodeficiency virus (HIV). The morbidity associated with these infections is principally due to the inflammatory response, the development of lesions, and scarring. Chemokines have become an important aspect in understanding the host immune response to microbial pathogens due in part to the timing of expression. In this paper, we will explore the current understanding of chemokine production as it relates to the orchestration of the immune response to HSV infection.
1. Introduction
1.1 General properties of herpes simplex viruses
Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) are neurotropic viruses that are members of the family alphaherpesviridae [42]. Both types of HSV are transmissible from person to person via infectious mucosal secretions which come in contact with mucosal epithelia that line surface apertures of the body [9, 42, 57]. Herpes simplex viruses can cause a variety of diseases including keratitis, cold sores, encephalitis, genital herpes, cutaneous herpes, and meningitis [12, 42]. HSV-1 and HSV-2 enter the epithelium of the host and initiate a lytic replicative cycle [18, 40–42, 57, 70]. HSV enters its target cell through a multistep process which includes envelope glycoproteins (g) that surround the viral particle [42, 64]. The initial interaction begins with the binding of gC and gB to heparin sulfate proteoglycans that are found on the surface of target cells [42, 64]. After the attachment of the viral particle to the host cell, another viral glycoprotein, gD, interacts with other host cell surface receptors, including herpesvirus entry mediator A, which is a TNF receptor family member, and nectins, that allow for the fusion of the virion envelope to the cell’s plasma membrane via gB, gD, gH, and gL [42, 64]. Local replication commences with transcription of viral lytic genes [18, 33, 40–42, 57, 64, 70]. Following a lytic replicative cycle, the virus enters sensory nerve endings in the basal aspect of the epithelium and undergoes retrograde transport to associated sensory ganglia [18, 42, 64, 70]. Within the sensory ganglia, HSV undergoes a second stage of lytic infection. Depending on the extent of the infection, HSV may travel further to the central nervous system. Following acute infection of sensory ganglia, the virus establishes latency in a subpopulation of neurons [18, 42, 64, 70]. Periodic reactivation from latency during periods of stress or immune suppression results in the re-infection of the initial port of entry [18, 42, 70].
Clearance of the virus from the host is dependent on both the host’s innate and adaptive immune responses. Polymorphonuclear cells (PMNs) are the first and most predominant cell to infiltrate the area of infection releasing a number of soluble factors including cytokines, chemokines, and tissue degrading enzymes including matrix metalloproteinases [6, 22, 45, 96, 97]. Likewise, natural killer (NK) cells and subsequently, macrophages and T cells are recruited to the site of inflammation. Chemokine expression has become an interest in the scientific community as it relates to the immune response to infectious agents and the pathology that develops from this response.
1.2 Herpes simplex virus type 1
The cornea is a transparent, avascular tissue composed of a surface epithelium, corneal stroma, and endothelium that covers the anterior portion of the eye [70]. It is the avascular nature of the cornea that preserves the visual axis providing a translucent conduit for subsequent processing of an image by the lens and retina of the eye. However, experimental evidence suggests ocular HSV-1 can limit the visual axis through neovascularization and infiltration of leukocytes attracted to the infection through the production of chemokines [103]. Experimental infection of the cornea initiates in the surface epithelium in the outermost squamous layer of cells. HSV-1 spreads from cell-to-cell in a polarized fashion to the next layer of cells, wing cells [70]. The virus is able to travel to the sensory nerve endings that can be found in the basal aspect of the epithelium [70].
One clinically significant disease that is caused by HSV-1 is herpetic stromal keratitis (HSK) an intense inflammatory response triggered by the viral infection of the corneal stroma [3, 45, 54]. If left untreated, the chronic inflammatory response leads to the formation of lesions, scarring, and eventually blindness.
1.3 Herpes simplex virus type 2
HSV-2 is the causative agent of genital herpes of which approximately 500,000 new cases arise annually [33]. It has been estimated that 33% of the adult population is seropositive for this sexually transmitted disease, making HSV-2 the most common sexually transmitted pathogen worldwide [33, 73, 78, 92]. Genital herpes infection can result in complications including urinary retention and meningoencephalitis [33, 73, 78, 92]. Approximately 3,500 births in the United States are impacted by HSV-2 infection which can lead to fatal infant encephalitis [18]. Even though a relatively large percentage of the population is seropositive for HSV-2, only a small percent are subjected to these complications. Hormones have been implicated in the susceptibility to infection in the female host [86]. Specifically, mice exposed to progesterone are rendered more susceptible to infection [37] whereas estradiol-treated mice are found to be resistant to infection [27]. Although the role of ovarian sex hormones in susceptibility to genital HSV-2 infection is not completely defined, immune suppression [27], changes in the vaginal epithelial thickness [72], and modulation of a cell membrane receptor, nectin-1-δ [48], may all influence the infectious process.
One common denominator in the recruitment process of leukocytes into the inflamed HSV-infected tissue is the expression of chemokines. Although a necessary process in attracting immune effector cells required to control replication and spread of the virus, chemokine expression and the ensuing inflammatory response has detrimental consequences to the host especially when considering the eye. Understanding the sequential expression of chemokines relative to ocular HSV-1 infection is pertinent to the development of a strategy that will ultimately control local inflammation and the collateral damage without rendering increased susceptibility to the host.
2. HSV-1 Infection of the Eye
2.1 Innate Immune Response to Ocular HSV-1 Infection
After initial infection of the virus into the cornea, an innate immune response is triggered to clear the pathogen. Toll-like receptors (TLR), a family of pattern recognition molecules, are known to respond to pathogens and serve as early warning molecules that induce the expression of proinflammatory molecules [5]. Of the twelve TLR subtypes found in the mouse, TLR2 and TLR9 are expressed by corneal epithelium [36]. HSV-1 stimulates TLR2 by unknown means resulting in the activation of NF-κB and production of IL-6 [46]. HSV-1 which contains CpG motifs [106] is recognized by TLR9 resulting in the expression of type I IFN [44]. In addition to the production of type I IFNs, the infected resident cells of the cornea as well as neighboring cells (most probably through TLR signaling and NF-κB activation) are known to release inflammatory cytokines including IL-1α, IL-6, and TNF-α [34, 88]. The absence or hindrance of these cytokines has been linked to a significant reduction in the incidence of HSK [6, 22, 101]. It is thought that IL-1α leads to the induction of IL-6 by resident corneal cells [6] that, in turn, elicits production of macrophage inflammatory protein-1α (CCL3) and -2 (CXCL2) [22] ultimately recruiting PMNs into the infected tissue. PMNs infiltrate the stroma underlying the infected epithelial cells contributing to clearance of the virus and limiting viral dissemination within 24 hr post infection [6, 96, 97]. PMNs are thought to be a rich source of iNOS and TNF-α [13], the latter of which up-regulates ICAM-1 expression [69] facilitating the adherence of leukocytes to the endothelium [89]. The administration of monoclonal antibody to ICAM-1 [15] or use of ICAM-1 deficient mice [67] has not been found to diminish the infiltration of cells or the clinical course of herpetic disease following corneal infection. However, ICAM-1 does play a key role in preventing herpetic encephalitis [15, 67] suggesting pathways independent of ICAM-1 expression are involved initially in the recruitment of cells into the cornea whereas controlling virus spread in the central nervous system involves ICAM-1 expression. After the initial infiltration of neutrophils, macrophages and NK cells infiltrate the area but PMNs remain the predominant cell type residing in the inflamed cornea up to the first 96 hr post infection [93].
2.2 Chemokine Expression during the Innate Immune Response to Ocular HSV-1 infection
Evidence for the expression of chemokines in the cornea following HSV-1 infection was first described using end-point PCR in which KC (CXCL1), CXCL2, IFN-γ-inducible protein 10 (CXCL10), monocyte chemoattractant protein-1 (CCL2), MIP-1β (CCL4), and regulated upon activation, normal T cell expressed (CCL5) were observed [90]. While trauma to the cornea in the form of scarification induced the expression, continued expression of CCL2, CCL5, and CXCL10 were noted out to 72 hr post infection whereas other chemokine mRNA levels precipitously dropped in both BALB/c and outbred ICR mice [14, 90]. Of the chemokines noted above, CXCL1 and CXCL2 specifically target neutrophils principally through the receptor, CXCR2 [11, 82, 99, 104]. Neutralization of CXCL2 with antibody leads to a reduction in PMN infiltration into the cornea [54, 104]. Likewise, CXCR2 knockout mice infected with HSV-1 show a minimal infiltration of PMNs into the cornea [3]. Even with a reduction in PMN influx, HSK still develops in the CXCR2 deficient mice which is thought to be due to an increase in IL-6 expression driven by elevated virus titers ultimately facilitating angiogenesis [3]. Although evidence suggest IL-6 can drive neovascularization through vascular endothelial growth factor (VEGF) in the cornea, the kinetics of expression of VEGF during the infectious process in this model suggests other dynamics are involved including T cells that are known to contribute to HSK [16, 83] and are a source of VEGF [63].
Whereas CXCL2 is thought to be induced by IL-6 [57], another CXC chemokine, CXCL10 has been found to be the only chemokine that is constitutively expressed in the cornea as determined by PCR [14, 90] and ELISA [10]. CXCL10 levels rapidly rise in the cornea following HSV-1 infection and neutralization of the chemokine dramatically reduces corneal edema and infiltrating cells [10]. The lone receptor for CXCL10 is CXCR3 expressed by NK cells, macrophages, dendritic cells, and activated T cells [21, 23, 47, 77, 94]. However, CXCR3 knockout mice ocularly infected with HSV-1 show a transient suppression of PMN (Gr-1+CD11b+Mac-3−) recruitment into the cornea [Carr, unpublished observation] calling into question the role of CXCR3 and its ligands in PMN recruitment. However, other studies at different anatomical sites have described PMN infiltration as a result of CXCL10 expression [8, 105]. It is tempting to speculate that CXCL10 may up-regulate CD11a on PMNs enhancing the adhesion to the endothelium as has been reported for Th1 cells [2] facilitating diapedesis into the stroma of the cornea. However, formal proof of this notion requires additional studies.
Of the CC chemokine ligands expressed during ocular HSV-1 infection, CCL2 is strongly expressed throughout the initial course of acute infection as measured by PCR [14, 90]. The role of CCL2 in the development of HSK may be peripheral to its effects on the recruitment of leukocytes into the cornea since the administration of neutralizing antibody to CCL2 has no effect on the incidence of HSK in HSV-1-infected mice [99]. In constrast, the administration of anti-CCL3 antibody significantly reduces the severity of corneal opacity [99]. The kinetics of CCL3 expression suggest it is not a stimulus for the recruitment of leukocytes into the cornea until 7–10 days post infection, at time which seems to correlate with the onset of HSK [99]. Consistent with this finding, mice deficient in CCL3 expression reportedly show little cellular infiltration in the cornea throughout the time course of infection with low to undetectable levels of Th1 cytokines including IL-2 and IFN-γ [98] normally found during acute ocular infection [93]. Ironically, the CCL3 knockout mice clear the virus at the same time as wild type control animals [99] which calls into question the mechanism of virus clearance. Since there is apparently little leukocyte infiltration including PMNs that are known to control HSV-1 replication in the eye [97] with a paucity of CD4+ T cells or IFN-γ present as well [99], it is puzzling what mechanism(s) controls the virus.
Similar to CCL2, CCL5 is also expressed throughout the course of acute HSV-1 infection [14]. CCL5 operating through its receptor CCR5 is a strong chemoattractant for T cells and NK cells [53, 80] but also influences PMN recruitment as well [71]. It is interesting to note that while HSV-1 tends to subvert immune activation, CCL5 is induced by HSV-1 through NFκB and IFN regulatory factor 3 pathways [56].
The plethora of chemokines and pro-inflammatory cytokines produced in the cornea during the innate immune response (i.e., 0–5 days post infection) may be generated from several sources. With the exception of CXCL10, CXCL1, CXCL2, CXCL9, CCL2, CCL3, and CCL5 are not constitutively expressed in the cornea (Fig. 1). Analysis by confocal microscopy has found the endothelial layer of the cornea expresses very modest amounts of the CXCL10 in uninfected mice (Carr, unpublished observation). Consistent with previous results [90], scarification of the cornea (a process typically employed to infect mice) alone elicits a rise in CCL3, CCL5, and CXCL10 expression (Fig. 1). Following infection, CXCL1, CCL2, CCL5, CXCL9, and CXCL10 are induced or up-regulated within 36 hr. Analysis of CCL5 and CXCL10 expression by confocal microscopy show two different patterns of expression. CCL5 is expressed in the epithelial layers of the eye co-localizing with HSV-1 antigen as well as within the stroma of the cornea [Carr, Wuest, Tomanek, Ramsey, Ash, Lane, and Kuziel, submitted]. By comparison, CXCL10 expression chiefly co-localizes with HSV-1 antigen expression in the epithelial layers of the cornea with punctate staining in the endothelium (Carr, unpublished observation). The expression profile of CCL5 and CXCL10 suggest the resident population generates most if not all of the CXCL10 within the first 24 hr post infection whereas CCL5 is produced principally by resident cells but may also be provided by the infiltrating PMNs that are found within the stroma 24 hr post infection [Carr et al., submitted]. It is likely that as the infection spreads over the next several hours, chemokines generated including CCL2, CCL5, CXCL1, CXCL2, CXCL9, and CXCL10 are produced by multiple sources including the resident fibroblasts, epithelial, and endothelial cells as well as infiltrating PMNs, macrophages, NK cells, and dendritic cells [11, 25, 82, 87, 100]. Collectively, the initial cascade of chemokine expression is complex but may be divided into two principal pathways involving CXCL10 and IL-6 (Fig. 2).
Figure 1.
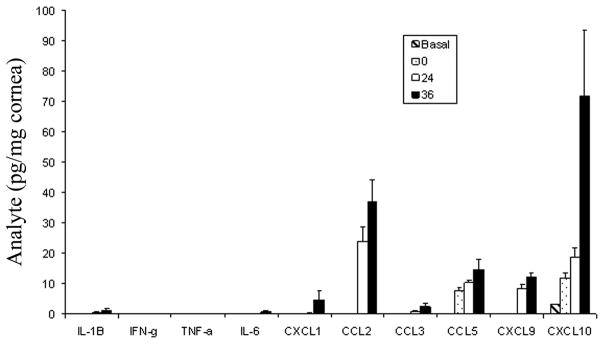
Expression of inflammatory cytokines and chemokines in the cornea of HSV-1 infected mice. C57BL/6 female mice (n=6/timepoint) were left alone (basal) or scarified (0) and infected with HSV-1 (McKrae strain, 1000 plaque forming units (PFU)/eye. Twenty-four to thirty-six hours postinfection, the mice were euthanized, perfused, and the cornea was removed and homogenized in a buffer containing a cocktail of protease inhibitors. The supernatant was clarified (10,000 × g, 5 min) and assayed for cytokine/chemokine content by ELISA. Bars represents mean ± SEM for each analyte under measure.
Figure 2.
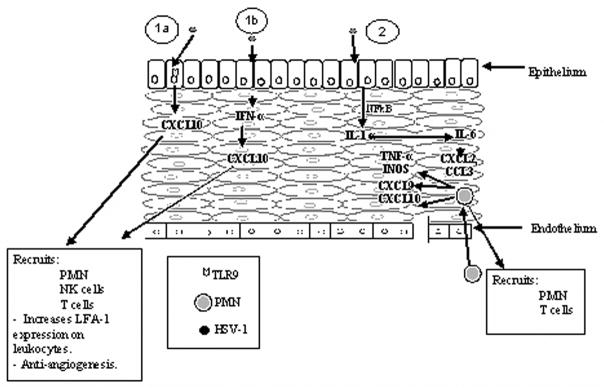
Chemokine expression in the cornea following HSV-1 infection. Three different scenarios can operate in the production of chemokines within the cornea following ocular HSV-1 infection. In 1a, HSV-1 DNA CpG motifs bind to the intracellular toll-like receptor (TLR)9 eliciting the production of CXCL10 through NFκB activation. In 1b, HSV-1 enters the epithelial cell and following transcription induces the production of IFN-α which induces CXCL10 production. In 2, HSV-1 activation of NFκB stimulates IL-1α synthesis leading to IL-6 production resulting in CXCL2 and CCL3 expression. These chemokines draw in PMNs and T cells. PMNs can secrete CXCL9 and CXCL10 which can recruit additional leukocytes including macrophages, dendritic cells, NK cells, and T cells.
The delayed expression of CCL3 in the cornea is associated with a secondary wave of PMNs and some T cells into the stroma (day 10 post infection) [99]. Since CCL3 targets monocytes, T cells, natural killer (NK) cells, basophils, eosinophils, dendritic cells (DCs), and hematopoietic progenitors [11, 12, 82], it is currently unknown what events transpire to recruit the subsequent wave of cells. However, CCL3 is central to the effect since neutralizing this chemokine with antibody or suppressing expression with IL-10 reduces leukocyte recruitment into the cornea [99].
2.3 Adaptive Immune Response to Ocular HSV-1 Infection
Following the innate response to infection, preferential recruitment of Th1 CD4+ T cells into the cornea is observed [35, 65]. Although it is currently unknown why there is a preferential recruitment of CD4+ T cells into the cornea of HSV-1-infected mice, the expression of CXCR3 and CCR5 on activated T cells and the presence of CCL5 and CXCL9 in the cornea may influence the recruitment process [80, 81, 102]. The presence of CD4+ T cells is crucial in controlling local virus replication and spread [7, 26] as well as the development of HSK [25, 58]. However, bystander activation of CD4+ T cells in addition to virus antigen stimulation may also contribute to HSK development [24]. The continued expression of chemokines including CXCL2, CCL2, CCL3, CCL4, CCL5, and CXCL10 in the cornea would also provide the maintenance of leukocytes in the tissue recruited from the periphery and facilitate collateral damage to the cornea stroma [84]. Collectively, chemokines are instrumental in the initial trafficking of cells into the infected anterior segment of the eye as well as the development of HSK. Blockinig their expression could lead preserve the visual axis pending local virus replication is controlled. A summary of chemokines expressed during the acute HSV-1 ocular infection are found in Table 1.
Table 1.
Chemokine Expression in the Cornea During Acute HSV-1 Infection
| Group | Name | Detection | Reference |
|---|---|---|---|
| CXC | |||
| CXCL1 | RT-PCR and ELISA | 90, Fig. 1 | |
| CXCL2 | RT-PCR and ELISA | 3, 6, 10, 22, 54, 90, 98, 104 | |
| CXCL9 | ELISA | 10, Fig. 1 | |
| CXCL10 | RT-PCR and ELISA | 10, 14, 90, Fig. 1 | |
| CC | |||
| CCL2 | RT-PCR and ELISA | 14, 90, 98, 99, Fig. 1 | |
| CCL3 | RT-PCR and ELISA | 10, 22, 90, 98, 99, Fig. 1 | |
| CCL5 | RT-PCR and ELISA | 10, 14, 90, Fig. 1 | |
| RANTES | CCL5 | CCR1, CCR4, CCR5 |
2.4 HSV-1 Latency in the Trigeminal Ganglion
After the successful infection of the cornea by HSV-1, a series of events occur that can lead to a stable latent neuronal infection in the trigeminal ganglion (TG) within one to two weeks postinfection [39, 41, 49]. Following an initial round of replication in the corneal epithelium, the virus is able to enhance its ability to access the axonal termini through mechanisms that are not understood, and through retrograde axonal transport enter the neuronal cell bodies in which another stage of lytic replication begins [49, 70]. After this brief replication cycle in the neuronal cell bodies, the lytic cycle genes are repressed and latency is established with minimal viral gene expression [49]. Infectious HSV-1 can consistently be detected in the TG out to approximately ten days postinfection [12]. By day thirty postinfection, latency is established as defined by the lack of detectable infectious virions [12]. Even though infectious virions are not readily detected during latency, HSV-1 latency associated transcripts or LATs can be detected in the TG, and an associated local immune response is evident [28, 49] With latency established, the immune system continually surveys the area with CD8+ T cells as the principal cell type that is thought to prevent reactivation [39]. Along these lines, CD8+ T cells greatly outnumber CD4+ T cells in the TG [49] and are thought to control the infection through non-cytolytic mechanisms using cytokines such as IFN-γ and TNF-α with minimal destruction to neurons [38, 50, 51, 95].
During latent infection, real time PCR detection of CXCR3 and CCR5 expression have been reported [12]. Although unproven, it is likely these chemokine receptors are found on the CD8+ T cells present in the TG during latency [29, 38]. Although ligands for CXCR3 including CXCL9 and CXCL10 have not been evaluated during latency, one ligand for CCR5, CCL5, has been detected [28]. Exposing latently infected mice to the potent anti-viral compound acyclovir has been found to reduce CCL5 expression in the TG, the continued presence of CD8 cells suggest additional signals provide a stimulus for retainment of these effector cells within the tissue [29].
2.5 Reactivation of HSV-1
Due to a variety of environmental cues including UV light, stress, and immunosuppression, the virus is able to reactivate in the latently infected neurons of the TG. Through antegrade transport, the virus can again be detected in the corneal epithelium and stroma [68, 70]. The reactivation cycle can be repeated eliciting chronic and episodic immune activation which leads to progressive scarring of the cornea resulting in decreased vision, glaucoma, iritis, cataract, and necrotizing retinitis [70]. While there is experimental evidence to suggest regulatory T cells may control ocular pathogenesis [91], how these cells impact on local chemokine expression is not understood.
3.0 HSV-2 infection of the genitalia
3.1 Immune response to genital HSV-2 infection
During initial infection of the mucosa of the vagina with HSV-2, the virus begins to replicate in the epithelium, typically restricted to the epidermis or cervicovaginal epithelium [43]. The initial host response to infection includes the induction of type I IFNs (i.e., IFN-α species) through TLR9 recognition of HSV-2 CpG motifs [52]. The IFN-responsive pathway, double-stranded RNA-dependent protein kinase but not 2′,5′-oligoadenylate synthetases is essential for resistance to infection as mice deficient in this pathway are highly susceptible to HSV-2-mediated mortality (Tomanek, Silverman, Williams, and Carr, manuscript in preparation). In addition to type I IFN production, IL-12, 1L-15, IL-18, NK cells, and PMNs are important first lines of defense against HSV-2 replication and spread [1, 31, 59]. Current evidence suggests the resident populations of Langerhans cells [19] do not traffic to the inguinal/iliac lymph nodes with most migrating cells consisting of B lymphocytes [40]. T lymphocytes including γδ T cells are essential components of the adaptive immune response in controlling genital infection with HSV-2 [55, 60, 66, 73]. CD4+ T cells produce the majority of IFN-γ in response to genital HSV-2 infection [32, 61]. Neutralization of IFN-γ leads to an increase in virus titer and a decrease in T cell recruitment into the vaginal tissue [61, 73]. B cell production of antibody is initiated in the draining lymph nodes and appears to have only a modest impact on HSV-2 titers suggesting a limited role for B lymphocytes in the control of genital HSV-2 infection [17, 62, 75]. Manifestations of genital herpes include macules, papules, and vesicles resulting in the development of ulcers in the genital region [33]. Due to these ulcerations, other pathogens are able to enter into the vaginal mucosa. Recent studies have shown that patients who are infected with HSV-2 have a higher risk of contracting HIV-1 than patients who are HSV-2 seronegative with a two to three fold increase in susceptibility [79].
3.2 Chemokines and HSV-2
The recruitment of leukocytes into the vaginal tissue following HSV-2 infection appears to include IFN-γ induction of the adhesion molecules intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 [76] since neutralizing IFN-γ diminishes lymphocyte infiltration into the infected tissue [74]. The expression of IFN-γ has also been associated with CCL5 production [30] found in the vagina following HSV-2 infection [4, 33]. The role of CCL5 expression in recruiting leukocytes into the infected tissue has not been described. However, plasmid DNA containing CCL5 has been found to enhance survival of HSV-2 infected mice [85]. Manipulating local expression of selective chemokines including CXCL2 and CCL3 using plasmid DNA suggests these chemokines may also play a significant role in protection for the host during genital virus infection by facilitating CD4+ T cell immunity and elevating IFN-γ production by NK cells [20]. However, there are a number of unresolved questions that remain as to those chemokines that initiate the inflammatory cascade as well as those that are critical for resistance to genital HSV-2.
4. Perspective
Chemokines are a significant group of soluble factors that contribute in the clearance of HSV-1 and HSV-2 pathogens from the host. Although necessary for an optimal immune response to the virus, chemokines initiate a frank inflammatory response that can result in a significant detrimental outcome to the host as it pertains to preservation of the visual axis. This chapter highlights the role of chemokines as they relate to the innate and adaptive immune response following ocular HSV-1 infection. Evidence suggests that curtailing expression of selective chemokines during HSV-1 infection of the eye may favor preservation of sight without consequences to controlling virus replication and spread. This observation suggests that while many chemokines are redundant in function and/or promiscuous in binding multiple receptors, selectivity in tissue expression of chemokines and targeting specific effector cells by those chemokines expressed in a given tissue may ultimately dictate the inflammatory response of the host and outcome of the infection. Understanding this process will prove beneficial in developing anti-inflammatory therapies for individuals experiencing chronic HSV reactivation.
Acknowledgments
The work was supported by a USPHS NIH grant, EY015566 and a Jules and Doris Stern RPB Research Professorship to DJJC.
Abbreviations
- HSV
Herpes simplex virus
- PMN
polymorphonuclear cell
- NK
natural killer
- HSK
herpetic stromal keratitis
- IL
interleukin
- DC
dendritic cell
- Th1
T helper 1 cell
- TG
trigeminal ganglion
- IFN
interferon
- TNF
tumor necrosis factor
- TLR
Toll-like receptor
- VEGF
vascular endothelial growth factor
References
- 1.Ashkar AA, Rosenthal KL. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J Virol. 2003;77:10168–10171. doi: 10.1128/JVI.77.18.10168-10171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atarashi K, Hirata T, Matsumoto M, Kanemitsu N, Miyasaka M. Rolling of Th1 cells via p-selectin glycoprotein 1 stimulates LFA-1-mediated cell binding to ICAM-1. J Immunol. 2005;174:1424–1432. doi: 10.4049/jimmunol.174.3.1424. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee K, Biswas PS, Kim B, Lee S, Rouse BT. CXCR2−/− mice show enhanced susceptibility to herpetic stromal keratitis: A role for IL-6-induced neovascularization. J Immunol. 2004;172:1237–1245. doi: 10.4049/jimmunol.172.2.1237. [DOI] [PubMed] [Google Scholar]
- 4.Benencia F, Gamba G, Cavalieri H, Courreges MC, Benedetti R, Villam SM, Massouh EJ. Nitric oxide and HSV vaginal infection in BALB/c mice. Virology. 2003;309:75–84. doi: 10.1016/s0042-6822(02)00057-0. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharjee R, Akira S. Toll-like receptor signaling: Emerging Opportunities in Human Diseases and Medicine. Curr Immunol Rev. 2005;1:81–90. [Google Scholar]
- 6.Biswas PS, Banerjee K, Kim B, Rouse BT. Mice transgenic for IL-1 receptor antagonist protein are resistant to herpetic stromal keratitis: Possible role for IL-1 in herpetic stromal keratitis Pathogenesis. J Immunol. 2004;172:3736–3744. doi: 10.4049/jimmunol.172.6.3736. [DOI] [PubMed] [Google Scholar]
- 7.Bouley DM, Kanangat S, Wire W, Rouse BT. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-γ knockout mice. J Immunol. 1995;155:3964–3971. [PubMed] [Google Scholar]
- 8.Boztug K, Carson MJ, Pham-Mitchell N, Asensio VC, DeMartino J, Campbell IL. Leukocyte infiltration, but not neurodegeneration, in the CNS of transgenic mice with astrocyte production of the CXC chemokine ligand 10. J Immunol. 2002;169:1505–1515. doi: 10.4049/jimmunol.169.3.1505. [DOI] [PubMed] [Google Scholar]
- 9.Brandt CR. The role of viral and host genes in corneal infection with herpes simplex virus type 1. Exp Eye Research. 2005;80:607–621. doi: 10.1016/j.exer.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Carr DJJ, Chodosh J, Ash J, Lane TE. Effect of Anti-CXCL10 Monoclonal Antibody on Herpes Simplex Virus Type 1 Keratitis and Retinal Infection. J Virol. 2003;77:10037–10046. doi: 10.1128/JVI.77.18.10037-10046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chensue SW. Molecular Machinations: Chemokine Signals in Host-Pathogen Interactions. Clin Micro Rev. 2001;14:821–835. doi: 10.1128/CMR.14.4.821-835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook WJ, Kramer MF, Walker RM, Burwell TJ, Holman HA, Coen DM, Knipe DM. Persistent expression of chemokine and chemokine receptor RNAs at primary and latent sites of herpes simplex virus I infection. Virol Journal. 2004;1:5. doi: 10.1186/1743-422X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daheshia M, Kanangat S, Rouse BT. Production of key molecules by ocular neutrophils early after herpetic infection of the cornea. Exp Eye Res. 1998;67:619–624. doi: 10.1006/exer.1998.0565. [DOI] [PubMed] [Google Scholar]
- 14.Daigle J, Carr DJJ. Androstenediol antagonizes herpes simplex virus type 1-induced encephalitis through the augmentation of type 1 IFN production. J Immunol. 1998;160:3060–3066. [PubMed] [Google Scholar]
- 15.Dennis RF, Siemasko KF, Tang Q, Hendricks RL, Finnegan A. Involvement of LFA-1 and ICAM-1 in the herpetic disease resulting from HSV-1 corneal infection. Curr Eye Res. 1994;14:55–62. doi: 10.3109/02713689508999914. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande S, Zheng M, Lee S, Banerjee K, Gangappa S, Kumaraguru U, Rouse BT. Bystander activation involving T lymphocytes in herpetic stromal keratitis. J Immunol. 2001;167:2902–2910. doi: 10.4049/jimmunol.167.5.2902. [DOI] [PubMed] [Google Scholar]
- 17.Dudley KL, Bourne N, Milligan GN. Immune protection against HSV-2 in B-cell-deficient mice. Virology. 2000;270:454–463. doi: 10.1006/viro.2000.0298. [DOI] [PubMed] [Google Scholar]
- 18.Duerst RJ, Morrison LA. Innate Immunity to Herpes Simplex Virus Type 2. Viral Immunol. 2003;16:475–490. doi: 10.1089/088282403771926300. [DOI] [PubMed] [Google Scholar]
- 19.Edwards JNT, Morris HB. Langerhans cells and lymphocyte subsets in the female genital tract. Br J Obstetrics and Gynaecol. 1985;92:974–982. doi: 10.1111/j.1471-0528.1985.tb03080.x. [DOI] [PubMed] [Google Scholar]
- 20.Eo SK, Lee S, Chun S, Rouse BT. Modulation of Immunity against Herpes Simplex Virus Infection via Mucosal Genetic Transfer of Plasmid DNA Encoding Chemokines. J Virol. 2001;75:569–578. doi: 10.1128/JVI.75.2.569-578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farber JM. Mig and IP-10. CXC chemokines that target lymphocytes. J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 22.Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with Neutrophil Chemoattractant Expression in Virus-Induced Ocular Inflammation. Invest Ophthalmol. 2002;43:737–743. [PubMed] [Google Scholar]
- 23.Foley JF, Yu CR, Solow R, Yacobucci M, Peden KW, Farber JM. Roles for CXC chemokine ligands 10 and 11 in recruiting CD4+ T cells to HIV-1-infected monocyte-derived macrophages, dendritic cells, and lymph nodes. J Immunol. 2005;174:4892–900. doi: 10.4049/jimmunol.174.8.4892. [DOI] [PubMed] [Google Scholar]
- 24.Gangappa S, Babu JS, Thomas J, Daheshia M, Rouse BT. Virus-induced immunoinflammatory lesions in the absence of viral antigen recognition. J Immunol. 1998;161:4289–4300. [PubMed] [Google Scholar]
- 25.Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H, Murphy M, Liao F, Farber J, Cassatella MA. Gene expression and production of the monokine induced by IFN-γ (MIG), IFN-inducible T cell α chemoattractant (I-TAC), and IFN-γ-inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunol. 1999;162:4928–4937. [PubMed] [Google Scholar]
- 26.Ghiasi H, Cai S, Perng GC, Nesburn AB, Wechsler SL. Both CD4+ and CD8+ T cells are involved in protection against HSV-1 induced corneal scarring. Br J Ophthalmol. 2000;84:408–412. doi: 10.1136/bjo.84.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillgrass AE, Fernandez SA, Rosenthal KL, Kaushic C. Estradiol regulates susceptibility following primary exposure to genital herpes simplex virus type 2, while progesterone induces inflammation. J Virol. 2005;79:3107–3116. doi: 10.1128/JVI.79.5.3107-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halford WP, Gebhardt BM, Carr DJJ. Persistent Cytokine Expression in Trigeminal Ganglion Latently Infected with Herpes Simplex Virus Type 1. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 29.Halford WP, Gebhardt BM, Carr DJJ. Acyclovir blocks cytokine gene expression in trigeminal ganglia latently infected with herpes simplex virus type 1. Virology. 1997;238:53–63. doi: 10.1006/viro.1997.8806. [DOI] [PubMed] [Google Scholar]
- 30.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Protective vaccination against genital herpes simplex virus type (HSV-2) infection in mice is associated with a rapid induction of local IFN-gamma-dependent RANTES production following a vaginal viral challenge. Am J Reprod Immunol. 2001;46:420–424. doi: 10.1034/j.1600-0897.2001.d01-34.x. [DOI] [PubMed] [Google Scholar]
- 31.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immunity. J Virol. 2001;75:6705–6709. doi: 10.1128/JVI.75.14.6705-6709.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol. 2001;82:845–853. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- 33.Harle P, Noisakran S, Carr DJJ. The Application of a Plasmid DNA Encoding IFN-α1 Postinfection Enhances Cumulative Survival of Herpes Simplex Virus Type 2 Vaginally Infected Mice. J Immunol. 2001;166:1803–1812. doi: 10.4049/jimmunol.166.3.1803. [DOI] [PubMed] [Google Scholar]
- 34.He J, Ichimura H, Iida T, Minami M, Kobayashi K, Kita M, Sotozono C, Tagawa Y-I, Iwakura Y, Imanishi J. Kinetics of cytokine production in the cornea and trigeminal ganglion of C57BL/6 mice after corneal HSV-1 infection. J Interferon Cytokine Res. 1999;19:609–615. doi: 10.1089/107999099313749. [DOI] [PubMed] [Google Scholar]
- 35.Hendricks RL, Janowicz M, Tumpey TM. Critical role of corneal Langerhans cells in the CD4+ mediated but not CD8+ mediated immunopathology in herpes simplex virus-1 infected corneas. J Immunol. 1992;148:2522–2529. [PubMed] [Google Scholar]
- 36.Johnson AC, Heinzel FP, Diaconu E, Sun Y, Hise AG, Golenbock D, Lass JH, Pearlman E. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Vis Sci. 2005;46:589–595. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- 37.Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khanna KM, Lepisto AJ, Decman V, Hendricks RL. Immune control of herpes simplex virus during latency. Curr Opin Immunol. 2004;16:463–469. doi: 10.1016/j.coi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 40.King NJC, Parr EL, Parr MB. Migration of Lymphoid Cells from Vaginal Epithelium to Iliac Lymph Nodes in Relation to Vaginal Infection by Herpes Simplex Virus Type 2. J Immunol. 1998;160:1173–1180. [PubMed] [Google Scholar]
- 41.Kodukula P, Liu T, Rooijen NV, Jager MJ, Hendricks RL. Macrophage Control of Herpes Simplex Virus Type 1 Replication in the Peripheral Nervous System. J Immunol. 1999;162:2895–2905. [PubMed] [Google Scholar]
- 42.Koelle DM, Corey L. Recent Progress in Herpes Simplex Virus Immunobiology and Vaccine Research. Clin Micro Rev. 2003;16:96–113. doi: 10.1128/CMR.16.1.96-113.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koelle DM, Gonzalez JC, Johnson AS. Homing in on the Cellular Immune Response to HSV-2 in Humans. Am J Reprod Immunol. 2005;53:172–181. doi: 10.1111/j.1600-0897.2005.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 45.Kumaraguru U, Davis I, Rouse BT. Chemokines and ocular pathology caused by corneal infection with herpes simplex virus. J NeuroVirol. 1999;5:42–47. doi: 10.3109/13550289909029744. [DOI] [PubMed] [Google Scholar]
- 46.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci USA. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang A, Nikolich-Zugich J. Development and Migration of Protective CD8+ T Cells into the Nervous System following Ocular Herpes Simplex Virus-1 Infection. J Immunol. 2005;174:2919–2925. doi: 10.4049/jimmunol.174.5.2919. [DOI] [PubMed] [Google Scholar]
- 48.Linehan MM, Richman S, Krummenacher C, Eisenberg RJ, Cohen GH, Iwasaki A. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. J Virol. 2004;78:2530–2536. doi: 10.1128/JVI.78.5.2530-2536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu T, Tang Q, Hendricks RL. Inflammatory Infiltration of the Trigeminal Ganglion after Herpes Simplex Virus Type 1 Corneal Infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu T, Khanna KM, Chen XP, Fink DJ, Hendricks RL. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu T, Khanna KM, Carriere BN, Hendricks RL. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J Virol. 2001;75:11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AEI, Plachy J, Bruhl H, Frink M, Anders HJ, Vielhauer V, Pfirstinger J, Stangassinger M, Schlondorff D. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol. 2001;166:4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- 54.Maertzdorf J, Osterhaus ADME, Verjans GMGM. IL-17 Expression in Human Herpetic Stromal Keratitis: Modulatory Effects on Chemokine Production by Corneal Fibroblasts. J Immunol. 2002;169:5897–5903. doi: 10.4049/jimmunol.169.10.5897. [DOI] [PubMed] [Google Scholar]
- 55.McDermott MR, Goldsmith CH, Rosenthal KL, Brais LJ. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J Infect Dis. 1989;159:460–466. doi: 10.1093/infdis/159.3.460. [DOI] [PubMed] [Google Scholar]
- 56.Melchjorsen J, Paludan SR. Induction of RANTES/CCL5 by herpes simplex virus is regulated by nuclear factor κB and interferon regulatory factor 3. J Gen Virol. 2003;84:2491–2495. doi: 10.1099/vir.0.19159-0. [DOI] [PubMed] [Google Scholar]
- 57.Melchjorsen J, Pedersen FS, Mogensen SC, Paludan SR. Herpes Simplex Virus Selectively Induces Expression of the CC Chemokine RANTES/CCL5 in Macrophages through a Mechanism Dependent on PKR and ICP0. J Virol. 2002;76:2780–2788. doi: 10.1128/JVI.76.6.2780-2788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mercadal CM, Bouley DM, DeStephano D, Rouse BT. Herpetic stromal keratitis in the reconstituted scid mouse model. J Virol. 1993;67:3404–3408. doi: 10.1128/jvi.67.6.3404-3408.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milligan GN. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J Virol. 1999;73:6380–6386. doi: 10.1128/jvi.73.8.6380-6386.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milligan GN, Bernstein DI. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology. 1995a;212:481–489. doi: 10.1006/viro.1995.1506. [DOI] [PubMed] [Google Scholar]
- 61.Milligan GN, Bernstein DI. Interferon-γ enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 62.Milligan GN, Bernstein DI. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology. 1995b;206:234–241. doi: 10.1016/s0042-6822(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 63.Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol. 2004;172:4618–4623. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 64.Mossman KL, Macgregor PF, Rozmus JJ, Goryachev AB, Edwards AM, Smiley JR. Herpes Simplex Virus Triggers and Then Disarms a Host Antiviral Response. J Virol. 2001;75:750–758. doi: 10.1128/JVI.75.2.750-758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niemialtowski MG, Rouse BT. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992;149:3035–3039. [PubMed] [Google Scholar]
- 66.Nishimura H, Yahima T, Kagimoto Y, Ohata M, Watase T, Kishihara K, Goshima F, Nishiyama Y, Yoshikai Y. Intraepithelial γδ T Cells May Bridge a Gap between Innate Immunity and Acquired Immunity to Herpes Simplex Virus Type 2. J Virol. 2004;78:4927–4930. doi: 10.1128/JVI.78.9.4927-4930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noisakran S, Härle P, Carr DJJ. ICAM-1 is required for resistance to herpes simplex virus type 1 but no interferon-α1 transgene efficacy. Virology. 2001;283:69–77. doi: 10.1006/viro.2001.0858. [DOI] [PubMed] [Google Scholar]
- 68.Noisakran S, Halford WP, Veress L, Carr DJJ. Role of the Hypothalamic Pituitary Adrenal Axis and IL-6 in Stress-Induced Reactivation of Latent Herpes Simplex Virus Type 1. J Immunol. 1998;160:5441–5447. [PubMed] [Google Scholar]
- 69.Norris DA. Cytokine modulation of adhesion molecules in the regulation of immunologic cytotoxicity of epidermal targets. J Invest Dermatol. 1990;95:111S–120S. doi: 10.1111/1523-1747.ep12874977. [DOI] [PubMed] [Google Scholar]
- 70.Ohara PT, Chin MS, LaVail JH. The Spread of Herpes Simplex Virus Type 1 from Trigeminal Neurons to the Murine Cornea: an Immunoelectron Microscopy Study. J Virol. 2000;74:4776–4786. doi: 10.1128/jvi.74.10.4776-4786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan ZZ, Parkyn L, Ray A, Ray P. Inducible lung-specific expression of RANTES preferential recruitment of neutrophils. Am J Physiol Cell Mol Physiol. 2000;279:L658–L666. doi: 10.1152/ajplung.2000.279.4.L658. [DOI] [PubMed] [Google Scholar]
- 72.Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–380. [PubMed] [Google Scholar]
- 73.Parr MB, Parr EL. Mucosal Immunity to Herpes Simplex Virus Type 2 Infections in the Mouse Vagina is Impaired by In Vivo Depletion of T Lymphocytes. J Virol. 1998;72:2677–2685. doi: 10.1128/jvi.72.4.2677-2685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parr MB, Parr EL. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. 1999;258:282–294. doi: 10.1006/viro.1999.9739. [DOI] [PubMed] [Google Scholar]
- 75.Parr MB, Harriman GR, Parr EL. Immunity to vaginal HSV-2 infection in immunoglobulin A knockout mice. Immunology. 1998;95:208–213. doi: 10.1046/j.1365-2567.1998.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parr MB, Parr EL. Interferon-γ up-regulates intercellular adhesion molecule-1 and vascular adhesion molecule-1 and recruits lymphocytes into the vagina of immune mice challenged with herpes simplex virus type 2. Immunology. 2000;99:540–545. doi: 10.1046/j.1365-2567.2000.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piali L, Weber C, LaRosa G, Mackay CR, Springer TA, Clark-Lewis I, Moser B. The chemokine receptor CXCR3 mediates rapid and shearing resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur J Immunol. 1998;28:961–972. doi: 10.1002/(SICI)1521-4141(199803)28:03<961::AID-IMMU961>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 78.Posavad CM, Huang MI, Barcy S, Koelle DM, Corey L. Long Term Persistence of Herpes Simplex Virus-Specific CD8+ CTL in Persons with Frequently Recurring Genital Herpes. J Immunol. 2000;165:1146–1152. doi: 10.4049/jimmunol.165.2.1146. [DOI] [PubMed] [Google Scholar]
- 79.Posavad CM, Koelle DM, Shaughnessy MF, Corey L. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci USA. 1997;94:10289–10294. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Ko AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rabin RL, Alston MA, Sircus JC, Knollmann-Ritschel B, Moratz C, Ngo D, Farber JM. CXCR3 is induced early on the pathway of CD4+ T cell differentiation and bridges central and peripheral functions. J Immunol. 2003;171:2812–2824. doi: 10.4049/jimmunol.171.6.2812. [DOI] [PubMed] [Google Scholar]
- 82.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 83.Russell RG, Nasisse MP, Larsen S, Rouse BT. Role of T-lymphocytes in the pathogenesis of herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 1984;25:938–944. [PubMed] [Google Scholar]
- 84.Seo SK, Park HY, Choi JH, Kim WY, Kim YH, Jung HW, Kwon B, Lee HW, Kwon BS. Blocking 4-1BB/4-1BB ligand interactions prevents herpetic stromal keratitis. J Immunol. 2003;171:576–583. doi: 10.4049/jimmunol.171.2.576. [DOI] [PubMed] [Google Scholar]
- 85.Sin J, Kim JJ, Pachuk C, Satishchandran C, Weiner DB. DNA Vaccines Encoding Interleukin-8 and RANTES Enhance Antigen-Specific Th1-Type CD4+ T-Cell-Mediated Protective Immunity against Herpes Simplex Virus Type 2 In Vivo. J Virol. 2000;74:11173–11180. doi: 10.1128/jvi.74.23.11173-11180.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sonnex G. Influence of ovarian hormones on urogenital infection. Sex Transm Infect. 1998;74:11–19. doi: 10.1136/sti.74.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sonoda KH, Sasa Y, Qiao H, Tsutsumi C, Hisatomi T, Komiyama S, Kubota T, Sakamoto T, Kawano Y-I, Ishibashi T. Immunoregulatory role of ocular macrophages: the macrophage produce RANTES to suppress experimental autoimmune uveitis. J Immunol. 2003;171:2652–2659. doi: 10.4049/jimmunol.171.5.2652. [DOI] [PubMed] [Google Scholar]
- 88.Staats HF, Lausch RN. Cytokine expression in vivo during murine herpetic stromal keratis. J Immunol. 1993;151:277–283. [PubMed] [Google Scholar]
- 89.Stewart MR, Cabanas C, Hogg N. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J Immunol. 1996;156:1810–1817. [PubMed] [Google Scholar]
- 90.Su YH, Yan XT, Oakes JE, Lausch RN. Protective Antibody Therapy is Associated with Reduced Chemokine Transcripts in Herpes Simplex Virus Type 1 Corneal Infection. J Virol. 1996;70:1277–1281. doi: 10.1128/jvi.70.2.1277-1281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 92.Svensson A, Nordstrom I, Sun JB, Eriksson K. Protective Immunity to Genital Herpes Simplex Virus Type 2 Infection is Mediated by T-bet. J Immunol. 2005;174:6266–6273. doi: 10.4049/jimmunol.174.10.6266. [DOI] [PubMed] [Google Scholar]
- 93.Tang Q, Chen W, Hendricks RL. Proinflammatory functions of IL-2 in herpes simplex virus corneal infection. J Immunol. 1997;158:1275–1283. [PubMed] [Google Scholar]
- 94.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human-interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion and endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. Latent Herpesvirus Infection in Human Trigeminal Ganglia Causes Chronic Immune Response. Am J Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thomas J, Gangappa S, Kanangat S, Rouse BT. On the Essential Involvement of Neutrophils in the Immunopathology Disease. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 97.Tumpey TM, Chen S, Oakes JE, Lausch RN. Neutrophil-Mediated Suppression of Virus Replication after Herpes Simplex Virus Type 1 Infection of the Murine Cornea. J Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tumpey TM, Cheng H, Cook DN, Smithies O, Oakes JE, Lausch RN. Absence of Macrophage Inflammatory Protein-1α Prevents the Development of Blinding Herpes Stromal Keratitis. J Virol. 1998;72:3705–3710. doi: 10.1128/jvi.72.5.3705-3710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tumpey TM, Cheng H, Yan X, Oakes JE, Lausch RN. Chemokine synthesis in the HSV-1-infected cornea and its suppression by interleukin-10. J Leukoc Biol. 1998;63:486–492. doi: 10.1002/jlb.63.4.486. [DOI] [PubMed] [Google Scholar]
- 100.Van Damme J, Decock B, Bertini R, Conings R, Lenaerts JP, Put W, Opdenakker G, Mantovani A. Production and identification of natural monocyte chemotactic protein from virally infected murine fibroblasts. Relationship with the product of the mouse competence (JE) gene. Eur J Biochem. 1991;199:223–229. doi: 10.1111/j.1432-1033.1991.tb16113.x. [DOI] [PubMed] [Google Scholar]
- 101.Wasmuth S, Bauer D, Yang Y, Steuhl KP, Heiligenhaus A. Topical treatment with antisense oligonucleotides targeting tumor necrosis factor-α in herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 2003;44:5228–5234. doi: 10.1167/iovs.03-0312. [DOI] [PubMed] [Google Scholar]
- 102.Whiting D, Hsieh G, Yun JJ, Banerji A, Yao W, Fishbein MC, Belperio J, Strieter RM, Bonavida B, Ardehali A. Chemokine monokine induced by IFN-γ/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production. J Immunol. 2004;172:7417–7424. doi: 10.4049/jimmunol.172.12.7417. [DOI] [PubMed] [Google Scholar]
- 103.Wickham S, Carr DJJ. Molecular mimicry versus bystander activation: herpetic stromal keratitis. Autoimmunity. 2004;37:393–397. doi: 10.1080/08916930410001713106. [DOI] [PubMed] [Google Scholar]
- 104.Yan XT, Tumpey TM, Kunkel SL, Oakes JE, Lausch RN. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Invest Ophthalmol Vis Sci. 1998;39:1854–1862. [PubMed] [Google Scholar]
- 105.Zeng X, Moore TA, Newstead MW, Deng JC, Lukacs NW, Standiford TJ. IP-10 mediates selective mononuclear cell accumulation and activation in response to intrapulmonary transgenic expression during adenovirus-induced pulmonary inflammation. J Interferon Cytokine Res. 2005;25:103–112. doi: 10.1089/jir.2005.25.103. [DOI] [PubMed] [Google Scholar]
- 106.Zheng M, Klinman DM, Gierynska M, Rouse BT. DNA containing CpG motifs induces angiogenesis. Proc Natl Acad Sci USA. 2002;99:8944–8949. doi: 10.1073/pnas.132605599. [DOI] [PMC free article] [PubMed] [Google Scholar]


