Abstract
Rationale
The efflux capacity of HDL with cultured macrophages associates strongly and negatively with CAD status, indicating that impaired sterol efflux capacity might be a marker—and perhaps mediator—of atherosclerotic burden. However, the mechanisms that contribute to impaired sterol efflux capacity remain poorly understood.
Objective
To determine the relationship between myeloperoxidase-mediated oxidative damage to apoA-I, the major HDL protein, and the ability of HDL to remove cellular cholesterol by the ABCA1 pathway.
Methods and Results
We quantified both site-specific oxidation of apoA-I and HDL’s ABCA1 cholesterol efflux capacity in control subjects and subjects with stable coronary artery disease or acute coronary syndrome. The CAD and ACS subjects had higher levels of chlorinated tyrosine-192 and oxidized methionine-148 than the control subjects. In contrast, plasma levels of MPO did not differ between the groups. HDL from the CAD and ACS subjects was less able to accept cholesterol from cells expressing ABCA1 than HDL from control subjects. Levels of chlorinated tyrosine and oxidized methionine associated inversely with ABCA1 efflux capacity and positively with atherosclerotic disease status. These differences remained significant after adjusting for HDL-cholesterol levels.
Conclusions
Our observations indicate that MPO may contribute to the generation of dysfunctional HDL with impaired ABCA1 efflux capacity in humans with atherosclerosis. Quantification of chlorotyrosine and oxidized methionine in circulating HDL might be useful indicators of the risk of cardiovascular disease that are independent of HDL-cholesterol.
Keywords: Acute coronary syndrome, cardiovascular diseases, 3-chlorotyrosine, mass spectrometry, myeloperoxidase, selected reaction monitoring
INTRODUCTION
Clinical and epidemiological studies show a robust, inverse association of high density lipoprotein cholesterol (HDL-C) levels with cardiovascular disease risk 1. In mouse models of atherosclerosis, HDL’s ability to remove cholesterol from macrophages in the artery wall is a key cardioprotective function 2, 3. A well-established pathway involves apolipoprotein A-I (apoA-I), HDL’s major protein 4. Lipid-free apoA-I promotes cholesterol efflux from macrophages via ABCA1, a membrane-associated transporter 5, 6. Humans lacking ABCA1 suffer from Tangier disease 7, which is characterized by low levels of HDL-C, the accumulation of cholesterol-loaded macrophage in many different tissues 8, 9. Ablation of ABCA1 in mouse myeloid cells promotes atherosclerosis in hypercholesterolemic mice without affecting HDL-C levels 10, 11, indicating that cholesterol efflux by the ABCA1 pathway is of central importance in the cardioprotective effects of HDL. Inflammation impairs macrophage sterol efflux in animal models, rendering HDL dysfunctional 12-14. However, the underlying mechanisms and relevance of these findings to human atherosclerosis remain unclear.
Many lines of evidence indicate that oxidative stress plays a role in the pathogenesis of cardiovascular disease 15, 16. One important mechanism may involve damage to HDL by myeloperoxidase (MPO), a heme protein expressed at high levels by inflammatory macrophages in human atherosclerotic tissue 14, 17. The enzyme uses hydrogen peroxide (H2O2) and chloride ion to produce hypochlorous acid (HOCl), which converts tyrosine (Tyr) to 3-chlorotyrosine 18-20. It also oxidizes methionine residues 21. When lipid-free apoA-I is oxidized by MPO in vitro, its ability to remove excess cellular cholesterol by the ABCA1 pathway is decreased 22-24. Moreover, in vitro studies with a mutated form of apoA-I and biochemical studies with methionine sulfoxide reductase suggest that chlorination of Tyr192 in concert with oxidation of methionine (Met) impairs ABCA1 transport activity 25. Oxidation of Met in apoA-I also impairs its ability to activate lecithin-cholesterol acyltransferase (LCAT) 26, which plays a key role in maturation of HDL. Elevated levels of chlorotyrosine have been detected in HDL isolated from coronary disease subjects 22, 24 and Tyr192 is the major chlorination site in apoA-I of HDL isolated from human atherosclerotic lesions 27. These observations suggest that oxidation might impair human HDL’s cardioprotective functions, such as cholesterol clearance from macrophages in the artery wall.
Inflammation and metabolic disorders have been proposed to convert HDL to a dysfunctional form lacking anti-atherogenic properties 12, 13, 28. Consistent with this proposal, the ability of serum HDL to promote sterol efflux from cultured macrophages varies markedly, despite similar levels of HDL-C and apoA-I 29. Therefore, HDL-C is not necessarily the major determinant of HDL’s macrophage sterol efflux capacity. One proposed factor is HDL’s activity with the ABCA1 pathway 29. Importantly, the efflux capacity of serum HDL with cultured macrophages associated strongly and negatively with CAD status 30, and that association was independent of HDL-C and apoA-I levels. Taken together, these observations suggest that serum HDL’s capacity to promote sterol efflux from macrophages reflects its functionality, raising the possibility that this function of HDL provides insight into the relationship between HDL biology and CAD risk.
The factors that control the efflux capacity of serum HDL remain poorly understood. In the current study, we isolated HDL from control subjects and subjects with stable coronary artery disease 31 or acute coronary syndrome 32, and used tandem mass spectrometry to explore the relationship between site-specific oxidation of apoA-I and the protein’s ability to promote cholesterol efflux by the ABCA1 pathway. We show that levels of apoA-I chlorinated and oxidized by MPO are elevated in CAD and ACS subjects, and that they correlate inversely with HDL’s cholesterol efflux capacity. Moreover, HDL from the CAD and ACS subjects was less able to accept sterol from cells expressing ABCA1 than HDL from control subjects. Thus, MPO may help generate dysfunctional HDL in humans.
Methods
Subjects
Plasma samples were collected prospectively over 10 months from three groups of subjects at the University of Washington: (i) control subjects (n=20), (ii) stable CAD subjects (n=20), and (iii) ACS subjects (n=20). The control subjects, who were recruited by advertisement, had no clinical history of cardiovascular disease, no family history of premature CAD, and were not receiving lipid-lowering therapy. The stable CAD subjects had clinically established atherosclerotic vascular disease but had been stable for at least 3 months. CAD subjects also had at least one ≥50% stenotic lesion on coronary angiography or a history of myocardial infarction, percutaneous coronary intervention or coronary artery bypass grafting. All ACS subjects exhibited acute ECG changes consistent with myocardial ischemia and/or elevated troponin levels. ACS was confirmed with urgent coronary angiography; all subjects had at least one ≥50% stenotic lesion with ruptured plaque and/or thrombus. All subjects provided signed informed consent and all protocols were approved by the University of Washington Institutional Review Board.
Plasma levels of lipids, high-sensitivity CRP (hs-CRP) and MPO
Lipid profiles and high sensitivity CRP (hs-CRP) were determined by the Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington. All lipid analyses were standardized using the Centers for Disease Control and Prevention Reference Methods. MPO levels in plasma were measured by the Cleveland Heart Lab (Cleveland, OH).
HDL isolation from plasma
Blood was collected from overnight fasted subjects into ice-cold tubes containing EDTA (6 mM final concentration). To protect Met residues from oxidation 33, plasma was prepared immediately by centrifugation (2500 g for 15 min) and frozen at −80°C until analysis. HDL (density 1.063-1.210 g/mL) was isolated by sequential ultracentrifugation from freshly thawed plasma 27, using buffers supplemented with 100 μM diethylenetriaminepentaacetic acid (DTPA), 100 μM butylated hydroxytoluene (BHT), and a protease inhibitor mixture (Sigma, St. Louis, MO). All studies were performed on samples that had been stored for <12 months, and freshly prepared Met (10 mM final) was added to each sample prior to work-up for MS analysis.
Isotope-labeled apoA-I
[15N]apoA-I was prepared by growing bacteria that stably express human apoA-I in minimal medium supplemented with [15N]ammonium chloride 34.
Mass spectrometric (MS) analysis with selected reaction monitoring (SRM)
The protein concentration of HDL was determined using the Lowry assay (BioRad), with albumin as the standard. Following the addition of freshly prepared Met (10 mM final concentration), proteins were reduced with dithiothreitol and alkylated with iodoacetamide 27. Following digestion of apoA-I with sequencing grade modified trypsin (Promega) or endoproteinase Glu-C (from Staphylococcus aureus V8, Roche Applied Science), site-specific oxidation of apoA-I was quantified by SRM, a quantitative and sensitive MS/MS technique for detecting peptides and their post-translational modifications 27, 35, in peptide digests of HDL with a nano-LC-MS/MS as described 27. Additional details are provided in Supplemental Materials.
Ex vivo oxidation of HDL
Preliminary studies demonstrated that Tyr chlorination was negligible in recombinant apoA-I and reconstituted HDL (rHDL) analyzed by tandem MS, indicating that sample work-up and MS analysis did not contribute significantly to ex vivo oxidation. However, when rHDL disks made from apoA-I and phospholipid containing linoleic acid (which is highly susceptible to oxidation) were isolated by ultracentrifugation, oxidation of Met86 and Met112 in apoA-I increased significantly (Supplemental Fig. I). In contrast, there was no evidence of increased oxidation of Met148 (Supplemental Fig. I). Moreover, there was no additional oxidation of Met148 in 15N-apoA-I when rHDL (made from 15N-apoA-I) was incubated in plasma and then isolated by ultracentrifugation (Supplemental Fig. II). Addition to plasma of azide (10 mM), a potent inhibitor of MPO, did not affect Met oxidation or Tyr chlorination of rHDL or HDL (Supplemental Fig. II, III). These data suggest that lipid oxidation products, but not plasma MPO or other heme proteins, can contribute to ex vivo oxidation of Met86 and Met112 – but not Met148 – as previously described 36.
ABCA1 efflux capacity of serum HDL and in vitro oxidized HDL
Serum was derived from plasma by adding calcium 29. Polyethylene glycol was then used to precipitate lipoproteins containing apolipoprotein B, and the supernatant was centrifuged to generate serum HDL. ABCA1-specific sterol efflux to serum HDL was quantified using baby hamster kidney (BHK) cells expressing mifepristone-inducible human ABCA1 30, 37. HDL isolated by ultracentrifugation was oxidized with HOCl and reduced with pilB as described 25, 26. ABCA1-specific efflux capacity of oxidized and reduced HDL was measured as previously described 25, 37. Additional details are provided in Supplemental Materials.
Statistical analysis
Continuous variables are presented as means and SDs and categorical variables as frequencies and percentages. Because levels of chloroTyr192 and Met(O)148 exhibited a non-normal distribution in our study population, we used logarithmic transformation in all analyses. Linear regression analysis with continuous variables used Pearson’s coefficient. Multiple logistic regression was used to estimate the association between 3-chloroTyr192, Met(O)148, cholesterol efflux capacity, and cardiovascular disease status after adjustment for HDL-C levels. Odds ratios are reported for 1-SD change for continuous variables. Significant P values were α<0.05 on two-tailed analysis. Statistical analyses were performed with SPSS (Windows version 19, Chicago, IL) or OriginPro (version 8.6, Origin Lab, Northampton, MA).
RESULTS
The subjects’ clinical characteristics and lipid values are shown in Table 1. Analysis with one-way ANOVA indicated a significant difference in HDL-C levels among the groups (P=0.0003). Further analysis with Fisher’s least significance difference [LSD] test demonstrated that the stable CAD (42±11 mg/dL, n=20) and ACS groups (47±17 mg/dL, n=20) had significantly lower HDL-C levels (P=0.0001 and P=0.003, respectively) than the healthy control group (61±16 mg/dL, n=20). LDL-C levels were similar in the control and ACS groups but lower in the CAD group (P=0.009, Fisher’s LSD test, CAD vs. control), likely because all CAD subjects were on statin therapy. Plasma levels of MPO were similar in the control and CAD groups, but higher in the ACS group. However, the differences in MPO levels between the groups were not significant by one-way ANOVA. In contrast, the ACS and CAD groups had significantly higher levels of hs-CRP than the control group (P=0.02 and P<0.0001, respectively, Fisher’s LSD test).
Table 1.
Clinical Characteristics of Study Subjects
| Control (n=20) | Stable CAD (n=20) | ACS (n=20) | |
|---|---|---|---|
| Age (years) | 56 ± 8 | 64 ± 11* | 63 ± 12 |
| Male (%) | 15 (75%) | 14 (70%) | 12 (60%) |
| Hypertensive patients (%) | 2 (10%) | 15 (75%) *** | 10 (50%) *** |
| Diabetic patients (%) | 0 (0%) | 3 (15%) | 9 (45%) *** |
| Smokers (%) | 4 (20%) | 13 (65%) *** | 12 (60%) ** |
| Total cholesterol (mg/dL) | 203 ± 35 | N/A | 186 ± 41 |
| HDL cholesterol (mg/dL) | 61 ± 16 | 42 ± 11*** | 47 ± 17 *** |
| LDL cholesterol (mg/dL) | 117 ± 25 | 94 ± 52 | 114 ± 42 |
| Triglycerides (mg/dL) | 123 ± 118 | 158 ± 93 | 126 ± 72 |
| MPO (pmol/L) | 809 ± 1,431 | 920 ± 2,033 | 1610 ± 2,267 |
| hs-CRP (μg/dL) | 88 ± 92 | 290 ± 406 * | 1,369 ± 3,442 *** |
Plus-minus values are means and SDs. MPO and hs-CRP values were log transformed before one-way ANOVA analysis. NA, not available.
P-value less than 0.05, 0.01 or 0.001, respectively, versus control subjects by Fisher’s least significant differences test (after one-way ANOVA with P<0.05).
Stable CAD and ACS subjects have elevated levels of chlorinated and oxidized apoA-I
To quantify oxidation sites in HDL isolated by ultracentrifugation from human plasma of the control, CAD and ACS subjects, we supplemented the HDL with [15N]apoA-I chlorinated with reagent HOCl before digesting the proteins with trypsin or Glu-C. Levels of 3-chlorotyrosine and Met(O) were quantified by tandem mass spectrometry with SRM and isotope dilution 27, 35. This sensitive and quantitative approach detected all seven peptides that contain Tyr, all three peptides that contain Met, and the corresponding chlorinated and oxidized products.
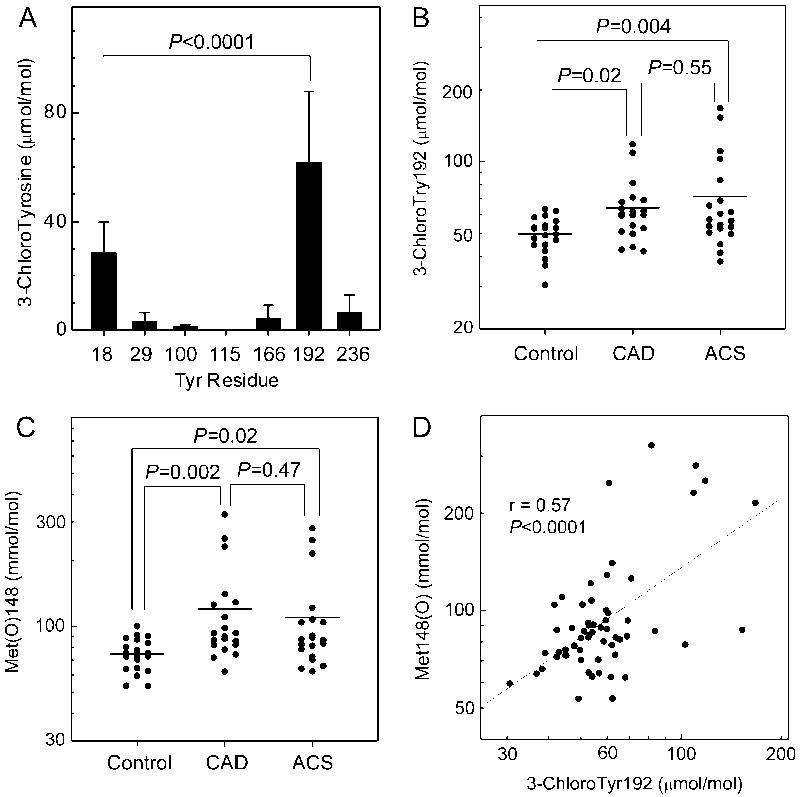
Chlorinated peptides were readily detectable in the proteolytic digests of apoA-I prepared from HDL isolated from human plasma (Fig. 1A). Moreover, we identified Tyr192 as the major chlorination site in apoA-I of HDL isolated from both the diseased and control subjects (P<0.0001 vs. Tyr-18, Student’s t-test), as previously demonstrated for HDL isolated from human atherosclerotic lesions 27. The average level (mean±SD) of 3-chloroTyr192 in all the subjects was 62±26 μmol/mol Tyr. Tyr18 was the second major site of chlorination, with an average level of 29±11 μmol/mol Tyr. Lower levels of 3-chlorotyrosine (<10 μmol/mol Tyr) were detected at the other five Tyr residues.
Figure 1. Site-specific tyrosine chlorination and methionine oxidation of apoA-I in control subjects, CAD subjects, and ACS subjects.
(A) Chlorination of Tyr residue in apoA-I for all subjects. (B) Levels of chloroTyr192 in control subjects, CAD subjects, and ACS subjects. (C) Levels of Met(O)148 in control subjects, CAD subjects, and ACS subjects. (D) Correlation of Tyr chlorination and Met oxidation in all subjects. HDL was isolated by ultracentrifugation from freshly prepared plasma of CAD, ACS, or healthy subjects. Following the addition of oxidized [15N]apoA-I as internal standard, HDL was subjected to proteolytic digestion. Peptide digests were analyzed by tandem MS with SRM. Values for chloroTyr192 and Met(O)148 were logarithmically transformed to achieve a normal distribution.
To determine whether the healthy and diseased groups had different levels of 3-chlorotyrosine in the apoA-I from their plasma HDL, we calculated the overall levels of Tyr chlorination for all seven Tyr residues. The total level of protein-bound 3-chlorotyrosine in the control group was 14±2.5 μmol/mol Tyr (n=20). In the CAD (n=20) and ACS (n=20) groups, it was 17±3.9 μmol/mol Tyr and 20±8.2 μmol/mol Tyr, respectively. Analysis by one-way ANOVA indicated total chloroTyr levels differed among the groups (P=0.009). Total levels of 3-chlorotyrosine in apoA-I of HDL from the CAD or ACS subjects were significantly higher than in the healthy control subjects (P=0.04 and P=0.002, Fisher’s LSD test). Levels of 3-chlorotyrosine ranged from 10 to 19 μmol/mol Tyr in the control group, 11 to 24 μmol/mol Tyr in the CAD group, and 10 to 41 μmol/mol Tyr in the ACS group.
CAD and ACS subjects have elevated levels of chlorinated Tyr192 and oxidized Met148
Because Tyr192 was the major chlorination site in apoA-I of HDL isolated from human plasma, we determined whether CAD or ACS subjects had higher levels of that chlorinated residue (Fig. 1B). The average level of 3-chloroTyr192 in the control group was 50±8.5 μmol/mol Tyr. After log transformation of 3-chloroTyr192 values, levels differed significantly among the groups (P=0.01, one-way ANOVA). Levels were significantly higher (Fisher’s LSD test) in the CAD subjects (64±20 μmol/mol Tyr, P=0.02) and ACS subjects (71±36 μmol/mol Tyr, P=0.004) than in the control subjects. However, the difference between the CAD and ACS levels was not significant.
We quantified levels of oxidation of all 3 Met residues in apoA-I (Supplemental Fig. IV). However, we focused on Met(O)148 because oxidation of that residue strongly associates with impaired ABCA1-mediated cholesterol efflux activity and reduced ability to activate LCAT 25, 26. Importantly, preliminary studies indicated that Met148 was resistant to ex vivo oxidation (Methods and Supplemental Fig. I, II). In the control group, the average level of Met(O)148 was 75±13 mmol/mol Met (Fig. 1C). After log transformation of Met(O)148 values, levels were significantly higher in the CAD subjects (120±69 mmol/mol Met, P=0.002) and ACS subjects (110±62 mmol/mol Met, P=0.02) than in the control subjects.
Studies of MPO-deficient mice strongly suggest that MPO is the only generator of 3-chlorotyrosine during acute inflammation 38. In contrast, other oxidative pathways can produce methionine sulfoxide 21. To determine whether MPO might be an important pathway for promoting methionine oxidation in humans, we assessed the relationship between 3-chloroTyr192 levels and Met(O)148 levels (Fig. 1D) in all of the subjects (n=60). Linear regression analysis demonstrated a strong correlation (Pearson’s r=0.57; P<0.0001) The levels of 3-chloroTyr192 also correlated with levels of Met(O)86 (r=0.52, P<0.0001, Supplemental Fig. IV D) and Met(O)112 (r=0.58, P<0.0001, Supplemental Fig. IV E). Our observations suggest that MPO contributes to Met oxidation in vivo in human apoA-I.
We also assessed the relationship between plasma MPO levels and levels of 3-chloroTyr192 and oxidized Met residues in HDL (Supplemental Fig. V). Linear regression analysis revealed that MPO levels were modestly correlated with levels of 3-chloroTyr192 (r=0.34, P=0.008) but failed to correlate with levels of Met(O)148 (r= 0.24, P=0.06). Supplementation of plasma with 10 mM azide, a potent inhibitor of MPO, had no effect on Met oxidation or Tyr chlorination (Supplemental Material, Fig. II, III). These observations suggest that MPO in plasma has little influence on HDL oxidation.
Correlations of 3-chloroTyr192 and Met(O)148 with traditional CAD risk factors
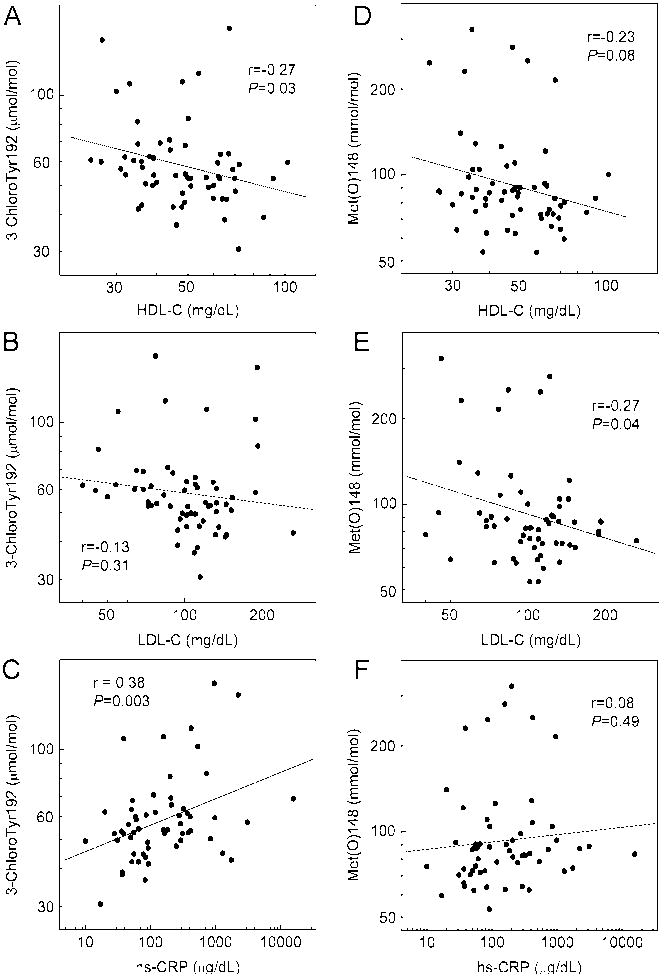
We examined the relationship between 3-chloroTyr192 levels in HDL and traditional CAD risk factors in this cohort of control and high-risk patients (Fig. 2). Levels of 3-chloroTyr192 did not associate with sex or hypertension; they also failed to correlate with total cholesterol, non-HDL-C, age, or triglycerides (data not shown). However, they were significantly related to smoking status (53.4 mol/mol vs. 70.6 mol/mol, non-smoker vs. smoker, P=0.007, two-tailed Student’s t-test) and diabetes (56.4 mol/mol vs. 83.2 mol/mol, non-diabetic vs. diabetic subjects, P=0.001). Levels of 3-chloroTyr192 correlated inversely with HDL-C levels (r=-0.27; P=0.03, Fig. 2A) but not with LDL-C levels (r=-0.13; P=0.31, Fig. 2B). Levels of 3-chloroTyr192 also correlated modestly with levels of hs-CRP (r=0.38; P=0.003, Fig. 2C).
Figure 2. Correlation of 3-chloroTyr192 and Met(O)148 with cardiovascular risk factors.
Levels of 3-chloroTyr192 and Met(O)148 were quantified as described in the legends of Fig. 1. Relationships between 3-chloroTyr192 or Met(O)148 and HDL-C (A, D), LDL-C (B, E), and hs-CRP (C, F) were linear regression analyses using Pearson’s correlation coefficient. Where indicated, values were logarithmically transformed.
We next examined the relationship between Met(O)148 levels and traditional CAD risk factors. Sex, age, hypertension, smoking status, total cholesterol, non-HDL-C, and triglyceride did not associate with levels of Met(O)148. However, Met(O)148 associated significantly with diabetes (92 mmol/mol Met vs. 138 mmol/mol Met, non-diabetic subjects vs. diabetic subjects, P=0.01). Unlike 3-chloroTyr192, Met(O)148 was not correlated with HDL-C (r=-0.23; P=0.08, Fig. 2D) but inversely correlated with LDL-C (r=-0.27; P=0.04, Fig. 2E). The correlation between Met(O)148 and hs-CRP was not significant (r=0.08; P=0.49, Fig. 2F).
Cholesterol efflux capacity of serum HDL with the ABCA1 pathway is impaired in CAD and ACS subjects
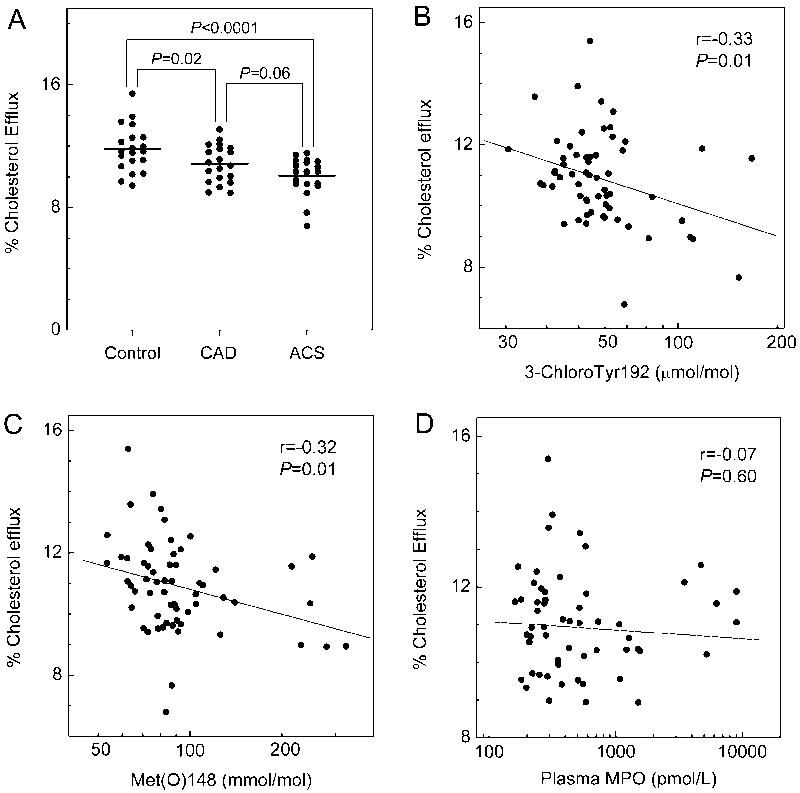
We assessed the capacity of serum HDL to promote cholesterol efflux by the ABCA1 pathway with BHK cells expressing or not expressing mifepristone-inducible human ABCA1. Analysis by one-way ANOVA indicated that serum HDL efflux capacity differed among the groups (P=0.0004). Serum HDL of CAD and ACS subjects had significantly less cholesterol efflux capacity (10.8% and 10.0% sterol efflux, P=0.02 and P<0.0001, Fisher’s LSD test) than that of control subjects (11.8% sterol efflux) when cells expressing ABCA1 were cholesterol donors (Fig. 3A). There was a trend towards greater impairment of sterol efflux from ABCA1 expressing cells to serum HDL of ACS subjects than CAD subjects (P=0.06).
Figure 3. Cholesterol efflux capacity of serum HDL in control subjects, CAD subjects, and ACS subjects.
Serum HDL was obtained by polyethylene glycol (PEG) precipitation of serum derived from plasma. Cholesterol efflux from serum HDL to ABCA1-expressing BHK cells (A) was measured as described in Methods. Correlations between 3-chloroTyr192 (B), Met(O)148 (C), or MPO levels in plasma (D) and ABCA1 efflux capacity were determined by linear regression analysis using Pearson’s correlation coefficient.
Levels of 3-chloroTyr192 and Met(O)148 inversely correlate with serum HDL’s ABCA1 cholesterol efflux capacity
We investigated the relationship between levels of oxidized residues in apoA-I and the cholesterol efflux capacity of serum HDL, using cells that expressed ABCA1. Linear regression analysis of all the subjects (n=60) revealed an inverse correlation with levels of both 3-chloroTyr192 (r=−0.33, P=0.01, Fig. 3B) and Met(O)148 (r=−0.32, P=0.01, Fig. 3C). The cholesterol efflux capacity of serum HDL also inversely correlated with levels of Met(O)86 and Met(O)112 (Supplemental Fig. VI).
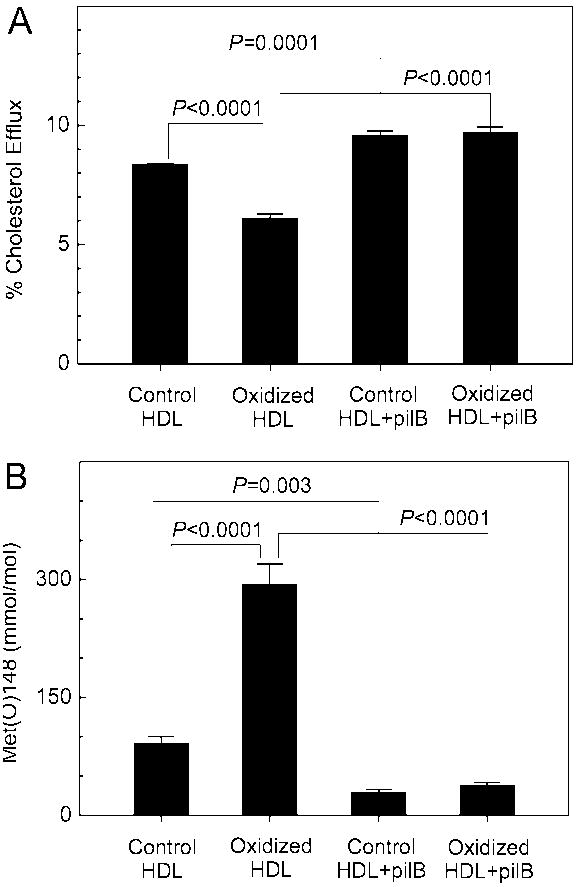
To investigate the potential contribution of Met oxidation in vivo to impaired sterol efflux, we exposed isolated HDL to low levels of HOCl. When 29% of Met148 of apoA-I was converted to Met(O)148, we observed ~30% loss of cholesterol efflux by the ABCA1 pathway (Fig. 4). Met(O)148 was almost completely reduced back to native Met when oxidized HDL was incubated with pilB, a methionine sulfoxide reductase that reduces both epimers of Met(O) 39. Reduction of Met(O) to Met completely restored the efflux capacity of HOCl-oxidized HDL (Fig. 4). The efflux capacity of control HDL, which contained ~9% Met(O)148, was also increased by incubation with pilB. Collectively, these observations provide strong evidence that the levels of Met(O) observed in HDL isolated from coronary artery disease subjects are likely high enough to contribute to impaired sterol efflux by the ABCA1 pathway.
Figure 4. Methionine oxidation and cholesterol efflux capacity of HDL exposed to HOCl in vitro.
(A) Cholesterol efflux capacity of HDL exposed to HOCl and reduced by pilB; (B) Levels of Met(O)148 in HDL exposed to HOCl and reduced by pilB. HDL (1 mg/mL) was oxidized by HOCl (180 μM) at 37°C for 1 h. Where indicated, HDL (0.5 mg/mL) was incubated subsequently with the methionine sulfoxide reductase pilB (4:1, HDL/pilB, w/w) at 37°C for 2 h. Cholesterol efflux from ABCA1-expressing BHK cells to control HDL, HOCl-oxidized HDL, and pilB-reduced HDL was measured as described in Methods. Levels of Met(O)148 were quantified by tandem MS with SRM. Results are means ± SDs of three determinations and are representative of two independent experiments.
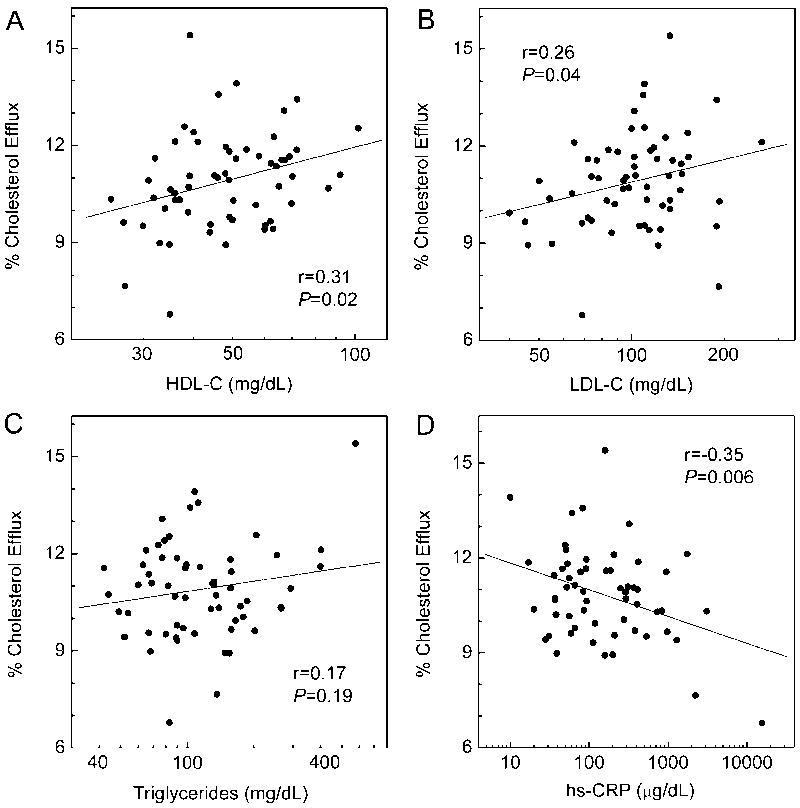
Serum HDL’s cholesterol efflux capability with macrophages associates with both HDL-C levels 30 and the functionality of the HDL particles 40. We therefore determined the relationship between HDL-C levels and the ABCA1 activity of serum HDL for all of the subjects (n=60), using linear regression analysis (Fig. 5A). Efflux capacity correlated with HDL-C levels (P=0.02) and accounted for approximately the same variance in the data (r=0.31) as was observed for apoA-I chlorination (r=−0.33). Thus, the impaired cholesterol efflux in the CAD and ACS groups appears partially due to lower HDL-C levels. After we controlled for HDL-C level, logistic regression analysis revealed that the inverse correlations between 3-chloroTyr192 or Met(O)148 with efflux capacity remained significant (r=−0.27, P=0.04 and r=−0.27, P=0.04, respectively). Therefore, modification of apoA-I by MPO correlated with impaired HDL cholesterol efflux capacity via the ABCA1 pathway, even after adjusting for HDL-C levels. In contrast, we observed no correlation of the efflux capacity of serum HDL with plasma levels of MPO (r=-0.07, P=0.60, Fig. 3D).
Figure 5. Correlation of ABCA1 cholesterol efflux capacity with cardiovascular risk factors.
Serum ABCA1 cholesterol efflux was measured as described in the legend of Fig. 3. Correlations between serum efflux and HDL-C (A), LDL-C (B), triglyceride (C), and hs-CRP (D) were determined by linear regression analysis using Pearson’s correlation coefficient. Where indicated, values were logarithmically transformed.
We also examined the relationship between ABCA1 cholesterol efflux capacity and traditional risk factors for atherosclerosis in our subjects (Fig. 5). Cholesterol efflux correlated weakly with HDL-C (r=0.31, P=0.02, Fig. 5A), consistent with the observation that lipid-free or poorly lipidated apolipoproteins are the major ligands for ABCA1 6. Efflux capacity also correlated with LDL-C (r=0.26, P=0.04, Fig. 5B) but adjusting for HDL-C levels attenuated that relationship (r=0.24, P=0.07). Cholesterol efflux did not correlate with triglyceride level (r=0.17, P=0.19, Fig. 5C). However, after we controlled for HDL-C levels, that relationship became significant (r=0.49, P=0.0001). Efflux did not associate with gender or hypertension. Efflux did not correlate with age or total cholesterol. However, it related significantly to diabetes (11.2% vs. 10.0%, non-diabetic vs. diabetic, P=0.01, two-tailed Student’s t-test). Efflux also associated with smoking status (11.3% vs. 10.6%, non-smoker vs. smoker, P=0.047). The efflux capacity of serum HDL also correlated with hs-CRP levels (r=-0.35, P=0.006, Fig. 5D).
3-ChloroTyr192, Met(O)148, and efflux capacity associate with cardiovascular disease status
We investigated the relationships among apoA-I oxidation, ABCA1 efflux capacity, traditional cardiovascular risk factors, and cardiovascular disease (CVD) status (Fig. 6). To increase power, and because the levels of oxidized amino acids and sterol efflux capacity of HDL were similar in CAD and ACS subjects, we included both CAD and ACS subjects in the CVD group.
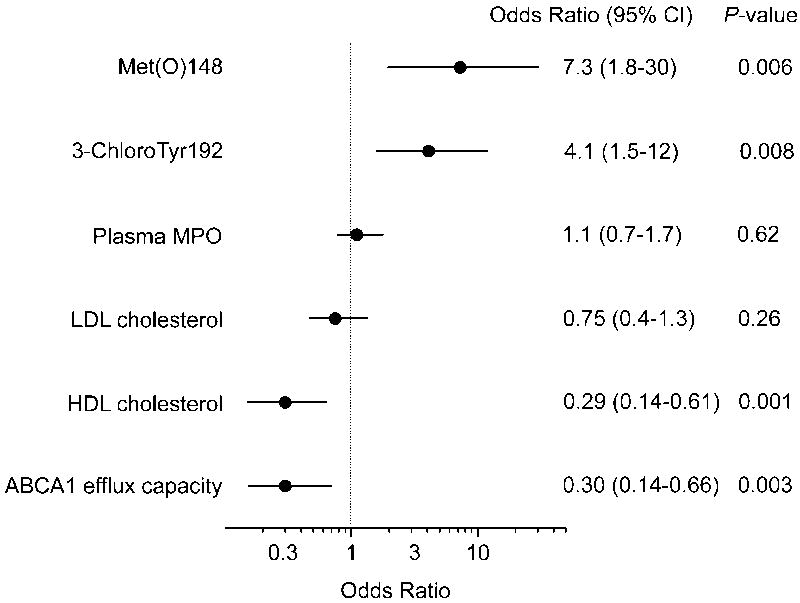
Figure 6. Odds ratios for cardiovascular disease status for 3-chloroTyr192, Met(O)148, plasma MPO, HDL-C, LDL-C and ABCA1 sterol efflux capacity.
Cardiovascular disease included both ACS and CAD subjects (n=40). Bars represent 95% confidence intervals for each odds ratio. Odds ratios are per 1-SD increase.
Compared with control subjects, CVD subjects had significantly lower levels of HDL-C (mean values for case and control subjects, 45 mg/dL and 61 mg/dL, respectively; P = 0.0003). Cholesterol efflux capacity of serum HDL was significantly lower in case group compared with control group (mean values for case and control subjects, 10.4% and 11.8%, respectively; P = 0.001). Increased efflux capacity by the ABCA1 pathway associated with a decreased risk of CVD (odds ratio [OR] per 1-SD change, 0.30; 95% confidence interval (CI), 0.14-0.66; P=0.003). This association remained robust after adjusting for HDL-C levels (adjusted OR, 0.33; 95% CI, 0.15-0.76; P=0.009). Elevated levels of 3-chloroTyr192 and Met(O)148 of apoA-I in HDL were also highly significantly associated with CVD status (ORs 4.1 and 7.3, P=0.008 and 0.006, respectively). In contrast, plasma levels of MPO and LDL-C were not associated with CVD status (Fig. 6).
DISCUSSION
Understanding the pathways that impair sterol efflux by HDL may lead to new diagnostic and therapeutic approaches to atherosclerosis. In this study, we found that levels of 3-chloroTyr192 and Met(O)148 were significantly higher in apoA-I of HDL isolated from CAD or ACS subjects than in HDL from apparently healthy control subjects. We also found that serum HDL of CAD and ACS subjects was significantly less able to promote cellular sterol efflux by the ABCA1 pathway. There was a strong inverse association between ABCA1 efflux capacity with CVD status, and this association persisted after adjustment for HDL-C. Levels of 3-chloroTyr192 and Met(O)148 positively associated with CVD status. Moreover, serum HDL’s ability to promote sterol efflux by the ABCA1 pathway correlated inversely with levels of 3-chloroTyr192 and Met(O)148. Because chlorination of Tyr192 in concert with oxidation of Met residues in vitro impairs the ABCA1 activity of apoA-I 23, 25, and 3-chlorotyrosine is a characteristic chemical fingerprint of MPO 38, our observations suggest that MPO contributes to the generation of dysfunctional HDL with impaired ABCA1 efflux capacity in humans with atherosclerotic vascular disease.
A key issue is whether the levels of oxidized apoA-I we detected in apoA-I of HDL of CAD and ACS subjects were high enough to potentially reduce HDL’s sterol efflux capacity. When HDL oxidized in vitro with HOCl contained levels of Met(O) similar to those observed in vivo, its ability to promote sterol efflux by the ABCA1 pathway was significantly impaired. Importantly, the ability of oxidized HDL to promote sterol efflux by the ABCA1 pathway was completely restored when Met[O] was converted to Met by methionine sulfoxide reductase. In contrast, the levels of 3-chlorotyrosine we detected in HDL were low and unlikely to contribute directly to impaired sterol efflux capacity by the ABCA1 pathway. Collectively, these observations provide strong evidence that the levels of Met(O) observed in HDL isolated from coronary artery disease subjects likely impair sterol efflux. In future studies, it will be important to determine if other modifications of apoA-I, such as lipid adduction 32 and tryptophan oxidation 41, contribute to impaired sterol efflux by the ABCA1 pathway.
Although methionine can be oxidized by lipid hydroperoxides in HDL as well as other pathways 42, 43, the correlation between levels of Met(O)148 and levels of 3-chloroTyr192 (a unique product of MPO 38) indicate that MPO likely contributes to oxidation of methionine residues in vivo. In contrast to our observations with the ABCA1 pathway and LCAT activation 25, 26, oxidation of Met residues in apoA-I does not impair sterol efflux from human monocyte-derived macrophages 36. However, the contribution of the ABCA1 pathway to efflux in these cells is uncertain, and the clinical relevance of these observations is unclear, because there is no evidence that impaired efflux with human macrophages associates with CVD status. In a secondary analysis, we also found that levels of 3-chloroTyr192 and Met(O)148 associated with smoking and diabetes status. Indeed, increased levels of oxidized Met residues in apoA-I of isolated HDL were observed in a study of type 1 diabetic subjects 44. In future studies, it will be of interest to determine if smoking and diabetes—two major risk factors for CAD—associate with impaired sterol efflux capacity and HDL modification by MPO.
There was a strong trend (P=0.06) towards greater impairment of serum HDL’s efflux capacity with the ABCA1 pathway in ACS subjects than in CAD subjects. However, the two groups contained similar levels of 3-chlorotyrosine and Met(O) in their HDLs. These observations suggest that additional factors impair sterol efflux in ACS subjects. One important contributor may be acute inflammation 12-14, 45, a prominent component of unstable angina and acute myocardial infarction 45. Consistent with this suggestion, we observed a significant, inverse correlation between hs-CRP levels and the sterol efflux capacity of serum HDL in our cohort. Acute inflammation alters the concentration of HDL-associated enzymes and remodels the human HDL proteome 46, and animal models and human studies provide strong support for the hypothesis that inflammation converts HDL into a dysfunctional form 12-14, 28, 47. Cholesterol efflux to HDL may be one important target because lipopolysaccharide, a potent inducer of acute inflammation, impairs cholesterol efflux from macrophages in mice 13.
Our detection of higher levels of 3-chloroTyr192 and Met(O)148 in plasma HDL from CAD and ACS subjects might seem surprising, given that blood is richly endowed with antioxidants that scavenge HOCl. Moreover, levels of the oxidized residues were similar in the two groups, suggesting that acute inflammation is unlikely to promote oxidation of circulating HDL. Thus, the oxidized HDL in plasma might be generated elsewhere, perhaps in a microenvironment that is depleted of antioxidants but enriched in macrophages, MPO and HOCl 17. Many lines of evidence indicate that atherosclerotic lesions provide such a milieu 17. One intriguing possibility is that HDL is oxidized in atherosclerotic lesions (or other inflamed tissues rich in leukocytes 45), resulting in a feedback loop in which reduced sterol efflux due to damage to HDL prompts macrophages to become foam cells, accelerating atherosclerosis. In turn, these events promote additional HDL oxidation and the appearance of MPO-oxidized HDL in blood. Regardless of whether it originates in blood or lesions, however, 3-chloroTyr and Met(O) in circulating HDL might be a useful indicator of the risk of cardiovascular disease and the efficacy of antioxidant interventions.
The levels of chloroTyr192 and Met(O)148 were similar in ACS and stable CAD patient, raising the possibility that the ACS subjects had elevated levels of the two oxidized amino acids before they developed clinical symptoms. In future studies, it will be of great interest to determine if levels of MPO-oxidized HDL can identify apparently healthy subjects who are at increased risk of CAD, and whether HDL oxidation is enhanced in conditions that predispose humans to clinically significant atherosclerosis.
Supplementary Material
Novelty and Significance.
What Is Known?
The ability of HDL to accept cholesterol from macrophages is negatively associated with cardiovascular disease status.
HDL receives cholesterol from ABCA1, a membrane protein that exports the sterol from macrophages.
HDL can be damaged by myeloperoxidase, which oxidizes methionine residues and chlorinates Tyr192 of apolipoprotein A-I (apoA-I) in HDL impairing the ability of HDL to accept cholesterol from the ABCA1 pathway.
What New Information Does This Article Contribute?
Subjects with stable coronary artery disease (CAD) or acute coronary syndrome (ACS) have elevated levels of oxidized HDL.
Levels of chlorinated Tyr192 and oxidized Met148 in apoA-I strongly associate with cardiovascular disease status and the degree to which HDL is unable to accept cholesterol from cells via the ABCA1 pathway.
Because chlorinated Tyr192 and oxidized Met148 are produced by myeloperoxidase, the enzyme may help generate dysfunctional HDL in humans.
The ability of HDL to remove cholesterol from artery wall macrophages is one of its key cardioprotective functions. The sterol efflux capacity of HDL shows a robust negative association with CAD status, suggesting that loss of efflux capacity contributes to HDL dysfunction. However, the underlying mechanisms remain poorly understood. We found that subjects with CAD or ACS have higher levels of oxidized and chlorinated HDL than control subjects, and that the oxidation pattern—chlorinated Tyr192 and oxidized Met148—matches that produced by myeloperoxidase. HDL from the CAD and ACS subjects was also less efficient in promoting sterol efflux by the ABCA1 pathway. Converting oxidized methionine residues of HDL back to methionine improved sterol efflux capacity in vitro. Importantly, the levels of oxidized and chlorinated HDL were inversely correlated with ABCA1 efflux capacity and positively associated with atherosclerotic status. These observations indicate that dysfunctional HDL with impaired sterol efflux capacity could be generated by myeloperoxidase. Thus, inhibiting HDL oxidation by myeloperoxidase in vivo might be a new approach for treating atherosclerosis.
Acknowledgments
Mass spectrometry experiments were performed by the Mass Spectrometry Resource, Department of Medicine, University of Washington and the Quantitative and the Functional Proteomics Core of the Diabetes Research Center, University of Washington.
SOURCES OF FUNDING
This work was supported by grants from the National Institutes of Health (HL086798, HL091055, HL092969, HL108897, HL112625) and Pfizer. B.S. is supported by a K99/R00 Award (R00HL091055), a Beginning Grant-in-Aid (13BGIA17290026) from the American Heart Association, and a Pilot and Feasibility Award from the University of Washington Diabetes Research Center (DRC; P30DK017047). C.T. is supported by a Scientist Development Grant (10SDG3860011) from the American Heart Association. Mass spectrometric studies were performed by the Quantitative and Functional Proteomics Core of the Diabetes Research Center. None of the sponsors had any role in the study design, data analysis, or reporting of the results.
Nonstandard Abbreviations and Acronyms
- ABCA1
ATP-binding cassette transporter A1
- ACS
acute coronary syndrome
- apoA-I
apolipoprotein A-I
- BHK
baby hamster kidney
- BHT
butylated hydroxytoluene
- CAD
coronary artery disease
- CVD
cardiovascular disease
- DTPA
diethylenetriaminepentaacetic acid
- H2O2
HDL, high density lipoprotein
- rHDL
reconstituted HDL
- HDL-C
high density lipoprotein cholesterol
- HOCl
hypochlorous acid
- hs-CRP
high-sensitivity C-reactive protein
- LC
liquid chromatography
- LCAT
lecithin-cholesterol acyltransferase
- LDL-C
low density lipoprotein cholesterol
- LSD
Fisher’s least significance difference
- Met(O)
methionine sulfoxide
- MPO
myeloperoxidase
- MS
mass spectrometry
- MS/MS
tandem MS
- OR
odds ratio
- PEG
Polyethylene glycol
- SRM
selected reaction monitoring
Footnotes
DISCLOSURES
Dr. Heinecke is named as a coinventor on patents from the US Patent Office on the use of oxidation markers to predict the risk of cardiovascular disease. Dr. Heinecke has served as a consultant for Merck, Amgen, Bristol Meyer Squibb and Insilicos. Dr. Zhao is the recipient of research support from Bristol Meyer Squibb and Kowa. The other authors report no conflicts.
References
- 1.Gordon DJ, Rifkind BM. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 2.Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab. 2008;7:365–375. doi: 10.1016/j.cmet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Rader DJ. Molecular regulation of macrophage reverse cholesterol transport. Curr Opin Cardiol. 2007;22:368–372. doi: 10.1097/HCO.0b013e3281ec5113. [DOI] [PubMed] [Google Scholar]
- 4.Davidson WS, Thompson TB. The structure of apolipoprotein A-I in high density lipoproteins. J Biol Chem. 2007;282:22249–22253. doi: 10.1074/jbc.R700014200. [DOI] [PubMed] [Google Scholar]
- 5.Freeman MW. Effluxed lipids: Tangier Island’s latest export. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10950–10952. doi: 10.1073/pnas.96.20.10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: A cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 7.Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest. 1999;104:R25–31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assmann G, von Eckardstein A, Brewer HB. Familial high density lipoprotein deficiency: Tangier disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. New York: McGraw-Hill; 1995. pp. 2053–2072. [Google Scholar]
- 9.Ferrans VJ, Fredrickson DS. The pathology of Tangier disease. A light and electron microscopic study. Am J Pathol. 1975;78:101–158. [PMC free article] [PubMed] [Google Scholar]
- 10.Aiello RJ, Brees D, Francone OL. ABCA1-deficient mice: insights into the role of monocyte lipid efflux in HDL formation and inflammation. Arterioscler Thromb Vasc Biol. 2003;23:972–980. doi: 10.1161/01.ATV.0000054661.21499.FB. [DOI] [PubMed] [Google Scholar]
- 11.van Eck M, Bos IS, Kaminski WE, Orso E, Rothe G, Twisk J, Bottcher A, Van Amersfoort ES, Christiansen-Weber TA, Fung-Leung WP, Van Berkel TJ, Schmitz G. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc Natl Acad Sci U S A. 2002;99:6298–6303. doi: 10.1073/pnas.092327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 13.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein. Chem Res Toxicol. 2010;23:447–454. doi: 10.1021/tx9003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 16.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 17.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazen SL, Hsu FF, Mueller DM, Crowley JR, Heinecke JW. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J Clin Invest. 1996;98:1283–1289. doi: 10.1172/JCI118914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurst JK, Barrette WC., Jr Leukocytic oxygen activation and microbicidal oxidative toxins. Crit Rev Biochem Mol Biol. 1989;24:271–328. doi: 10.3109/10409238909082555. [DOI] [PubMed] [Google Scholar]
- 20.Domigan NM, Charlton TS, Duncan MW, Winterbourn CC, Kettle AJ. Chlorination of tyrosyl residues in peptides by myeloperoxidase and human neutrophils. J Biol Chem. 1995;270:16542–16548. doi: 10.1074/jbc.270.28.16542. [DOI] [PubMed] [Google Scholar]
- 21.Winterbourn CC. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985;840:204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 22.Bergt C, Pennathur S, Fu X, Byun J, O’Brien K, McDonald TO, Singh P, Anantharamaiah GM, Chait A, Brunzell J, Geary RL, Oram JF, Heinecke JW. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:13032–13037. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao B, Bergt C, Fu X, Green P, Voss JC, Oda MN, Oram JF, Heinecke JW. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J Biol Chem. 2005;280:5983–5993. doi: 10.1074/jbc.M411484200. [DOI] [PubMed] [Google Scholar]
- 24.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao B, Oda MN, Bergt C, Fu X, Green PS, Brot N, Oram JF, Heinecke JW. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J Biol Chem. 2006;281:9001–9004. doi: 10.1074/jbc.C600011200. [DOI] [PubMed] [Google Scholar]
- 26.Shao B, Cavigiolio G, Brot N, Oda MN, Heinecke JW. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc Natl Acad Sci U S A. 2008;105:12224–12229. doi: 10.1073/pnas.0802025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao B, Pennathur S, Heinecke JW. Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J Biol Chem. 2012;287:6375–6386. doi: 10.1074/jbc.M111.337345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feingold KR, Grunfeld C. The acute phase response inhibits reverse cholesterol transport. J Lipid Res. 2010;51:682–684. doi: 10.1194/jlr.E005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 32.Szapacs ME, Kim HY, Porter NA, Liebler DC. Identification of proteins adducted by lipid peroxidation products in plasma and modifications of apolipoprotein A1 with a novel biotinylated phospholipid probe. J Proteome Res. 2008;7:4237–4246. doi: 10.1021/pr8001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, Schoneich C, Borchardt RT. Chemical instability of protein pharmaceuticals: Mechanisms of oxidation and strategies for stabilization. Biotechnol Bioeng. 1995;48:490–500. doi: 10.1002/bit.260480511. [DOI] [PubMed] [Google Scholar]
- 34.Ryan RO, Forte TM, Oda MN. Optimized bacterial expression of human apolipoprotein A-I. Protein Expr Purif. 2003;27:98–103. doi: 10.1016/s1046-5928(02)00568-5. [DOI] [PubMed] [Google Scholar]
- 35.Anderson L, Hunter CL. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol Cell Proteomics. 2006;5:573–588. doi: 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Panzenbock U, Kritharides L, Raftery M, Rye KA, Stocker R. Oxidation of methionine residues to methionine sulfoxides does not decrease potential antiatherogenic properties of apolipoprotein A-I. J Biol Chem. 2000;275:19536–19544. doi: 10.1074/jbc.M000458200. [DOI] [PubMed] [Google Scholar]
- 37.Shao B, Tang C, Heinecke JW, Oram JF. Oxidation of apolipoprotein A-I by myeloperoxidase impairs the initial interactions with ABCA1 required for signaling and cholesterol export. J Lipid Res. 2010;51:1849–1858. doi: 10.1194/jlr.M004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaut JP, Yeh GC, Tran HD, Byun J, Henderson JP, Richter GM, Brennan ML, Lusis AJ, Belaaouaj A, Hotchkiss RS, Heinecke JW. Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxidants during sepsis. Proc Natl Acad Sci U S A. 2001;98:11961–11966. doi: 10.1073/pnas.211190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowther WT, Brot N, Weissbach H, Honek JF, Matthews BW. Thiol-disulfide exchange is involved in the catalytic mechanism of peptide methionine sulfoxide reductase. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6463–6468. doi: 10.1073/pnas.97.12.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atger V, de la Llera Moya M, Bamberger M, Francone O, Cosgrove P, Tall A, Walsh A, Moatti N, Rothblat G. Cholesterol efflux potential of sera from mice expressing human cholesteryl ester transfer protein and/or human apolipoprotein AI. J Clin Invest. 1995;96:2613–2622. doi: 10.1172/JCI118326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng DQ, Brubaker G, Wu Z, Zheng L, Willard B, Kinter M, Hazen SL, Smith JD. Apolipoprotein A-I tryptophan substitution leads to resistance to myeloperoxidase-mediated loss of function. Arterioscler Thromb Vasc Biol. 2008;28:2063–2070. doi: 10.1161/ATVBAHA.108.173815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garner B, Waldeck AR, Witting PK, Rye KA, Stocker R. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J Biol Chem. 1998;273:6088–6095. doi: 10.1074/jbc.273.11.6088. [DOI] [PubMed] [Google Scholar]
- 43.Cascio G, Schiera G, Di Liegro I. Dietary fatty acids in metabolic syndrome, diabetes and cardiovascular diseases. Curr Diabetes Rev. 2012;8:2–17. doi: 10.2174/157339912798829241. [DOI] [PubMed] [Google Scholar]
- 44.Brock JW, Jenkins AJ, Lyons TJ, Klein RL, Yim E, Lopes-Virella M, Carter RE, Thorpe SR, Baynes JW. Increased methionine sulfoxide content of apoA-I in type 1 diabetes. J Lipid Res. 2008;49:847–855. doi: 10.1194/jlr.M800015-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med. 2000;343:1139–1147. doi: 10.1056/NEJM200010193431602. [DOI] [PubMed] [Google Scholar]
- 46.Alwaili K, Bailey D, Awan Z, Bailey SD, Ruel I, Hafiane A, Krimbou L, Laboissiere S, Genest J. The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochimica et biophysica acta. 2012;1821:405–415. doi: 10.1016/j.bbalip.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–2767. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.