Abstract
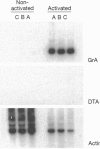
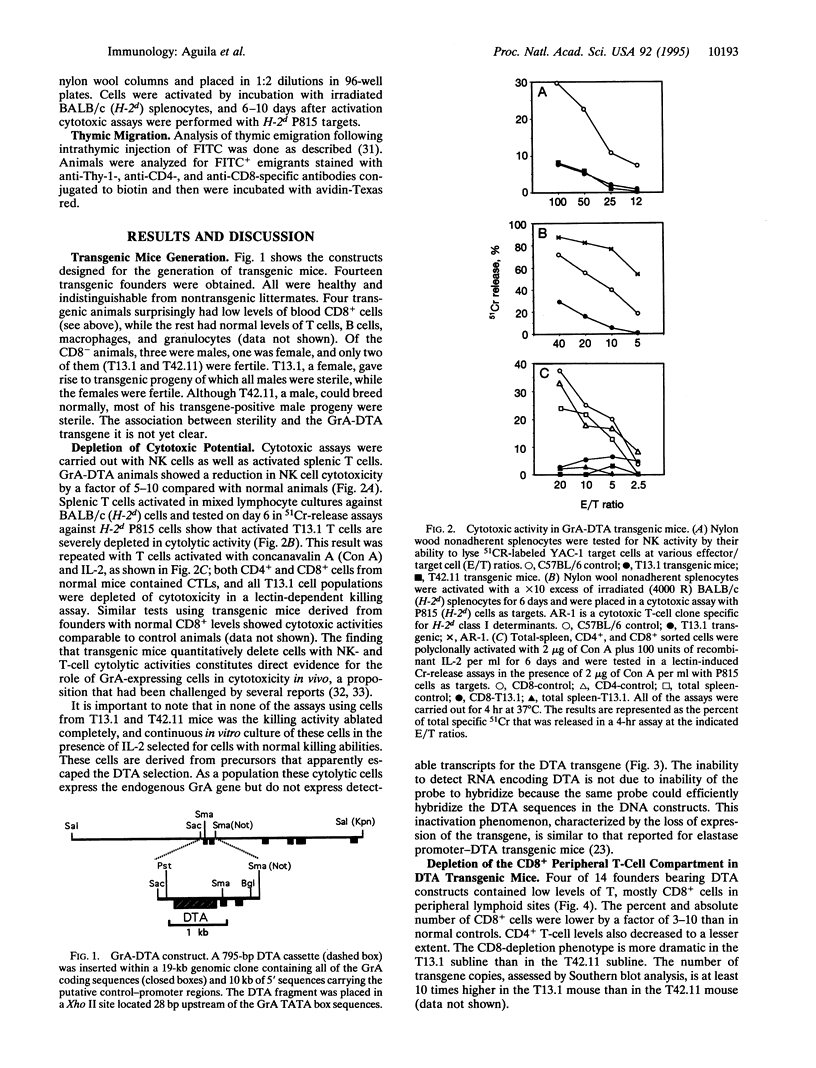
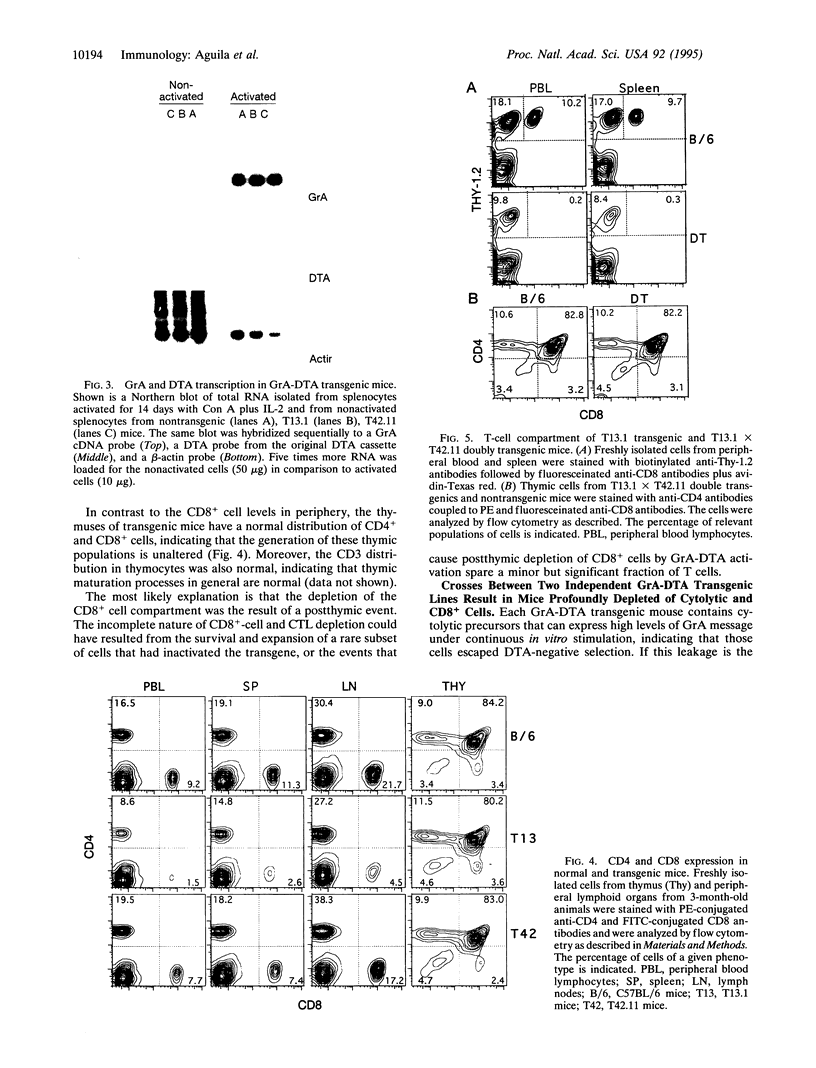
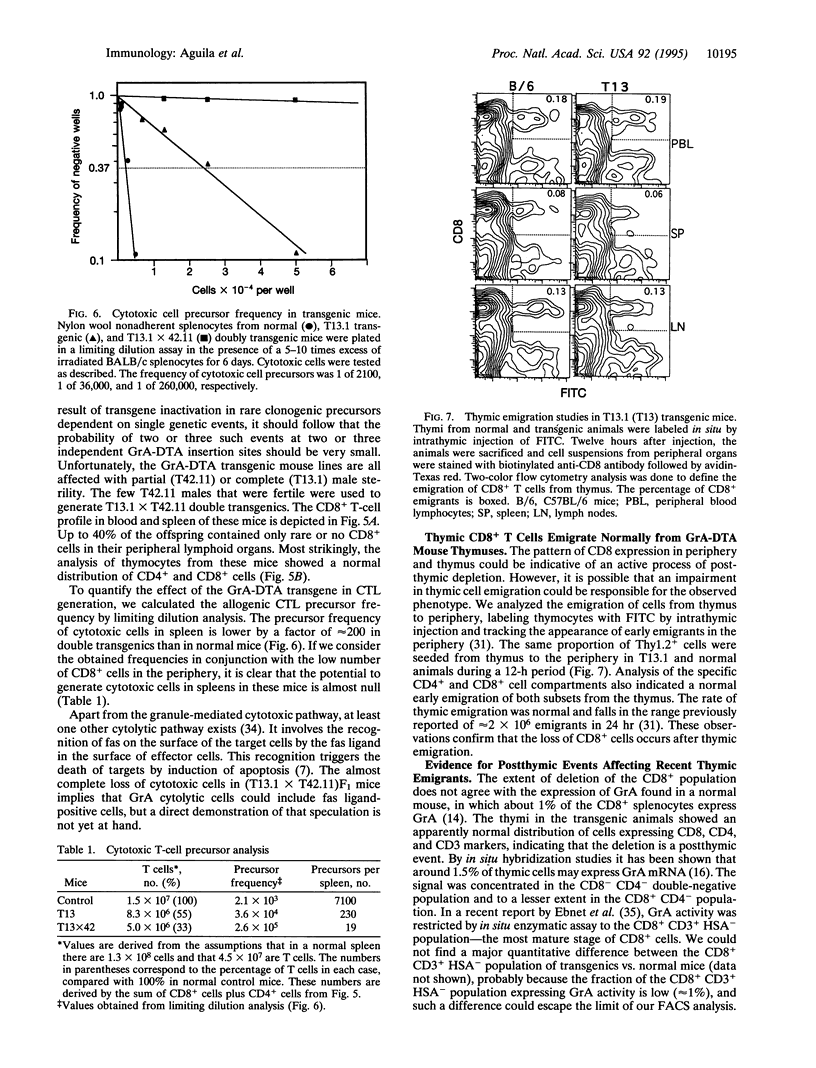
We have generated transgenic mice bearing the diphtheria toxin A chain (DTA) gene under the control of granzyme A (GrA) promoter sequences (GrA-DTA). GrA is expressed in activated cytotoxic cells but not in their immediate progenitors. These GrA-DTA mice are deficient in cytotoxic functions, indicating that most cytotoxic cells express GrA in vivo. Surprisingly, one founder strain containing a multicopy GrA-DTA insert show a marked and selective deficiency in CD8+ cells in peripheral lymphoid organs. This depletion was not observed in thymus, where the distribution of CD4+ and CD8+ cells is normal. Moreover, the emigration of T cells from thymus is normal, indicating that the depletion occurs in the periphery. GrA-DTA mice should be useful as models to dissect the role of cytotoxic cells in immune responses and as recipients of normal and neoplastic hematopoietic cells. The selective depletion of CD8+ cells in one founder strain could have implications for postthymic T-cell development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendelac A., Matzinger P., Seder R. A., Paul W. E., Schwartz R. H. Activation events during thymic selection. J Exp Med. 1992 Mar 1;175(3):731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke G., Rosen D. Highly lytic in vivo primed cytolytic T lymphocytes devoid of lytic granules and BLT-esterase activity acquire these constituents in the presence of T cell growth factors upon blast transformation in vitro. J Immunol. 1988 Sep 1;141(5):1429–1436. [PubMed] [Google Scholar]
- Breitman M. L., Clapoff S., Rossant J., Tsui L. C., Glode L. M., Maxwell I. H., Bernstein A. Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science. 1987 Dec 11;238(4833):1563–1565. doi: 10.1126/science.3685993. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet J. F., Denizot F., Suzan M., Haas W., Mencia-Huerta J. M., Berke G., Luciani M. F., Golstein P. CTLA-1 and CTLA-3 serine esterase transcripts are detected mostly in cytotoxic T cells, but not only and not always. J Immunol. 1987 Jun 15;138(12):4102–4105. [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ebnet K., Levelt C. N., Tran T. T., Eichmann K., Simon M. M. Transcription of granzyme A and B genes is differentially regulated during lymphoid ontogeny. J Exp Med. 1995 Feb 1;181(2):755–763. doi: 10.1084/jem.181.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sanz J. A., MacDonald H. R., Jenne D. E., Tschopp J., Nabholz M. Cell specificity of granzyme gene expression. J Immunol. 1990 Nov 1;145(9):3111–3118. [PubMed] [Google Scholar]
- Gershenfeld H. K., Weissman I. L. Cloning of a cDNA for a T cell-specific serine protease from a cytotoxic T lymphocyte. Science. 1986 May 16;232(4752):854–858. doi: 10.1126/science.2422755. [DOI] [PubMed] [Google Scholar]
- Held W., MacDonald H. R., Mueller C. Expression of genes encoding cytotoxic cell-associated serine proteases in thymocytes. Int Immunol. 1990;2(1):57–62. doi: 10.1093/intimm/2.1.57. [DOI] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Reynolds C. W., Ortaldo J. R. Mechanism of cytotoxicity by natural killer (NK) cells. Annu Rev Immunol. 1986;4:651–680. doi: 10.1146/annurev.iy.04.040186.003251. [DOI] [PubMed] [Google Scholar]
- Hershberger R. J., Gershenfeld H. K., Weissman I. L., Su L. Genomic organization of the mouse granzyme A gene. Two mRNAs encode the same mature granzyme A with different leader peptides. J Biol Chem. 1992 Dec 15;267(35):25488–25493. [PubMed] [Google Scholar]
- Heusel J. W., Wesselschmidt R. L., Shresta S., Russell J. H., Ley T. J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994 Mar 25;76(6):977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Borrelli E., Lesley J., Anderson D., Richman D. D., Baird S. M., Hyman R., Evans R. M. Thymidine kinase obliteration: creation of transgenic mice with controlled immune deficiency. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2698–2702. doi: 10.1073/pnas.86.8.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kägi D., Ledermann B., Bürki K., Seiler P., Odermatt B., Olsen K. J., Podack E. R., Zinkernagel R. M., Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994 May 5;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kägi D., Vignaux F., Ledermann B., Bürki K., Depraetere V., Nagata S., Hengartner H., Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994 Jul 22;265(5171):528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Manyak C. L., Norton G. P., Lobe C. G., Bleackley R. C., Gershenfeld H. K., Weissman I. L., Kumar V., Sigal N. H., Koo G. C. IL-2 induces expression of serine protease enzymes and genes in natural killer and nonspecific T killer cells. J Immunol. 1989 May 15;142(10):3707–3713. [PubMed] [Google Scholar]
- Masson D., Tschopp J. A family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell. 1987 Jun 5;49(5):679–685. doi: 10.1016/0092-8674(87)90544-7. [DOI] [PubMed] [Google Scholar]
- Minami Y., Kono T., Miyazaki T., Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- Mueller C., Gershenfeld H. K., Weissman I. L. Activation of CTL-specific genes during cell-mediated cytolysis in vivo: expression of the HF gene analyzed by in situ hybridization. Immunol Rev. 1988 Mar;103:73–85. doi: 10.1111/j.1600-065x.1988.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Mueller C., Shelby J., Weissman I. L., Périnat-Frey T., Eichwald E. J. Expression of the protease gene HF as a marker in rejecting allogeneic murine heart transplants. Transplantation. 1991 Feb;51(2):514–517. doi: 10.1097/00007890-199102000-00046. [DOI] [PubMed] [Google Scholar]
- Müller C., Kägi D., Aebischer T., Odermatt B., Held W., Podack E. R., Zinkernagel R. M., Hengartner H. Detection of perforin and granzyme A mRNA in infiltrating cells during infection of mice with lymphocytic choriomeningitis virus. Eur J Immunol. 1989 Jul;19(7):1253–1259. doi: 10.1002/eji.1830190716. [DOI] [PubMed] [Google Scholar]
- Oehm A., Behrmann I., Falk W., Pawlita M., Maier G., Klas C., Li-Weber M., Richards S., Dhein J., Trauth B. C. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992 May 25;267(15):10709–10715. [PubMed] [Google Scholar]
- Palmiter R. D., Behringer R. R., Quaife C. J., Maxwell F., Maxwell I. H., Brinster R. L. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987 Jul 31;50(3):435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- Peters P. J., Borst J., Oorschot V., Fukuda M., Krähenbühl O., Tschopp J., Slot J. W., Geuze H. J. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991 May 1;173(5):1099–1109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Hengartner H., Lichtenheld M. G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- Rothenberg E. V. The development of functionally responsive T cells. Adv Immunol. 1992;51:85–214. doi: 10.1016/s0065-2776(08)60487-3. [DOI] [PubMed] [Google Scholar]
- Rouvier E., Luciani M. F., Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med. 1993 Jan 1;177(1):195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollay R. G., Butcher E. C., Weissman I. L. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980 Mar;10(3):210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- Stutman O. Postthymic T-cell development. Immunol Rev. 1986 Jun;91:159–194. doi: 10.1111/j.1600-065x.1986.tb01488.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C. I., Jenkins N. A., Copeland N. G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994 Mar 25;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Tough D. F., Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994 Apr 1;179(4):1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velotti F., Palmieri G., Morrone S., Piccoli M., Frati L., Santoni A. Granzyme A expression by normal rat natural killer (NK) cells in vivo and by interleukin 2-activated NK cells in vitro. Eur J Immunol. 1989 Mar;19(3):575–578. doi: 10.1002/eji.1830190328. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Hafen K. The life span of naive alpha/beta T cells in secondary lymphoid organs. J Exp Med. 1993 Apr 1;177(4):891–896. doi: 10.1084/jem.177.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]