Abstract
This report describes a model protein specifically tailored to electrochemically study the reduction potential of protein tyrosine radicals as a function of pH. The model system is based on the 67-residue α3Y three-helix bundle. α3Y contains a single buried tyrosine at position 32 and displays structural properties inherent to a protein. The present report presents differential pulse voltammograms obtained from α3Y at both acidic (pH 5.4) and alkaline (pH 8.3) conditions. The observed Faradaic response is uniquely associated with Y32, as shown by site-directed mutagenesis. This is the first time voltammetry is successfully applied to detect a redox-active tyrosine residing in a structured protein environment. Tyrosine is a proton coupled electron-transfer cofactor making voltammetry-based pH titrations a central experimental approach. A second set of experiments was performed to demonstrate that pH-dependent studies can be conducted on the redox-active tyrosine without introducing large-scale structural changes in the protein scaffold. α3Y was re-engineered with the specific aim to place the imidazole group of a histidine close to the Y32 phenol ring. α3Y-K29H and α3Y-K36H each contain a histidine residue which protonation perturbs the fluorescence of Y32. We show that these variants are stable and well-folded proteins whose helical content, tertiary structure, solution aggregation state and solvent-sequestered position of Y32 remain pH insensitive across a range of at least 3–4 pH units. These results confirm that the local environment of Y32 can be altered and the resulting radical site studied by voltammetry over a broad pH range without interference from long-range structural effects.
INTRODUCTION
Amino-acid radical enzymes use selected glycine, cysteine, tyrosine and/or tryptophan residues as “in-house” one-electron redox cofactors.1,2 Functional glycine and cysteine radicals tend to be generated near or at the active site and directly involved in the catalytic chemistry. The two aromatic residues are used in catalytic redox reactions as well as serving as intermediate components in radical-transfer chains spanning tens of ångströms. Well-known examples of the former type are the YZ tyrosine in photosystem II (PSII)3,4 and the cross-linked Tyr-His species in the active site of cytochrome c oxidase,5 while the triple tryptophan electron-transfer wire in E. coli DNA photolyase,6 the ~ 35 Å radical-transfer chain in E. coli ribonucleotide reductase (RNR),7,8 and the putative multi heme/tryptophan electron/radical transfer chain in the MauG/pre-methylamine dehydrogenase complex9 represent examples of the latter type.
Virtually nothing is known as to how the protein matrix influences the thermodynamic properties of biocatalytic amino-acid radicals or the free-energy profiles of radical-transfer chains. It is very challenging to measure the reduction potentials of these highly oxidizing redox cofactors and, consequently, only a few estimates are available in the literature. An apparent potential of 1.0 V (pH 7.6) was reported for the tyrosine Y-O•/Y-OH(122) redox pair in E. coli RNR,10 although poor equilibria between the redox mediators in the bulk solvent and the radical site make this value unreliable. The formal potential of YZ in the large, membrane-bound PSII enzyme cannot be obtained directly by standard techniques such as redox titration or voltammetry-based methods but a time-dependent “operating” potential has been estimated from kinetically derived equilibrium constants.3,11 Assuming an operating potential of 1.25 V for P680+/P680,11,12 the YZ-O•/YZ-OH potential falls between 1.13 and 1.22 V depending on the time elapsed after light excitation and the redox state of the adjacent Mn4Ca cluster (pH 6.5 range; 13 and references therein). Likewise, an operating potential of 0.83 V (pH 6.0) has been estimated for the PSII YD-O•/YD-OH redox couple.11 These indirectly derived, single-pH potentials are, to our knowledge, the only available estimates for unmodified tyrosine radical cofactors. Even less is known about the potentials of protein glycine, cysteine and tryptophan radical cofactors with experimental data available only for the two-electron heme/W191 redox reactions in cytochrome c peroxidase14 and the photolyase tryptophan-radical system.15 Thus, on the basis of existing experimental data it is not possible to make any firm predictions regarding how the protein environment influences the reduction potentials of amino-acid radical cofactors. The hydrogen-bonding status of the reduced and radical states, the chemical characteristics of hydrogen-bonding partners, electrostatic interactions, solvent accessibility and the effective dielectric of the protein environment are likely to play important roles3,16–19 but, at present, correlations between these parameters and amino-acid radical reduction potentials are unknown and unexplored.
The thermodynamic properties of aqueous tyrosine give rise to a few key predictions regarding tyrosine redox chemistry in proteins.2,3 The following half reactions, redox couples and acid dissociation constants are associated with tyrosine in water:
| Y-OH•+ + e− ⇔ Y-OH | Y-OH•+/Y-OH |
| Y-O• + e− + H+ ⇔ Y-OH | Y-O•/Y-OH |
| Y-O• + e− ⇔ Y-O− | Y-O•/Y-O− |
| Y-OH•+⇔ Y-O• + H+ | KoY = [Y-O•][H+]/[Y-OH•+] |
| Y-OH ⇔ Y-O− + H+ | KrY = [Y-O−][H+]/[Y-OH] |
The cation Y-OH•+/Y-OH redox couple dominates at pH below pKoY while the tyrosinate Y-O•/Y-O− redox couple is observed at pH above pKrY. There is no proton release or uptake associated with the change in redox state and thus the reduction potentials of the Y-OH•+/Y-OH and Y-O•/Y-O− redox pairs are pH independent. The neutral tyrosine Y-O•/Y-OH pair is the dominant redox couple in the pKoY < pH < pKrY region. Since tyrosine oxidation/reduction is a 1e−/1H+ event in this pH region, the potential follows the Nernst equation and decreases by 59 mV per pH unit at 25° C. The pKoY and pKrY values are about −2 and 10 for aqueous tyrosine, respectively.20 These values suggest that Y-O•/Y-OH is the dominant redox couple in a protein environment. Consequently, tyrosine oxidation/reduction reactions occurring inside proteins are expected to a large extent to involve proton-coupled electron transfer (PCET).
There are no electrochemical data of any kind available for a protein tyrosine radical, much less a full Pourbaix diagram representing the formal potential of this species over a significant pH range. The PCET characteristics of tyrosine and phenol redox systems predict that local interactions are critical for tuning their redox properties.3,8,21 Thus, in order to obtain a meaningful Pourbaix diagram it is essential that the measured potential is not strongly influenced by global changes occurring in the protein scaffold as a function of pH but rather reflects local conditions at the radical site. We have developed a tyrosine radical protein system that fulfills these criteria. The complexity of this task required a fairly extensive experimental approach including the use of voltammetry methods, protein design and engineering, absorption, fluorescence and CD spectroscopy as well as several NMR-based techniques. The obtained data are described and analyzed in two connected papers. In the current paper we describe the electrochemical and structural properties of a protein system designed to provide tyrosine Pourbaix diagrams according to the criteria listed above. In a follow-up study, tyrosine Pourbaix diagrams obtained from this protein system are described and discussed.22
The tyrosine radical protein system is based on the de novo designed, 67-residue α3Y three-helix bundle, which contains a single buried tyrosine at position 32 and originally no histidine residues.16 In the present report we show that high-quality voltammetry data can be obtained from α3Y at both acidic and alkaline conditions. We show that the observed Faradaic response is uniquely associated with Y32. This is the first time voltammetry is successfully applied to detect a redox-active tyrosine residing in a structured protein environment. We also demonstrate a high-level structural control over α3Y by changing the environment of its redox-active tyrosine and proving that the resulting variants exhibit the required structural characteristics to support voltammetry-based pH titrations in a stable protein background. This is significant since pH-based studies are central to characterizing proton-coupled ET reactions in proteins. More specifically, α3Y was re-engineered to make a histidine variant in which the imidazole ring of the introduced histidine interacts structurally with the phenol group of Y32. Our aim was to introduce an interaction at the site of the redox-active tyrosine and then specifically determine the effect of this interaction on the potential of Y32. This would demonstrate that the constructed tyrosine radical model system is capable of providing relevant radical site-specific information. Two variants of interest, α3Y-K29H and α3Y-K36H, were identified using protein modeling combined with optical and NMR-based spectroscopic screening. These two stable and well-structured proteins do not display any major changes in their secondary structures, tertiary structures or solution aggregation state across a range of 3–4 pH units. Their single tyrosine residue Y32 is located in a hydrophobic and structured environment. Moreover, protonation of the introduced histidines perturbs the fluorescence of Y32 suggesting that α3Y-K29H and α3Y-K36H contain an electrostatically coupled Y32/His pair. We conclude that α3Y-K29H and α3Y-K36H exhibit the appropriate characteristics for probing the potential of Y32 as a function of local interactions and the solution pH.
MATERIALS AND METHODS
Protein expression and purification
The α3Y proteins were made by QuikChange (Stratagene) using a modified α3W/pET32b (Novagen) vector as template.18 α3Y has the following amino-acid sequence: GSRVKALEEKVKALEEKVKALGGGGRIEELKKKYEELKKKIEELGGGGEVKKVEEE VKKLEEEIKKL in which the N-terminal GS residues form part of a thrombin cleavage site. These two residues are labeled as −2 and −1 to keep the amino-acid numbering consistent with the chemically synthesized 65-residue α3Y protein.16 α3Y and variants were expressed in LB or minimal media and purified following standard protocols for His-tagged proteins (Novagen). Transformed BL21(DE3)pLysS or BL21-CodonPlus(DE3)-RIL cells were harvested either after a 3–4 hour IPTG induction period at 37° C (LB cultures) or after an overnight induction at 30° C (minimal media cultures) and stored at −20° C. Cells were resuspended in 20 mM Tris-HCl, 500 mM NaCl, 5 mM imidazole, pH 7.9 and lysed by sonication. The lysate was clarified by centrifugation, passed over a His•bind (Novagen) nickel column, and the thioredoxin-α3Y fusion protein eluted by an imidazole gradient. Thrombin (T6634; Sigma) was added to the thioredoxin-α3Y protein fraction and the resulting mixture dialyzed against 50 mM Tris-HCl, 500 mM NaCl, 2.5 mM CaCl2, pH 8.0 at room temperature overnight. The digestion mixture was passed over a second nickel column to remove the His-tagged thioredoxin and any remaining undigested fusion protein. Target proteins were isolated by reverse-phase HPLC (semi-preparative TP2181010 column; Grace/Vydac) using an acetonitrile/water gradient containing 0.1% (w/v) trifluoroacetic acid and stored as lyophilized powder.
Absorption and fluorescence spectroscopy
Absorption spectra were collected on a Varian Cary 50 Bio or a Hitachi U-3000 UV/Vis spectrometer at room temperature. pH-titration samples were prepared by dissolving lyophilized protein in a 10 mM potassium phosphate, 10 mM HEPES, 10 mM borate, 10 mM CAPS, pH 7.0 buffer to an Abs276 of 0.2 (10 mm path). The solution was split in two equal fractions and the pH carefully adjusted with 12 M HCl or 10 M NaOH. pH titrations were performed by constant volume titration. The apparent tyrosinate/tyrosine pKa of Y32 was estimated by measuring at 293 nm (Absmax for deprotonated Y32) and 400 nm (baseline) as a function of pH and fitting the resulting pH-titration curve to a single pKa using the nonlinear curve fitting routines in KaleidaGraph (www.synergy.com).
Fluorescence spectra were collected on a Horiba Jobin Yvon Spex Fluorolog spectroflurometer at 23° C. pH-titration samples were prepared by dissolving lyophilized protein in a 10 mM sodium acetate, 10 mM potassium phosphate, 10 mm sodium borate (APB) pH 7.0 buffer to an Abs276 of 0.2 (10 mm path), dividing the sample in two equal fractions, and adjusting the pH with either concentrated phosphoric acid or 10 M NaOH. The experiments were performed by constant volume titration and using an λex = 276 nm and λem = 285–445 nm. The slit width for the excitation and emission light was 0.7 and 2.0 nm, respectively, and the averaging time for each 0.05 nm step was 0.2 seconds. Tyrosine emission center of mass was calculated as described in Ref. 23 and the resulting pH-titration curves fitted to a single pKa.
Circular dichroism spectroscopy
CD studies were conducted on an Aviv 202 CD spectrometer at 25° C. For the α-helical measurements, lyophilized protein was dissolved in 10 mM APB pH 8.2 buffer to a concentration of ~ 50 µM. To ensure accurate absorbance readings, the cuvette pathlength was 10 and 2 mm for the UV/Vis and CD measurements, respectively. The absolute degree of secondary structures was determined by using α3W as a reference (76 ± 1% α-helical between pH 4 and 10).16,18 pH titrations were conducted by constant volume titration and the samples prepared by dissolving protein powder in 10 mM APB pH 7.0 buffer, splitting the solution into two equal fractions, and adjusting the pH with either 12 M HCl or 10 M NaOH. Chemical denaturation was conducted by automated constant volume titration of a 10 M urea protein sample into a 0 M urea protein sample. Samples were prepared by diluting protein stock in 10 mM APB pH 8.2 buffer containing either 0 or 10 M urea. The final protein concentrations were 15–40 µM for the pH and urea measurements. The degree of α-helical content and changes in this parameter were monitored by measuring the mean residue ellipticity at 222 nm ([Θ]222). The chemical denaturation curves were fitted as described in Ref. 24.
Size-exclusion chromatography
Gel filtration was performed using an analytical Superdex™ 75 column (GE Healthcare) equilibrated with 10 mM APB pH 7.0 buffer containing 100 mM KCl. The protein loading concentration was 250 µM.
NMR spectroscopy
NMR data were collected using a Varian Inova 750 MHz spectrometer equipped with a conventional room temperature probe or a Bruker Advance III 750 MHz spectrometer fitted with a cryoprobe. pH-titration samples were prepared by dissolving lyophilized protein in a 10 mM deuterated sodium acetate, 10 mM sodium phosphate, 30 mM KCl, pH 4.0 buffer and in a 10 mM sodium phosphate, 10 mM sodium borate, 30 mM KCl, pH 10.0 buffer. Buffers were prepared in D2O, all samples were ~ 600 µM in protein and contained 500 µM 2,2-dimethyl-2-silapentane-5-sulfonate sodium salt (DSS) as a chemical-shift standard. pH titrations were conducted by equal volume titration and collecting one-dimensional (1D) spectra of the α3Y-His proteins at 25° C. The chemical shift of the imidazole ring Hε1 resonance25 was used to determine the apparent imidazole/imidazolium pKa values of the single histidine in the α3Y-His proteins. Isotope effects were corrected using the following relationship: pK(H2O) = 0.929 × pK(D2O) + 0.42.26 Two-dimensional (2D) 15N-HSQC spectra27 were obtained on ~ 250 µM 15N-labeled α3Y-K29H and α3Y-K36H dissolved in either 20 mM deuterated sodium acetate, 20 mM sodium phosphate or 20 mM deuterated Tris. All samples contained 20 mM NaCl and 8% D2O. 15N-HSQC spectra were acquired at 25° and 35° C using a spectral width of 14.0 ppm and 1024 complex points in the direct 1H dimension and 18.0 ppm and 64 complex points in the indirect 15N dimension. 2D 1H–1H NOESY spectra28 were obtained on ~ 200 µM (α3Y-K29H and α3Y-K36H dissolved in D2O containing 20 mM deuterated sodium acetate and 20 mM NaCl or 20 mM deuterated Tris and 20 mM NaCl. NOESY spectra were collected at 25° using a mixing time of 150 ms, a spectral width of 10.0 ppm and 2048 complex points in the direct 1H dimension and 10.0 ppm and 800 complex points in the indirect 1H dimension. NMR data processing was performed with the Felix95 software (Accelrys Inc., San Diego, CA).
Electrochemistry
Cyclic voltammetry29 and differential pulse voltammetry29,30 were performed using an Autolab PGSTAT12 potentiostat. The electrochemical workstation was equipped with a temperature-controlled, Faraday-cage protected three-electrode micro-cell (Princeton Applied Research) containing a Ag/AgCl reference electrode, a platinum wire counter electrode, and 3 mm glassy carbon working electrode (all purchased from Advanced Measurements Inc.). The reference and counter electrodes were stored dry and routinely replaced after a limited set of experiments. They were prepared by filling the reference electrode with a 3 M KCl/saturated AgCl solution and the counter electrode with the buffer solution in which the sample was prepared. The surface of the working electrode was carefully polished between each measurement using a 0.05 µm alumina/water slurry on a glass-plate mounted microcloth pad (Bioanalytical systems Inc.). The electrode was manually polished for 60 sec., rinsed with water, sonicated first in ethanol for about 60 sec. and then in milli-Q water for another 60 sec., and finally rinsed with an excess of milli-Q water directed against the surface of the electrode. This protocol was repeated 2–3 times until a reproducible Faradaic response was observed from the sample. This cyclic procedure was typically performed only at the beginning of the experimental day and once the working electrode was conditioned, only a single polish/sonicate/rinse step was required to activate the surface between measurements. Voltammetry measurements were performed immediately following the electrode-activation treatment. The performance of the assembled three-electrode cell was routinely checked by collecting differential pulse voltammograms from a standard sample containing 300 µM N-acetyl-tryptophanamide in 10 mM APB, 125 mM KCl, pH 3.0. All samples were prepared using ultra-pure chemicals and the measurements made at 23° C under an argon atmosphere. Data analysis was performed using the Autolab GPES software and PeakFit (Systat Software Inc.).
RESULTS
Voltammetry studies of α3Y
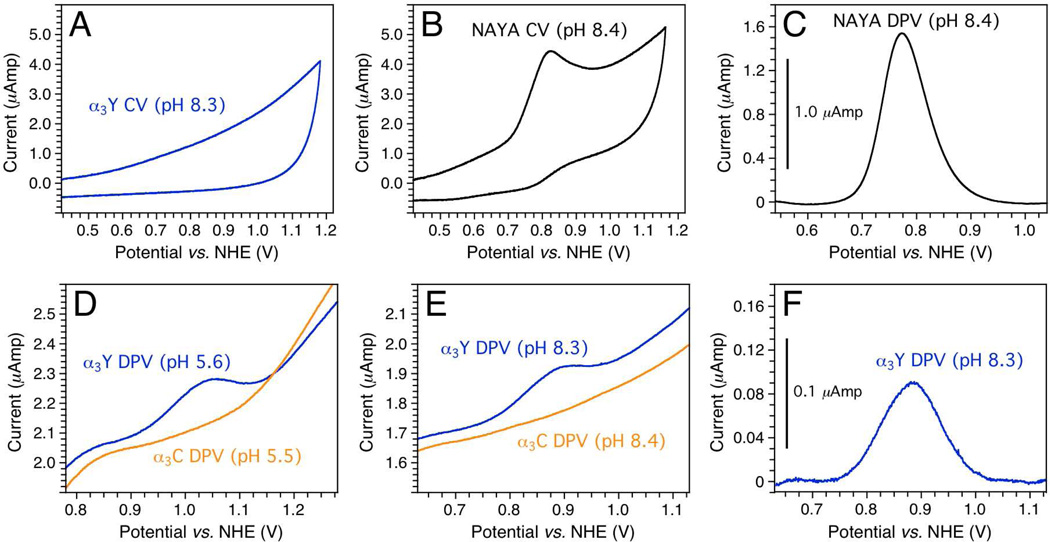
Panels A and B in Fig. 1 show cyclic voltammograms of α3Y and N-acetyl-L-tyrosinamide (NAYA), respectively. The two traces were obtained at very similar conditions, which are detailed in the figure legend. There is no observable Faradaic response from the protein sample while the NAYA sample gives rise to an easily detectable anodic waveform. As expected,31,32 the NAYA cyclic voltammogram is essentially completely irreversible. The poor response observed from α3Y when using cyclic voltammetry could not be improved by changing the protein concentration, buffer conditions or acquisition settings (e.g. see Fig. S1A in the supporting information). Thus unlike aqueous tyrosine31 and aqueous phenol,33 electrochemical analysis using cyclic voltammetry is at the present not a feasible approach for the α3Y system.
Fig. 1.
Electrochemical properties of α3Y and control samples. (A) Cyclic voltammogram of 210 µM α3Y in 20 mM potassium phosphate, 20 mM sodium borate, 40 mM KCl, pH 8.33; scan rate 200 mV/s, iR-compensation 103 ohm. (B) Cyclic voltammogram of 200 µM NAYA in 20 mM potassium phosphate, 20 mM sodium borate, 200 mM KCl, pH 8.37; scan rate 200 mV s−1, iR-compensation 103 ohm. (C) Differential pulse voltammogram of 200 µM NAYA in 20 mM potassium phosphate, 20 mM sodium borate, 200 mM KCl, pH 8.37; interval time 0.1 s, step potential 1.05 mV, scan rate 10.5 mV s−1, modulation time 8 ms, modulation amplitude 50 mV. The trace has been baseline corrected. (D) Differential pulse voltammograms of (blue) 210 µM α3Y in 20 mM sodium acetate, 20 mM potassium phosphate, 40 mM KCl, pH 5.56 and (orange) 200 µM α3C in 20 mM sodium acetate, 20 mM potassium phosphate, 40 mM KCl, pH 5.46; interval time 0.1 s, step potential 1.05 mV, scan rate 10.5 mV s−1, modulation time 5 ms, modulation amplitude 50 mV. (E) Differential pulse voltammograms of (blue) 210 µM α3Y in 20 mM potassium phosphate, 20 mM sodium borate, 40 mM KCl, pH 8.33 and (orange) 200 µM α3C in 20 mM potassium phosphate, 20 mM sodium borate, 40 mM KCl, pH 8.45; interval time 0.1 s, step potential 1.05 mV, scan rate 10.5 mV s−1, modulation time 8 ms, modulation amplitude 50 mV. (F) Baseline-corrected trace of the α3Y differential pulse voltammogram shown in Panel E.
However, a significant Faradaic response could be obtained from α3Y when using the more sensitive method of differential pulse voltammetry (DPV).29,30 Panels D and E in Fig. 1 show DP voltammograms obtained from α3Y (blue) and α3C (orange) at nearly identical conditions (see figure legend). α3C is a redox-inert Y32C variant of α3Y.19 At both acidic (panel D) and alkaline (panel E) conditions α3Y gives rise to a distinct oxidation wave while α3C does not. Differential pulse voltammograms recorded on samples containing α3C look essentially identical to baseline voltammograms collected on buffer samples (e.g. Fig. S1B). We conclude that there is no Faradaic response from the three-helix bundle scaffold when Y32 is removed. Thus, the α3Y voltammograms displayed in panels D and E represent the unique tyrosine residing in the hydrophobic core of α3Y.
Voltammograms obtained from aqueous samples at highly oxidizing potentials contain a prominent background current arising from water oxidation occurring at the surface of the working electrode. The magnitude of this current is influenced by the sample pH and by the type of working electrode used. Solvent oxidation effects are evident when comparing the raw and baseline-corrected α3Y pH 8.3 voltammogram displayed in panels E and F, respectively. α3Y DP voltammograms obtained at both acidic and alkaline pH display near symmetric waveforms with a well-defined peak potential and a width at half height of ~ 125 mV.
Panels C and F in Fig. 1 display baseline-corrected DPV traces of NAYA and α3Y, respectively. These traces were obtained at comparable sample conditions and using identical acquisition parameters (see figure legend). The only significant difference was the KCl concentration, which was 40 mM in the protein sample and 200 mM in the NAYA sample. At lower salt concentration the NAYA voltammogram displayed characteristics consistent with electrode absorption. α3Y gives rise to a current in the 0.1 µAmp range when using DPV while the NAYA sample provide a current on the 1.0 µAmp range. Thus, the peak current of the Y32 voltammogram is about one order of magnitude smaller relative to the peak current obtained from freely solvated NAYA.
We conclude that high-quality tyrosine DP voltammograms can be obtained from α3Y at both acidic and alkaline pH. The voltammogram collected at pH 5.6 is particularly impressive considering the high potential involved. Overall, these results represent the first successful use of voltammetry to detect a tyrosine radical located in a stable and well-folded protein environment. They also suggest that the α3Y system can be used to obtain tyrosine Pourbaix diagrams spanning a significant pH range.
Protein modeling
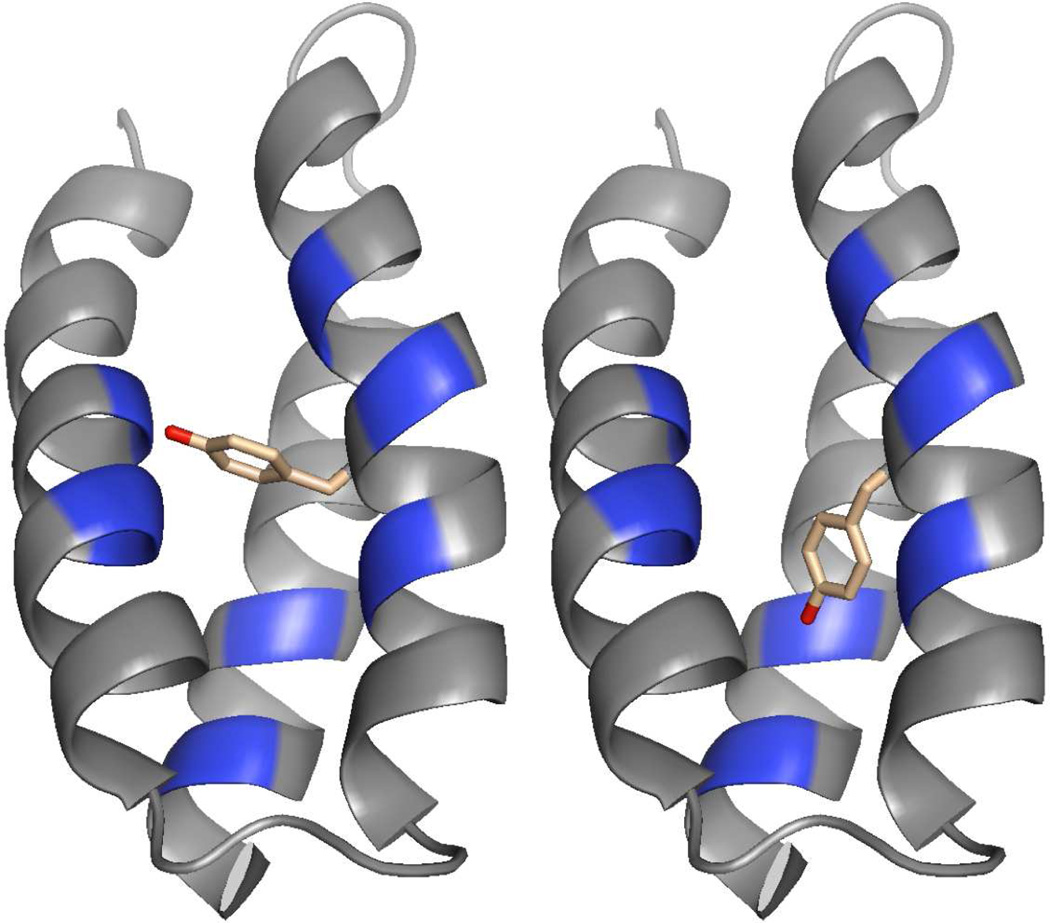
α3Y was re-engineered to make a histidine variant displaying a detectable interaction between the imidazole ring of the introduced histidine and the phenol head group of Y32. α3Y is a W32Y variant of the structurally characterized α3W three-helix bundle (see Fig. S2 in the supporting information).18 α3Y and α3W exhibit very similar structural properties including their α-helical content (~ 75%), pH stability (5–6 pH units), global stability (~ 4–5 kcal mol−1) and solution aggregation state (monomeric).16,18 Moreover, 2D 13C-HSQC spectra reflecting the environment of protein core residues in these two proteins display comparable spectral linewidths and chemical-shift dispersion. These observations show that large-scale structural changes do not occur in the three-helix bundle scaffold upon changing tryptophan 32 to a tyrosine. On this basis, α3Y models with various Y32 χ1 dihedral angles were made from the α3W NMR structure18 to identify sites where the incorporation of a histidine could place the imidazole ring within 5 Å of the Y32 phenol oxygen (Fig. 2). Histidine was modeled into each identified site and a broad range of Y32/His χ1 rotamer combinations were visually inspected. This analysis promoted the generation of the following eight proteins: α3Y-V9H, α3Y-L12H, α3Y-E13H, α3Y-K29H, α3Y-E33H, α3Y-K36H, α3Y-L58H and α3Y-I62H.
Fig. 2.
α3Y models illustrating two possible orientations of the Y32 side chain. The α-carbons of residues changed in the α3Y-His variants are shown in blue.
Screening for Y32/His interactions in the α3Y-His variants
It is long known that the optical properties of phenols are sensitive to the dielectric and hydrogen-bonding properties of solvating molecules.e.g.34 To provide an example, Fig. S3A in the supporting information displays the absorption spectra of α3Y (λmax 277.8 nm) and NAYA (λmax 275.3 nm) both obtained at neutral pH. The relative redshift of the α3Y spectrum indicates that the Y32 side chain is shielded from the bulk solvent. This is consistent with other characteristics of Y3216 and the sequestered position of the W32 residue (solvent accessible surface area of 2.6 ± 1.4% across the α3W NMR structural ensemble).18,35 Absorption and fluorescence spectra of the eight α3Y-His variants were compared to α3Y spectra obtained at corresponding conditions to search for changes in the microenvironment of Y32. Only minor shifts were detected in the Y32 absorption (Fig. S3B) while the fluorescence data provided more distinguishing information. We found α3Y-E13H, α3Y-E33H and α3Y-E58H excitation (data not shown) and emission (Fig. S4) spectra essentially identical to those of α3Y and these three proteins were excluded for further characterization. In contrast, excitation and/or emission spectra of α3Y-V9H, α3Y-L12H, α3Y-K29H, α3Y-K36H and α3Y-I62H are shifted relative to corresponding α3Y spectra (Fig. S5), which prompted further studies of these five proteins.
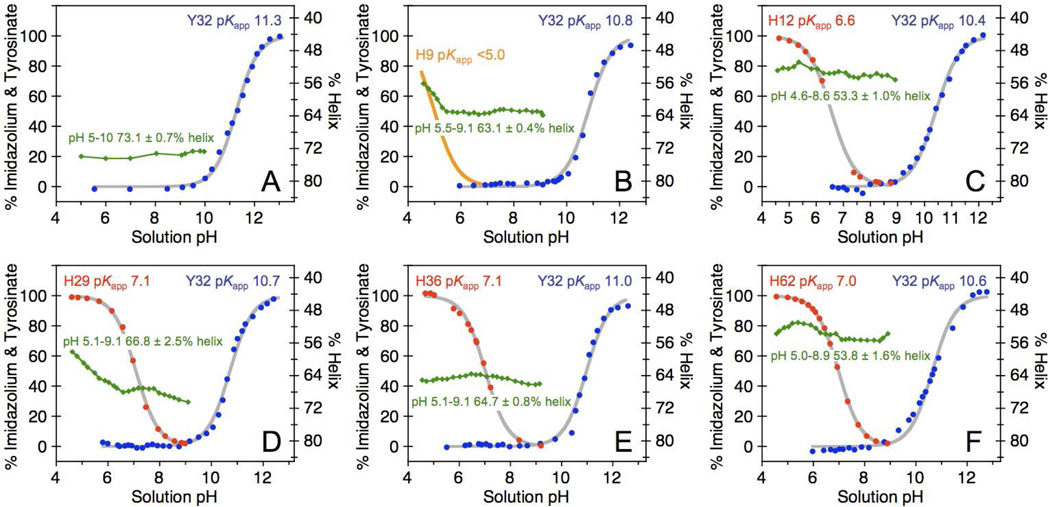
Alterations in the optical properties of Y32 may arise from a local change in the vicinity of the aromatic residue but may also reflect a global perturbation of the protein scaffold. A second screening step was performed to probe the structural integrity of the five selected α3Y-His proteins. pKapp values of Y32 and introduced histidines were determined optically via the tyrosinate absorbance at 293 nm (Fig. S6) and by following spectroscopically the NMR chemical shift of the imidazole ε1 proton (Fig. S7) as a function of pH. CD spectroscopy was used to determine absolute α-helical content, the pH-sensitivity in this parameter, and global protein stability. Only a minor decrease in the degree of helicity was observed for α3Y-V9H, α3Y-K29H and α3Y-K36H relative to α3Y (~ 5–10%; Fig. 3 and Table 1). These proteins also give rise to well-defined unfolding/folding transitions (Fig. S8A) from which the global protein stabilities could be determined (Table 1). In contrast, α3Y-L12H and α3Y-I62H show a loss of ~ 20% of their α-helical contents relative to α3Y (Table 1) and give rise to poorly defined denaturation curves (Fig. S8B). In addition, NMR resonances representing the H12 and H62 ring protons display relatively broad linewidths. This effect was particularly pronounced for α3Y-L12H with the imidazole Hε1 resonance broadened beyond detection between pH 6.3 and 7.4 (Fig. 3C). These observations are all consistent with structurally perturbed protein folds and no additional data were collected on α3Y-L12H and α3Y-I62H.
Fig. 3.
Physicochemical properties of α3Y and single-site histidine variants. pH titrations of Y32 (blue circles) and histidine residues (red circles) are shown for (A) α3Y, (B) α3Y-V9H, (C) α3Y-L12H, (D) α3Y-K29H, (E) α3Y-K36H, and (F) α3Y-I62H. pKapp values were derived by nonlinear curve fitting (grey lines; Table 1). α-helical contents as a function of pH are shown in green (diamonds).
Table 1.
Physicochemical properties of α3Y and α3Y-His variantsa
| Protein | pKapp Y32b | pKapp Hisb | Y32 Em(pH)c | [Θ]222d | % Helixe | ΔGf |
|---|---|---|---|---|---|---|
| α3Y | 11.3 | – | 8.1 | −21.9 | 73.1 ± 0.7 (pH 5.0–10.0) | −3.7 |
| α3Y-V9H | 10.8 | <5.0 | n.d. | −18.7 | 63.1 ± 0.4 (pH 5.5–9.1) | −3.0 |
| α3Y-L12H | 10.4 | 6.6 | n.d. | −16.0 | 53.3 ± 1.0 (pH 4.6–8.6) | n.d. |
| α3Y-K29H | 10.7 | 7.1 | 7.4 | −20.4 | 66.8 ± 2.5 (pH 5.1–9.1) | −2.8 |
| α3Y-K36H | 11.0 | 7.0 | 7.1 | −19.5 | 64.7 ± 0.8 (pH 5.1–9.1) | −2.4 |
| α3Y-I62H | 10.6 | 7.0 | n.d. | −16.5 | 53.8 ± 1.6 (pH 5.0–8.9) | n.d. |
Em (nm); [Θ]222 × 103 (deg cm2 dmol−1); ΔG (kcal mol−1); n.d., not determined
Apparent tyrosinate/tyrosine and imidazole/imidazolium pKa values of Y32 and histidine residues obtained by fitting the pH-titration curves in Fig. 3 to a single pKa. Statistical errors ≤ 0.1.
Apparent pKa obtained by fitting the pH-titration curves in Fig. 4 to a single pKa. Statistical errors ≤ 0.1.
Mean residue ellipticity measured at pH 8.2 and 25° C. The α3 W reference displays a [Θ]222 value of −22.6×103 deg cm2 dmol−1 at the same conditions.
Global protein stabilities obtained by fitting the urea-denaturation curves in Fig. S8A. Data recorded at pH 8.2 and 25° C. Fitting standard errors < 0.03 kcal mol−1.
A key requirement for the tyrosine radical model system is that pH-induced structural changes in the protein scaffold do not obscure detection and characterization of local interactions at the radical site. Thus for the histidine variants we wish to measure the potential of Y32 across the titratable pH range of the introduced histidine while avoiding large-scale structural changes. α3Y-K29H and α3Y-K36H meet this requirement well as they remain 66.8 ± 2.5 and 64.7 ± 0.8% α-helical, respectively, ± 2 pH units around the pKapp values of H29 and H36 (Table 1). Although the CD and NMR data collected on α3Y-V9H suggest a stable and well-folded protein, the pKapp of H9 is below the protein pH-stability range (Table 1). For this reason, α3Y-V9H was excluded from further characterization.
Spectroscopic evidence of Y32/His interactions in α3Y-K29H and α3Y-K36H
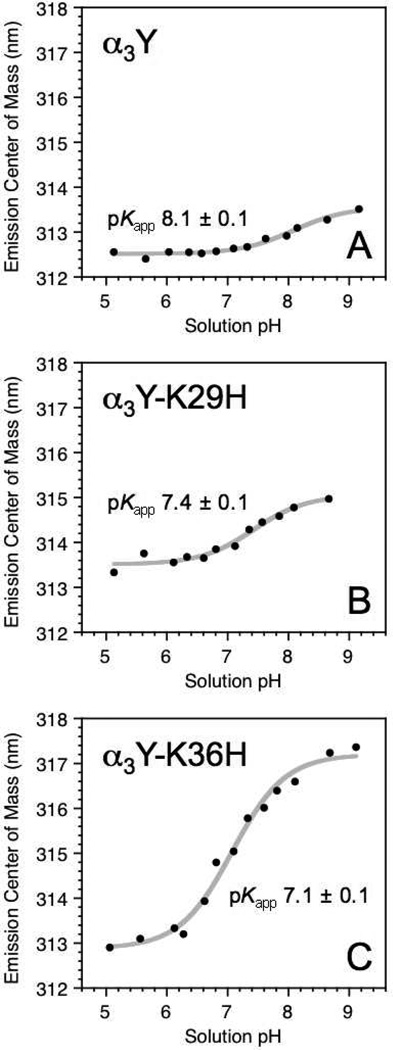
Fluorescence pH titrations were conducted to probe for more direct evidence of Y32/His interactions in α3Y-K29H and α3Y-K36H. Figure 4A shows the α3Y tyrosine emission center of mass as a function of pH. A 1.0 nm shift was observed between pH 5.1 and 9.1 and yielded a fitted single pKapp of 8.1 ± 0.1. This effect may arise from a pH-induced change in the electrostatic environment of the tyrosine, the hydrogen-bonding properties of the phenol OH group, or a combination thereof.36 A more substantial shift of 4.3 nm was observed for α3Y-K36H (Fig. 4C). The pH-induced spectral changes titrate with a pKapp of 7.1 ± 0.1, which is equivalent to the pKapp value determined for H36 via NMR (Table 1). The correlation between the two pKapp values suggests that the Y32 fluorescence is sensitive to the protonation state of H36 and that the two aromatic side chains are in close proximity. Likewise, the α3Y-K29H emission center of mass titrates with a pKapp of 7.4 ± 0.1 (Fig. 4B). This value is similar to the NMR-derived H29 pKapp of 7.1 ± 0.1 suggesting a structural connection between Y32 and H29. The observed changes in the Y32 fluorescence most likely reflect electrostatic interactions between Y32 and the introduced histidine residues. Alternatively, changing the protonation state of the histidine leads to a change in the hydrogen-bonding environment of the Y32 OH group. Either of these explanations suggests that the imidazole group of the histidine residue introduced in α3Y-K29H and α3Y-K36H reside near or at the Y32 site.
Fig. 4.
Fluorescence emission center of mass of Y32 in (A) α3Y, (B) α3Y-K29H and (C) α3Y-K36H as a function of pH. pKapp values were derived by nonlinear curve fitting (grey lines; Table 1).
Confirming the absence of pH-induced changes in aggregation state and tertiary structures
In preparation for voltammetry-based pH studies, it was essential to establish that α3Y-K29H and α3Y-K36H are monomeric and well structured across a significant pH range. Earlier work involving sedimentation equilibrium ultracentrifugation and analytical size-exclusion chromatography have demonstrated that the aggregation state of α3Y in solution is that of a monomeric protein over a concentration range of at least 4–850 µM.16 Moreover, 1D and 2D NMR spectra collected on α3Y in the mM concentration range display characteristics consistent with a non-aggregated, uniquely folded protein. On the basis of these earlier observations, α3Y was used here as a standard to determine the aggregation states of α3Y-K29H and α3Y-K36H. Fig. S9 in the supporting information shows size-exclusion chromatograms obtained from α3Y and α3Y-K29H at pH 7.0 and a protein loading concentration of 250 µM. The α3Y and α3Y-K29H chromatograms display profiles consistent with a single major species (95% and 91% of the total 220 nm absorbance, respectively) eluting with a retention volume of 13.1 and 12.9 ml, respectively. An equivalent chromatogram obtained on α3Y-K36H shows a single major species eluting at 13.1 ml (data not shown). We conclude that there are no significant differences in the hydrodynamic volume of the three proteins and, consequently, that they are all monomeric at neutral pH.
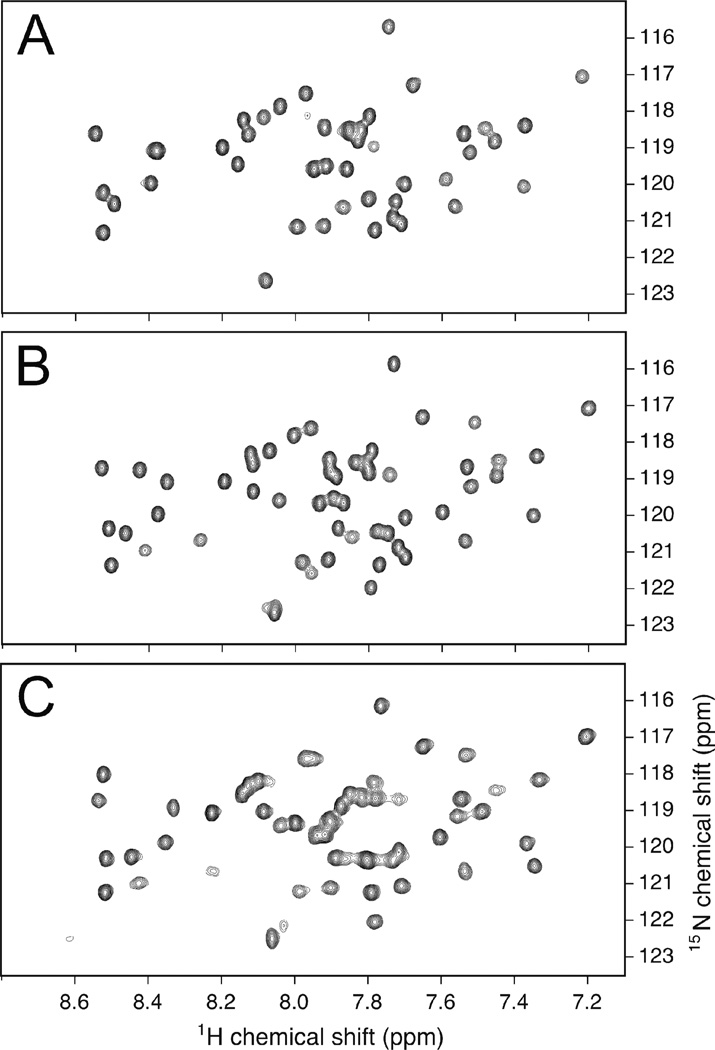
2D NMR spectroscopy was used to probe for changes in aggregation state and/or tertiary structure as a function of pH. 15N-HSQC spectra were collected from α3Y-K29H and α3Y-K36H at three different pH values (5.5, 7.0 and 8.5) and two different temperatures (25° and 35° C). Fig. 5B shows the α3Y-K29H 15N-HSQC spectrum obtained at pH 7.0 and 25° C. The displayed region of the 2D spectrum contains a set of 54 resolved 15N-1H cross peaks. In this spectral region we expect to detect ≤ 56 cross peaks arising from backbone N-H groups in the helical regions of the protein. Amide resonances associated with the eight glycines in loop regions (15N 107–109 ppm) and L65 at the C-terminus (15N 126.6 ppm) occur outside the displayed region. The observed narrow spectral linewidths, overall chemical-shift dispersion and absence of minor peaks confirm that α3Y-K29H is a monomeric, uniquely folded protein at these conditions. Moreover, when comparing panels A, B and C in Fig. 5 it is clear that there are no significant changes in the spectral linewidths as a function of pH, which, in turn, means that there is no significant change in the hydrodynamic volume of α3Y-K29H. pH-induced electrostatic and hydrogen-exchange effects influence peak positions and intensities as expected but the overall chemical-shift dispersion remains essentially the same. Thus, α3Y-K29H remains well folded across the pH 5.5 to 8.5 range. The same conclusions can be made from the 15N-HSQC pH 5.5, 7.0 and 8.5 spectra obtained from α3Y-K29H at 35° C (data not shown) and from α33Y-K36H at 25° C (data not shown) and 35° C (Fig. S10). There are no significant changes in spectral linewidths and chemical-shift dispersion as a function of protein, pH and temperature. We conclude that α3Y-K29H and α3Y-K36H are monomeric and well-structured proteins across a pH range of at least 5.5 to 8.5.
Fig. 5.
2D 15N-HSQC spectra of α3Y-K29H obtained at 25° C and with the pH at (A) 8.5, (B) 7.0 and (C) 5.5. These spectra, as well as those shown in Fig. S10, display a single set of peaks with no evidence of shadow peaks that would be indicative of minor conformers.
Confirming that Y32 is buried in α3Y-K29H and α3Y-K36H
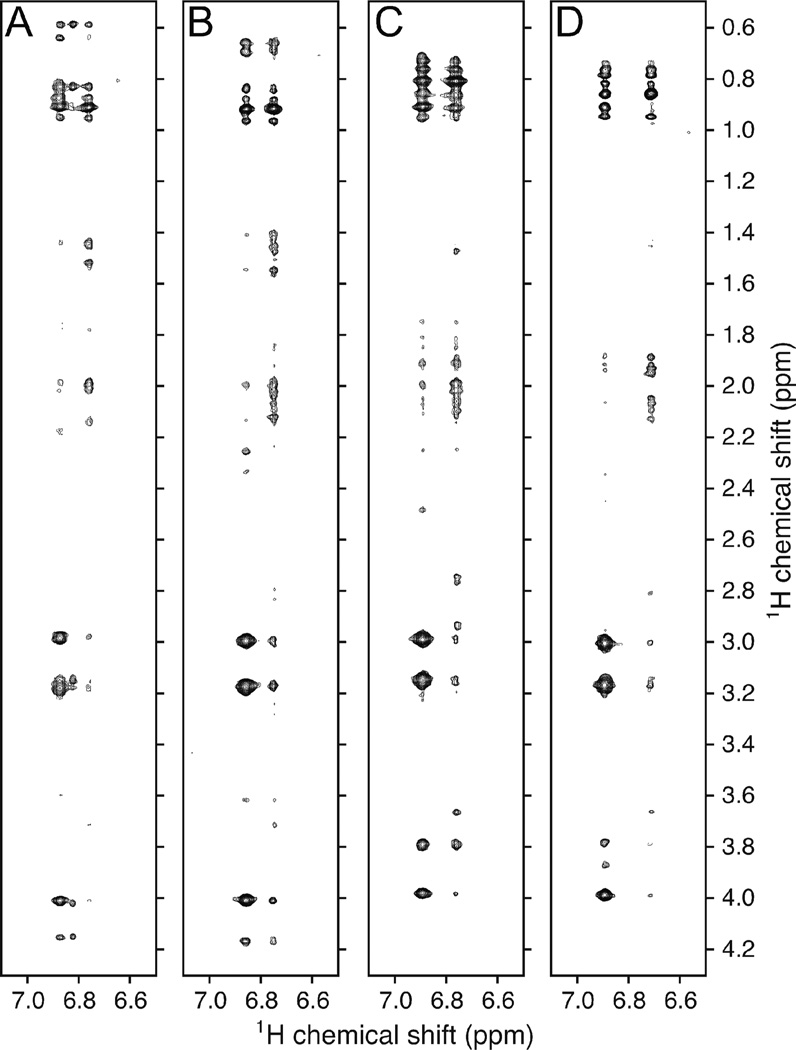
Figure 6 shows 1H-1H NOESY spectra obtained from α3Y-K29H at pH 8.5 and 5.6 (panels A and B) and α3Y-K36H at pH 8.4 and 5.6 (panels C and D). The selected region of the NOESY spectrum display NOEs between the Y32 ring-protons and aliphatic protons located within a distance of ~ 5 Å. The δ1 & δ2 (the meta positions of the aromatic ring) and ε1 & ε2 (ortho positions) ring-protons are unambiguously assigned on the basis of the observed intraresidue NOE patterns. For example, consider the α3Y-K36H pH 8.4 spectrum (Fig. 6C). The unresolved resonances of the δ1 & δ2 ring-protons at 6.89 ppm show strong NOE correlations to the Y32 β-protons at 2.99 and 3.15 ppm and Y32 α-proton at 3.98 ppm. The unresolved resonances of the ε1 & ε2 ring-protons at 6.76 also show intra-residue NOEs to the Y32 β- and α-protons but with weaker intensities corresponding to the longer 1H-1H distances. The same intra-residue Y32 NOE correlation patterns are observed in panels A, B and D.
Fig. 6.
2D 1H-1H-NOESY spectra obtained at 25° C and representing (A) α3Y-K29H at a measured pH of 8.5, (B) α3Y-K29H at pH 5.6, (C) α3Y-K36H at pH 8.4 and (D) α3Y-K36H at pH 5.6.
Figure 6 shows that the Y32 ring-protons are close to a dozen or more protons with resonances in the 0.59 to 0.97 ppm spectral region at both pH 8.5 and 5.6. The observed chemical shifts are consistent with resonances from methyl groups associated with valine, leucine and isoleucine residues. These types of residues form the hydrophobic packing layers above, below and at the level of W32 in the α3W structure.18,37 Depending on the pH, the α3Y-K29H aromatic ring-protons exhibit at least 12–26 additional inter-residue NOEs to aliphatic protons with resonances in the 1.40 to 4.17 ppm region (Figs. 6A and B). For α3Y-K36H at least 19–28 additional inter-residue NOEs are observed between the aromatic ring-protons and nearby aliphatic protons (Figs. 6C and D). These observations firmly suggest that Y32 is buried in the hydrophobic core of the two α3Y histidine variants, as expected on the basis of the protein design and the structural knowledge of α3W.
DISCUSSION
Key structural properties of the tyrosine radical protein system
Despite the fact that tyrosine radicals have been known to be involved in biological redox processes for more that three decades,38 a basic Pourbaix diagram describing the reduction potential of a protein tyrosine radical as a function of the solution pH is not available. In fact, information regarding reduction potentials associated with protein tyrosine radicals is limited to only two indirectly derived, single-pH estimates (vide supra). This situation suggests that studies limited to the natural systems will not provide basic thermodynamic information of these essential PCET cofactors in a timely manner, or even at all. For this reason, we adopted a model protein approach.
It is vital to stress that although the 7.5 kDa three-helix bundle scaffold used here is small relative to most naturally occurring tyrosine radical proteins, it nonetheless exhibits all of the key characteristics of a protein system. Its folding is driven by the hydrophobic effect.18,35,37 Its unfolding/folding transition is reversible and cooperative (e.g. Fig. S8A). Importantly, the ensemble of conformers that represent the solution state of the three-helix bundle proteins occupy narrow wells as shown by the excellent structural statistics of α3W (see Fig. S2 legend) and by the characteristics of their HSQC spectra (e.g. Figs. 5 and Fig. S10). Structural statistics of similar high quality were recently obtained for the NMR solution structures of two phenol-containing derivatives of α3Y (unpublished data). Finally, the tyrosine of interest Y32 is desolvated and maintained in a highly structured environment across a broad pH range. These structural properties uniquely separate the model system described here from all other tyrosine/phenol radical model systems described thus far in the literature. In these small-moleculee.g.39 and peptidee.g.32,40 model systems, the phenol side chain resides in a highly solvent-exposed and dynamic environment.
Electrochemical approach
Protein electrochemistry include classic redox titrations using chemical titrants and a redox cuvette,41 redox titrations using a thin-layer spectroelectrochemical cell controlled by a potentiostat,42 and protein voltammetry.43 Redox titrations require a mediator system that covers the potential range of interest and a distinct spectroscopic feature that reflects the redox state of the cofactor. The size and the complexity of the redox protein generally do not provide an experimental barrier as long as the redox center is in equilibrium with the electrode via the redox mediators and give rise to a detectable signal that occurs outside the spectral envelope of the added mediators. In contrast, protein voltammetry does not require redox mediators or a spectroscopic probe of the redox system. This technique is however highly sensitive to the size of the protein and its orientation on the electrode surface since there must be electronic contact between the redox cofactor and the working electrode. The experimental time scale differs between the two approaches as redox titrations are conducted on the hours time scale while voltammetry data are typically collected on the minutes to ms time scale.
The main characteristics of α3Y all suggest that voltammetry is the feasible approach. The small size of the protein is an advantage and predicts that a functional Y32/electrode electron-tunneling distance can occur in multiple α3Y/electrode spatial orientations. α3Y is a high-potential system and an experimental time scale in the minutes rather than hours range reduces the risk of oxidative damage. Moreover, the weak optical features of α3Y (ε276 1490 M−1 cm−1 for reduced α3Y, ε408 2750 M−1 cm−1 for oxidized tyrosine44) combined with spectral overlap of redox mediators typically used in the high-potential range,10,45 will make spectroscopic monitoring of the α3Y redox state challenging. Finally, and most importantly, voltammetry can provide kinetic and mechanistic information in addition to thermodynamic parameters.33,43 Thus, the key demonstration of a reproducible Faradaic response from Y32 sets the stage for more detailed studies as we continue to develop the α3Y system.
The main challenge in protein voltammetry is to identify a working electrode system that provides the potential range required, allows direct cofactor/electrode electron transfer and promotes the folded protein to interact in a favorably manner with the electrode surface without adsorptive denaturation. A variety of electrode preparation strategies have been employed in order to modulate the strength of the protein/electrode interactions to, ideally, generate either a diffusion-controlled system or a stable protein film.43
Electrochemical response of α3Y
In this study, our first aim was to demonstrate that Y32 is redox active and that voltammetry can be used to probe the potential of the tyrosine in the folded protein at both acidic and alkaline conditions. A glassy carbon electrode was chosen as the working electrode system since it has an anodic potential window that extends to about +1.3 V vs. NHE in aqueous media.29 The glassy carbon electrode was activated by polishing the surface in an Al2O3/water slurry, which is predicted to generate a hydrophilic surface with C–O functionalities such as hydroxyls, carbonyls, ethers and carboxylates.46 In a preliminary study on α3Y, a Faradaic response could not be obtained from the folded protein but only at sample conditions where the protein is unfolded.16 By refining the pretreatment of the working electrode (see Materials and Methods section), we have now obtained differential pulse voltammograms from α3Y at sample conditions where the protein is known to be monomeric, folded and well structured. Once the electrode pretreatment protocol was obtained, the electrochemical response from α3Y was compared at high and low pH allowing sample conditions and acquisition parameters to be refined in an iterative manner yielding typical voltammograms of the quality shown in Figs. 1D to F. We showed that the Faradaic response is uniquely associated with Y32 and that it is abolished when the tyrosine is replaced by site-directed mutagenesis. Importantly, this is the first time voltammetry is successfully applied to detect a redox-active tyrosine residing in a structured protein environment.
Structural properties of α3Y-K29H and α3Y-K36H
Our second major goal was to demonstrate that Pourbaix diagrams derived from the tyrosine radical protein system reflect the local environment of the redox-active tyrosine. Overall, this goal represents a quite challenging task and was divided into three connected projects including the following: 1) To engineer and demonstrate the presence of a specific structural interaction at the site of the redox-active tyrosine. 2) To demonstrate that the structural properties of the re-engineered α3Y variants are appropriate in order to conduct voltammetry-based pH titrations. Thus, effects on the tyrosine radical potential from global events occurring in the protein scaffold, such as pH-induced changes in secondary and tertiary structures, must be minimized. In addition, the aggregation state of the protein should be pH independent to avoid a situation in which the distance between the radical site and the surface of the working electrode varies as a function of pH. This can lead to changes in the shape and position of the voltammogram representing the redox-active tyrosine and to an overall loss of the Faradaic signal.47 3) To derive potential vs. pH diagrams from α3Y and variants and determine the effects of the specifically engineered interaction on the tyrosine redox system. This study completes the first two objectives.
We re-engineered α3Y with the specific aim to place the imidazole group of a histidine residue close to the phenol ring of Y32 (Figs. S2 and 2). Eight α3Y-His variants were generated of which two displayed promising spectroscopic and structural characteristics. α3Y-K29H and α3Y-K36H each contain a histidine residue which protonation perturbs the fluorescence of Y32 (Table 1; Fig. 4). This observation suggests that the engineered histidine and the redox-active Y32 residue are in close proximity. Moreover, we could show that α3Y-K29H and α3Y-K36H are stable (Fig. S8: Table 1) and well-folded proteins whose α-helical content (Fig. 3), tertiary structure (Figs. 5 and S10), solution aggregation state (Figs. 5, S9 and S10), and solvent-sequestered position of Y32 (Fig. 6) are pH independent or highly pH insensitive across a range of at least 3–4 pH units. These results demonstrate that we have achieved a tight structural control over the model protein hosting the redox-active tyrosine and that voltammetry measurements can be conducted across a broad pH range without large-scale structural changes occurring in the protein scaffold. Thus, the described model system is uniquely adapted to use voltammetry to study PCET reactions associated with tyrosine radical chemistry occurring in a solvent sequestered and well-structured protein environment. Such studies are described in a separate report.22
Supplementary Material
ACKNOWLEDGEMENTS
We are indebted to Drs. Josh Wand, Harry Gray and Jeff Warren for extensive and valuable discussions. Funding was provided by NIH grant GM079190 and by NIH predoctoral fellowship GM096756 to M.C.M.R.
Footnotes
SUPPORTING INFORMATION AVAILABLE
Figures showing cyclic and differential pulse voltammograms of α3Y and control samples; the α3W solution NMR structure; absorption and fluorescence spectra of NAYA, α3Y and single-site histidine variants; absorption spectra of α3Y at neutral and high pH; 1D NMR spectra of α3Y-K36H at acidic, neutral and alkaline pH; denaturation plots of α3Y and single-site histidine variants; size-exclusion chromatograms of α3Y and α3Y-K29H; 15N-HSQC spectra of α3Y-K36H. This material is available free of charge via the internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Stubbe J, van der Donk WA. Chem. Rev. 1998;98:705–762. doi: 10.1021/cr9400875. [DOI] [PubMed] [Google Scholar]; (b) Frey PA, Hegeman AD, Ruzicka FJ. Crit. Rev. Biochem. Mol. Biol. 2008;43:63–88. doi: 10.1080/10409230701829169. [DOI] [PubMed] [Google Scholar]
- 2.Hoganson CW, Tommos C. Biochim. Biophys. Acta. 2004;1655:116–122. doi: 10.1016/j.bbabio.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Tommos C, Babcock GT. Biochim. Biophys. Acta. 2000;1458:199–219. doi: 10.1016/s0005-2728(00)00069-4. [DOI] [PubMed] [Google Scholar]
- 4.(a) Umena Y, Kawakami K, Shen J-R, Kamiya N. Nature. 2011;473:55–60. doi: 10.1038/nature09913. [DOI] [PubMed] [Google Scholar]; (b) Kawakami K, Umena Y, Kamiya N, Shen J-R. J. Photochem. Photobiol. B. 2011;104:9–18. doi: 10.1016/j.jphotobiol.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 5.(a) Proshlyakov DA, Pressler MA, DeMaso C, Leykam JF, DeWitt DL, Babcock GT. Science. 2000;290:1588–1591. doi: 10.1126/science.290.5496.1588. [DOI] [PubMed] [Google Scholar]; (b) Hemp J, Robinson DE, Ganesan KB, Martinez TJ, Kelleher NL, Gennis RB. Biochemistry. 2006;45:15405–15410. doi: 10.1021/bi062026u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gorbikova EA, Belevich I, Wikström M, Verkhovsky MI. Proc. Nat. Acad. Sci. U.S.A. 2008;105:10733–10737. doi: 10.1073/pnas.0802512105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aubert C, Vos MH, Mathis P, Eker APM, Brettel K. Nature. 2000;405:586–590. doi: 10.1038/35014644. [DOI] [PubMed] [Google Scholar]
- 7.(a) Sjöberg BM. Struct. Bond. 1997;88:139–173. [Google Scholar]; (b) Stubbe J, Nocera DG, Yee CS, Chang MCY. Chem. Rev. 2003;103:2167–2201. doi: 10.1021/cr020421u. [DOI] [PubMed] [Google Scholar]
- 8.Reece SY, Hodgkiss JM, Stubbe J, Nocera DG. Phil. Trans. R. Soc. B. 2006;1472:1351–1364. doi: 10.1098/rstb.2006.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen LMR, Sanishvili R, Davidson VL, Wilmot CM. Science. 2010;327:1392–1394. doi: 10.1126/science.1182492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva KE, Elgren TE, Que L, Stankovich MT. Biochemistry. 1995;34:14093–14103. doi: 10.1021/bi00043a014. [DOI] [PubMed] [Google Scholar]
- 11.Rappaport F, Diner BA. Coord. Chem. Rev. 2008;252:259–272. [Google Scholar]
- 12.Grabolle M, Dau H. Biochim. Biophys. Acta. 2005;1708:209–218. doi: 10.1016/j.bbabio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 13.(a) Jeans C, Schilstra MJ, Klug DR. Biochemistry. 2002;41:5015–5023. doi: 10.1021/bi0118862. [DOI] [PubMed] [Google Scholar]; (b) Buchta J, Grabolle M, Dau H. Biochim. Biophys. Acta. 2007;1767:565–574. doi: 10.1016/j.bbabio.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Mondal MS, Fuller HA, Armstrong FA. J. Am. Chem. Soc. 1996;118:263–264. [Google Scholar]
- 15.Byrdin M, Lukacs A, Thiagarajan V, Eker APM, Brettel K, Vos MH. J. Phys. Chem. A. 2010;114:3207–3214. doi: 10.1021/jp9093589. [DOI] [PubMed] [Google Scholar]
- 16.Tommos C, Skalicky JJ, Pilloud DL, Wand AJ, Dutton PL. Biochemistry. 1999;38:9495–9507. doi: 10.1021/bi990609g. [DOI] [PubMed] [Google Scholar]
- 17.Tommos C. Phil. Trans. R. Soc. B. 2002;357:1383–1394. doi: 10.1098/rstb.2002.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai Q-H, Tommos C, Fuentes EJ, Blomberg MRA, Dutton PL, Wand AJ. J. Am. Chem. Soc. 2002;124:10952–10953. doi: 10.1021/ja0264201. [DOI] [PubMed] [Google Scholar]
- 19.Hay S, Westerlund K, Tommos C. Biochemistry. 2005;44:11891–11902. doi: 10.1021/bi050901q. [DOI] [PubMed] [Google Scholar]
- 20.Dixon WT, Murphy DJ. Chem. Soc. Faraday. Trans. II. 1976;72:1221–1230. [Google Scholar]
- 21.(a) Huynh MHV, Meyer TJ. Chem. Rev. 2007;107:5004–5064. doi: 10.1021/cr0500030. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Reece SY, Nocera DG. Annu. Rev. Biochem. 2009;78:673–699. doi: 10.1146/annurev.biochem.78.080207.092132. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Warren JJ, Tronic TA, Mayer JM. Chem. Rev. 2010;110:6961–7001. doi: 10.1021/cr100085k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Dempsey JL, Winkler JR, Gray HB. Chem. Rev. 2010;110:7024–7039. doi: 10.1021/cr100182b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry BW, Martínez-Rivera MC, Tommos C. Submitted to the Proceedings of the National Academy of Sciences USA [Google Scholar]
- 23.Ehrhardt MR, Erijman L, Weber G, Wand AJ. Biochemistry. 1996;35:1599–1605. doi: 10.1021/bi951267r. [DOI] [PubMed] [Google Scholar]
- 24.Santoro MM, Bolen DW. Biochemistry. 1988;27:8063–8068. doi: 10.1021/bi00421a014. [DOI] [PubMed] [Google Scholar]
- 25.Markley JL, Bax A, Arata Y, Hilbers CW, Kaptein R, Sykes BD, Wright PE, Wüthrich K. J. Mol. Biol. 1998;280:933–952. doi: 10.1006/jmbi.1998.1852. [DOI] [PubMed] [Google Scholar]
- 26.Krezel A, Bal W. J. Inorg. Biochem. 2004;98:161–166. doi: 10.1016/j.jinorgbio.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Cavanagh J, Fairbrother WJ, Palmer AG, Rance M, Skelton NJ. Protein NMR spectroscopy: Principles and practice. 2nd. USA: Elsevier; 2006. [Google Scholar]
- 28.Jeener J, Meier BH, Bachmann P, Ernst RR. (1979) Investigation of exchange processes by 2-dimensional NMR-spectroscopy. J. Chem. Phys. 1979;71:4546–4553. [Google Scholar]
- 29.Bard AJ, Faulkner LR. Electrochemical methods: Fundamentals and applications. 2nd. USA: John Wiley & Sons, Inc; 2001. [Google Scholar]
- 30.Parry EP, Osteryoung RA. Anal. Chem. 1965;37:1634–1637. [Google Scholar]
- 31.Harriman A. J. Phys. Chem. 1987;91:6102–6104. [Google Scholar]
- 32.DeFelippis MR, Murthy CP, Broitman F, Weinraub D, Faraggi M, Klapper MH. J. Phys. Chem. 1991;95:3416–3419. [Google Scholar]
- 33.Costentin C, Louault C, Robert M, Savéant J-M. Proc. Nat. Acad. Sci U.S.A. 2009;106:18143–18148. doi: 10.1073/pnas.0910065106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.(a) Strickland EH, Wilchek M, Horwitz J, Billups CJ. J. Biol. Chem. 1972;247:572–580. [PubMed] [Google Scholar]; (b) Lee JK, Ross RT. J. Phys. Chem. B. 1998;102:4612–4618. [Google Scholar]; (c) Noronha M, Lima JC, Lamosa P, Santos H, Maycock C, Ventura R, Macanita AL. J. Phys. Chem. A. 2004;108:2155–2166. [Google Scholar]
- 35.Berry BW, Elvekrog MM, Tommos C. J. Am. Chem. Soc. 2007;129:5308–5309. doi: 10.1021/ja068957a. [DOI] [PubMed] [Google Scholar]
- 36.(a) Lakowicz JR. Principles of Fluorescence Spectroscopy. New York, USA: Plenum Press; 1983. [Google Scholar]; (b) Willis KJ, Szabo AG. J. Phys. Chem. 1991;95:1585–1589. [Google Scholar]; (b) Lee JK, Ross RT, Thampi S, Leurgans S. J. Phys. Chem. 1992;96:9158–9162. [Google Scholar]
- 37.Westerlund K, Berry BW, Privett HK, Tommos C. Biochim. Biophys. Acta. 2005;1707:103–116. doi: 10.1016/j.bbabio.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Sjöberg B-M, Reichard P, Gräslund A, Ehrenberg A. J. Biol. Chem. 1977;252:536–541. [PubMed] [Google Scholar]
- 39.(a) Sjödin M, Styring S, Åkemark B, Sun L, Hammarström L. J. Am. Chem. Soc. 2000;122:3932–3936. [Google Scholar]; (b) Rhile IJ, Mayer JM. J. Am. Chem. Soc. 2004;126:12718–12719. doi: 10.1021/ja031583q. [DOI] [PubMed] [Google Scholar]; (c) Benisvy L, Bittl R, Bothe E, Garner CD, McMaster J, Ross S, Teutloff C, Nesse F. Angew. Chem. Int. Ed. 2005;44:5314–5317. doi: 10.1002/anie.200501132. [DOI] [PubMed] [Google Scholar]; (d) Costentin C, Robert M, Savéant J-M. J. Am. Chem. Soc. 2006;128:4552–4553. doi: 10.1021/ja060527x. [DOI] [PubMed] [Google Scholar]; (e) Markle TF, Rhile IJ, DiPasquale AG, Mayer JM. Proc. Nat. Acad. Sci. U.S.A. 2008;105:8185–8190. doi: 10.1073/pnas.0708967105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Moore GF, Hambourger H, Kodis G, Michl W, Gust D, Moore TA, Moore AL. J. Phys. Chem. B. 2010;114:14450–14457. doi: 10.1021/jp101592m. [DOI] [PubMed] [Google Scholar]; (g) Bonin J, Costentin C, Louault C, Robert M, Routier M, Savéant J-M. Proc. Nat. Acad. Sci. U.S.A. 2010;107:3367–3372. doi: 10.1073/pnas.0914693107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.(a) Sibert R, Jesowicz M, Porcelli F, Veglia G, Range K, Barry BA. J. Am. Chem. Soc. 2007;129:4393–4400. doi: 10.1021/ja068805f. [DOI] [PubMed] [Google Scholar]; (b) Sibert R, Jesowicz M, Barry BA. ACS Chem. Biol. 2010;58:1157–1168. doi: 10.1021/cb100138m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutton PL. Methods Enzymol. 1978;54:411–435. doi: 10.1016/s0076-6879(78)54026-3. [DOI] [PubMed] [Google Scholar]
- 42.(a) Moss D, Nabedryk E, Breton J, Mäntele W. Eur. J. Biochem. 1990;187:565–572. doi: 10.1111/j.1432-1033.1990.tb15338.x. [DOI] [PubMed] [Google Scholar]; (b) Rich PR, Iwaki M. Mol. Biosyst. 1997;3:398–407. doi: 10.1039/b702328f. [DOI] [PubMed] [Google Scholar]
- 43.(a) Rusling JF. Acc. Chem. Res. 1998;31:363–369. [Google Scholar]; (b) Armstrong FA, Wilson GS. Electrochim. Acta. 2000;45:2623–2645. [Google Scholar]; (c) Armstrong FA. Curr. Opin. Chem. Biol. 2005;9:110–117. doi: 10.1016/j.cbpa.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Feitelson J, Hayton E. J. Phys. Chem. 1973;77:10–15. [Google Scholar]
- 45.(a) Kálmál L, LoBrutto R, Allen JP, Williams JC. Nature. 1999;402:696–699. [Google Scholar]; (b) Bellér G, Lente G, Fábián I. Inorg. Chem. 2010;49:3968–3970. doi: 10.1021/ic902554b. [DOI] [PubMed] [Google Scholar]
- 46.(a) Chen P, McGreery RL. Anal. Chem. 1996;68:3958–3965. [Google Scholar]; (b) McGreery RL. In: Electrochemical properties of carbon surfaces, Interfacial chemistry. Wiechowski A, editor. USA: Dekker; 1999. [Google Scholar]
- 47.(a) Nicholson RS, Shain I. Anal. Chem. 1964;36:706–723. [Google Scholar]; (b) Laviron E. J. Electrochem. Chem. 1979;101:19–28. [Google Scholar]; (c) Tender L, Carter MT, Murray RW. Anal. Chem. 1994;66:3173–3181. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.