Abstract
Objectives
The in vivo efficacy of a cefotaxime-ciprofloxacin combination against Vibrio vulnificus and the effects on rtxA1 expression of commonly used antibiotics are unknown.
Methods
In vitro time-kill studies were performed to evaluate synergism. Female BALB/c mice were injected subcutaneously with 1×107 or 1×108 cfu of V. vulnificus. Antibiotic therapy was initiated at 2 h after inoculation in the following four therapy groups: cefotaxime; ciprofloxacin; cefotaxime-plus-ciprofloxacin; and cefotaxime-plus-minocycline. The cytotoxicity of V. vulnificus for HeLa cells was measured using the lactate dehydrogenase assay; rtxA1 transcription was measured in a transcriptional reporter strain using a β-galactosidase assay.
Results
In vitro time-kill assays exhibited synergism between cefotaxime and ciprofloxacin. In the animal experiments, the 96-h survival rate for the cefotaxime-plus-ciprofloxacin group (85%; 17/20) was significantly higher than that of the cefotaxime-plus-minocycline (35%; 7/20) and cefotaxime alone (0%; 0/20) groups (P<0.05 for both). Bacterial counts in the liver and spleen were significantly lower in the cefotaxime-plus-ciprofloxacin group 24 and 48 h after treatment, relative to the other groups. At sub-inhibitory concentrations, ciprofloxacin inhibited more effectively rtxA1 transcription and mammalian cell cytotoxicity than either minocycline or cefotaxime (P<0.05 for both).
Conclusions
Ciprofloxacin is more effective at reducing rtxA1 transcription and subsequent cytotoxicity than either minocycline or cefotaxime, and the combination of ciprofloxacin and cefotaxime was more effective in clearing V. vulnificus in vivo than previously used regimens. These data suggest that the combination of ciprofloxacin and cefotaxime is an effective option for the treatment of V. vulnificus sepsis in humans.
Introduction
Vibrio vulnificus is an opportunistic human pathogen that causes rapidly fatal sepsis, as well as skin and soft tissue infections, including necrotizing fasciitis. This pathogen is transmitted to humans through the exposure of wounds to seawater or the ingestion of seafood, and causes septicemia, especially in patients with immunosuppression and iron overload, as is the case in chronic liver disease [1], [2]. The mortality rate for V. vulnificus septicemia is ∼70% [1], and the major virulence factors of this pathogen include capsular polysaccharides, exotoxins such as the repeats-in-toxin A1 (RtxA1), hemolysin, metalloproteinase, and iron acquisition systems. Among these, RtxA1 is a potent cytotoxic virulence factor that plays important roles in the pathogenesis and lethality of V. vulnificus infections [3]–[10].
Immediate surgical intervention and the administration of effective antibiotics are essential to reduce the mortality rate for V. vulnificus sepsis [1], [2], [11]. Antibiotics that are known to be effective include beta-lactams, tetracyclines, and quinolones. Among these, the combinations of a third-generation cephalosporin with tetracycline or quinolone monotherapy are most often recommended, based on the results of in vitro [12] and in vivo research [13], [14] and several retrospective clinical studies [15], [16]. However, the mortality rates remain high, suggesting the need for adjuvant therapies [17], [18] and more effective antibiotic regimens.
A combination of cefotaxime and a quinolone has a synergistic effect on enteric Gram-negative pathogens, including Escherichia coli [19] and Salmonella species [20], [21]. It has also been shown that the combination of cefotaxime and ciprofloxacin has synergism and superior in vitro bactericidal activity against V. vulnificus than currently recommended regimens, such as ciprofloxacin alone or cefotaxime plus doxycycline [22]. However, the in vivo efficacy of this combination remains unclear. Moreover, although RtxA1 plays a key role in the virulence of V. vulnificus and mortality in V. vulnificus-related sepsis, the effects of commonly used therapeutic antibiotics at sub-inhibitory concentrations on rtxA1 expression have not been evaluated. To address these issues, we evaluated the in vitro synergism and in vivo efficacy of the combination of ciprofloxacin and cefotaxime against V. vulnificus sepsis, as well as the effect of commonly used antibiotics on rtxA1 expression.
Materials and Methods
Ethics
All animal experiments were carried out in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee (IACUC) of Chonnam National University and the guidelines for animal experiments set forth by the Korean Food and Drug Administration (KFDA) [23]. The study protocol was approved by the IACUC of Chonnam National University Hwasun Hospital.
Bacterial strains and in vitro time-kill assay
V. vulnificus CMCP6, which is a clinical isolate from Chonnam National University Hospital for which full genome sequence is available (GenBank accession nos. AEO16795 and AEO16796) [24], was used in the time-kill study and animal infection study. V. vulnificus strains MO6-24/O and CMM770 (MO6-24/O background with a deletion mutation in the rtxA1 gene) [25] were used in the cytotoxicity assay and transcriptional reporter assay.
The minimal inhibitory concentrations (MICs) of cefotaxime, minocycline, and ciprofloxacin were determined by the microdilution method according to the guidelines of the Clinical and Laboratory Standards Institute [26]. In vitro time-kill studies were performed to evaluate synergy, as described previously [22]. Cefotaxime (Chong Kun Dang Pharmaceutical, Seoul, Republic of Korea), minocycline (Sigma-Aldrich, St. Louis, MO) and ciprofloxacin (Ildong Pharmaceutical, Seoul, Republic of Korea) were used throughout the study. Synergy was defined as a ≥2–log10 cfu/mL increase in killing at 24 h using the combination therapy compared with the level of killing achieved with the most active single drug.
In vivo animal study
Female, specific pathogen free, 8-week-old BALB/c mice (Samtako, Osan, Republic of Korea) with an average weight of 20 g were used throughout the study. The inocula were prepared as described previously [14]. We chose 1×107 and 1×108 cfu as the initial inocula, based on previous studies [12], [14] and our preliminary results. To induce an iron-overload status, which increases susceptibility to V. vulnificus and more closely represents the iron-overloaded condition seen clinically, 900 µg ferric ammonium citrate was administered intra-peritoneally (i.p.) 30 min before V. vulnificus inoculation [27]. Next, 1×107 or 1×108 cfu V. vulnificus were injected subcutaneously into the area over the right thigh [12], [14].
Each experiment consisted of five groups: a control group and groups treated with cefotaxime, ciprofloxacin, cefotaxime-plus-ciprofloxacin, and cefotaxime-plus-minocycline. All antibiotics were initially given i.p. beginning 2 h after the animal was infected. Cefotaxime (30 mg/kg body weight [BW] i.p.) was given every 6 h. Minocycline (loading dose of 4 mg/kg BW, followed by a maintenance dose of 2 mg/kg BW i.p.) was given every 12 h. Ciprofloxacin (8 mg/kg BW, i.p.) was given every 12 h, as described previously [12], [14]. Control mice received 0.1 mL sterile saline every 6 h. Antibiotics were given for a total of 42 h. The condition of animals was monitored every 6 h. Humane endpoints were used during the survival study; animals were euthanized using ether when they exhibited a combined clinical criteria (≥8 points), according to KFDA guidelines [23]. Clinical endpoints were defined using the following scoring system: change in body weight, 0–3 points; hair coat, 0–2 points; eye opening, 0–2 points; activity, 0–2 points; posture 0–3 points.
In addition, we counted the numbers of viable bacteria in the livers and spleens 24 and 48 h of infected mice after initiation of antibiotic treatment using an initial inoculum of 1×107 cfu. Mice were humanely euthanized using ether at 24 and 48 h, and the livers and spleens were homogenized. Then, the homogenized tissues were serially diluted and plated onto Brain-Heart infusion agar, to quantify the bacteria.
Effects of sub-inhibitory concentrations of antibiotics on V. vulnificus cytotoxicity for HeLa cells
V. vulnificus cytotoxicity for HeLa cells was measured using the CytoTox96 non-radioactive cytotoxicity assay kit (Promega, Madison, WI), as described elsewhere [28], [29]. HeLa cells were seeded in 48-well culture plates and cultured at 37°C in 5% CO2. After incubation for 24 h, the cells were washed twice with pre-warmed serum-free Dulbecco’s modified Eagle’s medium (DMEM). Overnight-cultured V. vulnificus bacteria were incubated in fresh 2.5% NaCl heart infusion (HI) broth for 3 h. The logarithmically growing culture was harvested by centrifugation, washed with PBS, and re-suspended in PBS. Then, 2×105 HeLa cells/mL in 250 µL of DMEM were infected with 5×105 cfu/mL V. vulnificus with or without 1/4 MICs of antibiotics for 120 min. Lactate dehydrogenase (LDH) released into the supernatant fluid was assayed as a marker of cytotoxicity, in accordance with the manufacturer’s protocol.
Effects of sub-inhibitory concentrations of antibiotics on rtxA1 expression
A chromosomal PrtxA1::lacZ transcriptional reporter strain of V. vulnificus MO6–24/O was constructed as described previously [29], [30]. An overnight culture of the reporter strain was inoculated in 2.5% NaCl HI broth at a concentration of 5×105 cfu/mL with or without 1/4 MICs of antibiotics for 120 min. The culture was lysed in lysis buffer, and β-galactosidase activity was assayed using 2×β-galactosidase substrate (0.2 M sodium phosphate buffer, 2 mM MgCl2, 100 mM mercaptoethanol, 1.33 mg/mL O-nitrophenyl-β-D-galactopyranoside), following the method described previously [30]. All experiments were performed three or more times.
Statistical analyses
The Kolmogorov–Smirnov goodness-of-fit test was used to determine the distribution of each set of data for normality before analysis, and continuous variables were compared using Student’s t-test. Survival analysis was performed using the Kaplan–Meier method and log-rank test. All tests of significance were two-tailed, and P-values≤0.05 were deemed to indicate statistical significance. Statistical analyses of the data were performed using the SPSS ver. 19.0 software (SPSS, Chicago, IL) and the GraphPad Prism ver. 5.0 program (GraphPad Software, La Jolla, CA).
Results
In vitro time-kill assay
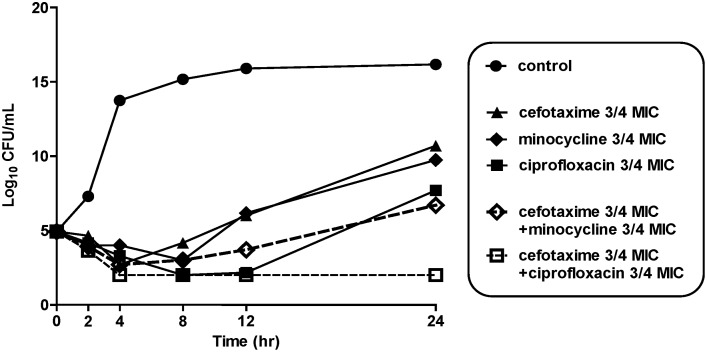
The MICs of cefotaxime, minocycline, and ciprofloxacin for V. vulnificus CMCP6 were 0.0625, 0.0625, and 0.03 mg/L, respectively. In the time-kill assay using 3/4 MICs of each antibiotic, the cefotaxime-ciprofloxacin combination was found to have synergistic activity against V. vulnificus CMCP6, and the bacterial colony counts after 24 h of in vitro treatment were lower for cefotaxime-plus-ciprofloxacin than for cefotaxime, ciprofloxacin, minocycline or cefotaxime-plus-minocycline (Fig. 1).
Figure 1. Time-kill curves for V. vulnificus CMCP6 after incubation with 3/4 MICs of cefotaxime alone, minocycline alone, ciprofloxacin alone, cefotaxime-plus-ciprofloxacin or cefotaxime-plus-minocycline.
CFU, colony-forming unit; MIC, minimum inhibitory concentration.
Survival rates of mice with V. vulnificus sepsis treated with various antibiotic regimens
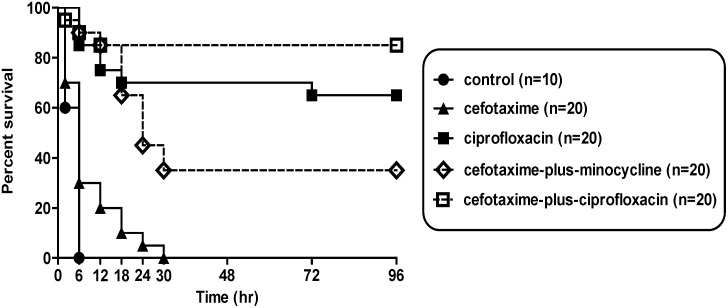
Figure 2 presents the survival rates of each treatment group after inoculation with 1×108 cfu V. vulnificus CMCP6. All 10 control mice died within 12 h. The 96-h survival rate was significantly higher in the cefotaxime-plus-ciprofloxacin group (85%, 17/20) compared with the cefotaxime (0%, 0/20) and cefotaxime-plus-minocycline groups (35%, 7/20) (P<0.001, P = 0.003, respectively). The 96-h survival rate in the cefotaxime-plus-ciprofloxacin group was also higher than that in the ciprofloxacin group (65%, 13/20), although the difference was not statistically significant (P = 0.148).
Figure 2. Survival rates of mice in each treatment group inoculated with 1×108 cfu V. vulnificus.
The 96-h survival rate of the cefotaxime-plus-ciprofloxacin group (85%, 17/20) was significantly higher than that of the cefotaxime (0%, 0/20) or the cefotaxime-plus-minocycline groups (35%, 7/20) (P<0.001 and P = 0.003, respectively; log-rank test).
In vivo clearance of V. vulnificus from mice treated with various antibiotic regimens
In experiments where 1×107 cfu V. vulnificus CMCP6 was used as the initial inoculum, all infected control mice (n = 6) died within 12 h, whereas all 48 of the infected mice treated with cefotaxime (n = 12), ciprofloxacin (n = 12), cefotaxime-plus-ciprofloxacin (n = 12), or cefotaxime-plus-minocycline (n = 12) were still alive after 96 h.
The viable bacterial counts in liver were lower in mice treated with cefotaxime-plus-ciprofloxacin than in those treated with cefotaxime alone (P<0.001 at 24 h and 48 h, each), ciprofloxacin alone (P = 0.030 at 24 h; P = 0.001 at 48 h) and cefotaxime-plus-minocycline (P = 0.044 at 24 h; P = 0.008 at 48 h). The viable bacterial counts in spleen were lower in mice treated with cefotaxime-plus-ciprofloxacin than those treated with cefotaxime alone (P<0.001) and ciprofloxacin alone (P = 0.003) at 24 h and those treated with cefotaxime alone (P<0.001) and cefotaxime-plus-minocycline (P = 0.008) at 48 h (n = 9 mice per group; Table 1).
Table 1. Viable bacterial counts in the livers and spleens 24 and 48×107 cfu V. vulnificus (n = 9 per group).
| Bacterial counts (CFU/g) | Treatment group | P valuea | P valueb | P valuec | |||
| Cefotaxime | Ciprofloxacin | Cefotaxime-plus-minocycline | Cefotaxime-plus-ciprofloxacin | ||||
| Liver at 24 h | 19544±12097 | 1415±1144 | 911±516 | 473±309 | <0.001 | 0.030 | 0.044 |
| Liver at 48 h | 921±101 | 310±109 | 287±119 | 162±35 | <0.001 | 0.001 | 0.008 |
| Spleen at 24 h | 13180±5558 | 3234±1092 | 2617±1413 | 1813±514 | <0.001 | 0.003 | 0.128 |
| Spleen at 48 h | 1304±565 | 379±238 | 361±113 | 217±87 | <0.001 | 0.074 | 0.008 |
Bacterial counts were expressed as means ± SDs.
CFU, colony-forming unit.
Comparison of the cefotaxime and cefotaxime-plus-ciprofloxacin groups.
Comparison of the ciprofloxacin and cefotaxime-plus-ciprofloxacin groups.
Comparison of the cefotaxime-plus-minocycline and cefotaxime-plus-ciprofloxacin groups.
In vitro effect of sub-inhibitory concentrations of antibiotics on RtxA1-mediated cytotoxicity and the transcription of rtxA1
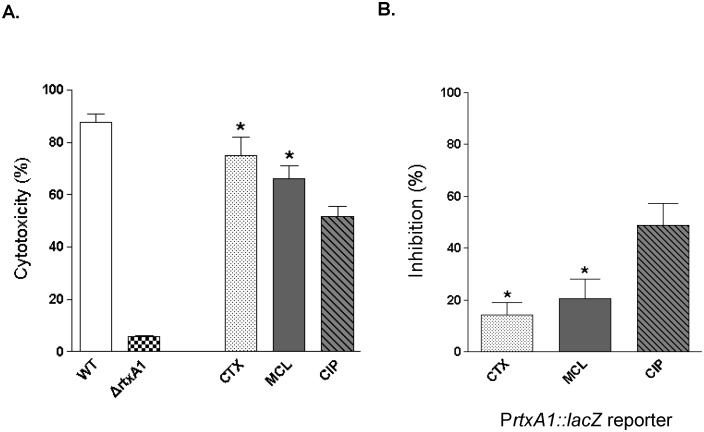
The MICs of cefotaxime, minocycline, and ciprofloxacin for V. vulnificus MO6–24/O were 0.0625, 0.0625, and 0.03 mg/L, respectively. The impaired cytotoxicity of CMM770 (ΔrtxA1), as compared with MO6–24/O (WT), indicates that the cytotoxicity noted at 120 min was mainly due to RtxA1 (Fig. 3A). V. vulnificus-induced cytotoxicity was inhibited more effectively by 1/4 MIC of ciprofloxacin than by 1/4 MIC of cefotaxime or 1/4 MIC of minocycline (P<0.05 for each). Similarly, the transcription of rtxA1 was inhibited more efficiently by 1/4 MIC of ciprofloxacin than by 1/4 MIC of cefotaxime or 1/4 MIC of minocycline (P<0.05 for each; Fig. 3B).
Figure 3. The effects of sub-inhibitory concentrations of antibiotics on V. vulnificus cytotoxicity and rtxA1 transcription.
A. Cytotoxicity assay. The impaired cytotoxicity of ΔrtxA1 compared with that of the WT strain shows that cytotoxicity at 120 min is due principally to RtxA1. V. vulnificus cytotoxicity is inhibited more markedly by 1/4 MIC of ciprofloxacin than by 1/4 MICs of cefotaxime or minocycline (n = 12 per group). B. Transcriptional reporter assay. The transcription of rtxA1 is more efficiently inhibited by 1/4 MIC of ciprofloxacin than by 1/4 MICs of cefotaxime or minocycline (n = 4 per group). WT, MO6-24/O; ΔrtxA1, CMM770 (MO6-24/O background with a deletion mutation in the rtxA1 gene); CTX, cefotaxime; CIP, ciprofloxacin; MCL, minocycline. *P<0.05 compared to the values for ciprofloxacin (Student’s t-test).
Discussion
A previous in vitro study demonstrated a synergistic bactericidal effect of cefotaxime-plus-ciprofloxacin against V. vulnificus ATCC 27562 [22]. In the present study, we demonstrated that the combination of ciprofloxacin-plus-cefotaxime acts synergistically, and exhibits more potent bactericidal activity in vitro against V. vulnificus CMCP6 than either cefotaxime-plus-minocycline or ciprofloxacin alone. In vitro synergism for the combination of cefotaxime-plus-ciprofloxacin against enteric Gram-negative pathogens also has been reported for E. coli [19], Serratia marcescens [31], Pseudomonas aeruginosa [32], and Salmonella enterica serotypes Typhi and Paratyphi [20], [21], [33]. One possible mechanism underlying this synergism may be the interaction of quinolones with the outer membrane, with the quinolones acting as chelating agents, thereby increasing its permeability to β-lactam antibiotics [19].
We performed a survival analysis using 108 cfu V. vulnificus as the initial inoculum, as a previous study showed in vivo synergism for cefotaxime and minocycline [14]. In that study, the mortality rates of cefotaxime-treated and cefotaxime-plus-minocycline-treated mice were 0% and 40%, respectively, similar to our results of 0% and 35%, respectively. However, the efficacy of quinolones was not directly compared with the other regimens using a high inoculum (108 cfu), as a subsequent study using quinolones [13] was performed with a lower inoculum (1.5×107 cfu) of V. vulnificus. Here, the survival rate was higher among mice treated with ciprofloxacin-based regimens than among mice treated with the cefotaxime-minocycline combination, although the in vivo V. vulnificus clearance rates for ciprofloxacin monotherapy and cefotaxime-plus-minocycline therapy were similar (P = 0.25, 0.32, 0.68, 0.84 in liver at 24 h, spleen at 24 h, liver at 48 h, spleen at 48 h).
To compare the efficacies of the treatment regimens on host survival and bacterial numbers in the organs, we infected mice with 1×107 cfu V. vulnificus. Although all infected control mice died rapidly, all of the mice in the treatment groups survived, regardless of the regimen used, including cefotaxime monotherapy. Similar results were reported by Tang et al. [13], who compared the efficacies of fluoroquinolone and other agents using 1.5×107 cfu V. vulnificus; they found that the survival rates of the mice were excellent at >80%, regardless of the administered regimen. Kim et al. [30] performed a survival analysis after antibiotic therapy using 108 cfu V. vulnificus as the initial inoculum [34], based on the finding that none of the mice that were inoculated with 107 cfu V. vulnificus and treated with antibiotics died, regardless of the regimen (personal communication). We considered it unfeasible to compare the survival rates for different antibiotic regimens when <108 cfu/mL V. vulnificus were used as the initial inoculum, given that many samples would be needed to show a statistically significant difference if the survival rate was >80% for all regimens used. Therefore, we enumerated the bacteria in the organs of the inoculated and treated mice and found that cefotaxime-ciprofloxacin was the most effective regimen in terms of clearing V. vulnificus. These data provide further evidence for the superior efficacy of cefotaxime-plus-ciprofloxacin regimens in clearing V. vulnificus in vivo.
Sub-inhibitory concentrations of antibiotics interfere with the processes of host-parasite interactions, such as phagocytosis, adherence, and toxin production. For this reason, the anti-toxin effects of sub-inhibitory levels of antibiotics have been studied specifically in pathogens that cause rapidly fatal toxin-related diseases, such as necrotizing skin and soft tissue infections, in which anti-toxin efficacy is therapeutically important [35]–[38]. In our previous studies, we showed that RtxA1 is a major virulence factor of V. vulnificus [3], [7], and that deletion of rtxA1 or its regulators decrease the cytotoxicity and increase the LD50 significantly in mice [6], [25], [28], [30], [39]. We also showed that RtxA1 can be suppressed by certain compounds [29], [40] and sub-inhibitory concentrations of chloramphenicol [25]. However, although RtxA1 is known as a major virulence element in V. vulnificus, and V. vulnificus causes rapidly fatal necrotizing skin and soft tissue infections, the effects of sub-inhibitory concentration of antibiotics that are frequently used in therapy on RtxA1 production have not been evaluated to date. In the present study, we showed that ciprofloxacin more effectively suppressed rtxA1 transcription and protected host cells than either minocycline or cefotaxime. In a previous study, suppressing RtxA1 production per se without direct killing of bacteria was shown to reduce the mortality rate of mice infected with V. vulnificus [29]. The more potent inhibitory effect of ciprofloxacin on RtxA1-induced cytotoxicity may explain the improved survival of mice treated with ciprofloxacin-based regimens, compared with mice treated with regimens using cefotaxime or doxycycline.
Certain conclusions that we can draw from this study are limited, as we examined only the combination of ciprofloxacin-plus-cefotaxime. Further studies will be needed to evaluate the efficacy of combinations of ciprofloxacin or minocycline and other third-generation cephalosporins, including ceftazidime and cefrtriaxone.
In summary, we found that, at sub-inhibitory concentrations, ciprofloxacin is more effective at reducing rtxA1 transcription and subsequent cytotoxicity than either minocycline or cefotaxime. Moreover, we demonstrated that the combination of ciprofloxacin and cefotaxime is more effective in clearing V. vulnificus in vivo than commonly used regimens, which suggests that this combination is a good candidate for the treatment of V. vulnificus sepsis in humans.
Acknowledgments
Presented in part: 52nd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, September 9–12, 2012 (abstract no. B-1298).
Funding Statement
This work was supported by a grant from the Research Institute of Medical Sciences, Chonnam National University (2011-CURIMS-DR005). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dechet AM, Yu PA, Koram N, Painter J (2008) Nonfoodborne Vibrio infections: an important cause of morbidity and mortality in the United States, 1997–2006. Clin Infect Dis 46: 970–976. [DOI] [PubMed] [Google Scholar]
- 2. Klontz KC, Lieb S, Schreiber M, Janowski HT, Baldy LM, et al. (1988) Syndromes of Vibrio vulnificus infections. Clinical and epidemiologic features in Florida cases, 1981–1987. Ann Intern Med 109: 318–323. [DOI] [PubMed] [Google Scholar]
- 3. Chung KJ, Cho EJ, Kim MK, Kim YR, Kim SH, et al. (2010) RtxA1-induced expression of the small GTPase Rac2 plays a key role in the pathogenicity of Vibrio vulnificus. J Infect Dis 201: 97–105. [DOI] [PubMed] [Google Scholar]
- 4. Kwak JS, Jeong HG, Satchell KJ (2011) Vibrio vulnificus rtxA1 gene recombination generates toxin variants with altered potency during intestinal infection. Proc Natl Acad Sci U S A 108: 1645–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lo HR, Lin JH, Chen YH, Chen CL, Shao CP, et al. (2011) RTX toxin enhances the survival of Vibrio vulnificus during infection by protecting the organism from phagocytosis. J Infect Dis 203: 1866–1874. [DOI] [PubMed] [Google Scholar]
- 6. Jeong KC, Jeong HS, Rhee JH, Lee SE, Chung SS, et al. (2000) Construction and phenotypic evaluation of a Vibrio vulnificus vvpE mutant for elastolytic protease. Infect Immun 68: 5096–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YR, Lee SE, Kang IC, Nam KI, Choy HE, et al.. (2013) A Bacterial RTX Toxin Causes Programmed Necrotic Cell Death Through Calcium-Mediated Mitochondrial Dysfunction. J Infect Dis. [DOI] [PubMed]
- 8. Yokochi N, Tanaka S, Matsumoto K, Oishi H, Tashiro Y, et al. (2013) Distribution of virulence markers among Vibrio vulnificus isolates of clinical and environmental origin and regional characteristics in Japan. PLoS One 8: e55219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morrison SS, Williams T, Cain A, Froelich B, Taylor C, et al. (2012) Pyrosequencing-based comparative genome analysis of Vibrio vulnificus environmental isolates. PLoS One 7: e37553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sugiyama H, Kashimoto T, Ueno S, Ehara H, Kodama T, et al. (2011) Relationship between localization on cellular membranes and cytotoxicity of Vibrio vulnificus hemolysin. PLoS One 6: e26018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen SC, Chan KS, Chao WN, Wang PH, Lin DB, et al. (2010) Clinical outcomes and prognostic factors for patients with Vibrio vulnificus infections requiring intensive care: a 10-yr retrospective study. Crit Care Med 38: 1984–1990. [DOI] [PubMed] [Google Scholar]
- 12. Chuang YC, Liu JW, Ko WC, Lin KY, Wu JJ, et al. (1997) In vitro synergism between cefotaxime and minocycline against Vibrio vulnificus. Antimicrob Agents Chemother 41: 2214–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang HJ, Chang MC, Ko WC, Huang KY, Lee CL, et al. (2002) In vitro and in vivo activities of newer fluoroquinolones against Vibrio vulnificus. Antimicrob Agents Chemother 46: 3580–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chuang YC, Ko WC, Wang ST, Liu JW, Kuo CF, et al. (1998) Minocycline and cefotaxime in the treatment of experimental murine Vibrio vulnificus infection. Antimicrob Agents Chemother 42: 1319–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu JW, Lee IK, Tang HJ, Ko WC, Lee HC, et al. (2006) Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch Intern Med 166: 2117–2123. [DOI] [PubMed] [Google Scholar]
- 16. Chen SC, Lee YT, Tsai SJ, Chan KS, Chao WN, et al. (2012) Antibiotic therapy for necrotizing fasciitis caused by Vibrio vulnificus: retrospective analysis of an 8 year period. J Antimicrob Chemother 67: 488–493. [DOI] [PubMed] [Google Scholar]
- 17. Kim DM, Cho HS, Kang JI, Kim HS, Park CY (2008) Deferasirox plus ciprofloxacin combination therapy after rapid diagnosis of Vibrio vulnificus sepsis using real-time polymerase chain reaction. J Infect 57: 489–492. [DOI] [PubMed] [Google Scholar]
- 18. Kim CM, Park RY, Choi MH, Sun HY, Shin SH (2007) Ferrophilic characteristics of Vibrio vulnificus and potential usefulness of iron chelation therapy. J Infect Dis 195: 90–98. [DOI] [PubMed] [Google Scholar]
- 19. Chapman JS, Georgopapadakou NH (1988) Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother 32: 438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neupane GP, Kim DM, Kim SH, Lee BK (2010) In vitro synergism of ciprofloxacin and cefotaxime against nalidixic acid-resistant Salmonella enterica serotypes Paratyphi A and Paratyphi B. Antimicrob Agents Chemother. 54: 3696–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim DM, Neupane GP, Jang SJ, Kim SH, Lee BK (2010) In vitro efficacy of the combination of ciprofloxacin and cefotaxime against nalidixic acid-resistant Salmonella enterica serotype Typhi. Int J Antimicrob Agents 36: 155–158. [DOI] [PubMed] [Google Scholar]
- 22. Kim DM, Lym Y, Jang SJ, Han H, Kim YG, et al. (2005) In vitro efficacy of the combination of ciprofloxacin and cefotaxime against Vibrio vulnificus. Antimicrob Agents Chemother 49: 3489–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korea Food and Drug Administration (2011) Guidelines for animal experiments and IACUC standard opreation. Available: http://www.mfds.go.kr/labanimal/index.do?mid=56&seq=11795&cmd=v. Accessed 7 June 2014.
- 24. Kim HU, Kim SY, Jeong H, Kim TY, Kim JJ, et al. (2011) Integrative genome-scale metabolic analysis of Vibrio vulnificus for drug targeting and discovery. Mol Syst Biol 7: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim YR, Lee SE, Kook H, Yeom JA, Na HS, et al. (2008) Vibrio vulnificus RTX toxin kills host cells only after contact of the bacteria with host cells. Cell Microbiol 10: 848–862. [DOI] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute (2009) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-eighth edition. Wayne: Clinical and Laboratory Standards Institute. M07–A8.
- 27. Lee SE, Kim SY, Kim CM, Kim MK, Kim YR, et al. (2007) The pyrH gene of Vibrio vulnificus is an essential in vivo survival factor. Infect Immun 75: 2795–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim YR, Lee SE, Kim CM, Kim SY, Shin EK, et al. (2003) Characterization and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infect Immun 71: 5461–5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim JR, Cha MH, Oh DR, Oh WK, Rhee JH, et al. (2010) Resveratrol modulates RTX toxin-induced cytotoxicity through interference in adhesion and toxin production. Eur J Pharmacol 642: 163–168. [DOI] [PubMed] [Google Scholar]
- 30. Kim SY, Lee SE, Kim YR, Kim CM, Ryu PY, et al. (2003) Regulation of Vibrio vulnificus virulence by the LuxS quorum-sensing system. Mol Microbiol 48: 1647–1664. [DOI] [PubMed] [Google Scholar]
- 31. Otsuki M, Nishino T (1996) The synergic effects of quinolones and oral cephem antibiotics on Serratia marcescens. J Antimicrob Chemother 38: 771–776. [DOI] [PubMed] [Google Scholar]
- 32. Piccoli L, Guerrini M, Felici A, Marchetti F (2005) In vitro and in vivo synergy of levofloxacin or amikacin both in combination with ceftazidime against clinical isolates of Pseudomonas aeruginosa. J Chemother 17: 355–360. [DOI] [PubMed] [Google Scholar]
- 33. Chang CM, Chuang YC, Wu JJ, Wang LR, Lee HC, et al. (2009) In vitro combination of cefotaxime and ciprofloxacin against non-typhoid salmonellae. Int J Antimicrob Agents 33: 593–594. [DOI] [PubMed] [Google Scholar]
- 34. Neupane GP, Kim DM, Yun NR, Shin SH, Lim SC, et al. (2012) Quantitative PCR and in vivo efficacy of antibiotics in the treatment of Vibrio vulnificus infection in a mouse model. Eur J Clin Microbiol Infect Dis 31: 2461–2467. [DOI] [PubMed] [Google Scholar]
- 35. Gemmell CG, Ford CW (2002) Virulence factor expression by Gram-positive cocci exposed to subinhibitory concentrations of linezolid. J Antimicrob Chemother 50: 665–672. [DOI] [PubMed] [Google Scholar]
- 36. Dumitrescu O, Boisset S, Badiou C, Bes M, Benito Y, et al. (2007) Effect of antibiotics on Staphylococcus aureus producing Panton-Valentine leukocidin. Antimicrob Agents Chemother 51: 1515–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohlsen K, Ziebuhr W, Koller KP, Hell W, Wichelhaus TA, et al. (1998) Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 42: 2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saleh-Mghir A, Dumitrescu O, Dinh A, Boutrad Y, Massias L, et al. (2012) Ceftobiprole efficacy in vitro against Panton-Valentine leukocidin production and in vivo against community-associated methicillin-resistant Staphylococcus aureus osteomyelitis in rabbits. Antimicrob Agents Chemother 56: 6291–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee SE, Ryu PY, Kim SY, Kim YR, Koh JT, et al. (2004) Production of Vibrio vulnificus hemolysin in vivo and its pathogenic significance. Biochem Biophys Res Commun 324: 86–91. [DOI] [PubMed] [Google Scholar]
- 40. Na HS, Cha MH, Oh DR, Cho CW, Rhee JH, et al. (2011) Protective mechanism of curcumin against Vibrio vulnificus infection. FEMS Immunol Med Microbiol 63: 355–362. [DOI] [PubMed] [Google Scholar]