Abstract
Objectives
Lactation may influence future progression to type 2 diabetes after gestational diabetes mellitus (GDM). However, biomarkers associated with progression to glucose intolerance have not been examined in relation to lactation intensity among postpartum women with previous GDM. This study investigates whether higher lactation intensity is related to more favorable blood lipids, lipoproteins and adipokines after GDM pregnancy independent of obesity, socio-demographics and insulin resistance.
Methods
The Study of Women, Infant Feeding, and Type 2 Diabetes (SWIFT) is a prospective cohort study that recruited 1,035 women diagnosed with GDM by the 3-hour 100 g oral glucose tolerance tests (OGTTs) after delivery of a live birth in 2008–2011. Research staff conducted 2-hour 75 gram OGTTs, and assessed lactation intensity, anthropometry, lifestyle behaviors and socio-demographics at 6–9 weeks postpartum (baseline). We assayed fasting plasma lipids, lipoproteins, non-esterified free fatty acids, leptin and adiponectin from stored samples obtained at 6–9 weeks postpartum for in 1,007 of the SWIFT participants who were free of diabetes at baseline. Mean biomarker concentrations were compared among lactation intensity groups using multivariable linear regression models.
Results
Increasing lactation intensity showed graded monotonic associations with fully adjusted mean biomarkers: 5–8% higher high-density lipoprotein cholesterol (HDL-cholesterol), 20–28% lower fasting triglycerides, 15–21% lower leptin (all trend P-values<0.01), and with 6% lower adiponectin, but only after adjustment for insulin resistance (trend P-value=0.04).
Conclusion
Higher lactation intensity was associated with more favorable biomarkers for type 2 diabetes, except for lower plasma adiponectin, after GDM delivery. Long-term follow-up studies are needed to assess whether these effects of lactation persist to predict progression to glucose intolerance.
Keywords: Adipokines, Insulin Resistance, Diabetes Mellitus, Gestational Diabetes Mellitus (GDM), Breastfeeding, Postpartum, Metabolism
Introduction
Clinical studies in lactating women have consistently reported lower early postpartum fasting triglycerides,(1;2) and higher HDL-cholesterol.(3;4) Lactation duration has been associated with higher plasma HDL-cholesterol up to 2 years postpartum,(5;6) but others found no association with adipokines (leptin and adiponectin) at 3 years postpartum. (7;8) In women with recent gestational diabetes mellitus (GDM), lactation also has been associated with more favorable metabolic parameters during the postpartum period,(9–13) including lower plasma glucose (fasting and 2-hour), improved glucose tolerance, improved pancreatic β-cell function and higher HDL-cholesterol. Evidence is limited and conflicting about whether lactation exerts persistent effects on metabolism that may reduce future risk of type 2 diabetes. One retrospective study of women with a history of GDM reported no association,(14) and a prospective study in antibody negative women with GDM reported a 45% lower incidence of diabetes for longer lactation.(15) However, the first study relied on self-report of type 2 diabetes, and both studies may be affected by recall bias, or potential residual confounding from postpartum lifestyle behaviors. Another 20-year study of black and white women that collected repeated measures (anthropometry, glycemia, and fasting lipids), both before and after pregnancies, found a graded inverse association between lactation duration and incidence of the metabolic syndrome in midlife among women with a history of GDM, independent of lifestyle changes, weight gain and socio-demographics.(6)
Previous studies have not utilized a quantitative measure of lactation intensity to assess the “dose-response” relationship with maternal metabolism, except for our large cohort of women with recent GDM. Our previous study found that higher lactation intensity was associated in a graded manner with lower fasting plasma glucose and insulin, as well as lower prevalence of prediabetes, even within obese women.(12) To our knowledge, biomarkers of insulin resistance, such as adiponectin and leptin, have never been assessed in relation to any measures of lactation (intensity or duration) among women with previous GDM.
We sought to determine whether increasing lactation intensity was associated with less atherogenic lipids and lipoproteins, and with lower free fatty acids (FFA) and leptin. Because the lactogenic hormone, prolactin, may suppress adiponectin levels,(16) we hypothesized that adiponectin would be inversely related to lactation intensity among insulin-resistant women with a history of GDM.
Materials and Methods
Research Design, Study Population
The sample for this analysis includes participants in the Study of Women, Infant Feeding, and Type 2 Diabetes After GDM Pregnancy (SWIFT), an ongoing prospective, observational cohort that enrolled 1,035 women with a GDM pregnancy between 20–37 weeks gestation, diagnosed by Carpenter and Coustan criteria,(17) delivered a live birth ≥ 35 weeks gestation from September 2008 and December 2011 at a Kaiser Permanente Northern California (KPNC) hospital, and intending to intensively breastfeed or intensively formula feed their infant. Women provided written, informed consent to participate in three in-person exams during the interval from 6–9 weeks postpartum to two years postpartum, and met the study eligibility criteria as previously described (18) and as shown in the Supplemental Table 1. The overall response rate among pregnant women with GDM was 46%, and the enrollment rate was 68% among women who met the study eligibility criteria.
For this analysis, we excluded 19 women due to elevated fasting glucose ≥126 mg/dL, or 2-hour glucose ≥200 mg/dL at 6–9 weeks postpartum diagnostic of diabetes, one woman who dropped out at baseline, and 8 women who did not have stored plasma specimens. The sample consisted of 1,007 participants who had fasting plasma specimens available and were free of diabetes at the baseline exam at 6–9 weeks postpartum. The Kaiser Permanente Institutional Review Board approved the study protocol. Written, informed consent was obtained from each participant for all study procedures that were conducted in accordance with the ethical standards of the institution.
Data Collection
Research assistants interviewed women about infant feeding intentions and practices from late pregnancy through 9 weeks postpartum via two telephone interviews, mailed infant feeding diaries to record formula supplementation and breastfeeding (duration, frequency per 24 hours, and amount of formula), and one in-person exam at 6–9 weeks postpartum. Women were classified into one of two groups at the 3–5 weeks telephone eligibility screening: 1) intensively breastfeeding (exclusive; 0 ounces of formula, or mostly; ≤6 ounces of formula per 24 hours), or 2) intensively formula feeding (exclusive; non-breastfeeding, or mostly; ≥14 ounces of formula per 24 hours), and stated their intent to maintain the same feeding practices for at least 4 months postpartum. By design, women were not enrolled if they reported supplementation with formula ranging from 7 to 13 ounces per day (mixed feeding) at the screening interview.(18) Research assistants mailed women instructions on how to prepare for the oral glucose tolerance test (OGTT) (consume adequate carbohydrate for 3 days, fast for at least 10 hours the night before), and advised women to express their breast milk before the test so they could feed expressed breast milk to their infant during the 2-hour 75 g OGTT.
At the 6–9 weeks postpartum exam, women provided written, informed consent prior to the collection of the blood specimens, administration of the 2-hour 75 g OGTT (annually for two years), medical and lifestyle questionnaires, and anthropometric measurements as previously described.(18) The fasting plasma samples collected at baseline (6–9 weeks postpartum) were shipped within one month to the University of Washington, Northwest Lipid Metabolism and Diabetes Research Laboratory for the glucose and insulin assays. Additional fasting plasma collected from the baseline exam was separated into 1.8 mL aliquots and stored at −70° C until it was shipped to the University of Washington in 2012 for the assays (lipids, lipoproteins, FFA, adipokines).
At the baseline exam, research staff queried women again about their frequency of breastfeeding, number of expressed breast milk feedings by bottle, and formula supplementation (quantity) per 24 hours during the past 7 days. We classified women into one of five lactation intensity groups: 1) exclusive breastfeeding; 0 ounces of formula or other milk feeds, 2) mostly breastfeeding; ≤ 6 ounces of formula per 24 hours, 3) mixed (breast milk and formula >6 to ≤ 17 ounces per 24 hours) or inconsistent feeding method, 4) mostly formula feeding >17 ounces per 24 hours, and 5) exclusive formula feeding.(12) The mixed/inconsistent group included women who had transitioned to higher formula supplementation between the 4–5 weeks postpartum screening interview and the 6–9 weeks postpartum exam.
Trained research assistants asked women to recall the duration of fasting and breastfeeding during the fasting period prior to the OGTT, recorded breastfeeding episodes during the 2-hour 75 g OGTT as described previously,(19) and measured weight, height and waist circumference via standardized methods using calibrated research quality scales (Tanita WB-100A, Tanita Corp, Toyko, Japan), stadiometer equipment (SECA, Model 67029, United Kingdom, www.seca.com), and measuring tapes (Gulick, 67019, Country Technology, Inc., United States). Interviewer and self-administered questionnaires collected information on socio-demographics, enrollment in Women’s, Infants and Children (WIC) special supplemental nutrition program, medical history, contraception, depression, and lifestyle behaviors. We utilized KPNC electronic medical records to obtain prenatal laboratory results and dates of GDM diagnosis (3-hour 100 g OGTT), GDM treatment, as well as maternal body weights. Gestational weight gain (kg) was defined as the last weight before delivery minus reported pre-pregnancy weight. Postpartum weight loss (kg) was calculated as the difference between the woman’s weight measured at 6–9 weeks postpartum and her last weight before delivery.
Biochemical Assays
For this analysis, we utilized fasting plasma collected at 6–9 weeks postpartum (baseline in-person exam) to measure lipids, lipoproteins, non-esterified FFA and adipokines (adiponectin, leptin). Assays were performed on thawed frozen fasting plasma stored at −70° C from the SWIFT baseline. The specimens were shipped to the University of Washington, Northwest Lipid Metabolism and Diabetes Research Laboratory and analyzed for insulin, glucose, lipids, lipoproteins, non-esterified FFA, leptin and adiponectin. Plasma glucose was analyzed enzymatically using Roche reagent on a Roche Modular P autoanalyzer. The method is based on the combined catalytic activities of hexokinase and glucose-6-phosphate-dehydrogenase. Total immunoreactive insulin (µU/mL) was assayed using a double-antibody radioimmunoassay with high precision. The assay is a 48-hour Polyethylene glycol (PEG)-accelerated assay involving a primary antibody, guinea pig anti-human insulin, and a secondary antibody, goat anti-guinea pig immunoglobulin.
Measurements of total cholesterol in plasma and cholesterol in the lipoprotein fractions and triglycerides were performed enzymatically using Roche reagent on the Roche Modular P autoanalyzer by methods standardized to the Centers for Disease Control and Prevention Reference Methods. Determination of HDL-cholesterol was performed after precipitation of apo B-containing particles by dextran sulfate Mg2+. LDL-cholesterol was calculated by the Friedewald equation. The Friedewald equation for the estimation of LDL-cholesterol is inaccurate when triglycerides are >400 mg/dL. In this case, a complete lipoprotein separation by ultracentrifugation, which allows quantitation of the individual lipoprotein classes, was performed using the Lipid Research Clinics Beta Quantification procedure. The inter-assay coefficients of variation (CVs) are consistently <1.5% for total cholesterol and triglycerides and <2% for HDL-cholesterol. Analysis for non-esterified FFA in plasma was performed on a Roche Hitachi Modular P analyzer. The range of linearity of this method is up to 4.0mEq/L. The minimum detectable level of the method is estimated to be 0.0014 mEq/L. Intra and Inter-assay CVs are 0.75% and 3.7% respectively. Total bilirubin levels up to 10 mg/dL have negligible interference in the measurement of results.
Analysis of adiponectin in plasma samples was performed using a latex particle-enhanced turbidimetric immunoassay (Otsuka Pharmaceuticals, Ltd) performed on Roche Modular P analyzer. The analysis uses latex beads-immobilized with an anti-adiponectin antibody. The assay sensitivity is 0.5 µg/mL and the analytical range is 0.5–25 µg/mL. Inter assay CVs for high and low adiponectin level samples are 1.9 % and 2.5% respectively.
The leptin analysis was performed using a commercially available radioimmunoassay kit (EMD Millipore, Inc., St Charles, MO). The assay uses 125I-labeled human leptin and a rabbit anti human leptin antibody and is highly specific and shows no cross reactivity to human leptin fragments. The assay sensitivity is 0.5 ng/mL and the assay linearity is up to 100 ng/mL. Intra- and inter-assay CVs for the leptin radioimmunoassay (RIA) are 3.53% and 5.2% respectively.
Indices of insulin resistance (homeostatic model assessment of insulin resistance; (HOMA-IR), insulin sensitivity (insulin sensitivity index; ISI0, 120) and homeostatic model assessment of insulin secretion (HOMA-β) were calculated using measures obtained from 0 and 120 minutes during the OGTT.(20)
HOMA-IR = (G0 × I0)/22.5;
ISI0, 120 = (m/MPG)/log MPI
- HOMA-β = (20 × I0)/(G0 − 3.5)
- G0 = fasting glucose, I0 = fasting insulin,
- G120 = glucose post 2-hour OGTT, I120 = insulin post 2-hour OGTT
- m = [75,000 mg + (G0 − I120) × 0.19 × body weight]/120 min;
- MPG = (G0 + G120)/2
- MPI = (I0 + I120)/2
- MPG = mean plasma glucose
- MPI = mean plasma insulin
Glucose Tolerance Classification
Glucose tolerance was defined as normal, glucose intolerant [e.g., prediabetes; impaired fasting glucose (IFG) 100–125 mg/dL, and/or impaired glucose tolerance (IGT) for 2-hour 75 g post-glucose 140–199 mg/d], or diabetes based on the American Diabetes Association diagnostic criteria for the 2-hour 75 g OGTT [fasting ≥126 mg/dL and/or 2-hour ≥200 mg/dL] and a repeat OGTT for women with elevated values. (21)
Statistical Methods
Lactation intensity group characteristics were contrasted using chi-square statistics for categorical variables (race, education, participation in the Special Supplemental Nutrition Program for Women, Infants and Children [WIC], contraception, glucose tolerance groups) and by comparison of means for continuous variables (fasting blood glucose, insulin, HOMA-IR, HOMA-β, ISI0, 120, triglycerides, lipoproteins, FFA, adiponectin and leptin) using F-statistics. Given skewness in distributions, adiponectin and leptin concentrations were log transformed in all analyses, with exponentiation of crude and adjusted means, and associated confidence intervals, from linear regression analyses to obtain point and interval estimation of geometric means. Insulin and triglycerides concentrations were assessed for skewness, but transformation was not necessary. All P-values are for two-sided tests; and statistical significance was set at P<0.05. Significance probabilities and confidence intervals for the pair-wise comparisons were corrected for multiple comparisons using the Dunnett’s procedure.
Unadjusted and adjusted means and 95% confidence intervals (95%CI) and group differences (95%CI) for mean fasting parameters (exclusive or mostly formula feeding, referent group) were estimated from multivariable linear regression models. All analyses were conducted using SAS for Windows 9.1.3 (SAS Institute Inc., Cary, NC, USA). Covariates were evaluated as potential confounders based on a priori hypotheses and included in regression models based on 10% change in one or more of the lactation intensity groups regression coefficients.(22) Trends in means across lactation intensity groups were assessed by assigning scores of 1, 2, 3 and 4 across increasing intensity groups, and treating the score as a continuous variable in the regression model. HOMA-IR was added to covariate-adjusted models to assess mediation within the lactation intensity associations with triglycerides, FFA, lipoproteins and adipokines. Age, race and ethnicity, pre-pregnancy BMI, glucose tolerance groups and HOMA-IR were also assessed as effect modifiers of the lactation intensity and biomarker associations (significance level P<0.10).
Results
Participants’ age ranged from 21–45 years with a mean (SD) age of 33.5 (4.8) years (median 33.5). Overall, 77% were women of color: 36% Asian, 8% non-Hispanic Black, 31% Hispanic, and 2% other. Of the 1,007 women, 437 (44%) were exclusively breastfeeding, 183 (18%) mostly breastfeeding, 128 (12%) mixed or inconsistent breast milk and formula, 100 (10%) mostly formula feeding and 159 (16%) as exclusively formula feeding. Mostly and exclusively formula feeding groups were combined into a single referent group as metabolic parameters were similar for these two groups (data not shown).
Exclusive and mostly breastfeeding (BF) groups compared with mixed feeding, and exclusive or mostly formula feeding (FF) groups were more likely to report non-Hispanic white race, to be multiparous, less obese, and slightly older, as well as having attained a higher education and income level (not enrolled in WIC), and to deliver by C-section (Table 1). At 6–9 weeks postpartum, higher intensity lactation (exclusive BF and mostly BF groups) was associated with lower fasting glucose and insulin, lower 2-hour glucose and insulin, higher insulin sensitivity index, lower insulin resistance and insulin secretion indices, as well as being less obese, less likely to be glucose intolerant, and having a smaller waist girth (Table 2). Exclusively BF women reported breastfeeding for a longer period during the fasting period and were more likely to have breastfed during the 2-hour OGTT.
Table 1.
Prenatal and Socio-demographic Characteristics by Lactation Intensity Groups at 6–9 weeks Postpartum (Total n=1,007) Women with Recent GDM in SWIFT 1
| Prenatal Socio-demographic Characteristics |
Exclusive BF n= 437 |
Mostly BF n= 183 |
Inconsistent/ Mixed n= 128 |
Exclusive or Mostly FF n= 259 |
P- value |
|---|---|---|---|---|---|
| n (Col %) | |||||
| Race/ethnicity | |||||
| Non-Hispanic white | 125 (28.6) | 29 (15.9) | 20 (15.6) | 60 (23.2) | <.001 |
| Non-Hispanic black | 24 (5.5) | 11 (6.0) | 13 (10.2) | 30 (11.6) | |
| Hispanic | 117 (26.8) | 70 (38.3) | 33 (25.8) | 89 (34.4) | |
| Asian | 165 (37.8) | 71 (38.8) | 60 (46.9) | 72 (27.8) | |
| Other | 6 (1.4) | 2 (1.1) | 2 (1.6) | 8 (3.1) | |
| Parity | |||||
| 1 | 161 (36.8) | 56 (30.6) | 57 (44.5) | 93 (35.9) | 0.21 |
| 2 | 158 (36.2) | 77 (42.1) | 47 (36.7) | 95 (36.7) | |
| 3 or more | 118 (27.0) | 50 (27.3) | 24 (18.8) | 71 (27.4) | |
| Education | |||||
| High School or less | 69 (15.8) | 53 (29.0) | 24 (18.8) | 94 (36.6) | <.001 |
| Some college | 124 (28.4) | 45 (24.6) | 44 (34.4) | 77 (30.0) | |
| College ≥4 years | 244 (55.8) | 85 (46.5) | 60 (46.9) | 86 (33.5) | |
| WIC Participant (yes) | 81 (18.5) | 58 (31.7) | 28 (21.9) | 94 (36.3) | <.001 |
| Family History of Diabetes (yes) | 213 (48.7) | 99 (54.1) | 53 (41.4) | 125 (48.3) | 0.16 |
| Mean (SD) | |||||
| Age (yrs) | 33.5 (4.6) | 33.2 (4.9) | 32.7 (5.0) | 33.2 (5.0) | 0.42 |
| Prepregnancy BMI (kg/m2) | 28.7 (6.5) | 30.1 (7.0) | 28.5 (7.1) | 31.3 (8.3) | <.001 |
| Total gestational weight gain (kg) | 10.2 (6.6) | 10.0 (7.2) | 11.0 (5.1) | 10.5 (7.9) | 0.60 |
| Prenatal 3 hr 100 g OGTT, glucose (mg/dL) | |||||
| Fasting | 90.9 (12.8) | 92.6 (12.0) | 92.6 (11.5) | 92.6 (11.4) | 0.19 |
| One hour | 199.0 (24.0) | 199.8 (23.6) | 201.0 (22.1) | 200.1 (22.9) | 0.84 |
| Two hour | 176.5 (26.4) | 176.4 (31.4) | 175.2 (29.9) | 176.8 (25.4) | 0.96 |
| Three hour | 125.1 (32.9) | 126.3 (35.6) | 129.5 (28.3) | 126.6 (34.5) | 0.63 |
| GDM diagnosis gestational age (wks) | 26.0 (6.7) | 25.5 (6.9) | 25.7 (7.1) | 24.9 (7.5) | 0.26 |
| Delivery gestational age (wks) | 38.7 (1.2) | 38.5 (1.2) | 38.9 (1.2) | 38.8 (1.1) | 0.05 |
| GDM Treatment | n (col %) | ||||
| Diet Only | 306 (70.0) | 123 (67.2) | 92 (71.9) | 176 (68.0) | 0.83 |
| Oral hypoglycemic | 115 (26.3) | 53 (29.0) | 34 (26.6) | 76 (29.3) | |
| Insulin | 16 (3.7) | 7 (3.8) | 2 (1.6) | 7 (2.7) | |
| C-Section Delivery | 126 (28.8) | 53 (29.0) | 38 (29.7) | 104 (40.2) | 0.01 |
BF, breastfeeding; FF, formula feeding; GDM, gestational diabetes mellitus; OGTT, oral glucose tolerance test; SWIFT, Study of Women, Infant Feeding and Type 2 Diabetes after GDM Pregnancy; WIC, Special supplemental nutrition program for Women, Infant and Children.
Table 2.
Postpartum Characteristics by Lactation Intensity Groups at 6–9 weeks Postpartum for n=1,007 women with GDM in SWIFT1
| Characteristics At 6–9 weeks Postpartum |
Exclusive BF n= 437 |
Mostly BF n= 183 |
Inconsistent/ Mixed n= 128 |
Exclusive or Mostly FF n= 259 |
P- value |
|---|---|---|---|---|---|
| Mean (SD) | |||||
| BMI (kg/m2) | 29.0 (6.0) | 30.4 (7.1) | 29.5 (6.5) | 32.0 (7.6) | <.001 |
| Waist circumference (cm) | 88.2 (13.2) | 89.6 (14.4) | 87.9 (14.1) | 93.2 (15.8) | <.001 |
| Weight Loss from delivery (kg) | −9.4 (3.4) | −8.8 (3.7) | −8.7 (3.8) | −8.8 (3.8) | 0.10 |
| Time since delivery (weeks) | 6.8 (1.1) | 7.1 (1.2) | 6.9 (1.1) | 7.2 (1.3) | 0.004 |
| Duration of fasting before OGTT (h) | 11.8 (1.5) | 11.7 (1.5) | 11.8 (1.6) | 12.0 (1.7) | 0.20 |
| BF during fasting period (min) | 57.6 (35.5) | 54.2 (39.5) | 43.1 (42.8) | 10.1 (23.2) | <.001 |
| Amount of formula fed (ounces/24 h) | 0 (0) | 3.2 (1.6) | 10.8 (3.7) | 28.7 (8.0) | <.001 |
| 2-hour 75 g OGTT | |||||
| Fasting: Glucose, mg/dL | 92.7 (8.2) | 93.9 (8.1) | 96.7 (11.0) | 97.8 (9.1) | <.001 |
| Insulin, µU/mL | 19.1 (11.1) | 22.6 (13.0) | 23.4 (14.9) | 29.7 (19.5) | <.001 |
| 2 hour: Glucose, mg/dL | 111.3 (30.0) | 112.8 (31.4) | 121.2 (29.2) | 111.9 (26.2) | 0.008 |
| Insulin, µU/mL | 84.5 (69.0) | 98.7 (94.0) | 113.2 (66.6) | 119.2 (77.3) | <.001 |
| HOMA-IR | 4.5 (3.0) | 5.4 (3.4) | 5.8 (4.2) | 7.3 (5.1) | <.001 |
| HOMA-β | 236 (129) | 264 (131) | 251 (141) | 317 (214) | <.001 |
| ISI0,120 | 1.7 (0.5) | 1.6 (0.4) | 1.5 (0.4) | 1.5 (0.4) | <.001 |
| n (col %) | |||||
| BMI (kg/m2) Categories: | <.001 | ||||
| Normal (<25) | 112 (25.7) | 36 (19.7) | 39 (30.5) | 42 (16.2) | |
| Overweight (25–29.9) | 157 (35.9) | 77 (42.1) | 52 (40.6) | 147 (56.8) | |
| Obese (≥30) | 168 (38.4) | 70 (38.2) | 37 (28.9) | 70 (27.0) | |
| Glucose Tolerance: | <.001 | ||||
| Normal | 323 (73.9) | 121 (66.1) | 72 (56.3) | 149 (57.5) | |
| IFG only | 53 (12.1) | 29 (15.9) | 32 (25.0) | 70 (27.0) | |
| IGT only | 43 (9.8) | 24 (13.1) | 13 (10.2) | 16 (6.2) | |
| IFG and IGT | 18 (4.1) | 9 (4.9) | 11 (8.6) | 24 (9.3) | |
| Contraception Methods: | 0.52 | ||||
| Norplant or Depo-provera | 8 (1.8) | 6 (3.3) | 5 (3.9) | 8 (3.1) | |
| Intrauterine Device | 38 (8.7) | 21 (11.5) | 9 (7.0) | 27 (10.4) | |
| Oral contraceptives | 49 (11.2) | 26 (14.2) | 13 (10.2) | 37 (14.3) | |
| Barrier methods or None | 342 (78.3) | 130 (71.0) | 101 (78.9) | 187 (72.2) | |
BF, breastfeeding; FF, formula feeding; GDM, gestational diabetes mellitus; HOMA-β; homeostatic model assessment of insulin secretion; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; ISI0, 120, insulin sensitivity index; OGTT, oral glucose tolerance test; SWIFT, Study of Women, Infant Feeding and Type 2 Diabetes after GDM Pregnancy.
Increasing lactation intensity was associated with a monotonic graded trend with decreasing mean (95%CI) fasting triglycerides (mg/dL) by 20–28%, and increasing mean (95%CI) fasting HDL-cholesterol (mg/dL) by 5–8%, all P-values for trend <0.01 (Table 3). The group mean differences by lactation intensity for exclusive BF, and mostly BF groups compared with the mostly or exclusive FF group remained significant in the fully adjusted models including pre-pregnancy BMI, race/ethnicity, education, postpartum weeks, and time period of fasting, except for mean HDL-cholesterol for the mostly BF group which was attenuated to borderline significance. C-section was not a potential confounder of these associations because of its strong association with maternal BMI which was included in the fully adjusted models. We evaluated HOMA-IR as a mediator of the lactation intensity associations with HDL-cholesterol and triglycerides concentrations and it modestly attenuated the associations (data not shown). Fasting LDL-cholesterol was associated with lactation intensity in unadjusted models, but not in fully adjusted models, or models including HOMA-IR.
Table 3.
Fasting Plasma Lipoproteins and Lipids by Lactation Intensity Groups at 6–9 weeks Postpartum in Women with Recent GDM (n=1,007)1,2
|
Biomarker Fasting Plasma |
Exclusive BF n=437 |
Mostly BF n=183 |
Inconsistent/ Mixed n= 127 |
Exclusive or Mostly FF n=257 |
Trend P- value |
Exclusive BF vs. Exclusive or Mostly FF |
Mostly BF vs. Exclusive or Mostly FF |
Mixed vs. Exclusive or Mostly FF |
|---|---|---|---|---|---|---|---|---|
| HDL-cholesterol, mg/dL | Mean (95% CI) | Mean Group Differences (95% CI) | ||||||
| Unadjusted | 55.3 (54.0,56.6) | 52.7 (50.7, 54.7) | 52.5 (50.1,54.9) | 48.2 (46.5,49.8) | <.001 | 7.2 ** (4.6, 9.7) | 4.5 * (1.4, 7.7) | 4.4* (0.8, 7.9) |
| Fully Adjusted3 | 54.2 (52.7,55.7) | 53.1 (51.0, 55.2) | 51.8 (49.4,54.2) | 50.4 (48.5,52.3) | <.01 | 3.9 * (1.0, 6.7) | 2.7 (−0.5, 6.0) | 1.4 (−2.1, 4.9) |
| LDL-cholesterol, mg/dL | ||||||||
| Unadjusted | 128.7 (125.9, 131.6) | 126.3 (121.8, 130.7) | 119.7 (114.4, 125.0) | 123.7 (120.0, 127.5) | 0.03 | 5.0 (−0.7, 10.6) | 2.5 (−4.4, 9.5) | −4.0 (−11.8, 3.8) |
| Fully Adjusted3 | 126.9 (123.5, 130.4) | 126.5 (121.8, 131.2) | 118.3 (112.8, 123.8) | 122.8 (118.4, 127.1) | 0.08 | 4.2 (−2.4, 10.7) | 3.7 (−3.8, 11.2) | −4.5 (−12.6, 3.6) |
| Total Cholesterol, mg/dL | ||||||||
| Unadjusted | 204.7 (201.5, 208.0) | 202.8 (197.7, 207.8) | 199.0 (193.0, 205.0) | 202.9 (198.7, 207.1) | 0.57 | 1.8 (−4.6, 8.2) | −0.1 (−8.1, 7.8) | −3.9 (−12.8, 5.0) |
| Fully Adjusted3 | 201.2 (197.3, 205.0) | 201.8 (196.5, 207.1) | 195.5 (189.3, 201.7) | 200.6 (195.7, 205.5) | 0.76 | 0.6 (−6.8, 7.9) | 1.2 (−7.2, 9.6) | −5.1 (−14.2, 4.1) |
| Triglycerides, mg/dL | ||||||||
| Unadjusted | 102.8 (95.3, 110.3) | 118.8 (107.2, 130.3) | 132.5 (118.6, 146.3) | 155.5 (145.8, 165.2) | <.001 | −52.7** (−67.4, −37.9) | −36.7** (−54.9, −18.6) | −23.0 (−43.4, −2.6) |
| Fully Adjusted3 | 99.1 (90.1, 108.0) | 110.4 (98.2, 122.7) | 125.8 (111.5, 140.1) | 138.1 (126.8, 149.4) | <.001 | −39.0** (−56.0, −22.1) | −27.7* (−47.0, −8.3) | −12.2 (−33.3, 8.8) |
| Free Fatty Acids, µmol/L | ||||||||
| Unadjusted | 448.2 (433.1, 463.2) | 418.7 (395.4, 442.0) | 430.7 (402.7, 458.7) | 432.2 (412.6, 451.7) | 0.22 | 16.0 (−13.8, 45.8) | −13.4 (−50.1, 23.2) | −1.5 (−42.6, 39.7) |
| Fully Adjusted3 | 439.0 (420.7, 457.2) | 408.0 (383.0, 432.9) | 424.1 (394.9, 453.2) | 437.3 (414.2, 460.3) | 0.90 | 1.7 (−32.8, 36.2) | −29.3 (−68.7, 10.1) | −13.2 (−56.0, 29.6) |
P-values are adjusted for multiple comparisons using the Dunnett’s test (*** <0.001 ** <0.01 * < 0.05).
Breastfeeding (BF), Formula Feeding (FF)
Fully adjusted: Race/ethnicity, education, WIC, time postpartum (weeks), pre-pregnancy BMI, and minutes BF during fasting period.
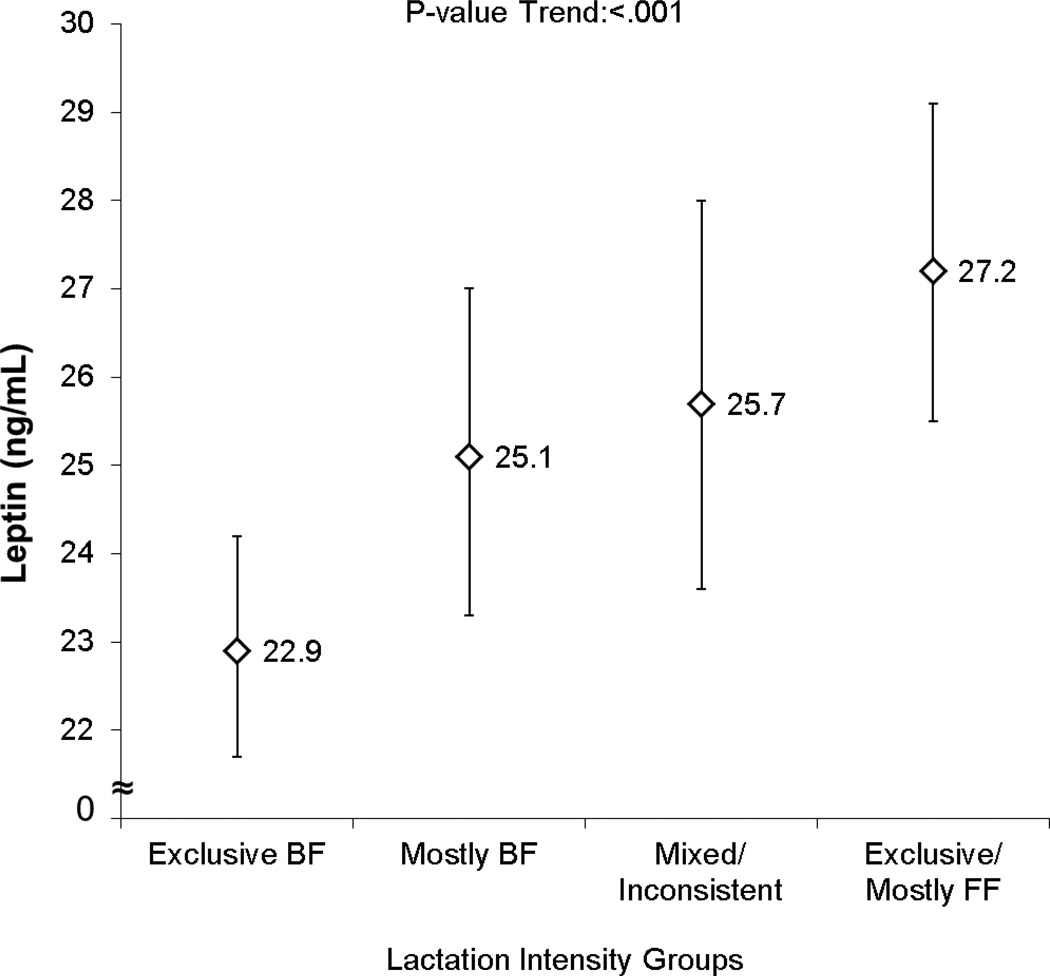
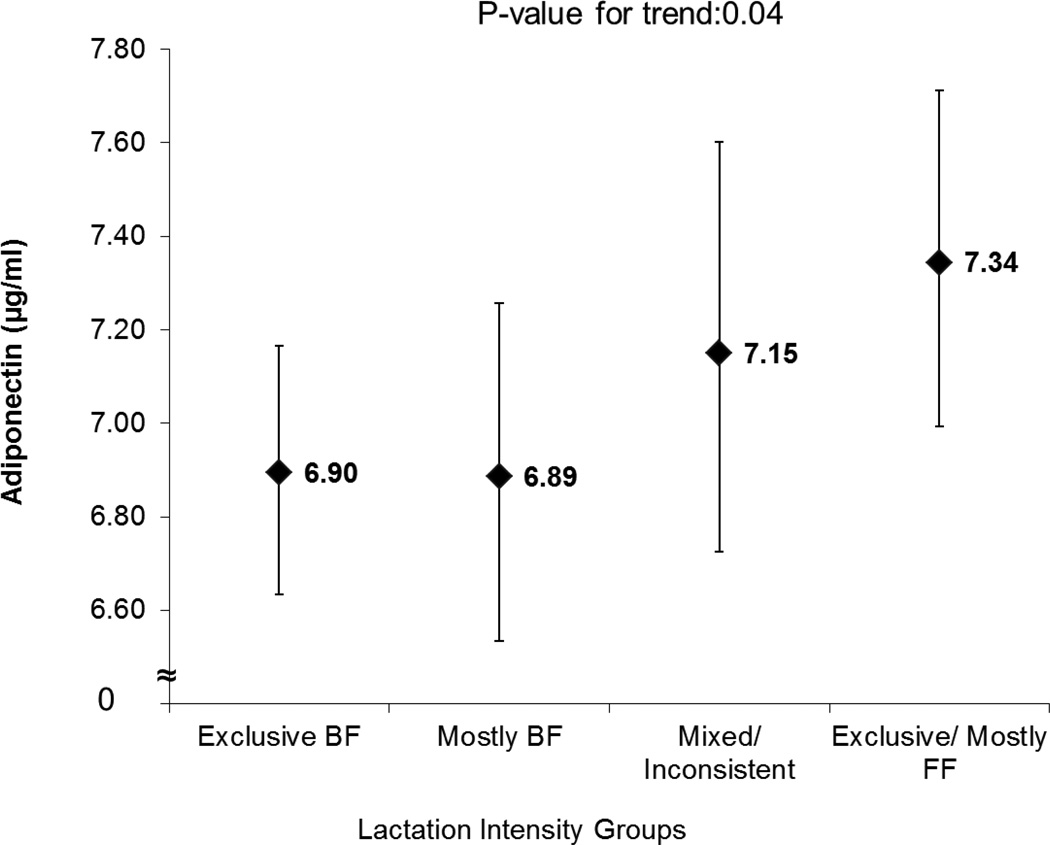
Unadjusted and fully adjusted mean leptin (ng/mL) concentrations were inversely associated with lactation intensity (lower by 15–21%) independent of pre-pregnancy BMI and other covariates (Figure 1). Mean adiponectin showed a graded inverse association with lactation intensity (lower by 6%), which was significant only after adjustment for HOMA-IR (P-value for trend = 0.04), (Figure 2).
Figure 1.
Lactation Intensity at 6–9 weeks Postpartum and Mean (95% CI) Plasma Leptin (ng/mL) in Women with Recent GDM adjusted for race/ethnicity, education, WIC enrollment, postpartum time (weeks), pre-pregnancy BMI and minutes breastfeeding during the fasting period and HOMA-IR1,2
1Means (95% CI) exponentiated from natural log transformed variable.
2 GDM, gestational diabetes mellitus; WIC, Special Supplemental Nutrition Program for Women, Infants and Children.
Figure 2.
Lactation Intensity Groups at 6–9 weeks Postpartum and Mean (95% CI) Plasma Adiponectin (µg/mL) in Women with Recent GDM adjusted for race/ethnicity, education, WIC enrollment, time postpartum (weeks), pre-pregnancy BMI and minutes breastfeeding during fasting period and HOMA-IR1,2
1Means (95% CI) exponentiated from natural log transformed variable.
2 GDM, gestational diabetes mellitus; WIC, Special Supplemental Nutrition Program for Women, Infants and Children.
Weight loss from delivery to 6–9 weeks postpartum, and gestational weight gain did not differ significantly among lactation intensity groups, and therefore, were not included in any of the adjusted models, because they did not meet the criteria for confounding. Weight loss was not correlated with adiponectin and leptin (Pearson correlation coefficients = 0.03 and 0.05) reflecting the maternal lean mass and fluid losses that occur during the early postpartum period.(23) We also examined the two-way interactions for pre-pregnancy BMI, parity, age, glucose tolerance groups, HOMA-IR and race/ethnicity groups to detect effect modification in the associations between lactation intensity groups and postpartum plasma biomarkers. The racial/ethnic group interaction reached statistical significance (P-value=0.05), that reflected a slightly weaker association between HDL-cholesterol and lactation intensity groups (data not shown) among Hispanics.
Discussion
Increasing lactation intensity was associated with lower fasting triglycerides, and leptin as well as higher HDL-cholesterol in a graded monotonic relationship independent of maternal obesity, clinical, and socio-demographic characteristics. Higher lactation intensity was also associated with lower adiponectin levels independent of the same risk factors as well as insulin resistance. Severity of gestational glucose intolerance, gestational weight gain, and postpartum weight loss did not confound these associations. The 3.9 mg/dL higher average plasma HDL-cholesterol for the exclusively lactating group in the present study is consistent with previous cross-sectional studies in non-GDM lactating women,(24) and Latinas with recent GDM that reported a 4 mg/dL higher mean HDL-cholesterol for any lactation versus none.(9) However, Kjos et al. found no difference in fasting triglycerides adjusted for age, BMI and prenatal insulin use,(9) which may be related to more formula use among the lactating women, or the greater variability in the postpartum time interval (i.e., 1 to 4 months). However, lower fasting triglycerides among lactating versus non-lactating women have been reported in non-GDM cohorts.(1;2) Fasting triglycerides increase by 200% during gestation (25) and decline rapidly post-delivery.(2;26) One study reported a more rapid fall in plasma triglycerides among lactating compared with women who never established lactation.(2) Greater adipose tissue lipolysis and higher levels of lipoprotein lipase by the lactating breast (27) are consistent with lower maternal triglyceride concentrations. A previous study reported no differences for blood triglycerides in the range of 40–60 mg/dL,(24) considerably lower than 100 mg/dL in our study.
We also found that higher lactation intensity was associated with lower plasma leptin and adiponectin in our GDM cohort independent of maternal pre-pregnancy obesity, race, weight loss, socio-demographics, and an index of insulin resistance (HOMA-IR). Previous studies in “healthy” women reported no differences in plasma leptin changes from 3 to 6 months postpartum for lactating versus non-lactating groups, or a null association between lactation duration and leptin measured only at 3 years postpartum. (8) However, these studies did not assess changes in lactation intensity over time, nor did they adjust for glucose tolerance, or insulin resistance in women. In our study, the inverse association between plasma leptin and lactation intensity remained after adjustment for pre-pregnancy obesity and postpartum insulin resistance. Gestational weight gain and postpartum weight loss did not confound the association. Leptin is a marker of body adiposity, (28) and secretion from the mammary adipose tissue is regulated by prolactin.(29) During lactation, prolactin suppresses leptin, however, the hormonal mechanisms are not clearly understood.(30) For postpartum women, lower leptin concentrations may be necessary to allow greater fluctuations in insulin secretion that may enhance gluconeogenesis during lactation, or other metabolic adaptations for milk production.
In the present study, plasma adiponectin was inversely associated with lactation intensity in women with GDM after accounting for differences in insulin resistance (i.e., HOMA-IR). These findings suggest that hormonal mechanisms to support lactation regulate adiponectin independent of obesity and insulin resistance.(31) Our finding that adiponectin concentrations were lowest for the highest intensity group is consistent with findings that lactation is associated with lower insulin secretion.(32) A study of lean lactating women reported that adiponectin decreased by 50% from early pregnancy to post-delivery, and that prolactin inhibited the production and secretion of adiponectin from human adipocytes.(16) Another study reported that duration of exclusive lactation was related in a non-linear fashion to adiponectin levels at 3 years postpartum independent of body weight and other confounders for postpartum women without GDM.(8)
Our findings for lower leptin and adiponectin with higher lactation intensity are consistent with the actions of prolactin, although mechanism(s) through which lactation may affect progression to glucose intolerance are unclear. Adiponectin is an adipocyte-derived protein that has been correlated with insulin sensitivity and pancreatic β-cell function and proliferation.(33) In suppressing circulating leptin and adiponectin,(34) prolactin may reduce insulin secretion and enhance sensitivity of the pancreatic β-cells.(32) Lower circulating adiponectin concentrations during lactation are unlikely to adversely affect insulin sensitivity, because glucose uptake by the mammary gland is supported by non-insulin mediated mechanisms. Thus, lower insulin secretion (35) during lactation may help unload on the β-cells.
Our findings suggest that lactation has favorable short-term influences on biomarkers for diabetogenesis, except for plasma adiponectin. The less atherogenic blood lipid profile associated with higher lactation intensity is accompanied by greater insulin sensitivity and lower fasting blood glucose.(12) Epidemiologic studies consistently report that higher adiponectin predicts lower incidence of type 2 diabetes among adults in general,(36;37) and better insulin sensitivity independent of adiposity.(38;39) Adiponectin has been inversely associated with risks of both GDM (40–42) and type 2 diabetes.(43;44) Thus, lower circulating adiponectin during lactation would appear to conflict with evidence that lactation lowers risk of type 2 diabetes in the long-term.(14;15) However, lower adiponectin may be beneficial because higher concentrations have been associated with weight gain in healthy women of reproductive age.(45) Thus, temporary suppression of plasma adiponectin in lactating women might may affect adipose tissue metabolism or postpartum weight retention that contribute to future diabetes risk.(46) In future analyses, changes in adiponectin and leptin among lactation intensity groups would be necessary to confirm any potential link with future weight changes.
Our study had some limitations, including the lack of longitudinal measures to assess the lasting effects of lactation on biomarkers post-weaning, and no measurements of prolactin. Very few studies have examined short- or long-term effects of lactation on metabolic parameters among women with previous GDM. To our knowledge, studies of women with prior GDM have never assessed circulating leptin and adiponectin concentrations by lactation status or intensity. Moreover, insulin resistance has not been examined in relation to lactation intensity and adiponectin, a key predictor of progression to type 2 diabetes following GDM delivery. Our study findings provide evidence of biologic plausibility that lactation may influence risk factors for diabetes and metabolic diseases after GDM pregnancy.(6;15)
The SWIFT cohort is the first study of women with GDM, to our knowledge, to report that higher lactation intensity is associated with lower circulating triglycerides, adiponectin and leptin, and higher HDL-cholesterol. The cohort is comprised of a racially and ethnically diverse sample of women with GDM (75% women of color) and thus, our findings are highly generalizable, particularly given that Asian subgroups and Hispanic women experience much higher rates of GDM than Caucasian women.(47) Longitudinal studies are necessary to determine whether these effects attributed to lactation persist long-term, as well as whether plasma adiponectin increases post-weaning to influence later insulin sensitivity. Others have hypothesized that lactation may lower type 2 diabetes risk later in life through physiologic hyperprolactinemia that may confer lasting benefits to pancreatic β-cells.(30) However, direct evidence to support this hypothesis is unavailable. Ultimately, prospective studies that assess lactation intensity changes over time, as well as lifestyle behaviors are essential to confirm the epidemiologic evidence that lactation may prevent progression to glucose intolerance in women with a history of GDM. A direct link between lactation, both intensity and duration, and changes in biochemical risk factors for type 2 diabetes mellitus after GDM pregnancy remains to be investigated in future studies.
Supplementary Material
Acknowledgments
None
Funding support: The assays for the biomarkers were funded by the Centers for Disease Control and Prevention, Atlanta, Georgia. The SWIFT study was funded by the National Institute of Child Health and Human Development (NICHD), R01 HD050625, R01 HD050625-03S1, and R01 HD050625-05S. This project was also supported in part by the National Institutes of Health National Center for Research Resources UCSF-CTSI UL1 RR024131. “Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the Centers for Disease Control and Prevention."
List of abbreviations
- BF
breastfeeding
- CARDIA
Coronary Artery Risk Development in Young Adults
- CV
Coefficient of Variation
- FF
formula feeding
- FFA
free fatty acids
- GDM
gestational diabetes mellitus
- HOMA-β
homeostatic model assessment of insulin secretion
- ISI0, 120
insulin sensitivity index
- KPNC
Kaiser Permanente Northern California
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- OGTT
oral glucose tolerance test
- RIA
Radio Immunoassay
- PEG
Polyethylene glycol
- SWIFT
Study of Women, Infant Feeding and Type 2 Diabetes After GDM Pregnancy
- WIC
Women’s, Infants and Children nutrition program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have no potential conflict of interest to disclose.
Author Contributions: EPG designed the study and had primary responsibility for the final content. CK provided consultation on the data analysis and critical comments on the written manuscript. SM provided technical expertise for the laboratory assays and quality control as well as methods description for the manuscript. CPQ provided biostatistical consultation and data interpretation. YC, DW, RAA, GF, CE, SY, NS, and ML provided consultation and support regarding patient recruitment at the Santa Clara, Oakland, Roseville and Sacramento, South Sacramento, North Valley, Fremont/Hayward, Richmond, and the San Jose field sites, respectively. JCL provided consultation for screening via the 2-hour OGTT. XN conducted the data analysis and comments on the manuscript. KGD provided consultation on the assessment of lactation intensity, and critical comments on the manuscript. All authors read and approved the final manuscript.
Reference List
- 1.Darmady JM, Postle AD. Lipid metabolism in pregnancy. Br J Obstet Gynaecol. 1982 Mar;89(3):211–215. doi: 10.1111/j.1471-0528.1982.tb03616.x. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi IA, Xi XR, Limbu YR, Bin HY, Chen MI. Hyperlipidaemia during normal pregnancy, parturition and lactation. Ann Acad Med Singapore. 1999 Mar;28(2):217–221. [PubMed] [Google Scholar]
- 3.Erkkola R, Viikari J, Irjala K, Solakivi-Jaakkola T. One-year follow-up of lipoprotein metabolism after pregnancy. Biol Res Pregnancy Perinatol. 1986;7(2):47–51. [PubMed] [Google Scholar]
- 4.Knopp RH, Walden CE, Wahl PW, Bergelin R, Chapman M, Irvine S, et al. Effect of postpartum lactation on lipoprotein lipids and apoproteins. J Clin Endocrinol Metab. 1985 Mar;60(3):542–547. doi: 10.1210/jcem-60-3-542. [DOI] [PubMed] [Google Scholar]
- 5.Gunderson EP, Lewis CE, Wei GS, Whitmer RA, Quesenberry CP, Sidney S. Lactation and changes in maternal metabolic risk factors. Obstet Gynecol. 2007 Mar;109(3):729–738. doi: 10.1097/01.AOG.0000252831.06695.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunderson EP, Jacobs DR, Jr, Chiang V, Lewis CE, Feng J, Quesenberry CP, Jr, et al. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: a 20-Year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults) Diabetes. 2010 Feb;59(2):495–504. doi: 10.2337/db09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stuebe AM, Kleinman K, Gillman MW, Rifas-Shiman SL, Gunderson EP, Rich-Edwards J. Duration of lactation and maternal metabolism at 3 years postpartum. J Womens Health (Larchmt) 2010 May;19(5):941–950. doi: 10.1089/jwh.2009.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuebe AM, Mantzoros C, Kleinman K, Gillman MW, Rifas-Shiman S, Gunderson EP, et al. Duration of lactation and maternal adipokines at 3 years postpartum. Diabetes. 2011 Apr;60(4):1277–1285. doi: 10.2337/db10-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjos SL, Henry O, Lee RM, Buchanan TA, Mishell DR., Jr The effect of lactation on glucose and lipid metabolism in women with recent gestational diabetes. Obstet Gynecol. 1993 Sep;82(3):451–455. [PubMed] [Google Scholar]
- 10.O'Reilly MW, Avalos G, Dennedy MC, O'Sullivan EP, Dunne F. Atlantic DIP: high prevalence of abnormal glucose tolerance post partum is reduced by breast-feeding in women with prior gestational diabetes mellitus. Eur J Endocrinol. 2011 Dec;165(6):953–959. doi: 10.1530/EJE-11-0663. [DOI] [PubMed] [Google Scholar]
- 11.McManus RM, Cunningham I, Watson A, Harker L, Finegood DT. Beta-cell function and visceral fat in lactating women with a history of gestational diabetes. Metabolism. 2001 Jun;50(6):715–719. doi: 10.1053/meta.2001.23304. [DOI] [PubMed] [Google Scholar]
- 12.Gunderson EP, Hedderson MM, Chiang V, Crites Y, Walton D, Azevedo RA, et al. Lactation Intensity and Postpartum Maternal Glucose Tolerance and Insulin Resistance in Women With Recent GDM: The SWIFT cohort. Diabetes Care. 2012 Jan 1;35(1):50–56. doi: 10.2337/dc11-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chouinard-Castonguay S, Weisnagel SJ, Tchernof A, Robitaille J. Relationship between lactation duration and insulin and glucose response among women with prior gestational diabetes. Eur J Endocrinol. 2013 Apr;168(4):515–523. doi: 10.1530/EJE-12-0939. [DOI] [PubMed] [Google Scholar]
- 14.Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005 Nov 23;294(20):2601–2610. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler AG, Wallner M, Kaiser I, Rossbauer M, Harsunen MH, Lachmann L, et al. Long-term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes. 2012 Dec;61(12):3167–3171. doi: 10.2337/db12-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asai-Sato M, Okamoto M, Endo M, Yoshida H, Murase M, Ikeda M, et al. Hypoadiponectinemia in lean lactating women: Prolactin inhibits adiponectin secretion from human adipocytes. Endocr J. 2006 Aug;53(4):555–562. doi: 10.1507/endocrj.k06-026. [DOI] [PubMed] [Google Scholar]
- 17.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2000 Jan;23(Suppl 1):S4–S19. [PubMed] [Google Scholar]
- 18.Gunderson EP, Matias SL, Hurston SR, Dewey KG, Ferrara A, Quesenberry CP, Jr, et al. Study of Women, Infant Feeding, and Type 2 diabetes mellitus after GDM pregnancy (SWIFT), a prospective cohort study: methodology and design. BMC Public Health. 2011 Dec 23;11(1):952. doi: 10.1186/1471-2458-11-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunderson EP, Crites Y, Chiang V, Walton D, Azevedo RA, Fox G, Elmasian C, Young S, Salvador N, Lum M, Hedderson M, Quesenberry CP, Lo JC, Ferrara A, Sternfeld B. Influence of Brestfeeding During the Postpartum Oral Glucose Tolerance Test on Plasma Glucose and Insulin. Obstetrics & Gynecology. 2012;120(1):136–143. doi: 10.1097/AOG.0b013e31825b993d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanley AJ, Williams K, Gonzalez C, D'Agostino RB, Jr, Wagenknecht LE, Stern MP, et al. Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes. 2003 Feb;52(2):463–469. doi: 10.2337/diabetes.52.2.463. [DOI] [PubMed] [Google Scholar]
- 21.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010 Jan;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989 Jan;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 23.Gunderson EP, Abrams B, Selvin S. Does the pattern of postpartum weight change differ according to pregravid body size? Int J Obes Relat Metab Disord. 2001 Jun;25(6):853–862. doi: 10.1038/sj.ijo.0801631. [DOI] [PubMed] [Google Scholar]
- 24.Knopp RH, Bergelin RO, Wahl PW, Walden CE. Effects of pregnancy, postpartum lactation, and oral contraceptive use on the lipoprotein cholesterol/triglyceride ratio. Metabolism. 1985 Oct;34(10):893–899. doi: 10.1016/0026-0495(85)90134-9. [DOI] [PubMed] [Google Scholar]
- 25.Piechota W, Staszewski A. Reference ranges of lipids and apolipoproteins in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1992 Jun 16;45(1):27–35. doi: 10.1016/0028-2243(92)90190-a. [DOI] [PubMed] [Google Scholar]
- 26.Montelongo A, Lasuncion MA, Pallardo LF, Herrera E. Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes. 1992 Dec;41(12):1651–1659. doi: 10.2337/diab.41.12.1651. [DOI] [PubMed] [Google Scholar]
- 27.Rebuffe-Scrive M, Enk L, Crona N, Lonnroth P, Abrahamsson L, Smith U, et al. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J Clin Invest. 1985 Jun;75(6):1973–1976. doi: 10.1172/JCI111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butte NF, Hopkinson JM, Nicolson MA. Leptin in human reproduction: serum leptin levels in pregnant and lactating women. J Clin Endocrinol Metab. 1997 Feb;82(2):585–589. doi: 10.1210/jcem.82.2.3731. [DOI] [PubMed] [Google Scholar]
- 29.Feuermann Y, Mabjeesh SJ, Niv-Spector L, Levin D, Shamay A. Prolactin affects leptin action in the bovine mammary gland via the mammary fat pad. J Endocrinol. 2006 Nov;191(2):407–413. doi: 10.1677/joe.1.06913. [DOI] [PubMed] [Google Scholar]
- 30.Ben Jonathan N, Hugo ER, Brandebourg TD, LaPensee CR. Focus on prolactin as a metabolic hormone. Trends Endocrinol Metab. 2006 Apr;17(3):110–116. doi: 10.1016/j.tem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Baratta R, Amato S, Degano C, Farina MG, Patane G, Vigneri R, et al. Adiponectin relationship with lipid metabolism is independent of body fat mass: evidence from both cross-sectional and intervention studies. J Clin Endocrinol Metab. 2004 Jun;89(6):2665–2671. doi: 10.1210/jc.2003-031777. [DOI] [PubMed] [Google Scholar]
- 32.Tigas S, Sunehag A, Haymond MW. Metabolic adaptation to feeding and fasting during lactation in humans. J Clin Endocrinol Metab. 2002 Jan;87(1):302–307. doi: 10.1210/jcem.87.1.8178. [DOI] [PubMed] [Google Scholar]
- 33.Retnakaran R, Hanley AJ, Raif N, Hirning CR, Connelly PW, Sermer M, et al. Adiponectin and beta cell dysfunction in gestational diabetes: pathophysiological implications. Diabetologia. 2005 May;48(5):993–1001. doi: 10.1007/s00125-005-1710-x. [DOI] [PubMed] [Google Scholar]
- 34.Lenz S, Kuhl C, Hornnes PJ, Hagen C. Influence of lactation on oral glucose tolerance in the puerperium. Acta Endocrinol (Copenh) 1981 Nov;98(3):428–431. doi: 10.1530/acta.0.0980428. [DOI] [PubMed] [Google Scholar]
- 35.Lee YH, Magkos F, Mantzoros CS, Kang ES. Effects of leptin and adiponectin on pancreatic beta-cell function. Metabolism. 2011 Dec;60(12):1664–1672. doi: 10.1016/j.metabol.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Mather KJ, Funahashi T, Matsuzawa Y, Edelstein S, Bray GA, Kahn SE, et al. Adiponectin, change in adiponectin, and progression to diabetes in the Diabetes Prevention Program. Diabetes. 2008 Apr;57(4):980–986. doi: 10.2337/db07-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daimon M, Oizumi T, Saitoh T, Kameda W, Hirata A, Yamaguchi H, et al. Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese Population: the Funagata study. Diabetes Care. 2003 Jul;26(7):2015–2020. doi: 10.2337/diacare.26.7.2015. [DOI] [PubMed] [Google Scholar]
- 38.Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S, et al. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes. 2003 Feb;52(2):239–243. doi: 10.2337/diabetes.52.2.239. [DOI] [PubMed] [Google Scholar]
- 39.Matsubara M, Katayose S, Maruoka S. Decreased plasma adiponectin concentrations in nondiabetic women with elevated homeostasis model assessment ratios. Eur J Endocrinol. 2003 Mar;148(3):343–350. doi: 10.1530/eje.0.1480343. [DOI] [PubMed] [Google Scholar]
- 40.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002 Jul;51(7):2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 41.Kautzky-Willer A, Pacini G, Tura A, Bieglmayer C, Schneider B, Ludvik B, et al. Increased plasma leptin in gestational diabetes. Diabetologia. 2001 Feb;44(2):164–172. doi: 10.1007/s001250051595. [DOI] [PubMed] [Google Scholar]
- 42.Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004 Apr;83(4):341–347. doi: 10.1111/j.0001-6349.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 43.Winzer C, Wagner O, Festa A, Schneider B, Roden M, Bancher-Todesca D, et al. Plasma adiponectin, insulin sensitivity, and subclinical inflammation in women with prior gestational diabetes mellitus. Diabetes Care. 2004 Jul;27(7):1721–1727. doi: 10.2337/diacare.27.7.1721. [DOI] [PubMed] [Google Scholar]
- 44.Saucedo R, Zarate A, Basurto L, Hernandez M, Puello E, Galvan R, et al. Relationship between circulating adipokines and insulin resistance during pregnancy and postpartum in women with gestational diabetes. Arch Med Res. 2011 May;42(4):318–323. doi: 10.1016/j.arcmed.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Hivert MF, Sun Q, Shrader P, Mantzoros CS, Meigs JB, Hu FB. Higher adiponectin levels predict greater weight gain in healthy women in the Nurses' Health Study. Obesity (Silver Spring) 2011 Feb;19(2):409–415. doi: 10.1038/oby.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahin-Efe A, Katsikeris F, Mantzoros CS. Advances in Adipokines. Metabolism. 2012 Dec;61(12):1659–1665. doi: 10.1016/j.metabol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol. 2010 Sep 1;24(5):441–448. doi: 10.1111/j.1365-3016.2010.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.