Highlights
-
•
Coronaviruses (CoV) emergence has caused two major outbreaks within the past decade.
-
•
Systems biology has identified host components critical to CoV pathogenesis.
-
•
CoV disease is influenced by host genetic polymorphisms.
-
•
Systems genetics drives a broader insight into viral disease outcomes.
-
•
Both approaches provide transitions into therapeutics and human disease.
Abstract
Coronaviruses comprise a large group of emergent human and animal pathogens, including the highly pathogenic SARS-CoV and MERS-CoV strains that cause significant morbidity and mortality in infected individuals, especially the elderly. As emergent viruses may cause episodic outbreaks of disease over time, human samples are limited. Systems biology and genetic technologies maximize opportunities for identifying critical host and viral genetic factors that regulate susceptibility and virus-induced disease severity. These approaches provide discovery platforms that highlight and allow targeted confirmation of critical targets for prophylactics and therapeutics, especially critical in an outbreak setting. Although poorly understood, it has long been recognized that host regulation of virus-associated disease severity is multigenic. The advent of systems genetic and biology resources provides new opportunities for deconvoluting the complex genetic interactions and expression networks that regulate pathogenic or protective host response patterns following virus infection. Using SARS-CoV as a model, dynamic transcriptional network changes and disease-associated phenotypes have been identified in different genetic backgrounds, leading to the promise of population-wide discovery of the underpinnings of Coronavirus pathogenesis.
Current Opinion in Structural Biology 2014, 6:61–69
This review comes from a themed issue on Viral pathogenesis
Edited by Mark Heise
For a complete overview see the Issue and the Editorial
Available online 17th May 2014
http://dx.doi.org/10.1016/j.coviro.2014.04.007
1879-6257/© 2014 Elsevier B.V. All rights reserved.
Introduction
Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) emerged in Guangdong province, China, in 2002, causing a global epidemic that resulted in about 8000 reported cases and an overall mortality rate of ∼10% [1]. The virus was initially present in horseshoe bat populations, and either evolved mutations that allowed transition to Palm Civets and Raccoon Dogs before emerging in human populations, or was directly transmitted from bats to humans and subsequently amplified through intermediate hosts [2, 3, 4]. From there, SARS-CoV rapidly spread across the globe, with focal outbreaks in China, Singapore, Vietnam, Taiwan and Canada [1]. More recently, the antigenically distinct Middle East Respiratory Syndrome (MERS-CoV) emerged in 2012 and is still currently circulating in animal and human populations in the Middle East, resulting in 184 cases and 80 deaths to date (http://www.promed.org). MERS-CoV most likely emerged from circulating bat strains and appears to also replicate efficiently in camels [5, 6]. Both pathogens cause a respiratory disease, with many severely impacted individuals transitioning into an acute respiratory distress syndrome (ARDS) [7, 8, 9, 10]. Although the SARS-CoV outbreak was controlled by epidemiological measures, the recent identification of SARS-like bat-CoVs that can recognize human angiotensin 1 converting enzyme 2 receptors and replicate efficiently in primate cells documents the inevitability of a SARS-CoV-like virus re-emergence event in the near future [11•]. Together, these data highlight prototypical outbreak concerns for the 21st century, where increased travel and community pressures on wildlife areas present numerous opportunities for novel viral disease emergence followed by rapid spread worldwide, sometimes within a matter of months [12, 13, 14]. Rapid response platforms are clearly needed to maximize public health preparedness against emerging viruses.
A fundamental problem in dealing with emerging infectious disease control is both the limited accessibility to and the limited number of biological samples associated with an expanding epidemic, confounding insights into susceptibility and mechanistic disease processes which are critical for rational antiviral and vaccine design strategies. In order to advance our understanding of those disease processes at work, novel approaches have been evolved that utilize newly developed state-of-the-art techniques and technologies. Systems biology [15] utilizes an integration of traditional pathogenesis approaches, as well as high-throughput molecular profiling, and computational modeling to identify key host genes and pathways involved in pathogenesis. In a related way [16], systems genetics integrates molecular profiling and pathogenesis readouts within genetically complex populations to identify genes and pathways that contribute to disease variation across genetically diverse populations. Integration of both platforms provides unparalleled power in identifying and studying host susceptibility networks that contribute to disease outcomes. The common feature of both discovery platforms is that they seek to understand viral disease as part of complex, interacting systems with multiple genes and response pathways. While fundamentally different from standard reductionist strategies, these approaches still rely on standard genetic, molecular biology, biochemical and immunologic strategies to validate the role of targeted genes and networks in disease processes. Using these approaches, there is hope that model systems and platform approaches can be utilized to identify critical regulators of disease across genetically diverse human populations, and to transition these findings into prophylactic and therapeutic drugs.
Systems biology approaches
Over the past decade, a series of important technological advances, genome wide molecular screening platforms and computational strategies have emerged that provide new opportunities for rapid response against newly emerging viral disease threats, globally. The paradigm of these systems biology approaches [15, 17] is that (Figure 1 ) a model system or systems (e.g. tissue culture model, in vivo animal model, or even human challenge model and vaccine studies) are perturbed, in our case by viral challenge, preferably resulting in a spectra of disease severities (e.g., lethal vs sub-lethal) to maximize contrast for downstream data mining and modeling. Over a time course, multiple global measures of the system's performance are taken in response to infection, including high-throughput molecular measures (transcriptome, proteome, metabolome, etc.), as well as a variety of virologic, immunologic and pathologic measures (e.g. weight loss, respiratory function, inflammatory response, mortality and histopathological damage). A variety of computation methodologies ([18, 19, 20••, 21•] and reviewed more fully in [22]) and network approaches are then used to de novo identify regulatory networks, with these networks and their kinetic responses then being correlated to different disease outcomes in the system. Following these initial descriptions, there are a series of continuing cycles of testing and perturbations (host gene knockout, virus mutant or therapeutic intervention) designed to further validate and then refine the model and to elucidate the mechanistic underpinnings of the systems’ performance as a function of infection and disease severity.
Figure 1.
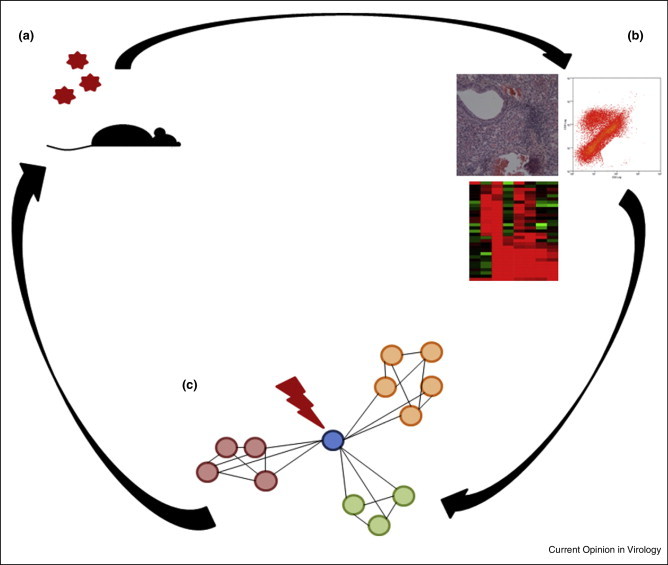
The Systems Biology Paradigm. Systems Biology focuses on an iterative cycle of experiments. In model system (a) mouse is infected. (b) Measurements of molecular (e.g. whole transcriptome, proteome) and disease related phenotypes (histopathology and flow cytometry) are taken at multiple timepoints and contrasted with mock infected animals. (c) Transcriptional (or proteomic) data are assembled into networks of interacting and coexpressed transcripts. These networks are then correlated back to specific disease pathologies. These data are then fed into new sets of experiments where key members of networks (e.g. the blue gene central to the network) are then disrupted to alter pathologic outcomes in a predicted manner.
Modeling algorithms are rapidly evolving in response to the emergence of these complex and comprehensive systems wide datasets and are beyond the focus of this review (but see [22] for more information); however, many of these approaches de novo assemble the networks, independent of annotated pathways or interactions. By allowing this de novo assembly within the context of infection, new relationships between genes (or the breaking of previously annotated relationships) emerge that allow for the identification of critical subnetworks. Such a method was recently successfully used to identify critical components of SARS-CoV induced pathogenesis following infection of mice [20••]. A de novo assembled network approach was used to identify Serpine1 and other members of the Urokinase pathway as high priority candidates in regulating severe disease outcomes following lethal vs sub-lethal infections. Subsequent study of Serpine1 knockouts as well as knockouts from other pathway members confirmed a protective role for these Urokinase pathway members in regulating severe SARS-CoV disease outcomes. Illustrating the power of these de novo computational algorithms, it seems unlikely that this pathway would have been otherwise implicated in SARS-CoV infection. These approaches can become even more powerful by integrating analyses across multiple large-scale datasets. Gibbs et al. [19] were able to further refine these approaches by independently assembling transcriptional and proteomic networks and then cross-contrasting these two network types. This method was able to clarify network membership and connections, as well as enhance the relationship between these joint networks and aspects of SARS-induced lung pathology. In addition, such approaches also resulted in highly prioritized list of regulators with conserved behavior for SARS-CoV and influenza A viruses (IAV) via a combined analyses, which provide valuable candidates for downstream experimental validations and therapeutic intervention [21•].
Iterative rounds of perturbation are another key component of the systems biology paradigm. These iterative perturbations are utilized in order to refine and re-evaluate networks when key members of these networks are modified. While perturbations are typically thought of as host perturbations, in some cases they can also be viral perturbations. In this way, SARS-CoV ORF6 [23] was identified as a key inhibitor of multiple antiviral cell intrinsic host genetic responses by blocking the import of targeted clusters of transcription factors into the nucleus during infection and thereby reprogramming host response networks following infection. Chromosome immunoprecipitation studies further validated the role of ORF6 expression in the nuclear import and DNA binding of select transcription factors, and loss of ORF6 attenuated virus pathogenesis. In a parallel example, the SARS-CoV E protein is a known virulence determinant [24]. Using systems biology, E protein was found to suppress the expression of 25 stress related proteins and specifically down-regulated the inositol-requiring enzyme 1 (IRE-1) signaling pathway of unfolded protein responses. In the absence of E protein, an increase in stress responses and the reduction of inflammation likely contributed to the attenuation of rSARS-CoV-ΔE, validating the systems wide predictions. In other cases, contrasting SARS-CoV with immune stimulatory molecules (e.g. interferon stimulation) or different pathogens can be used for cross-comparison. In this way, Danesh et al. [25] were able to show that in contrast to a strict interferon response in a ferret model of SARS-CoV infection, a wider variety of cell migratory and inflammatory genes were induced.
Population-wide variation in coronavirus responses
Population-wide variation in disease responses is known to occur for many pathogens, and there was notable variability within the disease severity and clinical outcomes after SARS-CoV and MERS-CoV infections, most notably in the elderly population. For SARS-CoV, systems approaches were used to differentiate resolution from fatality in a patient cohort [26]. This study showed that although initial immune responses were fairly uniform, fatal cases of SARS-CoV infection exhibited aberrant interferon stimulation, persistent chemokine responses and disregulated adaptive immune networks. Similarly, MERS-CoV infections have mostly clustered in men, and those with underlying medical conditions, although this may represent a gender difference in accessibility to health care in the Middle East [9]. However, as is often the case with heterogeneous human populations, while clear trends can be observed in disease responses, it is unclear whether those observed differentiating pathologic/response classes are due to underlying genetic variation within the population, or due to other factors, such as environmental factors, demography or exposure histories. For example, SARS-CoV exhibited a ∼10% mortality throughout the outbreak, but this mortality rate rose to ∼50% in the aged population [1, 12]. A mouse model of this phenomenon suggested a genetic link, in that increased disease severity correlates with aberrant PGD(2) expression that impairs respiratory DC migration and associated reduced T cell responses [27].
However, in the human population, the extent to which this disease variation is due to genetic versus non-genetic causes remains unclear. It is clear from studies following the SARS-CoV outbreak that host genetic variants do have significant associations with variant immune phenotypes following SARS-CoV infection, although the clinical relevance of these polymorphisms and their connections to pathologic outcomes are less understood [28, 29, 30, 31]. More generally, it is well accepted that host genetic variants play key roles in onset, severity and resolution of viral infection (reviewed in [32]). Despite the presence of several well-known and highly penetrant susceptibility genes of large effect (e.g. CCR5 and HIV [33], FUT2 in norovirus and perhaps rotavirus infections [34, 35]), there is an increasing awareness that responses to viral pathogens are likely regulated by complex interactions involving multiple variant genes and their corresponding expression networks that are activated following infection [36]. However, identification of these polymorphic genes and their associated pathways and outcomes is confounded by the large controlled cohorts typically needed to detect moderate to small effect alleles in association studies [37]. Therefore, novel approaches are needed to aid in the discovery of those polymorphic networks which contribute to viral pathogenesis in the cases of emerging pathogens with limited human samples
Systems genetics approaches
While genome wide association studies within human populations can provide powerful insight into disease responses, both the absence of large human cohorts to conduct such association studies, and the difficulty in transitioning such associations into mechanisms of pathologic or protective outcomes provide roadblocks for direct human studies. In answer to such needs, systems genetics approaches utilize genetically diverse experimental models to recapitulate the population-wide variation seen across the human population and attempt to disentangle complex traits, such as immune responses [38, 39]. Specifically, by integrating not only pathologic and high-throughput molecular data, but also explicit information on the genetic composition of the experimental population, systems genetics seeks to identify genes and pathways of polymorphic genes that directly contribute to variation in responses to infection across genetically diverse populations, as well as for to further disentangle the underlying molecular signatures and pathways associated with various disease outcomes (Figure 2 ). Furthermore, by explicitly contrasting the high-throughput molecular and phenotypic data across unique genetic backgrounds, robust virus-response signatures can be identified across host genetic backgrounds, attaining a better resolution of the dynamic and host regulatory responses that act in host-genetic background specific manners during infection.
Figure 2.
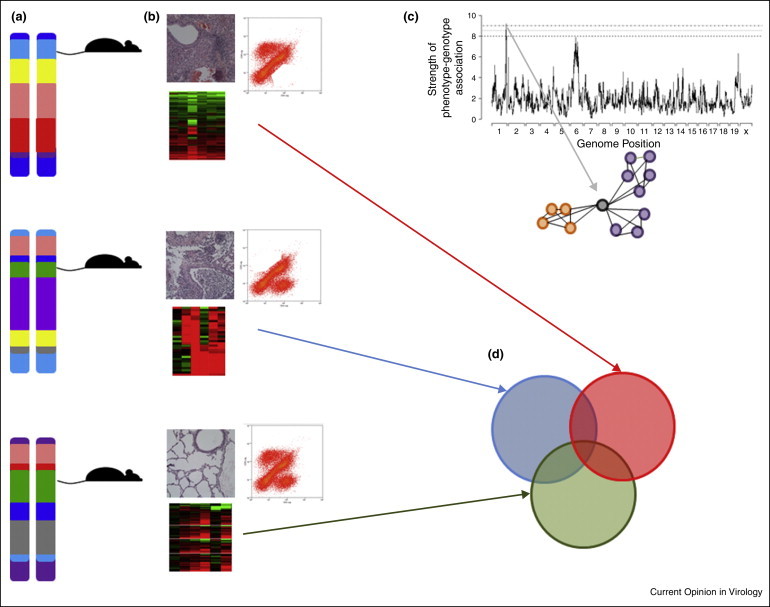
Systems Genetics integrates systems biology and genetic complexity. Here sets of genetically well-defined yet distinct mouse strains (a) are challenged with a pathogen and a variety (b) of disease and molecular phenotypes are collected. Integration of genetic variants within this population and disease phenotypes (c) can identify host genome regions containing polymorphisms controlling disease phenotypes (QTL mapping), and contrasting the expression profiles of individuals with variant polymorphisms at this loci can identify those groups of transcripts that are up-regulated (orange) or down-regulated (purple) due to polymorphisms at this genome location, highlighting mechanisms of virus induced pathology. Furthermore, by contrasting in a strain-specific manner all of those transcripts that are differentially expressed during infection (d), specific transcriptional subsets can be associated with variant disease outcomes. Here each of the three mouse strains have a pool of differentially expressed transcripts (colored circles) following infection. Therefore, the union of red, blue and green describes those transcripts commonly differentially regulated across all genotypes in response to infection. Similarly, the intersection of red and blue transcripts (excluding green transcripts) describes those transcripts differentially regulated in genotypes with severe lung pathologies.
The field of viral pathogenesis has long used a limited number of mouse strains for in vivo pathogenesis studies [40, 41]. These lines (e.g. C57Bl/6J or Balb/cJ) have played critical roles in the development of animal models and reagents that are useful for the study of host responses; however, they do not recapitulate the genetic variation present within the outbred human population, which is critical to disease responses. Recently, newly developed mouse resources were explicitly designed for systems genetics analysis as well as better capturing the genetic variation seen within human populations. Specifically the Collaborative Cross (CC) [42] recombinant inbred panel and Diversity Outbred (DO) [43] population are novel mouse resources which combine the utility of experimental mouse models with the genetic variability critical to contrasting experimental models with human responses. The CC and DO are complimentary resources (Figure 3 ) with levels of natural genetic variation roughly consistent with common variants segregating across the human population (∼107 single nucleotide polymorphisms and ∼106 small insertion/deletions), and characterized by relatively uniform distributions of variation across the genome. The large number of CC lines, and the continual generation of novel genomes of DO mice give rise to an incredibly large number of combinations of genetic variants across those genomes. These attributes are critical for first, mapping of genetic variants associated with infectious outcomes, second, creating novel genetic background with which to study transcriptional and regulatory networks, third, describing new models of virus diseases and pathologies, and fourth, accurate modeling of the human population's genetic composition while maintaining experimentally tractable systems [44]. Importantly for systems genetics approaches, the CC and the DO not only facilitate initial discovery, but by allowing for the generation of new crosses and animals with similar allele frequencies but in new combinations, they also allow for the validation of the role of specific polymorphic genes and further mechanistic study (Figure 3).
Figure 3.
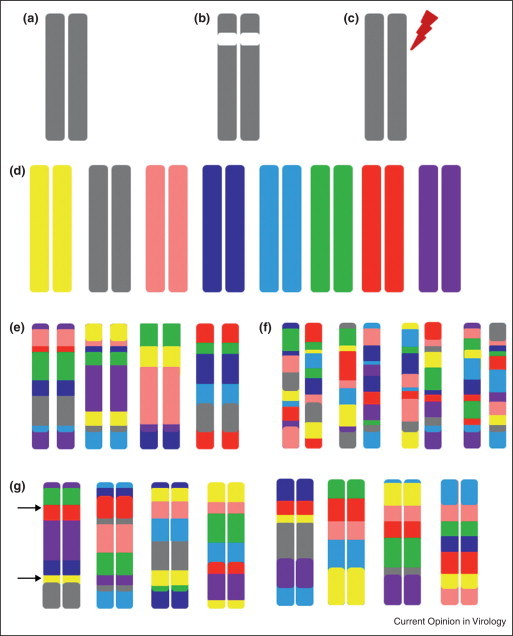
Platforms for Systems genetics discovery and validation. Traditionally, classical inbred strains such as C57BL/6J (a) have been used for systems biology approaches. These classical systems have utilized (b) gene knockouts or (c) the introduction of functional changing mutations as perturbation/validation systems. The Collaborative Cross (CC) and DO (DO) populations were derived from a set of eight genetically diverse founders whose genomes are represented by the following colors (d): A/J (yellow), C57BL/6J (gray), 129s1/SvImJ (pink), NOD/ShiLtJ (dk. blue), NZO/HILtJ (lt. blue), CAST/EiJ (green), PWK/PhJ (red), and WSB/EiJ (purple). CC lines (e) have inbred genomes that are mosaics of these eight founders (with the founder contributions keeping the color coding of D). CC lines have well-characterized genomes and being inbred are an infinitely reproducible population. Similarly (f) the Diversity Outbred (DO) is a completely outbred population of animals derived from the same eight founder strains. While this population is not reproducible, the genetic architecture of the population can be reproduced. In these ways, both the CC and DO facilitate systems genetics approaches. The CC and DO, by virtue of the large number of unique genomes, can be used (f) to create a variety of validation crosses, or sets of lines with unique genetic combinations for further mechanistic study of polymorphisms of interest. Here, a panel of CC lines is being used to contrast the PWK/PhJ (red) and 129S1/SvImJ (pink) alleles at Locus 1, while simultaneously being used to contrast A/J (yellow) and WSB/EiJ (purple) alleles at Locus 2.
Systems genetics approaches have been used extensively in studying the responses to influenza [44, 45, 46, 47••]. Overall, these studies have found that multiple host polymorphisms contribute to differential disease outcomes following influenza infection, that some of these polymorphisms act in virus strain-specific manners, and that different subsets of transcripts associate with specific disease responses following these infections. Furthermore, by integrating these systems genetics approaches throughout multiple timepoints, Nedelko et al. [47••] were able to show that polymorphisms worked at specific points throughout the infection process, pointing to further complexity in the role of genetic regulation underlying differential disease outcomes. Together, these studies highlight the incredible power and precision that systems genetics approaches can provide, especially when blended with systems biology and computational modeling.
Systems approaches have classically used traditional transcriptome profiling, such as microarray and mRNA seq. However, there is increasing evidence that non-coding RNAs play roles in regulating immune responses [48, 49], and can have direct impact on viral infection [50]. Relevant to Coronavirus pathogenesis, two studies of contrasting IAV and SARS-CoV induced long [51] and small [52] non-coding RNAs were recently conducted within a subset of the founder animals of the CC, focusing on founder lines from the three genetically distant subspecies of Mus musculus, which have distinct responses to both SARS-CoV and IAV infection. Both of these studies found that there were pervasive changes in the expression levels of these noncoding transcripts during infections. Importantly for systems genetics approaches, they showed that these two pathogens led to differential regulation of these noncoding RNAs and that the levels of differential expression for these noncoding RNAs vary depending on host genetic background. This work highlights that unique interactions between specific viral infections and host genetic variation drive differential disease outcomes, and through the use of systems genetics approaches, host responses and the critical pathways causing various pathologic outcomes can be defined. With a growing appreciation for the overall roles of noncoding RNAs in regulating immune responses and pathogenesis [53], as well as evidence that polymorphisms within noncoding RNAs can directly impact pathologic outcomes during infection, such as clearance of Hepatitis B infection [54], the investigation and detection of noncoding RNAs in future systems genetics approaches will provide a rich investigative environment for investigating how host genetic variation shapes immune responses and pathologic outcomes.
Future prospects
As illustrated throughout this study, the integration of systems approaches in traditional studies on viral pathogenesis provides immensely powerful tools with which to identify the host factors critical for pathologic or protective outcomes following viral infections in experimental systems. A key challenge for the field is to transition targets generated by systems approaches into therapeutics and prophylactics. Recently this has been seen for both MERS-CoV [55••], and H7N9 avian influenza [56], using cell culture models. In both cases, application of systems approaches and contrasting infections (MERS-CoV and SARS-CoV; H7N9 and H3N2 influenza) were used to identify pathways differentially regulated between related pathogens, and then this information was applied to select and test potential antiviral compounds which were able to inhibit both the target and related virus in the case of Coronaviruses [55••], or just the specific H7N9 target virus but not the related H3N2 virus [56]. Future approaches in these veins, and transitioning such results to in vivo systems genetic platforms such as the CC will further improve our capacity to combat conventional and new viral diseases of the future.
A longstanding divide in the scientific community has been bridging the gap between experimental systems and human populations. Indeed, some commonalities exist between murine and human immune responses [57, 58], such as the role of IFITM3 in both human and mouse responses to influenza [58]. However, there are other studies highlighting discordance between humans and mice [59]. While systems approaches identify key genes, both their focus on pathways and systemic responses, and the explicit integration of genetic variation will allow for more robust descriptions of how pathogens cause variant disease responses within and across species. These results will increase the likelihood that, while individual genes might not be key regulators of disease across species, there will be commonly identified pathways regulating disease that can be identified in experimental models and transitioned into human systems. In support of this hope, Mitchell [21•] was able to show common transcriptional signatures between human cells and mice following highly pathogenic flu and SARS infections. Similarly, Sims [23] found conserved signals between immortalized Calu3 cells and primary airway epithelial cultures. Furthermore, systems based approaches studying influenza vaccine responses within humans were able to identify the CaMKIV kinase pathway as critical for these responses, and this molecule was validated in murine knockout systems [57]. The further advancement and refinement of such approaches in experimental systems, combined with state-of-the-art experimental approaches such as gene editing [60], as well as molecular profiling and disease data gathered from human cohorts [61], hold keys for transitioning bench-top findings to clinical results. Given the expanding nature of viral emergences, due to increased connectivity and ease of travel, the continuing refinement and further development of systems approaches combined with the advanced methodological approaches being developed should provide novel avenues with which to quickly address the added complexity of host genetic variation in combatting emerging pathogens.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We acknowledge SP and RG for assistance with figures. The authors were supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases under award number U19AI100625. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Peiris J.S., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li W., Wong S.K., Li F., Kuhn J.H., Huang I.C., Choe H., Farzan M. Animal origins of the severe acute respiratory syndrome coronavirus: insight from ACE2-S-protein interactions. J Virol. 2006;80:4211–4219. doi: 10.1128/JVI.80.9.4211-4219.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau S.K., Woo P.C., Li K.S., Huang Y., Tsoi H.W., Wong B.H., Wong S.S., Leung S.Y., Chan K.H., Yuen K.Y. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci USA. 2005;102:14040–14045. doi: 10.1073/pnas.0506735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L., Luo S.W., Li P.H., Zhang L.J., Guan Y.J. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 5.Haagmans B.L., Al Dhahiry S.H., Reusken C.B., Raj V.S., Galiano M., Myers R., Godeke G.J., Jonges M., Farag E., Diab A. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14:140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Memish Z.A., Mishra N., Olival K.J., Fagbo S.F., Kapoor V., Epstein J.H., Alhakeem R., Durosinloun A., Al Asmari M., Islam A. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19:1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peiris J.S. Severe Acute Respiratory Syndrome (SARS) J Clin Virol. 2003;28:245–247. doi: 10.1016/j.jcv.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Memish Z.A., Zumla A.I., Al-Hakeem R.F., Al-Rabeeah A.A., Stephens G.M. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 9.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A., Flemban H., Al-Nassir W.N., Balkhy H.H., Al-Hakeem R.F. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A., Alabdullatif Z.N., Assad M., Almulhim A., Makhdoom H. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]; By isolating novel Coronaviruses from wild bats, and showing that these viruses (a) use the same ACE2 receptor as SARS-CoV and (b) can specifically utilize the human ACE2, this paper highlights the need for vigilance and the development of methodologies to quickly respond to novel disease outbreaks.
- 12.Cherry J.D. The chronology of the 2002–2003 SARS mini pandemic. Paediatr Respir Rev. 2004;5:262–269. doi: 10.1016/j.prrv.2004.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong N.S., Wong G.W. Epidemiology of severe acute respiratory syndrome (SARS): adults and children. Paediatr Respir Rev. 2004;5:270–274. doi: 10.1016/j.prrv.2004.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipkin W.I. The changing face of pathogen discovery and surveillance. Nat Rev Microbiol. 2013;11:133–141. doi: 10.1038/nrmicro2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aderem A., Adkins J.N., Ansong C., Galagan J., Kaiser S., Korth M.J., Law G.L., McDermott J.G., Proll S.C., Rosenberger C. A systems biology approach to infectious disease research: innovating the pathogen–host research paradigm. MBio. 2011;2:e00325–e00410. doi: 10.1128/mBio.00325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Threadgill D.W., Miller D.R., Churchill G.A., de Villena F.P. The collaborative cross: a recombinant inbred mouse population for the systems genetic era. ILAR J. 2011;52:24–31. doi: 10.1093/ilar.52.1.24. [DOI] [PubMed] [Google Scholar]
- 17.Law G.L., Korth M.J., Benecke A.G., Katze M.G. Systems virology: host-directed approaches to viral pathogenesis and drug targeting. Nat Rev Microbiol. 2013;11:455–466. doi: 10.1038/nrmicro3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott J.E., Shankaran H., Eisfeld A.J., Belisle S.E., Neuman G., Li C., McWeeney S., Sabourin C., Kawaoka Y., Katze M.G., Waters K.M. Conserved host response to highly pathogenic avian influenza virus infection in human cell culture, mouse and macaque model systems. BMC Syst Biol. 2011;5 doi: 10.1186/1752-0509-5-190. 190-0509-5-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbs D.L., Gralinski L., Baric R.S., McWeeney S.K. Multi-omic network signatures of disease. Front Genet. 2014;4:309. doi: 10.3389/fgene.2013.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Gralinski L.E., Bankhead A., 3rd, Jeng S., Menachery V.D., Proll S., Belisle S.E., Matzke M., Webb-Robertson B.J., Luna M.L., Shukla A.K. Mechanisms of severe acute respiratory syndrome coronavirus-induced acute lung injury. MBio. 2013;4 doi: 10.1128/mBio.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; By utilizing de novo network assembly approaches, and a series of escalating doses of SARS-CoV, the authors were able to identify and validate the role of serpine1 and the urokinase pathway as protective in SARS-CoV infection. This study highlighted the ability of systems biology approaches that can be used not only in vitro, but also in dissecting in vivo Coronavirus pathogenesis responses.
- 21•.Mitchell H.D., Eisfeld A.J., Sims A.C., McDermott J.E., Matzke M.M., Webb-Robertson B.J., Tilton S.C., Tchitchek N., Josset L., Li C. A network integration approach to predict conserved regulators related to pathogenicity of influenza and SARS-CoV respiratory viruses. PLoS ONE. 2013;8:e69374. doi: 10.1371/journal.pone.0069374. [DOI] [PMC free article] [PubMed] [Google Scholar]; By explicitly integrating multiple pathogens and multiple pathogen strains in this analysis, the authors were able to identify sets of transcripts and key regulators that acted in virus-specific and pan-virus ways. Furthermore, they were able to show that these approaches could be used to predict responses derived from in vitro systems into ex vivo primary human airway cultures.
- 22.Diercks A., Aderem A. Systems approaches to dissecting immunity. Curr Top Microbiol Immunol. 2013;363:1–19. doi: 10.1007/82_2012_246. [DOI] [PubMed] [Google Scholar]
- 23.Sims A.C., Tilton S.C., Menachery V.D., Gralinski L.E., Schafer A., Matzke M.M., Webb-Robertson B.J., Chang J., Luna M.L., Long C.E. Release of severe acute respiratory syndrome coronavirus nuclear import block enhances host transcription in human lung cells. J Virol. 2013;87:3885–3902. doi: 10.1128/JVI.02520-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeDiego M.L., Nieto-Torres J.L., Jimenez-Guardeno J.M., Regla-Nava J.A., Alvarez E., Oliveros J.C., Zhao J., Fett C., Perlman S., Enjuanes L. Severe acute respiratory syndrome coronavirus envelope protein regulates cell stress response and apoptosis. PLoS Pathog. 2011;7:1002315. doi: 10.1371/journal.ppat.1002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danesh A., Cameron C.M., Leon A.J., Ran L., Xu L., Fang Y., Kelvin A.A., Rowe T., Chen H., Guan Y. Early gene expression events in ferrets in response to SARS coronavirus infection versus direct interferon-alpha2b stimulation. Virology. 2011;409:102–112. doi: 10.1016/j.virol.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cameron M.J., Ran L., Xu L., Danesh A., Bermejo-Martin J.F., Cameron C.M., Muller M.P., Gold W.L., Richardson S.E., Poutanen S.M. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol. 2007;81:8692–8706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao J., Zhao J., Legge K., Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao K., Wang H., Wu C. The immune responses of HLA-A*0201 restricted SARS-CoV S peptide-specific CD8(+) T cells are augmented in varying degrees by CpG ODN, PolyI:C and R848. Vaccine. 2011;29:6670–6678. doi: 10.1016/j.vaccine.2011.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S.F., Chen K.H., Chen M., Li W.Y., Chen Y.J., Tsao C.H., Yen M.Y., Huang J.C., Chen Y.M. Human-leukocyte antigen class I Cw 1502 and class II DR 0301 genotypes are associated with resistance to severe acute respiratory syndrome (SARS) infection. Viral Immunol. 2011;24:421–426. doi: 10.1089/vim.2011.0024. [DOI] [PubMed] [Google Scholar]
- 30.Chan K.Y., Ching J.C., Xu M.S., Cheung A.N., Yip S.P., Yam L.Y., Lai S.T., Chu C.M., Wong A.T., Song Y.Q. Association of ICAM3 genetic variant with severe acute respiratory syndrome. J Infect Dis. 2007;196:271–280. doi: 10.1086/518892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan K.Y., Xu M.S., Ching J.C., Chan V.S., Ip Y.C., Yam L., Chu C.M., Lai S.T., So K.M., Wong T.Y. Association of a single nucleotide polymorphism in the CD209 (DC-SIGN) promoter with SARS severity. Hong Kong Med J. 2010;16:37–42. [PubMed] [Google Scholar]
- 32.Ferris M.T., Heise M.T. Quantitative genetics in the study of virus-induced disease. Adv Virus Res. 2014;88:193–225. doi: 10.1016/B978-0-12-800098-4.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y., Paxton W.A., Wolinsky S.M., Neumann A.U., Zhang L., He T., Kang S., Ceradini D., Jin Z., Yazdanbakhsh K. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 34.Imbert-Marcille B.M., Barbe L., Dupe M., Le Moullac-Vaidye B., Besse B., Peltier C., Ruvoen-Clouet N., Le Pendu J. A FUT2 gene common polymorphism determines resistance to rotavirus A of the P[8] genotype. J Infect Dis. 2014;209:1227–1230. doi: 10.1093/infdis/jit655. [DOI] [PubMed] [Google Scholar]
- 35.Lindesmith L., Moe C., Marionneau S., Ruvoen N., Jiang X., Lindblad L., Stewart P., LePendu J., Baric R. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 36.Nozawa Y., Umemura T., Joshita S., Katsuyama Y., Shibata S., Kimura T., Morita S., Komatsu M., Matsumoto A., Tanaka E., Ota M. KIR, HLA, and IL28B variant predict response to antiviral therapy in genotype 1 chronic hepatitis C patients in Japan. PLoS ONE. 2013;8:e83381. doi: 10.1371/journal.pone.0083381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou L., Zhao H. A review of post-GWAS prioritization approaches. Front Genet. 2013;4:280. doi: 10.3389/fgene.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Threadgill D.W., Churchill G.A. Ten years of the collaborative cross. G3 (Bethesda) 2012;2:153–156. doi: 10.1534/g3.111.001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blair R.H., Kliebenstein D.J., Churchill G.A. What can causal networks tell us about metabolic pathways? PLoS Comput Biol. 2012;8:e1002458. doi: 10.1371/journal.pcbi.1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts A., Deming D., Paddock C.D., Cheng A., Yount B., Vogel L., Herman B.D., Sheahan T., Heise M., Genrich G.L. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3:e5. doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts A., Lamirande E.W., Vogel L., Jackson J.P., Paddock C.D., Guarner J., Zaki S.R., Sheahan T., Baric R., Subbarao K. Animal models and vaccines for SARS-CoV infection. Virus Res. 2008;133:20–32. doi: 10.1016/j.virusres.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collaborative Cross Consortium The genome architecture of the Collaborative Cross mouse genetic reference, population. Genetics. 2012;190:389–401. doi: 10.1534/genetics.111.132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svenson K.L., Gatti D.M., Valdar W., Welsh C.E., Cheng R., Chesler E.J., Palmer A.A., McMillan L., Churchill G.A. High-resolution genetic mapping using the mouse diversity outbred population. Genetics. 2012;190:437–447. doi: 10.1534/genetics.111.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferris M.T., Aylor D.L., Bottomly D., Whitmore A.C., Aicher L.D., Bell T.A., Bradel-Tretheway B., Bryan J.T., Buus R.J., Gralinski L.E. Modeling host genetic regulation of influenza pathogenesis in the collaborative cross. PLoS Pathog. 2013;9:e196–e1003. doi: 10.1371/journal.ppat.1003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boon A.C., deBeauchamp J., Hollmann A., Luke J., Kotb M., Rowe S., Finkelstein D., Neale G., Lu L., Williams R.W., Webby R.J. Host genetic variation affects resistance to infection with a highly pathogenic H5N1 influenza A virus in mice. J Virol. 2009;83:10417–10426. doi: 10.1128/JVI.00514-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bottomly D., Ferris M.T., Aicher L.D., Rosenzweig E., Whitmore A., Aylor D.L., Haagmans B.L., Gralinski L.E., Bradel-Tretheway B.G., Bryan J.T. Expression quantitative trait loci for extreme host response to Influenza A in pre-collaborative cross mice. Genes Genomes Genet. 2012;2:213–221. doi: 10.1534/g3.111.001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47••.Nedelko T., Kollmus H., Klawonn F., Spijker S., Lu L., Hessmann M., Alberts R., Williams R.W., Schughart K. Distinct gene loci control the host response to influenza H1N1 virus infection in a time-dependent manner. BMC Genomics. 2012;13:411. doi: 10.1186/1471-2164-13-411. [DOI] [PMC free article] [PubMed] [Google Scholar]; Utilizing the genetically diverse, recombinant inbred BxD panel of mice, the authors were able to show that host responses to influenza A virus were under the control of multiple polymorphisms. Importantly for both systems genetics approaches, and the study of host polymorphisms in human populations, many of these polymorphisms acted at specific times post-infection.
- 48.Podshivalova K., Salomon D.R. MicroRNA regulation of T-lymphocyte immunity: modulation of molecular networks responsible for T-cell activation, differentiation, and development. Crit Rev Immunol. 2013;33:435–476. doi: 10.1615/critrevimmunol.2013006858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z., Chao T.C., Chang K.Y., Lin N., Patil V.S., Shimizu C., Head S.R., Burns J.C., Rana T.M. The long noncoding RNA THRIL regulates TNFalpha expression through its interaction with hnRNPL. Proc Natl Acad Sci USA. 2014;111:1002–1007. doi: 10.1073/pnas.1313768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swaminathan G., Navas-Martin S., Martin-Garcia J. MicroRNAs and HIV-1 Infection: antiviral activities and beyond. J Mol Biol. 2014;426:1178–1197. doi: 10.1016/j.jmb.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 51.Peng X., Gralinski L., Armour C.D., Ferris M.T., Thomas M.J., Proll S., Bradel-Tretheway B.G., Korth M.J., Castle J.C., Biery M.C. Unique signatures of long noncoding RNA expression in response to virus infection and altered innate immune signaling. MBio. 2010;1:6–10. doi: 10.1128/mBio.00206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng X., Gralinski L., Ferris M.T., Frieman M.B., Thomas M.J., Proll S., Korth M.J., Tisoncik J.R., Heise M., Luo S. Integrative deep sequencing of the mouse lung transcriptome reveals differential expression of diverse classes of small RNAs in response to respiratory virus infection. MBio. 2011;2:10. doi: 10.1128/mBio.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou A., Li S., Wu J., Khan F.A., Zhang S. Interplay between microRNAs and host pathogen recognition receptors (PRRs) signaling pathways in response to viral infection. Virus Res. 2014;184C:1–6. doi: 10.1016/j.virusres.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 54.Cheong J.Y., Shin H.D., Kim Y.J., Cho S.W. Association of polymorphism in MicroRNA 219-1 with clearance of hepatitis B virus infection. J Med Virol. 2013;85:808–814. doi: 10.1002/jmv.23551. [DOI] [PubMed] [Google Scholar]
- 55••.Josset L., Menachery V.D., Gralinski L.E., Agnihothram S., Sova P., Carter V.S., Yount B.L., Graham R.L., Baric R.S., Katze M.G. Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. MBio. 2013;4 doi: 10.1128/mBio.00165-13. e00165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors utilize a systems biology approach and contrasting cell culture infections of SARS-CoV and MERS-CoV to identify critical networks controlling these infections. Most importantly, they were able to identify kinase inhibitors which attenuated growth of both viruses, highlighting the ability to transition systems approaches to therapeutics.
- 56.Josset L., Zeng H., Kelly S.M., Tumpey T.M., Katze M.G. Transcriptomic characterization of the novel avian-origin Influenza A (H7N9) virus: specific host response and responses intermediate between Avian (H5N1 and H7N7) and human (H3N2) viruses and implications for treatment options. MBio. 2014;5 doi: 10.1128/mBio.01102-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakaya H.I., Wrammert J., Lee E.K., Racioppi L., Marie-Kunze S., Haining W.N., Means A.R., Kasturi S.P., Khan N., Li G.M. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Everitt A.R., Clare S., Pertel T., John S.P., Wash R.S., Smith S.E., Chin C.R., Feeley E.M., Sims J.S., Adams D.J. IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 2012;484:519–523. doi: 10.1038/nature10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seok J., Warren H.S., Cuenca A.G., Mindrinos M.N., Baker H.V., Xu W., Richards D.R., McDonald-Smith G.P., Gao H., Hennessy L. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siggs O.M. Dissecting mammalian immunity through mutation. Immunol Cell Biol. 2014 doi: 10.1038/icb.2014.8. Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaas A.K., Chen M., Varkey J., Veldman T., Hero A.O., 3rd, Lucas J., Huang Y., Turner R., Gilbert A., Lambkin-Williams R. Gene expression signatures diagnose influenza and other symptomatic respiratory viral infections in humans. Cell Host Microbe. 2009;6:207–217. doi: 10.1016/j.chom.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


