Abstract
Background
Negative symptoms represent an unmet therapeutic need in many patients with schizophrenia. In an extension to our previous voxel-based morphometry findings, we employed a more specific, vertex-based approach to explore cortical thinning in relation to persistent negative symptoms (PNS) in non-affective first-episode of psychosis (FEP) patients to advance our understanding of the pathophysiology of primary negative symptoms.
Methods
This study included 62 non-affective FEP patients and 60 non-clinical controls; 16 patients were identified with PNS (i.e., at least 1 primary negative symptom at moderate or greater severity sustained for at least 6 consecutive months). Using cortical thickness analyses, we explored for differences between PNS and non-PNS patients as well as between each patient group and healthy controls; cut-off threshold was set at p<0.01, corrected for multiple comparisons.
Results
A thinner cortex prominently in the right superior temporal gyrus extending into the temporo-parietal junction (TPJ), right parahippocampal gyrus, and left orbital frontal gyrus was identified in PNS patients vs. non-PNS patients. Compared with healthy controls, PNS patients showed a thinner cortex prominently in the right superior temporal gyrus, right parahippocampal gyrus, and right cingulate; non-PNS patients showed a thinner cortex prominently in the parahippocampal gyrus bi-laterally.
Conclusion
Cortical thinning in the early stages of non-affective psychosis is present in the frontal and temporo-parietal regions in patients with PNS. With these brain regions strongly related to social cognitive functioning, our finding suggests a potential link between primary negative symptoms and social cognitive deficits through common brain etiologies.
Introduction
Cortical thinning in fronto-temporal regions has become a well-documented finding in schizophrenia [1], [2]. However, with the pivotal confounds associated with illness chronicity more recent studies have turned to exploring for morphological abnormalities in first-episode of psychosis (FEP) samples [3]–[5]. Although studies have shown cortical thinning related to symptomology during these early stages of illness, the relationship with negative symptoms remains vague with some studies identifying an association [6]–[8] and others not [3], [9]. The ambiguity of these findings may be due to the fact that not all studies explicitly explored primary negative symptoms.
Primary and enduring negative symptoms are symptoms intrinsic to schizophrenia [10] that are readily studied in people with either deficit syndrome (DS) or persistent negative symptoms (PNS). For DS, 2 out of 6 items on the Schedule for the Deficit Syndrome (SDS) [11] need to be present for a minimum of 12 months and can only be measured using the SDS [10], [12]. In contrast, for PNS, only 1 item of the 6 on the needs to be present for a minimum of 6 months; symptoms that can be measured using ratings scales other than the SDS (e.g., SANS or PANSS). Furthermore, PNS can easily characterized in FEP samples, removing such potential confounds such as illness or medication chronicity. These key characteristics have lead to a recent increase in studies exploring primary negative symptoms in PNS [10], [12]–[18].
As an extension to our VBM study exploring PNS in a FEP sample [15], we wanted to see if cortical thickness analyses would identify the same regions of interest (right frontal medial-orbital and right parahippocampal gyri). Moreover, since cortical thickness has not been used to examine PNS in FEP patients, we set out to determine if other regions of interest could be identified using this more precise technique [19]. At the methodological level, VBM analyses capture the volume of structures by the totality of voxels it encompasses or by examining gray matter density; in contrast, cortical thickness analyses examine MRIs at a subvoxel level to provide a direct measurement in millimeters of gray matter morphology, an anatomically more meaningful measure reflecting cortical laminar structure and integrity. With VBM and cortical thickness becoming easily accessible imaging techniques, comparability of results between the two methods is a topic of great interest [19]–[22] and has been cited as a necessary step when investigating the pathophysiology of disorders such as schizophrenia [23].
Materials and Methods
2.1 Participants & Treatment Setting
All patients were recruited and treated through the Prevention and Early Intervention Program for Psychoses (PEPP-Montreal), a specialized early intervention service at the Douglas Mental Health University Institute serving a local catchment area in Montreal, Canada. People aged 14 to 35 years experiencing an affective or non-affective first-episode of psychosis who had not previously taken antipsychotic medication for more than one month with an IQ higher than 70 were consecutively admitted to the program as either in- or out-patients. For complete program details see [24] or http://www.douglas.qc.ca/pages/view?section_id=165&locale=en. Only those with a non-affective diagnosis who were over the age of 18 years were included in the analysis.
Patients were identified as having ‘persistent negative symptoms’ if they had a global rating of moderate (value of 3) or more on at least one negative symptom (affective flattening, alogia, avolition-apathy, or anhedonia-asociality) as measured with the Scale for the Assessment of Negative Symptoms (SANS) [25]. Of note, if a global score of 3 or more was given on affective flattening and alogia entirely as a result of inappropriate affect and poverty of content of speech, respectively, these symptoms were not used in classifying PNS. Next, to ensure PNS were indeed primary negative symptoms, PNS patients had to have a global rating of mild (value of 2) or less on all positive symptoms as measured with the Scale for the Assessment of Positive Symptoms (SAPS) [26], a total score of 4 or less on the Calgary Depression Scale for Schizophrenia (CDSS) [27], and extrapyramidal symptoms that were absent or too mild to require treatment with anticholinergic medications. Finally, all scores had to be maintained for a period of at least 6 consecutive months (between month 6 and 12 after admission, in our case). See Hovington et al [12] for further details regarding the adapted criteria used for identifying PNS.
In all, 62 non-affective FEP patients were subsequently separated into two groups: PNS (n = 16, 25.8%) and non-PNS (n = 46, 74.2%). Among the 46 non-PNS patients, eight displayed PNS but were excluded from the PNS group because of clinically relevant positive (n = 6) and depressive symptoms (n = 2); none were excluded due to extrapyramidal symptoms. Diagnoses included: schizophrenia (PNS = 11; non-PNS = 33), schizoaffective disorder (PNS = 4; non-PNS = 7), schizophreniform disorder (non-PNS = 1), and psychosis NOS (PNS = 1; non-PNS = 5) according to the Structured Clinical Interview for DSM-IV [28] confirmed between two senior research psychiatrists (A.M. & R.J.).
Sixty non-clinical controls were recruited through advertisements in local newspapers and were included only if they had no current or past history of 1) any Axis I disorders, 2) any neurological diseases, 3) head trauma causing loss of consciousness, and 4) a first-degree family member suffering from schizophrenia or related schizophrenia spectrum psychosis.
2.2 Ethics Statement
All research was conducted according to the guidelines laid out by the Declaration of Helsinki, and was approved by the Research Ethics Board of the Douglas Mental Health University Institute and the McGill University Faculty of Medicine Research Ethics Board. All participants provided written informed consent prior to engaging in any research-related activity, and were free to withdraw from the study at any time; verbal consent was not considered adequate. Particular to the patients, for the collection and disposition of clinical-based data, if a client was under 18 years of age or deemed incapable to properly represent themselves, written informed consent was obtained from the next of kin, caretaker, or legal guardian. The capacity for individual clients to provide consent was determined by the individual treating team (psychiatrist, case manager, and clinical evaluator) and confirmed by either of the two senior staff psychiatrists (A.K.M. & R.J.). For the collection and disposition of the neuroimaging data, only those aged 18 years and over were recruited from the PEPP clinic, and only after obtaining written informed consent for the collection and disposition of clinical-based data. Finally, after a comprehensive description of the neuroimaging study was provided and the patient displayed a complete understanding, written informed consent was obtained.
2.3 Data Collection
2.3.1 Symptom, Medication, and Socio-demographic Data
The SANS, SAPS, CDSS, and anticholinergic data were obtained at first assessment and at months 1, 2, 3, 6, 9, and 12 after first assessment; first assessment was conducted, on average, within one month after admission (in days; mean = 25.5, s.d. = 9.3, range = 4.8–51.0). Evaluators at PEPP have established an ICC of 0.74 on the SAPS and 0.71 on the SANS; all raters participated in inter-rater reliability sessions at least once a year to avoid rater drift. The type and dosage of antipsychotic taken were also recorded and subsequently converted into chlorpromazine equivalents [29]–[31]. Medication adherence, based on a 5-point scale ranging from 0 (never) to 4 (fully), was obtained from patients or, when possible, from family members; method was validated elsewhere [32]. Additionally, the following data were acquired at first assessment: education level (number of school years completed), Full Scale IQ with the Wechsler Adult Intelligence Scale [33], parental socio-economic status (SES) with the Hollingshead two-factor index [34], and handedness with the Edinburgh Handedness Inventory [35].
2.3.2 MRI Data Acquisition
Scanning was carried out at the Montreal Neurological Institute on a 1.5 T Siemens whole body MRI system. Structural T1 volumes were acquired for each participant using a three-dimensional (3D) gradient echo pulse sequence with sagittal volume excitation (repetition time = 22 ms, echo time = 9.2 ms, flip angle = 30°, 180 1 mm contiguous sagittal slices). The rectangular field-of-view for the images was 256 mm (SI)×204 mm (AP). Patient groups did not differ as to when sessions took place past entry (weeks; PNS mean = 15.9, s.d. = 5.8; non-PNS mean = 19.9, s.d. = 7.8; t = 1.82, df = 60, p = 0.07).
2.4 Statistical Analyses
2.4.1. Measurement of Cortical Thickness
MRIs were submitted to the CIVET processing pipeline (Version 1.1.9) (http://wiki.bic.mni.mcgill.ca/index.php/CIVET) [36], [37]. Native T1-weighted images were first registered to the ICBM152 template using linear transformation [38], [39] and simultaneously corrected for non-uniformity artifacts using N3 [40]. The transformed images were then segmented into grey matter, white matter, cerebral spinal fluid and background using a neural net classifier (INSECT) [37]. Grey matter and white matter surfaces were extracted using CLASP algorithm [41]–[43]. A spherical-mesh deformation algorithm was used to produce a surface mesh of 81 920 polygons (40 962 nodes or vertices) for each hemisphere. Nonlinear registration of both cortical surfaces to a high resolution average surface template generated from the ICBM152 data set was performed to establish inter-subject correspondence of vertices [44], [45]. Reverse linear transformation of volumes was performed to allow vertex-based corticometric (VBC) measurements in native space for each subject’s MRI [46]. The deformation algorithm first fits the white matter surface and then expands to the outer GM and cerebral spinal fluid intersection. From these surfaces, cortical thickness was computed in native space using the t-link method [47], which determines the linked distance between the inner and outer cortical surfaces at each of 40 962 vertices. Each participant’s cortical thickness map was subsequently blurred using a 20-mm full-width at half-maximum surface-based diffusion smoothing kernel [48].
Statistics were performed at all 40 962 vertices using three difference contrasts: PNS vs. non-PNS, PNS vs. Controls, and non-PNS vs. Controls. Total intracranial volume was not included as a covariate as cortical thickness and brain volume are poorly correlated [46], [49]. Statistical maps were thresholded and multiple comparisons were taken in to account using the false discovery rate procedure, with q = 0.05 [50]; results were considered significant at t = 2.64 (p<0.01).
2.4.2 Whole-brain Tissue Volumes
Finally, whole-brain GM, WM, and CSF volumes were estimated using VBM8 (http://dbm.neuro.uni-jena.de/vbm/download/) for each participant and were summed for an estimation of total intracranial volume (TIV); the four volumes were compared among the three groups using an ANOVA (post-hoc Tukey’s HSD test).
2.4.3 Behavioral Analyses
Among the three groups, age at scan, education level, and Full Scale IQ were compared using a one-way ANOVA (post-hoc Tukey’s HSD test), parental SES with a Kruskall-Wallis H-test (post-hoc Mann-Whitney U-test), and sex (male vs. female) and handedness (right vs. other) with cross tabulation and Chi-square tests. Between patient groups, independent t-tests were used to compare antipsychotic dosage and symptom totals and Mann-Whitney U-tests to compare medication adherence at first assessment, month 6, and month 12. CDSS ratings were log-transformed while SAPS ratings and antipsychotic total dosage were square-root transformed to achieve normal distribution; all other variables were normally distributed. All analyses were conducted using PASW Statistics 18 (SPSS Inc., 2009, Chicago, IL, USA) and were two-tailed with a critical p-value of 0.05.
Results
3.1 Socio-demographic and Clinical Characteristics
The groups did not significantly differ in age, parental SES, sex, or handedness. PNS and non-PNS patients had fewer years of education and a lower Full Scale IQ compared to controls; the patient groups did not significantly differ (Table 1). Patient groups did not significantly differ in negative symptoms at first assessment but the PNS patients showed significantly higher totals at month 6 and 12, as expected. The two groups did not significantly differ in positive or depressive symptoms, total antipsychotic dosage (in chlorpromazine equivalents), and medication adherence at any time point (Table 2).
Table 1. Socio-demographic characteristics and whole-brain tissue volumes for PNS patients, non-PNS patients, and controls.
| PNS (n = 16) | non-PNS (n = 46) | Controls (n = 60) | p | |
| Socio-demographic variable | ||||
| Age at scan (years) | 24.2±4.3 | 23.7±3.4 | 24.8±3.3 | 0.285 |
| Parental SESa | 3.4±1.0 | 3.4±1.2 | 3.1±1.1 | 0.394 |
| Education levelb | 11.2±2.0 | 12.1±2.6 | 14.4±2.5 | <0.001 |
| Full Scale IQc | 97.6±18.2 | 95.5±12.2 | 107.9±14.9 | <0.001 |
| Handed, Right/Other | 12/4 | 40/6 | 55/5 | 0.193 |
| Sex, Male/Female | 13/3 | 32/14 | 40/20 | 0.529 |
| Whole-brain tissue volumes (ml) | ||||
| Grey matter | 624±56 | 643±60 | 658±71 | 0.161 |
| White matter | 605±65 | 596±64 | 618±71 | 0.264 |
| Cerebral-spinal fluid | 201±27 | 197±27 | 203±35 | 0.656 |
| Total intracranial | 1430±127 | 1437±121 | 1479±151 | 0.220 |
Abbreviations: PNS, persistent negative symptoms.
Hollingshead parental socioeconomic status: 1 = highest and 5 = lowest.
Education level measured as number of years completed; post-hoc tests revealed: PNS = non-PNS (p = 0.285); PNS < controls (p<0.001); non-PNS < controls (p<0.001).
Full Scale IQ measured with the WAIS-III (data were available for only 58 controls); post-hoc tests revealed: PNS = non-PNS (p = 0.870); PNS < controls (p = 0.034); non-PNS < controls (p<0.001).
Table 2. Clinical characteristics for PNS patients and non-PNS patients.
| PNS (n = 16) | non-PNS (n = 46) | p | |
| Negative symptom total (SANS) | |||
| First Assessment | 31.2±13.6 | 27.4±12.5 | 0.308 |
| Month 6 | 30.1±11.3 | 16.6±11.7 | <0.001 |
| Month 12 | 30.3±14.7 | 14.9±10.0 | <0.001 |
| Positive symptom total (SAPS) | |||
| First Assessment | 34.5±10.7 | 35.0±17.9 | 0.909 |
| Month 6 | 10.9±9.2 | 9.7±11.9 | 0.710 |
| Month 12 | 14.7±14.2 | 10.5±17.9 | 0.399 |
| Depressive symptom total (CDSS) | |||
| First Assessment | 4.1±4.3 | 4.9±5.2 | 0.552 |
| Month 6 | 3.4±3.7 | 1.8±3.3 | 0.105 |
| Month 12 | 1.9±2.5 | 1.9±3.2 | 0.920 |
| Antipsychotic dosage (mg/day) a | |||
| First Assessment | 151.5±116.1 | 170.7±161.8 | 0.665 |
| Month 6 | 178.4±164.9 | 198.7±199.6 | 0.717 |
| Month 12 | 104.7±65.7 | 206.4±253.4 | 0.119 |
| Medication adherence b | |||
| First Assessment | 3.3±1.5 | 3.2±1.5 | 0.591 |
| Month 6 | 3.1±1.2 | 3.0±1.4 | 0.951 |
| Month 12 | 2.3±1.9 | 3.2±1.5 | 0.126 |
Abbreviations: PNS, persistent negative symptoms; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; CDSS, Calgary Depression Scale for Schizophrenia.
Antipsychotic totals presented in chlorpromazine equivalents.
Medication adherence: 0 (never adherent) to 4 (fully adherent).
3.2 Cortical Thickness
3.2.1 PNS patients vs. non-PNS patients
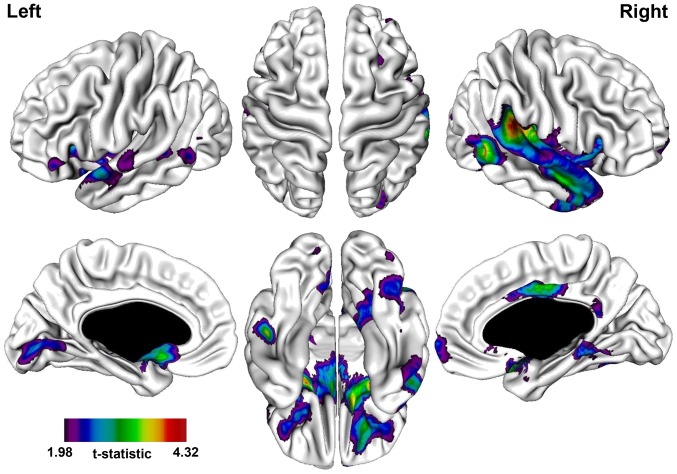
A significantly thinner cortex in the PNS patients was observed in the following regions: bilateral frontal, temporal, fusiform, and occipital gyri, right parahippocampal gyrus, bi-lateral anterior cingulate, and right middle and posterior cingulate (Table 3; Figure 1). The most prominent difference was observed in the right superior temporal gyrus extending into the temporo-parietal junction (near the angular gyrus).
Table 3. Areas of cortical thinning in PNS patients compared to non-PNS patients.
| Region (Brodmann Area) | Coordinates in MNI space | |||
| x | y | z | t-value | |
| Right hemisphere | ||||
| Medial frontal gyrus (10) | 6 | 66 | −4 | 2.49 |
| Orbital frontal gyrus (47) | 20 | 32 | −23 | 3.25 |
| Anterior cingulate (24) | 3 | 30 | −7 | 2.13 |
| Parahippocampal gyrus (34) | 26 | 7 | −17 | 4.19 |
| Inferior temporal gyrus (20) | 50 | 0 | −34 | 3.09 |
| Anterior/middle cingulate (24/23) | 3 | −3 | 38 | 3.34 |
| Middle temporal gyrus (21) | 50 | −5 | −20 | 3.30 |
| Middle temporal gyrus (39) | 56 | −55 | 5 | 4.22 |
| Superior temporal gyrus (41) | 41 | −34 | 17 | 4.32 |
| Posterior cingulate (30) | 3 | −47 | 19 | 2.38 |
| Fusiform gyrus (37) | 42 | −65 | −16 | 2.54 |
| Middle occipital gyrus (19) | 30 | −85 | 18 | 2.38 |
| Left hemisphere | ||||
| Inferior frontal gyrus (47) | −54 | 35 | −1 | 2.36 |
| Middle frontal gyrus (11) | −23 | 27 | −17 | 2.69 |
| Subgenual cingulate (25) | −3 | 11 | −10 | 3.39 |
| Inferior frontal gyrus (47) | −19 | 8 | −19 | 3.97 |
| Superior temporal gyrus (22) | −59 | 3 | −7 | 2.89 |
| Fusiform gyrus (20) | −48 | −28 | −25 | 3.74 |
| Middle temporal gyrus (22) | −51 | −41 | 2 | 2.44 |
| Middle temporal gyrus (21) | −56 | −58 | 1 | 2.41 |
| Middle temporal gyrus (39) | −49 | −69 | 11 | 2.07 |
| Cuneus (17) | −7 | −83 | 2 | 2.80 |
| Lingual gyrus (18) | −13 | −88 | −12 | 2.30 |
Abbreviations: PNS, persistent negative symptoms.
Figure 1. t-statistical brain maps showing cortical thinning in patients with persistent negative symptoms compared to patients without persistent negative symptoms.
Most pronounced differences in the right temporo-parietal junction, right superior temporal gyrus, right parahippocampal gyrus, and left inferior frontal gyrus. The colour bar indicates the t-value. All areas shown exceed a FDR corrected statistical threshold of P<0.01.
3.2.2 PNS patients vs. Controls
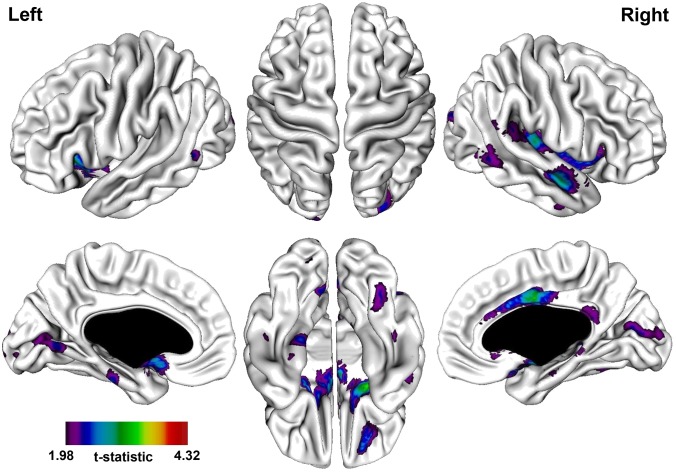
A significantly thinner cortex in the PNS patients was observed in the following regions: bi-lateral frontal, temporal, fusiform, parahippocampal, and occipital gyri, bi-lateral anterior cingulate, and right middle and posterior cingulate (Table 4; Figure 2). The most prominent difference was observed in the right parahippocampal gyrus.
Table 4. Areas of cortical thinning in PNS patients compared to controls.
| Region (Brodmann Area) | Coordinates in MNI space | |||
| x | y | z | t-value | |
| Right hemisphere | ||||
| Middle frontal gyrus (11) | 18 | 48 | −20 | 2.66 |
| Anterior cingulate (32) | 3 | 23 | −8 | 2.25 |
| Parahippocampal gyrus (34) | 22 | 5 | −17 | 3.45 |
| Inferior temporal gyrus (20) | 49 | −3 | −32 | 2.38 |
| Anterior/middle cingulate (24/23) | 2 | −4 | 35 | 3.23 |
| Superior temporal gyrus (21) | 52 | −5 | −15 | 2.98 |
| Parahippocampal gyrus (28) | 24 | −18 | −20 | 2.38 |
| Fusiform gyrus (20) | 43 | −30 | −20 | 2.19 |
| Parahippocampal gyrus (27) | 16 | −36 | −3 | 2.37 |
| Posterior cingulate (30) | 3 | −45 | 22 | 2.34 |
| Middle temporal gyrus (21) | 58 | −56 | 1 | 2.48 |
| Fusiform gyrus (37) | 37 | −58 | −16 | 2.36 |
| Middle temporal gyrus (37) | 45 | −63 | −2 | 2.64 |
| Cuneus (18) | 5 | −78 | 12 | 2.41 |
| Cuneus (19) | 26 | −91 | 21 | 2.47 |
| Left hemisphere | ||||
| Anterior cingulate (25) | −4 | 10 | −10 | 2.79 |
| Inferior frontal gyrus (47) | −18 | 9 | −19 | 2.79 |
| Parahippocampal gyrus (36) | −29 | −13 | −30 | 2.25 |
| Parahippocampal gyrus (35) | −22 | −24 | −26 | 2.52 |
| Fusiform gyrus (20) | −48 | −28 | −26 | 2.11 |
| Middle temporal gyrus (21) | −55 | −56 | 2 | 2.39 |
| Cuneus (30) | −6 | −66 | 4 | 2.42 |
| Lingual gyrus (18) | −15 | −84 | −15 | 2.01 |
| Middle occipital gyrus (18) | −16 | −102 | 11 | 2.51 |
Abbreviations: PNS, persistent negative symptoms.
Figure 2. t-statistical brain maps showing cortical thinning in patients with persistent negative symptoms compared to healthy controls.
Most pronounced differences in the right temporal gyrus, right parahippocampal gyrus, and right anterior/middle cingulate. The colour bar indicates the t-value. All areas shown exceed a FDR corrected statistical threshold of P<0.01.
3.2.3 non-PNS patients vs. Controls
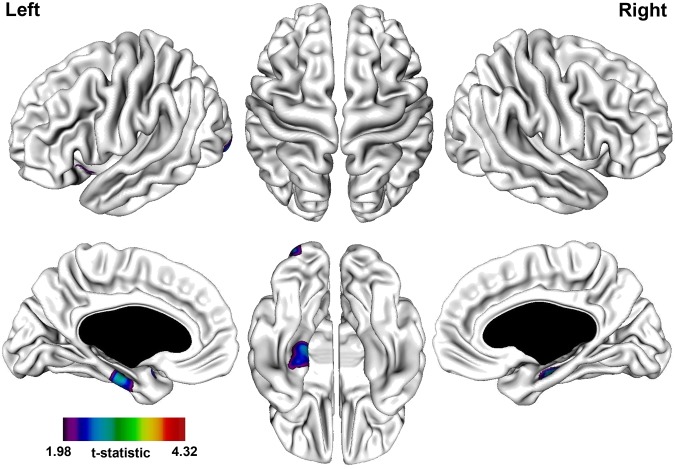
A significantly thinner cortex in the non-PNS patients was observed in the following regions: bi-lateral parahippocampal gyrus, left superior temporal gyrus, and left inferior occipital gyrus (Table 5; Figure 3). The most prominent differences were observed in the bi-lateral parahippocampal gyrus.
Table 5. Areas of cortical thinning in non-PNS patients compared to controls.
| Region (Brodmann Area) | Coordinates in MNI space | |||
| x | y | z | t-value | |
| Right hemisphere | ||||
| Parahippocampal gyrus (34) | 30 | −19 | −19 | 2.92 |
| Left hemisphere | ||||
| Superior temporal gyrus (38) | −34 | 7 | −21 | 2.64 |
| Parahippocampal gyrus (28) | −19 | −19 | −23 | 2.92 |
| Inferior occipital gyrus (18) | −28 | −96 | −13 | 2.72 |
Abbreviations: PNS, persistent negative symptoms.
Figure 3. t-statistical brain maps showing cortical thinning in patients without persistent negative symptoms compared to healthy controls.
Most pronounced difference in the parahippocampal gyrus bi-laterally. The colour bar indicates the t-value. All areas shown exceed a FDR corrected statistical threshold of P<0.01.
Discussion
The present study used cortical thickness - a more precise method that directly measures gray matter morphology in millimeters reflecting cortical laminar structure and integrity - to explore the neural correlates of persistent negative symptoms (PNS) in non-affective first-episode of psychosis (FEP) patients using a well-established criteria for PNS.
We found a thinner cortex (less grey matter) in the right medial-orbital gyrus and right parahippocampal gyrus in the PNS patients compared to non-PNS patients, supporting of our previous VBM findings [15]. However, we were also able to identify cortical thinning in the PNS patients in additional frontal (cingulate cortex bilaterally) and temporal (temporal gyrus and fusiform gyrus bilaterally) regions, with the largest area of thinning extending into the temporo-parietal junction (TPJ). As well, when compared to controls, PNS patients showed greater cortical thinning overall, more notably in the anterior cingulate bilaterally, temporal gyrus bilaterally, and left parahippocampal gyrus. Given these findings, it is clear that the neural correlates of PNS involve multiple cortical and subcortical regions.
4.1 Negative Symptoms and the Temporal Lobe
4.1.1 Superior Temporal Gyrus and Temporo-parietal Junction
To date, this is the first study, to the best of our knowledge, to identify a thinner cortex in the superior temporal gyrus (STG) extending into the temporo-parietal junction (TPJ) in FEP patients with PNS.
Previous imaging studies have identified significantly less grey matter in the STG in relation to primary and enduring negative symptoms [6], [8], [51]–[53]. However, other studies [54], [55] including our previous VBM analysis [15] did not demonstrate this relationship. Moreover, other studies have shown a correlation with positive symptoms [56], [57], supporting its significant role in auditory and language processing [58], [59]. The involvement of the STG as related to PNS appears somewhat unclear [60]. Alternatively, cortical thinning in the STG extending into the TPJ could be related to the social cognitive deficits that define non-affective psychoses, such as schizophrenia [61].
People with PNS are defined by a lack of social skills from not smiling (flat affect) to not talking (poverty of speech) with many withdrawing from society (asociality) or choosing not to engage in everyday activities (avolition). These “missing” social skills are innate to their presentation of psychosis. Our strongest finding was in the right TPJ and this region has received increasing attention concerning its role in social cognition, empathy, and social salience. The TPJ has been associated with various social cognitive processes [62]–[65] with the right TPJ specifically related to the attribution of thoughts in others compared to the attribution of appearance or bodily-sensations about a person [66], [67]. The TPJ has also been implicated in identification and reorientation towards salient events in the sensory environment [68], [69]. However, one study demonstrated that TPJ activation is limited to choice deliberation about the nature of the upcoming decision in a social context rather than external social stimuli itself [70]. Nevertheless, the TPJ is believed to play a critical role in coordinating behavior in a dynamic, social environment. We as humans must be able to make adaptive socially-correct decisions in a social context and the TPJ is central to this ability [61], [70].
Of course, the TPJ has been generally associated with the positive symptoms of schizophrenia based on the plethora of studies that either evoke [71], [72] or disrupt [73]–[76] activation of the TPJ leading to the induction or alleviation of auditory-related symptoms, respectively. However, there is a new direction that suggests schizophrenia as a social communication disorder with the TPJ as a central structure of interest [61]. Our findings support this idea if we equate primary negative symptoms to a diminished social cognitive ability (flat affect, poverty of speech, asociality, or avolition). Needless to say, we know that structure size does not correlate with function, but a lack of grey matter in the STG and TPJ could help us better understand the relationship among negative symptoms, socializing, and psychosis. Moreover, it could point to a region of interest in developing new treatments for this with primary negative symptoms.
4.1.2 Parahippocampus
Reduced grey matter in the parahippocampus has been identified as a consistent finding in schizophrenia [77]–[79] with these reductions associated with negative symptom severity [51], [52], [80], [81]. However, these associations have been left-lateralized [52] or bilateral [51], [80], [81]. In contrast, our analyses examining PNS patients vs. non-PNS patients identified a thinner cortex as well as a reduced volume specific to the right side [15]. Yet, PNS patients showed a thinner cortex bilaterally compared to controls. As such, the association of negative symptoms with laterality is unclear or perhaps associations exist related to specific negative symptoms. For example, our group identified in FEP patients a significant correlation between higher social withdrawal ratings and reduced parahippocampal grey matter consistently on the right side [80], [81] whereas, in people with chronic schizophrenia, we revealed a significant positive correlation between flat affect ratings and parahippocampal activity bilaterally [82]. Although these studies did not explicitly investigate primary negative symptoms, a neurobiological association may exist with specific negative symptoms that was not explored in the abovementioned studies. Further investigations are needed to explore the neurobiological basis of individual negative symptoms.
4.2 Cortical Thickness Vs. Voxel-Based Morphometry (VBM)
Recent studies have emerged using both cortical thickness and VBM to study various populations including healthy-aging controls [19], Alzheimer’s [20], late-life depression [21], and schizophrenia [23]. In the study examining late-life depression, results revealed cortical thickness was more sensitive in detecting group differences than VBM [21]. Similarly, in the healthy-aging sample, the authors revealed both methods yield similar results but with cortical thickness more sensitive to grey matter decline [19]. Hutton et al elaborated on this by mentioning “[cortical thickness] is expected to be more sensitive than [VBM]… if there is a prior hypothesis that grey matter changes are mainly due to changes in cortical thickness and also if there is any correlation between the effect of interest and the total brain volume” [19]. Interestingly, for the Alzheimer’s study comparing posterior cortical atrophy, similar results were found using both techniques [20]. Finally, for the schizophrenia study, Palaniyappan and Liddle concluded, “while VBM may be more sensitive in identifying the regions with gray matter abnormalities, studies investigating the pathophysiology of illnesses such as schizophrenia are better informed when both [cortical thickness] and VBM analyses are performed concurrently” [23]. In fact, Hutton et al drew a similar conclusion stating that both techniques should be used together to better separate and understand the underlying grey matter changes.
From our analyses, the cortical thickness analysis appeared more sensitive than our VBM analysis [15] in detecting group differences regarding negative symptoms in non-affective FEP patients. What could count for these difference considering that the same sample was examined? First, we must reiterate the fact that cortical thickness specifically measures the cortex thickness in millimeters while VBM measures grey matter differences in local surface area and cortical folding [19]. This leads to the possible reasons why VBM fails to detect more grey matter differences related to: (1) the changes in the shape or displacement of structures during spatial normalization [83]–[86] or (2) the variability of gyrification [87] that has been shown to be present in schizophrenia [88]–[92]. Furthermore, the blurring of cortical thickness data takes place in a topologically correct manner along the cortical surface, whereas VBM blurring is 3-dimensional, meaning it does not respect boundaries between tissue classes, leading to an increased likelihood of diluting existing signal or misinterpreting boundary shift as signal [22].
Importantly, we must highlight that our previous VBM analysis used a statistical threshold of p<0.05, family-wise error (FWE) corrected for multiple comparisons [15] while the current cortical thickness analysis used a statistical threshold of p<0.01, false-discovery rate (FDR) corrected. Although FWE is prone to more false negatives and FDR (considered a less stringent correction than FWE) is prone to more false positives [93], studies examining these corrections (using VBM) have shown results to be similar [94], [95]. So, any observed differences in sensitivity between the techniques should not be solely attributed to the correction method employed. In addition, by using a cut-off of p<0.01 in the cortical thickness analysis we reduced the number of possible false positives and, effectively, the number of identifiable regions. Yet, more grey matter differences were still identified using this technique.
Taken together, it would appear that cortical thickness may be more sensitive in detecting grey matter anomalies in schizophrenia or related psychoses compared to VBM. This may help to explain why the cortical thickness analysis was able to detect more differences between all of the contrasts investigated and was able to detect more regions of interest related to primary negative symptoms. But for a more complete understanding of group differences in grey matter both techniques should be used to complement each other.
4.3 Conclusions
Our results along with previous studies investigating primary negative symptoms highlight neural abnormalities in two key regions: the frontal and temporal areas [6], [8], [15], [51], [52], [54] supporting the proposed prefronto-temporolimbic model of negative symptoms [60], [96]. Moreover, in the PNS patients, the largest area of cortical thinning was found in the right superior temporal gyrus extending into the temporo-parietal junction-core structures related to social cognitive functioning [61], [62]–[65]. With both social cognitive deficits and negative symptoms characterizing schizophrenia, this area could be explored further in the development of more effective treatments. In fact, treatments utilizing transcranial magnetic stimulation have targeted both the frontal and temporal areas, but the temporal region has been generally targeted in the hope of alleviating positive symptoms [76]. So, perhaps the temporo-parietal junction could be targeted in the hope of alleviating negative symptoms or even the social cognitive difficulties expressed in people with schizophrenia [61]. Finally, it must be stressed when investigating between-group grey matter differences in disorders like schizophrenia, multiple fully-automated techniques should be employed to provide a better understanding of the results [23].
4.4 Limitations
There are several limitations in our study. First, although we employed a first- episode sample in an attempt to reduce the effect of antipsychotic exposure on brain morphology [97], [98], the majority of patients were still treated with antipsychotics possibly affecting our results. Second, avolition and anhedonia were more prevalent than alogia and blunted affect in our sample and, as such, our results may not be generalizable to all primary negative symptoms. Furthermore, the categorical approach of this study did not allow us to specify which negative symptoms contributed the most to the structural differences identified; future studies need to examine these symptoms separately. Third, at the time of analysis, clinical data was only available for the first 12 months of treatment in our sample as it has been shown that PNS categorization is more consistent after the first year of treatment [17]. Fourth, our PNS patient group was relatively small, limiting the generalization (interpretation) of our results. Additionally, we could not examine whether the structural differences related to PNS were specific to one diagnosis or not because we were limited by the small number of patients with PNS that did not allow for any meaningful diagnosis specific between-group comparisons. Lastly, the non-PNS group were prescribed, on average, almost double the dosage of antipsychotics [in chlorpromazine equivalents (mg/day)] by month 12. Because treatment is determined on an individual basis at our clinic, we cannot provide any particular reason as to why the individuals of the PNS group were prescribed such a lower dosage. This is noteworthy as antipsychotics have been shown to affect brain morphology [97], [99]. However, with scanning completed 18 weeks, on average, after the start of antipsychotic treatment only minimal effects, if any at all, were expected regarding the frontal and temporal regions [100].
Acknowledgments
We thank PEPP-Montreal research staff for their help with recruitment and for conducting the clinical assessments and the M. Lepage staff for acquiring the MRI scans. Finally, we are grateful to all the people who participated in the study.
Funding Statement
The study was supported by operating grants from CIHR (#68961) and the Sackler Foundation to Drs. M. Lepage/A.K Malla; Fonds de la Recherche en Santé du Québec (FRSQ) (salary award to M.L.); Canada Research Chairs Program (to A.K.M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schultz CC, Koch K, Wagner G, Nenadic I, Schachtzabel C, et al. (2012) Reduced anterior cingulate cognitive activation is associated with prefrontal-temporal cortical thinning in schizophrenia. Biol Psychiatry 71: 146–153. [DOI] [PubMed] [Google Scholar]
- 2. Nesvag R, Bergmann O, Rimol LM, Lange EH, Haukvik UK, et al. (2012) A 5-year follow-up study of brain cortical and subcortical abnormalities in a schizophrenia cohort. Schizophr Res 142: 209–216. [DOI] [PubMed] [Google Scholar]
- 3. Crespo-Facorro B, Roiz-Santianez R, Perez-Iglesias R, Rodriguez-Sanchez JM, Mata I, et al. (2011) Global and regional cortical thinning in first-episode psychosis patients: relationships with clinical and cognitive features. Psychol Med 41: 1449–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, et al. (2010) Reduced cortical thickness in first episode schizophrenia. Schizophr Res 116: 204–209. [DOI] [PubMed] [Google Scholar]
- 5. Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, et al. (2005) Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex 15: 708–719. [DOI] [PubMed] [Google Scholar]
- 6. Cascella NG, Fieldstone SC, Rao VA, Pearlson GD, Sawa A, et al. (2010) Gray-matter abnormalities in deficit schizophrenia. Schizophr Res 120: 63–70. [DOI] [PubMed] [Google Scholar]
- 7. Venkatasubramanian G, Jayakumar PN, Gangadhar BN, Keshavan MS (2008) Automated MRI parcellation study of regional volume and thickness of prefrontal cortex (PFC) in antipsychotic-naive schizophrenia. Acta Psychiatr Scand 117: 420–431. [DOI] [PubMed] [Google Scholar]
- 8. Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, et al. (2000) Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry 57: 471–480. [DOI] [PubMed] [Google Scholar]
- 9. Roiz-Santianez R, Perez-Iglesias R, Quintero C, Tordesillas-Gutierrez D, Mata I, et al. (2010) Insular cortex thinning in first episode schizophrenia patients. Psychiatry Res 182: 216–222. [DOI] [PubMed] [Google Scholar]
- 10. Buchanan RW (2007) Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull 33: 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr (1989) The Schedule for the Deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res 30: 119–123. [DOI] [PubMed] [Google Scholar]
- 12. Hovington CL, Bodnar M, Joober R, Malla AK, Lepage M (2012) Identifying persistent negative symptoms in first episode psychosis. BMC Psychiatry 12: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jhamnani K, Shivakumar V, Kalmady S, Rao NP, Venkatasubramanian G (2013) Successful Use of Add-on Minocycline for Treatment of Persistent Negative Symptoms in Schizophrenia. J Neuropsychiatry Clin Neurosci 25: E06–07. [DOI] [PubMed] [Google Scholar]
- 14. Galderisi S, Mucci A, Bitter I, Libiger J, Bucci P, et al. (2013) Persistent negative symptoms in first episode patients with schizophrenia: Results from the European First Episode Schizophrenia Trial. Eur Neuropsychopharmacol 23: 196–204. [DOI] [PubMed] [Google Scholar]
- 15. Benoit A, Bodnar M, Malla AK, Joober R, Lepage M (2012) The structural neural substrates of persistent negative symptoms in first-episode of non-affective psychosis: a voxel-based morphometry study. Front Psychiatry 3: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buchanan RW, Panagides J, Zhao J, Phiri P, den Hollander W, et al. (2012) Asenapine versus olanzapine in people with persistent negative symptoms of schizophrenia. J Clin Psychopharmacol 32: 36–45. [DOI] [PubMed] [Google Scholar]
- 17. Chang WC, Hui CL, Tang JY, Wong GH, Lam MM, et al. (2011) Persistent negative symptoms in first-episode schizophrenia: a prospective three-year follow-up study. Schizophr Res 133: 22–28. [DOI] [PubMed] [Google Scholar]
- 18. Malla AK, Norman RM, Takhar J, Manchanda R, Townsend L, et al. (2004) Can patients at risk for persistent negative symptoms be identified during their first episode of psychosis? J Nerv Ment Dis 192: 455–463. [DOI] [PubMed] [Google Scholar]
- 19. Hutton C, Draganski B, Ashburner J, Weiskopf N (2009) A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage 48: 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lehmann M, Crutch SJ, Ridgway GR, Ridha BH, Barnes J, et al. (2011) Cortical thickness and voxel-based morphometry in posterior cortical atrophy and typical Alzheimer’s disease. Neurobiol Aging 32: 1466–1476. [DOI] [PubMed] [Google Scholar]
- 21. Colloby SJ, Firbank MJ, Vasudev A, Parry SW, Thomas AJ, et al. (2011) Cortical thickness and VBM-DARTEL in late-life depression. J Affect Disord 133: 158–164. [DOI] [PubMed] [Google Scholar]
- 22.Buchy L, Ad-Dab’bagh Y, Malla A, Lepage C, Bodnar M, et al.. (2010) Cortical thickness is associated with poor insight in first-episode psychosis. J Psychiatr Res Epub ahead of print. [DOI] [PubMed]
- 23. Palaniyappan L, Liddle PF (2012) Differential effects of surface area, gyrification and cortical thickness on voxel based morphometric deficits in schizophrenia. Neuroimage 60: 693–699. [DOI] [PubMed] [Google Scholar]
- 24. Malla A, Norman R, McLean T, Scholten D, Townsend L (2003) A Canadian programme for early intervention in non-affective psychotic disorders. Aust N Z J Psychiatry 37: 407–413. [DOI] [PubMed] [Google Scholar]
- 25.Andreasen NC (1984) Modified Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa.
- 26.Andreasen NC (1984) Scale for the Assessment of Positive Symptoms (SAPS). Iowa City: University of Iowa.
- 27.Addington D, Addington J, Maticka-Tyndale E (1993) Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl: 39–44. [PubMed]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW (1998) Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P & SCID-I/NP), Version 2. New York: New York Psychiatric Institute, Biometrics Research.
- 29.Jensen B, Regier LD, editor (2012) Drug Comparison Charts. 9th Edition ed. Saskatoon, Saskatchewan: RxFiles Academic Detailing Program. 118 p. [Google Scholar]
- 30. Woods SW (2011) Chlorpromazine Equivalent Doses for Atypical Antipsychotics: An Update. 2003–2010: 1–8. [DOI] [PubMed] [Google Scholar]
- 31. Woods SW (2003) Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64: 663–667. [DOI] [PubMed] [Google Scholar]
- 32. Cassidy CM, Rabinovitch M, Schmitz N, Joober R, Malla A (2010) A comparison study of multiple measures of adherence to antipsychotic medication in first-episode psychosis. J Clin Psychopharmacol 30: 64–67. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D (1997) Wechsler Adult Intelligence Scale San Antonio, TX: The Psychological Corporation.
- 34.Hollingshead A (1965) Two-Factor Index of Social Position. New Haven, CN: Yale University Press.
- 35. Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- 36.Ad-Dab’bagh Y, Einarson D, Lyttelton O, Muehlboeck J-S, Mok K, et al.. (2006) The CIVET Image-Processing Environment: A Fully Automated Comprehensive Pipeline for Anatomical Neuroimaging Research. The 12th Annual meeting of the Organization for Human Brain Mapping (OHBM). Florence, Italy.
- 37. Zijdenbos AP, Forghani R, Evans AC (2002) Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging 21: 1280–1291. [DOI] [PubMed] [Google Scholar]
- 38. Collins DL, Neelin P, Peters TM, Evans AC (1994) Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- 39. Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, et al. (2006) Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv 9: 58–66. [DOI] [PubMed] [Google Scholar]
- 40. Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17: 87–97. [DOI] [PubMed] [Google Scholar]
- 41. Kabani N, Le Goualher G, MacDonald D, Evans AC (2001) Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage 13: 375–380. [DOI] [PubMed] [Google Scholar]
- 42. Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab’bagh Y, et al. (2005) Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage 27: 210–221. [DOI] [PubMed] [Google Scholar]
- 43. MacDonald D, Kabani N, Avis D, Evans AC (2000) Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage 12: 340–356. [DOI] [PubMed] [Google Scholar]
- 44. Lyttelton O, Boucher M, Robbins S, Evans A (2007) An unbiased iterative group registration template for cortical surface analysis. Neuroimage 34: 1535–1544. [DOI] [PubMed] [Google Scholar]
- 45.Robbins SM (2004) Anatomical standardization of the human brain in euclidean 3-space and on the cortical 2-manifold.: Monteal: School of Computer Science, McGill University, p. 315.
- 46.Ad-Dab’bagh Y, Singh V, Robbins S, Lerch J, Lyttelton O, et al.. (2005) Native-Space Cortical Thickness Measurement And The Absence of Correlation to Cerebral Volume. The 11th Annual meeting of the Organization for Human Brain Mapping (OHBM). Toronto, Canada.
- 47. Lerch JP, Evans AC (2005) Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage 24: 163–173. [DOI] [PubMed] [Google Scholar]
- 48. Chung MK, Worsley KJ, Robbins S, Paus T, Taylor J, et al. (2003) Deformation-based surface morphometry applied to gray matter deformation. Neuroimage 18: 198–213. [DOI] [PubMed] [Google Scholar]
- 49. Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, et al. (2007) Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex 17: 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- 51. Koutsouleris N, Gaser C, Jager M, Bottlender R, Frodl T, et al. (2008) Structural correlates of psychopathological symptom dimensions in schizophrenia: a voxel-based morphometric study. Neuroimage 39: 1600–1612. [DOI] [PubMed] [Google Scholar]
- 52. Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, et al. (2001) Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry 158: 234–243. [DOI] [PubMed] [Google Scholar]
- 53. Turetsky B, Cowell PE, Gur RC, Grossman RI, Shtasel DL, et al. (1995) Frontal and temporal lobe brain volumes in schizophrenia. Relationship to symptoms and clinical subtype. Arch Gen Psychiatry 52: 1061–1070. [DOI] [PubMed] [Google Scholar]
- 54. Galderisi S, Quarantelli M, Volpe U, Mucci A, Cassano GB, et al. (2008) Patterns of structural MRI abnormalities in deficit and nondeficit schizophrenia. Schizophr Bull 34: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Quarantelli M, Larobina M, Volpe U, Amati G, Tedeschi E, et al. (2002) Stereotaxy-based regional brain volumetry applied to segmented MRI: validation and results in deficit and nondeficit schizophrenia. Neuroimage 17: 373–384. [DOI] [PubMed] [Google Scholar]
- 56. Tang J, Liao Y, Zhou B, Tan C, Liu W, et al. (2012) Decrease in temporal gyrus gray matter volume in first-episode, early onset schizophrenia: an MRI study. PLoS One 7: e40247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Palaniyappan L, Balain V, Radua J, Liddle PF (2012) Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr Res 137: 169–173. [DOI] [PubMed] [Google Scholar]
- 58. Gernsbacher MA, Kaschak MP (2003) Neuroimaging studies of language production and comprehension. Annu Rev Psychol 54: 91–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Martin RC (2003) Language processing: functional organization and neuroanatomical basis. Annu Rev Psychol 54: 55–89. [DOI] [PubMed] [Google Scholar]
- 60. Hovington CL, Lepage M (2012) Neurocognition and neuroimaging of persistent negative symptoms of schizophrenia. Expert Rev Neurother 12: 53–69. [DOI] [PubMed] [Google Scholar]
- 61. Wible CG (2012) Schizophrenia as a disorder of social communication. Schizophr Res Treatment 2012: 920485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Saxe R, Kanwisher N (2003) People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage 19: 1835–1842. [DOI] [PubMed] [Google Scholar]
- 63. Hampton AN, Bossaerts P, O’Doherty JP (2008) Neural correlates of mentalizing-related computations during strategic interactions in humans. Proc Natl Acad Sci U S A 105: 6741–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, et al. (2008) Know your place: neural processing of social hierarchy in humans. Neuron 58: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chiao JY, Harada T, Oby ER, Li Z, Parrish T, et al. (2009) Neural representations of social status hierarchy in human inferior parietal cortex. Neuropsychologia 47: 354–363. [DOI] [PubMed] [Google Scholar]
- 66. Saxe R, Wexler A (2005) Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia 43: 1391–1399. [DOI] [PubMed] [Google Scholar]
- 67. Saxe R, Powell LJ (2006) It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci 17: 692–699. [DOI] [PubMed] [Google Scholar]
- 68. Corbetta M, Patel G, Shulman GL (2008) The reorienting system of the human brain: from environment to theory of mind. Neuron 58: 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chechlacz M, Rotshtein P, Hansen PC, Deb S, Riddoch MJ, et al. (2013) The central role of the temporo-parietal junction and the superior longitudinal fasciculus in supporting multi-item competition: Evidence from lesion-symptom mapping of extinction. Cortex 49: 487–506. [DOI] [PubMed] [Google Scholar]
- 70. Carter RM, Bowling DL, Reeck C, Huettel SA (2012) A distinct role of the temporal-parietal junction in predicting socially guided decisions. Science 337: 109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Arzy S, Seeck M, Ortigue S, Spinelli L, Blanke O (2006) Induction of an illusory shadow person. Nature 443: 287. [DOI] [PubMed] [Google Scholar]
- 72. Blanke O, Ortigue S, Landis T, Seeck M (2002) Stimulating illusory own-body perceptions. Nature 419: 269–270. [DOI] [PubMed] [Google Scholar]
- 73. Hoffman RE, Gueorguieva R, Hawkins KA, Varanko M, Boutros NN, et al. (2005) Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol Psychiatry 58: 97–104. [DOI] [PubMed] [Google Scholar]
- 74. Jardri R, Lucas B, Delevoye-Turrell Y, Delmaire C, Delion P, et al. (2007) An 11-year-old boy with drug-resistant schizophrenia treated with temporo-parietal rTMS. Mol Psychiatry 12: 320. [DOI] [PubMed] [Google Scholar]
- 75. Poulet E, Brunelin J, Bediou B, Bation R, Forgeard L, et al. (2005) Slow transcranial magnetic stimulation can rapidly reduce resistant auditory hallucinations in schizophrenia. Biol Psychiatry 57: 188–191. [DOI] [PubMed] [Google Scholar]
- 76. Freitas C, Fregni F, Pascual-Leone A (2009) Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res 108: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Williams LM (2008) Voxel-based morphometry in schizophrenia: implications for neurodevelopmental connectivity models, cognition and affect. Expert Rev Neurother 8: 1049–1065. [DOI] [PubMed] [Google Scholar]
- 78. Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, et al. (2000) Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 157: 16–25. [DOI] [PubMed] [Google Scholar]
- 79. Shenton ME, Dickey CC, Frumin M, McCarley RW (2001) A review of MRI findings in schizophrenia. Schizophr Res 49: 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bodnar M, Malla AK, Joober R, Lord C, Smith E, et al. (2012) Neural markers of early remission in first-episode schizophrenia: A volumetric neuroimaging study of the parahippocampus. Psychiatry Res 201: 40–47. [DOI] [PubMed] [Google Scholar]
- 81. Bodnar M, Harvey P-O, Malla AK, Joober R, Lepage M (2011) The parahippocampal gyrus as a neural marker of early remission in first-episode psychosis: a voxel-based morphometry study. Clin Schizophr Relat Psychoses 4: 217–228. [PubMed] [Google Scholar]
- 82. Lepage M, Sergerie K, Benoit A, Czechowska Y, Dickie E, et al. (2011) Emotional face processing and flat affect in schizophrenia: functional and structural neural correlates. Psychol Med 41: 1833–1844. [DOI] [PubMed] [Google Scholar]
- 83. Bookstein FL (2001) “Voxel-based morphometry” should not be used with imperfectly registered images. Neuroimage 14: 1454–1462. [DOI] [PubMed] [Google Scholar]
- 84. Davatzikos C (2004) Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage 23: 17–20. [DOI] [PubMed] [Google Scholar]
- 85.Pletson JE, editor (2007) Psychology and Schizophrenia. 1st ed. New York: Nova Publishers, Inc.
- 86.Thacker NA (2008) Tutorial: A Critical Analysis of Voxel Based Morphometry (VBM).
- 87. Park HJ, Levitt J, Shenton ME, Salisbury DF, Kubicki M, et al. (2004) An MRI study of spatial probability brain map differences between first-episode schizophrenia and normal controls. Neuroimage 22: 1231–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Harris JM, Yates S, Miller P, Best JJ, Johnstone EC, et al. (2004) Gyrification in first-episode schizophrenia: a morphometric study. Biol Psychiatry 55: 141–147. [DOI] [PubMed] [Google Scholar]
- 89. Kulynych JJ, Luevano LF, Jones DW, Weinberger DR (1997) Cortical abnormality in schizophrenia: an in vivo application of the gyrification index. Biol Psychiatry 41: 995–999. [DOI] [PubMed] [Google Scholar]
- 90. Schultz CC, Koch K, Wagner G, Roebel M, Nenadic I, et al. (2010) Increased parahippocampal and lingual gyrification in first-episode schizophrenia. Schizophr Res 123: 137–144. [DOI] [PubMed] [Google Scholar]
- 91. Wheeler DG, Harper CG (2007) Localised reductions in gyrification in the posterior cingulate: schizophrenia and controls. Prog Neuropsychopharmacol Biol Psychiatry 31: 319–327. [DOI] [PubMed] [Google Scholar]
- 92. Palaniyappan L, Liddle PF (2012) Aberrant cortical gyrification in schizophrenia: a surface-based morphometry study. J Psychiatry Neurosci 37: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hobbs N, Novak M (2008) VBM: Voxel-based morphometry 37.
- 94. Silver M, Montana G, Nichols TE (2011) False positives in neuroimaging genetics using voxel-based morphometry data. Neuroimage 54: 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, et al. (2008) False positives in imaging genetics. Neuroimage 40: 655–661. [DOI] [PubMed] [Google Scholar]
- 96.Voineskos AN, Foussias G, Lerch J, Felsky D, Remington G, et al.. (2013) Neuroimaging Evidence for the Deficit Subtype of Schizophrenia. JAMA Psychiatry: 1–9. [DOI] [PubMed]
- 97. Moncrieff J, Leo J (2010) A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med 40: 1409–1422. [DOI] [PubMed] [Google Scholar]
- 98. Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V (2011) Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 68: 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Puri BK (2011) Brain tissue changes and antipsychotic medication. Expert Rev Neurother 11: 943–946. [DOI] [PubMed] [Google Scholar]
- 100. Leung M, Cheung C, Yu K, Yip B, Sham P, et al. (2011) Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta-analyses with sample size weighting. Schizophr Bull 37: 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]