Abstract
Objective
Preliminary evidence suggests that omega-3 fatty acids may reduce hyperactivity in children with autism spectrum disorder (ASD). We sought to examine the feasibility of a novel, internet-based clinical trial design to evaluate the efficacy of this supplement.
Method
E-mail invitations were sent to parents of children aged 5-8 enrolled in the Interactive Autism Network. All study procedures, including screening, informed consent, and collection of outcome measures took place over the internet. The primary outcome measures were parent- and teacher-rated changes in hyperactivity on the Aberrant Behavior Checklist.
Results
During the 6-week recruitment period, 57 children from 28 states satisfied all eligibility criteria and were randomly assigned to 1.3 grams of omega-3 fatty acids or an identical placebo daily for 6 weeks. Outcome assessments were obtained from all 57 participants and 57 teachers, and the study was completed in 3 months. Children in the omega-3 fatty acid group had a greater reduction in hyperactivity (-5.3 points) compared to the placebo group (-2.6 points), but the difference was not statistically significant (1.9 point greater improvement in the omega-3 group, 95% CI -2.2 to 5.2). Side effects were rare and not associated with omega-3 fatty acids. Participant feedback was positive.
Conclusion
Internet-based randomized controlled trials of therapies in children with ASD are feasible and may lead to marked reductions in the time and cost of completing trials. A larger sample size is required to definitively determine the efficacy of omega-3 fatty acids.
Clinical trial registration information—Omega-3 Fatty Acids for Hyperactivity Treatment in Autism Spectrum Disorder; http://clinicaltrials.gov; NCT01694667.
Keywords: autism, nutritional supplement, alternative medicine, hyperactivity
Introduction
Autism spectrum disorders (ASDs) are estimated to affect as many as 1 in 50 children in the United States1 and are characterized by impairments in communication, social interaction, and repetitive behavior.2 Complementary and alternative medical (CAM) therapies, such as omega-3 fatty acids, digestive enzymes, and high-dose vitamins are widely used to treat ASD despite little or no evidence of efficacy and safety.3 Traditional, clinic-based, randomized controlled trials (RCTs) of therapies for ASD are expensive and slow. We sought to determine whether it would be feasible to conduct a RCT of an intervention for ASD entirely over the internet with the goal of developing a platform for rapidly evaluating promising therapies. We selected omega-3 fatty acids as the intervention in this first, internet-based randomized controlled trial (IB-RCT) in ASD because hyperactivity is a common problem among children with ASD, and standard pharmacological treatments have unpredictable effects and more side effects in children with ASD.4, 5
Two prior small pilot studies found non-significant trends suggesting that omega-3 fatty acids may reduce hyperactivity in children with ASD.6, 7 Amminger et al. randomly assigned 13 children with ASD to 6 weeks of omega-3 fatty acids vs. placebo and found that the hyperactivity score on the Aberrant Behavior Checklist (ABC-H) was reduced by 4.0 points in the treatment group compared to an increase of 3.0 points in the placebo group (p=0.098), a non-significant result with an effect size of 0.71.6 Bent et al. randomly assigned 27 children with ASD to 12-weeks of omega-3 fatty acids vs. placebo and found that hyperactivity was reduced by 2.7 points on the ABC-H in the omega-3 group compared to 0.3 points in the placebo group (p=0.4), a statistically non-significant difference (effect size = 0.38).7 Both prior studies were small and had insufficient power to definitively determine efficacy but showed trends favoring the omega-3 fatty acid group.
Other evidence suggesting possible efficacy comes from the use of omega-3 fatty acids in different disorders. A recent systematic review found that omega-3 fatty acid treatment leads to modest improvements in overall symptoms and in inattention and hyperactivity in children with attention-deficit hyperactivity disorder (with an effect size = 0.31).8 A recent, large RCT of omega-3 fatty acids in 362 healthy schoolchildren aged 7-9 years found that it led to significant improvements in reading among children with low initial reading scores.9 Preliminary studies also suggest that omega-3 fatty acids may have benefits in treating depression and schizophrenia.10, 11 Omega-3 fatty acids have been found to have a favorable safety profile.12 We therefore conducted an IB-RCT to examine the potential of this method and to further examine the safety and efficacy of omega-3 fatty acids in ASD.
Method
Participants
The study protocol was approved by the Committees on Human Research at the University of California, San Francisco and at Johns Hopkins University. The trial was registered prior to enrolling patients at clinicaltrials.gov (NCT01694667) and took place between September 18, 2012 and December 31, 2012.
Recruitment was limited to children between the ages of 5 and 8 with some verbal ability (as defined by question 1 of the Social Communication Questionnaire or SCQ) who were enrolled in the Interactive Autism Network (IAN), an on-line registry and longitudinal study of over 13,000 families of children affected by ASD. Children were defined as having an accurate diagnosis of ASD if they met two criteria: 1) parents reported that their child was diagnosed by a professional, and 2) a score of > 12 on the SCQ. A prior validation study within IAN found that 99% of children meeting these two criteria were confirmed to have an ASD diagnosis based on in-person clinical testing with the Autism Diagnostic Observation Schedule (ADOS) or the Autism Diagnostic Interview-Revised (ADI-R).13
E-mail invitations were sent to the 863 registered IAN members who met the above criteria and had given prior consent to be contacted about research opportunities. Interested parents completed an initial screening questionnaire by clicking on an embedded link in the e-mail. Children were required to have elevated levels of hyperactivity, defined as a score of > 20 on the hyperactivity subscale of the Aberrant Behavior Checklist (ABC-H), which is one standard deviation above the mean score. Children were also required to have a parent (or caregiver) and a teacher willing to complete baseline and outcome assessments by e-mail. Children were excluded if they had used omega-3 fatty acids in the last 6 months, had a known bleeding disorder, an allergy to fish or seafood, or a major medical illness. Parents of children who passed the eligibility screen were asked to complete an online informed consent process which was confirmed by their electronic signature. All participants were given the option of speaking with an investigator by phone prior to signing informed consent, and the online informed consent process was approved by both participating institutional review boards. After completion of online baseline measures, participating parents received follow-up e-mails weekly to report medication adherence, new medical problems, and outcome assessments (3 and 6 weeks).
Internet-based clinical trial platform
The flow of participants through each step of the study was managed by an automated internet-based clinical trial platform developed by experts at IAN (P.L. and J.N.). Once the study launched, all steps of the study proceeded automatically with oversight from IAN staff.
All participants received a phone call from a study investigator within 3 days of randomization to welcome them to the study, to reinforce study instructions, and to answer questions. The data collection system met U.S. Food and Drug Administration (FDA), 21CFR Part11, HL7, and Health Insurance Portability and Accountability Act (HIPAA) compliance criteria for the capture and security of electronic data. Adverse events were monitored by both the study principal investigator (S.B.) and the IAN site principal investigator (P.L.). As soon as a new adverse event was entered by a participant into the online platform, an e-mail message was sent to both principal investigators, who conferred about the adverse event and called the parent to obtain and document all information. Further details of the platform will be provided in a subsequent publication.
Intervention
Eligible children were randomly assigned to 6 weeks of treatment with omega-3 fatty acids or an identical placebo, which was sent by overnight mail. Omega-3 fatty acids were provided as orange-flavored pudding packets (Coromega®, Vista, CA) containing 650 mg of omega-3 fatty acids, including 350mg of eicosapentanoic acid (EPA) and 230mg of docosahexanoic acid (DHA), given twice daily for a daily dose of 1.3 gms of omega-3 fatty acids (and 1.1 gms of DHA + EPA). The six-week duration of study medication was selected because this duration was used in the earlier Amminger study,6 which found the largest effect size. The fixed dose is the same as used in the previous Bent study and similar to that used in the Amminger study.6, 7 Placebo packets had the same orange-flavored pudding with an identical appearance and taste but included safflower oil instead of the fish oil. Safflower oil was used because it has a similar texture and is comprised of fatty acids (but not omega-3 fatty acids), and this allowed for an examination of the unique effects of omega-3 fatty acids compared to “non-omega-3” fatty acids.
Objectives and Outcomes
The two primary goals of this study were to determine the feasibility of conducting an IB-RCT in children with ASD and to obtain further information regarding the safety and efficacy of omega-3 fatty acids. The primary outcome measure was defined a priori as the change in hyperactivity on the ABC-H (parent and teacher) over the six-week treatment period.
Secondary outcomes of the study included changes in the other subscales of the ABC and the Social Responsiveness Scale (SRS). Parents were also asked to complete a parent version of the Clinical Global Impression-Improvement (CGI-I) scale, which was identical to the scale designed for clinicians. Medication adherence was reported by parents through weekly e-mail reminders. Teachers were asked to complete the ABC at baseline and at 6 weeks.
Randomization and Blinding
Eligible participants were randomized in equal proportions using a computer-generated randomization list (the ralloc.ado procedure in Stata, a software module used to design randomized controlled trials).14 The randomization list was prepared by a person who was not involved with the study, and each new participant was assigned to the next randomized sequence, entered on the list, and mailed the appropriate study medication (omega-3 or placebo) by a person external to the study team. All participants, families, and study personnel were blinded to group assignment and the randomized sequence list for the entire study, including the data analysis. Participants were given an emergency contact telephone number, which was staffed by the study's principal investigator (S.B.) at all times. If an emergency required breaking of the study blind, plans were in place for the principal investigator to emergently contact the individual holding the randomization list to reveal the treatment assignment for a given participant.
Statistical Methods
The study was designed to test the IB-RCT method in ASD and did not have sufficient resources to enroll enough patients to have adequate power to identify clinically important changes in hyperactivity. The proposed total enrollment goal was 30, which was almost doubled (57) during the 6-week enrollment period. We estimate that 190 patients (95 in each group) would be required to provide 90% power to detect a clinically important difference of 4 points in the ABC-H between the omega-3 and placebo groups, based on an alpha of 0.05, a standard deviation of change in the ABC-H of 8, and a drop-out rate of 10%.
We compared baseline characteristics of the omega-3 and placebo groups using the student's t-test for continuous variables and chi-square tests for categorical variables. We assessed the statistical significance of the differences in changes in ABC-hyperactivity ratings between the omega-3 and placebo groups using linear mixed-effects models15 with SAS, version 9.3.16 These models include random intercepts to accommodate the repeated measures gathered from each study participant as well as terms for the fixed effects of time, study group, and time-by-group interaction, the effects of interest in our analyses. These analyses were bootstrapped because the residuals from some variables were non-normally distributed. All reported p-values are two-sided and not adjusted for multiple testing.17 If patients discontinued study medication, they were asked to complete all outcome assessments at 6 weeks, and they were analyzed in their assigned treatment group (intention-to-treat analysis).
Results
During the six-week recruitment period, e-mail recruitment notices were sent to 863 IAN members meeting initial age and ASD diagnostic criteria, and 118 families expressed interest and completed the online screening form. Fifty-seven children from 28 states were deemed eligible and randomly assigned to study treatment. After the study was completed, it was discovered that a programming error had resulted in three participants being included who were not eligible: one patient in the omega-3 group had used omega-3 fatty acids in the past 6 months, and one patient in each treatment group had hyperactivity scores below 21 (Figure 1). All patients were included in the overall intention-to-treat analysis. All 57 participants and 57 teachers provided complete outcome assessment at baseline and six weeks for a 100% completion rate. Eight patients in the omega-3 group and 4 patients in the placebo group discontinued the study medication due to dislike of the taste but remained in the study to complete all outcome assessments. Medication adherence was not statistically different between the two treatment groups (69% of doses completed in the omega-3 group vs. 83% in the placebo group, p=0.36). Baseline characteristics of the treatment groups were similar (Table 1). Children in the study had a mean age of 86.6 months (7.2 years), and 50 out of 57 (88%) were male. The baseline parent rating of hyperactivity on the ABC was similar in both groups (28.1 in the placebo group vs. 28.4 in the omega-3 group). This degree of hyperactivity is similar to children enrolled in previous studies of the pharmaceutical drugs atomoxetine (baseline ABC-H = 25.0)18 and methylphenidate (baseline ABC-H = 33.2)19 in children with ASD.
Figure 1. Flow Diagram Showing Distribution of Participants at Each Stage.
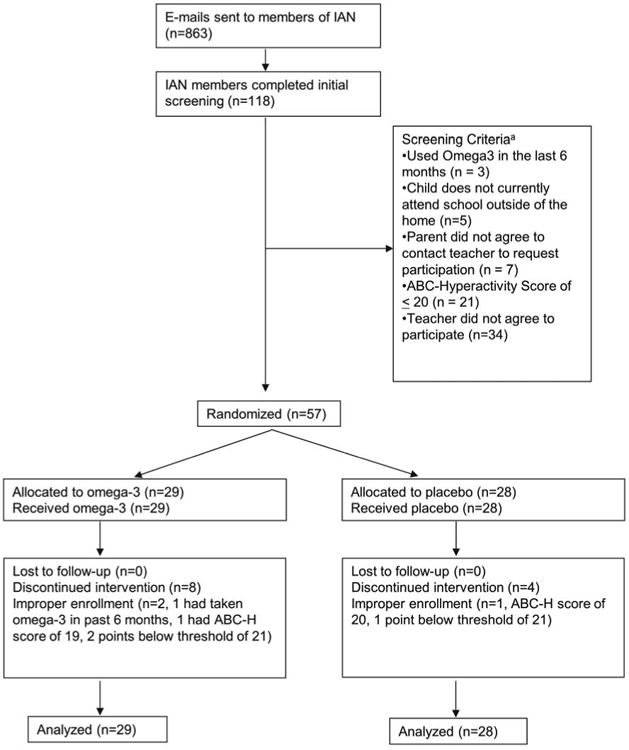
aNote: Participants can screen fail for multiple reasons
Table 1. Baseline Characteristics.
| Characteristic | Group A: Placebo (n=28) mean (SD) | Group B: Omega 3 (n=29) mean (SD) | Total (N=57) mean (SD) |
|---|---|---|---|
| DEMOGRAPHICS: | |||
| Age (months) | 85.0 (13.2) | 88.2 (12.3) | 86.6 (12.7) |
| Gender (M/F) | 24/4 | 26/3 | 50/7 |
| Race | |||
| -White | 23 | 24 | 47 |
| -Asian | 1 | 1 | 2 |
| -Black | 3 | 1 | 4 |
| -American Indian or Alaskan | 0 | 1 | 1 |
| -Other | 1 | 2 | 3 |
| MEDICATION USE: | |||
| -None | 16 | 18 | 34 |
| -Antipsychotic | 3 | 5 | 8 |
| -Stimulant | 6 | 2 | 8 |
| -Antidepressant | 6 | 4 | 10 |
| -Benzodiazepine | 2 | 1 | 3 |
| -Anticonvulsant | 1 | 3 | 4 |
| PARENT RATINGS: | |||
| ABC – Hyperactivity | 28.1(7.6) | 28.4(8.4) | 28.2(8.0) |
| ABC – Irritability | 16.8(8.3) | 20.0(8.9) | 18.4(8.7) |
| ABC – Stereotypy | 5.4(4.7) | 8.0(6.0) | 6.7(5.5) |
| ABC – Lethargy | 8.8(4.2) | 12.2(8.8) | 10.5(7.1) |
| ABC – Inapp. Speech | 5.8(2.8) | 7.0(3.4) | 6.4(3.2) |
| SRS Total | 88.3(9.7) | 89.7(12.7) | 89.0(11.2) |
| SRS Social Awareness | 78.9(8.1) | 79.5(11.4) | 79.2(9.9) |
| SRS Social Cognition | 83.1(10.6) | 84.8(11.2) | 84.0(10.8) |
| SRS Social Communication | 83.8(10.3) | 84.9(11.2) | 84.4(10.7) |
| SRS Social Motivation | 75.9(9.6) | 76.1(14.4) | 76.0(12.2) |
| SRS Autistic Mannerisms | 92.5(13.0) | 94.7(16.9) | 93.6(15.1) |
| CGI-S | 4.0(.79) | 3.8(1.0) | 3.9(.93) |
| TEACHER RATINGS: | |||
| ABC – Hyperactivity | 14.6(10.4) | 18.1(12.0) | 16.4(11.2) |
| ABC – Irritability | 9.4(9.3) | 14.3(9.8) | 11.9(9.8) |
| ABC – Stereotypy | 3.7(4.1) | 5.1(4.8) | 4.4(4.5) |
| ABC – Lethargy | 8.3(7.1) | 9.9(8.9) | 9.1(8.0 |
| ABC – Inapp. Speech | 3.1(3.3) | 4.2(3.9) | 3.7(3.7) |
Note: ABC = Aberrant Behavior Checklist; CGI-S = Clinical Global Impression – Severity; SRS = Social Responsiveness Scale; SSRI = Selective Serotonin Reuptake Inhibitor.
Both groups showed an improvement in hyperactivity as indicated by a decrease in the parent ABC-H score over the six-week study period (Table 2 and Figure 2). In the omega-3 group, the change in the mean parent ABC-H was -5.3 points compared to -3.4 points in the placebo group. The greater improvement in the omega-3 group was not statistically significant (omega-3 group improvement = 1.9 points greater than placebo, 95% CI: -2.2 to 5.2, p=0.38). Teachers rated children less hyperactive than parents at baseline, and the greater improvement in the mean teacher ABC-H in the omega-3 group was not statistically significant.
Table 2. Changes in Primary and Secondary Outcome Measures.
| Group A: Placebo Change (SD)a n=28 | Group B: Omega 3 (SD) a n=29 | Difference in change between groupsb | 95% Confidence Interval of Difference | Standardized Effect Size c | p-valued | |
|---|---|---|---|---|---|---|
| Primary Outcome: | ||||||
|
| ||||||
| ABC Hyperactivity | ||||||
| -parent | -3.4(7.5) | -5.3(7.2) | 1.9 | -2.2, 5.2 | 0.26 | 0.38 |
| -teacher | -1.0(10.0) | -2.6(8.0) | 1.7 | -3.4, 5.8 | 0.18 | 0.50 |
|
| ||||||
| Secondary Outcomes: | ||||||
|
| ||||||
| ABC-Irritability | ||||||
| -parent | -2.1(4.4) | -2.0(6.9) | -0.1 | -3.3, 2.8 | 0.02 | 0.91 |
| -teacher | -0.2(6.8) | -1.3(8.6) | 1.1 | -2.9, 4.9 | 0.14 | 0.57 |
|
| ||||||
| ABC-Stereotypy | ||||||
| -parent | -0.5(2.6) | -2.0(3.7) | 1.6 | 0.0, 3.2 | 0.49 | 0.05 |
| -teacher | -0.7(3.2) | -1.0(4.2) | 0.3 | -1.5, 2.3 | 0.08 | 0.73 |
|
| ||||||
| ABC-Lethargy | ||||||
| -parent | 0.1(2.6) | -2.1(4.2) | 2.2 | 0.5, 4.1 | 0.64 | 0.01 |
| -teacher | -1.6(5.5) | -2.5(7.9) | 0.9 | -2.4, 4.7 | 0.13 | 0.60 |
|
| ||||||
| ABC–Inapp. Speech | ||||||
| -parent | -0.9(2.2) | -0.6(2.7) | -0.3 | -1.4, 1.0 | 0.10 | 0.73 |
| -teacher | -0.1(2.8) | -1.0(2.6) | 0.9 | -0.5, 2.2 | 0.32 | 0.22 |
|
| ||||||
| SRS Total | -6.1(7.8) | -2.6(8.3) | -3.5 | -7.8, 0.5 | -0.43 | 0.09 |
|
| ||||||
| SRS Social Awareness | -3.3(8.5) | -1.8(7.4) | -1.5 | -5.5, 2.9 | -0.18 | 0.50 |
|
| ||||||
| SRS Social Cognition | -3.8(8.3) | -1.8(9.5) | -1.9 | -6.7, 2.8 | -0.22 | 0.36 |
|
| ||||||
| SRS Social Communication | -5.2(8.4) | -2.1(9.1) | -3.1 | -8.0, 1.0 | -0.35 | 0.15 |
|
| ||||||
| SRS Social Motivation | -5.3(8.4) | -2.5(9.0) | -2.8 | -7.6, 1.5 | -0.32 | 0.20 |
|
| ||||||
| SRS Autistic Mannerisms | -8.6(11.4) | -2.9(12) | -5.7 | -11.5, 0.7 | -0.49 | 0.08 |
Note: ABC = Aberrant Behavior Checklist; SRS = Social Responsiveness Scale.
All changes within the omega-3 and placebo group are shown as negative when there was an improvement in the score and positive when there was a worsening of the score.
The difference in change between groups is positive when the omega-3 change was greater (better) and negative when the placebo change was greater (better).
Effect sizes are positive when the change in the omega-3 group is greater than in the placebo group and negative when the change in placebo group is greater than in the omega-3 group.
p-values were obtained from linear mixed effect models.
Figure 2. Change in Mean Aberrant Behavior Checklist-Hyperactivity (ABC-H) Subscale Scores.
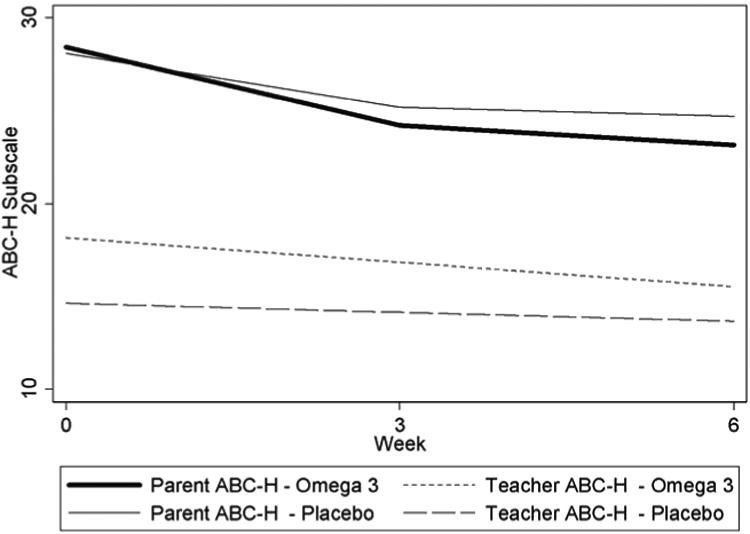
Note: During the six week study, mean ABC-H decreased (improved) more in the omega-3 group compared to the placebo group, as rated by both parents (top two lines) and teachers (bottom two lines), but the differences between treatment groups were not statistically significant.
In an a priori defined subgroup of participants with greater than 80% adherence to study medications (n=23 in the placebo group vs. n=17 in the omega-3 group), the parent ABC-H showed similar changes to the intention-to-treat analysis (1.5 points greater improvement in the omega-3 group compared to the placebo group, 95% CI: -3.7 to 6.3, p=0.54) but the teacher ABC-H showed a greater, non-significant improvement in the omega-3 group (4.3 points greater improvement in the omega-3 group compared to the placebo group, 95% CI: -0.6 to 9.8, p=0.09).
The stereotypy and lethargy subscales of the ABC showed statistically significant improvements in the omega-3 group compared to the placebo group (Table 2). There were no statistically significant differences in the comparisons of changes between treatment groups in social function as measured by the SRS.
Using the parent CGI-I, 12/29 (41%) of parents in the omega-3 group rated their child as having any improvement (improved, much improved, or very much improved) compared to 10/28 (36%) in the placebo group (p=0.66). Also, 4/29 (14%) of parents in the omega-3 group rated their child as much improved or very much improved compared to 2/28 (7%) in the placebo group (p=0.41).
Omega-3 fatty acids were well tolerated. No serious adverse events were reported (Table 3), and there was no statistical difference in the total number of adverse events (p=0.25).
Table 3. Adverse Events.
| Event | Omega-3 | Placebo |
|---|---|---|
| Asthma Flare | 0 | 1 |
| Croup | 1 | 0 |
| Elective Tonsillectomy | 1 | 0 |
| Gastrointestinal Virus | 1 | 0 |
| Increased Stimming | 1 | 0 |
| Increased Tantrums | 2 | 0 |
| Mild Cough | 0 | 1 |
| Nosebleed | 1 | 0 |
| Pink Eye | 0 | 1 |
| Pneumonia | 0 | 1 |
| Rash | 1 | 0 |
| Sinus Infection | 0 | 1 |
| Viral URI | 0 | 1 |
| Vomiting | 2 | 0 |
| TOTAL | 10 | 6 |
Note: URI = Upper respiratory infection.
The adequacy of blinding was assessed by asking parents whether they believed their child was taking omega-3 fatty acids or placebo. At 6 weeks, 17% of parents (5/29) in the omega-3 group believed their child was taking the omega-3 fatty acids compared to 14% (4/28) in the placebo group (p=1.0).
Participating families were very positive about their participation in this unique, online clinical trial format. Most families (54/57, or 95%) reported no problems with using the computer or entering study-related information. In response to open-ended questions about the experience with the study, many families highlighted the flexibility of being able to answer questions during their free time without having to go to a clinic, the ability to participate in a trial from a rural area, and the prompt receipt of a phone call from a study investigator when they reported a medical problem online.
Discussion
This study demonstrates the feasibility of conducting an IB-RCT in children with ASD. In just six weeks, 57 children from 28 states completed all eligibility criteria and were enrolled. The response rate (118 responses to 863 e-mail invitations, or 13.7%) is a statistic that is most often reported for survey responses and not for acceptance to invitations for clinical trials. Although the response rate in this study is relatively low compared to published surveys, it is similar to or higher than response rates for mailed invitations to clinical trials.20 The entire study was completed in just over 3 months from the start of enrollment. The rapid nature of the study compares favorably to traditional, clinic-based trials, including the landmark study of risperidone in 101 children with autism, which took 22 months to complete.21 Although precise cost information from prior studies is not available, the cost of the IB-RCT method is likely substantially lower. The IB-RCT method has many other potential advantages, including the ability for families to participate from virtually any location and with limited interruption of their schedules. The 100% study completion rate and the positive feedback from parents indicate initial enthusiasm from the ASD community for this method of participating in clinical trials.
We are aware of only one prior IB-RCT of a supplement, which used a broader model of recruitment.22 In that study of using the herbs kava and valerian for treatment of anxiety and insomnia, adult participants were recruited through both an e-mail campaign and banner advertisements on web-sites. The study recruited many more participants (391 participants enrolled in 8 weeks) but had a lower study completion rate (82%). A potential reason for the 100% completion rate in the current study is that participants were already part of an online community and as such had an established relationship with members of the study team.
This study did not find a statistically significant improvement in hyperactivity in children who were taking omega-3 fatty acids. This is not surprising, since the study was primarily designed to test the feasibility of the IB-RCT method and did not have sufficient power to detect small-to-moderate improvements in hyperactivity. Our sample size calculations indicate that 190 participants would be needed to definitively determine the efficacy of omega-3 fatty acids for reducing hyperactivity in ASD, but funding limitations only allowed us to recruit for 6 weeks. Despite this short recruitment period, the study exceeded the target recruitment goal of 30 and enrolled 57 participants, an average of almost 10 new participants per week. Children in the omega-3 fatty acid group did have greater mean improvements in hyperactivity compared to the placebo group (a 5.3 point reduction in the ABC-H in the omega-3 group vs. 3.4 in the placebo group), although this difference was not significant. The magnitude of this nonsignificant effect size (0.26) is smaller than the effect sizes observed in the two previous studies of omega-3 fatty acids in ASD by Amminger6 (effect size = 0.71) and Bent7 (effect size = 0.38). One potential reason for the much smaller effect size in the current study compared to the Amminger study is that the baseline hyperactivity score was lower in the current study (ABC-H = 28.2) compared to the Amminger study (baseline ABC-H of 33.3 in the omega-3 group).
With regards to secondary outcome measures, this study found statistically significant improvements in the omega-3 group in two secondary outcome measures, the stereotypy and lethargy subscales of the ABC. Since this is a new finding that was not reported in the two previous small, randomized controlled trial of omega-3 fatty acids,6, 7 it should be viewed as preliminary and deserving of further investigation. We are not aware of any hypothesized mechanism whereby omega-3 fatty acids would have a greater effect on stereotypy or lethargy than hyperactivity. There were no statistically significant changes in the SRS, although it is interesting to note that the improvements in each of the five subscales of the SRS were greater in the placebo group. Changes in measures of social function should be monitored in future studies of omega-3 fatty acids in children with autism.
This study did not include direct clinical observation, which potentially impacts the validity of both the diagnosis of ASD and the outcome assessments. However, a prior study among IAN participants found that 99% of children were accurately diagnosed with ASD using the criteria employed in this study.13 This very high rate of diagnostic accuracy may be related to several factors that are unique to the IAN population and the diagnostic criteria that were evaluated in the validation study, including the requirements that children were verbally fluent, that parents were capable and willing to enroll online, that the parents had obtained formal medical documentation of an ASD diagnosis, and that children lived within a specific catchment area close to autism centers. Other studies suggest that reliance solely on parent-completed screening tools such as the SCQ or the SRS in community-based samples of children suspected of having ASD may result in much lower diagnostic accuracy.23 While current evidence suggests that the methods used in the current study result in a high degree of diagnostic validity within the IAN population, future studies to improve methods for the efficient identification of children with ASD would improve the application of this internet-based method to other populations.
With respect to outcome assessments, the parent or teacher-completed ABC has been used as the primary18, 19, 21, 24-26 or secondary27 outcome measure in most prior clinical trials of drug therapies for ASD, indicating that it is the standard measure of assessing meaningful change in behavior in ASD. In the current study, the use of teacher ABC ratings provides an observation of change from outside of the home. During the data analysis phase of the study, we discovered that 3 patients who did not meet all eligibility criteria were included in the study. We conducted a careful analysis of the system and discovered an error in the automated exclusion criteria coding. This prompted a complete review of the automated process and the development of new procedures for more stringent system testing as well as manual review of eligibility criteria prior to randomization. This error highlights the need to have careful pre-testing of any automated aspects of clinical trial systems. We measured adherence only by report from parents, a technique that may not be as accurate as other methods, including the measurement of serum levels of omega-3 fatty acids. Obtaining blood samples through an internet-based study is a potential extension of the current platform that has numerous possible benefits. Finally, the planned sample size was only 30 subjects, and although this target was almost doubled (57), the study still did not have sufficient power to make definitive conclusions regarding efficacy.
In conclusion, the IB-RCT method is a promising technique for evaluating treatments for autism that has many advantages, including low cost, rapid enrollment, high completion rate, convenience for participating families, and the ability to participate from virtually any location. In the current study, omega-3 fatty acids did not lead to a statistically significant reduction in hyperactivity, but a non-significant trend of a beneficial effect was observed. A larger study is needed to definitively determine the efficacy of this supplement.
Acknowledgments
This work was supported by a grant from the Simons Foundation (SFARI 206484, S.B.) and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131.
Mr. Kalb served as the statistical expert for this research.
The authors would like to express their deepest appreciation to the participating families, whose generous commitment of time and energy made this research possible.
Footnotes
Disclosures: Drs. Bent, Hendren, K. Law, and P. Law, and Ms. Zandi, Ms. Choi, Ms. Widjaja, Mr. Kalb, and Mr. Nestle report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Stephen Bent, University of California, San Francisco.
Dr. Robert L. Hendren, University of California, San Francisco.
Ms. Tara Zandi, Kennedy Krieger Institute.
Dr. Kiely Law, Kennedy Krieger Institute.
Ms. Jae-Eun Choi, University of California, San Francisco.
Ms. Felicia Widjaja, University of California, San Francisco.
Mr. Luther Kalb, Kennedy Krieger Institute.
Mr. Jay Nestle, Kennedy Krieger Institute.
Dr. Paul Law, Kennedy Krieger Institute.
References
- 1.Blumberg SJ, Bramlett MD, Kogan, et al. Changes in prevalence of parent-reported autism spectrum disorder in school-aged U S children: 2007 to 2011-2012. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 2.Lord C, Spence S. Autism spectrum disorders: phenotype and diagnosis. In: Moldin SO, Rubenstein JLR, editors. Understanding Autism: From Basic Neuroscience to Treatment. Boca Raton FL: CRC Press; 2006. [Google Scholar]
- 3.Lofthouse N, Hendren R, Hurt E, Arnold LE, Butter E. A review of complementary and alternative treatments for autism spectrum disorders. Autism Res Treat. 2012;2012:870391. doi: 10.1155/2012/870391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aman MG, Buican B, Arnold LE. J Child Adolesc Psychopharmacol. 1. Vol. 13. Spring; 2003. Methylphenidate treatment in children with borderline IQ and mental retardation: analysis of three aggregated studies; pp. 29–40. [DOI] [PubMed] [Google Scholar]
- 5.Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. Journal of autism and developmental disorders. 2000 Jun;30(3):245–255. doi: 10.1023/a:1005548619694. [DOI] [PubMed] [Google Scholar]
- 6.Amminger GP, Berger GE, Schafer MR, Klier C, Friedrich MH, Feucht M. Omega-3 fatty acids supplementation in children with autism: a double-blind randomized, placebo-controlled pilot study. Biological psychiatry. 2007 Feb 15;61(4):551–553. doi: 10.1016/j.biopsych.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Bent S, Bertoglio K, Ashwood P, Bostrom A, Hendren RL. A Pilot Randomized Controlled Trial of Omega-3 Fatty Acids for Autism Spectrum Disorder. Journal of autism and developmental disorders. 2011;41:545–554. doi: 10.1007/s10803-010-1078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry. 2011 Oct;50(10):991–1000. doi: 10.1016/j.jaac.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson AJ, Burton JR, Sewell RP, Spreckelsen TF, Montgomery P. Docosahexaenoic acid for reading, cognition and behavior in children aged 7-9 years: a randomized, controlled trial (the DOLAB Study) PloS one. 2012;7(9):e43909. doi: 10.1371/journal.pone.0043909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amminger GP, Schafer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Archives of general psychiatry. 2010 Feb;67(2):146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 11.Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006 Dec;67(12):1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 12.Bent S, Bertoglio K, Hendren RL. Omega-3 Fatty Acids for Autistic Spectrum Disorder: A Systematic Review. Journal of autism and developmental disorders. 2009;39:1145–1154. doi: 10.1007/s10803-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee H, Marvin AR, Watson T, et al. Accuracy of phenotyping of autistic children based on Internet implemented parent report. Am J Med Genet B Neuropsychiatr Genet. 2010 Sep;153B(6):1119–1126. doi: 10.1002/ajmg.b.31103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stata Statistical Software [computer program] Version 12.0. College Station, TX: Stata Corporation; [Google Scholar]
- 15.McCulloch CE, Searle SR. Generalized, Linear, and Mixed Models. New York, N.Y.: Wiley; 2000. [Google Scholar]
- 16.SAS. Version 9.3. Vol. 9. Cary, NC: SAS Institute; p. 3. computer program. [Google Scholar]
- 17.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990 Jan;1(1):43–46. [PubMed] [Google Scholar]
- 18.Arnold LE, Aman MG, Cook AM, et al. Atomoxetine for hyperactivity in autism spectrum disorders: placebo-controlled crossover pilot trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2006 Oct;45(10):1196–1205. doi: 10.1097/01.chi.0000231976.28719.2a. [DOI] [PubMed] [Google Scholar]
- 19.Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Archives of general psychiatry. 2005 Nov;62(11):1266–1274. doi: 10.1001/archpsyc.62.11.1266. [DOI] [PubMed] [Google Scholar]
- 20.Wong AD, Kirby J, Guyatt GH, Moayyedi P, Vora P, You JJ. Randomized controlled trial comparing telephone and mail follow-up for recruitment of participants into a clinical trial of colorectal cancer screening. Trials. 2013;14:40. doi: 10.1186/1745-6215-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCracken JT, McGough J, Shah B, et al. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002 Aug 1;347(5):314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs BP, Bent S, Tice JA, Blackwell T, Cummings SR. An internet-based randomized, placebo-controlled trial of kava and valerian for anxiety and insomnia. Medicine. 2005 Jul;84(4):197–207. doi: 10.1097/01.md.0000172299.72364.95. [DOI] [PubMed] [Google Scholar]
- 23.Warren Z, Vehorn A, Dohrmann E, Nicholson A, Sutcliffe JS, Veenstra-Vanderweele J. Accuracy of phenotyping children with autism based on parent report: what specifically do we gain phenotyping “rapidly”? Autism Res. 2012 Feb;5(1):31–38. doi: 10.1002/aur.230. [DOI] [PubMed] [Google Scholar]
- 24.Marcus RN, Owen R, Kamen L, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. JAm Acad Child Adolesc Psychiatry. 2009 Nov;48(11):1110–1119. doi: 10.1097/CHI.0b013e3181b76658. [DOI] [PubMed] [Google Scholar]
- 25.Owen R, Sikich L, Marcus RN, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009 Dec;124(6):1533–1540. doi: 10.1542/peds.2008-3782. [DOI] [PubMed] [Google Scholar]
- 26.Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004 Nov;114(5):e634–641. doi: 10.1542/peds.2003-0264-F. [DOI] [PubMed] [Google Scholar]
- 27.King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Archives of general psychiatry. 2009 Jun;66(6):583–590. doi: 10.1001/archgenpsychiatry.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]


