ABSTRACT
Recent advances in the cryopreservation of mouse sperm have resulted in dramatically improved in vitro fertilization (IVF) rates, but the biological mechanisms underlying the techniques remain unclear. Two different classes of compounds have been widely utilized to improve the IVF rates of cryopreserved mouse sperm: antioxidants and cyclodextrins. To determine how cryopreservation reduces mouse sperm IVF and how antioxidants and cyclodextrins mitigate this effect, we examined sperm function and oxidative damage after cryopreservation, with and without treatments, in mouse strains important for biomedical research. Our investigation revealed mouse strain-specific effects on IVF by modulation of oxidative stress and cholesterol efflux of cryopreserved sperm. Antioxidants improved IVF rates of C57Bl6/J cryopreserved mouse sperm by reducing hydrogen peroxide produced by sperm mitochondria and ameliorating peroxidative damage to the sperm acrosome. Enhancing cholesterol efflux with cyclodextrin restored capacitation-dependent sperm function and IVF after cryopreservation of C57Bl/6J, C57Bl/6N, and 129X1 mouse sperm. Our results highlight two accessible pathways for continued development of IVF techniques for mouse sperm and provide novel endpoints prognostic of IVF success. These insights may improve sperm cryopreservation methods of other mouse strains and species.
Keywords: acrosome reaction, cryopreservation, in vitro fertilization (IVF), oxidative stress, sperm capacitation
Mitochondrial hydrogen peroxide and defective cholesterol efflux prevent in vitro fertilization by mouse sperm after cryopreservation in a strain-dependent manner.
INTRODUCTION
Researchers attempting to cryopreserve sperm for archival of genetically modified mouse models have long been hampered by the severely reduced in vitro fertilization (IVF) rates for the inbred mouse strains most commonly used for genetic manipulation, C57Bl/6J (B6/J) and 129 substrains [1–5]. As a result, a wealth of new cryopreservation techniques to improve IVF rates has been published in the last few years [6–11]. The range of approaches utilized in these protocols is broad, but the two dominant methods include antioxidant supplementation [7–9] and the use of the high-affinity cholesterol acceptor macromolecule methyl-beta-cyclodextrin (CD) [3, 6, 8].
Improving IVF rates of B6/J sperm with antioxidants, during or after cryopreservation, suggests that some form of oxidative stress is interfering with IVF. Cryopreserved sperm of many species have shown evidence of oxidative stress, including elevated reactive oxygen species (ROS) production [12–15], reduced antioxidant capacity [16–18], membrane lipid peroxidation [18–21], and oxidative DNA damage [22–24]. Sperm are highly vulnerable to attack from ROS due to low cytoplasmic antioxidant capacity and membrane high polyunsaturated fatty acid content [25, 26]. While sperm generate ROS as an important component of signal transduction-stimulating capacitation [27], excessive levels of ROS impair sperm function by reducing sperm motility [28] and preventing normal acrosome reaction [29], and have been associated with male-factor infertility [30].
Similarly, increased efficiency of cholesterol removal by CD from cryopreserved B6/J sperm membranes is associated with higher IVF rates [6]. Efflux of membrane cholesterol is an essential initiation step of sperm activation and capacitation [31, 32], and CD has been shown to potently mediate these processes [33, 34]. The cooling and subsequent warming during cryopreservation disorganizes membrane lipids [35–37] and reduces membrane fluidity [38], potentially interfering with capacitation-associated membrane remodeling and cholesterol dynamics.
Due to the widespread use of the B6/J mouse strain for genetically modified mouse models, a substantial amount of research has been done to understand why these sperm lose fertilization ability after cryopreservation. The consensus is that cryopreserved B6/J sperm fail to penetrate the zona pellucida of the oocyte [5], based upon the improvement of IVF by removing, thinning, or drilling holes in the zona pellucida [5, 39], or performing intracytoplasmic sperm injection [40]. To successfully penetrate the zona pellucida, sperm must locate and pass through the extracellular matrix of the cumulus-oocyte complex (COC), undergo the acrosome reaction, and possess hyperactivated motility for sufficient mechanical force [41, 42]. Cryopreserved B6/J sperm are not deficient in percentage or progressive motility and are able to effectively bind the zona pellucida [1, 5, 43]. High-magnification electron microscopy of B6/J sperm during cryopreservation has shown damage to the sperm head membrane overlying the acrosome [5]. These lesions were reduced in mouse strains and IVF rates were unaffected by cryopreservation, suggesting an important role in IVF [5]. Incubation with glutathione (GSH) results in increased thiol content of the zona pellucida and expansion of the zona pellucida after IVF with cryopreserved B6/J sperm [8, 9], but the relationship of these changes to fertilization is unclear. Combined, this evidence suggests that oxidative stress is somehow interfering with zona pellucida penetration of B6/J mice.
Insufficient attention has been given to the biological mechanisms underpinning how these new protocols improve IVF rates. Understanding the biological basis for these improvements should aid in the development of protocols for cryopreservation of sperm where current techniques are unsatisfactory. Furthermore, it may be possible to apply these IVF techniques to other mouse strains commonly used for genetic manipulation and to improve the outcomes of B6/J mouse sperm cryopreservation beyond current levels. In this study we expand the testing of antioxidants and CD to cryopreserved 129X1 and C57Bl/6N (B6/N) mice, and use mouse strain-specific responses in IVF rates to show what aspects of oxidative damage and sperm function are determinant of IVF rate after cryopreservation.
MATERIALS AND METHODS
Media, Reagents, and Animals
All animals used in this study were obtained from Jackson Laboratories or were bred in our animal facilities and were used in accordance with all guidelines of the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. Fluorescent dyes SYTOX Blue, propidium iodide, Alexa488-conjugated soybean trypsin inhibitor, and BODIPY 581/591 were purchased from Invitrogen. Boronate-derived hydrogen peroxide (H2O2) fluorescent sensors PF6-AM, MitoPY1, and PG1 were synthesized in the laboratory of Dr. Chris Chang at the University of California at Berkeley. All chemicals not otherwise mentioned were obtained from Sigma. Human tubal fluid (HTF) was purchased from Irvine Scientific and was supplemented with 4 mg/ml fatty acid-free bovine serum albumin (BSA) before use. Experiments utilizing HTF were carried out in an incubator at 37°C with 5% CO2 in air added. Reduced GSH was added to HTF medium for sperm capacitation and IVF where indicated at 1 mM, and was allowed to equilibrate overnight before usage [9, 11]. CD, when used, was added to HTF at 0.75 mM along with 1 mg/ml of polyvinyl alcohol, and was only used for a 30-min sperm capacitation period [6].
Sperm Cryopreservation
Sperm were cryopreserved from B6129XF1, FVB/NJ, B6/J, B6/N, and 129X1/J male mice from 3–6 mo of age housed individually for at least 1 wk before sperm collection. Each cryopreserved sample utilized cauda epididymides, and vas deferentia were removed from two mice. Sperm were cryopreserved by the Nakagata method [2] by nicking each cauda epididymis five times and allowing 2 min for sperm to disperse into cryoprotectant medium composed of 18% raffinose and 3% skim milk with or without 477 μM monothioglycerol (MTG) [7]. The osmolarity of the cryoprotectant medium was verified to be within 485–495 mOsm with an Advanced Micro Osmometer (Advanced Instruments). Sperm were cryopreserved in 0.25 ml insemination straws by exposure to lipid nitrogen vapor as described by Stacy et al. [44] and stored under liquid nitrogen until use.
In Vitro Fertilization
Cryopreserved sperm were prepared for IVF by thawing straws with 37°C water and diluting the thawed sample into 200 μl of HTF. Freshly collected sperm for IVF experiments were prepared similarly to cryopreservation, except HTF was used in place of cryopreservation medium. Sperm suspensions were diluted and analyzed for motile concentration by computer-assisted sperm analysis (CASA; see Sperm Motility following this section) and added to 500-μl IVF drops to a final concentration of 0.2 million motile sperm per milliliter. Sperm were capacitated 60 min before IVF. Female B6129XF1 mice aged 8 to 16 wk were superovulated with 5 IU of equine chorionic gonadotropin followed by 5 IU of human chorionic gonadotropin (HCG) 48 h later. COCs were collected from female reproductive tracts 13 h after HCG administration, and two to four COCs were added to each IVF. IVF reactions were allowed to proceed 4–6 h, and oocytes were washed through four 200-μl drops of HTF. Fertilization was assessed as formation of 2-cell embryos 24 h after initiation of the IVF.
Sperm Motility
For studies of sperm motility, CASA was performed on Hamilton Thorne Biosciences IVOS with software version 12.3B using parameters for mouse sperm previously described [45]. Sperm were prepared for analysis by dilution of 10 μl of concentrated suspensions, either cryopreserved or freshly collected, into 1 ml of HTF and incubated 90 min before loading into 100-μm-deep Leja sperm analysis chambers. The CASAnova software algorithm was used for classification of sperm motility patterns [45]. For examination of capacitation-dependent motility patterns necessary for fertilization, the CASAnova hyperactive and intermediate patterns were combined.
Sperm Zona Pellucida Binding Ability
Sperm were allowed to incubate with COCs under IVF conditions for 1 h, at which time COCs were removed from the IVF reaction and treated with 0.1% hyaluronidase for 5–10 min to remove any adherent cumulus cells. Sperm-oocyte complexes were then washed four times to remove any loosely bound sperm and fixed by 1.25% glutaraldehyde in PBS with 1 mg/ml polyvinylpyrrolidone (PVP; PBS-PVP) for 10 min. Sperm and oocyte DNA were then stained by 1 ug/ml DAPI (4′,6-diamidino-2-phenylindole) in PBS-PVP for 10 min and subsequently washed. Sperm-oocyte complexes were mounted with Slow Fade Gold anti-fade mounting reagent (Invitrogen) on slides within 120-μm Secure Seal adhesive spacers (Invitrogen). Bound sperm were counted by differential interference contrast and DAPI fluorescent microscopy at 400× magnification on a Zeiss AxioImager M2 (Carl Zeiss).
Acrosome Reaction within the COC Extracellular Matrix
Given that in a recent report mouse sperm during in vitro fertilization primarily underwent acrosome reaction as they passed through the outer vestments of the COC rather than at the surface of the zona pellucida [46], we assessed the ability of cryopreserved sperm to undergo this process. Sperm were diluted into HTF containing 5 μg/ml Hoechst 33342 to stain sperm DNA, allowed to capacitate 60 min, and then placed in an IVF reaction with 2 μg/ml Alexa488-conjugated soybean trypsin inhibitor to label acrosome-reacted sperm [47, 48]. The IVF reaction was allowed to proceed for 30 min, as longer IVF times resulted in a significant proportion of the oocytes being penetrated at the time of assessment, altering their ability to bind acrosome-reacted sperm [48]. COCs were then washed in HTF and fixed with 2% paraformaldehyde in PBS-PVP for 60 min. Sperm counts within the COC were performed on a Zeiss AxioImager M2 at 250× magnification using Hoechst fluorescence for total sperm numbers and Alexa488 fluorescence for acrosome-reacted sperm (Supplemental Fig. S1; all supplemental data are available online at www.biolreprod.org).
Flow Cytometry Measurements
Flow cytometry analyses were performed with a Beckman-Coulter CyAn (Dako), with ten thousand sperm-specific events collected and nonsperm events gated out by forward- and side-scatter properties. SYTOX Blue staining was assessed using the 405-nm laser and 450/50 detector. PF6-AM [49] and MitoPY1 [50] were measured with the 488-nm laser coupled with the 530/40 detector. HEPES-buffered BWW medium [51] supplemented with 4 mg/ml BSA was used in lieu of HTF for all flow-cytometric analyses, except for BODIPY 581/591 staining, in which 1 mg/ml polyvinyl alcohol was used in place of BSA.
Measurement of Hydrogen Peroxide Levels
We employed a variety of fluorescent H2O2 probes that allowed measurements in several different cellular contexts. The family of probes used generates fluorescence only after specific oxidation of a boronate group to a phenol by H2O2 [52–54]. The specificity comes from the accompanying groups attached to the fluorophore scaffold [52–54]. Flow cytometry was used for measurements of intracellular H2O2 using an acetoxymethyl-coupled fluorescent H2O2 probe called PF6-AM (Supplemental Fig. S2A) [49] and mitochondrial-specific H2O2 with a mitochondrial-targeted boronate-based probe, MitoPY1 (Supplemental Fig. S2B) [50]. Staining was carried out by incubation of sperm in 200 μl of BWW-HEPES with 5 μM of either PF6-AM or MitoPY1 for 30 min. Sperm were washed by addition of 1 ml of 37°C BWW-HEPES, centrifugation at 300 × g for 3 min, and resuspension in 500 μl of BWW-HEPES containing 1 μM SYTOX Blue. Flow cytometry was performed immediately following staining with MitoPY1. PF6-AM-stained sperm were incubated 30 min before analysis to allow probe activation by cellular esterases [50]. Sperm suspensions with 1 mM H2O2 added were used as a positive control. Results from these probes are presented as the mean fluorescence intensities of live sperm, with dead SYTOX Blue-stained sperm excluded.
To assess total extracellular and intracellular H2O2 present in sperm suspensions in HTF, we employed PG1, a fluorescent cell-permeable H2O2 probe that is active intracellularly and in the extracellular media (Supplemental Fig. S2C) [55]. Sperm were diluted into 200 μl of HTF lacking phenol red, analyzed for motile concentration, and added to a 250-μl reaction volume at 0.2 million motile sperm per milliliter to mimic IVF conditions. PG1 was then added to the sperm suspension at a final concentration of 5 μM and incubated 1 h. The fluorescence of stained sperm suspensions was measured in duplicate in clear-bottomed 96-well plates by a FLUOstar Optima plate reader (BMG Labtech). Results are presented as relative fluoresce units after the value of a media blank has been subtracted. Media with 400 μM H2O2 added was used as a positive control.
Detection of Lipid Peroxidation
To assess lipid peroxidation sperm, were stained in 200 μl BWW-HEPES containing 5 μM BODIPY 581/591 for 30 min at 37°C [56]. Sperm treated with 80 μM ferrous sulfate were used as a positive control [56]. All cryopreserved sperm possessed bright green staining on the midpiece, and the intensity of this staining by flow cytometry was not different among strains (data not shown). We then microscopically examined the localization of membrane lipid peroxidation by allowing BODIPY-stained sperm to swim up for 10 min into 40 μl of BWW-HEPES containing 10 μM propidium iodide. Sperm were then placed on warmed, charged slides, and at least 100 sperm without propidium iodide staining were immediately assessed for green lipid peroxidation staining on the sperm head or the principal piece (see Figs. 2A and 3C).
Statistical Analysis
Statistical analyses were performed by Microsoft Excel 2004 version 11.6.6 (Microsoft). Four biological replicates were performed for each strain and treatment for all assays. Statistical significance was determined by two-tailed unpaired t-tests, and differences with P < 0.05 were considered significant.
RESULTS
Effects of Cryopreservation on B6/J Mouse Sperm
Cryopreservation of B6/J sperm causes a substantial decrease in IVF rate. In contrast, a negative control strain, a B6/J 129X1 F1 hybrid (B6129XF1), did not have statistically significant declines after cryopreservation (Fig. 1A). Low fertilization rates of B6/J with protocols routinely successful with other mouse strains have led to the use of antioxidants (MTG and GSH) and CD to improve IVF rates [6–11]. We validated that these compounds significantly improved IVF rates of cryopreserved B6/J sperm (Fig. 1A), but also found that none of the compounds fully restored the IVF rate to levels similar to unfrozen B6/J sperm (Fig. 1A)
FIG. 1.
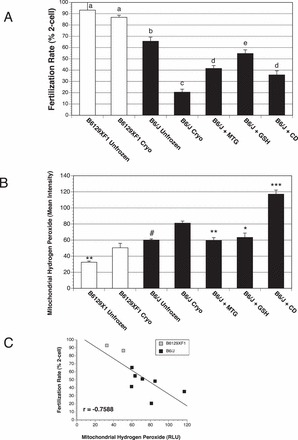
Decreased IVF rates of cryopreserved B6/J sperm are associated with increased mitochondrial H2O2, which is reduced by antioxidants. A) IVF rates measured by 2-cell embryo formation of freshly collected and cryopreserved sperm from B6/J and two control mouse strains. Letters indicate statistically distinct values (P < 0.05) by two-tailed, unpaired t-test of four biological replicates. B) Measurement of mitochondrial H2O2 levels of viable cryopreserved B6/J sperm by flow cytometry. Both antioxidants reduced mitochondrial H2O2 levels, and CD increased mitochondrial H2O2 levels. C) Correlation between IVF rate and mitochondrial H2O2 levels (r = −0.7588) indicates a strong relationship between these two endpoints for the control strains and B6/J. Differences were analyzed by two-tailed, unpaired t-test. Four biological replicates were performed for each group, and data are represented as the mean ± SEM. *, **, ***, and # indicate P < 0.05, 0.01, 0.001, and 0.001, respectively, by two-tailed, unpaired t-test compared to cryopreserved sperm of the same mouse strain.
Because of the observed improvement in IVF rates of B6/J cryopreserved sperm with antioxidants, we analyzed the dynamics of the long-lived cell-permeable ROS H2O2 in three different contexts: total (Supplemental Fig. S3A) [55], intracellular (Supplemental Fig. S3B) [49], and mitochondrial (Fig. 1B) [50]. Total and intracellular H2O2 levels were not elevated in B6/J sperm compared to the control strains (Supplemental Fig. S3, A and B). Total and intracellular H2O2 were only affected by antioxidant GSH and not MTG, despite the ability of MTG to improve IVF rate (Supplemental Fig. S3, A and B). Collectively, these results show that there is not a strong relationship between total and intracellular H2O2 and IVF rate.
Mitochondrial H2O2 levels were significantly higher in B6/J sperm than in the B6129XF1 control, both before (P < 0.0001) and after (P = 0.0011) cryopreservation (Fig. 1B). While there was a significant increase in H2O2 levels in the control strain after cryopreservation, these levels were still below that of unfrozen B6/J sperm (P < 0.05; Fig. 1B). Both antioxidants, MTG and GSH, reduced mitochondrial H2O2 in B6/J to precryopreservation levels, consistent with their ability to improve IVF rates (Fig. 1, A and B). Surprisingly, treating cryopreserved B6/J sperm with CD increased mitochondrial H2O2 (Fig. 1B). Although the trend for all of the data shows a strong inverse correlation between mitochondrial H2O2 and IVF rate for B6/J and B6129XF1 sperm (r = −0.7588; Fig. 1C), CD treatment does not fit this pattern, suggesting a downstream role (see discussion). For MTG and GSH, the inverse correlation suggests that reducing mitochondrial H2O2 by antioxidants is a mechanism for improving IVF rates of B6/J sperm.
We also investigated the location of lipid peroxidation on sperm plasma membranes after cryopreservation and its influence on IVF rate. Sperm membrane lipids are vulnerable targets of ROS, and their peroxidation can affect functions necessary for fertilization, including motility and the acrosome reaction [29, 57]. We found more frequent lipid peroxidation over the sperm head of cryopreserved B6/J sperm than that of the control strain (Fig. 2, A and B). Furthermore, both antioxidants were able to reduce the percentage of cryopreserved B6/J sperm with lipid peroxidation on the sperm head to levels similar to those of the control strain (Fig. 2B). CD did not have any effect on the presence of lipid peroxidation on B6/J sperm membranes after cryopreservation (Fig. 2B). We also assessed lipid peroxidation on the principal piece of cryopreserved sperm, but there was no statistical difference between B6129XF1 and B6/J (Supplemental Fig. S3C).
FIG. 2.
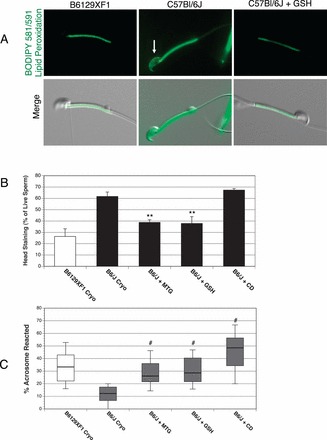
Lipid peroxidation on the head of B6/J cryopreserved sperm is associated with reduced acrosome reaction within COCs. A) Lipid peroxidation assessed by BODIPY 581/591 fluorescence is increased on the head of cryopreserved B6/J sperm (arrow) compared to that of B6129XF1. The membrane staining of at least 100 propidium iodide-negative sperm were assessed for staining of the sperm head (B). Increased staining on the head of cryopreserved B6/J sperm compared to that of the control strain was reduced by antioxidants, but unaffected by CD. Original magnification ×630. C) Assessment of acrosome reaction status of cryopreserved sperm within COC. The percentage of sperm with reacted acrosomes is lower for cryopreserved B6/J sperm, but restored with CD or antioxidant treatments. Differences were analyzed by two-tailed, unpaired t-test. Four biological replicates were performed for each group, and data are represented as the mean ± SEM. *, **, ***, and # indicate P < 0.05, 0.01, 0.001, and 0.001, respectively, compared to cryopreserved sperm of the same mouse strain.
After locating two sites of significant oxidative stress in cryopreserved B6/J sperm, we wanted to identify sperm functions damaged by cryopreservation. We first determined cryopreserved B6/J sperm were able to readily penetrate the extracellular matrix of the COC and bind the zona pellucida of the oocyte (Supplemental Fig. S4, A and B). This suggests cryopreserved B6/J sperm were unable to penetrate the zona pellucida, which could be due to an inability to achieve hyperactivated motility or undergo acrosome reaction. To assess sperm motility, we utilized CASA coupled with an algorithm for classifying the motility patterns [45]. We found cryopreserved B6/J sperm were not deficient in percentage, progressive, or hyperactivated motility compared to the control strain (Supplemental Fig. S5, A–C). We then assessed the ability of cryopreserved B6/J sperm to undergo acrosome reaction as they approached the oocyte through the cumulus cell matrix. Cryopreserved B6/J sperm within COCs possessed a reduced percentage of reacted acrosomes compared to the control strains, and both antioxidants and CD were able to increase this percentage (Fig. 2C). These data indicate that B6/J sperm are unable to effectively undergo acrosome reaction after cryopreservation, but this failure is eliminated with antioxidant and CD treatment.
Because of the divergent effects of CD and antioxidants on ROS and the report of increased IVF rates after combining GSH and CD in Takeo and Nakagata [8], we tested the effects of combinations of antioxidants and CD on IVF of cryopreserved B6/J sperm. Although Takeo and Nakagata [8, 11] added glutamine to the sperm cryopreservation medium and used TYH medium for sperm capacitation, we examined antioxidants and CD together with standard cryopreservation medium and standard IVF media to control for composition differences. In standard IVF media, both MTG with CD and GSH with CD failed to improve the IVF rate beyond the antioxidants alone (P > 0.05; Supplemental Fig. S6A). Combining CD with GSH eliminated its capacity to reduce cellular and mitochondrial H2O2 levels (Supplemental Fig. S6, B and C), and CD blunted mitochondrial H2O2 reduction by MTG (Supplemental Fig. S6C). These results suggest that glutamine and TYH medium may be responsible for the effectiveness of combining CD with an antioxidant.
Effects of Cryopreservation on B6/N Mouse Sperm
Because the B6/N substrain, which is increasingly being used for large-scale genetic knockout projects [58], has poor IVF rates after cryopreservation (Fig. 3A), we tested the ability of antioxidants and CD to improve IVF rates. After cryopreservation, IVF rates were unaffected by antioxidants, but were restored to unfrozen levels by CD (Fig. 3A). Consistent with the failure of antioxidants to improve IVF rates, mitochondrial (Fig. 3B), total (Supplemental Fig. S3A), and intracellular (Supplemental Fig. S3B) H2O2 levels for B6/N were similar to the control strain, FVB/N. The compounds that reduced lipid peroxidation on the head of cryopreserved sperm differed between B6/N and B6/J. MTG and CD reduced lipid peroxidation in B6/N (Fig. 3D), and MTG and GSH reduced levels in B6/J (Fig. 2B). Lipid peroxidative damage to the membranes of the B6/N sperm head after cryopreservation was associated with a reduction in acrosome-reacted sperm within COCs (Fig. 4A). CD increased the percentage of acrosome-reacted sperm after cryopreservation, but antioxidants did not (Fig. 4A). The data are consistent with loss of acrosome-reaction ability being the functional determinant of the IVF rate of these sperm.
FIG. 3.
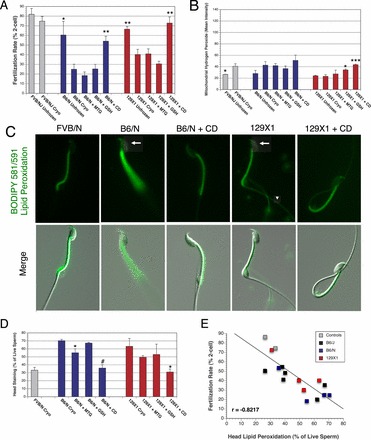
Restoration of IVF rates of cryopreserved B6/N and 129X1 sperm by CD is associated with reduced lipid peroxidation of the sperm head. A) IVF rates, measured by 2-cell embryo formation of freshly collected and cryopreserved sperm, from B6/N, 129X1, and control mouse strains FVB/NJ. CD fully restored IVF rates of B6/N and 129X1 cryopreserved sperm to precryopreservation levels, but antioxidants had no effect on IVF rates. B) Measurements of mitochondrial H2O2 in viable cryopreserved sperm by flow cytometry. H2O2 production was unchanged by cryopreservation in B6/N and 129X1 sperm, but increased in FVB/N. CD and GSH increased mitochondrial H2O2 production of 129X1 sperm to levels similar to those of FVB/N. C) Lipid peroxidation assessed by BODIPY 581/591 fluorescence is increased on the head of cryopreserved B6/N and 129X1 sperm (arrow) compared to B6129XF1, and was reduced by CD. Lipid peroxidation was also increased on the principal piece of 129X1 sperm (arrowhead). Original magnification ×630. D) The frequency of lipid peroxidation on the head of cryopreserved B6/N and 129X1 sperm was increased compared to that of FVB/N (P < 0.05), and was reduced by CD in both strains and MTG in B6/N sperm. E) Correlation between IVF rate and sperm head lipid peroxidation among all five strains in our study indicates a strong relationship between these two endpoints (r = −0.8217). Four biological replicates were performed for each group, and data are represented as the mean ± SEM. *, **, ***, and # indicate P < 0.05, 0.01, 0.001, and 0.001, respectively, by two-tailed, unpaired t-test compared to cryopreserved sperm of the same mouse strain.
FIG. 4.
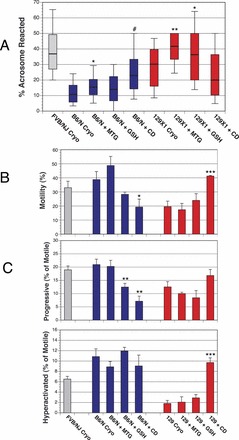
Mouse strain-specific inhibition of capacitation-dependent sperm function by cryopreservation of B6/N and 129X1 is rescued by CD. A) Assessment of acrosome reaction status of cryopreserved sperm within COC. The percentage of sperm with reacted acrosomes is lower for cryopreserved B6/N sperm than for FVB/N (P < 0.001), but increased by CD or MTG. MTG and GSH also increase the percentage of 129X1 sperm that underwent acrosome reaction. B) CASA assessment of percentage of motility shows CD increased the percentage of 129X1 cryopreserved sperm that are motile, but reduced the percentage of B6/N sperm that are motile. C) CASAnova classification of sperm motility patterns showed a decrease in progressive B6/N sperm treated with GSH or CD, and an increase in hyperactivated cryopreserved 129X1 sperm incubated with CD. Four biological replicates were performed for each group, and data are represented as the mean ± SEM. Differences between cryopreserved sperm of the same mouse strain were analyzed by two-tailed, unpaired t-test. *, **, ***, and # indicate P < 0.05, 0.01, 0.001, and 0.001, respectively.
Effects of Cryopreservation on 129X1 Mouse Sperm
Because of the importance of 129X1 mice for genetic manipulation and that mouse strain's previously characterized loss of IVF ability during cryopreservation, we wanted to examine whether this strain underwent damage resembling that of B6/J or B6/N sperm after cryopreservation, and if IVF rates could be restored by antioxidants or CD. We found the reduction in the IVF rate of cryopreserved 129X1 sperm was unaffected by antioxidants but the IVF rate was fully restored by incubation with CD, which is similar to the results in B6/N cryopreserved sperm (Fig. 3A). We next analyzed the H2O2 production of 129X1 cryopreserved sperm to determine if it bore any relationship to IVF rate. Intracellular and mitochondrial H2O2 production of 129X1 sperm were unchanged by cryopreservation (Fig. 3B and Supplemental Fig. S3B). However, CD and, unexpectedly, GSH increased mitochondrial and intracellular H2O2 production to levels comparable to those of the cryopreserved control strain (Fig. 3B and Supplemental Fig. S3B). Despite lower cellular production of H2O2, 129X1 cryopreserved sperm had more lipid peroxidation on the head and principal piece of the sperm tail than did the control strain (Fig. 3, C and D, and Supplemental Fig. S3C). CD reduced the percentage of cryopreserved 129X1 sperm with lipid peroxidation on both of these areas to levels found in the negative controls (Fig. 3, C and D, and Supplemental Fig. S4B), while MTG reduced lipid peroxidation only on the sperm principal piece (Supplemental Fig. S4B).
Next, we investigated how cryopreservation and CD affect 129X1 sperm function. In contrast to B6/J sperm, 129X1 acrosome reaction was not reduced by cryopreservation (Fig. 4A). Several aspects of motility were reduced after cryopreservation compared to the FVB/N control strain, including hyperactivation, progressive motility, and percentage of motility (Fig. 4, B–D). CD alleviated all of the reductions in 129X1 motility compared to the control strain, and produced significantly more motile and hyperactive sperm compared to 129X1 cryopreserved sperm alone. For all the strains in our study combined, the prevalence of lipid peroxidation on the sperm head showed a strong negative correlation with the fertilization rate (r = −0.8217; Fig. 3E).
DISCUSSION
Our investigation demonstrates two main factors contributing to reduced rates of IVF of cryopreserved mouse sperm: mitochondrial oxidative stress and membrane lipid peroxidation. Pharmacological mitigation of these factors in a strain-specific fashion can significantly restore IVF ability after cryopreservation. Membrane lipid peroxidation is present in all three strains exhibiting loss of IVF rate after cryopreservation in our study, but B6/J alone was also affected by mitochondrial oxidative stress (Table 1).
TABLE 1.
Summarized effects of antioxidants and CD on loss of IVF after cryopreservation.a
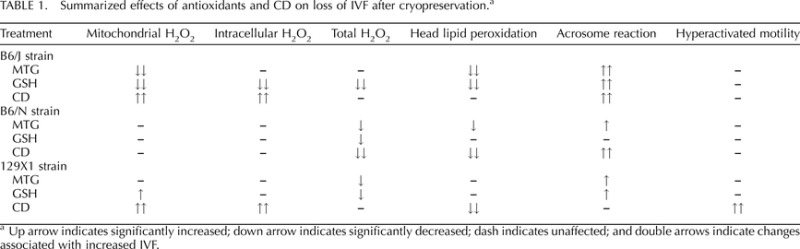
Up arrow indicates significantly increased; down arrow indicates significantly decreased; dash indicates unaffected; and double arrows indicate changes associated with increased IVF.
B6/J produced more mitochondrial H2O2 than any other strain tested, both before and after cryopreservation. The strong negative correlation between mitochondrial H2O2 and IVF rate is consistent with this ROS having a potent ability to interfere with IVF in B6/J. These data strongly support that reduction of mitochondrial H2O2 to precryopreservation levels is the mode of action of antioxidants GSH and MTG in improving IVF rates of B6/J sperm. Mitochondrial ROS have been implicated as the primary source of oxidative stress in human sperm, which is associated with male factor infertility [59] and characterized as an early event in the intrinsic apoptotic cascade of human sperm [59, 60].
Recent evidence in human sperm studies suggests mitochondrial ROS perpetuate oxidative cycling and lipid peroxidation [61]. Our data suggest a similar pathogenesis in cryopreserved B6/J sperm, where elevated mitochondrial H2O2 is associated with a high incidence of lipid peroxidation on the sperm head. H2O2 is a stable ROS capable of diffusing from its site of production in the sperm midpiece to the head [62], its site of membrane damage. The plasma and acrosomal membranes of the sperm head have long been understood to be highly susceptible to lipid peroxidation [63]. Electron microscopy studies of cryopreserved B6/J sperm have shown frequent membrane structural damage localized to the sperm head, corroborating our findings [5].
Peroxidation of the membranes present on the sperm head appears to be responsible for the reduced ability of cryopreserved B6/J sperm to undergo acrosome reaction. Reduction in head lipid peroxidation by antioxidants produced associated improvements in acrosome-reaction rates. This relationship has been demonstrated in human sperm by the ability of exogenous ROS to induce lipid peroxidation and reduce ionophore-induced acrosome reaction [29]. Furthermore, this study implicated H2O2 as the source of acrosome damage of human sperm. Catalase, which specifically detoxifies H2O2, was the only antioxidant capable of improving acrosome reaction of ROS-treated human sperm [29]. Only acrosome-reacted sperm are able to penetrate the zona pellucida of the oocyte [64], thereby explaining the previously observed defects in zona pellucida penetration of cryopreserved B6/J sperm [5].
The genetic similarity between the C57Bl/6 substrains, B6/J and B6/N, suggests a genetic basis for the elevated mitochondrial H2O2 phenotype of B6/J sperm. The genetic similarity between the two substrains is very high, with only five single nucleotide polymorphisms [65] and two genetic deletions [66, 67] between the two strains. One of the characterized deletions unique to B6/J mice is within the nicotinamide nucleotide transhydrogenase (Nnt) gene and results in a total loss of the protein [66, 68]. Nnt is a mitochondrial membrane protein involved in regeneration of mitochondrial antioxidants [69]. B6/N did not have the susceptibility to cryopreservation-induced mitochondrial oxidative stress exhibited by B6/J. The lack of Nnt in B6/J sperm mitochondria may impair regeneration of endogenous antioxidants GSH and thioredoxin, thereby impairing detoxification of mitochondrial H2O2 [66]. The deletion of Nnt in B6/J is associated with a variety of oxidative stress-based phenotypes, including impaired glucose tolerance [70], insulin resistance [71], and sensitization to genetic deletion of antioxidant proteins [66, 72]. Our study illustrates another instance in which the deletion of Nnt may play a role in an important phenotype of B6/J.
Our results that show MTG and GSH improved IVF for B6/J but not for 129X1 or B6/N contrast with other studies. Our results are largely due to the ability of antioxidants to mitigate the substantial increase in mitochondrial H2O2 that occurs in B6/J, but not in 129X1 or B6/N, after cryopreservation. Other strains not included in our study may suffer from increased mitochondrial H2O2 after cryopreservation and, like B6/J, benefit from MTG or GSH treatment. Using significantly smaller IVF volumes, Bath [9] examined IVF with GSH for cryopreserved mouse sperm and found that GSH increased IVF rates of B6/J, FVB/N, 129S1/SvImJ, and C3H/HeJ. However, when using conditions similar to ours, that study reported GSH increased IVF rates of only B6/J and FVB/N. These results suggest that H2O2 from cryopreserved sperm accumulates to inhibitory concentrations in small IVF volumes, and that these conditions impair a variety of mouse strains. Unlike Bath [9], we did not test GSH with FVB/N sperm, because their IVF rate was not significantly reduced by cryopreservation. Ostermeier et al. [7] reported that many strains, in addition to B6/J, benefited from new cryopreservation methodology that included MTG. However, with the exception of B6/J, these results are confounded by the other differences in their cryopreservation protocol.
CD improved IVF rates for 129X1, B6/N, and B6/J cryopreserved sperm, suggesting a common defect in the efflux of membrane cholesterol. CD has been shown to efficiently remove cholesterol from the B6/J sperm membranes after cryopreservation when albumin, which typically serves this function, is unable to do so [6]. Here we show that CD acts to allow normal capacitation-dependent sperm function, which is necessary for IVF in 129X1, B6/N, and B6/J cryopreserved sperm. Efflux of cholesterol is a key initiation event of sperm capacitation [73], and the potent ability of CD to induce capacitation of mouse sperm has been previously demonstrated [33, 34]. Cryopreservation has been shown to disorganize membrane lipids [35–37], and evidence suggests that CD removes cholesterol on a more indiscriminate basis than physiologically relevant albumin [74, 75], perhaps enabling it to remove cholesterol when lipid peroxidation has occurred or normal membrane structure is disrupted. These studies indicate that if capacitation-dependent sperm function is inhibited by cryopreservation, CD may improve IVF rates.
The type of capacitation-dependent sperm function rescued by CD after cryopreservation differed among mouse strains. CD alleviated cryopreservation-induced inhibition of acrosome reaction in B6/J and B6/N sperm, and hyperactivated the motility of 129X1 sperm. 129X1 sperm may be susceptible to loss of hyperactivated motility due to genetic polymorphisms in the PGK2 gene, resulting in lower baseline glycolytic rates [76, 77]. Microscopic evaluation of retention of membrane cholesterol in cryopreserved B6/J sperm showed pronounced staining over the acrosome [6], and removal of membrane cholesterol is necessary for acrosome reaction to proceed [78].
CD's IVF improvements after cryopreservation also differed between mouse strains. CD restored IVF rates with cryopreserved sperm to prefreezing levels in B6/N and 129X1, but produced only a moderate increase in B6/J sperm. In B6/J sperm, CD both hinders IVF by increasing mitochondrial H2O2 production and promotes IVF by improving the acrosome reaction. These antagonistic roles explain why CD treatment does not follow the strong negative correlation between mitochondrial H2O2 production and IVF rates in B6/J. Co-administering antioxidants with CD lowered mitochondrial H2O2 production and resulted in improved IVF rates compared to those in CD alone.
It is unclear why CD causes greater ROS production, but it could be due to elevated capacitation-dependent ROS production or the removal of BSA's antioxidant capacity in CD-containing media [79, 80]. When we co-administered CD and antioxidants to B6/J sperm, mitochondrial H2O2 remained above prefreezing levels, and precryopreservation IVF rates were not fully restored. This is contrary to the result reported in Takeo and Nakagata [8], where IVF rates were fully restored after addition of GSH and CD. That study, however, utilized TYH medium for sperm capacitation, rather than HTF. Other labs have analyzed the components in TYH and HTF and found that media calcium in HTF is detrimental to the IVF of cryopreserved B6/J sperm [10]. Examination of the effects of media calcium and ROS production of cryopreserved sperm warrants further investigation.
The second feature common to strains losing IVF rate after cryopreservation was lipid peroxidation of the sperm head membrane. The relationship of sperm head lipid peroxidation to loss of IVF of cryopreserved B6/J and B6/N sperm is readily explained by the associated loss of acrosome reaction in these sperm and the well-defined susceptibility of this organelle to oxidative stress [29, 63]. It is unclear, however, why 129X1 sperm head lipid peroxidation is not associated with a reduction in acrosome reaction. 129X1 sperm also had elevated lipid peroxidation on the principal piece of the flagellum, consistent with their loss of hyperactivation after cryopreservation.
While the source of membrane lipid peroxidation of B6/J sperm can readily be accounted for by high levels of mitochondrial H2O2, 129X1 and B6/N cryopreserved sperm exhibited lipid peroxidation without elevated H2O2, indicating a different pathogenesis. CD reduced lipid peroxidation in 129X1 and B6/N cryopreserved sperm, suggesting a direct relationship to the failure to remove membrane cholesterol. Efflux of sterols from sperm membranes is initiated by their oxidation [81], and this has been confirmed to occur in mouse sperm [75]. Retained products of sterol oxidation may be directly binding to the lipid peroxidation sensor, or they may serve as nucleation sites for peroxidation of adjacent lipid groups. It was additionally shown that CD removes oxidized sterols via a different mechanism than BSA [75], perhaps accounting for their divergent activities with cryopreserved mouse sperm. CD did not reduce lipid peroxidation in B6/J sperm, but this failure may be accounted for by the even greater burden of mitochondrial H2O2 produced by these sperm with CD present. The presence of lipid peroxidation of the head of cryopreserved mouse sperm indicates that incubation with CD prior to IVF will likely improve IVF rates.
Our findings illustrate the specific deficiencies of cryopreserved sperm that can be improved by CD and antioxidants, and expand use of these compounds to two additional mouse strains important for biomedical research. This knowledge will be informative when these technologies are applied to improving IVF rates of cryopreserved sperm from other species, mouse strains, or similarly susceptible individuals. Our studies additionally suggest that B6/J IVF rates may be improved further by reducing mitochondrial H2O2 to levels found in the control strains while simultaneously combating the cholesterol efflux defect of these sperm.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank Drs. Deborah O'Brien, Gary Klinefelter, Sally Darney, James Swenberg, Michael O'Rand, Andrew Fedoriw, and Mauro Calabrese for their critical discussions and reading of the manuscript, and the University of North Carolina at Chapel Hill School of Medicine Flow Cytometry Core Facility NCI Center Core Support Grant (P30CA06086) for their expertise in experimental design and technical support.
Footnotes
Supported by NIH grant D010924. B.C.D. is a Fellow of the Jane Coffin Childs Memorial Fund for Medical Research.
REFERENCES
- Sztein JM, Farley JS, Mobraaten LE. In vitro fertilization with cryopreserved inbred mouse sperm. Biol Reprod 2000; 63: 1774 1780. [DOI] [PubMed] [Google Scholar]
- Nakagata N. Cryopreservation of mouse spermatozoa. Mamm Genome 2000; 11: 572 576. [DOI] [PubMed] [Google Scholar]
- Liu L, Nutter LM, Law N, McKerlie C. Sperm freezing and in vitro fertilization in three substrains of C57BL/6 mice. J Am Assoc Lab Anim Sci 2009; 48: 39 43. [PMC free article] [PubMed] [Google Scholar]
- Bath ML. Simple and efficient in vitro fertilization with cryopreserved C57BL/6J mouse sperm. Biol Reprod 2003; 68: 19 23. [DOI] [PubMed] [Google Scholar]
- Nishizono H, Shioda M, Takeo T, Irie T, Nakagata N. Decrease of fertilizing ability of mouse spermatozoa after freezing and thawing is related to cellular injury. Biol Reprod 2004; 71: 973 978. [DOI] [PubMed] [Google Scholar]
- Takeo T, Hoshii T, Kondo Y, Toyodome H, Arima H, Yamamura K, Irie T, Nakagata N. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod 2008; 78: 546 551. [DOI] [PubMed] [Google Scholar]
- Ostermeier GC, Wiles MV, Farley JS, Taft RA. Conserving, distributing and managing genetically modified mouse lines by sperm cryopreservation. PLoS ONE 2008; 3: e2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo T, Nakagata N. Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-beta-cyclodextrin. Biol Reprod 2011; 85: 1066 1072. [DOI] [PubMed] [Google Scholar]
- Bath ML. Inhibition of in vitro fertilizing capacity of cryopreserved mouse sperm by factors released by damaged sperm, and stimulation by glutathione. PLoS ONE 2010; 5: e9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki-Migishima R, Hino T, Takabe M, Oda K, Migishima F, Morimoto Y, Yokoyama M. Marked improvement of fertility of cryopreserved C57BL/6J mouse sperm by depletion of Ca2+ in medium. J Reprod Dev 2009; 55: 386 392. [DOI] [PubMed] [Google Scholar]
- Takeo T, Nakagata N. Combination medium of cryoprotective agents containing L-glutamine and methyl-{beta}-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab Anim 2010; 44: 132 137. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Gagnon C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol Reprod Dev 2001; 59: 451 458. [DOI] [PubMed] [Google Scholar]
- Li Z, Lin Q, Liu R, Xiao W, Liu W. Protective effects of ascorbate and catalase on human spermatozoa during cryopreservation. J Androl 2010; 31: 437 444. [DOI] [PubMed] [Google Scholar]
- Peris SI, Bilodeau JF, Dufour M, Bailey JL. Impact of cryopreservation and reactive oxygen species on DNA integrity, lipid peroxidation, and functional parameters in ram sperm. Mol Reprod Dev 2007; 74: 878 892. [DOI] [PubMed] [Google Scholar]
- Kim SH, Yu DH, Kim YJ. Effects of cryopreservation on phosphatidylserine translocation, intracellular hydrogen peroxide, and DNA integrity in canine sperm. Theriogenology 2010; 73: 282 292. [DOI] [PubMed] [Google Scholar]
- Bilodeau JF, Chatterjee S, Sirard MA, Gagnon C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol Reprod Dev 2000; 55: 282 288. [DOI] [PubMed] [Google Scholar]
- Marti E, Marti JI, Muino-Blanco T, Cebrian-Perez JA. Effect of the cryopreservation process on the activity and immunolocalization of antioxidant enzymes in ram spermatozoa. J Androl 2008; 29: 459 467. [DOI] [PubMed] [Google Scholar]
- Alvarez JG, Storey BT. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J Androl 1992; 13: 232 241. [PubMed] [Google Scholar]
- Neild DM, Gadella BM, Chaves MG, Miragaya MH, Colenbrander B, Aguero A. Membrane changes during different stages of a freeze-thaw protocol for equine semen cryopreservation. Theriogenology 2003; 59: 1693 1705. [DOI] [PubMed] [Google Scholar]
- Thuwanut P, Axner E, Johanisson A, Chatdarong K. Detection of lipid peroxidation reaction in frozen-thawed epididymal cat spermatozoa using BODIPY(581/591) C11. Reprod Domest Anim 2009; 44 (suppl 2): 373 376. [DOI] [PubMed] [Google Scholar]
- Brouwers JF, Gadella BM. In situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radic Biol Med 2003; 35: 1382 1391. [DOI] [PubMed] [Google Scholar]
- Branco CS, Garcez ME, Pasqualotto FF, Erdtman B, Resveratrol Salvador M. and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology 2010; 60: 235 237. [DOI] [PubMed] [Google Scholar]
- Thomson LK, Fleming SD, Aitken RJ, De Iuliis GN, Zieschang JA, Clark AM. Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod 2009; 24: 2061 2070. [DOI] [PubMed] [Google Scholar]
- Zribi N. Feki Chakroun N, El Euch H, Gargouri J, Bahloul A, Ammar Keskes L. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil Steril 2010; 93: 159 166. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Curry BJ. Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal 2011; 14: 367 381. [DOI] [PubMed] [Google Scholar]
- Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol 2011; 7: 504 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford WC. Regulation of sperm function by reactive oxygen species. Hum Reprod Update 2004; 10: 387 399. [DOI] [PubMed] [Google Scholar]
- Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl 1987; 8: 338 348. [DOI] [PubMed] [Google Scholar]
- Griveau JF, Dumont E, Renard P, Callegari JP, Le Lannou D. Reactive oxygen species, lipid peroxidation and enzymatic defence systems in human spermatozoa. J Reprod Fertil 1995; 103: 17 26. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol 2008; 59: 2 11. [DOI] [PubMed] [Google Scholar]
- Osheroff JE, Visconti PE, Valenzuela JP, Travis AJ, Alvarez J, Kopf GS. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol Hum Reprod 1999; 5: 1017 1026. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Ning X, Fornes MW, Alvarez JG, Stein P, Connors SA, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol 1999; 214: 429 443. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm. Beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem 1999; 274: 3235 3242. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kondoh G. Mouse sperm undergo GPI-anchored protein release associated with lipid raft reorganization and acrosome reaction to acquire fertility. J Cell Sci 2011; 124: 2573 2581. [DOI] [PubMed] [Google Scholar]
- Ricker JV, Linfor JJ, Delfino WJ, Kysar P, Scholtz EL, Tablin F, Crowe JH, Ball BA, Meyers SA. Equine sperm membrane phase behavior: the effects of lipid-based cryoprotectants. Biol Reprod 2006; 74: 359 365. [DOI] [PubMed] [Google Scholar]
- Akhoondi M, Oldenhof H, Stoll C, Sieme H, Wolkers WF. Membrane hydraulic permeability changes during cooling of mammalian cells. Biochim Biophys Acta 2011; 1808: 642 648. [DOI] [PubMed] [Google Scholar]
- Oldenhof H, Friedel K, Sieme H, Glasmacher B, Wolkers WF. Membrane permeability parameters for freezing of stallion sperm as determined by Fourier transform infrared spectroscopy. Cryobiology 2010; 61: 115 122. [DOI] [PubMed] [Google Scholar]
- Giraud MN, Motta C, Boucher D, Grizard G. Membrane fluidity predicts the outcome of cryopreservation of human spermatozoa. Hum Reprod 2000; 15: 2160 2164. [DOI] [PubMed] [Google Scholar]
- Nakagata N, Okamoto M, Ueda O, Suzuki H. Positive effect of partial zona-pellucida dissection on the in vitro fertilizing capacity of cryopreserved C57BL/6J transgenic mouse spermatozoa of low motility. Biol Reprod 1997; 57: 1050 1055. [DOI] [PubMed] [Google Scholar]
- Szczygiel MA, Kusakabe H, Yanagimachi R, Whittingham DG. Intracytoplasmic sperm injection is more efficient than in vitro fertilization for generating mouse embryos from cryopreserved spermatozoa. Biol Reprod 2002; 67: 1278 1284. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Inoue N, Benham AM, Okabe M. Fertilization: a sperm's journey to and interaction with the oocyte. J Clin Invest 2010; 120: 984 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update 2008; 14: 647 657. [DOI] [PubMed] [Google Scholar]
- Sztein JM, Farley JS, Young AF, Mobraaten LE. Motility of cryopreserved mouse spermatozoa affected by temperature of collection and rate of thawing. Cryobiology 1997; 35: 46 52. [DOI] [PubMed] [Google Scholar]
- Stacy R, Eroglu A, Fowler A, Biggers J, Toner M. Thermal characterization of Nakagata's mouse sperm freezing protocol. Cryobiology 2006; 52: 99 107. [DOI] [PubMed] [Google Scholar]
- Goodson SG, Zhang Z, Tsuruta JK, Wang W, O'Brien DA. Classification of mouse sperm motility patterns using an automated multiclass support vector machines model. Biol Reprod 2011; 84: 1207 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci U S A 2011; 108: 4892 4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollner TL, Yudin AI, Cherr GN, Overstreet JW. Soybean trypsin inhibitor as a probe for the acrosome reaction in motile cynomolgus macaque sperm. Zygote 2000; 8: 127 137. [DOI] [PubMed] [Google Scholar]
- Baibakov B, Gauthier L, Talbot P, Rankin TL, Dean J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development 2007; 134: 933 943. [DOI] [PubMed] [Google Scholar]
- Dickinson BC, Peltier J, Stone D, Schaffer DV, Chang CJ. Nox2 redox signaling maintains essential cell populations in the brain. Nat Chem Biol 2011; 7: 106 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson BC, Chang CJ. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J Am Chem Soc 2008; 130: 9638 9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asquith KL, Baleato RM, McLaughlin EA, Nixon B, Aitken RJ. Tyrosine phosphorylation activates surface chaperones facilitating sperm-zona recognition. J Cell Sci 2004; 117: 3645 3657. [DOI] [PubMed] [Google Scholar]
- Miller EW, Albers AE, Pralle A, Isacoff EY, Chang CJ. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J Am Chem Soc 2005; 127: 16652 16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Pralle A, Isacoff EY, Chang CJ. A selective, cell-permeable optical probe for hydrogen peroxide in living cells. J Am Chem Soc 2004; 126: 15392 15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert AR, Van de Bittner GC, Chang CJ. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc Chem Res 2011; 44: 793 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat Chem Biol 2007; 3: 263 267. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Wingate JK, De Iuliis GN, McLaughlin EA. Analysis of lipid peroxidation in human spermatozoa using BODIPY C11. Mol Hum Reprod 2007; 13: 203 211. [DOI] [PubMed] [Google Scholar]
- Alvarez JG, Storey BT. Lipid peroxidation and the reactions of superoxide and hydrogen peroxide in mouse spermatozoa. Biol Reprod 1984; 30: 833 841. [DOI] [PubMed] [Google Scholar]
- Guan C, Ye C, Yang X, Gao J. A review of current large-scale mouse knockout efforts. Genesis 2010; 48: 73 85. [DOI] [PubMed] [Google Scholar]
- Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab 2008; 93: 3199 3207. [DOI] [PubMed] [Google Scholar]
- Koppers AJ, Mitchell LA, Wang P, Lin M, Aitken RJ. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J 2011; 436: 687 698. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Whiting S, De Iuliis GN, McClymont S, Mitchell LA, Baker MA. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J Biol Chem 2012; 287: 33048 33060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Jones KT, Robertson SA. Reactive oxygen species and sperm function–in sickness and in health. J Androl 2012; 33: 1096 1106. [DOI] [PubMed] [Google Scholar]
- Jones R, Mann T. Toxicity of exogenous fatty acid peroxides towards spermatozoa. J Reprod Fertil 1977; 50: 255 260. [DOI] [PubMed] [Google Scholar]
- Buffone MG, Ijiri TW, Cao W, Merdiushev T, Aghajanian HK, Gerton GL. Heads or tails? Structural events and molecular mechanisms that promote mammalian sperm acrosomal exocytosis and motility. Mol Reprod Dev 2012; 79: 4 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res 2004; 14: 1806 1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Naeemuddin M, Elchuri S, Yamaguchi M, Kozy HM, Carlson EJ, Epstein CJ. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum Mol Genet 2006; 15: 1187 1194. [DOI] [PubMed] [Google Scholar]
- Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, Caspi RR. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci 2012; 53: 2921 2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, Joseph S, Khan A, Epstein CJ, Sobel R, Huang TT. Enhanced expression of mitochondrial superoxide dismutase leads to prolonged in vivo cell cycle progression and up-regulation of mitochondrial thioredoxin. Free Radic Biol Med 2010; 48: 1501 1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydstrom J., Mitochondrial NADPH. transhydrogenase and disease. Biochim Biophys Acta 2006; 1757: 721 726. [DOI] [PubMed] [Google Scholar]
- Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 2006; 55: 2153 2156. [DOI] [PubMed] [Google Scholar]
- Freeman H, Shimomura K, Cox RD, Ashcroft FM. Nicotinamide nucleotide transhydrogenase: a link between insulin secretion, glucose metabolism and oxidative stress. Biochem Soc Trans 2006; 34: 806 810. [DOI] [PubMed] [Google Scholar]
- Huang YC, Hwang TL, Yang YL, Wu SH, Hsu MH, Wang JP, Chen SC, Huang LJ, Liaw CC. Acetogenin and prenylated flavonoids from Helminthostachys zeylanica with inhibitory activity on superoxide generation and elastase release by neutrophils. Planta Med 2010; 76: 447 453. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995; 121: 1129 1137. [DOI] [PubMed] [Google Scholar]
- Flesch FM, Brouwers JF, Nievelstein PF, Verkleij AJ, van Golde LM, Colenbrander B, Gadella BM. Bicarbonate stimulated phospholipid scrambling induces cholesterol redistribution and enables cholesterol depletion in the sperm plasma membrane. J Cell Sci 2001; 114: 3543 3555. [DOI] [PubMed] [Google Scholar]
- Boerke A, Brouwers JF, Olkkonen VM, van de Lest CH, Sostaric E, Schoevers EJ, Helms JB, Gadella BM. Involvement of bicarbonate-induced radical signaling in oxysterol formation and sterol depletion of capacitating mammalian sperm during in vitro fertilization. Biol Reprod 2013; 88: 21. [DOI] [PubMed] [Google Scholar]
- Vandeberg JL, Blohm SV. An allelic isozyme of mouse PGK-2 with low activity. J Exp Zool 1977; 201: 479 483. [DOI] [PubMed] [Google Scholar]
- Odet F, Gabel S, London RE, Goldberg E, Eddy EM. Glycolysis and mitochondrial respiration in mouse LDHC null sperm. Biol Reprod 2013; 88: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadella BM, Tsai PS, Boerke A, Brewis IA. Sperm head membrane reorganisation during capacitation. Int J Dev Biol 2008; 52: 473 480. [DOI] [PubMed] [Google Scholar]
- Armstrong JS, Rajasekaran M, Hellstrom WJ, Sikka SC. Antioxidant potential of human serum albumin: role in the recovery of high quality human spermatozoa for assisted reproductive technology. J Androl 1998; 19: 412 419. [PubMed] [Google Scholar]
- Ermilov A, Diamond MP, Sacco AG, Dozortsev DD. Culture media and their components differ in their ability to scavenge reactive oxygen species in the plasmid relaxation assay. Fertil Steril 1999; 72: 154 157. [DOI] [PubMed] [Google Scholar]
- Brouwers JF, Boerke A, Silva PF, Garcia-Gil N, van Gestel RA, Helms JB, van de Lest CH, Gadella BM. Mass spectrometric detection of cholesterol oxidation in bovine sperm. Biol Reprod 2011; 85: 128 136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


