ABSTRACT
Androgens/androgen receptor (AR) signaling is involved primarily in the development of male-specific phenotypes during embryogenesis, spermatogenesis, sexual behavior, and fertility during adult life. However, this signaling has also been shown to play an important role in development of female reproductive organs and their functions, such as ovarian folliculogenesis, embryonic implantation, and uterine and breast development. The establishment of the testicular feminization (Tfm) mouse model exploiting the X-linked Tfm mutation in mice has been a good in vivo tool for studying the human complete androgen insensitivity syndrome, but this mouse may not be the perfect in vivo model. Mouse models with various cell-specific AR knockout (ARKO) might allow us to study AR roles in individual types of cells in these male and female reproductive systems, although discrepancies are found in results between labs, probably due to using various Cre mice and/or knocking out AR in different AR domains. Nevertheless, no doubt exists that the continuous development of these ARKO mouse models and careful studies will provide information useful for understanding AR roles in reproductive systems of humans and may help us to develop more effective and more specific therapeutic approaches for reproductive system-related diseases.
Keywords: ARKO mice, androgens, androgen receptor, fertility, male sexual function, reproductive system, sex differentiation, spermatogenesis
Exploration of androgen receptor (AR) cell type- or tissue-specific roles in male and female reproductive systems using ARKO mouse models provides indications of AR function in the reproductive system in humans.
INTRODUCTION
Androgens are steroid hormones that control male sexual development and the adult male phenotype as well as maintenance of reproductive functions. Androgens exert most of their effects through genomic actions, which involve their binding to the androgen receptor (AR), leading to AR transactivation that in turn results in the modulation of AR downstream gene expressions [1]. Besides the genomic regulation, androgens are activated through nongenomic mechanisms [2, 3]. Therefore, several steroidal and nonsteroidal AR ligands have been developed for therapeutic use, including the treatment of male hypogonadism (AR agonists) and prostate diseases (AR antagonists) (for review, see [4]).
The ARs are expressed in male reproductive organs, such as efferent ductules, urogenital sinus, Wolffian ducts (WDs), epididymides, ductus deferens, seminal vesicles (SVs), coagulating glands, prostates, and bulbourethral glands, from Embryonic Day (E) 13 to Postnatal Day (P) 10 [5]. Androgens also influence female reproductive functions, including ovarian function through interaction with the AR during early follicular development [6, 7], and AR is expressed in various ovarian cell types [8, 9]. So, dysregulation of androgen/AR signaling is expected to perturb normal male and female reproductive development.
In vivo animal studies using androgen agonists/antagonists [10, 11] have revealed the roles of androgen/AR signaling in the male reproductive system. Importantly, the establishment of the testicular feminization (Tfm) mouse model exploiting the X-linked Tfm mutation in mice [12] provides an excellent in vivo model to study the human complete androgen insensitivity syndrome (CAIS), because both exhibit inactive AR and display female genital phenotypes, including cryptic testes, lack of male external genitalia, and absent accessary sex organs, despite differences in circulating levels of testosterone and estradiol (E2).
Importantly, the androgen effect may not be equal to the AR effect, and early data showed that the number of AR-positive cells in the human fetal penis was not changed after castration [13], suggesting that the mouse model with castration (loss of androgen) or total knockout of AR (loss of androgen and AR) may not be the perfect in vivo models to distinguish the androgen roles versus the AR roles in individual cell types within reproductive systems.
The development of various AR knockout (ARKO) mouse models with AR deleted in specific cell types provides an excellent tool to study AR physiological roles in selective cell types within reproductive systems. This review focuses on the AR roles in male and female reproductive systems, including those recent in vivo results generated from various ARKO mouse models concerning development and homeostasis of male and female reproductive systems. The mouse models are summarized in Table 1.
TABLE 1.
Summary of various ARKO mice studied in the reproductive systems.*
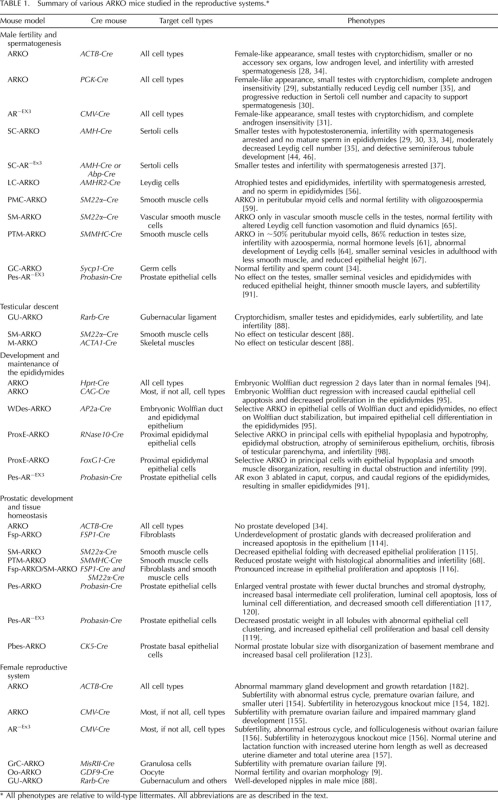
All phenotypes are relative to wild-type littermates. All abbreviations are as described in the text.
AR ROLES IN THE MALE REPRODUCTIVE SYSTEM
Sex determination in the human is a genetically controlled phenomenon that results in the commitment of an undifferentiated fetal gonad to either an ovary or a testis. Early human embryos develop both Wolffian (male) and Müllerian (female) genital ducts, with sex differentiation involving selective development of one duct and regression of the other. Testosterone levels begin to rise after differentiation of the interstitial cells (steroid-producing Leydig cells) of the fetal testis, which occurs by Embryonic Weeks 8–9. Testosterone controls development of WDs, accessory structures, and male external genitalia, including epididymis, SVs, and vas deferens via the conversion to the more active dihydrotestosterone (DHT) [14]. DHT is first detected between 9 and 13 wk of gestation and might become the mediator of differentiation and development of the prostate and prostatic urethra internally; differentiation of the external genitalia, including the penis, penile urethra, and scrotum; and testicular descent [15, 16].
These tissues and organs also contain multiple cell types with varying AR expression, so it is impossible to determine how the AR in each cell type regulates the developmental processes using these models in which the AR protein is inactive (Tfm) or completely lost (ARKO) in all cell types. Therefore, development of several cell-specific ARKO mouse models has been and continues to be essential to study androgen/AR signaling in various cell types during testicular spermatogenesis, testicular descent, and tissue homeostasis of the prostate and other auxiliary sexual organs in the mouse (Fig. 1, top).
FIG. 1.
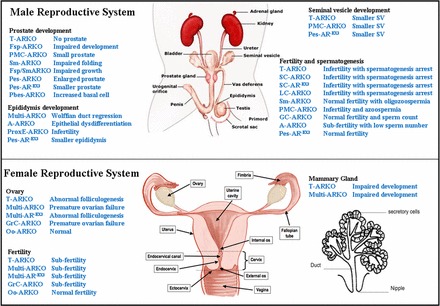
Effect of various tissue-specific ARKO on female and male reproductive systems in mice. In the male reproductive system (top), loss of the AR in specific types of cells can impair the fertility function, the spermatogenesis process, and the development of sex accessory organs, such as the epididymides, SV, and prostate. In the female reproductive system (bottom). the AR can play essential roles. ARKO in certain types of cells can cause abnormal development of the ovary and mammary gland, subfertility, and premature ovarian failure. T-ARKO is ARKO(ACTB-Cre), Multi-ARKO is ARKO(CMV-Cre), and Multi-AREx3 is AR-EX3(CMV-Cre) denoting total ARKO, multiple ARKO, and multiple AREX3 mice, respectively. The remaining abbreviations are as described in the text.
AR Roles in Male Fertility and Spermatogenesis
In mammals, testosterone and follicle-stimulating hormone (FSH) are the main regulators of the progression of spermatogenesis, which can be followed through various morphologically distinct stages in the testes [17]. The testis consists of two functional compartments, the seminiferous tubules and the interstitial space between them, with spermatogenesis arising in the seminiferous tubules and androgen biosynthesis in the interstitial Leydig cells [18]. The interstitial space consists of the testosterone-producing Leydig cells [19], macrophages, perivascular smooth muscle cells, and vascular endothelial cells.
The Sertoli cells provide the structural support for germ cell development [20, 21], including facilitation of germ cell movement and mature germ cell release [22], maintenance of the blood-testis barrier (BTB), and secretion of seminiferous tubular fluid [23], as well as various functional glycoproteins and peptides for nourishment of germ cells [24]. Thus, intimate functional and junctional communication between Sertoli and germ cells is essential for spermatogenesis [25].
Peritubular myoid cells, in conjunction with Sertoli cells, produce the basement membrane required to maintain normal tubule morphology. Several important functions of peritubular myoid cells have been proposed [26, 27].
The fact that spermatogenesis in Tfm [12] and various ARKO male [28–32] mice is arrested at the pachytene spermatocyte stage suggests the AR plays important roles during later stages of sperm formation and maturation by influencing the four major cell types: Sertoli, Leydig, peritubular myoid, and germ cells.
The AR in Sertoli cells is essential for spermatogenesis.
Sertoli cell-specific ARKO (SC-ARKO) male mice were generated [30, 33] by crossing exon 2-floxed AR mice [28, 29] with anti-Müllerian hormone (AMH) promoter-driven Cre (AMH-Cre) mice, which is specific for Sertoli cells in the testes and granulosa cells in the ovary [18]. Comparison of SC-ARKO mice with wild-type (Wt) mice indicated that SC-ARKO mice develop normally except for smaller testes (78.5% decline vs. Wt) [30, 33–36], smaller epididymides [29, 34, 36], and infertility, with spermatogenesis arrested at the diplotene premeiotic stage and no mature sperm detected in the epididymides [29, 33, 34]. Similar results were seen in SC-AR−EX3 (exon 3 is knocked out) mice despite the fact that a truncated AR with nongenomic function was still expressed in Sertoli cells and might influence Sertoli cell functions [37]. These observations suggest that in Sertoli cells, AR signaling, especially its genomic function, is critical for germ cell meiosis and spermatogenesis. Decreased germ cell (spermatogonia) proliferation [33] and increased apoptosis of pachytene and metaphase spermatocytes [29, 33, 34] were found in the testes of SC-ARKO mice compared to Wt mice. These findings support previous observations that testosterone regulates survival of spermatocytes and spermatids [38] and further suggest that AR signaling in Sertoli cells regulates survival of these cells. Serum testosterone levels decreased, whereas luteinizing hormone (LH) levels increased, in SC-ARKO mice compared to Wt mice [33, 36, 37]. The hypotestosteronemia in SC-ARKO mice might be due to increased expression of AMH [33], which regulates differentiation of Leydig cells and their production and secretion of testosterone. In contrast, elevated LH levels observed in these mice might be due to reduced feedback inhibition of their release by androgens. However, hypotestosteronemia and elevated LH levels in SC-ARKO mice were not observed in other studies [29, 35], and the discrepancy could be due in part to the use of different analyses [39, 40].
During spermatogenesis, the primary spermatocytes migrate from the basal compartment to the adluminal compartment, traveling across the BTB [41], with the highest AR-immunopositive nuclear staining in Sertoli cells occurring during stages VII–VIII [42, 43]. SC-ARKO testes displayed defective seminiferous tubule development, including delayed and defective Sertoli cell barrier formation with defective Sertoli cell maturation, reduced seminiferous tubule luminal volume, and faulty cytoskeletal development [36, 44]. At P10.5, seminiferous tubules in Wt testes began to form the central lumen, whereas this did not occur in SC-ARKO testes [36]. This lack of central lumen persisted to P50, at which the mice reach puberty. After puberty (P50), SC-ARKO testes exhibited obvious duplication of basal lamina in the seminiferous epithelium [36, 44]. Moreover, in tubules of SC-ARKO testes, the majority of spermatogenesis did not proceed beyond the pachytene primary spermatocyte stage [36].
Analysis of gene expression profiles at P10.5 or earlier displayed significant alterations in the expressions of vimentin and laminin α5 [36] as well as several other genes involved in tubular remodeling and junctional dynamics [44, 45]. Results of a subsequent study suggested that both Sertoli cell AR and the presence of differentiated germ cells are required for maintenance of the Sertoli cell organization within the seminiferous tubules [46]. One of the cytoskeletal components, beta-tubulin isotype Tubb3, an AR-target gene, was found to be differentially regulated between P10 and adulthood in SC-ARKO testes compared to Wt testes [47]. Early studies in Tfm mice have indicated that global inactivation of the AR resulted in reduction of Sertoli cell number in the testes [48], an observation confirmed in phosphoglycerate kinase promoter-driven Cre ARKO (ARKO[PGK-Cre]) mice [30]. In testes of the SC-ARKO [30, 39] and SC-AR−EX3 [37] mice, the Sertoli cell numbers were comparable to those in Wt testes, indicating that perinatal Sertoli cell proliferation is independent of Sertoli cell AR. However, a detailed examination indicated a disturbance of Sertoli cell nuclear maturation in SC-ARKO mice [33]. Comparison of gene expression between the testes of adult jsd (Utp14bjsd/jsd; juvenile spermatogonial depletion) and SC-ARKO/jsd mice suggested that some AR-regulated genes in Sertoli cells might be involved in the regulation of spermatogonial differentiation [49].
O'Shaughnessy et al. [40] generated mice lacking FSH receptor (FSHRKO), SC-ARKO, and FSHRKO/SC-ARKO double-knockout mice and investigated their AR role in Sertoli cell function and spermatogenesis. Sertoli cell numbers and total spermatogonia numbers were reduced in both FSHRKO/SC-ARKO and FSHRKO mice compared to Wt control mice, but no significant differences were found between the two mutant mice, supporting the role of FSH receptor in maintaining spermatogonia numbers and the lack of a role for Sertoli cell AR in spermatogonial development. In contrast, spermatocyte numbers were reduced in both FSHRKO and SC-ARKO testes and more substantially reduced in the FSHRKO/SC-ARKO testes, suggesting that both FSH receptor and Sertoli cell AR are involved in meiosis. O'Shaughnessy et al. [48] also compared testicular morphology and spermatogenesis among Wt, FSHRKO, SC-ARKO, FSHRKO/SC-ARKO, ARKO(PGK-Cre), and FSHRKO/ARKO(PGK-Cre) male mice. Analyses of testicular morphology and cell count showed that Sertoli cell numbers in ARKO(PGK-Cre) mice were decreased until adulthood, whereas those in FSHRKO mice were reduced slightly up to P20 and became more markedly reduced in adulthood. However, Sertoli or Leydig cell numbers were not altered in SC-ARKO mice. Leydig cell numbers in FSHRKO and ARKO(PGK-Cre) mice were normal from P1 to P5 but became significantly reduced by P20. Germ cell numbers were similar in all genotypes from P1 to P5 but became markedly reduced by P20 in FSHRKO and ARKO mice, and they were synergistically reduced when both receptors were knocked out. SC-ARKO mice had few changes in germ cell numbers but displayed synergistic effects with FSHRKO. Those authors concluded that FSH receptor and AR play age-dependent roles in testicular development and spermatogenesis.
The hypogonadal (hpg) mutant mice that lack circulating gonadotropins and intratesticular androgen production displayed disruption of spermatogenesis [50, 51]. To differentiate the direct roles of FSH from those mediated via androgen/AR, O'Shaughnessy et al. [52] generated hpg/SC-ARKO and hpg/ARKO(PGK-Cre) mice and investigated testicular morphology and spermatogenesis in response to FSH treatment. Both types of mice exhibited smaller testes, but hpg/ARKO(PGK-Cre) testes had fewer germ cells and Sertoli cells than hpg mice. Treatment with FSH increased testes sizes in both genotypes but increased intratesticular testosterone level only in hpg testes. It was concluded that FSH acted to stimulate spermatogenesis through increasing spermatogonial numbers and subsequent entry of these cells into meiosis but had no direct effect on the completion of meiosis, being partially dependent on androgen/AR signaling. In addition, the stimulatory effect of FSH on Leydig cell numbers was shown to be dependent on interstitial AR [53], and probably dependent on AR in Leydig cells [54].
Schauwaers et al. [55] generated a germline AR knock-in mouse model termed SPARKI in which mouse AR DNA-binding zinc finger 2 (zf2) sequence was replaced by glucocorticoid receptor second zinc finger sequence. These male SPARKI mice exhibited normal body weight, with normal body fat, muscle, and bone. Their serum testosterone and gonadotropin levels were also normal. However, male SPARKI mice exhibited smaller reproductive organs than Wt mice and were subfertile except for normal testicular descent. Germ cell analysis indicated that meiosis was not completely blocked, with the number of round and elongated spermatids reduced by 47% and 44%, respectively. This observation indicated that Sertoli cells of SPARKI mice could not fully support completion of meiosis.
In summary, the AR in Sertoli cells likely is essential for normal spermatogenesis and fertility and might play an important role in regulating the expression of several genes involved in seminiferous tubular restructuring, junctional dynamics, and nursery functions of germ cells, although the exact AR regulatory mechanisms remain to be elucidated.
The AR in Leydig cells may be essential for normal spermatogenesis.
Leydig cell-specific ARKO (LC-ARKO) male mice have been generated [56] by crossing of male mice carrying Leydig cell-specific AMH receptor 2 promoter-driven Cre recombinase (Amhr2-Cre) [57] with females carrying homozygous exon 2-floxed AR [28]. Male LC-ARKO mice exhibited normal appearance compared to Wt male mice except for atrophied testes and epididymides. LC-ARKO mice were infertile, with spermatogenesis found to be arrested predominately at the round spermatid stage and no sperm detected in epididymides. LC-ARKO mice also had lower serum testosterone levels and higher serum LH and FSH levels than Wt mice [56]. Hypotestosteronemia in LC-ARKO mice may not be attributed to the reduced numbers of Leydig cells but to the alterations in the expression levels of several key steroidogenic enzymes, including 17β-hydroxysteroid dehydrogenase III (17βHSD3), 3βHSD6, and cytochrome P450c17 (P450c17), which were also found to be altered in Leydig cells of adult Tfm mouse testes [58]. These observations suggested that a functional AR in Leydig cells might be essential to maintain normal spermatogenesis, testosterone production, and male fertility [56]. However, immunohistochemical staining of LC-ARKO testes indicated that AR was not knocked out in all Leydig cells, and AR loss was also observed in some Sertoli cells. Therefore, the phenotypes observed in LC-ARKO mice could be due to combined AR ablation effects in Leydig and Sertoli cells.
The AR in peritubular myoid cells in spermatogenesis is inconclusive.
Testicular peritubular myoid cell-specific ARKO (PMC-ARKO) mice have been generated [59] by crossing male smooth muscle protein 22α (SM22α)-Cre mice [60] with female mice carrying heterozygous exon 2-floxed AR gene [28]. Serum levels of testosterone, LH, and FSH in PMC-ARKO mice were comparable to those in Wt mice, and the distribution and apoptosis rates of germ cells in the testes were similar between PMC-ARKO and Wt mice. Except for smaller testes and lower epididymal sperm counts (76% and 42.7% of those in Wt mice, respectively), the PMC-ARKO mice, which are also designated smooth muscle ARKO (SM-ARKO) mice, displayed normal appearance, with normal external and internal genitalia, as well as normal fertility compared to Wt mice. Several Sertoli cell functional and junctional genes and peritubular myoid cell contractility-related genes were also altered in PMC-ARKO mice [59]. Apparently, lack of AR in peritubular myoid cells affected their regulatory effects on Sertoli cell function, thereby decreasing nursery functions of Sertoli cells, which in turn led to reduction in germ cell differentiation and maturation processes. These events resulted in decreased sperm numbers in the epididymides. Therefore, it was concluded that the AR in peritubular myoid cells is not essential for progression of spermatogenesis but is required for optimal sperm number production.
Welsh et al. [61] generated a different peritubular myoid ARKO (PTM-ARKO) mouse model by crossing smooth muscle myosin heavy chain (SMMHC) promoter-driven Cre (SMMHC-Cre) mice [62] or SM22α-Cre mice with exon 2-floxed AR mice [29]. The expression of SMMHC-Cre was claimed to be confined to vascular and nonvascular smooth muscle cells [63]. Those authors reported that testes of the PTM-ARKO mice generated with SMMHC-Cre mice expressed Cre and mediated partial ARKO in peritubular myoid cells, whereas the SM22α-Cre-generated PMC-ARKO (or SM-ARKO) mice expressed Cre recombinase only in vascular smooth muscle cells in the testes and did not have knockout of AR in peritubular myoid cells [63].
The PTM-ARKO mice [61] displayed phenotypes very similar to those of the PMC-ARKO mice [59]. The PTM-ARKO mice showed changes in Leydig cell function, vasomotion, and fluid dynamics due to ARKO in vascular smooth muscle cells and became azoospermic and infertile. The weights of PTM-ARKO testes were comparable to those of Wt testes at P12 but decreased progressively with age starting at P15, reaching 86% reduction after puberty compared to Wt testes. PTM-ARKO seminiferous tubules were smaller in diameter and exhibited reduced luminal volume, fewer germ cells, and disorganized Sertoli cells compared to Wt tubules, particularly in the adult testes. Total germ cell volume was significantly decreased with age-dependent progressive reduction in all germ cell types. Intratesticular testosterone and serum LH levels were elevated in their PTM-ARKO mice compared to Wt mice. Welsh et al. [61] concluded that AR signaling in peritubular myoid cells is essential for normal testis function, spermatogenesis and fertility.
Further studies on PTM-ARKO mice indicated that the development, ultrastructure, and functions of Leydig cells were affected by ARKO in peritubular myoid cells. Immunohistochemical analysis indicated both normal and abnormal populations of Leydig cells, yet total Leydig cell numbers were not affected. In abnormal Leydig cells, the expression levels of the AR and Leydig cell marker genes were reduced. Welsh et al. [64] concluded that peritubular myoid cells appear to provide a paracrine modulation of adult Leydig cell development and function.
The discrepancies between the above-mentioned studies [59, 61, 64, 65] might be due to different extents of AR ablation in peritubular myoid cells. The RT-PCR data did not show the ARKO band in the testes of the SM-ARKO mice of Welsh et al. [65], as opposed to the PMC-ARKO (SM-ARKO) mice of Zhang et al. [59], which clearly exhibited the knockout band. In addition, Welsh et al. [65] were unable to see Cre expression in SM-ARKO testes, whereas Zhang et al. [59] were able to observe SM22α-Cre-mediated recombination of Rosa26-LacZ. These differences indicate that weakened SM22α-Cre mice might have been used by Welsh et al. [61]. It is possible that the knockout extent in PMC-ARKO mice [59] was lower than that in the PTM-ARKO model of Welsh et al. [61], thereby leading to a milder phenotype and the reduction of peritubular myoid cell AR signaling in PTM-ARKO mice [61], reaching a threshold level sufficient to disrupt spermatogenesis and cause infertility. In contrast, although SMMHC-Cre is expressed strongly in the testis, due to the ectopic germline expression of Cre [63, 66], the Cre mice might be capable of mediating low levels of recombination in vascular smooth muscle cells and some smooth muscle cell-deficient tissues [63]. In fact, AR ablation in smooth muscle cells of SVs [67] and the prostate [68] have been reported in PTM-ARKO mice. Similarly, Cre leakage in PTM-ARKO testes [61] might lead to partial ARKO in Sertoli and Leydig cells that is not sufficient to alter their function [61, 64].
The AR in germ cells may not be essential for normal spermatogenesis.
Immunohistochemical localization of the AR in germ cells has been a subject of controversy. Results of several studies [69–71] have indicated the presence, whereas those of others [54, 72, 73] have suggested the absence, of the AR in testicular germ cells of different species. Nevertheless, male germ cell-specific ARKO (GC-ARKO) mice have been generated [34] by mating male mice carrying synaptonemal complex protein 1 gene promoter-driven Cre (Sycp1-Cre) [74] with homozygous exon 2-floxed AR females [28]. Young adult male GC-ARKO mice displayed normal external and internal genitalia and normal fertility as well as normal spermatogenesis. Their epididymal sperm counts, sperm motility, and serum testosterone levels were also comparable to those of Wt mice. These observations indicate that androgen/AR signaling in testicular germ cells may not be essential for spermatogenesis [34], and they corroborate the same conclusion derived from the early observations by Lyon et al. [75], studying normal sperm development with Tfm germ cells in chimeric mice, and by Johnston et al. [76], studying spermatogonial transplantation.
The above-mentioned studies have provided the AR roles by which each testicular cell type modulates spermatogenesis and are summarized in Figure 1 (top).
AR Roles in Testicular Descent
Testicular descent occurs in two distinct and sequential phases [77]. During the first, called the transabdominal phase, the gubernaculum develops and grows, thus pulling the testes toward the base of the abdomen. Each of the two phases of testicular descent is regulated via a hormone secreted by Leydig cells, and disrupted production of either hormone is responsible for a disorder called cryptorchidism, or undescended testes [78]. Androgen/AR signaling might regulate inguinoscrotal testicular descent via the control of release of calcitonin gene-related peptide (CGRP) from the genitofemoral nerve (GFN) [79]. In addition, androgen/AR signaling might also involve cell proliferation in the gubernacular tip in response to CGRP stimulation [80].
The AR is expressed in the parenchymal cells of the gubernaculum during fetal and postnatal life [81]. In 3-day-old rats treated with E2 to induce cryptorchidism and with human chorionic gonadotrophin (hCG), the testicular descent was evoked with increased numbers of AR-expressing fibroblasts located in the connective tissue of the gubernacular cord and between striated muscle fibers in the gubernacular bulb [82]. In hCG-treated rats, increases also were found in AR-expressing GFN motor nuclei versus untreated rats [83]. In contrast, prenatal androgen blockade in rats, which caused cryptorchidism, decreased CGRP-containing neurons in the GFN [84], suggesting that androgen/AR signaling modulates CGRP production. Both PMC-ARKO and SM-ARKO mice exhibited normal testicular descent [59, 65], suggesting that the AR ablation in gubernacular fibroblasts and smooth muscle cells in these models might not be critical for normal testicular descent. Various neuronal ARKO mice [85–87] also displayed normal testicular descent, although it is not certain whether the AR in GFN was knocked out in these mice.
Most recently, Kaftanovskaya et al. [88] generated gubernaculum-specific ARKO (GU-ARKO) mice by crossing male mice carrying retinoic acid receptor 2 promoter-driven Cre (Rarb-Cre) [89] with female mice carrying homozygous exon 2-floxed AR [29]. The Rarb-Cre transgene was shown to express mainly in various gubernacular cells in newborn males, with strongest expressions seen in the mesenchymal cells of the gubernacular bulb and the epithelial cells of the gubernaculum ligament. Compared to Wt littermates, GU-ARKO mice exhibited AR ablation in all parts of the gubernaculum and some stromal cells of the caudal epididymides. These mice developed all parts of the male reproductive system except for 50% smaller testes and epididymides when compared to Wt littermates. However, the testes did not descend to the scrotum and remained located in the abdominal cavity. Interestingly, these mice were shown to be subfertile when young (age 6–12 wk), and infertile when older (age, 4–12 mo) despite normal testosterone and LH levels. Analyses of testes sections of older GU-ARKO mice indicated the presence of abnormal seminiferous tubules with arrested spermatogenesis and vacuolization of the Sertoli cells, and the sperm counts in the epididymides were very low compared to age-matched Wt mice. Histological analyses indicated maldescent of the testes from P1 to P12 so that the processus vaginalis of GU-ARKO mice only reached half the distance of that in the Wt mice, a phenomenon very similar to what has been observed in Tfm male mice [90]. In addition, significant numbers of genes involved in extracellular signaling, matrix composition, and muscle differentiation were found to be misregulated in the gubernacular cremasteric sac of GU-ARKO mice. However, ablation of the AR in smooth or striated muscle cells using SM22α-Cre- or striated muscle-specific actin-Cre (ACAT1-Cre)-mediated ARKO mice (M-ARKO) did not affect testicular descent, suggesting that androgen/AR signaling in other cell types in the gubernaculum regulates testicular descent [88]. Thus, the GU-ARKO mouse provides a useful in vivo model for studying the mechanism of testicular descent and AR-related cryptorchidism and associated infertility.
AR Roles in SV Development and Function
The SVs also depend on androgen/AR signaling for their normal development and differentiation and for maintenance of structure and function. The AR is expressed in all cell types (epithelial, stromal, and smooth muscle) in adult SVs. Welsh et al. [67] examined the development and function of SVs in their SMMHC-Cre-mediated PTM-ARKO mice with smooth muscle-specific AR ablation [61]. They observed that Cre recombinase was specifically expressed in smooth muscle cells of PTM-ARKO SVs, so AR was knocked out in smooth muscle cells but not in the epithelial cells or outer stromal layer of SVs. Adult PTM-ARKO mice exhibited significantly smaller SVs with less smooth muscle and reduced epithelial cell height compared to Wt mice. Adult PTM-ARKO SVs also exhibited reduced epithelial cell proliferation and seminal proteins production. Treatment of mice with testosterone plus E2 resulted in increased size of SVs and epithelial cell proliferation. It was concluded that the smooth muscle cells play a vital role in androgen-driven stromal-epithelial interaction in the SVs, which influences epithelial structure and function and limits proliferative response of epithelial cells to exogenous E2.
Simanainen et al. [91] examined the probasin-Cre-mediated prostate epithelium-specific AR−EX3 (pes-AR−EX3) mice and found that AR exon 3 was also knocked out in other sex accessary organs, including the SVs, epididymides, and vas deferens. Despite the unexplained Cre expression in the SVs smooth muscle cells, pes-AR−EX3 mice displayed smaller SVs than Wt mice. The distal region of pes-AR−EX3 SVs exhibited normal epithelial morphology with occasional small foci of hyperplastic epithelial cells, decreased size of the acini, and thinner stromal smooth muscle layer. Moreover, the expression levels of two androgen-dependent markers of epithelial function of SVs, SVS2 and SVP99, in pes-AR−EX3 mice were reduced compared to Wt SVs. This observation indicates that the genomic function of SV smooth muscle AR might be important for normal SV development and function. However, the floxed AR mice used for generating the pes-AR−EX3 mice already had increased SV mass compared to Wt mice [92], so it is not clear whether the abnormal morphology observed in SVs epithelium of pes-AR−EX3 mice was inherited from the floxed AR mice.
AR Roles in Epididymal Development and Homeostasis
The epididymides are male sex accessory organs involved in androgen-regulated maturation and storage of sperm. Stabilization and subsequent differentiation of the WD is under the control of testicular androgen. Early studies with Tfm mice indicated that the activity of the AR is important for virilization of the WD [93]. Welsh et al. [94] observed that embryonic treatment of male mice with an antiandrogen plus an inhibitor of androgen production or ablation of the AR gene (ARKO) resulted in regression of the WD. These observations also support the importance of androgen/AR signaling in WD development and differentiation.
Murashima et al. [95] investigated the role of the AR in WD stabilization and masculinization in mouse embryos. At E12.5, the approximate onset of testicular androgen production, AR was detected in the periductal mesenchyme of both male and female WDs. The AR expression was detected in the cranial portion of the WD in E14.5 male embryos during WD stabilization and gradually increased in both the epithelium and mesenchyme during morphogenesis of the WD from E16.5 to E18.5. The WD of chicken beta-actin promoter-driven Cre ARKO (ARKO[CAG-Cre]) male embryos displayed aberrant regression from cranial to caudal direction with increased epithelial cell apoptosis, suggesting the AR plays a pivotal role in maintaining epithelial survival during WD stabilization. Male embryos of WD epithelium-specific ARKO (WDes-ARKO) mice, generated by mating activating enhancer-binding protein 2-alpha promoter-driven Cre (AP2α-Cre) [96] male and exon 1-floxed AR [97] female mice, showed selective ablation of the AR in WD epithelium. In male WDes-ARKO embryos, AR was undetectable in the majority of epithelial cells in the WD and the epididymides at E14.5 and E18.5, yet WDs persisted and coiled epididymides developed at E18.5. No differences were found in the number of epithelial cells undergoing apoptosis or proliferation (at E14.5) or in the expression of smooth muscle cell differentiation markers (at E18.5) between WDes-ARKO and Wt embryos. These observations indicated that the epithelial AR signaling is not essential for WD epithelial cell survival or stabilization or for mesenchymal cell differentiation during gestation. In contrast, postnatal differentiation of epithelial cells into basal and principal cells was retarded, because expression of the markers of these cells were reduced in WDes-ARKO epididymides.
Krutskikh et al. [98] generated a proximal epididymis-specific ARKO (ProxE-ARKO) mouse model using ribonuclease 10 promoter-driven Cre (Rnase10-Cre) to elicit ARKO from exon 2-floxed AR [29]. At the onset of Rnase10 expression (P20–P25), the AR gene became selectively inactivated in the principal cells of the proximal epididymides in these mice, leading to caput epithelial hypoplasia and hypotrophy. Upon subsequent onset of spermiation, epididymal obstruction occurred, resulting in infertility. O'Hara et al. [99] also generated a ProxE-ARKO mouse model using forkhead box G1 promoter-driven Cre (FoxG1-Cre) mice [100] and exon 2-floxed AR mice [29]. They observed specific ARKO in epithelial principal cells but not in basal cells or in caput epididymides. At P21–P100, these mice failed to develop initial segments and displayed significant decreases in epithelial height as well as disruption of the smooth muscle layer, resulting in smaller and irregular ductal shapes compared to Wt mice. Their effluent ducts were accumulated with cell debris, proteinaceous deposits, and spermatozoa that led to ductal obstruction and infertility. These observations agreed well with those of Krutskikh et al. [98], indicating that the AR in the epididymal epithelial principal cells is required for proper postnatal development and function of the proximal epididymides.
Simanainen et al. [91] observed smaller epididymides in pes-AR−EX3 mice compared to Wt littermates when AR exon 3 was knocked out in the epithelia of caput, corpus, and caudal regions of the epididymides. They suggested that the interaction of AR−zf2 protein with some AR coregulators might influence phenotype manifestations in pes-AR−EX3 mice.
In male SPARKI mice, all sex organs, including epididymides, were smaller than in Wt mice [55], suggesting that maturation of spermatozoa in the epididymides might be impaired. Kerkhofs et al. [101] further studied the effect of ablating the AR function via interrupting selective androgen-response elements (AREs) involved in the structure and function of the epididymides. The epididymides of male SPARKI mice were found to display abnormal morphology and had threefold lower sperm count than Wt mice. These observations indicate that the AR participates in regulating epididymal sperm maturation and, at least in part, involves its genomic function via the selective AREs.
AR Roles in Prostatic Development and Tissue Homeostasis
Prostatic stromal AR as stimulator to promote prostate growth.
Tissue recombination studies by Cunha and Lung [102], Donjacour and Cunha [103], and Cunha and Young [104] using urogenital sinus mesenchyme (UGM) and urogenital sinus epithelium indicated that the AR in the UGM provides signaling for prostatic ductal morphogenesis. The stromal-epithelial interaction continues to operate and remains dependent on androgen/AR signaling in the adult prostate to maintain cellular homeostasis of the organ [105, 106]. Castration of the adults resulted in prostate involution due to epithelial cell apoptosis [107], a change that could be reversed by androgen supplementation, which restored prostatic size and ductal morphology [108, 109]. Prostate tissues recombined from rat UGM plus Wt epithelium or from rat UGM plus Tfm epithelium exhibited similar rates of apoptosis in castrated hosts, and androgen supplementation inhibited apoptosis of these recombinants in a similar manner [110]. These observations suggest that survival of prostatic epithelial cells is dependent on stromal, but not epithelial, AR signaling.
Gao et al. [111] reported that human and rat prostatic epithelial cell organoids transplanted subcutaneously into intact nude mice were able to grow by attracting host mouse mesenchymal cells to form a stromal environment. However, transplantation of rat prostatic epithelial cells into castrated nude Tfm mice with or without testosterone supplementation invariably resulted in the rudimentary structure with very low epithelial proliferation and loss of epithelial AR. These observations indicate that stromal AR is required for prostatic epithelial proliferation and maintenance of epithelial AR expression.
During development, pleiotrophin, which plays important roles in cellular growth and differentiation, was expressed in the ventral mesenchymal pad and prostatic mesenchyme, and its protein was localized to the mesenchyme surrounding the epithelial tips of prostatic ducts undergoing branching morphogenesis as well as on the epithelial surface [112]. When added to the rat ventral prostate organ culture, human pleiotrophin stimulated branching morphogenesis and proliferation of stromal and epithelial cells as well as growth of fetal human prostate fibroblasts, prostate cancer-associated fibroblasts, and benign prostatic hyperplagia (BPH) epithelial cells. The expression levels of pleiotrophin were reduced in hypoxanthine-guanine phosphoribosyltransferase promoter-driven Cre ARKO (ARKO[Hprt-Cre]) mice (generated with exon 2-floxed AR mice [29]) compared to Wt mice, and testosterone stimulated the expression of pleiotrophin in fetal human prostate fibroblasts and female rat ventral mesenchymal pads in organ culture [112]. These observations suggest that androgen/AR signaling may modulate prostatic stromal and epithelial cell proliferation during prostatic development through regulation of pleiotrophin expression, because pleiotrophin might be the stromal paracrine growth factor that was implied by the study of Gao et al. [111].
The prostatic stroma consists of fibroblasts, smooth muscle cells, endothelial cells, and immune cells [113]. A recent study of fibroblast-specific protein 1 promoter-driven Cre-mediated ARKO (Fsp-ARKO) mice with the AR specifically knocked out in fibroblasts [114] resulted in underdevelopment of the prostate gland. Examination of male SM-ARKO [59] mice also showed a decreased epithelial infolding with decreased epithelial cell proliferation [115]. Welsh et al. [94] examined the prostate of their PTM-ARKO mice, which also displayed ARKO in prostatic smooth muscle cells, and found decreased prostatic weight with various histological abnormalities, including hyperplasia, inflammation, fibrosis, and altered expressions of various epithelial, smooth muscle, and stem cell markers. Male Fsp-ARKO/SM-ARKO double-knockout mice have also been generated and found to display more profound decreases in epithelial cell proliferation and increases in luminal epithelial cell apoptosis [116]. These observations are in good agreement with those of Gao et al. [111] and thus strongly suggest that prostatic stromal AR is important as a positive regulator of epithelial cell proliferation and survival and for prostatic stromal-epithelial interaction.
Prostatic epithelial AR as survival factor for luminal epithelial cells.
Prostatic epithelial AR also plays a role as a survival factor for luminal epithelial cells as revealed from studies of prostatic epithelial cell-specific ARKO (pes-ARKO) mice by Wu et al. [117]. After crossing exon 2-floxed AR mice [28] with probasin-Cre mice [118], the AR expression levels in prostatic epithelium of pes-ARKO mice were found to decrease gradually, starting at 6 wk of age, until becoming undetectable at 24 wk, along with increasing probasin expression. Upon histological analysis of tissues from 6- to 32-wk-old mice, the ventral prostate in pes-ARKO mice displayed progressive decrease in epithelial height, loss of glandular infolding, and increases in luminal epithelial cells apoptosis [119]. However, no significant apoptosis in basal cells was observed [120]. Instead, the p63-positive [119] or CK5/CK8 double-positive epithelial basal intermediate cell population in the prostate of pes-ARKO mice increased during puberty and then remained elevated [119, 120], whereas the CK8/CK18-positive luminal epithelial cell population declined, which was accompanied by decreased levels of various differentiation markers [119]. Thus, prostatic epithelial AR is believed to be an important survival factor for luminal epithelial cells.
AR in epithelial cells as a suppressor for basal intermediate cell proliferation.
The expansion of basal intermediate CK5/CK8-positive cell population observed in the ventral prostate of pes-ARKO mice studies [117] might be due to increased proliferation of CK5-positive basal and stem/progenitor cells. An expansion of CK5/CK8 double-positive basal intermediate cell population was found. Moreover, the prostates of pes-ARKO mice exhibited less ductal branches and a thinner smooth muscle layer, with fewer differentiated smooth muscle cells, than Wt mice. The stromal dystrophy in pes-ARKO mice appeared to be associated with a reduced level of transforming growth factor-β1 signaling, which is involved in stromal differentiation [120]. Male AR(T857A)/pes-ARKO double-transgenic mice, generated by crossing pes-ARKO mice with AR(T857A) transgenic mice carrying a constitutively active murine AR mutant [121], displayed the normal prostatic morphology and glandular histology [117]. Thus, the increase in basal intermediate cell proliferation, the loss of luminal epithelial cells, and stromal dystrophy in pes-ARKO mice are attributed to the loss of epithelial AR signaling.
Simanainen et al. [119] examined the prostate of their pes-AR−EX3 mutant mouse model, which was also generated with the probasin-Cre mice. At 8 wk of age, pes-AR−EX3 mice developed prostates with normal lobular structures and ductal branching. The weights of all prostate lobules and SVs in pes-AR−EX3 mice were significantly lower than those of Wt littermates, with the most pronounced reduction seen in anterior prostates. Histological analyses indicated the presence of abnormal epithelial cell clustering, increased epithelial cell proliferation, and increased basal epithelial cells. Thus, it appears that genomic function of the AR in prostate epithelium may be involved in suppression of epithelial cell proliferation, albeit via unknown effects of AR−zf2 mutant protein. They also examined the effect of castration and androgen resupplementation on prostate involution and regeneration in pes-AR−EX3 and Wt mice [122], observed that castration resulted in similar prostate involution and epithelial apoptosis, and concluded that prostatic epithelial involution in castrated mice is dependent primarily on AR-dependent apoptosis signals.
Lee et al. [123] have generated a prostatic basal epithelial cell-specific ARKO (pbes-ARKO) mice by crossing male CK5-Cre mice with female exon 2-floxed AR mice [28]. The pbes-ARKO mice exhibited similar body weights, testosterone levels, and prostate lobular sizes as Wt littermates. Histological analyses indicated increased proliferation of both CK5-positive and CK5/CK18 double-positive cells, without any difference in basal cell apoptosis, as compared to Wt mice. These observations indicate that expression of the AR in basal epithelial cells suppresses their proliferation, which is in agreement with the cell line studies showing suppression of proliferation in basal and stem/progenitor cells with ectopic expression of AR [123–125]. Together, these two opposite roles of luminal epithelial and basal epithelial ARs appear to contribute significantly to cellular homeostasis in the prostate.
AR ROLES IN THE FEMALE REPRODUCTIVE SYSTEM
One of the valuable lessons from ARKO mouse models is the discovery of AR roles in the female reproductive system, which remain largely unclear due to the lack of proper in vivo mouse models despite the fact that the AR is expressed in various female reproductive tissues, including the mammary gland [126], ovary [126–131], uterus [126, 129, 132, 133], fallopian tubes [134], and vagina [133], of various mammalian species. The AR roles in the female reproductive system implicated from the studies of ARKO mice are depicted in Figure 1 (bottom).
AR Roles in Ovarian Folliculogenesis and Fertility
In females, androgens are produced mainly in the ovaries and adrenal glands. In the ovary, testosterone is synthesized by theca cells in response to LH. It is generally believed that androgens directly influence ovarian function through interaction with AR during early follicular development yet serve as precursors for the synthesis of estrogens during late preovulatory development [6, 7].
In various mammalian species, the AR is expressed in various ovarian cell types, including theca, granulosa, stroma, and oocytes [129, 135–139]. Exposure of nonhuman primates [140] and women [141, 142] to high serum androgens resulted in development of large ovaries with increased numbers of antral follicles. Both testosterone and DHT promote mouse follicular growth in vitro [143, 144], and DHT treatment enhanced the ovulation rate in pigs [145]. Because the AR is expressed predominantly in granulosa cells of growing ovarian follicles, it is believed that androgen exerts direct actions on these cells. Thus, many of the differentiating actions of FSH on granulosa cells, including cholesterol metabolism, progesterone secretion, expression of steroidogenic enzymes, and induction of aromatase activity, were augmented by AR agonists [146]. It has been reported that androgen treatment up-regulated the expression of ovarian FSH receptor [146, 147] in various species as well as that of insulin-like growth factor (IGF)-I and IGF-I receptor (IGF-IR) in granulosa cells [148] and oocytes [149] of rhesus monkeys. In addition, it has been reported that DHT enhanced proliferation of porcine cumulus-oocyte complexes in small antral follicles stimulated by IGF-I and in large antral follicles stimulated by FSH [150], suggesting that the AR may function at different stages of follicular development. Androgen has been proposed to promote atresia during cyclic recruitment [151]. This notion is supported by the observation of high levels of atretic follicles in the ovaries of estrogen receptor beta (ERβ) knockout mice with overexpression of the AR in granulosa cells and restoration of healthy late antral follicles and corpora lutea in these mice after treatment with the antiandrogen hydroxyflutamide (HF) [152].
An early study using Tfm/Tfm AR mutant mice by Lyon and Glenister [153] showed that inactivating AR resulted in age-dependent premature ovarian failure and loss of fertility whereas Tfm/+ heterozygous females did not display any abnormal fertility. Similarly, studies with female human beta-actin promoter-driven Cre-generated (ARKO[ACTB-Cre]) [28, 154] or cytomegalovirus promoter-driven Cre-generated (ARKO[CMV-Cre]) [155] mice demonstrated that loss of the AR in female mice resulted in subfertility with prolonged estrous cycle, decreased litter numbers and sizes, and shortened reproductive life. Similar observations of subfertility and prolonged estrous cycle were made in female AR−EX3(CMV-Cre) mice in which the AR genomic function was ablated despite the fact that the affected cells still expressed the AR−zf2 protein with nongenomic function [156]. These observations clearly indicate that the AR genomic function is important for normal folliculogenesis and fertility in female mice.
Analyses of the ovaries of ARKO(ACTB-Cre) mice in comparison with Wt littermates indicated no differences in the numbers of growing follicles at an early age (4 wk), whereas at an older age (16 wk), the ARKO(ACTB-Cre) ovaries exhibited less corpora lutea than Wt ovaries, suggesting a defective luteinization [154]. Upon stimulation of superovulation in young mice with exogenous hormones, ARKO(ACTB-Cre) mice produced fewer oocytes than Wt mice. Morphological analyses of the ovaries indicated that preovulatory follicles in ARKO(ACTB-Cre) mice produced smaller and fewer corpora lutea than Wt mice after superovulation stimulation. In addition, in ARKO(ACTB-Cre) mice, the preovulatory follicles reached only the small luteal cell stage, with no luteolysis, suggesting a delayed or defective luteinization. Moreover, the cumulus-oocyte complexes in large, growing antral follicles in ARKO(ACTB-Cre) ovaries appeared to be defective. The decrease in granulosa cell numbers in ARKO(ACTB-Cre) preovulatory follicles appeared to be associated with increasing rates of cell apoptosis in primary, preantral, and antral follicles upon stimulation of superovulation [154]. Heterozygous ARKO(ACTB-Cre) female mice also displayed subfertility to some extent [28, 154], but no detailed study was made in these mice.
Shiina et al. [155] observed that female homozygous ARKO(CMV-Cre) mice in comparison with Wt female mice exhibited normal levels of serum hormones, including FSH, LH, testosterone, progesterone, and E2. However, ARKO ovaries displayed an age-dependent loss of all types of ovarian follicles and corpora lutea, and the mice became completely infertile. These observations clearly suggest that AR signaling is required to maintain normal ovarian folliculogenesis, luteinization, and ovulation and are in good agreement with results from studies on Tfm/Tfm female mice by Lyon and Glenister [153].
In contrast, Walters et al. [156] observed that heterozygous and homozygous AR−EX3(CMV-Cre) female mice exhibited age-dependent defects in late follicular development and subfertility but no complete ovarian failure even at 52 wk of age. In addition, no significant difference was found in follicular population numbers between the AR−EX3 and Wt mice from 12 to 52 wk of age. Those authors also performed ovarian transplantation in ovariectomized Wt and AR−EX3 hosts [157] and observed that AR−EX3 hosts transplanted with Wt ovaries exhibited abnormal estrous cycles, with 37.5% of them infertile. In contrast, Wt hosts transplanted with either Wt or AR−EX3 ovaries exhibited normal estrous cycles and fertility. The authors concluded that female AR−EX3 mice have both extraovarian neuroendocrine and ovarian functional defects, suggesting that the AR is involved in both ovarian and neuroendocrine functions.
Sen and Hammes [9] generated granulosa cell-specific and oocyte-specific ARKO (Grc-ARKO and Oo-ARKO, respectively) mice by crossing the exon 2-floxed AR mice [29] with Müllerian-inhibiting substance receptor II promoter-driven Cre (MisRII-Cre) [158], also known as Amhr2-Cre [57] mice, and growth differentiation factor-9 promoter-driven Cre (GDF9-Cre) [159] mice, respectively. Homozygous Grc-ARKO female mice displayed premature ovarian failure and subfertility, with longer estrous cycles and fewer ovulated oocytes than heterozygous Grc-ARKO and Wt littermates. Ovaries of homozygous Grc-ARKO mice contained more preantral and atretic follicles, with fewer antral follicles and corpora lutea than ovaries of Wt mice. These phenotype manifestations in homozygous Grc-ARKO mice are similar to those observed in ARKO(ACTB-Cre) mice [154]. The follicles isolated from homozygous Grc-ARKO mice also exhibited a slower in vitro growth rate than Wt mice. In contrast, Oo-ARKO mice displayed normal fertility, estrous cycles, and ovarian morphology. These observations strongly suggested that the AR in granulosa cells is an important regulator of androgen-mediated follicular growth and development and might contribute to the premature ovarian failure and subfertility.
Apparent phenotypic discrepancies are found among the results of several groups. Walters et al. [156] observed no premature ovarian failure in homozygous AR−EX3(CMV-Cre) mice; whereas Shiina et al. [155] found ovarian failure in ARKO(CMV-Cre) mice. Hu et al. [154] observed that a few ARKO(ACTB-Cre) female mice became infertile at 5–6 mo of age. The discrepancy might be in part due to different ARKO strategies. Both Hu et al. [154] and Shiina et al. [155] employed Cre-loxP recombination strategies to knock out AR exon 2 and exon 1, respectively, and created premature stop codons leading to the absence of AR protein expression [154, 155] and, hence, complete loss of AR function. In contrast, Walters et al. [156] employed a strategy of knocking out only AR exon 3, allowing the expression of a truncated AR−zf2 protein. Such a mutant AR protein with nearly 900 amino acids might retain AR nongenomic function. Both AR N-terminal domain and ligand-binding domain (LBD) are capable of binding to various AR coregulators [160]. It is possible that the mutant AR−zf2 protein of Walters et al. [156] and Simanainen et al. [122] retains the capability to complex with the AR or other nuclear receptors and coactivators to influence their functions. Moreover, AR and ERα are capable of interaction through the AR N-terminal domain and ERα LBD. Cotransfection of the AR with ERα resulted in inhibition of AR and ERα transactivation [161]. The inhibitory effect of the AR on ERα and vice versa might not occur in mouse ovaries when both receptors are intact and function normally and would be absent when no AR protein is expressed [154, 155], but it might become effective with the expression of mutant AR−zf2 [156, 157]. These differences in AR protein expression in different ARKO mouse models might contribute to the observed phenotype discrepancies and the differences in ovarian gene expression profiles reported in these studies.
Apparent discrepancies also exist regarding the involvement of the AR in regulation of the estrous cycle. The results of ovarian transplantation studies by Walters et al. [157] suggested that the AR is involved in an extraovarian neuroendocrine regulation. In contrast, the results of Sen and Hammes [9] indicated that loss of AR function in granulosa cells is sufficient to effect dysregulation of the estrous cycle, suggesting possible generation of a negative modulator of cycle regulation. Ovaries of various mammalian species are subjected to sympathetic innervation to form an intrinsic neural network, which changes with age and declines with reproduction aging [162]. If the putative ovarian negative regulator generated by the loss of granulosa AR acts through the ovarian neural reflex, such an effect would not be observed in Wt hosts transplanted with mutant AR−EX3 ovaries. Therefore, it remains possible that both extra- and intraovarian AR regulatory mechanisms involving the estrous cycle exist.
AR Roles in Uterine Development and Embryonic Implantation
Despite the presence of AR in the uteri of various species [126, 129, 132, 133], the AR function in uteri is not clear at present, although androgens have uterotrophic effects [163]. In a porcine uterine study, Kowalski et al. [164] suggested that AR and ERα likely are interactive partners requisite for regulating endometrial gene expression and uterine growth. In immature rat uterus, the antiandrogen HF could block E2-induced proliferation of uterine luminal epithelial cells [165], and in ovariectomized rats, treatment with nonmetabolizable androgens resulted in stimulation of uterine growth [166].
Interestingly, AR may also mediate the antiproliferative effect of antiprogestins on the growth of endometrium [167]. Abnormal endometrial homeobox A10 (HOXA10) expression is associated with infertility in patients with endometriosis, and embryo implantation failed to occur in HOXA10-deficient mice [168]. The expression of HOXA10 is regulated by testosterone [168]. Women with polycystic ovarian syndrome and hyperandrogenemia exhibited a reduced expression of HOXA10 mRNA in their endometrial biopsies [169], suggesting that AR may participate in regulation of uterine receptivity to embryo implantation.
Hu et al. [154] observed that female ARKO(ACTB-Cre) mice had smaller uteri than Wt mice. Upon superovulation stimulation, the Wt uteri exhibited hypertrophy in the endometrium and displayed a thicker smooth muscle layer than ARKO(ACTB-Cre) uteri, suggesting that androgen/AR signaling is involved in the growth of myometrium and endometrium. These observations were similar to the effects of DHT in rat and mouse uteri [166]. However, it is not clear how loss of uterine AR signaling could contribute to the subfertility seen in ARKO(ACTB-Cre) mice. Both Shiina et al. [155] and Walters et al. [156] observed little uterine differences between either their ARKO(CMV-Cre) or AR−EX3(CMV-Cre) and Wt mice. CMV enhancer-mediated Cre expression was relatively weaker in the uterus, ovary, and breast than in the testis, heart, and brain [170, 171]. The discrepancies between these studies and that of Hu et al. [154] might be due in part to insufficient AR deletion in uteri of ARKO(CMV-Cre) or AR−EX3(CMV-Cre) mice.
A subsequent study by Walters et al. [157] indicated that AR−EX3(CMV-Cre) mice exhibited longer uterine horn length, with decreased uterine diameter and total uterine area (including endometrial and myometrial areas) as measured at diestrus and estrus, suggesting an AR regulatory role in uterine growth. These observations appear to be in agreement with the results of Hu et al. [154]. In contrast, ablation of AR genomic function had no effect on uterine function with respect to the rate of implantation loss, gestation length, pup weights, and pup survival rates [157]. Given the conflicting reports of uterine phenotypes in AR−EX3(CMV-Cre) mice [156, 157], further studies using cell type- or tissue-specific ARKO models will be needed to delineate the AR roles in uterine growth, development, and function.
AR Roles in Breast Development
The ovary secretes testosterone and E2, and both hormones appear to play important roles in breast growth. E2/ERα stimulates mammary epithelial cell proliferation, whereas testosterone can be converted into either DHT or E2 and thus can act either directly via AR or indirectly through ERα to control breast growth. It is generally believed that androgen/AR signaling may function as a suppressor of breast growth.
During the menstrual cycle in premenopausal women, circulating levels of E2 and testosterone peak at midcycle, whereas testosterone levels decrease while E2 levels further increase in the transition to the luteal phase, during which breast epithelial cell proliferation is highest. In contrast, circulating levels of E2 reach nadir while testosterone levels stay constant during the follicular phase, in which breast epithelial apoptosis occurs at the highest rate [172]. These phenomena are also regarded as reflecting the AR and ERα roles in breast growth as a suppressor and a stimulator, respectively.
Early studies in mice and rats showed that androgens/AR suppressed the development of fetal mammary rudiment [173] through reciprocal interactions between mammary epithelium and the surrounding mesenchyme [174, 175]. Tissue recombination studies with mesenchyme and epithelial tissues isolated from mammary anlagen of Tfm and Wt mice also demonstrated that the mesenchymal androgen/AR signaling mediated mammary epithelial degeneration [176].
Short-term, low doses of several forms of androgens have been shown to inhibit the estrogen-induced proliferation of the mammary epithelial cells in adult female rats, mice, and monkeys [177, 178]. Similar inhibitory effects are also observed in human and rat mammary glands in vitro [179, 180]. This effect might be mediated through the suppression of ERα in mammary epithelial cells [181]. However, because both mammary stromal and epithelial cells express AR [126], it is uncertain in which cell type the AR signaling mediates such an inhibitory effect.
The development of the mammary glands in female ARKO(ACTB-Cre) mice has also been examined. Yeh et al. [182] observed that mammary glands of female ARKO(ACTB-Cre) mice had less extended ductal system, with reduced numbers and sizes of terminal end buds, accompanied by a 50% lower proliferation rate compared to glands of Wt mice, suggesting the development of mammary glands in these mice is retarded during the prepubertal and pubertal stages. The retarded mammary gland development in prepubertal female ARKO(ACTB-Cre) mice was associated with reduced IGF-IR expression, mitogen-activated protein kinase (MAPK) activation, and cyclin D1 expression, suggesting that a defective AR → IGF-I/IGF-IR → MAPK → cyclin D1 signaling pathway may be involved in modulating mammary gland development and epithelial proliferation [183–185].
In mice ovariectomized at 4 wk of age and treated with E2, the expressions of mammary ER target genes, estrogen-responsive ring finger protein [186], and hepatocyte growth factor [187], which are important factors for breast growth [188, 189], were reduced in the glands of ARKO(ACTB-Cre) mice compared to Wt glands, indicating reduced ER signaling in these mice with ARKO. Mammary glands of mature female ARKO(ACTB-Cre) mice were filled with large bloated ducts terminating with bloated ends and contained fewer secondary and tertiary ductal branches as compared to glands of age-matched Wt mice. During pregnancy, the retarded ductal branch numbers were partially restored but still contained less milk-producing alveoli than Wt glands. These observations indicated that mammary ductal morphogenesis was impaired during pubertal development, pregnancy, and lactation in ARKO(ACTB-Cre) mice, whereas AR functions as a stimulator of mammary ductal differentiation. Shiina et al. [155] also observed similar defective development of mammary glands in their female ARKO(CMV-Cre) mice. Therefore, in female mice, AR is thought to be required for normal development of the mammary glands by modulating ductal branching and epithelial cell proliferation. Because AR is expressed in both the stroma and epithelium of the mammary gland, it is possible that the ARs in these tissues play roles in modulating the development of mammary glands.
It should be mentioned that women with CAIS are genetic males but exhibit apparently normal breast development after puberty [190]. However, when breast development in female ARKO mice and Wt littermates was compared, the loss of AR resulted in abnormal mammary duct branching [191]. Therefore, the differential breast development observed in CAIS patients (breast enlargement) and female ARKO mice (breast differentiation) cannot be used as the only indication of the existence of different AR roles in the two species.
CONCLUSIONS AND FUTURE PROSPECTS
In summary, for the male reproductive system, we observed the following important points. First, the observations made with SC-ARKO testes clearly indicate that the AR in Sertoli cells is essential for normal spermatogenesis and fertility. Second, the results of LC-ARKO mice studies suggest an essential AR role in Leydig cells in maintaining normal spermatogenesis, testosterone production, and normal male fertility. However, the possibility exists that the phenotypes observed in LC-ARKO mice could be due to combined AR ablation in Leydig and Sertoli cells, and the development of a better mouse model that can study Leydig cells AR function specifically is needed. Third, discrepancies exist in the results of PMC-ARKO and SM-ARKO mice studies in confirming the AR role in peritubular myoid cells in regulating spermatogenesis. Fourth, the AR in germ cells is not essential for normal spermatogenesis based on studies in the GU-ARKO mouse model. Fifth, AR roles in SVs were studied in PTM-ARKO and pes-AR-EX3 mouse models and showed that AR might be important for normal SV development and function. Sixth, studies using WDes-ARKO and SPARKI mouse models indicate that AR also participates in regulation of epididymal sperm maturation process. Seventh, prostatic stromal AR was shown to promote prostate growth based on studies using Fsp-ARKO/SM-ARKO double-ARKO mouse models. Eighth, prostatic epithelial AR was shown to act as a survival factor for luminal epithelial cells but as a suppressor for basal epithelial intermediate cell proliferation using the pes-ARKO mouse model.
For the female reproductive system, based on studies using Tfm, total ARKO, Grc-ARKO, and Oo-ARKO mouse models, we observed the following points. First, AR may function at different stages of follicular development. Second, AR is involved in ovarian functions. Third, apparent discrepancies exist regarding the involvement of the AR in regulation of the estrous cycle. Fourth, AR may participate in the regulation of uterine receptivity to embryo implantation.
Overall, the studies of ARKO mouse models prove that the AR indeed plays many important roles in the development and functions of male and female reproductive systems. Table 1 summarizes various ARKO mouse models included in this review. Although various studies of the cell type- or tissue-specific ARKO mice have established AR roles in reproductive organs, many aspects of AR roles remain to be elucidated using these mouse models. More importantly, AR regulatory mechanisms involved in these specific AR roles remain unclear. It is also essential to develop more mouse models specific for other cell types in which AR roles have not yet been investigated. In addition, double-knockout mice models could be applied to further delineate the AR roles. For example, cell type-specific ARKO and AR/ER double-knockout mice are needed to determine AR roles and its possible interaction with the ER in ovarian folliculogenesis and other functions in the female reproductive system.
Supplementary Material
Footnotes
Supported by National Institutes of Health (NIH) grants CA127300 and CA156700 and Taiwan Department of Health Clinical Trial and Research Center of Excellence grant DOH99-TD-B-111-004 (China Medical University, Taichung).
REFERENCES
- Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 2007; 28: 778 808. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol 2008; 29: 169 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol 2002; 16: 2181 2187. [DOI] [PubMed] [Google Scholar]
- Patrao MT, Silva EJ, Avellar MC. Androgens and the male reproductive tract: an overview of classical roles and current perspectives. Arq Bras Endocrinol Metab 2009; 53: 934 945. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Young P, Cunha GR. Androgen receptor expression in developing male reproductive organs. Endocrinology 1991; 128: 2867 2873. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Tetsuka M. Role of androgens in follicle maturation and atresia. Baillieres Clin Obstet Gynaecol 1997; 11: 249 260. [DOI] [PubMed] [Google Scholar]
- Tetsuka M, Hillier SG. Differential regulation of aromatase and androgen receptor in granulosa cells. J Steroid Biochem Mol Biol 1997; 61: 233 239. [PubMed] [Google Scholar]
- Walters KA, Middleton LJ, Joseph SR, Hazra R, Jimenez M, Simanainen U, Allan CM, Handelsman DJ. Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility. Biol Reprod 2012; 87: 151, 1 11. [DOI] [PubMed] [Google Scholar]
- Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol 2010; 24: 1393 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaosa K, Kishimoto A, Kizu R, Nakagawa A, Shiratsuchi A, Nakanishi Y. Perturbation of spermatogenesis by androgen antagonists directly injected into seminiferous tubules of live mice. Reproduction 2007; 133: 21 27. [DOI] [PubMed] [Google Scholar]
- Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology 2006; 223: 144 155. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature 1970; 227: 1217 1219. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Sutherland RS, DiSandro MJ, Hayward SW, Lipschutz J, Cunha GR. The effect of testosterone on androgen receptors and human penile growth. J Urol 1997; 158: 1113 1118. [DOI] [PubMed] [Google Scholar]
- Desjardins C. Endocrine regulation of reproductive development and function in the male. J Anim Sci 1978; 47 (suppl 2): 56 79. [PubMed] [Google Scholar]
- Tong SY, Hutson JM, Watts LM. Does testosterone diffuse down the wolffian duct during sexual differentiation? J Urol 1996; 155: 2057 2059. [PubMed] [Google Scholar]
- Hutson JM, Hasthorpe S, Heyns CF. Anatomical and functional aspects of testicular descent and cryptorchidism. Endocr Rev 1997; 18: 259 280. [DOI] [PubMed] [Google Scholar]
- McLachlan RI, O'Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, Robertson DM. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res 2002; 57: 149 179. [DOI] [PubMed] [Google Scholar]
- Lecureuil C, Fontaine I, Crepieux P, Guillou F. Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis 2002; 33: 114 118. [DOI] [PubMed] [Google Scholar]
- Saez JM. Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr Rev 1994; 15: 574 626. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Pfeiffer DC, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol 2000; 63: 1 15. [DOI] [PubMed] [Google Scholar]
- De Franca LR, Bartke A, Borg KE, Cecim M, Fadden CT, Yagi A, Russell LD. Sertoli cells in testes containing or lacking germ cells: a comparative study of paracrine effects using the W (c-kit) gene mutant mouse model. Anat Rec 1994; 240: 225 232. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004; 25: 747 806. [DOI] [PubMed] [Google Scholar]
- Waites GM, Gladwell RT. Physiological significance of fluid secretion in the testis and blood-testis barrier. Physiol Rev 1982; 62: 624 671. [DOI] [PubMed] [Google Scholar]
- Griswold MD. Protein secretions of Sertoli cells. Int Rev Cytol 1988; 110: 133 156. [DOI] [PubMed] [Google Scholar]
- Jegou B. The Sertoli-germ cell communication network in mammals. Int Rev Cytol 1993; 147: 25 96. [PubMed] [Google Scholar]
- Norton JN, Skinner MK. Regulation of Sertoli cell function and differentiation through the actions of a testicular paracrine factor P-Mod-S. Endocrinology 1989; 124: 2711 2719. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Schlitz SM, Anthony CT. Regulation of Sertoli cell differentiated function: testicular transferrin and androgen-binding protein expression. Endocrinology 1989; 124: 3015 3024. [DOI] [PubMed] [Google Scholar]
- Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A 2002; 99: 13498 13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A 2004; 101: 1327 1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, Denolet E, Verhoeven G. The role of androgens in Sertoli cell proliferation and functional maturation: studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology 2005; 146: 2674 2683. [DOI] [PubMed] [Google Scholar]
- Notini AJ, Davey RA, McManus JF, Bate KL, Zajac JD. Genomic actions of the androgen receptor are required for normal male sexual differentiation in a mouse model. J Mol Endocrinol 2005; 35: 547 555. [DOI] [PubMed] [Google Scholar]
- Yu JJ, Jiang YC. Association of testicular p63 expression and spermatogenesis in androgen receptor knockout (ARKO) mice. Aging Male 2011; 14: 72 75. [DOI] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci U S A 2004; 101: 6876 6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Yeh SD, Wang RS, Yeh S, Zhang C, Lin HY, Tzeng CR, Chang C. Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc Natl Acad Sci U S A 2006; 103: 18975 18980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K, Atanassova N, Tan KA, de Franca LR, Parreira GG, McKinnell C, Sharpe RM, Saunders PT, Mason JI, Hartung S, Ivell R, Denolet E, et al. Development and function of the adult generation of Leydig cells in mice with Sertoli cell-selective or total ablation of the androgen receptor. Endocrinology 2005; 146: 4117 4126. [DOI] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, Wu CC, di Sant'Agnese PA, deMesy-Bentley KL, Tzeng CR, Chang C. Androgen receptor in Sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology 2006; 147: 5624 5633. [DOI] [PubMed] [Google Scholar]
- Lim P, Robson M, Spaliviero J, McTavish KJ, Jimenez M, Zajac JD, Handelsman DJ, Allan CM. Sertoli cell androgen receptor DNA binding domain is essential for the completion of spermatogenesis. Endocrinology 2009; 150: 4755 4765. [DOI] [PubMed] [Google Scholar]
- Ruwanpura SM, McLachlan RI, Meachem SJ. Hormonal regulation of male germ cell development. J Endocrinol 2010; 205: 117 131. [DOI] [PubMed] [Google Scholar]
- Johnston H, Baker PJ, Abel M, Charlton HM, Jackson G, Fleming L, Kumar TR, O'Shaughnessy PJ. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology 2004; 145: 318 329. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Morris ID, Huhtaniemi I, Baker PJ, Abel MH. Role of androgen and gonadotrophins in the development and function of the Sertoli cells and Leydig cells: data from mutant and genetically modified mice. Mol Cell Endocrinol 2009; 306: 2 8. [DOI] [PubMed] [Google Scholar]
- Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat 1977; 148: 313 328. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Maddocks S, Millar M, Kerr JB, Saunders PT, McKinnell C. Testosterone and spermatogenesis. Identification of stage-specific, androgen-regulated proteins secreted by adult rat seminiferous tubules. J Androl 1992; 13: 172 184. [PubMed] [Google Scholar]
- Hill CM, Anway MD, Zirkin BR, Brown TR. Intratesticular androgen levels, androgen receptor localization, and androgen receptor expression in adult rat Sertoli cells. Biol Reprod 2004; 71: 1348 1358. [DOI] [PubMed] [Google Scholar]
- Willems A, Batlouni SR, Esnal A, Swinnen JV, Saunders PT, Sharpe RM, Franca LR, De Gendt K, Verhoeven G. Selective ablation of the androgen receptor in mouse Sertoli cells affects Sertoli cell maturation, barrier formation and cytoskeletal development. PLoS ONE 2010; 5: e14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denolet E, De Gendt K, Allemeersch J, Engelen K, Marchal K, Van Hummelen P, Tan KA, Sharpe RM, Saunders PT, Swinnen JV, Verhoeven G. The effect of a Sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol Endocrinol 2006; 20: 321 334. [DOI] [PubMed] [Google Scholar]
- Wang G, Weng CC, Shao SH, Zhou W, de Gendt K, Braun RE, Verhoeven G, Meistrich ML. Androgen receptor in Sertoli cells is not required for testosterone-induced suppression of spermatogenesis, but contributes to Sertoli cell organization in Utp14bjsd mice. J Androl 2009; 30: 338 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gendt K, Denolet E, Willems A, Daniels VW, Clinckemalie L, Denayer S, Wilkinson MF, Claessens F, Swinnen JV, Verhoeven G. Expression of Tubb3, a beta-tubulin isotype, is regulated by androgens in mouse and rat Sertoli cells. Biol Reprod 2011; 85: 934 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Monteiro A, Abel M. Testicular development in mice lacking receptors for follicle stimulating hormone and androgen. PLoS ONE 2012; 7: e35136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang G, Small CL, Liu Z, Weng CC, Yang L, Griswold MD, Meistrich ML. Gene expression alterations by conditional knockout of androgen receptor in adult Sertoli cells of Utp14bjsd/jsd (jsd) mice. Biol Reprod 2011; 84: 400 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature 1977; 269: 338 340. [DOI] [PubMed] [Google Scholar]
- Scott IS, Charlton HM, Cox BS, Grocock CA, Sheffield JW, O'Shaughnessy PJ. Effect of LH injections on testicular steroidogenesis, cholesterol side-chain cleavage P450 mRNA content and Leydig cell morphology in hypogonadal mice. J Endocrinol 1990; 125: 131 138. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Monteiro A, Verhoeven G, De Gendt K, Abel MH. Effect of FSH on testicular morphology and spermatogenesis in gonadotrophin-deficient hypogonadal mice lacking androgen receptors. Reproduction 2010; 139: 177 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Verhoeven G, De Gendt K, Monteiro A, Abel MH. Direct action through the Sertoli cells is essential for androgen stimulation of spermatogenesis. Endocrinology 2010; 151: 2343 2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl 2002; 23: 870 881. [PubMed] [Google Scholar]
- Schauwaers K, De Gendt K, Saunders PT, Atanassova N, Haelens A, Callewaert L, Moehren U, Swinnen JV, Verhoeven G, Verrijdt G, Claessens F. Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc Natl Acad Sci U S A 2007; 104: 4961 4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Lin HY, Yeh SD, Yu IC, Wang RS, Chen YT, Zhang C, Altuwaijri S, Chen LM, Chuang KH, Chiang HS, Yeh S, et al. Infertility with defective spermatogenesis and steroidogenesis in male mice lacking androgen receptor in Leydig cells. Endocrine 2007; 32: 96 106. [DOI] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet 2002; 32: 408 410. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Johnston H, Willerton L, Baker PJ. Failure of normal adult Leydig cell development in androgen-receptor-deficient mice. J Cell Sci 2002; 115: 3491 3496. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yeh S, Chen YT, Wu CC, Chuang KH, Lin HY, Wang RS, Chang YJ, Mendis-Handagama C, Hu L, Lardy H, Chang C. Oligozoospermia with normal fertility in male mice lacking the androgen receptor in testis peritubular myoid cells. Proc Natl Acad Sci U S A 2006; 103: 17718 17723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci U S A 2002; 99: 7142 7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Saunders PT, Atanassova N, Sharpe RM, Smith LB. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J 2009; 23: 4218 4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin HB, Deng KY, Rishniw M, Ji G, Kotlikoff MI. Smooth muscle expression of Cre recombinase and eGFP in transgenic mice. Physiol Genomics 2002; 10: 211 215. [DOI] [PubMed] [Google Scholar]
- Frutkin AD, Shi H, Otsuka G, Leveen P, Karlsson S, Dichek DA. A critical developmental role for tgfbr2 in myogenic cell lineages is revealed in mice expressing SM22-Cre, not SMMHC-Cre. J Mol Cell Cardiol 2006; 41: 724 731. [DOI] [PubMed] [Google Scholar]
- Welsh M, Moffat L, Belling K, de Franca LR, Segatelli TM, Saunders PT, Sharpe RM, Smith LB. Androgen receptor signalling in peritubular myoid cells is essential for normal differentiation and function of adult Leydig cells. Int J Androl 2012; 35: 25 40. [DOI] [PubMed] [Google Scholar]
- Welsh M, Sharpe RM, Moffat L, Atanassova N, Saunders PT, Kilter S, Bergh A, Smith LB. Androgen action via testicular arteriole smooth muscle cells is important for Leydig cell function, vasomotion and testicular fluid dynamics. PLoS ONE 2010; 5: e13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutkin AD, Shi H, Otsuka G, Dichek DA. Targeted rearrangement of floxed alleles in smooth muscle cells in vivo. Circ Res 2007; 101: e124 e125. [DOI] [PubMed] [Google Scholar]
- Welsh M, Moffat L, Jack L, McNeilly A, Brownstein D, Saunders PT, Sharpe RM, Smith LB. Deletion of androgen receptor in the smooth muscle of the seminal vesicles impairs secretory function and alters its responsiveness to exogenous testosterone and estradiol. Endocrinology 2010; 151: 3374 3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Moffat L, McNeilly A, Brownstein D, Saunders PT, Sharpe RM, Smith LB. Smooth muscle cell-specific knockout of androgen receptor: a new model for prostatic disease. Endocrinology 2011; 152: 3541 3551. [DOI] [PubMed] [Google Scholar]
- Kimura N, Mizokami A, Oonuma T, Sasano H, Nagura H. Immunocytochemical localization of androgen receptor with polyclonal antibody in paraffin-embedded human tissues. J Histochem Cytochem 1993; 41: 671 678. [DOI] [PubMed] [Google Scholar]
- Vornberger W, Prins G, Musto NA, Suarez-Quian CA. Androgen receptor distribution in rat testis: new implications for androgen regulation of spermatogenesis. Endocrinology 1994; 134: 2307 2316. [DOI] [PubMed] [Google Scholar]
- Zhou X, Kudo A, Kawakami H, Hirano H. Immunohistochemical localization of androgen receptor in mouse testicular germ cells during fetal and postnatal development. Anat Rec 1996; 245: 509 518. [DOI] [PubMed] [Google Scholar]
- Galena HJ, Pillai AK, Terner C. Progesterone and androgen receptors in non-flagellate germ cells of the rat testis. J Endocrinol 1974; 63: 223 237. [DOI] [PubMed] [Google Scholar]
- Suarez-Quian CA, Martinez-Garcia F, Nistal M, Regadera J. Androgen receptor distribution in adult human testis. J Clin Endocrinol Metab 1999; 84: 350 358. [DOI] [PubMed] [Google Scholar]
- Vidal F, Sage J, Cuzin F, Rassoulzadegan M. Cre expression in primary spermatocytes: a tool for genetic engineering of the germ line. Mol Reprod Dev 1998; 51: 274 280. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Glenister PH, Lamoreux ML. Normal spermatozoa from androgen-resistant germ cells of chimeric mice and the role of androgen in spermatogenesis. Nature 1975; 258: 620 622. [DOI] [PubMed] [Google Scholar]
- Johnston DS, Russell LD, Friel PJ, Griswold MD. Murine germ cells do not require functional androgen receptors to complete spermatogenesis following spermatogonial stem cell transplantation. Endocrinology 2001; 142: 2405 2408. [DOI] [PubMed] [Google Scholar]
- Hutson JM. A biphasic model for the hormonal control of testicular descent. Lancet 1985; 326: 419 421. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ. What signals testis descent? Biol Reprod 2010; 83: 687 689. [DOI] [PubMed] [Google Scholar]
- Foresta C, Zuccarello D, Garolla A, Ferlin A. Role of hormones, genes, and environment in human cryptorchidism. Endocr Rev 2008; 29: 560 580. [DOI] [PubMed] [Google Scholar]
- Shenker NS, Huynh J, Farmer PJ, Hutson JM. A new role for androgen in testicular descent: permitting gubernacular cell proliferation in response to the neuropeptide, calcitonin gene-related peptide. J Pediatr Surg 2006; 41: 407 412. [DOI] [PubMed] [Google Scholar]
- Staub C, Rauch M, Ferriere F, Trepos M, Dorval-Coiffec I, Saunders PT, Cobellis G, Flouriot G, Saligaut C, Jegou B. Expression of estrogen receptor ESR1 and its 46-kDa variant in the gubernaculum testis. Biol Reprod 2005; 73: 703 712. [DOI] [PubMed] [Google Scholar]
- Vigueras RM, Reyes G, Moreno-Mendoza N, Merchant-Larios H. Gubernacular fibroblasts express the androgen receptor during testis descent in cryptorchid rats treated with human chorionic gonadotrophin. Urol Res 2004; 32: 386 390. [DOI] [PubMed] [Google Scholar]
- Vigueras RM, Moreno-Mendoza N, Reyes G, Merchant-Larios H. Androgen receptor and calcitonin gene-related peptide in neurons of the genitofemoral nerve during testicular descent induced with human chorionic gonadotropin. Arch Med Res 2003; 34: 166 170. [DOI] [PubMed] [Google Scholar]
- Goh DW, Middlesworth W, Farmer PJ, Hutson JM. Prenatal androgen blockade with flutamide inhibits masculinization of the genitofemoral nerve and testicular descent. J Pediatr Surg 1994; 29: 836 838. [DOI] [PubMed] [Google Scholar]
- Yu IC, Lin HY, Liu NC, Sparks JD, Yeh S, Fang LY, Chen L, Chang C. Neuronal androgen receptor regulates insulin sensitivity via suppression of hypothalamic NF-kappaB-mediated PTP1B expression. Diabetes 2013; 62: 411 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci 2009; 29: 4461 4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S, Harada N, Shah NM. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 2010; 66: 260 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaftanovskaya EM, Huang Z, Barbara AM, De Gendt K, Verhoeven G, Gorlov IP, Agoulnik AI. Cryptorchidism in mice with an androgen receptor ablation in gubernaculum testis. Mol Endocrinol 2012; 26: 598 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 2005; 132: 2809 2823. [DOI] [PubMed] [Google Scholar]
- Griffiths AL, Momose Y, Hutson JM. The gubernaculum in adult female, adult male and TFM male mice. Int J Androl 1993; 16: 380 384. [DOI] [PubMed] [Google Scholar]
- Simanainen U, McNamara K, Davey RA, Zajac JD, Handelsman DJ. Severe subfertility in mice with androgen receptor inactivation in sex accessory organs but not in testis. Endocrinology 2008; 149: 3330 3338. [DOI] [PubMed] [Google Scholar]
- MacLean HE, Chiu WS, Ma C, McManus JF, Davey RA, Cameron R, Notini AJ, Zajac JD. A floxed allele of the androgen receptor gene causes hyperandrogenization in male mice. Physiol Genomics 2008; 33: 133 137. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Wilson JD. Studies on the pathogenesis of the pseudohermaphroditism in the mouse with testicular feminization. J Clin Invest 1972; 51: 1647 1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, Sharpe RM, Walker M, Smith LB, Saunders PT. New insights into the role of androgens in wolffian duct stabilization in male and female rodents. Endocrinology 2009; 150: 2472 2480. [DOI] [PubMed] [Google Scholar]
- Murashima A, Miyagawa S, Ogino Y, Nishida-Fukuda H, Araki K, Matsumoto T, Kaneko T, Yoshinaga K, Yamamura K, Kurita T, Kato S, Moon AM, et al. Essential roles of androgen signaling in Wolffian duct stabilization and epididymal cell differentiation. Endocrinology 2011; 152: 1640 1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatee TL, Hammond BP, Arenkiel BR, Francis L, Frank DU, Moon AM. Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development 2003; 130: 6361 6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, et al. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A 2004; 101: 1673 1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutskikh A, De Gendt K, Sharp V, Verhoeven G, Poutanen M, Huhtaniemi I. Targeted inactivation of the androgen receptor gene in murine proximal epididymis causes epithelial hypotrophy and obstructive azoospermia. Endocrinology 2011; 152: 689 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara L, Welsh M, Saunders PT, Smith LB. Androgen receptor expression in the caput epididymal epithelium is essential for development of the initial segment and epididymal spermatozoa transit. Endocrinology 2011; 152: 718 729. [DOI] [PubMed] [Google Scholar]
- Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol 2000; 222: 296 306. [DOI] [PubMed] [Google Scholar]
- Kerkhofs S, Dubois V, De Gendt K, Helsen C, Clinckemalie L, Spans L, Schuit F, Boonen S, Vanderschueren D, Saunders PT, Verhoeven G, Claessens F. A role for selective androgen response elements in the development of the epididymis and the androgen control of the 5alpha reductase II gene. FASEB J 2012; 26: 4360 4372. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool 1978; 205: 181 193. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR. Assessment of prostatic protein secretion in tissue recombinants made of urogenital sinus mesenchyme and urothelium from normal or androgen-insensitive mice. Endocrinology 1993; 132: 2342 2350. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P. Inability of Tfm (testicular feminization) epithelial cells to express androgen-dependent seminal vesicle secretory proteins in chimeric tissue recombinants. Endocrinology 1991; 128: 3293 3298. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Haughney PC, Rosen MA, Greulich KM, Weier HU, Dahiya R, Cunha GR. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation 1998; 63: 131 140. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Del Buono R, Deshpande N, Hall PA. A functional model of adult human prostate epithelium. The role of androgens and stroma in architectural organization and the maintenance of differentiated secretory function. J Cell Sci 1992; 102 (pt 2): 361 372. [DOI] [PubMed] [Google Scholar]
- Isaacs JT. Antagonistic effect of androgen on prostatic cell death. Prostate 1984; 5: 545 557. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA. Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol Reprod 1986; 34: 973 983. [DOI] [PubMed] [Google Scholar]
- Kyprianou N, Isaacs JT. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology 1988; 122: 552 562. [DOI] [PubMed] [Google Scholar]
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol 2003; 253: 165 174. [DOI] [PubMed] [Google Scholar]
- Gao J, Arnold JT, Isaacs JT. Conversion from a paracrine to an autocrine mechanism of androgen-stimulated growth during malignant transformation of prostatic epithelial cells. Cancer Res 2001; 61: 5038 5044. [PubMed] [Google Scholar]
- Orr B, Vanpoucke G, Grace OC, Smith L, Anderson RA, Riddick AC, Franco OE, Hayward SW, Thomson AA. Expression of pleiotrophin in the prostate is androgen regulated and it functions as an autocrine regulator of mesenchyme and cancer associated fibroblasts and as a paracrine regulator of epithelia. Prostate 2011; 71: 305 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Young P. Role of stroma in oestrogen-induced epithelial proliferation. Epithelial Cell Biol 1992; 1: 18 31. [PubMed] [Google Scholar]
- Yu S, Yeh CR, Niu Y, Chang HC, Tsai YC, Moses HL, Shyr CR, Chang C, Yeh S. Altered prostate epithelial development in mice lacking the androgen receptor in stromal fibroblasts. Prostate 2012; 72: 437 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Zhang C, Lin CC, Niu Y, Lai KP, Chang HC, Yeh SD, Chang C, Yeh S. Altered prostate epithelial development and IGF-1 signal in mice lacking the androgen receptor in stromal smooth muscle cells. Prostate 2011; 71: 517 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KP, Yamashita S, Vitkus S, Shyr CR, Yeh S, Chang C. Suppressed prostate epithelial development with impaired branching morphogenesis in mice lacking stromal fibromuscular androgen receptor. Mol Endocrinol 2012; 26: 52 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CT, Altuwaijri S, Ricke WA, Huang SP, Yeh S, Zhang C, Niu Y, Tsai MY, Chang C. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci U S A 2007; 104: 12679 12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, Sangiorgi FO, Maxson RE, Sucov HM, Roy-Burman P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev 2001; 101: 61 69. [DOI] [PubMed] [Google Scholar]
- Simanainen U, Allan CM, Lim P, McPherson S, Jimenez M, Zajac JD, Davey RA, Handelsman DJ. Disruption of prostate epithelial androgen receptor impedes prostate lobe-specific growth and function. Endocrinology 2007; 148: 2264 2272. [DOI] [PubMed] [Google Scholar]
- Niu Y, Wang J, Shang Z, Huang SP, Shyr CR, Yeh S, Chang C. Increased CK5/CK8-positive intermediate cells with stromal smooth muscle cell atrophy in the mice lacking prostate epithelial androgen receptor. PLoS ONE 2011; 6: e20202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Buchanan G, Ittmann M, Harris JM, Yu X, Demayo FJ, Tilley W, Greenberg NM. Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proc Natl Acad Sci U S A 2005; 102: 1151 1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanainen U, McNamara K, Gao YR, Handelsman DJ. Androgen sensitivity of prostate epithelium is enhanced by postnatal androgen receptor inactivation. Am J Physiol Endocrinol Metab 2009; 296: E1335 E1343. [DOI] [PubMed] [Google Scholar]
- Lee SO, Tian J, Huang CK, Ma Z, Lai KP, Hsiao H, Jiang M, Yeh S, Chang C. Suppressor role of androgen receptor in proliferation of prostate basal epithelial and progenitor cells. J Endocrinol 2012; 213: 173 182. [DOI] [PubMed] [Google Scholar]
- Ling MT, Chan KW, Choo CK. Androgen induces differentiation of a human papillomavirus 16 E6/E7 immortalized prostate epithelial cell line. J Endocrinol 2001; 170: 287 296. [DOI] [PubMed] [Google Scholar]
- Whitacre DC, Chauhan S, Davis T, Gordon D, Cress AE, Miesfeld RL. Androgen induction of in vitro prostate cell differentiation. Cell Growth Differ 2002; 13: 1 11. [PubMed] [Google Scholar]
- Pelletier G. Localization of androgen and estrogen receptors in rat and primate tissues. Histol Histopathol 2000; 15: 1261 1270. [DOI] [PubMed] [Google Scholar]
- Ito A, Sato W, Mori Y. Identification and partial characterization of the cytoplasmic androgen receptor in bovine ovarian capsule. J Steroid Biochem 1985; 23: 27 31. [DOI] [PubMed] [Google Scholar]
- Horie K, Takakura K, Fujiwara H, Suginami H, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human ovary throughout the menstrual cycle in relation to estrogen and progesterone receptor expression. Hum Reprod 1992; 7: 184 190. [DOI] [PubMed] [Google Scholar]
- Hirai M, Hirata S, Osada T, Hagihara K, Kato J. Androgen receptor mRNA in the rat ovary and uterus. J Steroid Biochem Mol Biol 1994; 49: 1 7. [DOI] [PubMed] [Google Scholar]
- Slomczynska M, Duda M. Sl zak K. The expression of androgen receptor, cytochrome P450 aromatase and FSH receptor mRNA in the porcine ovary. Folia Histochem Cytobiol 2001; 39: 9 13. [PubMed] [Google Scholar]
- Vermeirsch H, Simoens P, Coryn M, Van den Broeck W. Immunolocalization of androgen receptors in the canine ovary and their relation to sex steroid hormone concentrations. Reproduction 2001; 122: 711 721. [DOI] [PubMed] [Google Scholar]
- Moutsatsou P, Sekeris CE. Steroid receptors in the uterus: implications in endometriosis. Ann N Y Acad Sci 2003; 997: 209 222. [DOI] [PubMed] [Google Scholar]
- Pelletier G, Luu-The V, Li S, Labrie F. Localization and estrogenic regulation of androgen receptor mRNA expression in the mouse uterus and vagina. J Endocrinol 2004; 180: 77 85. [DOI] [PubMed] [Google Scholar]
- Shao R, Ljungstrom K, Weijdegard B, Egecioglu E, Fernandez-Rodriguez J, Zhang FP, Thurin-Kjellberg A, Bergh C, Billig H. Estrogen-induced upregulation of AR expression and enhancement of AR nuclear translocation in mouse fallopian tubes in vivo. Am J Physiol Endocrinol Metab 2007; 292: E604 E614. [DOI] [PubMed] [Google Scholar]
- Horie K, Takakura K, Imai K, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Hum Reprod 1992; 7: 1461 1466. [DOI] [PubMed] [Google Scholar]
- Tetsuka M, Whitelaw PF, Bremner WJ, Millar MR, Smyth CD, Hillier SG. Developmental regulation of androgen receptor in rat ovary. J Endocrinol 1995; 145: 535 543. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Tetsuka M, Fraser HM. Location and developmental regulation of androgen receptor in primate ovary. Hum Reprod 1997; 12: 107 111. [DOI] [PubMed] [Google Scholar]
- Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, Bondy CA. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab 1998; 83: 2479 2485. [DOI] [PubMed] [Google Scholar]
- Cardenas H, Pope WF. Androgen receptor and follicle-stimulating hormone receptor in the pig ovary during the follicular phase of the estrous cycle. Mol Reprod Dev 2002; 62: 92 98. [DOI] [PubMed] [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest 1998; 101: 2622 2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha S, Pache TD, Huikeshoven JM, Brinkmann AO, van der Kwast TH. Androgen receptor expression in human ovarian and uterine tissue of long-term androgen-treated transsexual women. Hum Pathol 1994; 25: 1198 1204. [DOI] [PubMed] [Google Scholar]
- Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, Jacobs HS. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 1995; 10: 2107 2111. [DOI] [PubMed] [Google Scholar]
- Murray AA, Gosden RG, Allison V, Spears N. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil 1998; 113: 27 33. [DOI] [PubMed] [Google Scholar]
- Wang H, Andoh K, Hagiwara H, Xiaowei L, Kikuchi N, Abe Y, Yamada K, Fatima R, Mizunuma H. Effect of adrenal and ovarian androgens on type 4 follicles unresponsive to FSH in immature mice. Endocrinology 2001; 142: 4930 4936. [DOI] [PubMed] [Google Scholar]
- Cardenas H, Herrick JR, Pope WF. Increased ovulation rate in gilts treated with dihydrotestosterone. Reproduction 2002; 123: 527 533. [DOI] [PubMed] [Google Scholar]
- Daniel SA, Armstrong DT. Site of action of androgens on follicle-stimulating hormone-induced aromatase activity in cultured rat granulosa cells. Endocrinology 1984; 114: 1975 1982. [DOI] [PubMed] [Google Scholar]
- Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab 1999; 84: 2951 2956. [DOI] [PubMed] [Google Scholar]
- Vendola K, Zhou J, Wang J, Bondy CA. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum Reprod 1999; 14: 2328 2332. [DOI] [PubMed] [Google Scholar]
- Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod 1999; 61: 353 357. [DOI] [PubMed] [Google Scholar]
- Hickey TE, Marrocco DL, Gilchrist RB, Norman RJ, Armstrong DT. Interactions between androgen and growth factors in granulosa cell subtypes of porcine antral follicles. Biol Reprod 2004; 71: 45 52. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 1996; 17: 121 155. [DOI] [PubMed] [Google Scholar]
- Cheng G, Weihua Z, Makinen S, Makela S, Saji S, Warner M, Gustafsson JA, Hovatta O. A role for the androgen receptor in follicular atresia of estrogen receptor beta knockout mouse ovary. Biol Reprod 2002; 66: 77 84. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Glenister PH. Reduced reproductive performance in androgen-resistant Tfm/Tfm female mice. Proc R Soc Lond B Biol Sci 1980; 208: 1 12. [DOI] [PubMed] [Google Scholar]
- Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci U S A 2004; 101: 11209 11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, et al. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci U S A 2006; 103: 224 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters KA, Allan CM, Jimenez M, Lim PR, Davey RA, Zajac JD, Illingworth P, Handelsman DJ. Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology 2007; 148: 3674 3684. [DOI] [PubMed] [Google Scholar]
- Walters KA, McTavish KJ, Seneviratne MG, Jimenez M, McMahon AC, Allan CM, Salamonsen LA, Handelsman DJ. Subfertile female androgen receptor knockout mice exhibit defects in neuroendocrine signaling, intraovarian function, and uterine development but not uterine function. Endocrinology 2009; 150: 3274 3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol 2005; 288: 276 283. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan ZJ, Cooney AJ, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008; 319: 611 613. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev 2002; 23: 175 200. [DOI] [PubMed] [Google Scholar]
- Panet-Raymond V, Gottlieb B, Beitel LK, Pinsky L, Trifiro MA. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol Cell Endocrinol 2000; 167: 139 150. [DOI] [PubMed] [Google Scholar]
- Dees WL, Hiney JK, McArthur NH, Johnson GA, Dissen GA, Ojeda SR. Origin and ontogeny of mammalian ovarian neurons. Endocrinology 2006; 147: 3789 3796. [DOI] [PubMed] [Google Scholar]
- Armstrong DT, Moon YS, Leung PC. Uterotrophic effects of testosterone and 5alpha-dihydrotestosterone in intact and ovariectomized immature female rats. Biol Reprod 1976; 15: 107 114. [DOI] [PubMed] [Google Scholar]
- Kowalski AA, Vale-Cruz DS, Simmen FA, Simmen RC. Uterine androgen receptors: roles in estrogen-mediated gene expression and DNA synthesis. Biol Reprod 2004; 70: 1349 1357. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Ekman J, Almkvist A, Saji S, Wang L, Warner M, Gustafsson JA. Involvement of androgen receptor in 17beta-estradiol-induced cell proliferation in rat uterus. Biol Reprod 2002; 67: 616 623. [DOI] [PubMed] [Google Scholar]
- Nantermet PV, Masarachia P, Gentile MA, Pennypacker B, Xu J, Holder D, Gerhold D, Towler D, Schmidt A, Kimmel DB, Freedman LP, Harada S, et al. Androgenic induction of growth and differentiation in the rodent uterus involves the modulation of estrogen-regulated genetic pathways. Endocrinology 2005; 146: 564 578. [DOI] [PubMed] [Google Scholar]
- Brenner RM, Slayden OD, Critchley HO. Anti-proliferative effects of progesterone antagonists in the primate endometrium: a potential role for the androgen receptor. Reproduction 2002; 124: 167 172. [DOI] [PubMed] [Google Scholar]
- Zanatta A, Rocha AM, Carvalho FM, Pereira RM, Taylor HS, Motta EL, Baracat EC, Serafini PC. The role of the Hoxa10/HOXA10 gene in the etiology of endometriosis and its related infertility: a review. J Assist Reprod Genet 2010; 27: 701 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermik D, Selam B, Taylor HS. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab 2003; 88: 238 243. [DOI] [PubMed] [Google Scholar]
- Schmidt EV, Christoph G, Zeller R, Leder P. The cytomegalovirus enhancer: a pan-active control element in transgenic mice. Mol Cell Biol 1990; 10: 4406 4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notini AJ, Davey RA, McManus JF, Bate KL, Zajac JD. Genomic actions of the androgen receptor are required for normal male sexual differentiation in a mouse model. J Mol Endocrinol 2005; 35: 547 555. [DOI] [PubMed] [Google Scholar]
- Hickey TE, Robinson JL, Carroll JS, Tilley WD. Minireview: the androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene? Mol Endocrinol 2012; 26: 1252 1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao DJ, Dickson RB. Roles of androgens in the development, growth, and carcinogenesis of the mammary gland. J Steroid Biochem Mol Biol 2002; 80: 175 189. [DOI] [PubMed] [Google Scholar]
- Durnberger H, Kratochwil K. Specificity of tissue interaction and origin of mesenchymal cells in the androgen response of the embryonic mammary gland. Cell 1980; 19: 465 471. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol 2004; 67: 417 434. [DOI] [PubMed] [Google Scholar]
- Drews U. Regression of mouse mammary gland anlagen in recombinants of Tfm and wild-type tissues: testosterone acts via the mesenchyme. Cell 1977; 10: 401 404. [DOI] [PubMed] [Google Scholar]
- Pashko LL, Schwartz AG, Abou-Gharbia M, Swern D. Inhibition of DNA synthesis in mouse epidermis and breast epithelium by dehydroepiandrosterone and related steroids. Carcinogenesis 1981; 2: 717 721. [DOI] [PubMed] [Google Scholar]
- Jayo MJ, Register TC, Hughes CL, Blas-Machado U, Sulistiawati E, Borgerink H, Johnson CS. Effects of an oral contraceptive combination with or without androgen on mammary tissues: a study in rats. J Soc Gynecol Investig 2000; 7: 257 265. [PubMed] [Google Scholar]
- Finkelstein M, Geier A, Horn H, Levij IS, Ever-Hadani P. Effect of testosterone and estradiol-17beta on synthesis of DNA, RNA and protein in human breast in organ culture. Int J Cancer 1975; 15: 78 90. [DOI] [PubMed] [Google Scholar]
- Palekar L, Waldo ED, Seidman I. Hyperplasia of rat mammary gland in vitro. J Natl Cancer Inst 1975; 54: 235 240. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ng S, Adesanya-Famuiya O, Anderson K, Bondy CA. Testosterone inhibits estrogen-induced mammary epithelial proliferation and suppresses estrogen receptor expression. FASEB J 2000; 14: 1725 1730. [DOI] [PubMed] [Google Scholar]
- Yeh S, Hu YC, Wang PH, Xie C, Xu Q, Tsai MY, Dong Z, Wang RS, Lee TH, Chang C. Abnormal mammary gland development and growth retardation in female mice and MCF7 breast cancer cells lacking androgen receptor. J Exp Med 2003; 198: 1899 1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberg DL, Feldman M, Ruan W. IGF-I: an essential factor in terminal end bud formation and ductal morphogenesis. J Mammary Gland Biol Neoplasia 2000; 5: 7 17. [DOI] [PubMed] [Google Scholar]
- Bonnette SG, Hadsell DL. Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology 2001; 142: 4937 4945. [DOI] [PubMed] [Google Scholar]
- Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Tan J, Dey SK, Dotto GP, Weinberg RA. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell 2002; 3: 877 887. [DOI] [PubMed] [Google Scholar]
- Inoue S, Orimo A, Hosoi T, Kondo S, Toyoshima H, Kondo T, Ikegami A, Ouchi Y, Orimo H, Muramatsu M. Genomic binding-site cloning reveals an estrogen-responsive gene that encodes a RING finger protein. Proc Natl Acad Sci U S A 1993; 90: 11117 11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JG, Bell A, Liu Y, Zarnegar R. Transcriptional regulation of the hepatocyte growth factor gene by the nuclear receptors chicken ovalbumin upstream promoter transcription factor and estrogen receptor. J Biol Chem 1997; 272: 3928 3934. [DOI] [PubMed] [Google Scholar]
- Urano T, Saito T, Tsukui T, Fujita M, Hosoi T, Muramatsu M, Ouchi Y, Inoue S. Efp targets 14-3-3 sigma for proteolysis and promotes breast tumor growth. Nature 2002; 417: 871 875. [DOI] [PubMed] [Google Scholar]
- Niemann C, Brinkmann V, Spitzer E, Hartmann G, Sachs M, Naundorf H, Birchmeier W. Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis. J Cell Biol 1998; 143: 533 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo A, Lagana AS, Borrielli I, Dugo N. Complete androgen insensitivity syndrome: a rare case of disorder of sex development. Case Rep Obstet Gynecol 2013; 2013: 232696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar H, Cunha GR. Epithelial-stromal interactions in the mouse and human mammary gland in vivo. Endocr Relat Cancer 2004; 11: 437 458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


