ABSTRACT
Loss of pelvic organ support (i.e., pelvic organ prolapse) is common in menopausal women. Surgical reconstruction of pelvic organ prolapse is plagued with high failure rates. The objective of this study was to determine the effects of estrogen on biomechanical properties, lysyl oxidase (LOX), collagen content, and histomorphology of the vagina with or without surgical injury. Nulliparous ovariectomized guinea pigs were treated systemically with either 50 μg/kg/day estradiol (E2,) or vehicle. After 2 wk, vaginal surgery was performed, and animals were treated with either beta-aminopropionitrile (BAPN, an irreversible LOX inhibitor), or vehicle to determine the role of LOX in recovery of the vaginal wall from injury with or without E2. Estradiol resulted in (i) significant growth, increased smooth muscle, and increased thickness of the vagina, (ii) increased distensibility without compromise of maximal force at failure, and (iii) increased total and cross-linked collagen. In the absence of E2, BAPN resulted in decreased collagen and vaginal wall strength in the area of the injury. In contrast, in E2-treated animals, increased distensibility, maximal forces, and total collagen were maintained despite BAPN. Interestingly, LOX mRNA was induced dramatically (9.5-fold) in the injured vagina with or without E2 at 4 days. By 21 days, however, LOX levels declined to near baseline in E2-deprived animals. LOX mRNA levels remained strikingly elevated (12-fold) at 21 days in the estrogenized vagina. The results suggest that prolonged E2 induced increases in LOX, and collagen cross-links may act to sustain a matrix environment that optimizes long-term surgical wound healing in the vagina.
Keywords: collagen, estradiol/estradiol receptor, lysyl oxidase, matrix remodeling, menopause, pelvic organ prolapse, vagina
Estradiol not only increases baseline vaginal distensibility, vaginal epithelium and muscularis growth, and collagen content, but also prolonged increases in lysyl oxidase and collagen cross-links in the vaginal muscularis after injury.
INTRODUCTION
Pelvic organ prolapse is a distressing condition in which the uterus, cervix, bladder, rectum, and vaginal wall herniate through the vaginal introitus. This condition occurs with a high prevalence in women after menopause with a lifetime risk of surgery for prolapse or urinary incontinence of 11% [1, 2]. Every year, nearly 200 000 women undergo surgical intervention to repair prolapse, and this figure is likely to increase as the number of women over the age of 50 is expected to increase by 72% over the next 3 decades [3, 4].
The exact cause of pelvic organ prolapse is poorly understood and likely multifactorial, but several risk factors have been postulated including vaginal childbirth, aging, hypoestrogenism, menopause, genetic factors, connective tissue diseases, race, chronically increased intraabdominal pressure, smoking, and prior surgery [2, 5, 6]. Surgical failure has been attributed to impairment of surgical wound healing [7], suggesting a contributing molecular or genetic basis to the pathogenesis of the disease. The molecular defects in connective tissue that underlie initial failure of pelvic organ support may also contribute to failure of reconstructive pelvic surgery.
Wound healing is a highly regulated and dynamic series of events involving numerous mediators, blood factors, stromal cells, and extracellular matrix elements that ultimately culminate in collagen deposition and scar formation [8, 9]. The enzyme lysyl oxidase (LOX) is essential for cross-linking collagen for scar formation that imparts strength to wounds. LOX is inhibited by beta-aminopropionitrile (BAPN), and animals exposed to BAPN develop loss of connective tissue tensile strength and develop multiple diseases affecting mesenchymal tissues [10–13].
Estrogen may play an important role in the pathogenesis and progression of pelvic organ prolapse. Studies in this regard, however, are conflicting, and the effect of hormone treatment on pelvic floor support in postmenopausal women remains poorly understood [1, 6, 14–17]. Thus, the objective of the current investigation was to test the hypothesis that estrogen regulates wound healing of the vaginal wall through increased expression of LOX and biosynthesis of mature collagen. Further, we propose that LOX enzyme activity is crucial for optimal wound healing in the vaginal wall and restoration of pelvic organ support. Here, we used a novel guinea pig model to determine the effects of estrogen on collagen biosynthesis in the vaginal wall and vaginal wound healing in response to surgical injury.
MATERIALS AND METHODS
Guinea Pigs
All the guinea pigs were studied and euthanized in accordance with the standards of humane animal care described by the National Institutes of Health Guide for the Care and Use of Laboratory Animals, using protocols approved by the Institutional Animal Care and Use Committee of University of Texas Southwestern Medical Center. A total of 48 virginal female adult Hartley strain (Charles River Laboratories, Wilmington, MA) guinea pigs at 12 wk of age were housed in Institutional Animal Care and Use Committee-approved facilities under a 12L:12D cycle at 22°C.
After facility acclimation, all the animals underwent bilateral oophorectomy through 1 cm dorsal flank incisions. Prior to skin closure, Alzet osmotic minipumps (Model 2006; Durect Corporation, Cupertino, CA) containing either 50 μg/kg/day estradiol (E2) (n = 24), or polyethylene glycol as the vehicle control (n = 24) were inserted into the subcutaneous space. This dose of estrogen was chosen because it is well-known to deliver serum estrogen levels approximate to that of cycling rats (29–71 pg/ml) and causes increases in uterine weight similar to those observed in estrus [18–23]. After a 2-wk period to wash out endogenous estradiol and allow pump equilibration and steady state hormone levels, a modified vaginal posterior colpoperineorrhaphy was performed in all the guinea pigs (Fig. 1). Briefly, the posterior introitus was incised, and the posterior vaginal wall was undermined, with excision of a 2.0 × 0.3 cm strip of full-thickness posterior wall. The surgical wound was primarily reapproximated with absorbable suture, and the excised vaginal wall strip was snap-frozen in liquid N2 and stored at −80°C as baseline noninjured control tissue for hydroxyproline assays.
FIG. 1.
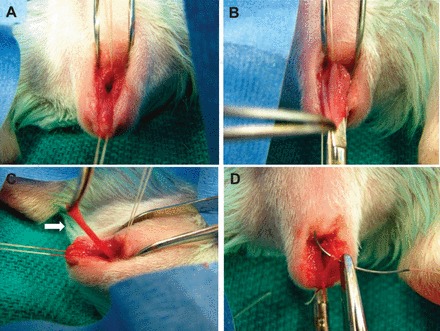
Vaginal surgical injury (posterior colpoperineorrhaphy) in the guinea pig model. A) Stay sutures placed in posterior vaginal wall at perineum and midvagina. B) Perineum incised and vaginal wall undermined. C) Full-thickness strip of posterior vaginal wall excised (arrow). D) Primary reapproximation of wound.
To gain insight into potential mechanisms of estrogen on wound healing, one-half of each group was treated with either oral 0.4% BAPN (n = 12) in a 5% sucrose solution or 5% sucrose alone (n = 12) on the day prior to vaginal surgery. These treatments were continued in the drinking water for the remainder of the study. Because BAPN is known to cause weight loss and reduced oral intake among test animals [12, 13], water intake was monitored daily and animals were weighed twice weekly (Supplemental Table S1, available online at www.biolreprod.org). At both 4 and 21 days after vaginal surgery, the animals were killed and the vaginal tube harvested for biomechanical testing, histomorphology, and determination of hydroxyproline content and mRNA levels (n = 6 for each group at each time point). In animals killed on Day 21, uterosacral ligaments were harvested from sacral attachments to insertion on the cervix.
Tissue Processing
After euthanasia, the right flank was examined to confirm pump placement and integrity, and the abdominal cavity was opened. The pubic symphysis was disarticulated, and the uterine horns, bladder, cervix, and vagina were dissected down to the perineal skin. In animals killed on Day 21 (n = 24), the ureters were identified bilaterally, dissected away from the uterosacral ligaments, which were harvested from sacral attachment sites to insertion on the cervix. Using microsurgical instruments and a dissection microscope, the perineal skin was removed, and the bladder and urethra were dissected from the anterior vaginal wall. Uterine horns and cervix were removed from the vaginal tube at the vaginal fornices. Wet weights of the pelvic organs were determined, vaginal tube length was measured, and the entire length of the posterior wall injury was identified. At the superior margin of the injury, the vagina was divided transversely into upper and lower vaginal segments. Microscopic examination of each segment confirmed an intact upper vagina and injured lower vagina. Rings (3 mm in width) were excised from each segment for biomechanical testing and histology. Thereafter, tissues from each segment were snap-frozen in liquid N2 and stored at −80°C.
Biomechanical Testing
Each vaginal ring was suspended loosely between two stainless steel wire mounts, fastened to a steel rod mounted with a calibrated mechanical drive on one end and to a force transducer (Grass FT.03C; Grass Instrument Company, Quincy, MA) on the other. Tissues were maintained in physiologic salt solution in water-jacketed baths at 37°C with 95% O2 and 5% CO2. After acclimation for 15 min, the ring diameter was equilibrated to slack length (i.e., length of initial resting tone) as measured by the calibrated mechanical drive and confirmed with digital calipers sensitive to 0.01 mm. Rings were then distended in 1 mm increments with 30-sec intervals between successive changes in length to allow forces to return to steady state before the next distention. This series of step-strains was continued until failure (ring breakage) or until force generation plateaued. Wet weights of vaginal rings were determined after testing. Stress (N/m2) was calculated as maximum force per unit area and plotted against strain (change in length divided by slack length), producing a sigmoid-shaped curve. The modulus of elasticity, an index of tissue stiffness, was calculated from the slope of the linear portion of the curve.
Histomorphology
Injured and noninjured vaginal rings were fixed in neutral buffer formalin (10%) for 24 h, and 5 μm cross-sections of the formalin-fixed, paraffin-embedded tissues were obtained at 0.1 mm intervals throughout the specimen. Tissue was stained with hematoxylin and eosin, Masson trichrome, and Hart stain. Quantification of elastic fibers was performed on Hart-stained sections in a blinded manner. Three to six images were captured from each slide and quantitated using National Institutes of Health ImageJ software [24]. Sections were set to a consistent threshold limit, and percentage elastic fiber pixel area over total vaginal stromal area were calculated for each image and averaged for each group.
Hydroxyproline Assay and Collagen Solubility
Collagen solubility measurements were used as an index of collagen structure within the tissue [25]. Briefly, weights of vaginal tissues were determined before and after lyophilization. Lyophilized tissue was extracted with 1 M NaOH plus protease inhibitors (pancreas extract 0.02 mg/ml, pronase 0.005 mg/ml, and thermolysin 0.0005 mg/ml) at 4°C for 24 h and centrifuged, and the supernatant was saved as fraction A containing newly synthesized noncross-linked collagen. The remaining insoluble residue was washed with water plus protease inhibitors and extracted with 0.5 M acetic acid plus protease inhibitors at 4°C for 24 h with gentle rotation. After centrifugation, the supernatant was saved as fraction B containing denatured collagen. The pellet was saved as fraction C containing mature, cross-linked collagen. Collagen content in fractions A, B, and C were determined by measurement of hydroxyproline content by the chloramine-T method [25] after hydrolysis in 6 M HCl overnight at 100°C. Hydroxyproline values were converted to collagen (× 7.6) and normalized to tissue wet weight. Collagen content in each fraction is reported as percent total collagen in which total collagen = A + B + C.
Quantitative PCR
Tissues were analyzed for cola1a, col3a1, tropoelastin, LOX, and TGFβ1 mRNA using quantitative PCR as described previously [26]. The housekeeping gene β-2 microglobulin (B2M) was used for normalization because it was not altered by E2 or BAPN. The primers are listed in Table 1.
TABLE 1.
Oligonucleotide primers used to quantify guinea pig mRNA using quantitative PCR.

Statistics
Data are presented as means ± SEM. A two-way ANOVA was used to compare treatments across time and between groups. The Student t-test was used to compare differences between two groups.
RESULTS
Effect of Estrogen on Gross and Histologic Morphology of the Female Reproductive Tract
The vaginal introitus of guinea pigs in nonestrus is characterized by the presence of a closed thin membrane of connective tissue that acts as a barrier and protects the vagina (Fig. 2A). In the presence of estrogen, however, the membrane absorbs, allowing vaginal access. In addition, the reproductive tract undergoes marked growth, as the vagina, uterus, and cervix demonstrated 3-, 5.6-, and 11.9-fold increases in wet weights, respectively, compared with vehicle-treated ovariectomized controls (Fig. 2B). In contrast, bladder wet weights were similar regardless of estrogen status (negative control). Although vaginal length was maintained, estrogen resulted in global circumferential growth of the female reproductive tract (Fig. 2C). In the absence of E2, vaginal epithelium was thin, being made up of approximately one cell layer with few mucin-laden cells (Fig. 3A, panel a). The muscularis was flattened with poor distinction between longitudinal and circular smooth muscle bundles. In contrast, epithelial thickness was increased in the vaginal wall from E2-treated animals with invaginating pseudostratified mucin-laden epithelium and clear increases in both circular and longitudinal smooth muscle in the muscularis (Fig. 3A, panel b). Quantification of tissue layers revealed that E2 induced significant proportionate increases in both the epithelium and muscularis (Fig. 3B).
FIG. 2.
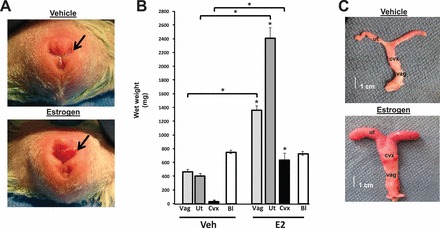
Effect of estrogen on growth of the female reproductive tract in ovariectomized guinea pigs. A) Vulva of ovariectomized guinea pigs treated with vehicle was characterized by a membrane covering the vaginal introitus (arrow). The membrane was dissolved in estrogen-treated animals. B) Vaginal (vag, light gray bar), uterine (ut, dark gray bar), and cervical (cvx, black bar) weight were increased significantly in estrogen-treated animals compared with vehicle-treated controls. Bladder (Bl, white bar) weights were similar between the two groups. Each bar represents mean ± SEM from six animals in each group. *P < 0.01 compared with corresponding organ from controls. C) Gross appearance of female reproductive tract in vehicle and estrogen-treated animals.
FIG. 3.
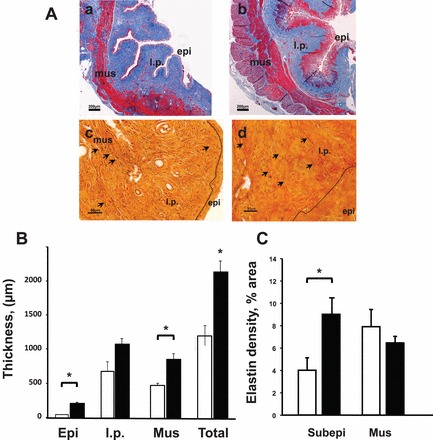
Effect of E2 on histology of the vaginal wall. A) Representative photographs of Masson (a, b) and Hart (c, d) -stained vaginal rings from ovariectomized animals treated with vehicle (a, c) or E2 (b, d) for 21 days. Note increased thickness of epithelium (epi) and muscularis (mus) in E2-treated animals. Sections were taken at midvagina. Dotted line delineates epithelium (epi) from underlying lamina propria (l.p.) and muscularis (mus). Arrows indicate elastic fibers. Bar = 200 μm for a, b; 50 μm for c, d. B) Thickness of epi, l.p., mus, and total wall (Total) in control (n = 6, open bar) and E2-treated (n = 6, closed bar) animals. *P < 0.05 C. Elastin density in subepithelium (within 500 μm of basal lamina) and muscularis of control (open bar) and E2-treated (closed bar) animals. Data represent mean ± SEM of noninjured animals with similar results 21 days after the injury. *P < 0.05 compared with control.
Using Hart stain, subepithelial-branching elastic fibers were increased in E2-treated animals (Fig. 3A, panel d). This increase in elastic fibers was limited to the subepithelium rather than the entire lamina propria. Further, elastic fiber density was similar in the muscularis from vehicle and E2-treated animals (Fig. 3C).
Effect of E2 on Expression of Collagen, Tropoelastin, TGFβ, and LOX mRNA in the Vaginal Wall
To gain insight regarding potential mechanisms by which E2 alters collagen content in the vaginal wall, mRNA levels of genes involved in collagen synthesis were quantified. In ovariectomized controls, both col1a2 and col3a1 gene expression was low, but increased modestly 4 days after injury, returning to baseline at 21 days (Fig. 4). Interestingly, E2 treatment resulted in 8- and 5-fold increases in col1a2 and col3a1 gene expression in the noninjured vaginal wall (at 4 days, i.e., 2 wk of E2 treatment). Injury did not result in substantial increases in collagen mRNA levels within 4 days. Nonetheless, E2 treatment for 5 wk resulted in dramatic increases in col1a2 and col3a1 gene expression that was further amplified after injury. Similar results were found with tropoelastin (Fig. 4C) in which E2 resulted in drastic changes in tropoelastin mRNA in the injured estrogenized vagina. These results indicate that E2 has a profound effect on collagen and tropoelastin gene expression in the noninjured and injured vagina that is time-dependent. Prolonged E2 treatment results in increased expression of both collagen type I and type III and tropoelastin mRNA and an augmented response to injury.
FIG. 4.
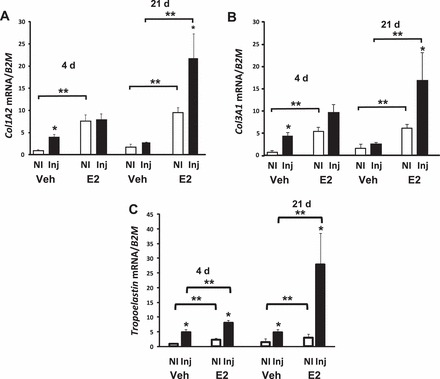
Effect of injury and E2 on collagen and tropoelastin mRNA in the vaginal wall. Col1A2 (A), Col3A1 (B), and tropoelastin (C) mRNA levels were quantified in vaginal tissues from noninjured (NI) or injured (Inj) ovariectomized vehicle- (Veh) or E2-treated animals at 4 and 21 days after injury. Data were normalized to B2M and represent the mean ± SEM of six animals in each group. *P < 0.05 compared with NI. **P < 0.05 compared with controls, two-way ANOVA.
Wound healing is associated with increased expression of TGFβ, which is known to be a major regulator of LOX gene expression [27]. To assess the effect of E2 on collagen maturation in the vaginal wall, TGFβ1 and LOX mRNA levels were quantified in the noninjured and injured vaginal wall from ovariectomized controls and E2-treated animals (Fig. 5). TGFβ1 and LOX mRNA were induced dramatically (P < 0.01) in the injured vagina with or without E2 at 4 days. By 21 days, however, levels declined to near baseline levels in controls. On the contrary, LOX mRNA levels remained strikingly elevated (12-fold) in the injured estrogenized vagina at 21 days.
FIG. 5.
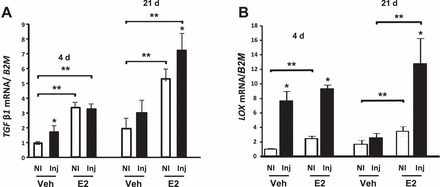
Effect of injury and E2 on TGFβ1 and LOX gene expression in the vaginal wall. TGFβ1 (A) and LOX (B) mRNA levels were quantified in vaginal tissues from noninjured (NI) or injured (Inj) ovariectomized vehicle- (Veh) or E2-treated animals at 4 and 21 days after injury. Data were normalized to B2M and represent mean ± SEM of six animals in each group. *P < 0.05 compared with NI. **P < 0.05 compared with controls, two-way ANOVA.
Effect of E2 on Collagen Content of the Vagina
Hydroxyproline assays were used to determine if these changes in collagen mRNA levels resulted in increased collagen content. Baseline collagen content of the noninjured vagina from ovariectomized vehicle-treated animals was decreased significantly compared with E2-treated animals (Fig. 6A). Injury in ovariectomized controls, however, resulted in significant increases in collagen content after 21 days. Estradiol treatment increased collagen content of the vaginal wall at baseline and augmented injury-induced effects on total collagen content (Fig. 6A). To determine the effect of E2 on maturation of collagen in the vaginal wall, hydroxyproline assays were conducted in fractions of vaginal homogenates representing immature, denatured, and mature cross-linked collagen (Fig. 6B). In noninjured ovariectomized animals, vaginal collagen content was low and only 58% was mature. After injury, although collagen content increased, the proportion of cross-linked collagen remained at 58% (Fig. 6B). Interestingly, E2-induced increases in total collagen content of the noninjured vaginal wall represented a striking increase in mature collagen (91%, Fig. 6B). After injury (21 days), there was an E2-associated increase in the quantity of collagen in each fraction compared to injured vehicle-treated animals. However, compared to both injured vehicle-treated and noninjured E2-treated animals, maturation of the collagen was incomplete. Specifically, injury-induced collagen content increased predominantly in the newly synthesized and denatured fractions, whereas the proportion of mature collagen was only 35% of the total (Fig. 6B). Although E2 induced significant effects on vaginal weight and collagen content of the vaginal wall, uterosacral ligaments were not affected. Specifically, uterosacral weights were not statistically different in vehicle and E2-treated animals (78.4 ± 11.6 mg vs. 120.4 ± 22 mg). Histologically, the uterosacral ligaments consisted predominantly of adipose tissue with interspersed strands of fibroblasts, smooth muscle bundles, and ganglia, especially at the sacral insertion site. As expected, the collagen content was low with only 3.1% ± 1.6% mature in vehicle-treated animals compared with 7.2% ± 2.3% in the E2-treated group.
FIG. 6.
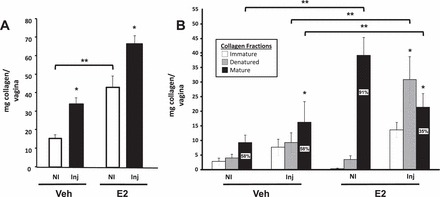
Effect of E2 and injury on collagen content of the vaginal wall. A) Effect of E2 on total collagen content in the vagina from ovariectomized vehicle- (Veh) or E2-treated noninjured (NI) or injured (Inj) animals after 21 days. *P < 0.05 compared with NI. **P < 0.05 compared with NI Veh. B) Effect of E2 on immature (open bar), denatured (grey bar), or mature (solid bar) collagen content in the vagina from ovariectomized Veh- or E2-treated NI or Inj animals. *P < 0.05 compared with NI, paired analysis; **P < 0.05 compared with controls. Data represent mean ± SEM of six animals in each group.
Taken together, these data indicate that although injury of the vaginal wall of ovariectomized vehicle-treated animals results in increased collagen synthesis and increased expression of TGFβ and LOX, these effects were transient. Estradiol treatment results in marked and prolonged increases in collagen type I, collagen type III, TGFβ, and LOX gene expression at baseline and after injury that was associated with dramatic increases in collagen content.
Effect of BAPN on Collagen Content in Control and E2-Treated Animals
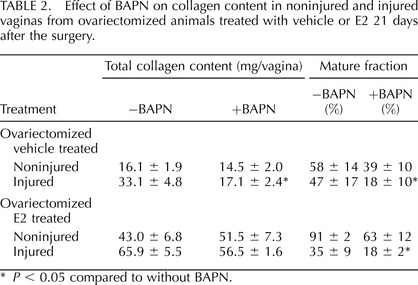
To determine if E2-induced increases in LOX enzymes were important in E2-induced increases in collagen content in the noninjured and injured vaginal wall, ovariectomized vehicle-treated and E2-treated animals were treated with the LOX inhibitor, BAPN, in drinking water after injury and the collagen content was determined (Table 2). Whereas BAPN had no effect on baseline collagen content in control or E2-treated animals, BAPN treatment abolished injury-induced increases in collagen content in controls and significantly mitigated injury-induced effects in E2-treated animals (Table 2). Although total collagen content did not change in control noninjured vaginal tissues, BAPN reduced the fraction of mature collagen from 58% to 39% in controls and from 91% to 63% in E2-treated animals, indicating that LOX activity is important in maintaining maturation of collagen in the adult vagina even in resting conditions. In the injured vagina, maturation of collagen was severely affected by BAPN with reductions from 47% to 18% in injured animals. Hence, LOX is crucial for injury-induced increases in mature collagen in both control and E2-treated animals. Because baseline mature collagen content was much greater in E2-treated animals, the impact of BAPN on loss of mature collagen after wounding was not as profound.
TABLE 2.
Effect of BAPN on collagen content in noninjured and injured vaginas from ovariectomized animals treated with vehicle or E2 21 days after the surgery.
P < 0.05 compared to without BAPN.
Effect of E2 on Biomechanical Properties of the Noninjured and Injured Vaginal Wall
To determine if E2-induced changes in collagen synthesis were associated with alterations in the biomechanical properties of the vaginal wall, maximal force at failure, stiffness, distention at maximal force, maximal length at failure, and strain at maximal force were quantified 21 days after the injury. Distention at maximal force, maximal length at failure and strain at maximal force were not changed appreciably by E2 treatment (data not shown). In ovariectomized controls, injury resulted in loss of tissue stiffness (Fig. 7), and BAPN amplified injury-induced loss of tissue stiffness even in the noninjured vagina. In controls, injury alone was not sufficient to alter the strength of the vaginal wall after 21 days (Fig. 7B), yet, BAPN resulted in loss of strength in control and injured vaginal tissues. Biomechanical properties of the estrogenized vaginal wall differed from those of controls. Specifically, tissue stiffness was less than that of controls, was similar with or without injury, and BAPN did not significantly alter this effect. Although less stiff (i.e., more distensible), estrogenized vaginal rings maintained strength similar to that of controls (Fig. 7B). Importantly, unlike controls, BAPN had no effect on vaginal tissue stiffness or strength in E2-treated animals (Fig. 7). Thus, E2 increased tissue distensibility and maintained tissue strength regardless of injury.
FIG. 7.
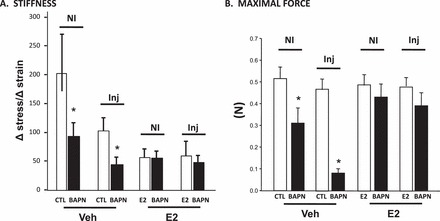
Effect of E2 and BAPN on biomechanical properties of the vaginal wall in noninjured and injured animals 21 days after the surgery. Stiffness and maximal force were determined in vaginal rings from noninjured (NI) or injured (Inj) ovariectomized vehicle- or E2-treated animals treated with control (CTL) or BAPN as described in Materials and Methods. Data represent mean ± SEM of six animals in each group. *P < 0.05 compared with corresponding control t-test.
DISCUSSION
Using a guinea pig model, we aimed to examine the effect of estrogen on reproductive tissues and determine its role in response to vaginal surgical wounding. The major findings of our study indicate that estrogen functions to increase the total amount of collagen in the vaginal wall with an increase in the amount of mature cross-linked collagen in both noninjured and injured animals compared to their respective controls. After injury, E2 caused a prolonged and sustained response, which ultimately led to larger increases in total collagen content. In addition, biomechanical testing revealed that E2 increased distensibility of the vagina while maintaining tissue strength, and neither surgical injury nor BAPN altered this pattern.
Effects of E2 on the Noninjured Vaginal Wall
A large proportion of reconstructive pelvic surgery for pelvic organ prolapse is performed in postmenopausal women. It has been suggested that hormone therapy may mitigate some of the effects of menopause on pelvic connective tissue by producing a more favorable ratio of collagen subtypes [14]. In noninjured animals, E2 increased total collagen content. After 2 wk, increased total collagen was reflected in an increase in all maturation fractions in similar proportions, suggesting that E2 acts on multiple steps in the biosynthetic process to increase collagen. In contrast, after 5 wk, E2-induced increases in total collagen were primarily due to increases in mature collagen, suggesting that E2 functions over time to ultimately increase mature cross-linked collagen in the absence of injury. Our findings agree with, and complement, studies in postmenopausal women in which estrogen supplementation increases collagen content of the skin, vasculature, and pelvic tissues [14–16] and with our studies demonstrating that estradiol increases both collagen and elastin synthesis in the vaginal wall of mice (data not shown). Other studies, however, suggest that estrogen may have negative effects on pelvic support [1, 6, 17, 28], underscoring the fact that hormone effects on pelvic floor tissues in postmenopausal women are not clearly understood [6].
Effects of E2 on the Injured Vaginal Wall
Much of our knowledge on wound healing and repair comes from cutaneous wounds, with little data available on the female reproductive tract. Wound healing is a dynamic process involving mediators, cytokines, parenchymal cells, and extracellular matrix, and can be divided into three overlapping phases: inflammation, tissue formation, and tissue remodeling. Our study mainly focused on the latter two phases. We chose to examine healing at 4 and 21 days based on one of the only animal studies on vaginal wound healing in which tensile strength nadir and recovery to baseline occurred at these time points, respectively [7]. In contrast with Abramov et al. [7], however, we did not find a significant difference in tensile strength between nonestrogenized and estrogenized animals at either 4 (not shown) or 21 days. While these authors studied vaginal wounds that healed by secondary intention, we chose to mimic the clinical situation by closing the wounds primarily, which may explain some of the differences in biomechanical testing between the studies. Nonetheless, our findings agree with previous studies in animals showing that administration of E2 had no effect on tissue tensile strength of cutaneous wounds compared with controls [29–33], although progressive increases in tensile strength have been demonstrated by other investigators [34–36]. These discrepancies may be explained by a number of factors, including different species and gender, hormones and delivery routes, and tissue type studied [8, 36]. Our biomechanical testing indicated that estrogen reduced tissue stiffness without compromise of the maximum force at failure, suggesting that estrogen may have a role in protecting the vagina from further injury by allowing tissues to stretch under traumatic stress while wound healing takes place.
Ashcroft et al. [8] found that ovariectomized rodents exhibited significant delays in acute cutaneous wound healing that was reversed by topical estrogen. It was suggested that the cellular mechanism underlying these changes involved estrogen-induced increases in latent TGFβ-1 by fibroblasts, which accelerates wound repair by stimulating collagen deposition during early wound healing [8]. A more recent study involving perioperative estrogen treatment in rabbits with surgical mesh implantation demonstrated increased deposition of collagen with enhanced host tissue incorporation in estrogen-treated animals, whereas collagen deposition was decreased in ovariectomized rabbits with more technically challenging procedures due to tissue atrophy [37]. Our results demonstrating E2-induced increases in TGFβ-1 mRNA and collagen biosynthesis in the vagina are compatible with these findings. Overall, we suggest that estrogen plays a major role in regulation of connective tissue remodeling, may play a therapeutic role in healing and repair, and may complement repair of pelvic tissues with various mesh materials.
Regulation of LOX Enzymes in the Vagina
Lysyl oxidases (LOX and LOXL1–4) are essential for cross-linking collagen. LOX enzymes are inhibited by seeds of the sweet pea Lathyrus oderatus. The active substance is BAPN, and animals consuming this sweet pea develop lathyrism, characterized by widespread loss of connective tissue tensile strength, hernias, aneurysms, and skeletal deformities. Prolapse of male genital organs has even been reported in BAPN-treated animals [12, 13], but there are no available studies examining the effects of BAPN on prolapse in females or wound healing in the female genital tract. Interestingly, tissue abnormalities in response to BAPN appear to be more pronounced in areas of stress or foci of anatomic weakness [12]. Thus, BAPN is an attractive tool to study the role of LOX enzymes in both prolapse and wound healing.
In our study, significant prolapse was not detected in response to surgical wounding in BAPN-treated animals. Although genetically altered mice with defects in proteins involved in elastogenesis develop pelvic organ prolapse [26], animals used in this study were genetically normal without preexisting connective tissue defects. Thus, impaired LOX activity in adults does not appear to be primarily involved in the pathogenesis of prolapse at least during short-term observations. Our biomechanical testing results indicate that E2 restores maximal force to near normal in BAPN-treated animals, suggesting that E2 may be able to overcome the action of BAPN. There is scant data in the literature examining the effects of both BAPN and E2 together, but our findings agree with one of the only studies available, demonstrating that estrogen therapy reduced the effect of BAPN on collagen and tensile properties of skin and the bony architecture [38].
Strengths and Limitations
The strengths of the present study include the ability to study the wound-healing process on an accelerated time scale through use of an animal model [39]. With the guinea pig model, we were able to standardize the type, size, shape, and depth of the wound [39], which would otherwise be difficult in humans. The vaginal wall of the guinea pig is muscular, expands at the vaginal fornices, and is large enough to conduct vaginal surgical procedures. Although the rabbit has been used as an animal model for vaginal surgery [7, 37, 40], it has a unique structure of striated muscle underlying the vaginal introitus that may be problematic for vaginal colpoperineorrhaphy used herein. In contrast to other studies on vaginal wall wounding, we tried to reproduce the real life situation as closely as possible by using a menopausal model and closing the surgical wounds by primary intention. In addition, our study design allowed us to examine multiple steps in the collagen biosynthetic pathway and make multiple comparisons between nonestrogenized and estrogenized animals. Further, use of BAPN, an inhibitor of collagen cross-linking, allowed us to confirm the results of our study and gain insights into mechanisms by which estrogen regulates wound healing in the vaginal wall.
Although a pure unoperated control or sham-operated control group was not used in this study, each animal was used as its own internal control by harvesting noninjured vaginal tissue from each animal for analysis. Wound healing was not studied beyond 21 days. Some investigators have suggested that maximum tensile strength and collagen content is not attained until the scar is fully mature, which may take months [35]. However, our findings suggest that the ultimate effect of estrogen on strength and collagen content may be underestimated. BAPN-induced inhibition of collagen accumulation after injury ensures that animals were receiving the chemical, but the dose and regularity of the drug are unknown (daily water intake was recorded to ensure ingestion of minimal amounts). Daily systemic injections and gavage are cumbersome, not feasible, and may introduce unnecessary stress to the animals.
Summary and Working Model
Wound healing in the remodeling phase is an orchestrated event involving a delicate balance between degradative and synthetic processes. Our results suggest that estrogen may act on multiple steps in the wound-healing process at the molecular level. In the absence of injury, E2-induced growth of the vagina with increases in both epithelial and muscularis layers. Estradiol also induced 5- to 10-fold increases in collagen gene expression. Simultaneous 2-fold increases in LOX gene expression ensured cross-linking of immature collagen precursors in the extracellular space, thereby contributing to increases in tissue strength.
In response to surgical insult, a similar growth is noted in the reproductive organs. However, E2 induced prolonged and marked (10- to 20-fold) increases in collagen, tropoelastin, and LOX gene expression, leading to enhanced collagen biosynthesis, increases in the amount of mature collagen that contribute to ultimate strength, and increased density of elastic fibers in the subepithelium. In addition, E2 appears to counteract the effects of the LOX inhibitor BAPN to enhance wound healing, either through gene upregulation and increased enzyme production or by direct competitive inhibition, or both. The data presented herein are compatible with this model. Because systemic E2 is associated with significant side-effects, further studies are warranted regarding treatment regimens that may enhance estrogen-signaling pathways in the vaginal wall without systemic hormonal therapy.
Supplementary Material
Footnotes
Supported by NIH AG028048 and HD064824. Presented in part at the 2011 annual meeting of the American Urogynecologic Society.
REFERENCES
- Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol 2002; 186: 1160–1166. [DOI] [PubMed] [Google Scholar]
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 1997; 89: 501–506. [DOI] [PubMed] [Google Scholar]
- Luber KM, Boero S, Choe JY. The demographics of pelvic floor disorders: current observations and future projections. Am J Obstet Gynecol 2001; 184: 1496–1501. discussion 1501–1503. [DOI] [PubMed] [Google Scholar]
- Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol 2003; 188: 108–115. [DOI] [PubMed] [Google Scholar]
- Schaffer JI, Wai CY, Boreham MK. Etiology of pelvic organ prolapse. Clin Obstet Gynecol 2005; 48: 639–647. [DOI] [PubMed] [Google Scholar]
- Nygaard I, Bradley C, Brandt D. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol 2004; 104: 489–497. [DOI] [PubMed] [Google Scholar]
- Abramov Y, Webb AR, Botros SM, Goldberg RP, Ameer GA, Sand PK. Effect of bilateral oophorectomy on wound healing of the rabbit vagina. Fertil Steril 2011; 95: 1467–1470. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol 1999; 155: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA. Regulation of fibroplasia in cutaneous wound repair. Am J Med Sci 1993; 306: 42–48. [DOI] [PubMed] [Google Scholar]
- Bulut T, Bilsel Y, Yanar H, Yamaner S, Balik E, Solakoglu S, Koser M. The effects of beta-aminopropionitrile on colonic anastomosis in rats. J Invest Surg 2004; 17: 211–219. [DOI] [PubMed] [Google Scholar]
- Norton PA. Pelvic floor disorders: the role of fascia and ligaments. Clin Obstet Gynecol 1993; 36: 926–938. [DOI] [PubMed] [Google Scholar]
- Lalich JJ, Angevine DM. Dysostosis in adult rats after prolonged beta-aminopropionitrile feeding. Arch Pathol 1970; 90: 22–28. [PubMed] [Google Scholar]
- Lalich JJ, Paik WC. Influence of cystine supplements on the incidence of aortic rupture in rats fed beta-aminopropionitrile. Proc Soc Exp Biol Med 1968; 127: 543–546. [DOI] [PubMed] [Google Scholar]
- Moalli PA, Talarico LC, Sung VW, Klingensmith WL, Shand SH, Meyn LA, Watkins SC. Impact of menopause on collagen subtypes in the arcus tendineous fasciae pelvis. Am J Obstet Gynecol 2004; 190: 620–627. [DOI] [PubMed] [Google Scholar]
- Baron YM, Galea R, Brincat M. Carotid artery wall changes in estrogen-treated and -untreated postmenopausal women. Obstet Gynecol 1998; 91: 982–986. [DOI] [PubMed] [Google Scholar]
- Brincat M, Versi E, Moniz CF, Magos A, de Trafford J, Studd JW. Skin collagen changes in postmenopausal women receiving different regimens of estrogen therapy. Obstet Gynecol 1987; 70: 123–127. [PubMed] [Google Scholar]
- Helvering LM, Adrian MD, Geiser AG, Estrem ST, Wei T, Huang S, Chen P, Dow ER, Calley JN, Dodge JA, Grese TA, Jones SA, et al. Differential effects of estrogen and raloxifene on messenger RNA and matrix metalloproteinase 2 activity in the rat uterus. Biol Reprod 2005; 72: 830–841. [DOI] [PubMed] [Google Scholar]
- Ren J, Hintz KK, Roughead ZK, Duan J, Colligan PB, Ren BH, Lee KJ, Zeng H. Impact of estrogen replacement on ventricular myocyte contractile function and protein kinase B/Akt activation. Am J Physiol Heart Circ Physiol 2003; 284: H1800–H1807. [DOI] [PubMed] [Google Scholar]
- Sarvari M, Kallo I, Hrabovszky E, Solymosi N, Toth K, Liko I, Molnar B, Tihanyi K, Liposits Z. Estradiol replacement alters expression of genes related to neurotransmission and immune surveillance in the frontal cortex of middle-aged, ovariectomized rats. Endocrinology 2010; 151: 3847–3862. [DOI] [PubMed] [Google Scholar]
- Li J, McMurray RW. Effects of cyclic versus sustained estrogen administration on peripheral immune functions in ovariectomized mice. Am J Reprod Immunol 2010; 63: 274–281. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jin X, Zeng Z, Liu W, Wang B, Wang H. Estrogen-replacement therapy promotes angiogenesis after acute myocardial infarction by enhancing SDF-1 and estrogen receptor expression. Microvasc Res 2009; 77: 71–77. [DOI] [PubMed] [Google Scholar]
- Mannino CA, South SM, Inturrisi CE, Quinones-Jenab V. Pharmacokinetics and effects of 17beta-estradiol and progesterone implants in ovariectomized rats. J Pain 2005; 6: 809–816. [DOI] [PubMed] [Google Scholar]
- Yeh JK, Evans JF, Chen MM, Aloia JF. Effect of hypophysectomy on the proliferation and differentiation of rat bone marrow stromal cells. Am J Physiol 1999; 276: E34–E42. [DOI] [PubMed] [Google Scholar]
- Budatha M, Roshanravan S, Zheng Q, Weislander C, Chapman SL, Davis EC, Starcher B, Word RA, Yanagisawa H. Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans. J Clin Invest 2011; 121: 2048–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EJ, Rhodes RK. Preparation and characterization of the different types of collagen. Methods Enzymol 1982; 82: 33–64. [DOI] [PubMed] [Google Scholar]
- Drewes PG, Yanagisawa H, Starcher B, Hornstra I, Csiszar K, Marinis SI, Keller P, Word RA. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am J Pathol 2007; 170: 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Lee DC, Yang SJ, Lee JJ, Bae EM, Kim DM, Min SH, Kim SJ, Kang DC, Sang BC, Myung PK, Park KC, et al. Lysyl oxidase like 4, a novel target gene of TGF-beta1 signaling, can negatively regulate TGF-beta1-induced cell motility in PLC/PRF/5 hepatoma cells. Biochem Biophys Res Commun 2008; 373 (4): 521–527. [DOI] [PubMed] [Google Scholar]
- DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol 2007; 109: 295–302. [DOI] [PubMed] [Google Scholar]
- Taylor FW, Dittmer TL, Porter DO. Wound healing and the steroids. Surgery 1952; 31: 683–690. [PubMed] [Google Scholar]
- Nyman S, Lindhe J, Zederfeldt B. Influence of estrogen and progesterone on tissue regeneration in oophorectomized rabbits. Acta Chir Scand 1971; 137: 131–139. [PubMed] [Google Scholar]
- Nyman S, Lindhe J, Zederfeldt B. The vascularity of wounded areas in estradiol and progesterone treated female rabbits. A microangiographic study. Acta Chir Scand 1971; 137: 631–637. [PubMed] [Google Scholar]
- Nyman S, Lindhe J, Zederfeldt B, Solvell L. Vascular permeability in wounded areas of estradiol and progesterone treated female rabbits. Acta Chir Scand 1971; 137: 709–714. [PubMed] [Google Scholar]
- Pallin B, Ahonen J, Zederfeldt B. Granulation tissue formation in oophorectomized rats treated with female sex hormones. II. Studies on the amount of collagen and on tensile strength. Acta Chir Scand 1975; 141: 710–714. [PubMed] [Google Scholar]
- Jorgensen O, Schmidt A. Influence of sex hormones on granulation tissue formation and on healing of linear wounds. Acta Chir Scand 1962; 124: 1–10. [PubMed] [Google Scholar]
- Schlaff WD, Cooley BC, Shen W, Gittlesohn AM, Rock JA. A rat uterine horn model of genital tract wound healing. Fertil Steril 1987; 48: 866–872. [DOI] [PubMed] [Google Scholar]
- Oestrogens Calvin M. and wound healing. Maturitas 2000; 34: 195–210. [DOI] [PubMed] [Google Scholar]
- Higgins EW, Rao A, Baumann SS, James RL, Kuehl TJ, Muir TW, Pierce LM. Effect of estrogen replacement on the histologic response to polypropylene mesh implanted in the rabbit vagina model. Am J Obstet Gynecol 2009; 201 (505): e501–e509. [DOI] [PubMed] [Google Scholar]
- Henneman DH. Inhibition by estradiol-17 beta of the lathyritic effect of beta-aminopropionitrile (BAPN) on skin and bone collagen. Clin Orthop Relat Res 1972; 83: 245–254. [DOI] [PubMed] [Google Scholar]
- Sartori-Valinotti JC, Iliescu R, Yanes LL, Dorsett-Martin W, Reckelhoff JF. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension 2008; 51: 1170–1176. [DOI] [PubMed] [Google Scholar]
- Abramov Y, Golden B, Sullivan M, Botros SM, Miller JJ, Alshahrour A, Goldberg RP, Sand PK. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen 2007; 15: 80–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


